Advancing Antimony(III) Adsorption: Impact of Varied Manganese Oxide Modifications on Iron–Graphene Oxide–Chitosan Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of FM @ GC Composite Materials
2.3. Batch Adsorption Experiment
2.4. Analytical Techniques
3. Results and Discussion
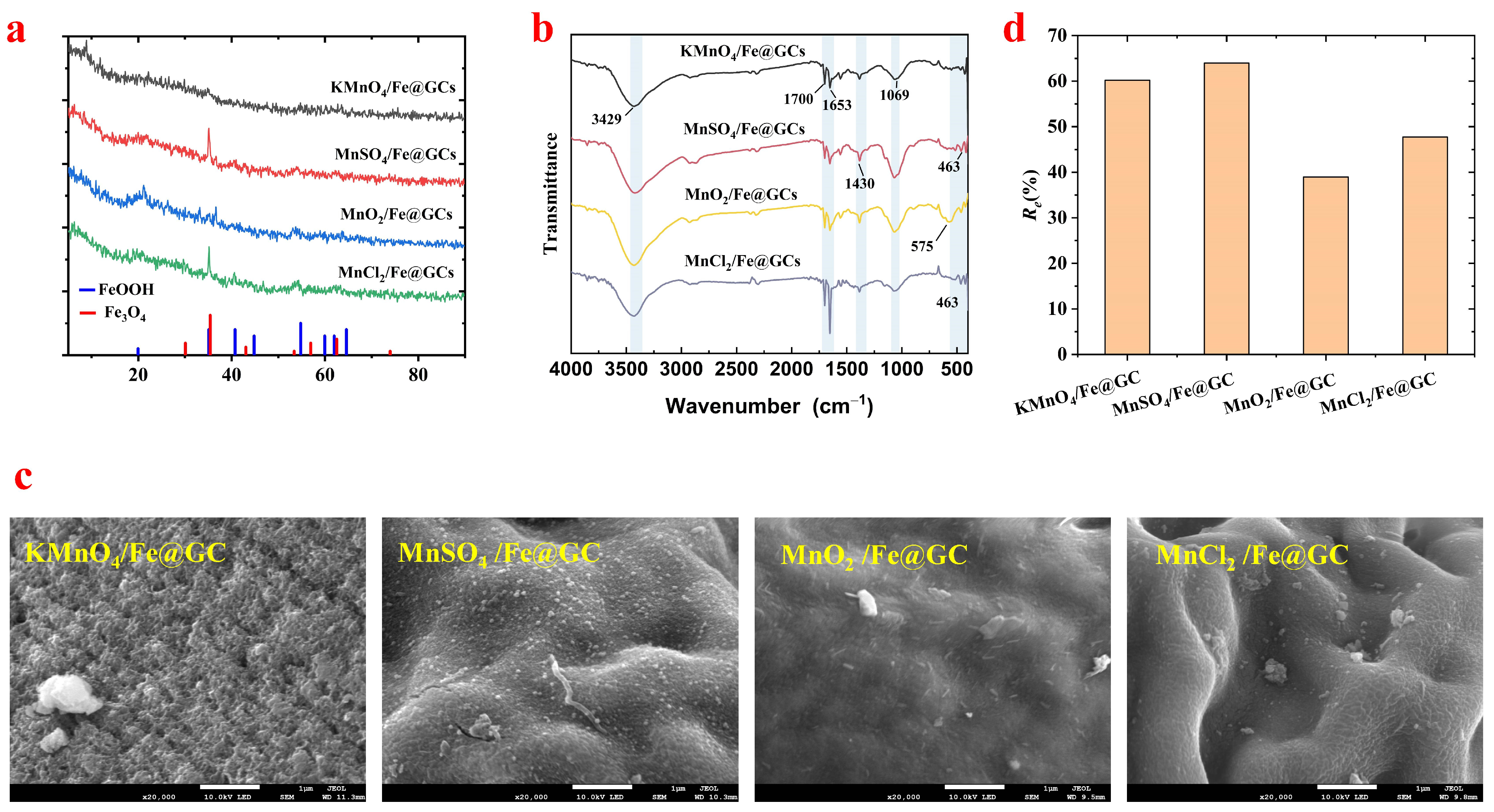
3.1. Characterization
3.1.1. XRD Analysis
3.1.2. FTIR Analysis
3.1.3. SEM Analysis
3.1.4. Adsorption Capacity Analysis
3.2. Influencing Factors
3.2.1. Influence of pH Value
3.2.2. Influence of Mass-to-Volume Ratio
3.2.3. Influence of Initial Solution Concentration
3.2.4. Influence of Adsorption Time
3.2.5. Influence of Coexisting Ions
3.3. Adsorption Characteristics
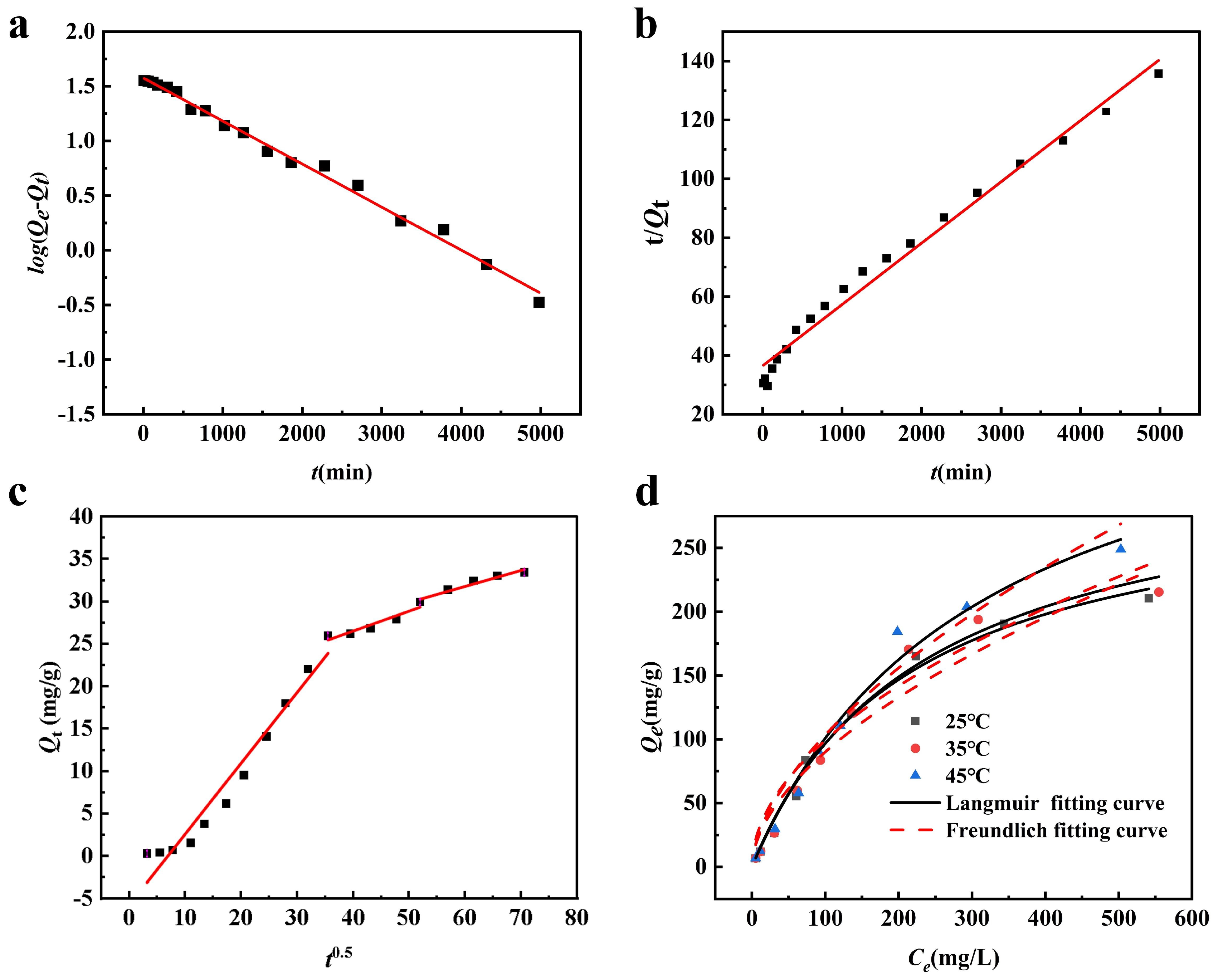
3.3.1. Adsorption Kinetic
3.3.2. Isothermal Adsorption
3.4. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, M.; Gu, Z.; Minale, M.; Xia, S.; Zhao, J.; Wang, X. Simultaneous Adsorption and Oxidation of Sb(III) from Water by the pH-Sensitive Superabsorbent Polymer Hydrogel Incorporated with Fe-Mn Binary Oxides Composite. J. Hazard. Mater. 2022, 423, 127013. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Liu, R.; Zhao, X.; Qu, J. The Mechanism of Antimony(III) Removal and Its Reactions on the Surfaces of Fe–Mn Binary Oxide. J. Colloid Interface Sci. 2011, 363, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, Z.; Chen, Z.; Weng, X.; Owens, G.; Chen, Z. Simultaneous Removal of Sb(III) and Sb(V) from Mining Wastewater by Reduced Graphene Oxide/Bimetallic Nanoparticles. Sci. Total Environ. 2022, 836, 155704. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Luo, X.; Crittenden, J.; Qu, J.; Bai, Y.; Peng, Y.; Li, J. Removal of Antimonite (Sb(III)) and Antimonate (Sb(V)) from Aqueous Solution Using Carbon Nanofibers That Are Decorated with Zirconium Oxide (ZrO2). Environ. Sci. Technol. 2015, 49, 11115–11124. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, H.; Chai, L.-Y.; Mirza, N.; Yang, Z.-H.; Pervez, A.; Tariq, M.; Shaheen, S.; Mahmood, Q. Antimony (Sb)—Pollution and Removal Techniques—Critical Assessment of Technologies. Toxicol. Environ. Chem. 2015, 97, 1296–1318. [Google Scholar] [CrossRef]
- Li, X.; Dou, X.; Li, J. Antimony(V) Removal from Water by Iron-Zirconium Bimetal Oxide: Performance and Mechanism. J. Environ. Sci. 2012, 24, 1197–1203. [Google Scholar] [CrossRef]
- Bulin, C.; Li, B.; Zhang, Y.; Zhang, B. Removal Performance and Mechanism of Nano α-Fe2O3/Graphene Oxide on Aqueous Cr(VI). J. Phys. Chem. Solids 2020, 147, 109659. [Google Scholar] [CrossRef]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Buthiyappan, A. Adsorption of Arsenic Using Chitosan Magnetic Graphene Oxide Nanocomposite. J. Environ. Manag. 2019, 246, 547–556. [Google Scholar] [CrossRef]
- Xiong, N.; Wan, P.; Zhu, G.; Xie, F.; Xu, S.; Zhu, C.; Hursthouse, A.S. Sb(III) Removal from Aqueous Solution by a Novel Nano-Modified Chitosan (NMCS). Sep. Purif. Technol. 2020, 236, 116266. [Google Scholar] [CrossRef]
- Shan, H.; Mo, H.; Liu, Y.; Zeng, C.; Peng, S.; Zhan, H. As(III) Removal by a Recyclable Granular Adsorbent through Dopping Fe-Mn Binary Oxides into Graphene Oxide Chitosan. Int. J. Biol. Macromol. 2023, 237, 124184. [Google Scholar] [CrossRef]
- Cao, D.; Zeng, H.; Yang, B.; Zhao, X. Mn Assisted Electrochemical Generation of Two-Dimensional Fe-Mn Layered Double Hydroxides for Efficient Sb(V) Removal. J. Hazard. Mater. 2017, 336, 33–40. [Google Scholar] [CrossRef]
- Kong, S.; Wang, Y.; Hu, Q.; Olusegun, A.K. Magnetic Nanoscale Fe–Mn Binary Oxides Loaded Zeolite for Arsenic Removal from Synthetic Groundwater. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 220–227. [Google Scholar] [CrossRef]
- Luo, J.; Hu, C.; Meng, X.; Crittenden, J.; Qu, J.; Peng, P. Antimony Removal from Aqueous Solution Using Novel α-MnO2 Nanofibers: Equilibrium, Kinetic, and Density Functional Theory Studies. ACS Sustain. Chem. Eng. 2017, 5, 2255–2264. [Google Scholar] [CrossRef]
- Yang, C.; Pang, Y.; Han, Y.; Zhan, X.; Wang, H.; Liu, J.; Gao, R.; Liu, H.; Shi, H. Removal of Trace Concentration Sb(V) in Textile Wastewater by Mn-Doped Fe3O4: The Mechanisms of Mn Affect Adsorption Performance. Microporous Mesoporous Mater. 2022, 343, 112150. [Google Scholar] [CrossRef]
- Zeng, J.; Qi, P.; Shi, J.; Pichler, T.; Wang, F.; Wang, Y.; Sui, K. Chitosan Functionalized Iron Nanosheet for Enhanced Removal of As(III) and Sb(III): Synergistic Effect and Mechanism. Chem. Eng. J. 2020, 382, 122999. [Google Scholar] [CrossRef]
- Liu, Y.; Shan, H.; Pang, Y.; Zhan, H.; Zeng, C. Iron Modified Chitosan/Coconut Shell Activated Carbon Composite Beads for Cr(VI) Removal from Aqueous Solution. Int. J. Biol. Macromol. 2023, 224, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.L.; Hameed, B.H. Insight into the Adsorption Kinetics Models for the Removal of Contaminants from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Zheng, Q.; Hou, J.; Hartley, W.; Ren, L.; Wang, M.; Tu, S.; Tan, W. As(III) Adsorption on Fe-Mn Binary Oxides: Are Fe and Mn Oxides Synergistic or Antagonistic for Arsenic Removal? Chem. Eng. J. 2020, 389, 124470. [Google Scholar] [CrossRef]
- Hasan, S.; Ghosh, A.; Race, K.; Schreiber, R.; Prelas, M. Dispersion of FeOOH on Chitosan Matrix for Simultaneous Removal of As(III) and As(V) from Drinking Water. Sep. Sci. Technol. 2014, 49, 2863–2877. [Google Scholar] [CrossRef]
- Yoon, Y.; Park, W.K.; Hwang, T.-M.; Yoon, D.H.; Yang, W.S.; Kang, J.-W. Comparative Evaluation of Magnetite–Graphene Oxide and Magnetite-Reduced Graphene Oxide Composite for As(III) and As(V) Removal. J. Hazard. Mater. 2016, 304, 196–204. [Google Scholar] [CrossRef]
- Xu, R.; Li, Q.; Nan, X.; Jiang, G.; Wang, L.; Xiong, J.; Yang, Y.; Xu, B.; Jiang, T. Simultaneous Removal of Antimony(III/V) and Arsenic(III/V) from Aqueous Solution by Bacteria–Mediated kaolin@Fe–Mn Binary (Hydr)Oxides Composites. Appl. Clay Sci. 2022, 217, 106392. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, Q.; Huang, X.; Li, X.; Wang, Y.; Liu, W.; Lin, Z. The High Efficient Sb(III) Removal by Cauliflower like Amorphous Nanoscale Zero-Valent Iron (A-nZVI). J. Hazard. Mater. 2022, 436, 129056. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Lin, Y.; Yu, P.; Luo, Y. Study of Aniline/ε-Caprolactam Mixture Adsorption from Aqueous Solution onto Granular Activated Carbon: Kinetics and Equilibrium. Chem. Eng. J. 2012, 187, 69–78. [Google Scholar] [CrossRef]
- Cheng, M.; Fang, Y.; Li, H.; Yang, Z. Review of Recently Used Adsorbents for Antimony Removal from Contaminated Water. Environ. Sci. Pollut. Res. 2022, 29, 26021–26044. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Cheng, J.; Cao, W.; Yang, C.; Chen, J.; Luo, Z. Removal of Heavy Metals from Aqueous Solution Using Chitosan-Combined Magnetic Biochars. J. Colloid Interface Sci. 2019, 540, 579–584. [Google Scholar] [CrossRef]
- Shan, H.; Zeng, C.; Zhao, C.; Zhan, H. Iron Oxides Decorated Graphene Oxide/Chitosan Composite Beads for Enhanced Cr(VI) Removal from Aqueous Solution. Int. J. Biol. Macromol. 2021, 172, 197–209. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, R.; Ren, B.; Hou, B.; Hursthouse, A. Preparation of a Novel Fe3O4/HCO Composite Adsorbent and the Mechanism for the Removal of Antimony (III) from Aqueous Solution. Sci. Rep. 2019, 9, 13021. [Google Scholar] [CrossRef]
- Li, M.; Kuang, S.; Kang, Y.; Ma, H.; Dong, J.; Guo, Z. Recent Advances in Application of Iron-Manganese Oxide Nanomaterials for Removal of Heavy Metals in the Aquatic Environment. Sci. Total Environ. 2022, 819, 153157. [Google Scholar] [CrossRef]
- Deng, S.; Ren, B.; Hou, B.; Deng, X.; Deng, R.; Zhu, G.; Cheng, S. Adsorption of Sb(III) and Pb(II) in Wastewater by Magnetic γ-Fe2O3-Loaded Sludge Biochar: Performance and Mechanisms. Chemosphere 2024, 349, 140914. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of Tetracycline by NaOH-Activated Carbon Produced from Macadamia Nut Shells: Kinetic and Equilibrium Studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Li, X.; He, K.; Pan, B.; Zhang, S.; Lu, L.; Zhang, W. Efficient As(III) Removal by Macroporous Anion Exchanger-Supported Fe–Mn Binary Oxide: Behavior and Mechanism. Chem. Eng. J. 2012, 193–194, 131–138. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.-J. Chitosan-Functionalized Graphene Oxide: A Novel Adsorbent an Efficient Adsorption of Arsenic from Aqueous Solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Bulin, C. Combination Mechanism of the Ternary Composite Based on Fe3O4-Chitosan-Graphene Oxide Prepared by Solvothermal Method. Int. J. Biol. Macromol. 2023, 231, 123337. [Google Scholar] [CrossRef]
- Simić, M.; Petrović, J.; Šoštarić, T.; Ercegović, M.; Milojković, J.; Lopičić, Z.; Kojić, M. A Mechanism Assessment and Differences of Cadmium Adsorption on Raw and Alkali-Modified Agricultural Waste. Processes 2022, 10, 1957. [Google Scholar] [CrossRef]
- Shan, H.; Peng, S.; Zhao, C.; Zhan, H.; Zeng, C. Highly Efficient Removal of As(III) from Aqueous Solutions Using Goethite/Graphene Oxide/Chitosan Nanocomposite. Int. J. Biol. Macromol. 2020, 164, 13–26. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, J.; Lou, Z.; Zhou, X.; Liu, Y.; Li, Y.; Ali Baig, S.; Xu, X. Removal of Sb(V) from Aqueous Solutions Using Fe-Mn Binary Oxides: The Influence of Iron Oxides Forms and the Role of Manganese Oxides. Chem. Eng. J. 2018, 354, 577–588. [Google Scholar] [CrossRef]
- Zhang, C.; He, M.; Ouyang, W.; Lin, C.; Liu, X. Influence of Fe(II) on Sb(III) Oxidation and Adsorption by MnO2 under Acidic Conditions. Sci. Total Environ. 2020, 724, 138209. [Google Scholar] [CrossRef]
- Sheshmani, S.; Akhundi Nematzadeh, M.; Shokrollahzadeh, S.; Ashori, A. Preparation of Graphene Oxide/Chitosan/FeOOH Nanocomposite for the Removal of Pb(II) from Aqueous Solution. Int. J. Biol. Macromol. 2015, 80, 475–480. [Google Scholar] [CrossRef]
- Min, X.; Guo, M.; Li, K.; Gu, J.; Guo, X.; Xue, Y.; Liang, J.; Hu, S.; Jia, J.; Sun, T. Enhancement of Toluene Removal over A@δ-MnO2 Composites Prepared via One-Pot by Modifying the Molar Ratio of KMnO4 to MnSO4·H2O. Appl. Surf. Sci. 2021, 568, 150972. [Google Scholar] [CrossRef]
- Qu, J.; Shi, L.; He, C.; Gao, F.; Li, B.; Zhou, Q.; Hu, H.; Shao, G.; Wang, X.; Qiu, J. Highly Efficient Synthesis of Graphene/MnO2 Hybrids and Their Application for Ultrafast Oxidative Decomposition of Methylene Blue. Carbon 2014, 66, 485–492. [Google Scholar] [CrossRef]


| Temperature (°C) | Pseudo First Order | Pseudo Second Order | ||||
|---|---|---|---|---|---|---|
| Qe (mg/g) | k1 | R2 | Qe (mg/g) | k2 | R2 | |
| 25 | 1.58 | −0.00039 | 0.9817 | 36.48 | 0.020 | 0.9923 |
| Temperature (°C) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| Qe (mg/g) | KL | R2 | KF | 1/n | R2 | |
| 25 | 178.89 | 0.0335 | 0.8430 | 24.55 | 0.34 | 0.9306 |
| 35 | 214.09 | 0.0223 | 0.8205 | 21.91 | 0.38 | 0.9139 |
| 45 | 226.35 | 0.0238 | 0.8293 | 24.58 | 0.37 | 0.9328 |
| Elements | C | Fe | O | Mn | Sb |
|---|---|---|---|---|---|
| Before adsorption | 22.82 | 34.99 | 39.99 | 0.48 | - |
| After adsorption | 18.36 | 55.60 | 19.35 | 1.19 | 5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, H.; Shan, H.; Xu, Y.; Liao, H.; Peng, S. Advancing Antimony(III) Adsorption: Impact of Varied Manganese Oxide Modifications on Iron–Graphene Oxide–Chitosan Composites. Molecules 2024, 29, 4021. https://doi.org/10.3390/molecules29174021
Mo H, Shan H, Xu Y, Liao H, Peng S. Advancing Antimony(III) Adsorption: Impact of Varied Manganese Oxide Modifications on Iron–Graphene Oxide–Chitosan Composites. Molecules. 2024; 29(17):4021. https://doi.org/10.3390/molecules29174021
Chicago/Turabian StyleMo, Huinan, Huimei Shan, Yuqiao Xu, Haimin Liao, and Sanxi Peng. 2024. "Advancing Antimony(III) Adsorption: Impact of Varied Manganese Oxide Modifications on Iron–Graphene Oxide–Chitosan Composites" Molecules 29, no. 17: 4021. https://doi.org/10.3390/molecules29174021
APA StyleMo, H., Shan, H., Xu, Y., Liao, H., & Peng, S. (2024). Advancing Antimony(III) Adsorption: Impact of Varied Manganese Oxide Modifications on Iron–Graphene Oxide–Chitosan Composites. Molecules, 29(17), 4021. https://doi.org/10.3390/molecules29174021






