Effects of Differently Processed Tea on the Gut Microbiota
Abstract
1. Introduction
2. Tea
2.1. Bioactive Compounds in Tea
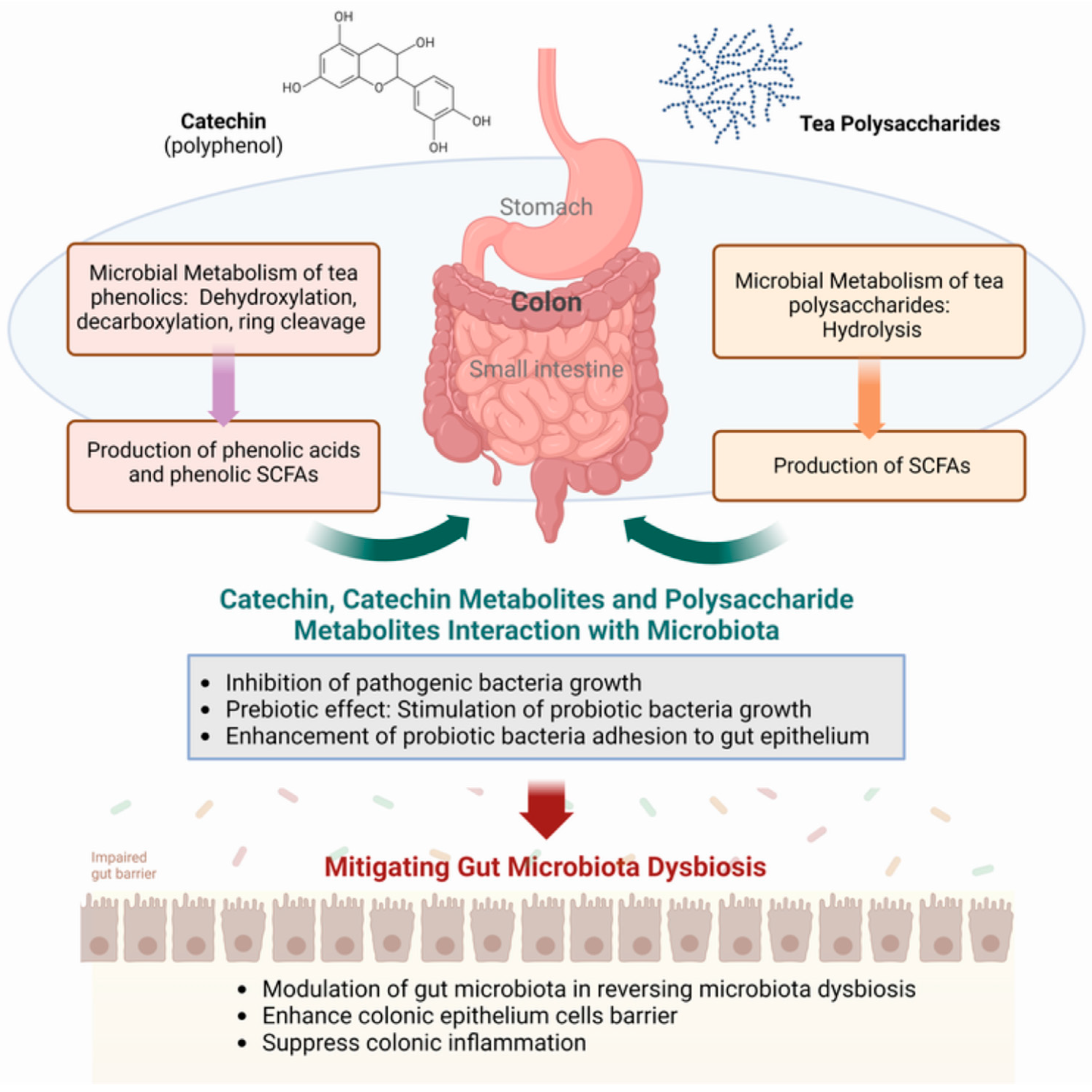
2.2. Biological Transformation and Utilization of Tea Polyphenols and Polysaccharides
3. Human Gut Microbiota
3.1. Diversity of Intestinal Flora
3.2. Effect of Tea Polyphenols and Polysaccharides on Intestinal Microbiota
3.3. Metabolic Influence of the Gut Microbiota
4. Interaction of Tea Polyphenols with the Gut Microbiota
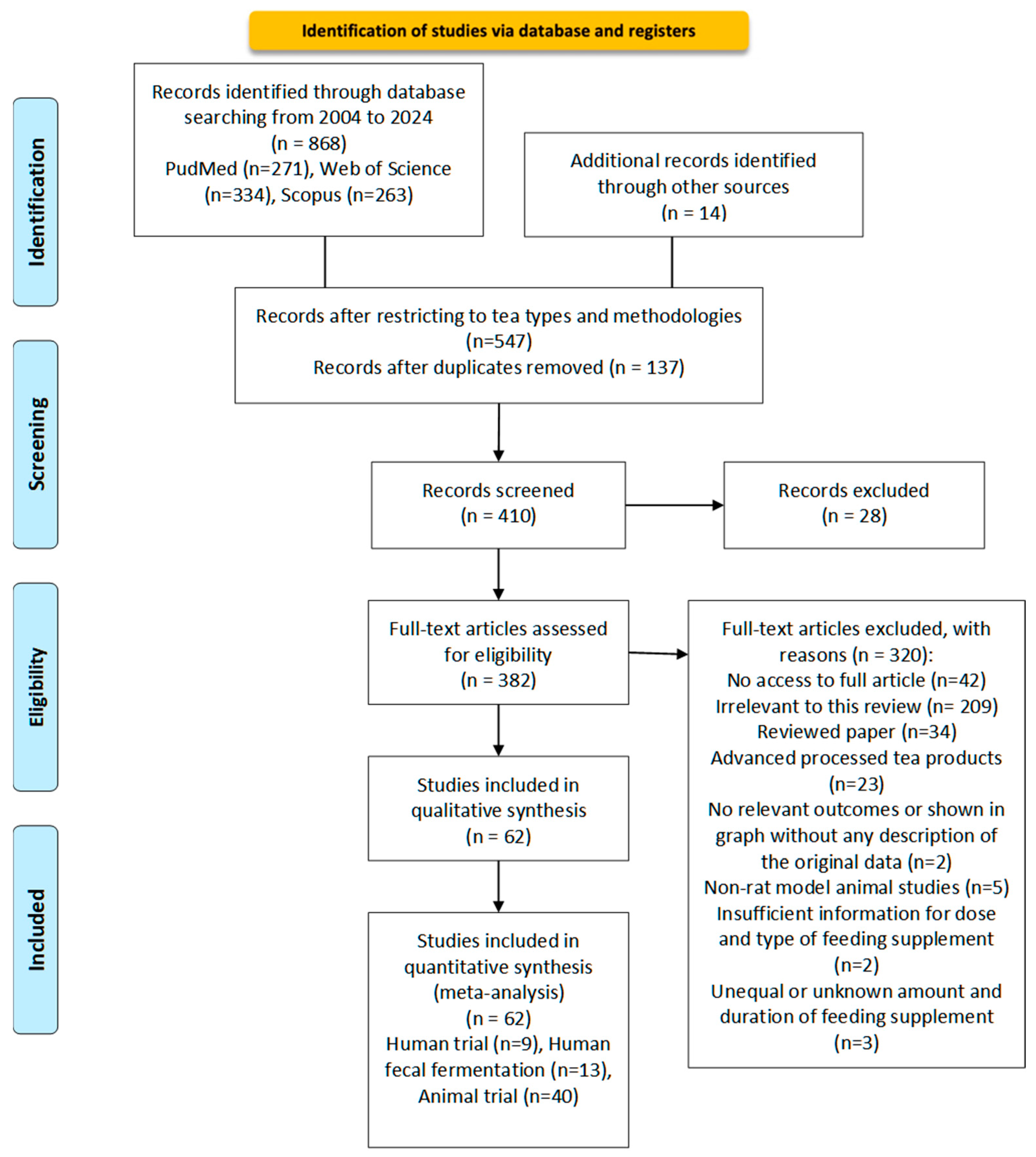
4.1. Literature Search Method
4.1.1. Search Strategy
4.1.2. Data Collection and Sorting
4.1.3. Eligibility Criteria
4.1.4. Data Extraction
4.1.5. Data Measures and Analysis
4.2. Rodent Feeding Trials
| Tea Type | Reference | Subjects | Treatment |
|---|---|---|---|
| Green Tea | [102] | Female ovariectomized Sprague-Dawley rats (six months old, Harlan Laboratories, Indianapolis, IN) | 0.5%, or 1.5% of (g/mL) green tea polyphenol extracts (decaffeinated) each day |
| [106] | Specific pathogen-free C57BL/6J mice | 0.05%, 0.2% and 0.8% (w/w) green tea polyphenols (commercial), added to the drinking water each day | |
| [127] | C57BL/6J mice | Decaffeinated 0.25% (g/g) Green tea polyphenol (ethanol extracts) added in diet every day | |
| [105] | Seven-week-old male C57/BL6 mice | 400 mg/kg green tea extracts (50% ethanol extracts dissolved in water) every day | |
| [136] | Five-week-old female db/db and wild-type mice | AIN-93M diet with 1% and 2% (w/w) dried green tea water extracts and green tea leaf powder (pulverized tea leaves) every day | |
| [137] | Male C57BL/6J mice (n = 50; 5 weeks old) | 2% (w/w) purified green tea extracts, 0.3% (w/w) EGCG (HF + EGCG), or 0.3% (w/w) CAT (HF + CAT)) every day | |
| [138] | Female albino hairless mice (Skh:HR-1, 6–8 weeks old) | 1% (w/w) green tea extracts (commercial) in diet each day | |
| [139] | C57BL/6 female mice (20 ± 2 g, 7–8 weeks) | 5 mg green tea water extract powder/kg bodyweight each day | |
| [140] | Germ-free C57BL/6J mice (6 weeks old) | 0.1% (w/w) Green Tea Polyphenols (water extracts) each day | |
| Oolong Tea | [114] | Young adult (6-week-old) male C57BL/6J mice | 0.1% (w/w) purified Oolong Tea Polyphenols (water extracts) each day |
| [115] | young adult (6-week-old) male C57BL/6J mice | 2% (w/v) purified Oolong tea polyphenols (water extracts) each day | |
| [121] | The 8-week-old C57BL/6J male mice | 200 mg/kg body weight Black tea powder (water extracts, concentrated, and dried) each day | |
| [112] | Sterile male mice C57BL/6J | 0.1% (w/w) Oolong tea polyphenols (water extracts) each day | |
| [110] | Six-week-old male C57BL/6J mice (6–8 weeks old, 23 ± 2 g) | 200 mg/kg body weight oolong tea polyphenols (purified) each day | |
| [116] | 6-week-old male C57BL/6J mice | 0.1% (w/w) oolong tea ground powder and purified EGCG3″Me each day | |
| [118] | 6-week-old male C57BL/6J mice | 0.1% w/w purified oolong tea polyphenols (water extracts) each day | |
| [117] | 6-week-old male C57BL/6J mice | 0.1% (w/w) oolong tea ground powder and purified EGCG3″Me each day | |
| [119] | Twenty-four 8-week-old cleaning Wistar male rats | 500 mg/kg oolong tea water extract added to drinking water each day | |
| [120] | Seventy-two male specific-pathogen-free grade Kunming mice, with a weight of 35–40 g at 6 weeks of age | 800 mg/kg·d oolong tea water extract, administered by gavage once a day for two consecutive weeks | |
| Black Tea | [126] | ICR mice, aged 8 weeks and weighing 22 ± 2 g (32M and 32F) | 25 mg/mL black tea water brewed each day |
| [121] | The 8-week-old C57BL/6J male mice | 200 mg/kg b.w. black tea extract powder (dried water extracts) each day | |
| [19] | The 8-week-old C57BL/6J male mice | 200 mg/kg b.w. black tea extract powder (dried water extracts) | |
| [125] | 6-week C57BL/6J male mice | 760 mg/Kg. Average energy intake. Purified black tea water extracts | |
| [127] | 48 male C57BL/6J mice at 6–7 weeks of age (body weight: 16–18 g) | 320 mg/kg body weight ethanol-water black tea extracts each day | |
| [124] | 48 C57BL/6 mice (male, 6 weeks) | 2.0% black tea ground powder [w/w] each day | |
| [123] | Male SD rats (6 weeks of age, n = 14) | 1.5 g/kg body weight black tea powder (suspended in distilled water) per day | |
| [120] | Seventy-two male specific-pathogen-free grade Kunming mice, with a weight of 35–40 g at 6 weeks of age | 800 mg/kg·d black tea water extracts (freeze-dried powder) | |
| [122] | Four-week-old male Wistar rats (n = 21) | 10 g/kg Black tea extracts (ethanol-aqueous freeze-dried powder) each day | |
| [128] | Eight-week-old male ICR mice weighing 35–40 g | 50 mg kg/1 Black tea theaflavins (commercial) every day | |
| Pu-erh tea | [133] | 3-week-old C57BL/6J male mice | 3 mg/mL instant Pu-erh tea (PT) water infusion (450 mg/kg/day) |
| [131] | 8-week-old male C57BL/6N mice | 0.4% and 1% (w/v) Ripened Pu-erh tea water extracts each day | |
| [132] | Male Wistar rats fed a high-fat and high-sugar diet (HFSD) to induce obesity | 0.15-g/kg and 0.4-g/kg body weight PTE (aqueous extracts) each day | |
| [141] | Male Sprague-Dawley rats (weighing 180–220 g) | Pu-erh tea water extracts 0.16 g/mL, 15 mL/kg/day | |
| [129] | 42 male C57BL/6 mice (6 weeks of age, body weight 20.4 ± 1.0 g) | 600 mg/kg/d and 300 mg/kg/d raw or ripened Pu-erh tea water extracts | |
| [135] | Six-week-old C57BL/6J male mice | Daily doses of 750 mg/kg of body weight Pu-erh tea (aqueous extraction) each day | |
| [134] | Female C57BL/6 mice (8 weeks old; 17–20 g) | 3%, 6% and 9% (w/w) Ripened Pu-erh tea water extracts each day | |
| [142] | 24 mice (12 males and 12 females) | 0.15 mL Pu-erh tea water extract each day | |
| [130] | Fifty C57BL/6J male mice | 400 mg/kg raw Pu-erh tea theabrownin (R-TB) or ripened Pu-erh tea theabrownin (F-TB) (purified) each day | |
| [143] | male C57BL/6J mice | 0.25% (w/v) Pu-erh tea (water extracts, freeze-dried powder) each day | |
| [144] | Pathogen-free C57BL/6 mice (8 weeks of age, weighing 20 ± 2 g) | 0.1 or 0.4% (1 or 4 g/L, w/v) Pu-erh tea freeze-dried water extracts each day |
4.3. Human Fecal Fermentation
| Tea Type | Reference | Subjects | Treatment |
|---|---|---|---|
| Green Tea | [145] | Fresh fecal samples were donated by three adults with metabolic syndrome (two males and one female) | 2% (w/v) GTE freeze-dried powder each day |
| [146] | Human Colon Microbiota (from human fecal samples of two healthy volunteers) | 0.67 mg/mL GTE freeze-dried powder (boiling water extracts) each day | |
| [147] | Fresh fecal collected from five healthy adults who had not received antibiotics or pre/probiotics in the preceding 3 months | 1% (w/v) freeze-dried Laphet (fermented green tea) ground powder each day | |
| [148] | Gut microbiota culture (Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, and Verrucomicrobia) | 0.5 mg/mL GTE dried green tea powder (70% ethanol extracts) each day | |
| [146] | Fecal materials from four healthy volunteers (three males and one female, 24–38 years) | 0.1 mmol/L catechin (EC, ECG, EGC, and EGCG) (purified) each day | |
| [155] | Fecal from ten healthy adult volunteers (six women and four men) aged between 33 and 70 years (average, 47 years) | Participants drank 1000 mL of green tea brew each day | |
| [150] | Fecal samples were obtained from 6 healthy volunteers (3 females and 3 males, aged 25–30 years) without recent antibiotic treatment or gastrointestinal disease | 1% (w/v) freeze-dried green tea polyphenols (purified) each day | |
| Oolong Tea | [150] | Fecal samples were obtained from 6 healthy volunteers (3 females and 3 males, aged 25–30 years) without recent antibiotic treatment or gastrointestinal disease | 1% (w/v) freeze-dried oolong tea polyphenols (purified) |
| [69] | Fecal samples were obtained from three healthy volunteers (one female and two males, ages 25–30) | 100 mg/L oolong tea EGCG, GCG, EGCG3”Me (purified) each day | |
| Black Tea | [150] | Fecal samples were obtained from 6 healthy volunteers (3 females and 3 males, aged 25–30 years) without recent antibiotic treatment or gastrointestinal disease | 1% (w/v) freeze-dried black tea polyphenols (purified) each day |
| [152] | Two healthy volunteers (one male and one female, aged 22–28 years) who had never had gastrointestinal disease and had not taken antibiotics in the past 3 months | 2% (w/v) black tea polyphenols (70% ethanol extracts) each day | |
| [151] | Identical fecal sample from a healthy human volunteer | 1000 mg black tea polyphenols each day | |
| [153] | Fresh human feces were collected from 8 healthy Chinese volunteers (18–24 years old; 4 males and 4 females) | 0.1 mg/mL black tea theaflavins (purified) each day |
4.4. Human Feeding Trials
| Tea Type | Reference | Subjects | Treatment |
|---|---|---|---|
| Green Tea | [157] | Fecal samples from adult volunteers aged between 27 and 46 years. (Healthy, normal weight (BMI 18–24 kg m2), or overweight/obese (BMI > 24 kg m2) | 400 g 7.5 g-Tea/L-Water green tea liquid each day |
| [163] | 58 Healthy Caucasians aged 18–50 years, either normal weight (BMI 18–25 kg/m2) or overweight/obese (BMI > 25 kg/m2), non-smokers, weight stable, and not on certain medications or specific medical conditions Clinical trial ID: NCT01556321 | GTE capsules (>0.56 g/day EGCG + 0.28 ∼ 0.45 g/day caffeine) | |
| [164] | 187 healthy postmenopausal women aged 50–60 years from the Minneapolis-St. Paul metropolitan area with a body mass index (BMI) between 19.3 and 36 kg/m2 and stable weight were enrolled | GTE Catechin Complex (Corban Complex GTB; Investigational New Drug #103,431) Intake: 1315 ± 115.0 mg catechins, including 843.0 ± 44.0 mg EGCG (capsules) each day | |
| [104] | A total of 85 participants (65.9% male; mean age: 43.3 years) without T2DM were included | The mean (SD) intakes of total green tea were 443 (417) mL/day, respectively. The median intakes of catechins and EGCG were 67.8 (23.2–150.1) and 9.5 (0.9–26.9) mg/day (green tea brew) | |
| [159] | 20 individuals with MetS and 20 age- and gender-matched healthy persons. Clinical trial ID: NCT03973996 | GTE (1g containing 890 mg of total catechins) confections each day | |
| Black Tea | [162] | Fecal from 72 Healthy males or females between 20 and 59 years of age. Clinical trial ID: UMIN000038168 | 3 cups of Black tea brew (polymerized polyphenols, 76.2 mg) each day |
| [161] | Fecal samples from a human volunteer | 1 cup of black tea brew (made from dry tea powder) each day (There is no prescribed time or dosage for drinking black tea) | |
| Oolong Tea | [160] | Fecal samples were obtained from 28 healthy adults, ranging in age from 20 to 50 years, whose weight had been stable for at least 3 years. Clinical trial ID: NKUIRB2022085 | 500 mL 5 mg/mL oolong tea water brew (Total phenolic content: 636.17 ± 8.54 μg GAE/mL) each day |
| Pu-erh tea | [165] | Thirteen healthy male volunteers, 24–32 years | 300 mL Pu-erh tea brew (powder) (50 mg/kg) each day |
5. Comparison among Different Teas in the Same Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, R.; Ho, C.; Zhang, X. Interaction between Tea Polyphenols and Intestinal Microbiota in Host Metabolic Diseases from the Perspective of the Gut–Brain Axis. Mol. Nutr. Food Res. 2020, 64, e2000187. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.-Y.; Meng, X.; Gan, R.-Y.; Zhao, C.-N.; Liu, Q.; Feng, Y.-B.; Li, S.; Wei, X.-L.; Atanasov, A.G.; Corke, H.; et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bruins, M.E.; Ni, L.; Vincken, J.-P. Green and Black Tea Phenolics: Bioavailability, Transformation by Colonic Microbiota, and Modulation of Colonic Microbiota. J. Agric. Food Chem. 2018, 66, 8469–8477. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. The Role of Tea in Human Health: An Update. J. Am. Coll. Nutr. 2002, 21, 1–13. [Google Scholar] [CrossRef]
- Zhou, B.; Ma, B.; Ma, C.; Xu, C.; Wang, J.; Wang, Z.; Yin, D.; Xia, T. Classification of Pu-erh ripened teas and their differences in chemical constituents and antioxidant capacity. LWT 2022, 153, 112370. [Google Scholar] [CrossRef]
- Piyasena, K.N.P.; Abayarathne, A.; Weerakoon, N.; Edirisinghe, E.; Jayasinghe, W.; Napagoda, M.; Hettiarachchi, L.; Abeysinghe, I. Chemical and biological characteristics of Sri Lankan white tea. Food Humanit. 2023, 1, 966–972. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Xiong, L.-G.; Chen, Y.-J.; Tong, J.-W.; Huang, J.-A.; Li, J.; Gong, Y.-S.; Liu, Z.-H. Tea polyphenol epigallocatechin gallate inhibits Escherichia coli by increasing endogenous oxidative stress. Food Chem. 2017, 217, 196–204. [Google Scholar] [CrossRef]
- Lee, W.; Lee, D.; Han, E.; Choi, J. Intake of green tea products and obesity in nondiabetic overweight and obese females: A systematic review and meta-analysis. J. Funct. Foods 2019, 58, 330–337. [Google Scholar] [CrossRef]
- Brimson, J.M.; Prasanth, M.I.; Kumaree, K.K.; Thitilertdecha, P.; Malar, D.S.; Tencomnao, T.; Prasansuklab, A. Tea Plant (Camellia sinensis): A Current Update on Use in Diabetes, Obesity, and Cardiovascular Disease. Nutrients 2022, 15, 37. [Google Scholar] [CrossRef]
- Hu, H.-Y.; Wu, B.-S.; Ou, Y.-N.; Ma, Y.-H.; Huang, Y.-Y.; Cheng, W.; Tan, L.; Yu, J.-T. Tea consumption and risk of incident dementia: A prospective cohort study of 377 592 UK Biobank participants. Transl. Psychiatry 2022, 12, 171. [Google Scholar] [CrossRef] [PubMed]
- Chieng, D.; Kistler, P.M. Coffee and tea on cardiovascular disease (CVD) prevention. Trends Cardiovasc. Med. 2022, 32, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, I. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem. 2022, 379, 132135. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P. Genomic techniques used to investigate the human gut microbiota. In Human Microbiome; IntechOpen: London, UK, 2021; pp. 1–22. [Google Scholar]
- Almeida, A.; Nayfach, S.; Boland, M.; Strozzi, F.; Beracochea, M.; Shi, Z.J.; Pollard, K.S.; Sakharova, E.; Parks, D.H.; Hugenholtz, P.; et al. A unified catalog of 204,938 reference genomes from the human gut microbiome. Nat. Biotechnol. 2021, 39, 105–114. [Google Scholar] [CrossRef]
- Castillo-Álvarez, F.; Marzo-Sola, M. Role of the gut microbiota in the development of various neurological diseases. Neurología (Engl. Ed.) 2022, 37, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Mao, Q.; Xiong, R.; Zhou, D.; Huang, S.; Saimaiti, A.; Shang, A.; Luo, M.; Li, H.; Li, H.; et al. Preventive Effects of Different Black and Dark Teas on Obesity and Non-Alcoholic Fatty Liver Disease and Modulate Gut Microbiota in High-Fat Diet Fed Mice. Foods 2022, 11, 3457. [Google Scholar] [CrossRef]
- Yang, G.; Wei, J.; Liu, P.; Zhang, Q.; Tian, Y.; Hou, G.; Meng, L.; Xin, Y.; Jiang, X. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism 2021, 117, 154712. [Google Scholar] [CrossRef]
- Yoshida, N.; Yamashita, T.; Hirata, K.-I. Gut Microbiome and Cardiovascular Diseases. Diseases 2018, 6, 56. [Google Scholar] [CrossRef]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut microbiota and cancer: From pathogenesis to therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Waubant, E.; Chehoud, C.; Kuczynski, J.; DeSantis, T.Z.; Warrington, J.; Venkatesan, A.; Fraser, C.M.; Mowry, E.M. Gut microbiota in multiple sclerosis: Possible influence of immunomodulators. J. Investig. Med. 2015, 63, 729–734. [Google Scholar] [CrossRef]
- Rolhion, N.; Chassaing, B. When pathogenic bacteria meet the intestinal microbiota. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150504. [Google Scholar] [CrossRef]
- Jeganathan, B.; Punyasiri, P.N.; Kottawa-Arachchi, J.D.; Ranatunga, M.A.; Abeysinghe, I.S.B.; Gunasekare, M.K.; Bandara, B.R. Genetic Variation of Flavonols Quercetin, Myricetin, and Kaempferol in the Sri Lankan Tea (Camellia sinensis L.) and Their Health-Promoting Aspects. Int. J. Food Sci. 2016, 2016, 6057434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ho, C.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and Biological Activities of Processed Camellia sinensis Teas: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef] [PubMed]
- Salman, S.; Öz, G.; Felek, R.; Haznedar, A.; Turna, T.; Özdemir, F. Effects of fermentation time on phenolic composition, antioxidant and antimicrobial activities of green, oolong, and black teas. Food Biosci. 2022, 49, 101884. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yang, G.; You, Q.; Sun, S.; Chen, R.; Lin, Z.; Simal-Gandara, J.; Lv, H. Updates on the chemistry, processing characteristics, and utilization of tea flavonoids in last two decades (2001–2021). Crit. Rev. Food Sci. Nutr. 2023, 63, 4757–4784. [Google Scholar] [CrossRef]
- Khokhar, S.; Magnusdottir, S.G.M. Total Phenol, Catechin, and Caffeine Contents of Teas Commonly Consumed in the United Kingdom. J. Agric. Food Chem. 2002, 50, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, Y.; Zhang, H.; Wang, J.; Liu, X.; Chen, Z.; Zheng, M.; Liu, B. Phenolic compounds and the biological effects of Pu-erh teas with long-term storage. Int. J. Food Prop. 2017, 20, 1715–1728. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Ma, Z.-Z.; Tu, P.-F. Comparison of the chemical constituents of aged pu-erh tea, ripened pu-erh tea, and other teas using HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2011, 59, 8754–8760. [Google Scholar] [CrossRef]
- LIczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Xie, G.; Ye, M.; Wang, Y.; Ni, Y.; Su, M.; Huang, H.; Qiu, M.; Zhao, A.; Zheng, X.; Chen, T.; et al. Characterization of pu-erh tea using chemical and metabolic profiling approaches. J. Agric. Food Chem. 2009, 57, 3046–3054. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.; Sirisena, S.; Ng, K. Phytochemical profile of differently processed tea: A review. J. Food Sci. 2022, 87, 1925–1942. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, M.; Qu, Z.; Xie, B. Compositional analysis and preliminary toxicological evaluation of a tea polysaccharide conjugate. J. Agric. Food Chem. 2007, 55, 2256–2260. [Google Scholar] [CrossRef] [PubMed]
- Takeo, T.; Unno, T.; Kinugasa, H.; Yayabe, F.; Motoyama, M. the chemical properties and functional effects of polysaccharides dissolved in green tea infusion. J. Jpn. Soc. Food Sci. Technol. 1998, 45, 270–272. [Google Scholar] [CrossRef]
- Xiao, J.; Huo, J.; Jiang, H.; Yang, F. Chemical compositions and bioactivities of crude polysaccharides from tea leaves beyond their useful date. Int. J. Biol. Macromol. 2011, 49, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, X.; Lu, X.; Wang, D.; Zhao, Y. Protective effects of Keemun black tea polysaccharides on acute carbon tetrachloride-caused oxidative hepatotoxicity in mice. Food Chem. Toxicol. 2013, 58, 184–192. [Google Scholar] [CrossRef]
- Chen, H.; Qu, Z.; Fu, L.; Dong, P.; Zhang, X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2009, 74, C469–C474. [Google Scholar] [CrossRef]
- Chen, L.; Cao, H.; Xiao, J. 2—Polyphenols: Absorption, bioavailability, and metabolomics. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 45–67. [Google Scholar]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Yin, Z.; Zheng, T.; Ho, C.-T.; Huang, Q.; Wu, Q.; Zhang, M. Improving the stability and bioavailability of tea polyphenols by encapsulations: A review. Food Sci. Hum. Wellness 2022, 11, 537–556. [Google Scholar] [CrossRef]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef] [PubMed]
- Grijalva, E.G.; Grijalva, P.G.; Pérez, D.L.A.; López, N.L.; López, R.I.C.; Heredia, J.B. Bioavailability of dietary phenolic compounds. Rev. Española Nutr. Humana Dietética 2016, 20, 140–147. [Google Scholar]
- Gan, R.-Y.; Li, H.-B.; Sui, Z.-Q.; Corke, H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Hu, T.; Zhao, H. Metabolic fate of tea polyphenols and their crosstalk with gut microbiota. Food Sci. Hum. Wellness 2022, 11, 455–466. [Google Scholar] [CrossRef]
- Neilson, A.P.; Hopf, A.S.; Cooper, B.R.; Pereira, M.A.; Bomser, J.A.; Ferruzzi, M.G. Catechin degradation with concurrent formation of homo- and heterocatechin dimers during in vitro digestion. J. Agric. Food Chem. 2007, 55, 8941–8949. [Google Scholar] [CrossRef]
- Slot, G.V.; Humpf, H.-U. Degradation and metabolism of catechin, epigallocatechin-3-gallate (EGCG), and related compounds by the intestinal microbiota in the pig cecum model. J. Agric. Food Chem. 2009, 57, 8041–8048. [Google Scholar] [CrossRef]
- Schantz, M.; Erk, T.; Richling, E. Metabolism of green tea catechins by the human small intestine. Biotechnol. J. 2010, 5, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Guergoletto, K.B.; Costabile, A.; Flores, G.; Garcia, S.; Gibson, G.R. In vitro fermentation of juçara pulp (Euterpe edulis) by human colonic microbiota. Food Chem. 2016, 196, 251–258. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef]
- Yadav, M.; Verma, M.K.; Chauhan, N.S. A review of metabolic potential of human gut microbiome in human nutrition. Arch. Microbiol. 2018, 200, 203–217. [Google Scholar] [CrossRef]
- Chen, G.; Chen, R.; Chen, D.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Tea polysaccharides as potential therapeutic options for metabolic diseases. J. Agric. Food Chem. 2018, 67, 5350–5360. [Google Scholar] [CrossRef]
- Li, H.; Fang, Q.; Nie, Q.; Hu, J.; Yang, C.; Huang, T.; Li, H.; Nie, S.-P. Hypoglycemic and hypolipidemic mechanism of tea polysaccharides on type 2 diabetic rats via gut microbiota and metabolism alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Laterza, L.; Rizzatti, G.; Gaetani, E.; Chiusolo, P.; Gasbarrini, A. The Gut Microbiota and Immune System Relationship in Human Graft-versus-Host Disease. Mediterr. J. Hematol. Infect. Dis. 2016, 8, e2016025. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the human gut microbiota by phenolics and phenolic fiber-rich foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1268–1298. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.E.; Weinstock, G.M.; Highlander, S.K.; Worley, K.C.; Creasy, H.H.; Wortman, J.R.; Rusch, D.B.; Mitreva, M.; Sodergren, E.; Chinwalla, A.T.; et al. A catalog of reference genomes from the human microbiome. Science 2010, 328, 994–999. [Google Scholar]
- Chakravorty, S.; Helb, D.; Burday, M.; Connell, N.; Alland, D. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J. Microbiol. Methods 2007, 69, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Sune, D.; Rydberg, H.; Augustinsson, N.; Serrander, L.; Jungeström, M.B. Optimization of 16S rRNA gene analysis for use in the diagnostic clinical microbiology service. J. Microbiol. Methods 2020, 170, 105854. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Ling, C.L.; Ciesielczuk, H.L.; Lockwood, J.; Hopkins, S.; McHugh, T.D.; Gillespie, S.H.; Kibbler, C.C. Detection and identification of bacteria in clinical samples by 16S rRNA gene sequencing: Comparison of two different approaches in clinical practice. J. Med. Microbiol. 2012, 61, 483–488. [Google Scholar] [CrossRef]
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in Chemical and Biological Methods to Identify Microorganisms—From Past to Present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Sun, Y.; Hu, B.; Sun, Y.; Jabbar, S.; Zeng, X. Fermentation in vitro of EGCG, GCG and EGCG3”Me isolated from Oolong tea by human intestinal microbiota. Food Res. Int. 2013, 54, 1589–1595. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J Physiol Pharmacol. 2012, 63, 497–503. [Google Scholar]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Hervert-Hernández, D.; Goñi, I. Dietary Polyphenols and Human Gut Microbiota: A Review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Stapleton, P.D.; Shah, S.; Ehlert, K.; Hara, Y.; Taylor, P.W. The beta-lactam-resistance modifier (−)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology 2007, 153 Pt 7, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Hamilton-Miller, J.M.T.; Shah, S. Activity of the tea component epicatechin gallate and analogues against methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2000, 46, 852–853. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Matsunaga, K.; Friedman, H. Protective effects of green tea catechins on alveolar macrophages against bacterial infections. BioFactors 2004, 21, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Song, D.; Cheng, L.; Zhang, X. Interactions of tea catechins with intestinal microbiota and their implication for human health. Food Sci. Biotechnol. 2019, 28, 1617–1625. [Google Scholar] [CrossRef]
- Xu, X.; Xu, P.; Ma, C.; Tang, J.; Zhang, X. Gut microbiota, host health, and polysaccharides. Biotechnol. Adv. 2013, 31, 318–337. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Wang, M.; Chen, G.; Chen, D.; Ye, H.; Sun, Y.; Zeng, X.; Liu, Z. Purified fraction of polysaccharides from Fuzhuan brick tea modulates the composition and metabolism of gut microbiota in anaerobic fermentation in vitro. Int. J. Biol. Macromol. 2019, 140, 858–870. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Li, W.; Yuan, G.; Pan, Y.; Chen, H. Preparation and characterization of a novel conformed bipolymer paclitaxel-nanoparticle using tea polysaccharides and zein. Carbohydr. Polym. 2016, 146, 52–57. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shim, J.S.; Lee, J.S.; Kim, J.K.; Yang, I.S.; Chung, M.-S.; Kim, K.H. Inhibition of pathogenic bacterial adhesion by acidic polysaccharide from green tea (Camellia sinensis). J. Agric. Food Chem. 2006, 54, 8717–8723. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Lei, Z.; Zeng, W.; Yang, J. Effect of tea polysaccharides on faecal microbiota and their short-chain fatty acid metabolic products. Int. Food Res. J. 2023, 30, 151–162. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Zhang, X. Modulation of Intestinal Flora by Dietary Polysaccharides: A Novel Approach for the Treatment and Prevention of Metabolic Disorders. Foods 2022, 11, 2961. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Yang, W.; Ren, D.; Zhao, Y.; Liu, L.; Yang, X. Fuzhuan Brick Tea Polysaccharide Improved Ulcerative Colitis in Association with Gut Microbiota-Derived Tryptophan Metabolism. J. Agric. Food Chem. 2021, 69, 8448–8459. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Z.-H.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X. An Insight into the roles of dietary tryptophan and its metabolites in intestinal inflammation and inflammatory bowel disease. Mol. Nutr. Food Res. 2021, 65, 2000461. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Watanabe, Y.; Tobe, K. The gut microbiome: A core regulator of metabolism. J. Endocrinol. 2023, 256, e220111. [Google Scholar] [CrossRef]
- Rivera-Chávez, F.; Zhang, L.F.; Faber, F.; Lopez, C.A.; Byndloss, M.X.; Olsan, E.E.; Xu, G.; Velazquez, E.M.; Lebrilla, C.B.; Winter, S.E.; et al. Depletion of Butyrate-producing Clostridia from the gut microbiota drives an aerobic luminal expansion of salmonella. Cell Host Microbe 2016, 19, 443–454. [Google Scholar] [CrossRef]
- Boets, E.; Gomand, S.V.; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M.; et al. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: A stable isotope study. J. Physiol. 2017, 595, 541–555. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Gérard, P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens 2013, 3, 14–24. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.-U.; Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- An, D.; Oh, S.F.; Olszak, T.; Neves, J.F.; Avci, F.Y.; Erturk-Hasdemir, D.; Lu, X.; Zeissig, S.; Blumberg, R.S.; Kasper, D.L. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 2014, 156, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Kishino, S.; Takeuchi, M.; Park, S.-B.; Hirata, A.; Kitamura, N.; Kunisawa, J.; Kiyono, H.; Iwamoto, R.; Isobe, Y.; Arita, M.; et al. Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc. Natl. Acad. Sci. USA 2013, 110, 17808–17813. [Google Scholar] [CrossRef]
- Prawitt, J.; Abdelkarim, M.; Stroeve, J.H.M.; Popescu, I.; Duez, H.; Velagapudi, V.R.; Dumont, J.; Bouchaert, E.; van Dijk, T.H.; Lucas, A.; et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 2011, 60, 1861–1871. [Google Scholar] [CrossRef]
- Trabelsi, M.-S.; Lestavel, S.; Staels, B.; Collet, X. Intestinal bile acid receptors are key regulators of glucose homeostasis. Proc. Nutr. Soc. 2017, 76, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Zhou, H.; Zhou, J.; Glenn, T.C.; Shen, C.-L.; Wang, J.-S. Long-term treatment with green tea polyphenols modifies the gut microbiome of female sprague-dawley rats. J. Nutr. Biochem. 2018, 56, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rahman, S.U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X.; et al. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar] [CrossRef]
- Ito, A.; Matsui, Y.; Takeshita, M.; Katashima, M.; Goto, C.; Kuriki, K. Gut microbiota-mediated associations of green tea and catechin intakes with glucose metabolism in individuals without type 2 diabetes mellitus: A four-season observational study with mediation analysis. Arch. Microbiol. 2023, 205, 191. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.W.; Kim, J.K.; Kim, A.Y.; Cho, D.; Lee, J.-H.; Choi, J.K.; Park, M.; Kim, W. Green tea encourages growth of Akkermansia muciniphila. J. Med. Food 2020, 23, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zeng, B.; Liu, Z.; Liao, Z.; Zhong, Q.; Gu, L.; Wei, H.; Fang, X. Green Tea Polyphenols Modulate Colonic Microbiota Diversity and Lipid Metabolism in High-Fat Diet Treated HFA Mice. J. Food Sci. 2018, 83, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Y.; Le Roy, C.; Hu, J.; Steves, C.J.; Bell, J.T.; Spector, T.D.; Gibson, R.; Menni, C.; Rodriguez-Mateos, A. Interplay between the (Poly)phenol Metabolome, Gut Microbiome, and Cardiovascular Health in Women: A Cross-Sectional Study from the TwinsUK Cohort. Nutrients 2023, 15, 1900. [Google Scholar] [CrossRef]
- Bond, T.; Derbyshire, E. Tea Compounds and the Gut Microbiome: Findings from Trials and Mechanistic Studies. Nutrients 2019, 11, 2364. [Google Scholar] [CrossRef]
- Khairudin, M.A.S.; Jalil, A.M.M.; Hussin, N. Effects of Polyphenols in Tea (Camellia sinensis sp.) on the Modulation of Gut Microbiota in Human Trials and Animal Studies. Gastroenterol. Insights 2021, 12, 202–216. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, X.; Hong, M.; Wu, Z.; Luo, S.; Cheng, K. Oolong tea polyphenols affect the inflammatory response to improve cognitive function by regulating gut microbiota. J. Funct. Foods 2023, 105, 105584. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Haq, I.-U.; Chen, A.; Majeed, F.; Feng, Q.; Li, M. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br. J. Nutr. 2020, 123, 1216–1226. [Google Scholar] [CrossRef]
- Yan, R.; Ho, C.-T.; Liu, Y.; Zhan, S.; Wu, Z.; Zhang, X. The modulatory effect of oolong tea polyphenols on intestinal flora and hypothalamus gene expression in a circadian rhythm disturbance mouse model. Food Sci. Hum. Wellness 2024, 13, 748–764. [Google Scholar] [CrossRef]
- Boulangé, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Ho, C.-T.; Zhang, X.; Cao, J.; Wang, H.; Shao, X.; Pan, D.; Wu, Z. Oolong Tea Polyphenols Ameliorate Circadian Rhythm of Intestinal Microbiome and Liver Clock Genes in Mouse Model. J. Agric. Food Chem. 2019, 67, 11969–11976. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.; Cheng, L.; Zheng, X.; Zhang, Z. The evaluation of the quality of Feng Huang Oolong teas and their modulatory effect on intestinal microbiota of high-fat diet-induced obesity mice model. Int. J. Food Sci. Nutr. 2018, 69, 842–856. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, X.; Miao, Y.; Cao, J.; Wu, Z.; Weng, P. The modulatory effect of (-)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3″Me) on intestinal microbiota of high fat diet-induced obesity mice model. Food Res. Int. 2017, 92, 9–16. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Zhu, J.; Zhang, M.; Ho, C.T.; Huang, Q.; Cao, J. Metagenomics Analysis of Gut Microbiota in a High Fat Diet–Induced Obesity Mouse Model Fed with (−)-Epigallocatechin 3-O-(3-O-Methyl) Gallate (EGCG3″Me). Mol. Nutr. Food Res. 2018, 62, 1800274. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Cheng, M.; Cao, J.; Wu, Z.; Weng, P.; Yan, M. Oolong Tea Polyphenols–Phospholipids Complex Reduces Obesity in High Fat Diet-Induced Mice Model. Eur. J. Lipid Sci. Technol. 2017, 119, 1600394. [Google Scholar] [CrossRef]
- Ye, X.; Tang, X.; Li, F.; Zhu, J.; Wu, M.; Wei, X.; Wang, Y. Green and Oolong Tea Extracts with Different Phytochemical Compositions Prevent Hypertension and Modulate the Intestinal Flora in a High-Salt Diet Fed Wistar Rats. Front. Nutr. 2022, 9, 892801. [Google Scholar] [CrossRef]
- Wu, D.; Chen, R.; Li, Q.; Lai, X.; Sun, L.; Zhang, Z.; Wen, S.; Sun, S.; Cao, F. Tea (Camellia sinensis) Ameliorates Hyperuricemia via Uric Acid Metabolic Pathways and Gut Microbiota. Nutrients 2022, 14, 2666. [Google Scholar] [CrossRef]
- Li, B.; Mao, Q.; Zhou, D.; Luo, M.; Gan, R.; Li, H.; Huang, S.; Saimaiti, A.; Shang, A.; Li, H. Effects of Tea against Alcoholic Fatty Liver Disease by Modulating Gut Microbiota in Chronic Alcohol-Exposed Mice. Foods 2021, 10, 1232. [Google Scholar] [CrossRef]
- Unno, T.; Osakabe, N. Green tea extract and black tea extract differentially influence cecal levels of short-chain fatty acids in rats. Food Sci. Nutr. 2018, 6, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xu, Y.; Yin, J. Black tea benefits short-chain fatty acid producers but inhibits genus Lactobacillus in the gut of healthy Sprague–Dawley rats. J. Sci. Food Agric. 2020, 100, 5466–5475. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Liu, S.; Chen, Y.; Kong, Y.; Wang, D.; Wang, Y.; Ling, T.; Xie, Z.; Khalilova, I.; Huang, J. Effects of Keemun and Dianhong Black Tea in Alleviating Excess Lipid Accumulation in the Liver of Obese Mice: A Comparative Study. Front. Nutr. 2022, 9, 849582. [Google Scholar] [CrossRef]
- Liu, X.; Hu, G.; Wang, A.; Long, G.; Yang, Y.; Wang, D.; Zhong, N.; Jia, J. Black Tea Reduces Diet-Induced Obesity in Mice via Modulation of Gut Microbiota and Gene Expression in Host Tissues. Nutrients 2022, 14, 1635. [Google Scholar] [CrossRef]
- Zhong, S.; Yang, Y.; Huo, J.; Sun, Y.; Ren, N.; Lu, Q.; Li, D.; Zhan, P.; Wu, W.; Chen, H.; et al. Dissection of gut microbiota and metabolites reveals the hypolipidemic effect of green mulberry leaf tea/black mulberry leaf tea in mice. J. Funct. Foods 2023, 111, 105906. [Google Scholar] [CrossRef]
- Henning, S.M.; Yang, J.; Hsu, M.; Lee, R.-P.; Grojean, E.M.; Ly, A.; Tseng, C.-H.; Heber, D.; Li, Z. Decaffeinated green and black tea polyphenols decrease weight gain and alter microbiome populations and function in diet-induced obese mice. Eur. J. Nutr. 2018, 57, 2759–2769. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, C.; Xiao, X.; Zhu, M.; Quan, W.; Liu, X.; Zhang, S.; Liu, Z. Theaflavins in Black Tea Mitigate Aging-Associated Cognitive Dysfunction via the Microbiota–Gut–Brain Axis. J. Agric. Food Chem. 2023, 71, 2356–2369. [Google Scholar] [CrossRef]
- Ye, J.; Zhao, Y.; Chen, X.; Zhou, H.; Yang, Y.; Zhang, X.; Huang, Y.; Zhang, N.; Lui, E.M.; Xiao, M. Pu-erh tea ameliorates obesity and modulates gut microbiota in high fat diet fed mice. Food Res. Int. 2021, 144, 110360. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, N.; Wang, Q.; Huang, Y.; Huang, Y.; Lin, Y.; Huang, M.; Zheng, F.; Xiao, M.; Ye, J. Theabrownin of raw and ripened pu-erh tea varies in the alleviation of HFD-induced obesity via the regulation of gut microbiota. Eur. J. Nutr. 2023, 62, 2177–2194. [Google Scholar] [CrossRef]
- Lu, X.; Liu, J.; Zhang, N.; Fu, Y.; Zhang, Z.; Li, Y.; Wang, W.; Li, Y.; Shen, P.; Cao, Y. Ripened Pu-erh Tea Extract Protects Mice from Obesity by Modulating Gut Microbiota Composition. J. Agric. Food Chem. 2019, 67, 6978–6994. [Google Scholar] [CrossRef]
- Xia, Y.; Tan, D.; Akbary, R.; Kong, J.; Seviour, R.; Kong, Y. Aqueous raw and ripe Pu-erh tea extracts alleviate obesity and alter cecal microbiota composition and function in diet-induced obese rats. Appl. Microbiol. Biotechnol. 2019, 103, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wang, S.; Zhao, A.; Zheng, X.; Zhang, Y.; Lei, S.; Ge, K.; Qu, C.; Zhao, Q.; Yan, C.; et al. Pu-erh Tea Regulates Fatty Acid Metabolism in Mice Under High-Fat Diet. Front. Pharmacol. 2019, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Q.; Mi, X.; Qiu, L.; Tao, X.; Zhang, Z.; Xia, J.; Wu, Q.; Wei, H. Ripened Pu-erh tea extract promotes gut microbiota resilience against dextran sulfate sodium induced colitis. J. Agric. Food Chem. 2021, 69, 2190–2203. [Google Scholar] [CrossRef]
- Gao, X.; Xie, Q.; Kong, P.; Liu, L.; Sun, S.; Xiong, B.; Huang, B.; Yan, L.; Sheng, J.; Xiang, H. Polyphenol- and caffeine-rich postfermented pu-erh tea improves diet-induced metabolic syndrome by remodeling intestinal homeostasis in mice. Infect. Immun. 2018, 86, e00601-17. [Google Scholar] [CrossRef]
- Wu, G.; Liu, A.B.; Xu, Y.; Wang, Y.; Zhao, L.; Hara, Y.; Lam, Y.Y.; Yang, C.S. The Effects of Green Tea on Diabetes and Gut Microbiome in Mice: Studies with Tea Extracts vs. Tea Powder. Nutrients 2021, 13, 3155. [Google Scholar] [CrossRef]
- Dey, P.; Olmstead, B.D.; Sasaki, G.Y.; Vodovotz, Y.; Yu, Z.; Bruno, R.S. Epigallocatechin gallate but not catechin prevents nonalcoholic steatohepatitis in mice similar to green tea extract while differentially affecting the gut microbiota. J. Nutr. Biochem. 2020, 84, 108455. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.S.; Park, H.M.; Hyun, S.M.; Shon, J.C.; Singh, D.; Liu, K.-H.; Whon, T.W.; Bae, J.-W.; Hwang, J.S.; Lee, C.H. The green tea modulates large intestinal microbiome and exo/endogenous metabolome altered through chronic UVB-exposure. PLoS ONE 2017, 12, e0187154. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, L.; Luo, Y.; Zhang, J.; Wang, X.; Sun, K.; Zeng, L. Prebiotic properties of green and dark tea contribute to protective effects in chemical-induced colitis in mice: A fecal microbiota transplantation study. J. Agric. Food Chem. 2020, 68, 6368–6380. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, M.; Zhang, X.; Cao, J.; Wu, Z.; Weng, P. Green tea polyphenols reduce obesity in high-fat diet-induced mice by modulating intestinal microbiota composition. Int. J. Food Sci. Technol. 2017, 52, 1723–1730. [Google Scholar] [CrossRef]
- Zheng, Y.; Zeng, X.; Chen, T.; Peng, W.; Su, W. Chemical profile, antioxidative, and gut microbiota modulatory properties of ganpu tea: A derivative of pu-erh tea. Nutrients 2020, 12, 224. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, B.; Gong, C.; Xu, K.; Zhu, H.; Wang, N.; Yang, Z.; Wan, H.; Wang, Y. A Comparison of the Gut Microbiota Modulatory Effect of Pu-Erh and Dian Hong Black Tea. Preprint 2021. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Y.; Zhao, S.; Sun, K.; Luo, L.; Zeng, L. Ripened pu-erh tea improved the enterohepatic circulation in a circadian rhythm disorder mice model. J. Agric. Food Chem. 2021, 69, 13533–13545. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, Y.; Wang, X.; Luo, L.; Sun, K.; Zeng, L. Gut Microbiome and metabolome response of pu-erh tea on metabolism disorder induced by chronic alcohol consumption. J. Agric. Food Chem. 2020, 68, 6615–6627. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, M.; Sun, X.; Shi, H.; Zhu, J. Green tea extract alters gut microbiota and their metabolism of adults with metabolic syndrome in a host-free human colonic model. Food Res. Int. 2022, 160, 111762. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yang, K.; Zhu, J. Monitoring the Diversity and Metabolic Shift of Gut Microbes during Green Tea Feeding in an In Vitro Human Colonic Model. Molecules 2020, 25, 5101. [Google Scholar] [CrossRef] [PubMed]
- Bo, B.; Seong, H.; Kim, G.; Han, N.S. Antioxidant and prebiotic activities of Laphet, fermented tea leaves in Myanmar, during in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Foods 2022, 95, 105193. [Google Scholar] [CrossRef]
- Choi, S.R.; Lee, H.; Singh, D.; Cho, D.; Chung, J.O.; Roh, J.H.; Kim, W.G.; Lee, C.H. Bidirectional Interactions between Green Tea (GT) Polyphenols and Human Gut Bacteria. J. Microbiol. Biotechnol. 2023, 33, 1317. [Google Scholar] [CrossRef]
- Liu, Z.; de Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.-P. Reciprocal Interactions between Epigallocatechin-3-gallate (EGCG) and Human Gut Microbiota In Vitro. J. Agric. Food Chem. 2020, 68, 9804–9815. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, Y.; Cheng, M.; Zhang, X.; Zheng, X.; Zhang, Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J. Food Sci. Technol. 2018, 55, 399–407. [Google Scholar] [CrossRef]
- Kemperman, R.A.; Gross, G.; Mondot, S.; Possemiers, S.; Marzorati, M.; Van de Wiele, T.; Doré, J.; Vaughan, E.E. Impact of polyphenols from black tea and red wine/grape juice on a gut model microbiome. Food Res. Int. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Liao, W.; Li, W.; Liu, S.; Tang, D.; Chen, Y.; Wang, Y.; Xie, Z.; Huang, J. Potential prebiotic effects of nonabsorptive components of Keemun and Dianhong black tea: An in vitro study. Food Sci. Hum. Wellness 2022, 11, 648–659. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Hu, K.; Li, D.; Guo, H.; Xie, Z. Microbial-Transferred Metabolites of Black Tea Theaflavins by Human Gut Microbiota and Their Impact on Antioxidant Capacity. Molecules 2023, 28, 5871. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.; Song, F.V.; Tian, K.V.; Su, H.; Chass, G.A. Systematic characterisation of the structure and radical scavenging potency of Pu’Er tea polyphenol theaflavin. Org. Biomol. Chem. 2019, 17, 9942–9950. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Touyama, M.; Hisada, T.; Benno, Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol. Immunol. 2012, 56, 729–739. [Google Scholar] [CrossRef]
- Janssens, P.L.; Hursel, R.; Westerterp-Plantenga, M.S. Nutraceuticals for body-weight management: The role of green tea catechins. Physiol. Behav. 2016, 162, 83–87. [Google Scholar] [CrossRef]
- Yuan, X.; Long, Y.; Ji, Z.; Gao, J.; Fu, T.; Yan, M.; Zhang, L.; Su, H.; Zhang, W.; Wen, X.; et al. Green Tea Liquid Consumption Alters the Human Intestinal and Oral Microbiome. Mol. Nutr. Food Res. 2018, 62, e1800178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, J.; Zhang, L.; Ge, Z.; Wang, X.; Hu, X.; Xu, T.; Li, P.; Xu, W. HS-β-cyclodextrin-functionalized Ag@Fe3O4@Ag nanoparticles as a surface-enhanced Raman spectroscopy substrate for the sensitive detection of butyl benzyl phthalate. Anal. Bioanal. Chem. 2019, 411, 5691–5701. [Google Scholar] [CrossRef]
- Hodges, J.K.; Zhu, J.; Yu, Z.; Vodovotz, Y.; Brock, G.; Sasaki, G.Y.; Dey, P.; Bruno, R.S. Intestinal-level anti-inflammatory bioactivities of catechin-rich green tea: Rationale, design, and methods of a double-blind, randomized, placebo-controlled crossover trial in metabolic syndrome and healthy adults. Contemp. Clin. Trials Commun. 2020, 17, 100495. [Google Scholar] [CrossRef]
- Li, A.; Kou, R.; Liu, H.; Chen, M.; Wang, J.; Liu, Q.; Xing, X.; Zhang, B.; Dong, L.; Wang, S. Multi-omics analyses reveal relationships among polyphenol-rich oolong tea consumption, gut microbiota, and metabolic profile: A pilot study. Food Chem. 2023, 426, 136653. [Google Scholar] [CrossRef]
- Mai, V.; Katki, H.A.; Harmsen, H.; Gallaher, D.; Schatzkin, A.; Baer, D.J.; Clevidence, B. Effects of a Controlled Diet and Black Tea Drinking on the Fecal Microflora Composition and the Fecal Bile Acid Profile of Human Volunteers in a Double-Blinded Randomized Feeding Study. J. Nutr. 2004, 134, 473–478. [Google Scholar] [CrossRef]
- Tomioka, R.; Tanaka, Y.; Suzuki, M.; Ebihara, S. The Effects of Black Tea Consumption on Intestinal Microflora—A Randomized Single-Blind Parallel-Group, Placebo-Controlled Study. J. Nutr. Sci. Vitaminol. 2023, 69, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.L.H.R.; Penders, J.; Hursel, R.; Budding, A.E.; Savelkoul, P.H.M.; Westerterp-Plantenga, M.S. Long-term green tea supplementation does not change the human gut microbiota. PLoS ONE 2016, 11, e0153134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, N.; Arikawa, A.Y.; Chen, C. Inhibitory effects of green tea polyphenols on microbial metabolism of aromatic amino acids in humans revealed by metabolomic analysis. Metabolites 2019, 9, 96. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Chen, R.; Ng, K. Effects of Differently Processed Tea on the Gut Microbiota. Molecules 2024, 29, 4020. https://doi.org/10.3390/molecules29174020
Zhao Z, Chen R, Ng K. Effects of Differently Processed Tea on the Gut Microbiota. Molecules. 2024; 29(17):4020. https://doi.org/10.3390/molecules29174020
Chicago/Turabian StyleZhao, Zimo, Ruofan Chen, and Ken Ng. 2024. "Effects of Differently Processed Tea on the Gut Microbiota" Molecules 29, no. 17: 4020. https://doi.org/10.3390/molecules29174020
APA StyleZhao, Z., Chen, R., & Ng, K. (2024). Effects of Differently Processed Tea on the Gut Microbiota. Molecules, 29(17), 4020. https://doi.org/10.3390/molecules29174020







