Wild Pepper (Piper laetispicum) Fruit Quality Traits at Different Developmental Stages
Abstract
1. Introduction
2. Results and Discussion
2.1. Crude Fat, Ash, Piperine and Protein Contents
2.2. VOCs
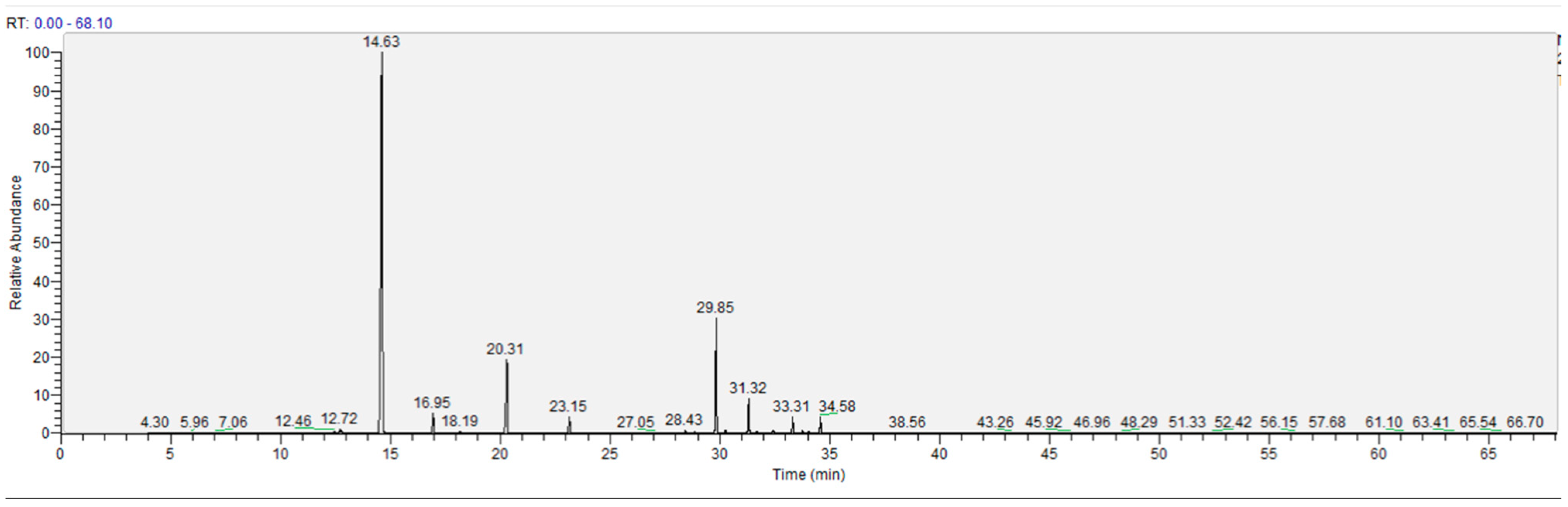
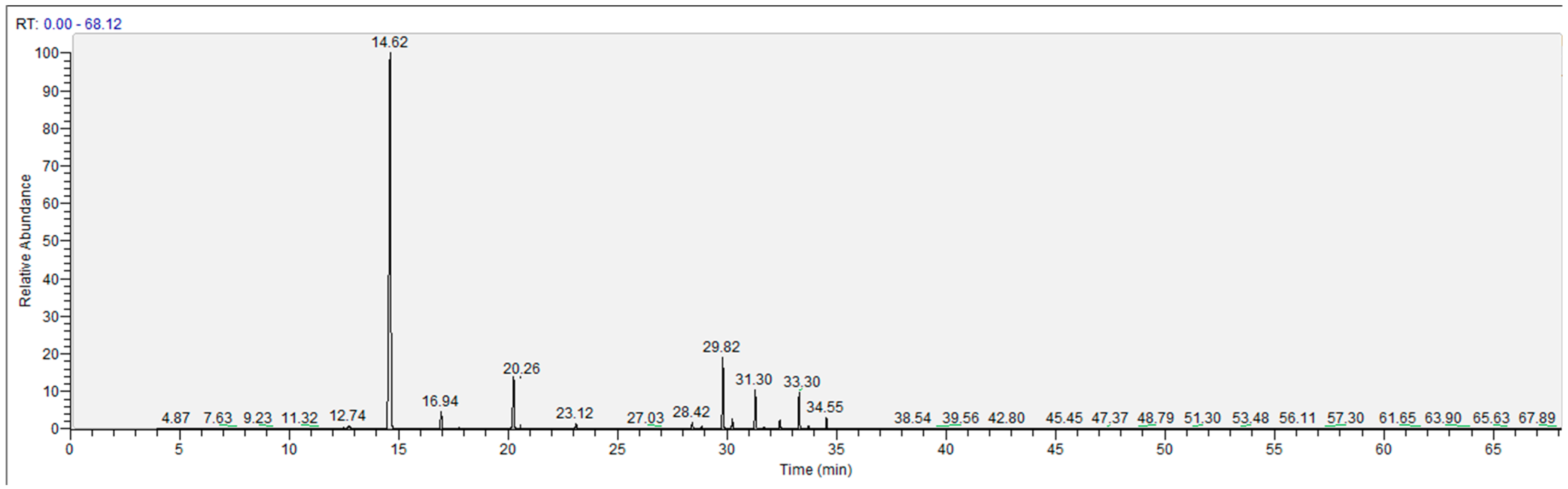
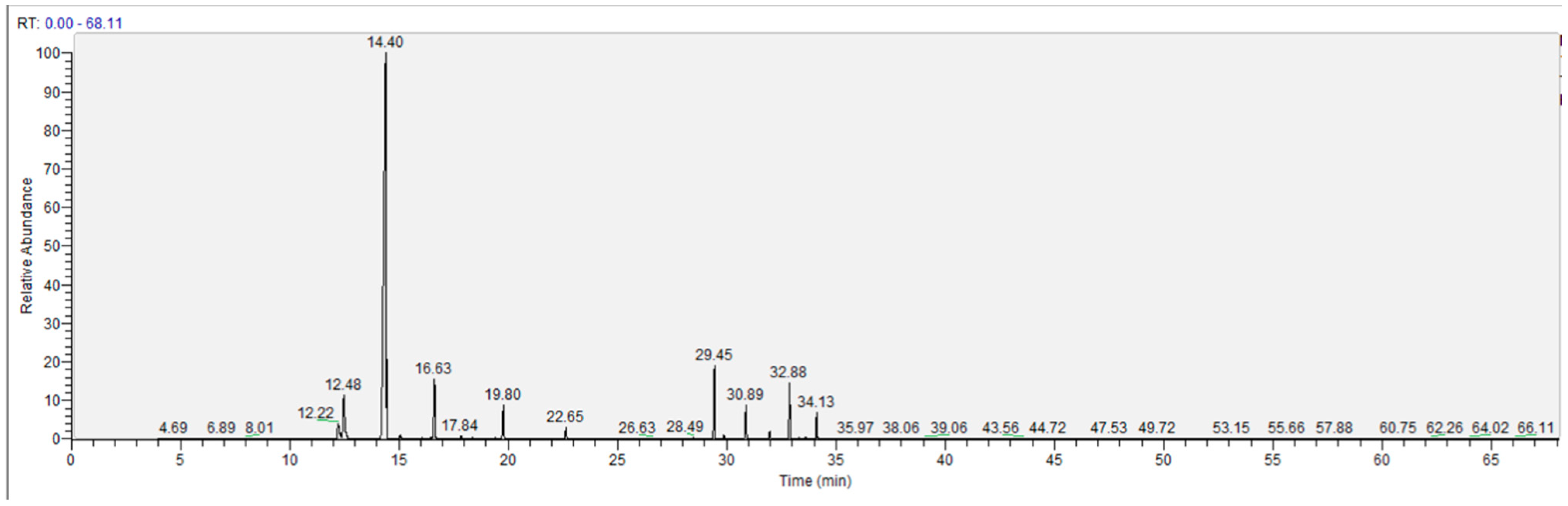
2.2.1. Steam Distillation Combined with GC-MS Analysis of VOCs
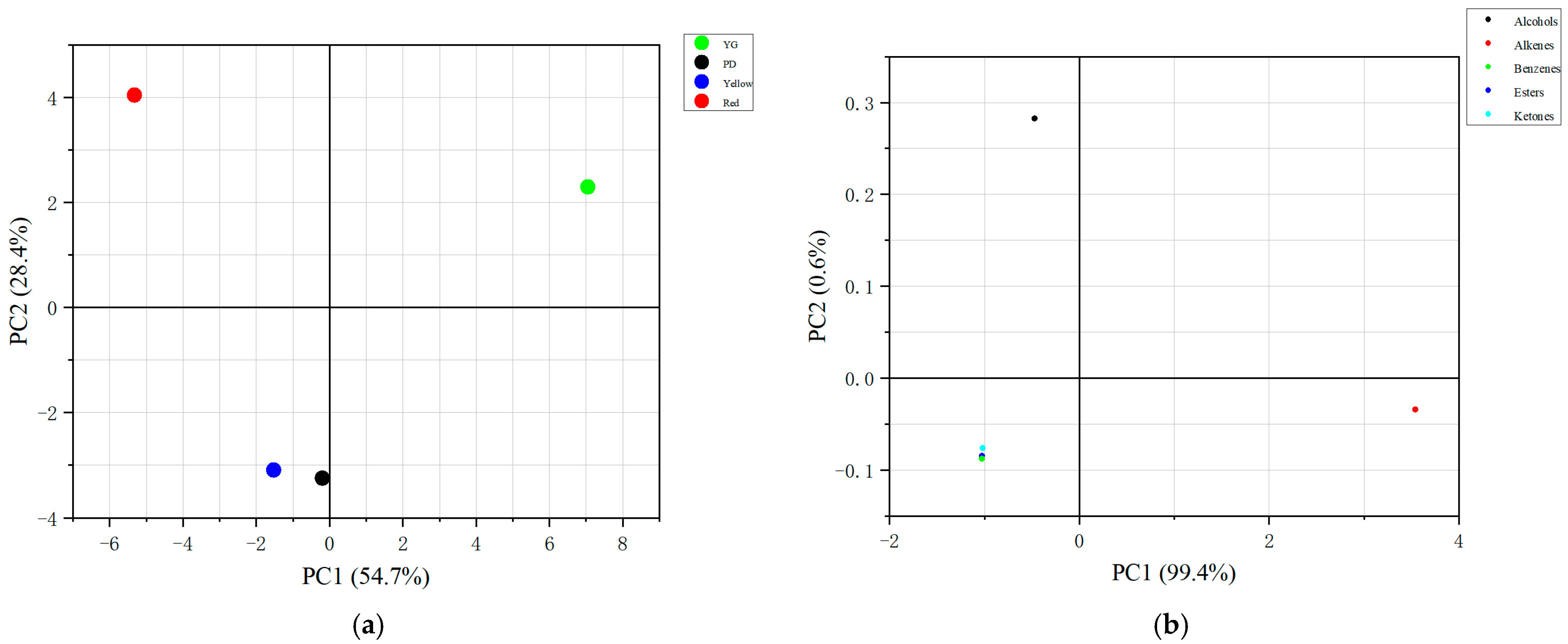
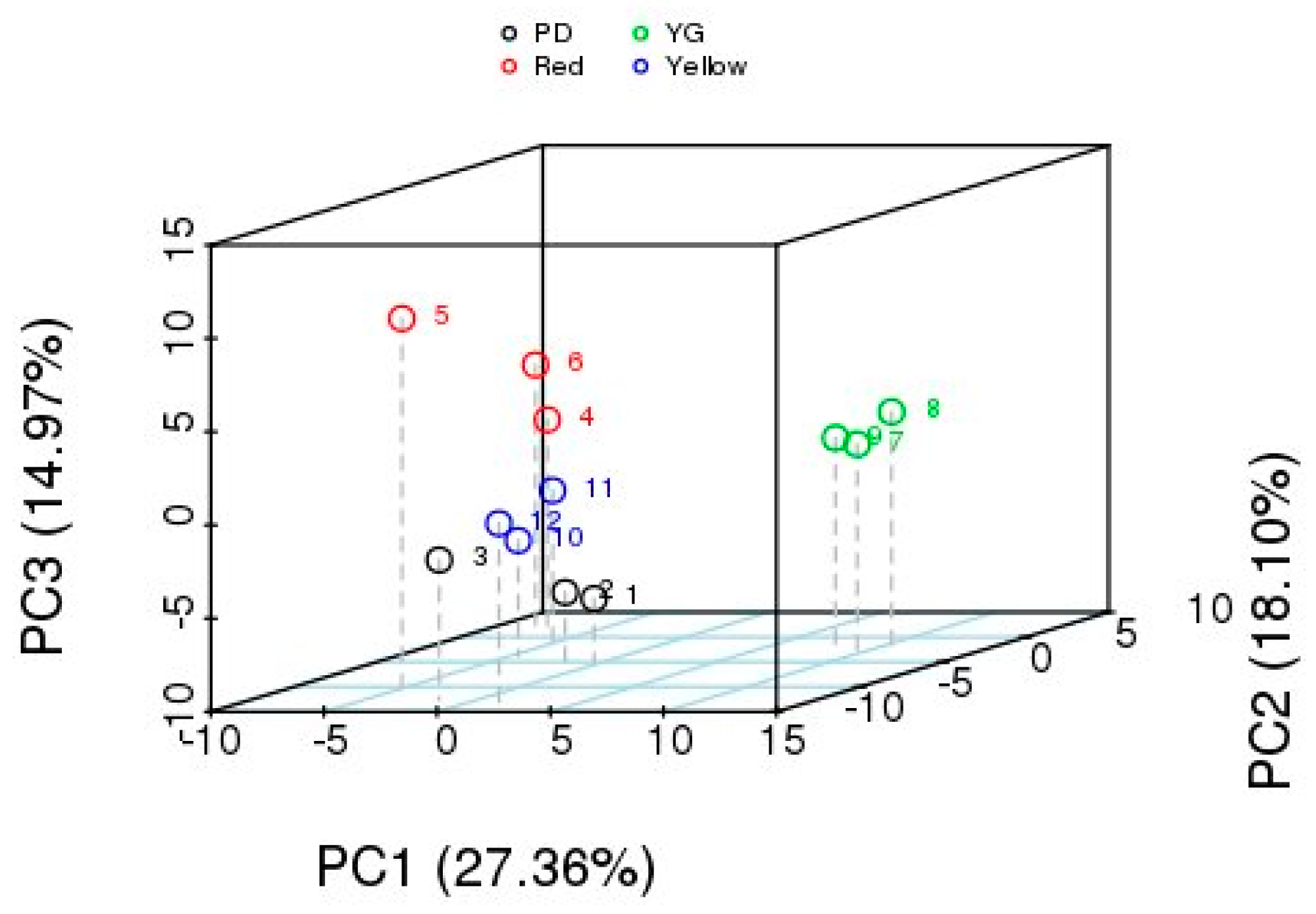
2.2.2. Principal Component Analysis (PCA) of VOCs
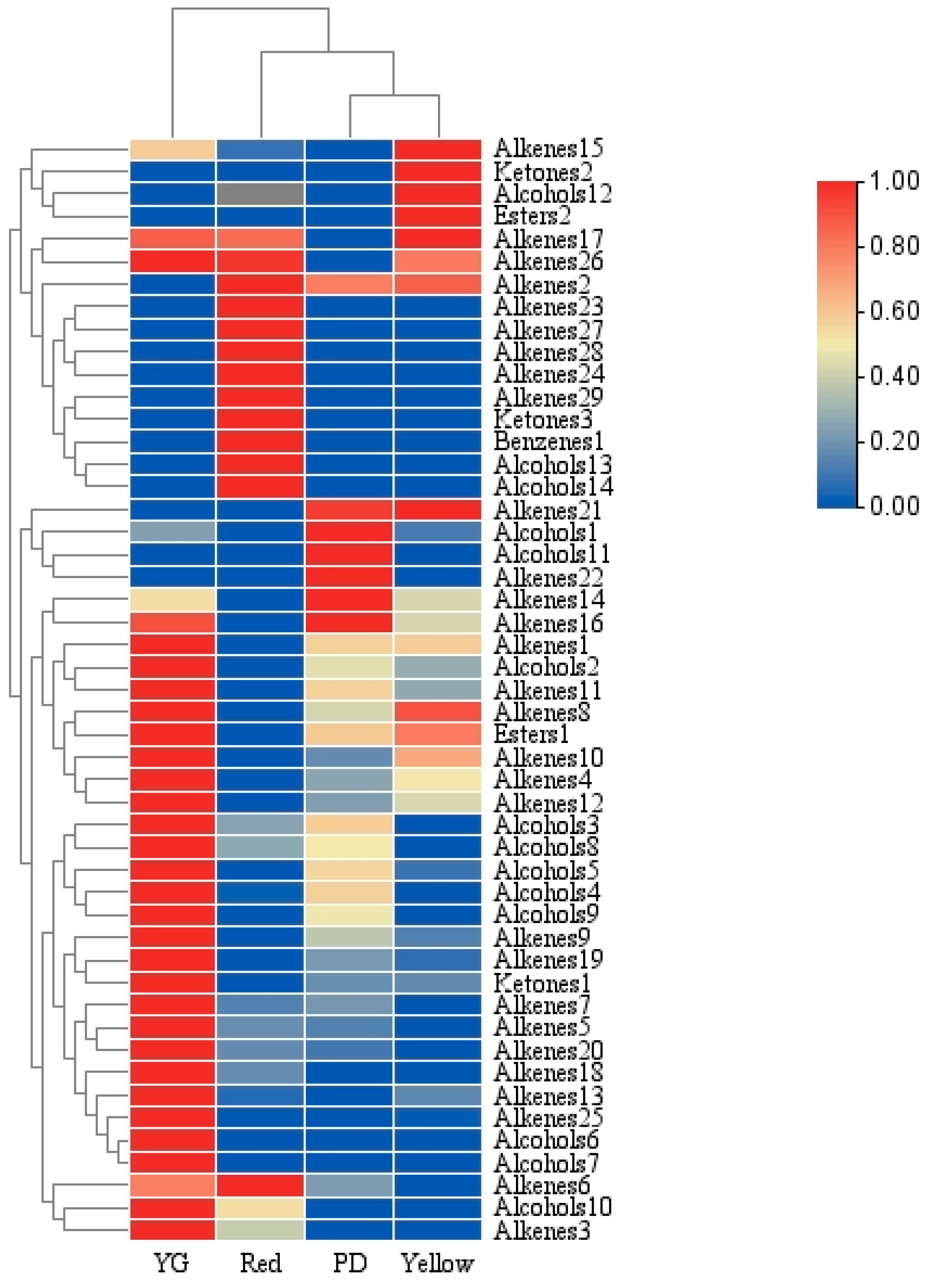
2.2.3. Cluster Analysis of VOCs
2.3. Aroma Metabolites
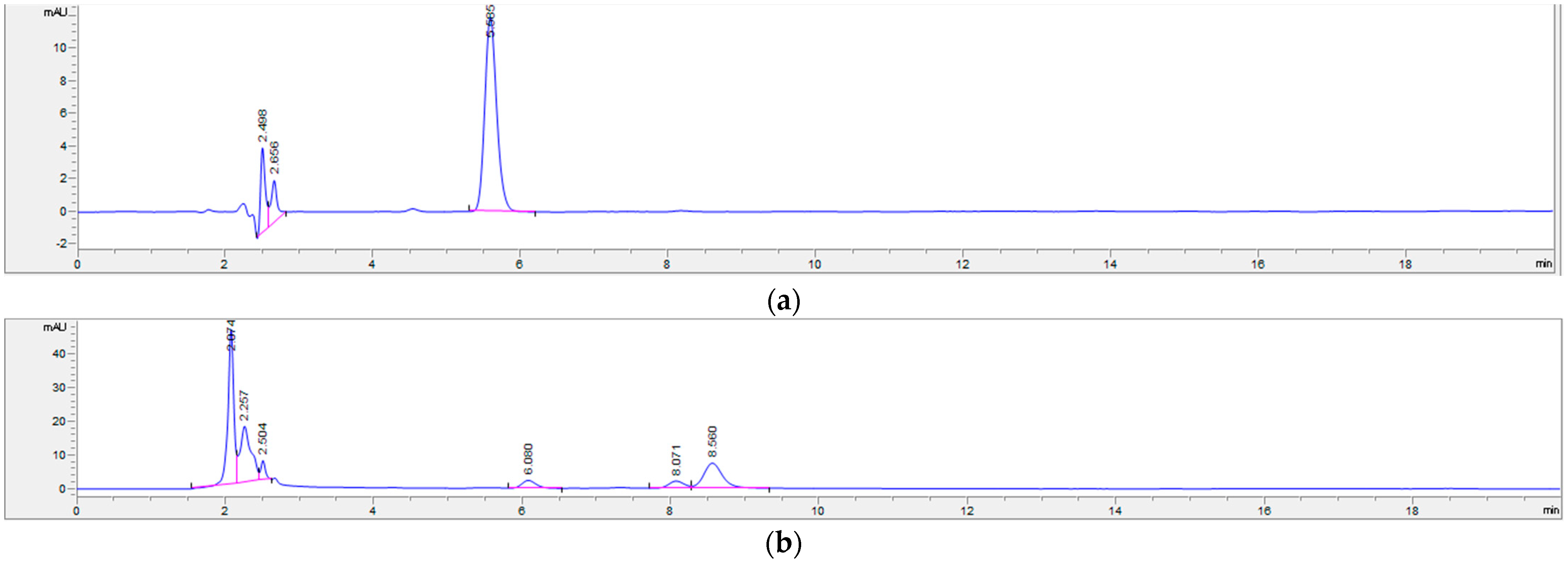
2.3.1. Identification of Aroma Metabolites
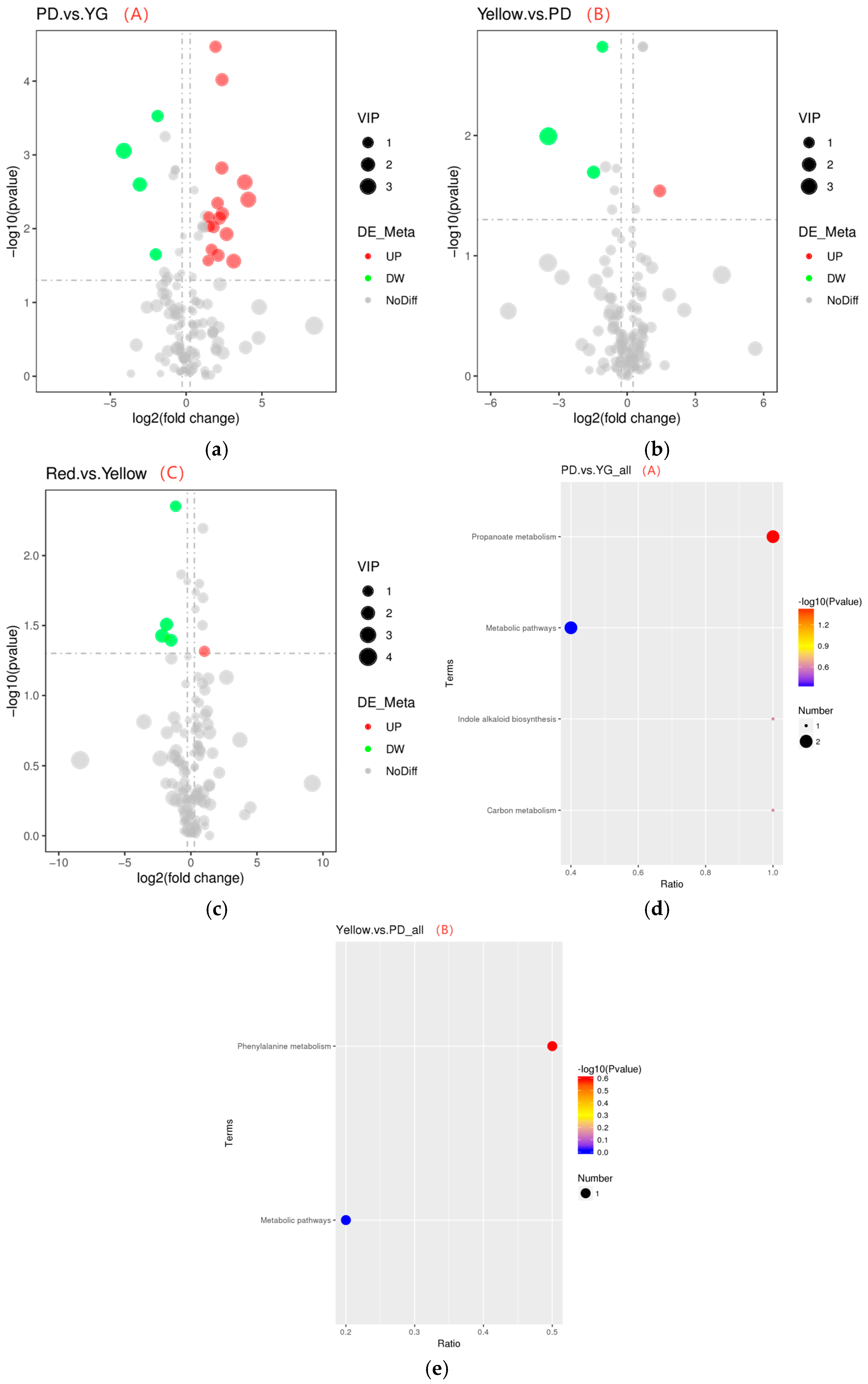
2.3.2. Identification and Analysis of Differential Aroma Metabolites
2.3.3. Metabolic Pathways of Differential Aroma Metabolites
3. Materials and Methods
3.1. Materials
3.2. Crude Fat Determination
3.3. Total Ash Content Measurement
3.4. Determination of Piperine Level
3.5. Steam Distillation Combined with GC-MS Analysis of Volatile Oil Compound (VOCs)
3.6. Solid-Phase Extraction Combined with GC-MS for the Analysis of Aroma Metabolites
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Category | LRI | Type ID | Component | Relative Percentage (%) ± SD Percentage | |||
|---|---|---|---|---|---|---|---|---|
| YG | PD | Yellow | Red | |||||
| 1 | 1160.95 | Alcohols8 | (Z)-p-2-menthen-1-ol | 0.08 ± 0.02 a | 0.04 ± 0.00 a | —— | 0.02 ± 0.00 a | |
| 2 | 1230.99 | Alcohols9 | (Z)-piperitol | 0.03 ± 0.01 a | 0.02 ± 0.00 a | —— | —— | |
| 3 | 1602.14 | Alcohols10 | (+)-viridiflorol | 0.04 ± 0.00 a | —— | —— | 0.02 ± 0.00 a | |
| 4 | 1623.13 | Alcohols11 | Nerolidol | —— | 0.03 ± 0.01 a | —— | —— | |
| 5 | 1585.47 | Alcohols12 | (±)-trans-nerolidol | —— | —— | 0.05 ± 0.00 a | —— | |
| 6 | 1584.46 | Alcohols13 | 1,7-dimethyl-4-propan-2-ylcyclodeca-2,7-dien-1-ol | —— | —— | —— | 0.01 ± 0.00 a | |
| 7 | 1656.94 | Alcohols14 | α-muurolol | —— | —— | —— | 0.01 ± 0.00 a | |
| 8 | 1421.97 | Alkenes25 | (−)-α-gurjunene | 0.06 ± 0.04 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b | |
| 9 | 1609.61 | Alkenes26 | Caryophyllene oxide | 0.07 ± 0.01 a | —— | 0.06 ± 0.01 a | 0.07 ± 0.00 a | |
| 10 | 945.62 | Alkenes27 | Camphene | —— | —— | —— | 0.03 ± 0.00 a | |
| 11 | 1348.95 | Alkenes28 | (−)-α-cubebene | —— | —— | —— | 0.08 ± 0.00 a | |
| 12 | 1438.85 | Alkenes29 | α-guaiene | —— | —— | —— | 0.01 ± 0.00 a | |
| 13 | 1264.51 | Esters2 | Linalyl anthranilate | —— | —— | 0.02 ± 0.00 a | —— | |
| 14 | 1184.43 | Ketones2 | 2,6,6-trimethyl-2,4-Cycloheptadien-1-one | —— | —— | 0.02 ± 0.00 a | —— | |
| 15 | 1172.96 | Ketones3 | Umbellulon | —— | —— | —— | 0.01 ± 0.00 a | |
| No. | Type ID | RT (min) | YG | PD | Yellow | Red |
|---|---|---|---|---|---|---|
| 1 | α-pinene, (+)- | 5.2686 | 2.61 ± 0.15 c | 3.81 ± 0.23 b | 3.91 ± 0.27 b | 5.04 ± 0.31 a |
| 2 | (−)-β-pinene | 7.38094 | 0.74 ± 0.05 b | 2.85 ± 0.19 a | 3.26 ± 0.38 a | 5.09 ± 0.49 a |
| 3 | Sabinene | 8.0155 | 1.61 ± 0.60 a | 10.26 ± 0.88 b | 9.18 ± 1.85 b | 8.45 ± 3.33 ab |
| 4 | Trans-2-pentenal | 8.30661 | 0.02 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.00 ± 0.00 a |
| 5 | Myrcene | 9.14548 | 1.81 ± 0.08 ab | 1.07 ± 0.32 bc | 1.07 ± 0.32 c | 1.14 ± 0.38 a |
| 6 | α-cubebene | 18.4893 | 2.04 ± 0.13 a | 0.71 ± 0.32 b | 0.87 ± 0.52 ab | 1.57 ± 0.96 ab |
| 7 | Copaene | 19.3107 | 9.11 ± 0.79 a | 6.07 ± 1.63 a | 4.02 ± 2.73 a | 10.65 ± 1.29 a |
| 8 | Linalool | 20.7709 | 17.17 ± 0.54 ab | 12.18 ± 3.20 b | 14.05 ± 0.98 a | 15.36 ± 1.04 ab |
| 9 | α-bergamotene, (e)-(−)- | 21.7403 | 1.17 ± 0.13 b | 1.05 ± 0.36 b | 1.52 ± 0.73 b | 2.17 ± 2.27 a |
| 10 | 4-terpineol, (−)- | 21.7968 | 0.86 ± 0.25 a | 2.28 ± 1.20 ab | 1.82 ± 0.59 b | 0.51 ± 0.00 b |
| 11 | 2-undecanone | 21.87925 | 4.43 ± 0.14 a | 3.90 ± 0.07 a | 4.59 ± 0.34 a | 4.04 ± 0.28 a |
| 12 | β-caryophyllene | 21.9228 | 1.66 ± 0.20 c | 2.24 ± 0.29 bc | 1.62 ± 0.8 b | 4.02 ± 0.57 a |
| 13 | α-terpineol | 23.8872 | 1.14 ± 0.09 a | 0.95 ± 0.69 a | 0.24 ± 0.15 a | 0.17 ± 0.15 a |
| 14 | Gamma-muurolene | 23.97825 | 1.14 ± 0.09 a | 0.47 ± 0.22 a | 0.97 ± 0.64 a | 0.62 ± 0.24 a |
| 15 | Germacrene D | 24.3739 | 1.31 ± 0.21 ab | 3.71 ± 0.84 a | 6.00 ± 2.54 ab | 12.68 ± 2.02 b |
| 16 | Bicyclogermacren | 24.9042 | 1.97 ± 0.37 a | 2.92 ± 0.05 b | 1.94 ± 1.23 b | 2.66 ± 1.19 a |
| 17 | β-cadinene | 25.6386 | 6.53 ± 0.54 a | 4.77 ± 2.00 ab | 7.64 ± 3.11 b | 8.3 ± 3.50 b |

References
- Wang, M.T.; Wang, J.H.; Zhao, K.K.; Zhu, Z.X.; Wang, H.F. Complete plastome sequence of Piper laetispicum (Piperaceae): An endemic plant species in South China. Mitochondrial DNA Part B Resour. 2018, 3, 1035–1036. [Google Scholar] [CrossRef]
- Xie, H.; Yan, M.C.; Jin, D.; Liu, J.J.; Yu, M.; Dong, D.; Cai, C.C.; Pan, S.L. Studies on antidepressant and antinociceptive effects of ethyl acetate extract from Piper laetispicum and structure-activity relationship of its amide alkaloids. Fitoterapia 2011, 82, 1086–1092. [Google Scholar] [CrossRef]
- Xie, H.; Jin, D.; Kang, Y.; Shi, X.; Liu, H.; Shen, H.; Chen, J.; Yan, M.; Liu, J.; Pan, S. The effect of Piper laetispicum extract (EAE-P) during chronic unpredictable mild stress based on interrelationship of inflammatory cytokines, apoptosis cytokines and neurotrophin in the hippocampus. BMC Complement. Altern. Med. 2015, 15, 240–250. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Xie, H.; Pan, S.L. Pharmacokinetics of laetispicine and its brain distribution in rats. Am. J. Chin. Med. 2010, 38, 895–907. [Google Scholar] [CrossRef]
- Abukawsar, M.M.; Saleh-e-In, M.M.; Ahsan, M.A.; Rahim, M.M.; Bhuiyan, M.N.H.; Roy, S.K.; Naher, S. Chemical, pharmacological and nutritional quality assessment of black pepper (Piper nigrum L.) seed cultivars. J. Food Biochem. 2018, 42, e12590. [Google Scholar] [CrossRef]
- Saleem, A.; Naureen, I.; Naeem, M.; Tasleem, G.; Ahmed, H.; Farooq, U. Therapeutic role of Piper Nigrum L. (Black Pepper) and pharmacological activities. Sch. Int. J. Biochem. 2022, 5, 15–21. [Google Scholar] [CrossRef]
- Wang, M.; Chittiboyina, A.G.; Parcher, J.F.; Ali, Z.; Ford, P.; Zhao, J.; Avula, B.; Wang, Y.H.; Khan, I.A. Piper nigrum oil—Determination of selected terpenes for quality evaluation. Planta Medica 2019, 85, 185–194. [Google Scholar]
- Hao, C.Y.; Fan, R.; Qin, X.W.; Hu, L.S.; Tan, L.H.; Xu, F.; Wu, B.D. Characterization of volatile compounds in ten Piper species cultivated in Hainan Island, South China. Int. J. Food Prop. 2018, 21, 633–644. [Google Scholar] [CrossRef]
- Yao, C.Y.; Wang, J.; Dong, D.; Qian, F.G.; Xie, J.; Pan, S.L. Laetispicine, an amide alkaloid from Piper laetispicum, presents antidepressant and antinociceptive effects in mice. Phytomedicine 2009, 16, 823–829. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- do Carmo, D.F.M.; Amaral, A.C.F.; Machado, G.M.; Leon, L.L.; Silva, J.R.D.A. Chemical and biological analyses of the essential oils and main constituents of Piper species. Molecules 2012, 17, 1819–1829. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Parker, J.K. Introduction to aroma compounds in foods. In Flavour Development, Analysis and Perception in Food and Beverages; Woodhead Publishing: Sawston, UK, 2015; pp. 3–30. [Google Scholar]
- Kim, S.; Kim, J.; Yun, E.J.; Kim, K.H. Food metabolomics: From farm to human. Curr. Opin. Biotechnol. 2016, 37, 16–23. [Google Scholar] [CrossRef]
- Kumar, A.; Mosa, K.A.; Ji, L.; Kage, U.; Dhokane, D.; Karre, S.; Madalageri, D.; Pathania, N. Metabolomics-assisted biotechnological interventions for developing plant-based functional foods and nutraceuticals. Crit. Rev. Food Sci. Nutr. 2017, 58, 1791–1807. [Google Scholar] [CrossRef]
- Sun, W.; Chen, Z.; Hong, J.; Shi, J. Promoting human nutrition and health through plant metabolomics: Current status and challenges. Biology 2021, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Rehman, S.; Noreen, H.; Zafar, M. Piper nigrum protects against Fe (II) mediated lipid peroxidation in phopholipids liposomes: Analytical and Biochemical Analysis. Lett. Appl. NanoBioSci 2021, 10, 2729–2741. [Google Scholar]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Floral scents and fruit aromas: Functions, compositions, biosynthesis, and regulation. Front. Plant Sci. 2022, 13, 860157–860179. [Google Scholar] [CrossRef]
- Raguso, R.A. More lessons from linalool: Insights gained from a ubiquitous floral volatile. Curr. Opin. Plant Biol. 2016, 32, 31–36. [Google Scholar] [CrossRef]
- Sköld, M.; Börje, A.; Harambasic, E.; Karlberg, A.T. Contact allergens formed on air exposure of linalool. Identification and quantification of primary and secondary oxidation products and the effect on skin sensitization. Chem. Res. Toxicol. 2004, 17, 1697–1705. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.A.; Gyawali, R.; Ibrahim, S.A.; Rajkovic, J.; Shinwari, Z.K.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857–112863. [Google Scholar] [CrossRef] [PubMed]
- Elisabetta, C.; Christian, B.; Sofia, M.; Serena, L.; Luigi, P.; Elena, D.; Hisanori, S.; Romana, F.; Giorgio, L. Involvement of mitochondrial permeability transition pore opening in alpha-bisabolol induced apoptosis. FEBS J. 2009, 276, 3990–4000. [Google Scholar]
- Akram, W.; Tagde, P.; Ahmed, S.; Arora, S.; Emran, T.B.; Babalghith, A.O.; Simal-Gandara, J. Guaiazulene and related compounds: A review of current perspective on biomedical applications. Life Sci. 2023, 316, 121389. [Google Scholar] [CrossRef] [PubMed]
- Kourounakis, A.P.; Rekka, E.A.; Kourounakis, P.N. Antioxidant activity of guaiazulene and protection against paracetamol hepatotoxicity in rats. J. Pharm. Pharmacol. 1997, 49, 938–942. [Google Scholar] [CrossRef]
- Wang, J.; Fan, R.; Zhong, Y.; Luo, H.; Hao, C. Effects of Cabya (Piper retrofractum Vahl.) fruit developmental stage on VOCs. Foods 2002, 11, 2528. [Google Scholar] [CrossRef]
- Saltveit Jr, M.E. Effect of alcohols and their interaction with ethylene on the ripening of epidermal pericarp discs of tomato fruit. Plant Physiol. 1989, 90, 167–174. [Google Scholar] [CrossRef]
- Ciftci, O.; Ozdemir, I.; Tanyildizi, S.; Yildiz, S.; Oguzturk, H. Antioxidative effects of curcumin, β-myrcene and 1, 8-cineole against 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced oxidative stress in rats’ liver. Toxicol. Ind. Health 2011, 27, 447–453. [Google Scholar] [CrossRef]
- Wu, H.; Huang, W.; Chen, Z.; Chen, Z.; Shi, J.; Kong, Q.; Yan, S. GC–MS-based metabolomic study reveals dynamic changes of chemical compositions during black tea processing. Food Res. Int. 2019, 120, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Hatai, M.; Horiyama, S.; Yoshikawa, N.; Kinoshita, E.; Kagota, S.; Shinozuka, K.; Nakamura, K. trans-2-Pentenal, an active compound in cigarette smoke, identified via its ability to form adducts with glutathione. Chem. Pharm. Bull. 2019, 67, 1000–1005. [Google Scholar] [CrossRef]
- Hatch, M.D.; Stumpf, P.K. Fat metabolism in higher plants: XVIII. Propionate metabolism by plant tissues. Arch. Biochem. Biophys. 1962, 96, 193–198. [Google Scholar] [CrossRef]
- Koukol, J.; Conn, E.E. The metabolism of aromatic compounds in higher plants: IV. Purification and properties of the phenylalanine deaminase of Hordeum vulgare. J. Biol. Chem. 1961, 236, 2692–2698. [Google Scholar] [CrossRef] [PubMed]
- Rychter, A.M.; Rao, I.M. Role of phosphorus in photosynthetic carbon metabolism. Handb. Photosynth. 2005, 2, 123–148. [Google Scholar]
- Omar, F.; Tareq, A.M.; Alqahtani, A.M.; Dhama, K.; Sayeed, M.A.; Emran, T.B.; Simal-Gandara, J. Plant-based indole alkaloids: A comprehensive overview from a pharmacological perspective. Molecules 2021, 26, 2297. [Google Scholar] [CrossRef]
- GB/T 5009.4-2016; Determination of Ash in Food. Health and Family Planning Commission, PRC: Beijing, China, 2016. Available online: https://www.chinesestandard.net/PDF.aspx/GB5009.4-2016 (accessed on 7 May 2024).
- GB/T 17528-2009; Determination of Piperine Content by High Performance Liquid Chromatography. General Administration of Quality Supervision, Inspection and Quarantine of the People‘s Republic of China: Beijing, China, 2009. Available online: https://www.chinesestandardslibrary.com/p/chinese-standard-gb-t-17528-2009/ (accessed on 7 May 2024).
- GB/T 17527-2009; Determination of Essential oil Content of Pepper. General Administration of Quality Supervision, Inspection and Quarantine of the People‘s Republic of China: Beijing, China, 2009. Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT17527-2009 (accessed on 7 May 2024).

| Matter (%) | YG | PD | Yellow | Red |
|---|---|---|---|---|
| Crude fat | 13.9 | 14.34 | 11.15 | 7.31 |
| Ash | 10.97 | 9.56 | 6.59 | 6.89 |
| Piperine | 0.00 | 0.00 | 0.08 | 0.00 |
| Protein | 20.24 | 19.57 | 11.57 | 13.71 |
| No. | Category | LRI | Type ID | Component | Relative Percentage (%) ± SD Percentage | |||
|---|---|---|---|---|---|---|---|---|
| YG | PD | Yellow | Red | |||||
| 1 | Alcohols | 1084.48 | Alcohols1 | Trans-β-Terpineol | 0.19 ± 0.07 a | 0.49 ± 0.32 a | 0.14 ± 0.00 a | 0.09 ± 0.03 a |
| 2 | 1624.20 | Alcohols2 | Linalool | 15.70 ± 2.43 a | 8.54 ± 0.00 b | 6.24 ± 0.16 b | 2.42 ± 0.02 c | |
| 3 | 1624.20 | Alcohols3 | (E)-p-2-menthen-1-ol | 0.10 ± 0.03 a | 0.06 ± 0.00 ab | —— | 0.03 ± 0.00 b | |
| 4 | 1140.63 | Alcohols4 | 4-terpineol | 2.87 ± 0.37 a | 1.94 ± 0.06 b | 0.70 ± 0.01 c | 0.75 ± 0.01 c | |
| 5 | 1624.20 | Alcohols5 | α-terpineol | 0.30 ± 0.07 a | 0.18 ± 0.10 ab | 0.06 ± 0.00 c | 0.04 ± 0.00 c | |
| 6 | 1296.34 | Alcohols6 | (Z)-linalool oxide (furanoid) | 0.13 ± 0.05 a | —— | —— | —— | |
| 7 | 1624.20 | Alcohols7 | α-bisabolol | 0.21 ± 0.17 a | —— | —— | —— | |
| 8 | Alkenes | 936.87 | Alkenes1 | (−)-α-pinene | 0.57 ± 0.07 a | 0.33 ± 0.02 b | 0.33 ± 0.01 b | —— |
| 9 | 979.49 | Alkenes2 | Sabinene | 34.83 ± 8.19 b | 67.52 ± 0.43 a | 70.64 ± 1.24 a | 76.14 ± 0.38 a | |
| 10 | 997.47 | Alkenes3 | β-pinene | 0.62 ± 0.28 a | —— | —— | 0.24 ± 0.01 a | |
| 11 | 1010.10 | Alkenes4 | α-phellandrene | 0.15 ± 0.07 a | 0.08 ± 0.00 a | 0.11 ± 0.01 a | 0.06 ± 0.00 a | |
| 12 | 1022.66 | Alkenes5 | Terpinolene | 0.29 ± 0.15 a | 0.09 ± 0.02 ab | 0.06 ± 0.01 b | 0.10 ± 0.00 ab | |
| 13 | 1036.45 | Alkenes6 | α-pinene | 3.72 ± 1.73 a | 2.16 ± 0.12 a | 1.51 ± 0.85 a | 4.31 ± 0.08 a | |
| 14 | 1067.00 | Alkenes7 | γ-terpinene | 0.96 ± 0.22 a | 0.25 ± 0.00 b | 0.06 ± 0.00 b | 0.19 ± 0.02 b | |
| 15 | 1347.75 | Alkenes8 | 1-ethenyl-1-methyl-2,4-bis(1-methylethylidene)-cyclohexane | 0.61 ± 0.06 a | 0.33 ± 0.01 b | 0.56 ± 0.10 a | 0.12 ± 0.00 c | |
| 16 | 1390.39 | Alkenes9 | α-copaene | 19.51 ± 3.52 a | 10.53 ± 0.17 b | 7.06 ± 0.03 bc | 5.11 ± 0.02 c | |
| 17 | 1402.87 | Alkenes10 | β-cubebene | 1.36 ± 0.89 a | 0.32 ± 0.01 a | 0.95 ± 0.02 a | 0.09 ± 0.10 a | |
| 18 | 1407.01 | Alkenes11 | 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl)-cyclohexane | 0.32 ± 0.24 a | 0.20 ± 0.16 a | 0.12 ± 0.01 a | 0.04 ± 0.00 a | |
| 19 | 1436.62 | Alkenes12 | trans-caryophyllene | 6.57 ± 0.21 a | 3.38 ± 0.00 c | 4.20 ± 0.12 b | 2.37 ± 0.01 d | |
| 20 | 1448.41 | Alkenes13 | (E)-α-bergamotene | 0.99 ± 0.56 a | —— | 0.16 ± 0.00 a | 0.06 ± 0.00 a | |
| 21 | 1452.23 | Alkenes14 | Aromadendrene | 0.06 ± 0.03 a | 0.12 ± 0.07 a | 0.05 ± 0.03 a | —— | |
| 22 | 1472.29 | Alkenes15 | α-caryophyllene | 0.88 ± 0.04 b | 0.44 ± 0.01 c | 1.19 ± 0.06 a | 0.51 ± 0.02 c | |
| 23 | 1494.59 | Alkenes16 | α-amorphene | 0.11 ± 0.09 a | 0.12 ± 0.08 a | 0.06 ± 0.02 a | 0.02 ± 0.00 a | |
| 24 | 1500.00 | Alkenes17 | Germacrene D | 3.74 ± 1.32 a | 1.30 ± 0.29 b | 4.10 ± 0.32 a | 3.64 ± 0.05 a | |
| 25 | 1518.92 | Alkenes18 | α-muurolene | 0.31 ± 0.16 a | —— | —— | 0.06 ± 0.00 a | |
| 26 | 1525.34 | Alkenes19 | β-bisabolene | 0.35 ± 0.01 a | 0.16 ± 0.01 b | 0.12 ± 0.01 c | 0.10 ± 0.00 c | |
| 27 | 1542.91 | Alkenes20 | (+)-δ-cadinene | 3.69 ± 0.22 a | 1.49 ± 0.07 bc | 1.22 ± 0.06 c | 1.65 ± 0.02 b | |
| 28 | 998.16 | Alkenes21 | β-myrcene | —— | 0.13 ± 0.00 a | 0.13 ± 0.00 a | —— | |
| 29 | 1347.45 | Alkenes22 | Santolina triene | —— | 0.16 ± 0.11 a | —— | —— | |
| 30 | 925.35 | Alkenes23 | 4-methyl-1-(1-methylethyl) bicyclo[3.1.0]hexane didehydro deriv | —— | —— | —— | 1.42 ± 0.01 a | |
| 31 | 1686.12 | Alkenes24 | Guaiazulene | —— | —— | —— | 0.01 ± 0.00 a | |
| 32 | Esters | 1239.44 | Esters1 | 2-ethylhexyl acrylate | 0.10 ± 0.02 a | 0.06 ± 0.00 a | 0.08 ± 0.00 a | —— |
| 33 | Ketones | 1293.80 | Ketones1 | 2-undecanone | 0.52 ± 0.23 a | 0.14 ± 0.00 b | 0.13 ± 0.01 b | 0.05 ± 0.00 b |
| 34 | Benzenes | 1025.12 | Benzenes1 | o-cymene | —— | —— | —— | 0.10 ± 0.01 a |
| Principal Component | Initial Eigenvalues | Extraction Sums of Squared Loadings | ||||
|---|---|---|---|---|---|---|
| Total | Variance Contribution Rate (%) | Cumulative (%) | Total | Variance Contribution Rate (%) | Cumulative (%) | |
| 1 | 26.79 | 54.67 | 54.67 | 26.79 | 54.67 | 54.67 |
| 2 | 13.90 | 28.36 | 83.02 | 13.90 | 28.36 | 83.02 |
| 3 | 8.32 | 16.98 | 100.00 | 8.32 | 16.98 | 100.00 |
| VOCs | PC1 | PC2 | PC3 |
|---|---|---|---|
| Alcohols2 | 0.998 | −0.019 | −0.064 |
| Alcohols4 | 0.914 | 0.098 | −0.393 |
| Alcohols5 | 0.949 | 0.038 | −0.314 |
| Alcohols9 | 0.937 | 0.134 | −0.322 |
| Alcohols6 | 0.908 | 0.410 | 0.089 |
| Alcohols7 | 0.908 | 0.410 | 0.089 |
| Alcohols11 | −0.025 | −0.581 | −0.814 |
| Alcohols12 | −0.197 | −0.553 | 0.81 |
| Alcohols14 | −0.686 | 0.723 | −0.085 |
| Alkenes1 | 0.949 | −0.293 | 0.112 |
| Alkenes2 | −0.971 | −0.239 | −0.03 |
| Alkenes4 | 0.938 | −0.027 | 0.345 |
| Alkenes5 | 0.859 | 0.511 | −0.042 |
| Alkenes6 | 0.026 | 0.976 | −0.216 |
| Alkenes7 | 0.895 | 0.433 | −0.109 |
| Alkenes9 | 0.984 | 0.132 | −0.122 |
| Alkenes11 | 0.980 | −0.081 | −0.179 |
| Alkenes25 | 0.909 | 0.403 | 0.105 |
| Alkenes12 | 0.956 | 0.036 | 0.290 |
| Alkenes15 | 0.291 | −0.273 | 0.917 |
| Alkenes16 | 0.772 | −0.479 | −0.419 |
| Alkenes17 | 0.022 | 0.447 | 0.894 |
| Alkenes19 | 0.964 | 0.263 | −0.036 |
| Alkenes20 | 0.857 | 0.515 | −0.014 |
| Alkenes21 | −0.195 | −0.98 | 0.029 |
| Esters1 | 0.854 | −0.43 | 0.293 |
| Esters2 | −0.197 | −0.553 | 0.810 |
| Ketones1 | 0.969 | 0.232 | 0.085 |
| Ketones3 | −0.686 | 0.723 | −0.085 |
| Benzenes1 | −0.686 | 0.723 | −0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Fang, Y.; Zhang, D.; Wang, J.; Huang, Y.; Hao, C.; Fan, R. Wild Pepper (Piper laetispicum) Fruit Quality Traits at Different Developmental Stages. Molecules 2024, 29, 4008. https://doi.org/10.3390/molecules29174008
Zhao Z, Fang Y, Zhang D, Wang J, Huang Y, Hao C, Fan R. Wild Pepper (Piper laetispicum) Fruit Quality Traits at Different Developmental Stages. Molecules. 2024; 29(17):4008. https://doi.org/10.3390/molecules29174008
Chicago/Turabian StyleZhao, Zhican, Yiming Fang, Dan Zhang, Jue Wang, Yanli Huang, Chaoyun Hao, and Rui Fan. 2024. "Wild Pepper (Piper laetispicum) Fruit Quality Traits at Different Developmental Stages" Molecules 29, no. 17: 4008. https://doi.org/10.3390/molecules29174008
APA StyleZhao, Z., Fang, Y., Zhang, D., Wang, J., Huang, Y., Hao, C., & Fan, R. (2024). Wild Pepper (Piper laetispicum) Fruit Quality Traits at Different Developmental Stages. Molecules, 29(17), 4008. https://doi.org/10.3390/molecules29174008





