Ionic Liquid-Based Extraction Strategy for the Efficient and Selective Recovery of Scandium
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening of ILs
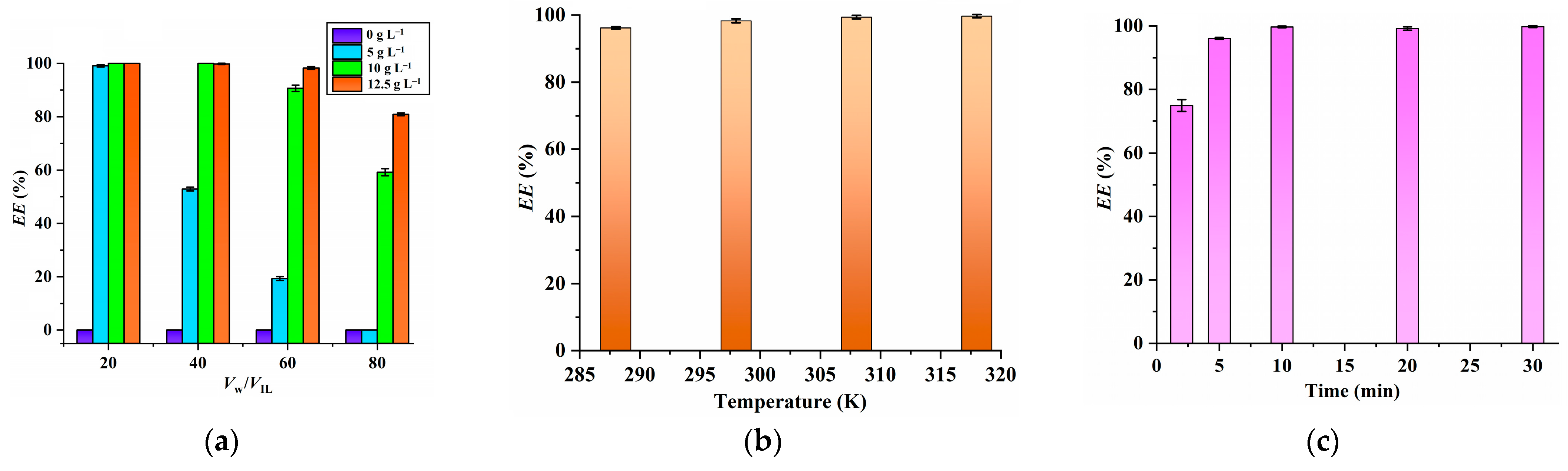
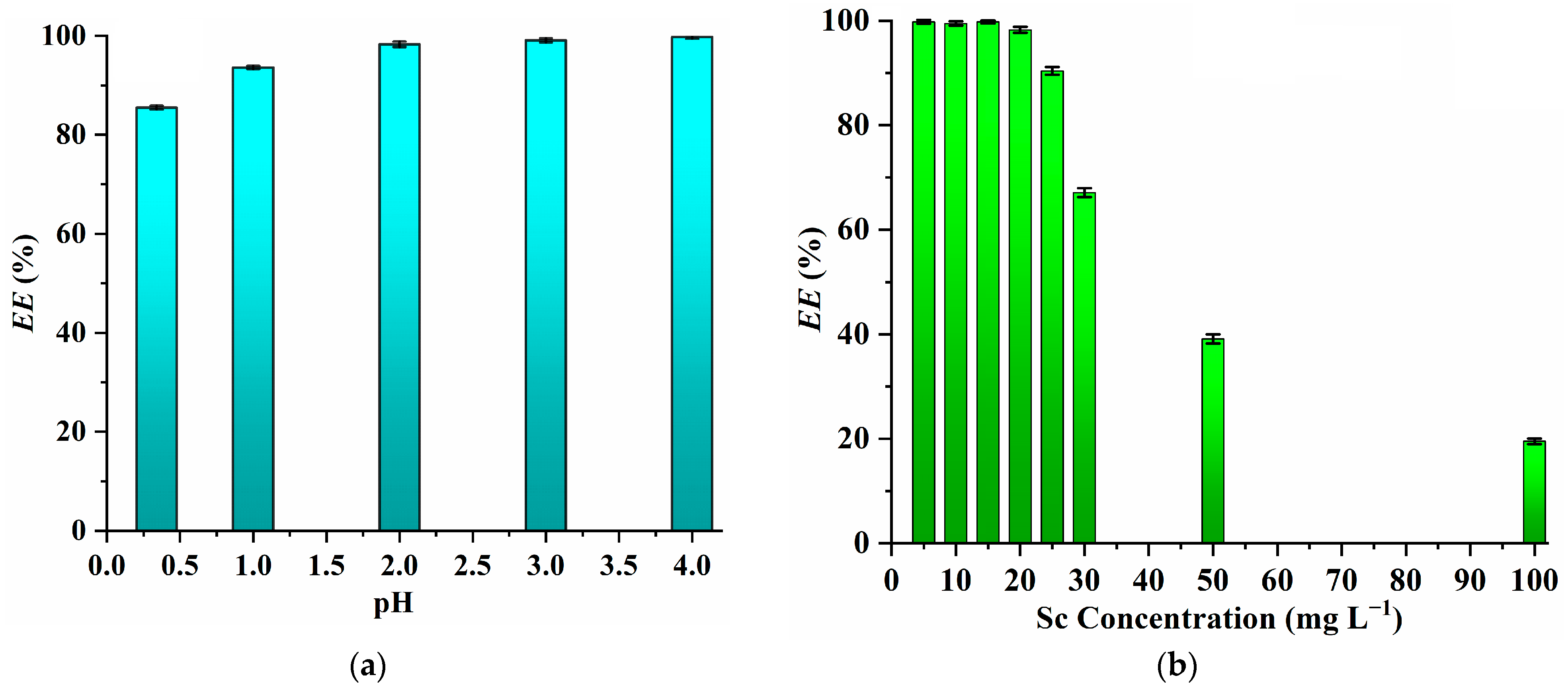
2.2. Effect of the PPAH Concentration, Temperature, Extraction Time, Initial pH Value of Aqueous Phase and Sc Concentration
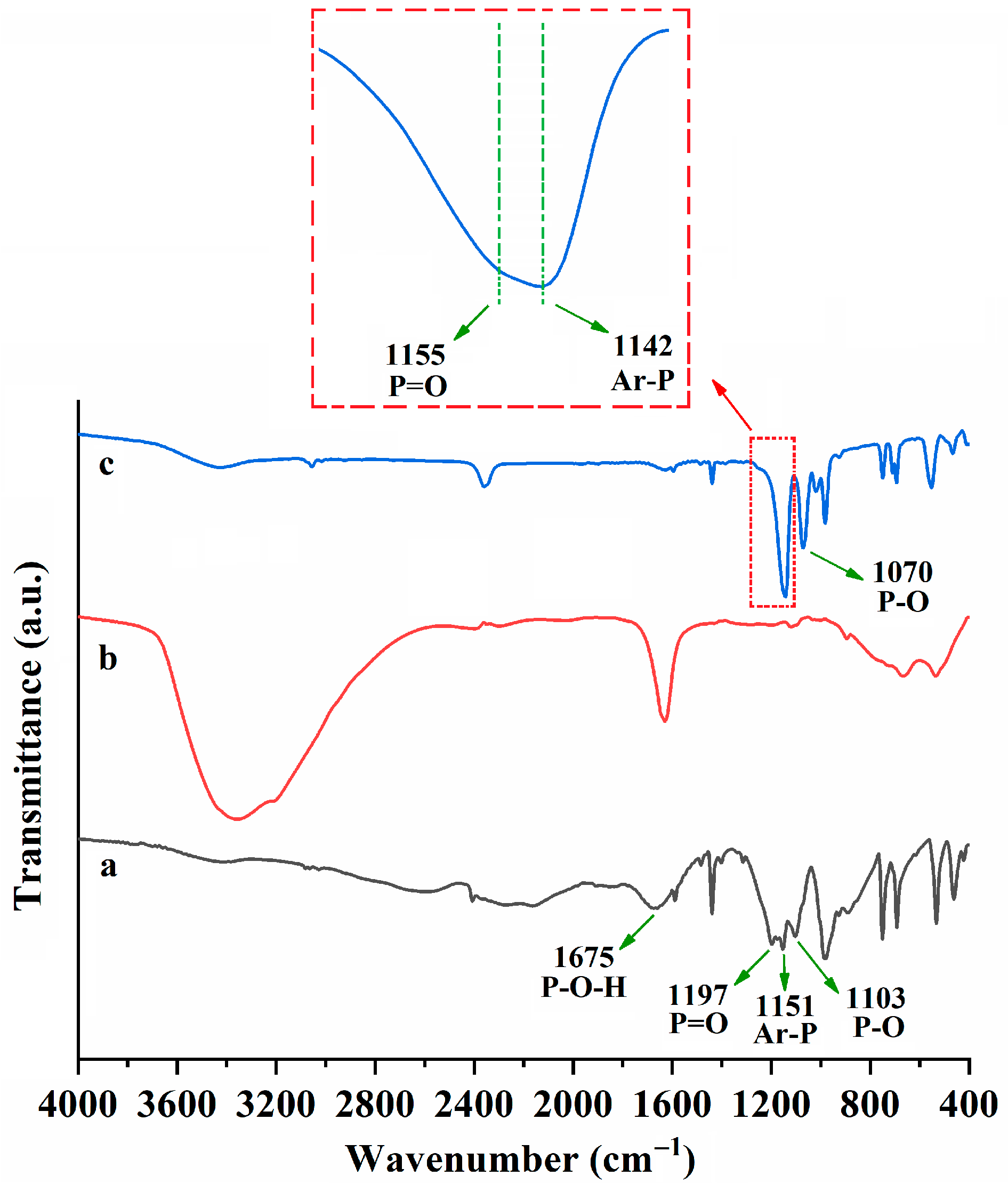
2.3. Extraction Mechanism
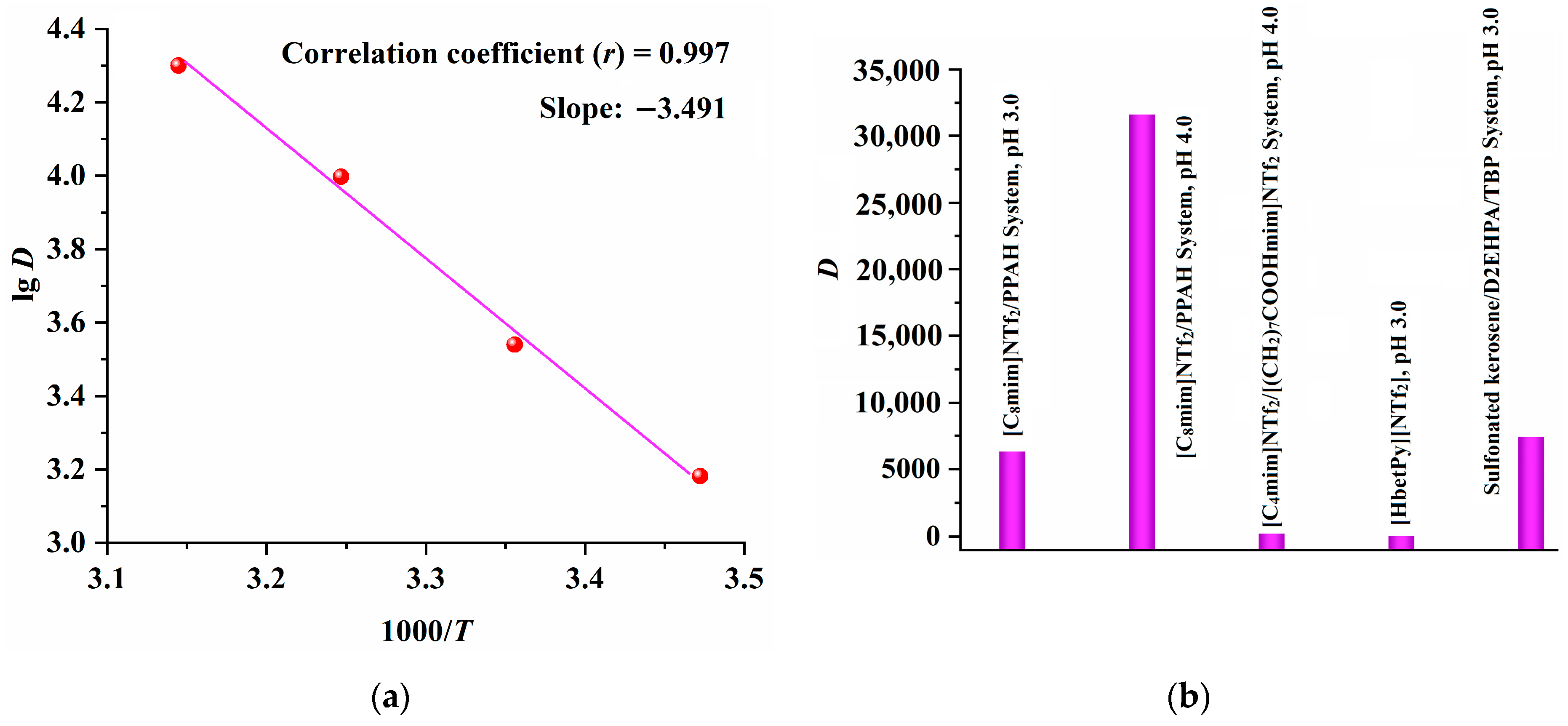
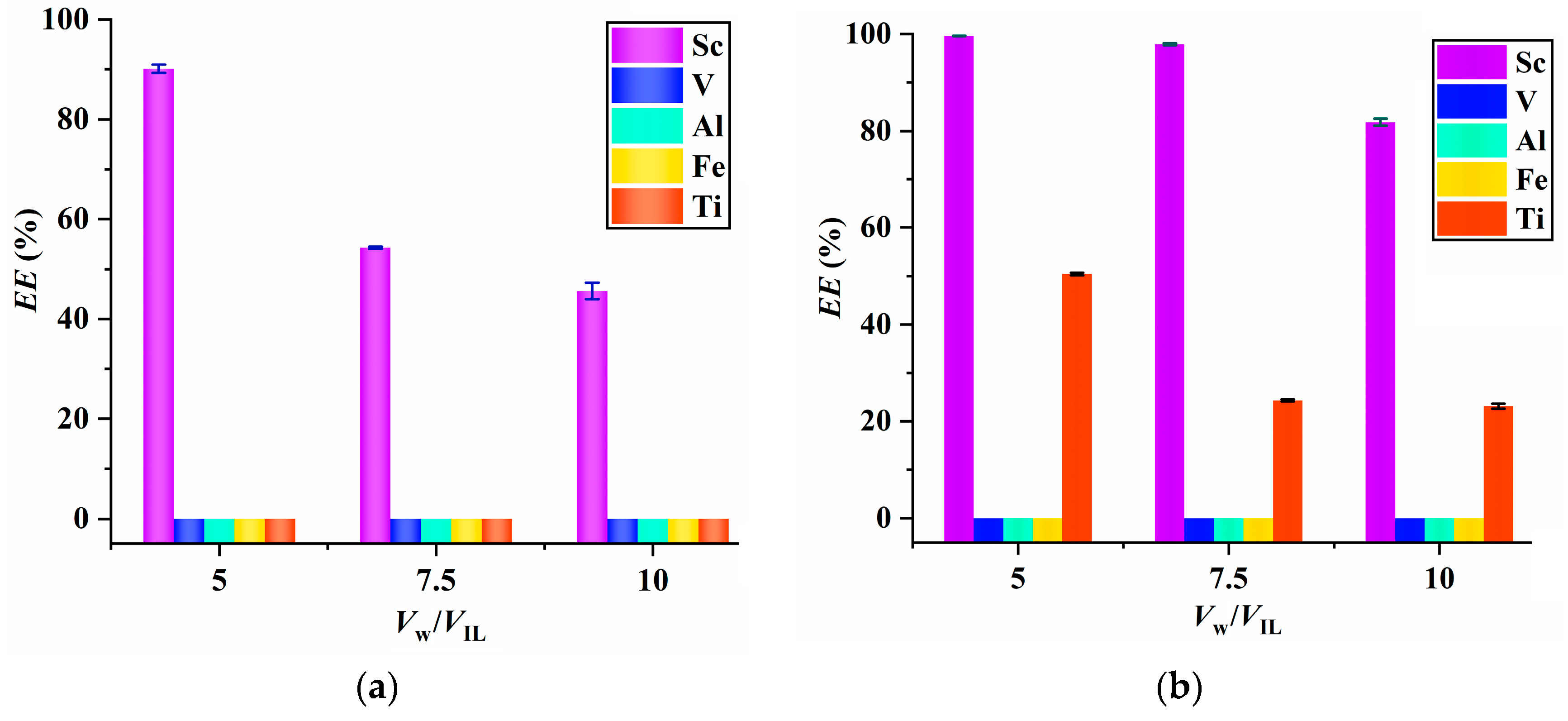
2.4. Comparison of [C8mim]NTf2/PPAH with the Reported Extraction Systems
2.5. Practical Application
3. Materials and Methods
3.1. Materials
3.2. Determination of the Polarities and Viscosities of the ILs
3.3. Determination of the Solubilities of PPAH in ILs
3.4. Extraction Procedure
3.5. Determination of the Stability Constant of Sc (PPA)3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garapati, M.S.; Sundara, R. Highly efficient and ORR active platinum-scandium alloy-partially exfoliated carbon nanotubes electrocatalyst for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2019, 44, 10951–10963. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, L.; Ding, M.; Bao, N.; Wu, X.; Narsu, B. Chemical-structural coupling in magnesium-scandium alloys. Results Phys. 2023, 50, 106582. [Google Scholar] [CrossRef]
- Loveless, C.S.; Blanco, J.R.; Diehl, G.L., III; Elbahrawi, R.T.; Carzaniga, T.S.; Braccini, S.; Lapi, S.E. Cyclotron production and separation of scandium radionuclides from natural titanium metal and titanium dioxide targets. J. Nucl. Med. 2021, 62, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Huclier-Markai, S.; Alliot, C.; Kerdjoudj, R.; Mougin-Degraef, M.; Chouin, N.; Haddad, F. Promising scandium radionuclides for nuclear medicine: A review on the production and chemistry up to in vivo proofs of concept. Cancer Biother. Radiopharm. 2018, 33, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczak, R.; Huclier-Markai, S.; Alliot, C.; Haddad, F.; Szikra, D.; Forgacs, V.; Garnuszek, P. Production of scandium radionuclides for theranostic applications: Towards standardization of quality requirements. EJNMMI Radiopharm. Chem. 2021, 6, 19. [Google Scholar] [CrossRef]
- Vind, J.; Malfliet, A.; Bonomi, C.; Paiste, P.; Sajó, I.E.; Blanpain, B.; Tkaczyk, A.H.; Vassiliadou, V.; Panias, D. Modes of occurrences of scandium in greek bauxite and bauxite residue. Miner. Eng. 2018, 123, 35–48. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, B.; Wang, C.; Chen, Y.; Wang, L. Separation and recovery of scandium from high pressure sulfuric acid leach liquor of limonitic laterite. Process Saf. Environ. Prot. 2022, 165, 161–172. [Google Scholar] [CrossRef]
- Yu, Q.; Ning, S.; Zhang, W.; Wang, X.; Wei, Y. Recovery of scandium from sulfuric acid solution with a macro porous TRPO/SiO2-P adsorbent. Hydrometallurgy 2018, 181, 74–81. [Google Scholar] [CrossRef]
- Remmen, K.; Schäfer, R.; Hedwig, S.; Wintgens, T.; Wessling, M.; Lenz, M. Layer-by-layer membrane modification allows scandium recovery by nanofiltration. Environ. Sci. Water Res. Technol. 2019, 5, 1683–1688. [Google Scholar] [CrossRef]
- Salman, A.D.; Juzsakova, T.; Jalhoom, M.G.; Abdullah, T.A.; Le, P.C.; Viktor, S.; Domokos, E.; Nguyen, X.C.; La, D.D.; Nadda, A.K.; et al. A selective hydrometallurgical method for scandium recovery from a real red mud leachate: A comparative study. Environ. Pollut. 2022, 308, 119596. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, D.; Dehaen, W.; Binnemans, K. Solvent extraction of scandium(III) by an aqueous biphasic system with a nonfluorinated functionalized ionic liquid. Ind. Eng. Chem. Res. 2015, 54, 8988–8996. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Pei, Y.; Wang, J. Selective separation of scandium (III) from rare earth metals by carboxyl-functionalized ionic liquids. Sep. Purif. Technol. 2017, 178, 261–268. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Guo, W.; Liu, D.; Ding, Y. Enhanced homogeneous liquid−liquid extraction for the selective recovery of Sc(III) by novel UCST-type ionic liquids. ACS Sustain. Chem. Eng. 2021, 9, 9932–9940. [Google Scholar] [CrossRef]
- Namazian, M.; Halvani, S. Calculations of pKa values of carboxylic acids in aqueous solution using density functional theory. J. Chem. Thermodyn. 2006, 38, 1495–1502. [Google Scholar] [CrossRef]
- Citra, M.J. Estimating the pKa of phenols, carboxylic acids and alcohols from semi-empirical quantum chemical methods. Chemosphere 1999, 38, 191–206. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, M.; Xie, Y.; Song, J.; Huang, T.; Li, X.M.; He, T. From trace to pure: Recovery of scandium from the waste acid of titanium pigment production by solvent extraction. Process Saf. Environ. Prot. 2019, 121, 118–124. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Zhang, G.; Zeng, L.; Cao, Z.; Guan, W.; Wang, L. Separation and recovery of scandium and titanium from spent sulfuric acid solution from the titanium dioxide production process. Hydrometallurgy 2018, 178, 1–6. [Google Scholar] [CrossRef]
- Soeta, T.; Takashita, S.; Sakata, Y.; Ukaji, Y. Phosphinic acid-promoted addition reaction of isocyanides to (Z)-hydroximoyl chlorides: Efficient synthesis of α-(hydroxyimino)amides. Org. Biomol. Chem. 2016, 14, 694–700. [Google Scholar] [CrossRef]
- Elstone, N.S.; Shimizu, K.; Shaw, E.V.; Lane, P.D.; D’Andrea, L.; Demé, B.; Mahmoudi, N.; Rogers, S.E.; Youngs, S.; Costen, M.L.; et al. Understanding the liquid structure in mixtures of ionic liquids with semiperfluoroalkyl or alkyl chains. J. Phys. Chem. B 2023, 127, 7394–7407. [Google Scholar] [CrossRef]
- Moschovi, A.M.; Dracopoulos, V. Structure of protic (HCnImNTf2, n = 0–12) and aprotic (C1CnImNTf2, n = 1–12) imidazolium ionic liquids: A vibrational spectroscopic study. J. Mol. Liq. 2015, 210, 189–199. [Google Scholar] [CrossRef]
- Watanabe, S.; Pilkington, G.A.; Oleshkevych, A.; Pedraz, P.; Radiom, M.; Welbourn, R.; Glavatskih, S.; Rutland, M.W. Interfacial structuring of non-halogenated imidazolium ionic liquids at charged surfaces: Effect of alkyl chain length. Phys. Chem. Chem. Phys. 2020, 22, 8450–8460. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Onghena, B.; Li, X.; Zhang, Z.; Binnemans, K. Enhancing metal separations using hydrophilic ionic liquids and analogues as complexing agents in the more polar phase of liquid–liquid extraction systems. Ind. Eng. Chem. Res. 2019, 58, 15628–15636. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Torkaman, R. Extraction of dysprosium from waste neodymium magnet solution with ionic liquids and ultrasound irradiation procedure. Korean J. Chem. Eng. 2022, 39, 134–145. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Feng, D.; Kang, X.; Sun, Y.; Zhao, L.; Liang, H. Enhancing extraction ability by rational design of phosphoryl functionalized ionic liquids and mechanistic investigation on neodymium (III) extraction. J. Rare Earths 2016, 34, 83–90. [Google Scholar] [CrossRef]
- Can, E.; Jalal, A.; Zirhlioglu, I.G.; Uzun, A.; Yildirim, R. Predicting water solubility in ionic liquids using machine learning towards design of hydro-philic/phobic ionic liquids. J. Mol. Liq. 2021, 332, 115848. [Google Scholar] [CrossRef]
- de Melo, E.B. A structure–activity relationship study of the toxicity of ionic liquids using an adapted Ferreira–Kiralj hydrophobicity parameter. Phys. Chem. Chem. Phys. 2015, 17, 4516–4523. [Google Scholar] [CrossRef]
- Mulia, K.; Fauzia, F.; Krisanti, E.A. Polyalcohols as hydrogen-bonding donors in choline chloride-based deep eutectic solvents for extraction of xanthones from the pericarp of Garcinia mangostana L. Molecules 2019, 24, 636. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Su, S.G.; Gung, S.T.; Lin, M.W.; Lin, Y.C.; Ou-Yang, W.C.; Sun, I.W.; Lai, C.A. Synthesis and characterization of protic ionic liquids containing cyclic amine cations and tetrafluoroborate anion. J. Iran. Chem. Soc. 2011, 8, 149–165. [Google Scholar] [CrossRef]
- Carmichael, A.J.; Seddon, K.R. Polarity study of some 1-alkyl-3-methylimidazolium ambient-temperature ionic liquids with the solvatochromic dye, Nile Red. J. Phys. Org. Chem. 2000, 13, 591–595. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.; Yan, L.; Li, J.; Hua, S.; Song, L.; Wang, R.; Sha, S. Enhanced extraction of antioxidants from aqueous solutions by ionic liquids. Sep. Purif. Technol. 2017, 172, 480–488. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Z.; Guo, H.; Gao, P.; Lu, R.; Zhou, W.; Zhang, S.; Gao, H. Ionic liquid-based totally organic solvent-free emulsification microextraction coupled with high performance liquid chromatography for the determination of three acaricides in fruit juice. Talanta 2013, 115, 556–562. [Google Scholar] [CrossRef]
- Fan, Y.; Cai, D.; Yang, L.; Chen, X.; Zhang, L. Extraction behavior of nicotinic acid and nicotinamide in ionic liquids. Chem. Eng. Res. Des. 2019, 146, 336–343. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.; Song, L.; Li, J.; Zhang, L. Effective extraction of quinine and gramine from water by hydrophobic ionic liquids: The role of anion. Chem. Eng. Res. Des. 2017, 119, 58–65. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, G.H.; He, L.; Yong, F.; Zhu, Q.H.; Zhang, L.; Qin, S.; Tao, G.H. Nanopockets with a thermoresponsive nitrate ionic liquid for highly efficient uranium extraction at high acidity. ACS Appl. Nano Mater. 2022, 5, 14893–14901. [Google Scholar] [CrossRef]
- Wu, X.; Wang, K.; Xiong, Z.; Ye, X. Solubility of α-calcium sulfate hemihydrate in Ca−Mg−K chloride salt solution at (353.0 to 371.0) K. J. Chem. Eng. Data 2013, 58, 48–54. [Google Scholar] [CrossRef]
- Zhu, X.; Ma, C.; Li, W. Sustainable selective recovery of sulfuric acid and vanadium from acidic wastewater with two-step solvent extraction. ACS Omega 2023, 8, 27127–27138. [Google Scholar] [CrossRef] [PubMed]
- Kajihara, K.; Takahashi, D.; Kobayashi, H.; Mandai, T.; Imai, H.; Kanamura, K. Phenylphosphonate surface functionalisation of MgMn2O4 with 3D open-channel nanostructures for composite slurry-coated cathodes of rechargeable magnesium batteries operated at room temperature. RSC Adv. 2021, 11, 19076–19082. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Chen, J.; Zou, D.; Deng, Y.; Li, D. Application of P507 and isooctanol extraction system in recovery of scandium from simulated red mud leach solution. J. Rare Earth. 2019, 37, 1002–1008. [Google Scholar] [CrossRef]
- Donner, E.M.; Kennedy, G.L., Jr.; Stadler, J.S. Four week feeding toxicity study with phenylphosphinic acid in rats. Drug Chem. Toxicol. 2003, 26, 125–133. [Google Scholar] [CrossRef]
- Pelletier, G.; Rigden, M.; Wang, G.S.; Caldwell, D.; Siddique, S.; Leingartner, K.; Kosarac, I.; Cakmak, S.; Kubwabo, C. Comparison of tris(2-ethylhexyl) phosphate and di(2-ethylhexyl) phosphoric acid toxicities in a rat 28-day oral exposure study. J. Appl. Toxicol. 2020, 40, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Carrington, C.D.; Lapadula, D.M.; Othman, M.; Farr, C.; Nair, R.S.; Johannsen, F.; Abou-Donia, M.B. Assessment of the delayed neurotoxicity of tributyl phosphate, tributoxyethyl phosphate, and dibutylphenyl phosphate. Toxicol. Ind. Health 1990, 6, 415–423. [Google Scholar] [CrossRef]
- Bukowsky, H.; Uhlemann, E.; Gloe, K.; Miihl, P. The separation of calcium and magnesium fromlithium chloride by liquid-liquid extraction with di(2-ethylhexyl)phosphoric acid. Hydrometallurgy 1992, 28, 323–329. [Google Scholar] [CrossRef]
- Apelblat, A.; Hornik, A. Gas-chromatographic studies of the system uranyl nitrate + TBP + diluent + water. Trans. Faraday Soc. 1967, 63, 185–194. [Google Scholar] [CrossRef]
- Sigma-Aldrich Chemical Reagents. Available online: https://www.sigmaaldrich.cn/CN/en/life-science/sigma-aldrich?utm_campaign=Brand%20Zone%20-%20China&utm_medium=Sigma-Aldrich_brandzone&utm_source=baidu&utm_content=description&utm_term=description (accessed on 4 August 2024).
- Zhang, L.; Qian, K.; Wang, X.; Zhang, F.; Shi, X.; Jiang, Y.; Liu, S.; Jaroniec, M.; Liu, J. Yolk–shell-structured aluminum phenylphosphonate microspheres with anionic core and cationic shell. Adv. Sci. 2016, 3, 1500363. [Google Scholar] [CrossRef] [PubMed]
- Amatucci, G.G.; Safari, A.; Shokoohi, F.K.; Wilkens, B.J. Lithium scandium phosphate-based electrolytes for solid state lithium rechargeable microbatteries. Solid State Ion. 1993, 60, 357–365. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Wang, N.; Gu, H. Selective extraction of rare earth elements from red mud using oxalic and sulfuric acids. J. Environ. Chem. Eng. 2022, 10, 108650. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Xing, B.; Zhang, Y. Extraction of scandium from red mud by acid leaching with CaF2 and solvent extraction with P507. J. Rare Earths 2020, 38, 1003–1008. [Google Scholar] [CrossRef]

| IL | Water Solubility (g L−1) | Viscosity (mPa s) | Polarity (ENR, kcal mol−1) d | PPAH Solubility in IL (g L−1) d |
|---|---|---|---|---|
| [C6mim]ClO4 | 32 a | 107 a | 51.7 | 27.5 |
| [C6mim]NTf2 | 2.6 b | 50.8 b | 52.2 | 12.0 |
| [C8mim]ClO4 | 9.2 a | 170 a | 51.9 | 24.3 |
| [C8mim]NTf2 | 1.0 b | 70.1 b | 52.3 | 12.5 |
| [C10mim]ClO4 | 2.4 c | 363 d | 52.1 | 22.4 |
| [C10mim]NTf2 | 0.7 b | 72.0 b | 52.2 | 10.0 |
| [C4C8im]ClO4 | 3.2 e | 333 d | 52.0 | 21.6 |
| [C4C8im]NTf2 | 0.31 e | 44.2 d | 52.2 | 11.4 |
| [C4C10im]ClO4 | 0.93 e | 479.3 d | 52.0 | 21.5 |
| [C4C10im]NTf2 | 0.086 e | 47.7 d | 52.4 | 12.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Yan, Y.; Zhou, Q.; Fan, Y. Ionic Liquid-Based Extraction Strategy for the Efficient and Selective Recovery of Scandium. Molecules 2024, 29, 4007. https://doi.org/10.3390/molecules29174007
Zhang S, Yan Y, Zhou Q, Fan Y. Ionic Liquid-Based Extraction Strategy for the Efficient and Selective Recovery of Scandium. Molecules. 2024; 29(17):4007. https://doi.org/10.3390/molecules29174007
Chicago/Turabian StyleZhang, Sheli, Yuerong Yan, Qiang Zhou, and Yunchang Fan. 2024. "Ionic Liquid-Based Extraction Strategy for the Efficient and Selective Recovery of Scandium" Molecules 29, no. 17: 4007. https://doi.org/10.3390/molecules29174007
APA StyleZhang, S., Yan, Y., Zhou, Q., & Fan, Y. (2024). Ionic Liquid-Based Extraction Strategy for the Efficient and Selective Recovery of Scandium. Molecules, 29(17), 4007. https://doi.org/10.3390/molecules29174007






