Abstract
Stimulus-responsive materials hold significant promise for antitumor applications due to their variable structures and physical properties. In this paper, a series of peptides with a responsive viologen derivative, Pep-CnV (n = 1, 2, 3) were designed and synthesized. The process and mechanism of the interaction were studied and discussed. An ultraviolet–visible (UV) spectrophotometer and fluorescence spectrophotometer were used to study their redox responsiveness. Additionally, their secondary structures were measured by Circular Dichroism (CD) in the presence or absence of the reductant, Na2SO3. DPPC and DPPG liposomes were prepared to mimic normal and tumor cell membranes. The interaction between Pep-CnV and biomembranes was investigated by the measurements of surface tension and cargo leakage. Results proved Pep-CnV was more likely to interact with the DPPG liposome and destroy its biomembrane under the stimulus of the reductant. And the destruction increased with the length of the hydrophobic tail chain. Pep-CnV showed its potential as an intelligent antitumor agent.
1. Introduction
Antimicrobial peptides (AMPs) are a kind of short peptide with the ability of killing bacteria [1,2,3]. These peptides achieve their function by forming pores in the cell membrane, which leads to leakage of entocytes and further cell death [4,5]. This mechanism overcomes the resistance of traditional drugs and also possesses the antitumor function. AMPs have become the focus of attention in biomedicine. Up to now, over 2600 AMPs have been cataloged in the antimicrobial peptide database, and several have been used in clinics [6].
However, natural antimicrobial peptides have certain drawbacks, including susceptibility to enzymolysis and low specificity, which restrict their applications [7,8]. Researchers started developing synthetic antimicrobial peptides by modifying natural ones or designing them independently. Based on their action mechanism, the basic structural characteristics of AMPs were summarized as electropositivity and hydrophobicity [9,10,11]. Therefore, researchers designed and synthesized antimicrobial peptides utilizing these structural features. In 2019, researchers at the University of British Columbia synthesized a series of Innate Defense Regulator (IDR) peptides with enhanced properties. One such peptide, IDR-1018, was found to have broad-spectrum antimicrobial activity, including multi-drug-resistant bacteria [12]. A magainin analog, Pexiganan, was developed and tested in two Phase III clinical trials by a company called Dipexium Pharmaceuticals for the treatment of mild infections of diabetic foot ulcers [13]. It was synthesized as a topical cream and showed promising results against both Gram-positive and Gram-negative bacteria.
Look at these antimicrobial peptides; hydrophobicity is the common and important feature to realize their antimicrobial/antitumor functions. It determines AMPs’ destruction of abnormal cells. The high hydrophobicity is useful especially for the penetration in the biological membranes, which leads to the leakage of intracellular contents and ultimate cell death [9]. However, it raised another question. Exposed hydrophobic tail chains would produce non-specific toxicity to normal cells [14,15]. How to reduce the toxicity to normal cells and increase the toxicity to tumor cells?
In solid tumors, the complex tumor micro-environment was characterized by acidity [16], hyperthermia [17], low oxygen concentration [18], high reductant [19], and elevated H2O2 [20]. Based on this, environmentally responsive materials became our focus [21,22]. Shan et al. synthesized a series of pH-responsive antimicrobial peptides equipped with efficient bacterial killing activity at pH 6.5 and inactivity at pH 7.4 [23]. Li et al. developed a prototype antimicrobial peptide capable of achieving high activity exclusively at low environmental pH to target bacterial species like Streptococcus mutans [24]. Redox responsiveness was also a common method. And viologen is a well-known reduction material. It has been explored for various applications due to its unique redox properties. For instance, viologen-based materials can be engineered to release therapeutic agents in response to specific redox conditions [25]. Viologens are also employed in the development of biosensors due to their electron transfer capabilities and sensitivity to changes in the redox environment [26]. The conversion between hydrophilicity and hydrophobicity under the stimulus of Na2SO3 is crucial for realizing its antitumor potential, which will enhance its safety in clinical applications [27,28]. Based on the above, viologen was used in our study and played an important role.
In this work, a series of Pep-CnV with different alkane chain lengths were designed and synthesized, in which Pep was obtained from our previous study and held the α-helical structure [29]. As shown in Scheme 1, in the normal cellular environment, Pep-CnV were hydrophilic so that they were hypotoxic. Under the stimulus of the reductant, Na2SO3, viologen changed to the reduced state, which was hydrophobic, showing its aggression to biomembranes. Compared with mimic normal cells, Pep-CnV had better affinity for mimic tumor cells in an acidic condition, which was based on their electrostatic effect between Pep and biomembrane. And hydrophobicity became an important factor affecting Pep-CnV’s function. Pep-CnV showed their potential as antitumor agents for their aggregation/destruction to biomembranes under the stimulus of reduction. This work provided a theoretical basis for the development of synthetic AMPs.
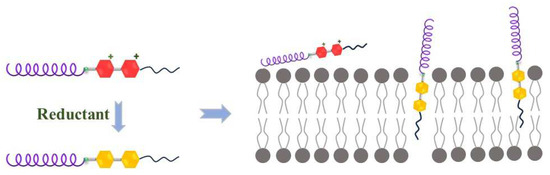
Scheme 1.
Mechanism of Pep-CnV with redox responsiveness.
2. Results and Discussions
2.1. Properties of Pep-CnV
2.1.1. Fundamental Structures
Pep-CnV was obtained by chemical synthesis, and their structures were confirmed using 1H NMR and FTIR. As shown in Figure 1, the Mal-CnV was confirmed for the peaks at δ9.164 (d, 2H, Aryl-H), δ8.908 (d, 2H, Aryl-H), δ8.641 (d, 2H, Aryl-H), δ8.096 (d, 2H, Aryl-H), δ7.055 (s, 2H, -CH=CH-), δ5.724 (s, 2H, -OOCCH2N-), δ4.389 (s, 3H, -NCH3), δ 4.326 (t, 2H, -CH2OOC-), and δ3.699 (t, 2H, -NCH2-).
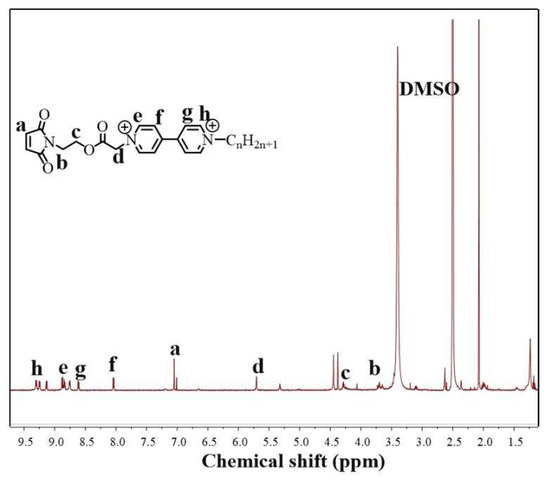
Figure 1.
H’NMR of Mal-CnV.
Figure 2 shows the FTIR spectra of Pep-CnV. The compound Mal-CnV exhibited a characteristic peak of -C=C-H (3000~3100 cm−1) in 3037.6 cm−1 wavenumbers. After the sulfhydryl addition occurred between the sulfydryl group of Pep and the maleimide group of Mal-CnV, a new characteristic peak emerged in 2929.6 cm−1 wavenumbers, which can be attributed to the molecular structure of –C-C-H in the maleimide group. Thus, the results convincingly confirmed the cross-linking between the Pep and Mal-CnV. This result confirmed that Pep-CnV was successfully synthesized.
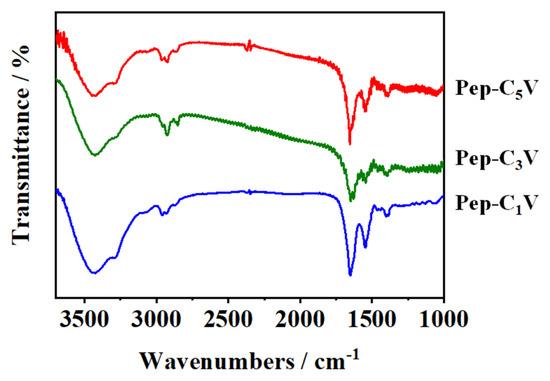
Figure 2.
FTIR of Pep-CnV (n = 1, 3, 5).
2.1.2. Secondary Structures
Peptides with α-helical structures have shown superior activities of interacting with biomembranes, which leads to disruption to the target cells. Here, the secondary structures of Pep-CnV with reductant or not were studied by CD spectra. As shown in Figure 3, Pep-CnV adopted a typical α-helix structure that possessed characteristic negative bands at 208 nm, 222 nm, and a positive band at 192 nm. On the contrary, the α-helix of reductive Pep-CnV (rePep-CnV) was weakened and gradually changed to random. Peptide’s α-helical structure was affected by hydrophobic force and electrostatic interaction [30]. Under the stimulus of Na2SO3, the state of viologen changed from oxidative V2+ to reductive V, accompanied by changes in secondary structures. It indicated that the reduced electrostatic interaction and enhanced hydrophobic interaction induced by CnV collectively influenced the secondary structure of Pep.
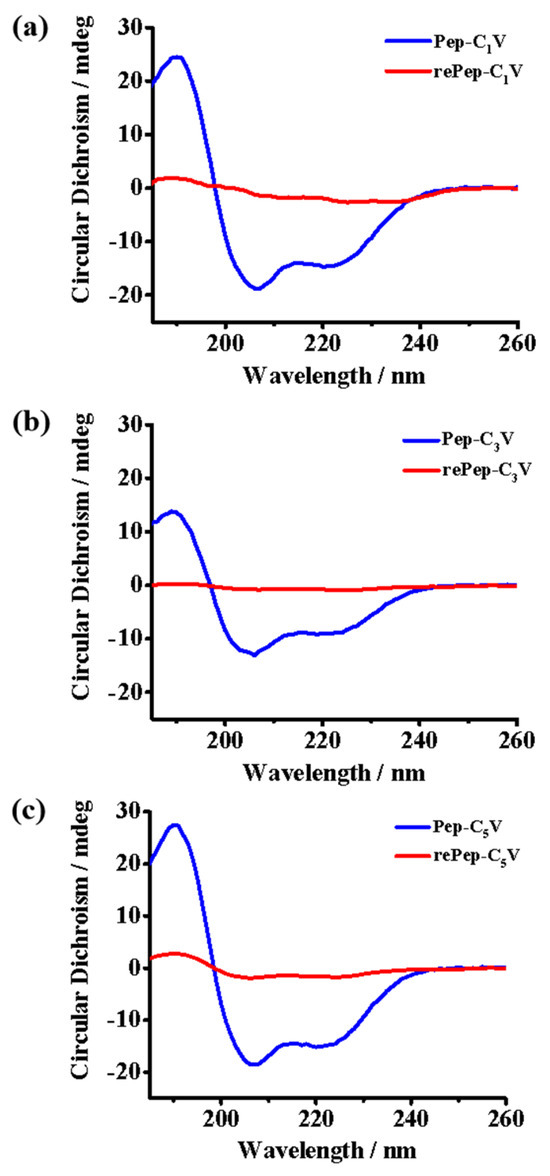
Figure 3.
CD spectra of (a) Pep-C1V, (b) Pep-C3V and (c) Pep-C5V with reductant or not.
2.2. Redox Responsiveness of Pep-CnV
Viologens are typical redox molecules; they are positively charged and capable of being reversibly reduced to a radical cationic or neutral species by means of chemical or electrical reductions. To confirm the redox responsiveness of Pep-CnV, UV–vis absorption spectroscopy and fluorescence spectra were measured.
As shown in Figure 4, for Pep-CnV, their UV–vis absorption peaks were at around 260 nm. Under the stimulus of a reductant, the absorption peak of viologen (260 nm) disappeared, indicating the presence of reduced viologen. It could be considered that the hydrophilic cationic V2+ had turned into hydrophobic uncharged V triggered by Na2S2O3.
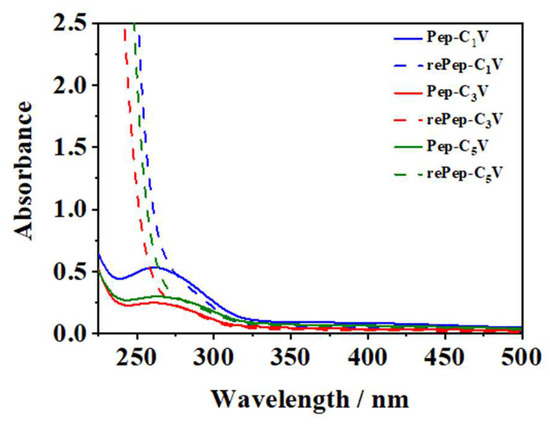
Figure 4.
UV-vis spectra of Pep-CnV with reductant or not.
Fluorescence spectra were also measured to further confirm the redox responsiveness in Figure 5. Pep-CnV showed strong fluorescence intensity at the wavelength of 510 nm. Upon adding the Na2S2O3, the fluorescence intensity was quenched significantly, owing to the change in viologens from V2+ to V. Pep-CnV showed their great redox responsiveness under the action of Na2S2O3.
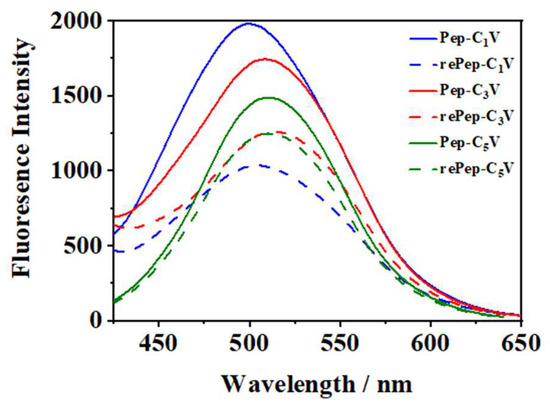
Figure 5.
Fluorescence spectra of Pep-CnV with reductant or not.
2.3. Interaction between Pep-CnV and Biomembranes
2.3.1. Surface Tension
To investigate the interaction between Pep-CnV and biomembranes, surface tensions of liposome solutions were measured. Here, negatively charged DPPG liposomes were used as mimic tumor cells, and neutral DPPC liposomes were used as normal cells. We investigated the interaction between Pep-CnV and DPPC/DPPG liposomes with reductant or not at 25 °C, as shown in Figure 6.
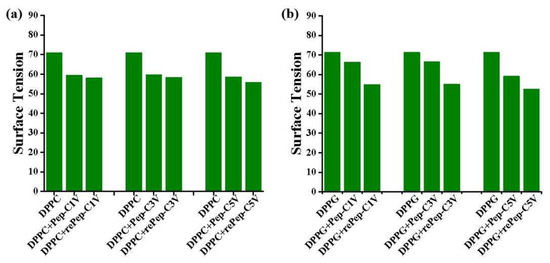
Figure 6.
Surface tension of (a) DPPC and (b) DPPG liposomes under the interaction of Pep-CnV with reductant or not.
For the DPPC liposome (Figure 6a), the addition of Pep-CnV reduced its surface tension. However, the presence of Na2S2O3 almost had no effect on surface tension. It indicated that the change in surface tension of the DPPC liposome was not induced by viologen. Surface tension could be affected by ions in the previous reports [31,32]. The significant reduction in surface tension was induced by Pep-CnV’s surface charge, and the slight change was induced by hydrophobic uncharged V under the stimulus of a reductant.
For DPPG liposomes (Figure 6b), the addition of Pep-CnV also reduced the surface tension. Different from DPPC liposomes, the rePep-CnV played an important role in further reducing the surface tension, confirming the function of viologen. The change in surface tension could be owed to the display of hydrophobicity. Under the stimulus of a reductant, driven by hydrophobic forces, the reduced viologens would insert into or even form pores in the membrane of the DPPG liposome. Studies have shown that the interfacial freedom of liposome membranes affects the surface tension of liposome solutions [33,34]. The inserted rePep-CnV reduced the interfacial freedom of the liposome membrane, resulting in a reduction in the surface tension of the solution. This result proved Pep-CnV had better affinity to mimic tumor cells under the stimulus of reduction. And this affinity was expected to be a potential antitumor agent.
2.3.2. Cargo Leakage
Rhodamine 6G was loaded into liposomes to mimic the cytoplasm, and its leakage kinetics incubating with Pep-CnV under the stimulus of reductant or not were investigated for discussing their aggression to biomembranes in Figure 7.
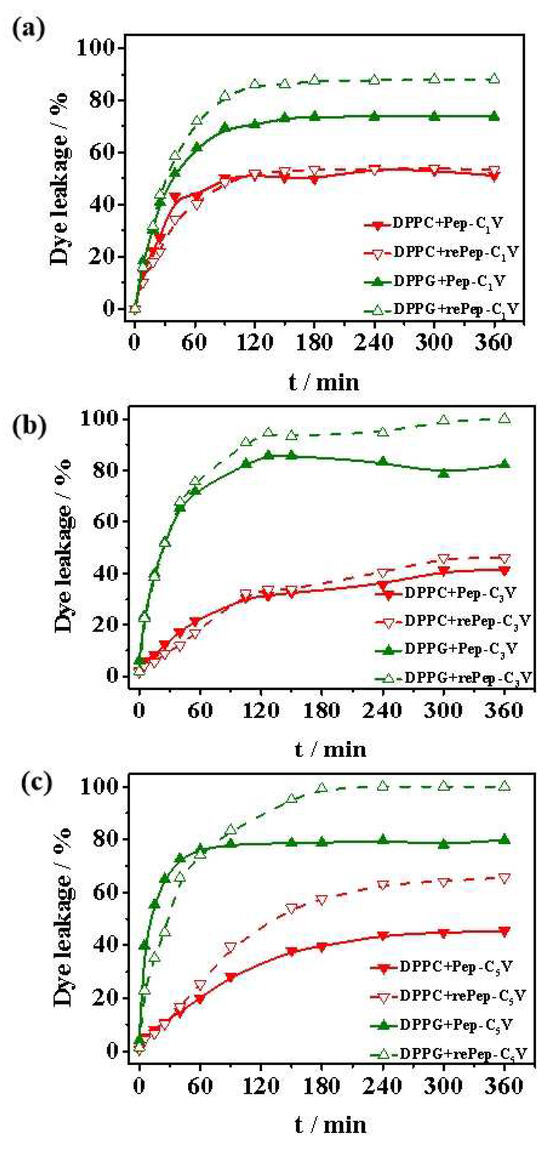
Figure 7.
Cargo leakage from DPPC and DPPG liposomes induced by (a) Pep-C1V, (b) Pep-C3V and (c) Pep-C5V with reductant or not.
Firstly, biocompatibility was investigated. For DPPC liposomes, the cargo leakages induced by Pep-CnV were all less than these from DPPG liposomes. And the reductant did not have much effect on cargo leakages for rePep-C1V and rePep-C3V. For Pep-C5V, the stimulus of reductant improved the cargo leakage for its longer hydrophobic tails. This result proved Pep-CnV almost had no destructive effect on DPPC liposomes. It exhibited some cytotoxicity only when the hydrophobicity was strong and the hydrophobic tail chain was exposed. This result proved exposed hydrophobic tail chains would produce non-specific toxicity to normal cells.
Then, aggression to biomembranes was studied. For DPPG liposomes, the behavior of cargo leakages was different. The cargo leakages were all improved with the addition of Pep-CnV. It proved the interaction between Pep-CnV and DPPG liposomes was stronger, owing to the electrostatic interaction between them. Then, the cumulative leakages reached the maximum under the stimulus of reductant, indicating the insertion of hydrophobic reduced CnV. This result was consistent with the results of surface tension in Section 2.3.1.
Finally, the destruction of Pep-CnV to biomembranes with hydrophobic tails of different lengths was compared. In the reduced states, the cargo leakage from DPPG liposomes induced by rePep-C3V and rePep-C5V reached almost 100%. It was 87.1% for rePep-C1V. The function of rePep-CnV increased with the increase in hydrophobic tails, proving the role of hydrophobicity in realizing tumor cells’ destruction. The stronger the hydrophobicity, the stronger the effect on the biomembranes. Combined with the above biocompatibility studies, Pep-C3V possessed significant advantages with biocompatibility for normal cells and destructiveness for tumor cells. Pep-CnV would be a potential antitumor agent under the stimulus of a reductant.
Scheme 2 concluded the process of interaction between Pep-CnV and biomembranes. On the basis of electrostatic interaction, Pep-CnV absorbed more on the surface of DPPG liposomes, proving its potential selectivity of tumor cells. Then, under the stimulus of reductant, the hydrophilic V2+ changed to the hydrophobic molecule V. Driven by hydrophobic forces, the adsorbed Pep-CnV further inserted into the biomembrane, inducing cell death. And the stronger the hydrophobicity, the stronger the effect on the biomembranes. Pep-CnV showed its potential as a novel antitumor agent.
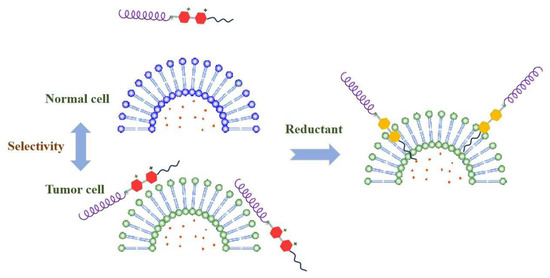
Scheme 2.
Pep-CnV’s selectivity and destructiveness for DPPG liposomes under the stimulus of reductant.
3. Materials and Methods
3.1. Materials and Reagents
Peptides with the sequence of NH2-C-VAQLEVK-VAQLESK-VSKLESK-VSSLESK-COOH, named Pep, were synthesized by Top-Peptide Co., Ltd. (purity > 95%) (Shanghai, China). Furthermore, 4,4-bipyridine and relevant alkyl halide were obtained from J&K Scientific (purity > 98%) (Beijing, China). Moreover, 2-bromoisobutyryl bromide and N-(2-hydroxyethyl) maleimide were obtained from Aladdin Industrial Corporation (purity > 98%) (Shanghai, China); 1,2-dipalmitoyl-sn-glycerol-3-phosphocholine (DPPC, purity > 98%) and 1,2-dipalmitoyl-sn-glycerol-3-phospho-(1-rac-glyerol) (DPPG, purity > 98%) were obtained from Lipoid (Ludwigshafen, Germany); Rhodamine 6G was purchased from J&K Scientific (Beijing, China); Sephadex G-25 was purchased from Shanghai Haoran Biological Technology Co. Ltd. (Shanghai, China). Other chemical reagents and solvents were of analytical grade and obtained from commercial suppliers.
3.2. Synthesis of Pep-CnV
The synthesis procedures of Pep-CnV were performed according to the literature previously reported [35]. There were four steps from 4,4-bipyridine to Pep conjugation, as shown in Figure 8.
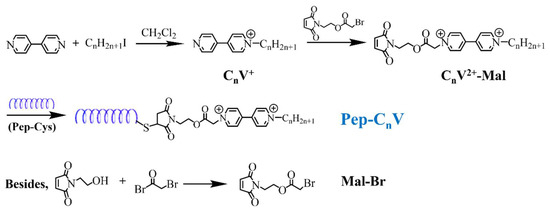
Figure 8.
Synthetic procedures of Pep-CnV.
Synthesis of Mal-Br. Mal-Br was obtained from the reaction between N-(2-hydroxyethyl) maleimide and trimethylamine (molar ratio: 1/1.5) in CH2Cl2 solution under an ice-water bath for 30 min and then at room temperature for another 12 h. Then, after processing extraction, concentration, and drying, Mal-Br was obtained.
Synthesis of CnV+. CnV+ was obtained from the reaction between 4,4-bipyridine and alkyl halide in the inert condition, avoiding light. Solvents and reaction temperatures were different for the different reactivity of alkyl halides. When n = 1, the reaction condition was room temperature for 24–48 h with light avoidance, and the reaction solvent was dichloromethane. When n = 3, the condition remained unchanged. When n = 5, the reaction condition became more stringent with the temperature of 70–80 °C, and the reaction solvent changed to anhydrous acetonitrile.
Synthesis of CnV2+-Mal. CnV+ and Mal-Br (molar ratio: 1/1.2) were mixed in anhydrous acetonitrile and refluxed at 90 °C for 72 h. Then, the solid precipitated and was filtered/washed three times with acetonitrile. Then, the precipitation was dried in a vacuum oven to obtain CnV2+-Mal.
Conjugation of CnV2+-Mal to Pep. The conjugation was achieved by the reaction between the thiol group at the C-terminus of Pep and the maleimide group of CnV2+-Mal. Because of the water solubility of Pep and CnV2+-Mal, the reaction was in Tris–HCl buffer at 25 °C for 12 h. After the reaction was completed, the mixture was put into the dialysis bag (MWCO:3500), dialyzing for 48 h. The dialysate was changed every 6 h to remove unbound peptides. Finally, Pep-CnV were obtained.
1H NMR (AVANCE III 400, Brucker, Leipzig, Germany) and FT-IR (Nicolet 5700, Thermo Fisher, Waltham, MA, USA) were used to characterize the structure of the above products.
3.3. Secondary Structure Studies
To analyze the secondary structure of Pep-CnV, Circular Dichroism (CD) scans were applied using a Circular Dichroism Spectrometer (Chirascan, Applied Photophysics, Leatherhead, UK). A certain amount of Pep-CnV was dissolved in the deionized water. Then, the CD spectrum was obtained with reductant or not over a wavelength range of 190 to 260 nm at the speed of 1 nm/s and bandwidth of 1.0 nm.
3.4. Responsiveness Studies
Redox responsiveness of Pep-CnV was studied by UV spectrophotometer (UV-2450, Shimadzu, Kyoto, Japan) and fluorescence spectrophotometer (F-4500, Hitachi, Tokyo, Japan). A UV spectrophotometer was recorded with reductant or not, and Pep-CnV’s redox responsiveness was judged by comparing the positions of the peaks. Similarly, the fluorescence intensity (λex = 360 nm, λem = 490 nm, 10 nm slits) were also measured under the action of reductant or not. A comparison of the intensity of peaks was used to confirm its redox responsiveness.
3.5. Construction of Cell Models
The cytoplasmic membrane of normal mammals is well known to contain several kinds of lipids containing phosphatidylcholine (PC), sphingomyelin, and cholesterol. PC has been reported to be enriched in the outer monolayer. For tumor cells whose membrane surfaces are electronegative, phosphatidylglycerol (PG) was chosen as the abnormal cell membrane mimicking system. Hence, DPPC and DPPG were used in this study to construct a model cell membrane system. A thin film hydration method was used to prepare liposomes as normal and tumor cell membranes. DPPC and DPPG were dissolved in the methanol/chloroform mixture, and the mixtures were evaporated. Then, the lipid films were hydrated using 5 mL Tris–HCl buffer (10 mM, pH 7.4) to obtain the liposomes (2 mg/mL). To obtain liposomes with good stability and uniform size, they were extruded several times through 200 nm polycarbonate membranes by a mini-extruder (LiposoFast-Basic, Avestin, Ottawa, Canada). Sizes and zeta potentials of the liposomes were measured using dynamic light scattering (DLS) (Zetasizer Nano-ZS, Malvern, UK) at 25 °C.
3.6. Surface Tension Studies
Surface tension was related to the surface adsorption concentration. It was performed in this work to investigate the interaction between biomembranes and Pep-CnV. The surface tensions of mimic cells mixed with Pep-CnV were measured using a surface tensiometer (Sigma 703D, Biolin, Espoo, Finland) at 25 °C. The mixtures of mimic cells (DPPC/DPPG liposomes) and Pep-CnV were prepared by mixing and stirring at room temperature for 6 h with reductant or not. Then, the surface tensions were measured and compared.
3.7. Cargo Leakage Studies
Rhodamine 6G@liposomes were prepared in which Rhodamine 6G was chosen as the fluorescent dye to mimic the cytoplasm of cells, and its leakage behavior was assayed to investigate the interaction between Pep-CnV and biomembranes. Cargo leakage was performed at 37 °C by the dialysis method. The amount of Pep-CnV with reductant or not was mixed with Rhodamine 6G@liposome. The mixture was put into a 2000 kDa molecular weight cut-off dialysis tube and dialyzed against Tris–HCl buffer (2 Mm, pH = 7.4). At various time points, the buffer was withdrawn and measured by fluorescence spectrophotometry (F-4500, Hitachi, Japan) (λex = 525 nm, λem = 550 nm, and 10 nm of both slit width). The fluorescence intensity of the buffer at every time point was defined as At. The initial total amount of Rhodamine 6G loaded in the liposome was defined as A0 after disrupting liposomes with 10% (v/v) Triton X-100 solution. The cumulative leakage amount of Rhodamine 6G was calculated as At/A0.
4. Conclusions
In this paper, we designed and synthesized a series of peptide derivatives Pep-CnV with alkane chains of different lengths. Pep-CnV had shown their redox responsiveness under the stimulus of reductant, Na2S2O3. And their secondary structures changed triggered by the reductant. Surface tension studies suggested the interaction between Pep-CnV and biomembranes, which was mainly controlled by the hydrophobic force. Cargo leakage studies confirmed Pep-CnV’s function on biomembranes. Pep-CnV showed their good biocompatibility and held preferential destructiveness to mimic tumor cells accompanied by the addition of reductant. Preferably, Pep-C3V showed its potential as an antitumor agent with low side effects and high antitumor efficiency. Based on the above results, the design of Pep-CnV involved incorporating alkane chains of different lengths to modulate the hydrophobic interactions with biomembranes, enhancing their efficacy. Additionally, the redox-responsive nature of Pep-CnV allowed for function activation in the presence of reductants. This approach minimizes off-target effects and enhances efficacy. This paper not only provides a theoretical foundation for the development of novel antitumor agents but also suggests a practical approach to designing molecules with enhanced selectivity and reduced toxicity for clinical applications.
Author Contributions
Methodology, L.Z.; Formal analysis, H.X.; Data curation, Y.L.; Writing-original draft, S.W.; Writing-review & editing, S.X. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support for this work was provided by the National Natural Science Foundation of China (No. 22208089) and the Natural Science Foundation of Henan Province (No. 242300420559).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no competing financial interests.
References
- Li, X.; Zuo, S.Y.; Wang, B.; Zhang, K.Y.; Wang, Y. Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules 2022, 27, 2675. [Google Scholar] [CrossRef]
- Magana, M.; Pushpanathan, M.; Santos, A.L.; Leanse, L.; Fernandez, M.; Ioannidis, A.; Giulianotti, M.A.; Apidianakis, Y.; Bradfute, S.; Ferguson, A.L.; et al. The value of antimicrobial peptides in the age of resistance. Lancet Infect. Dis. 2020, 20, E216–E230. [Google Scholar] [CrossRef]
- Bhopale, G.M. Antimicrobial Peptides: A Promising Avenue for Human Healthcare. Curr. Pharm. Biotechnol. 2020, 21, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Chen, C.H.; Hu, D.; Ulmschneider, M.B.; Ulmschneider, J.P. Spontaneous formation of structurally diverse membrane channel architectures from a single antimicrobial peptide. Nat. Commun. 2016, 7, 13535. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, L.; Wan, M.W.; Song, J.J.; Gao, L.H.; Fang, W.H. Peripheral Antimicrobial Peptide Gomesin Induces Membrane Protrusion, Folding, and Laceration. Langmuir 2019, 35, 13233–13242. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.; Maisetta, G.; Batoni, G.; Tavanti, A. Insights into the Antimicrobial Properties of Hepcidins: Advantages and Drawbacks as Potential Therapeutic Agents. Molecules 2015, 20, 6319–6341. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Rinaldi, A.C. Beyond natural antimicrobial peptides: Multimeric peptides and other peptidomimetic approaches. Cell. Mol. Life Sci. 2011, 68, 2255–2266. [Google Scholar] [CrossRef]
- Zhang, M.H.; Ouyang, J.H.; Fu, L.; Xu, C.; Ge, Y.K.; Sun, S.Q.; Li, X.Y.; Lai, S.; Ke, H.T.; Yuan, B.; et al. Hydrophobicity Determines the Bacterial Killing Rate of α-Helical Antimicrobial Peptides and Influences the Bacterial Resistance Development. J. Med. Chem. 2022, 65, 14701–14720. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.N.; Zhang, J.; Hu, X.Z.; Li, Z.Y.; Fa, K.; Liu, H.Y.; Waigh, T.A.; McBain, A.; Lu, J.R. Hydrophobic Control of the Bioactivity and Cytotoxicity of de Novo-Designed Antimicrobial Peptides. ACS Appl. Mater. Interfaces 2019, 11, 34609–34620. [Google Scholar] [CrossRef]
- Ye, Z.; Aparicio, C. Modulation of supramolecular self-assembly of an antimicrobial designer peptide by single amino acid substitution: Implications on peptide activity. Nanoscale Adv. 2019, 1, 4679–4682. [Google Scholar] [CrossRef]
- Haney, E.F.; Barbosa, S.C.; Baquir, B.; Hancock, R.E.W. Influence of Non-natural Cationic Amino Acids on the Biological Activity Profile of Innate Defense Regulator Peptides. J. Med. Chem. 2019, 62, 10294–10304. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Simonetti, O.; Pierpaoli, E.; Barucca, A.; Ghiselli, R.; Orlando, F.; Pelloni, M.; Minardi, D.; Trombettoni, M.M.C.; Guerrieri, M.; et al. Enhanced Efficacy of Combinations of Pexiganan with Colistin Versus Acinetobacter Baumannii in Experimental Sepsis. Shock 2016, 46, 219–225. [Google Scholar] [CrossRef]
- Li, J.; Yap, S.Q.; Chin, C.F.; Tian, Q.; Yoong, S.L.; Pastorin, G.; Ang, W.H. Platinum(IV) prodrugs entrapped within multiwalled carbon nanotubes: Selective release by chemical reduction and hydrophobicity reversal. Chem. Sci. 2012, 3, 2083–2087. [Google Scholar] [CrossRef]
- Dorn, S.B.; Degen, G.H.; Bolt, H.M.; van der Louw, J.; van Acker, F.A.A.; van den Dobbelsteen, D.J.; Lommerse, J.P.M. Some molecular descriptors for non-specific chromosomal genotoxicity based on hydrophobic interactions. Arch. Toxicol. 2008, 82, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B.L.; Jiang, J.; Wu, Y.; Song, A.N.; Wang, X.Y.; Yao, C.L.; Dai, H.X.; Xu, J.L.; Zhang, Y.; et al. SnSe Nanosheets Mimic Lactate Dehydrogenase to Reverse Tumor Acid Microenvironment Metabolism for Enhancement of Tumor Therapy. Molecules 2022, 27, 8552. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Deng, J.; Sun, J.H.; Ma, Y.L. Hyperthermia Targeting the Tumor Microenvironment Facilitates Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 595207. [Google Scholar] [CrossRef] [PubMed]
- Milotti, E.; Stella, S.; Chignola, R. Pulsation-limited oxygen diffusion in the tumour microenvironment. Sci. Rep. 2017, 7, 39762. [Google Scholar] [CrossRef]
- Yu, Q.L.; Wei, Z.Y.; Qin, X.; Qin, L.M.; Li, Y.S.; Shi, J.L.; Niu, D.C. Reductant-Free Synthesis of MnO2 Nanosheet-Decorated Hybrid Nanoplatform for Magnetic Resonance Imaging-Monitored Tumor Microenvironment-Responsive Chemodynamic Therapy and Near-Infrared-Mediated Photodynamic Therapy. Small Struct. 2021, 2, 2100116. [Google Scholar] [CrossRef]
- Yu, Y.; Xie, B.R.; Liu, X.H.; Ye, J.J.; Cheng, H.; Zhong, Z.L.; Zhang, X.Z. A H2O2-responsive theranostic platform for chemiluminescence detection and synergistic therapy of tumors. J. Mater. Chem. B 2022, 10, 1634–1640. [Google Scholar] [CrossRef]
- Chang, S.S.; Weng, Z.Z.; Zhang, C.M.; Jiang, S.H.; Duan, G.G. Cellulose-Based Intelligent Responsive Materials: A Review. Polymers 2023, 15, 3905. [Google Scholar] [CrossRef]
- Chang, J.; Zhang, L.B.; Wang, P. Intelligent environmental nanomaterials. Environ. Sci. Nano 2018, 5, 811–836. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Li, J.; Shang, L.; Li, J.; Chou, S.; Lyu, Y.; Shan, A. pH-Responsive Antimicrobial Peptide with Selective Killing Activity for Bacterial Abscess Therapy. J. Med. Chem. 2022, 65, 5355–5373. [Google Scholar] [CrossRef]
- Li, L.; He, J.; Eckert, R.; Yarbrough, D.; Lux, R.; Anderson, M.; Shi, W. Design and characterization of an acid-activated antimicrobial peptide. Chem. Biol. Drug Des. 2010, 75, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, H.; Qi, F.L.; Xia, T.; Xia, Y.; Xu, J.F.; Zhang, X. An Activatable Host–Guest Conjugate as a Nanocarrier for Effective Drug Release through Self-Inclusion. ACS Appl. Mater. Interfaces 2021, 13, 33962–33968. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Cosnier, S.; Almeida, M.G.; Moura, J. An efficient poly(pyrrole–viologen)-nitrite reductase biosensor for the mediated detection of nitrite. Electrochem. Commun. 2004, 6, 404–408. [Google Scholar] [CrossRef]
- Sathyamoorthi, S.; Kanagaraj, M.; Kathiresan, M.; Suryanarayanan, V.; Velayutham, D. Ethyl viologen dibromide as a novel dual redox shuttle for supercapacitors. J. Mater. Chem. A 2016, 4, 4562–4569. [Google Scholar] [CrossRef]
- Ohira, A.; Funaki, T.; Ishida, E.; Kim, J.D.; Sato, Y. Redox-Flow Battery Operating in Neutral and Acidic Environments with Multielectron-Transfer-Type Viologen Molecular Assembly. ACS Appl. Energy Mater. 2020, 3, 4377–4383. [Google Scholar] [CrossRef]
- Wang, S.; Han, X.; Liu, D.; Li, M.; Xu, S.; Liu, H. Melting Behavior of Zipper-Structured Lipopeptides in Lipid Bilayer. Langmuir 2017, 33, 1478–1485. [Google Scholar] [CrossRef]
- Shen, W.; Lammertink, R.G.H.; Sakata, J.K.; Kornfield, J.A.; Tirrell, D.A. Assembly of an artificial protein hydrogel through leucine zipper aggregation and disulfide bond formation. Macromolecules 2005, 38, 3909–3916. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhan, W.Q.; Yi, H.; Zhao, Y.L.; Song, S.X. Molecular dynamics simulations study for the effect of cations hydration on the surface tension of the electrolyte solutions. Colloids Surf. A 2018, 539, 80–84. [Google Scholar] [CrossRef]
- Wang, C.Y.; Morgner, H. The dependence of surface tension on surface properties of ionic surfactant solution and the effects of counter-ions therein. Phys. Chem. Chem. Phys. 2014, 16, 23386–23393. [Google Scholar] [CrossRef]
- Ortiz, A.; Teruel, J.A.; Eapuny, A.J.; Marqués, A.; Manresa, Á.; Aranda, F.J. Interactions of a bacterial biosurfactant trehalose lipid with phosphatidylserine membranes. Chem. Phys. Lipids 2009, 158, 46–53. [Google Scholar] [CrossRef]
- Aranda, E.; Teruel, J.A.; Ortiz, A.; Pérez-Cárceles, M.D.; Rodríguez-López, J.N.; Aranda, F.J. 3,4,5-Trimethoxybenzoate of Catechin, an Anticarcinogenic Semisynthetic Catechin, Modulates the Physical Properties of Anionic Phospholipid Membranes. Molecules 2022, 27, 2910. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.M.; Li, S.H.; Cui, Y.L.; Yu, J.; Liu, Y. Tunable Nanosupramolecular Aggregates Mediated by Host–Guest Complexation. Angew. Chem. Int. Ed. 2016, 55, 11452–11456. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).