Study on Soot and NOx Formation Characteristics in Ammonia/Ethylene Laminar Co-Flow Diffusion Flame
Abstract
1. Introduction
2. Results and Discussion
2.1. Experimental Results and Discussion
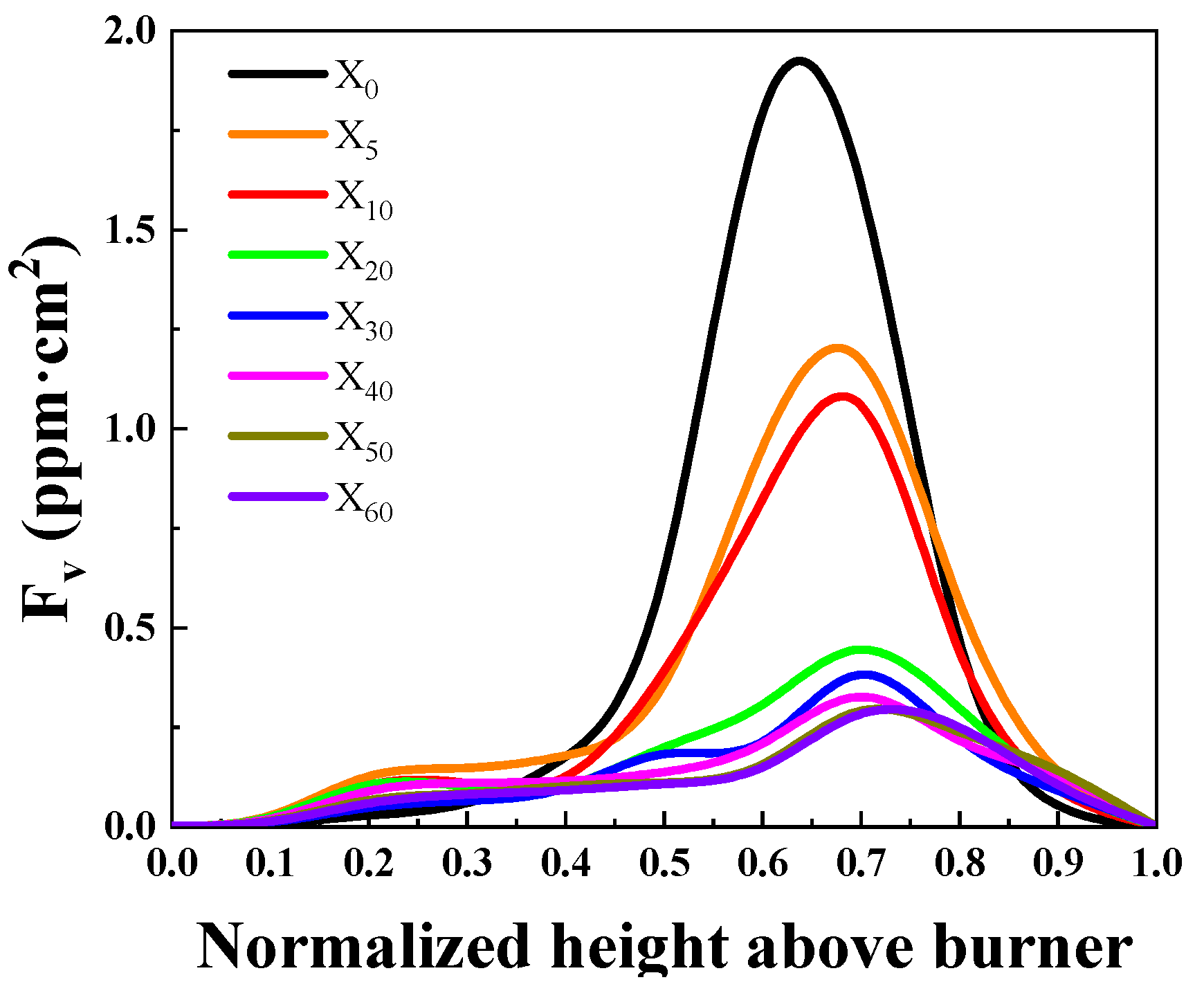
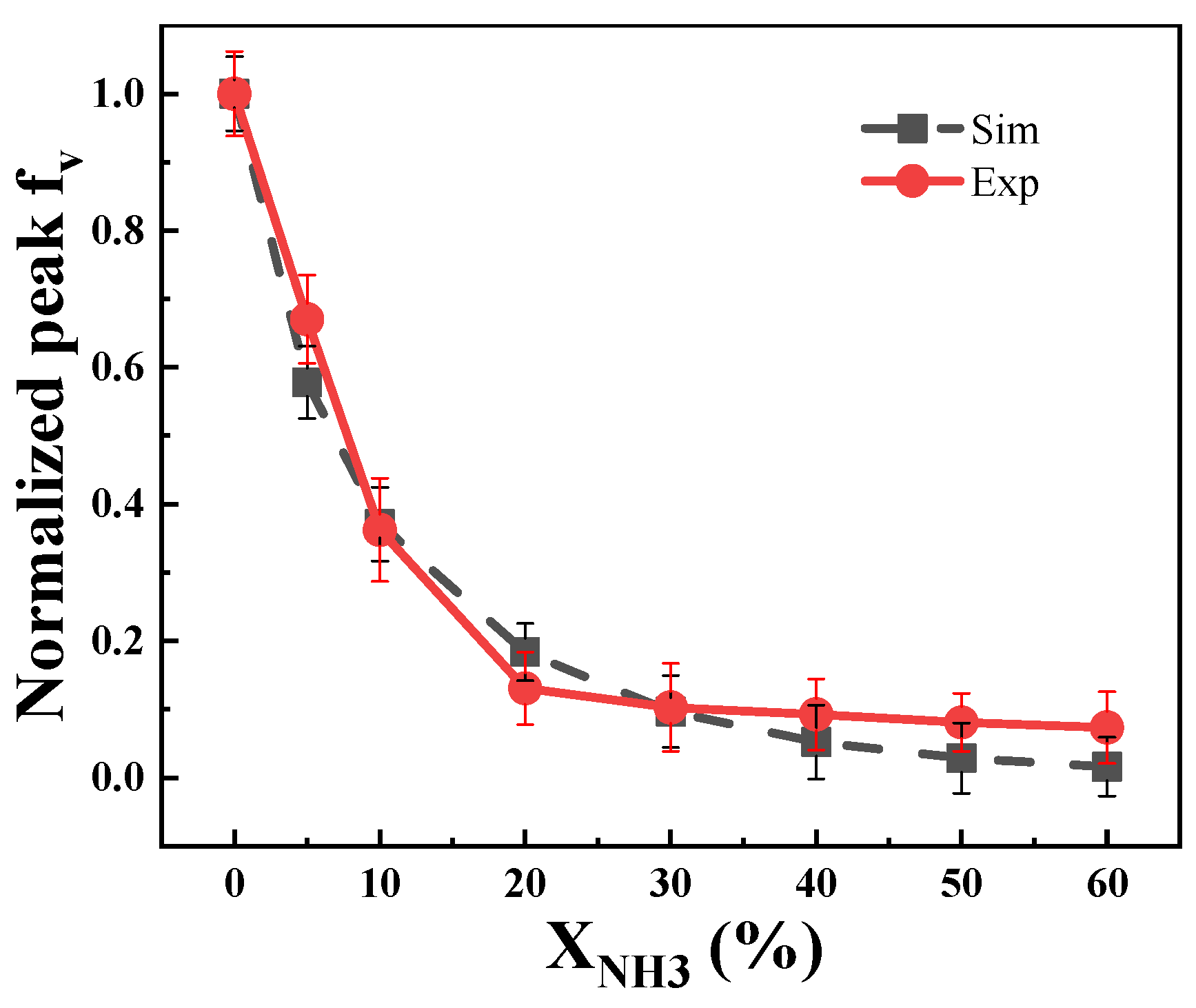
2.1.1. SVF Results and Analysis
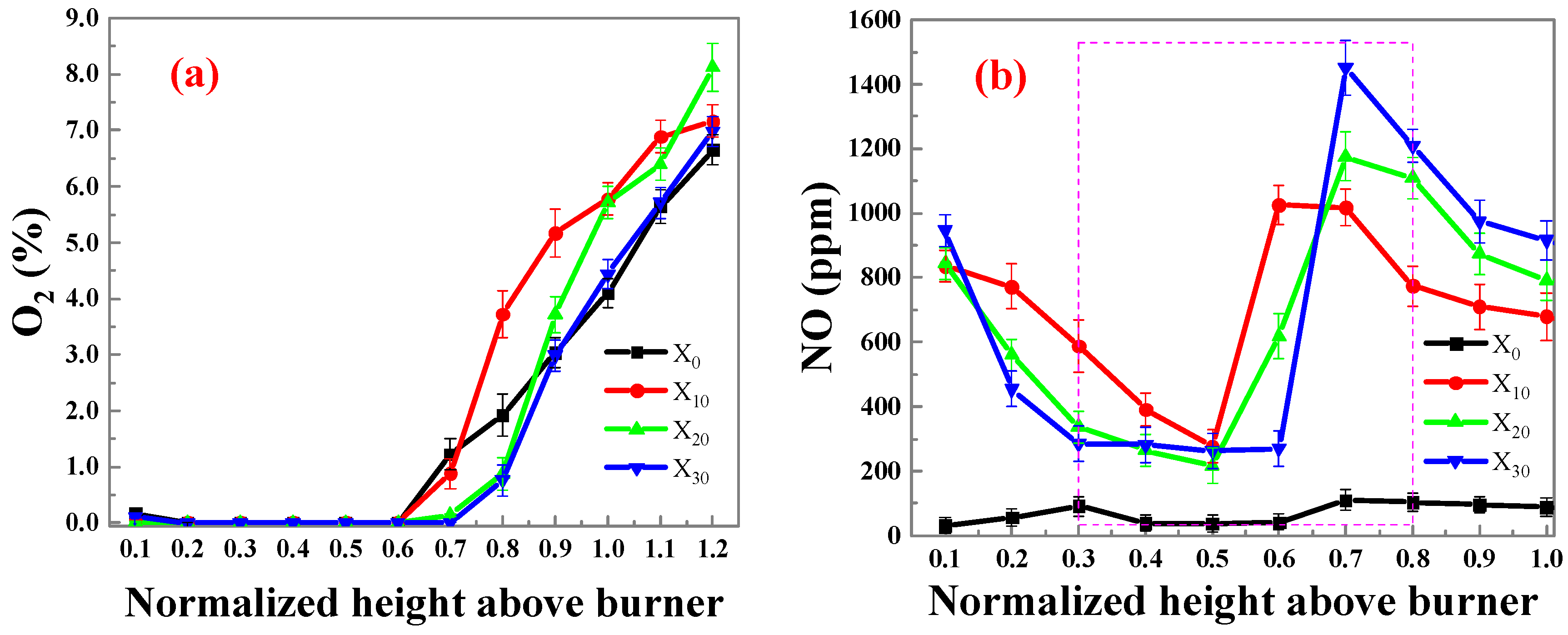
2.1.2. NOx Results and Analysis
2.2. Numerical Results and Discussion
2.2.1. The Effect of Ammonia on Soot Formation
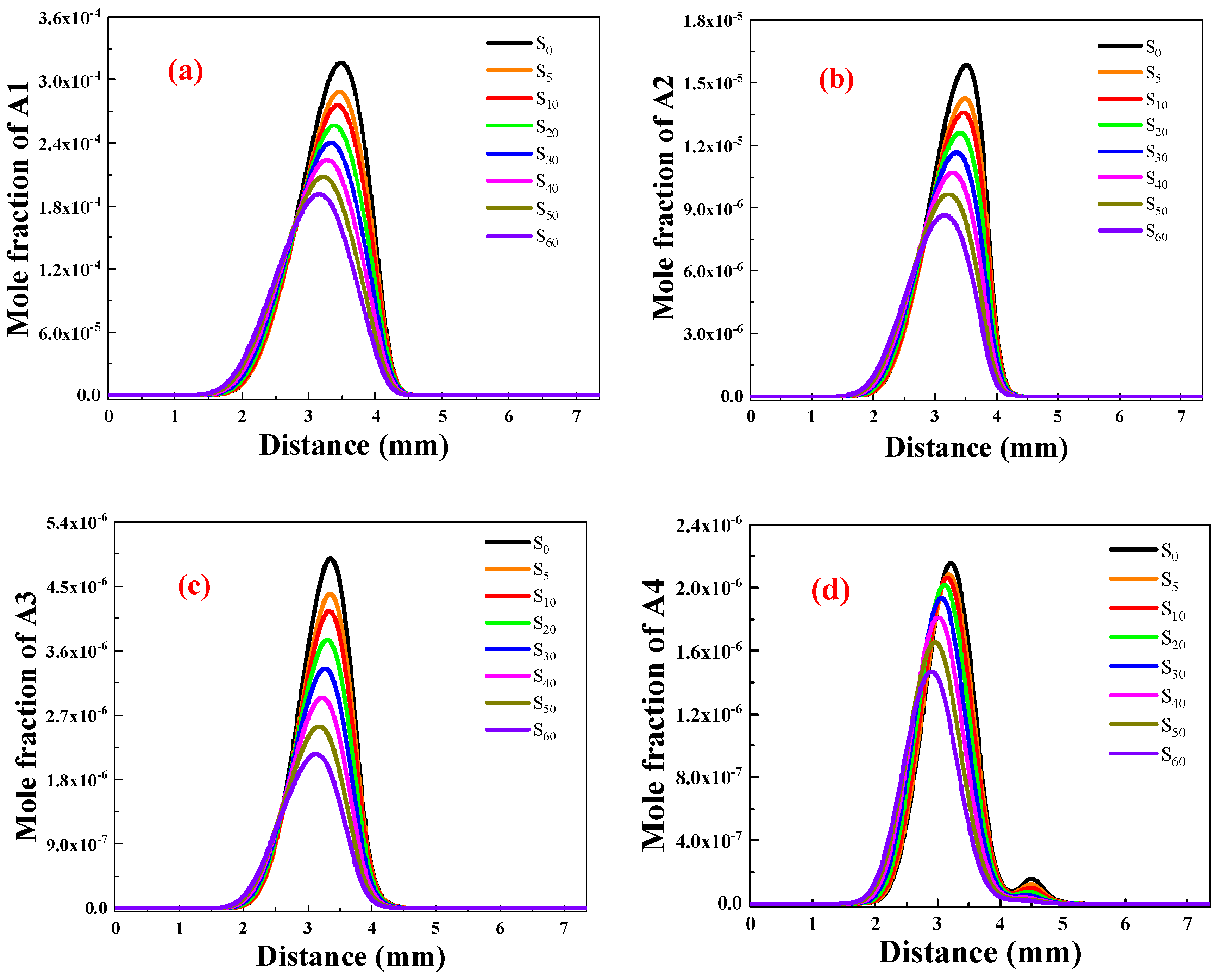
PAHs Generation Concentration Analysis
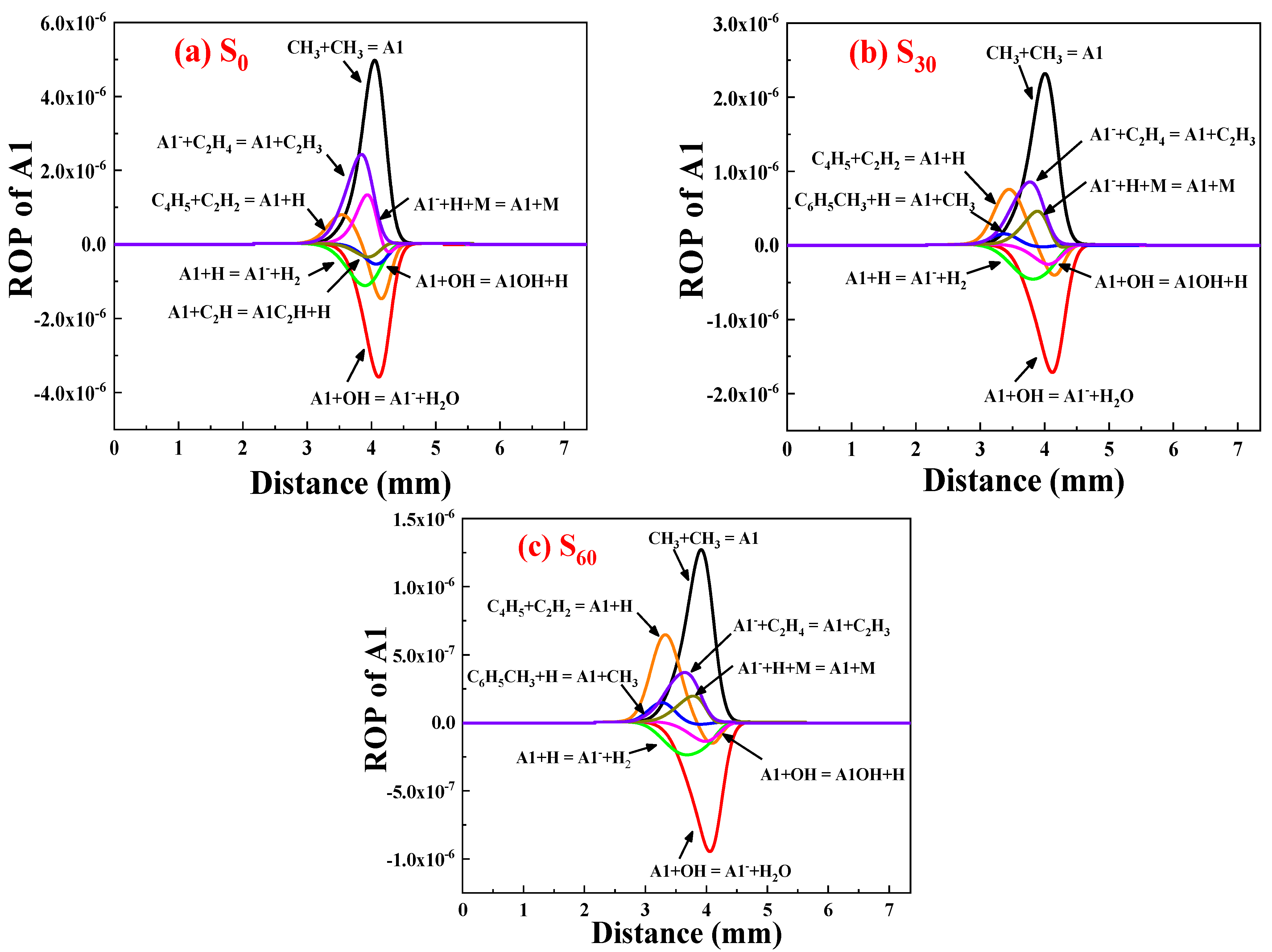
ROP Analysis of A1
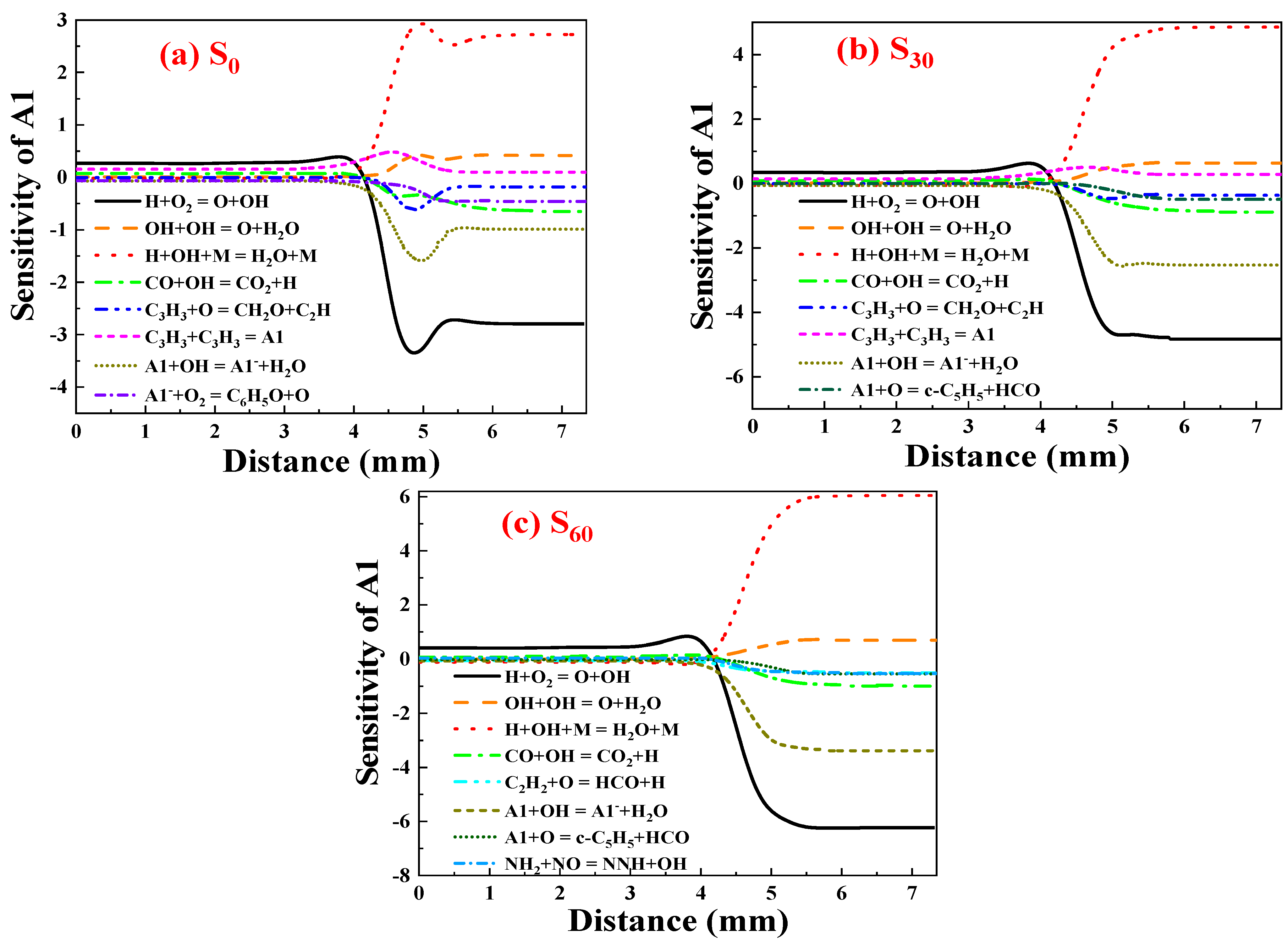
Sensitivity Analysis of A1
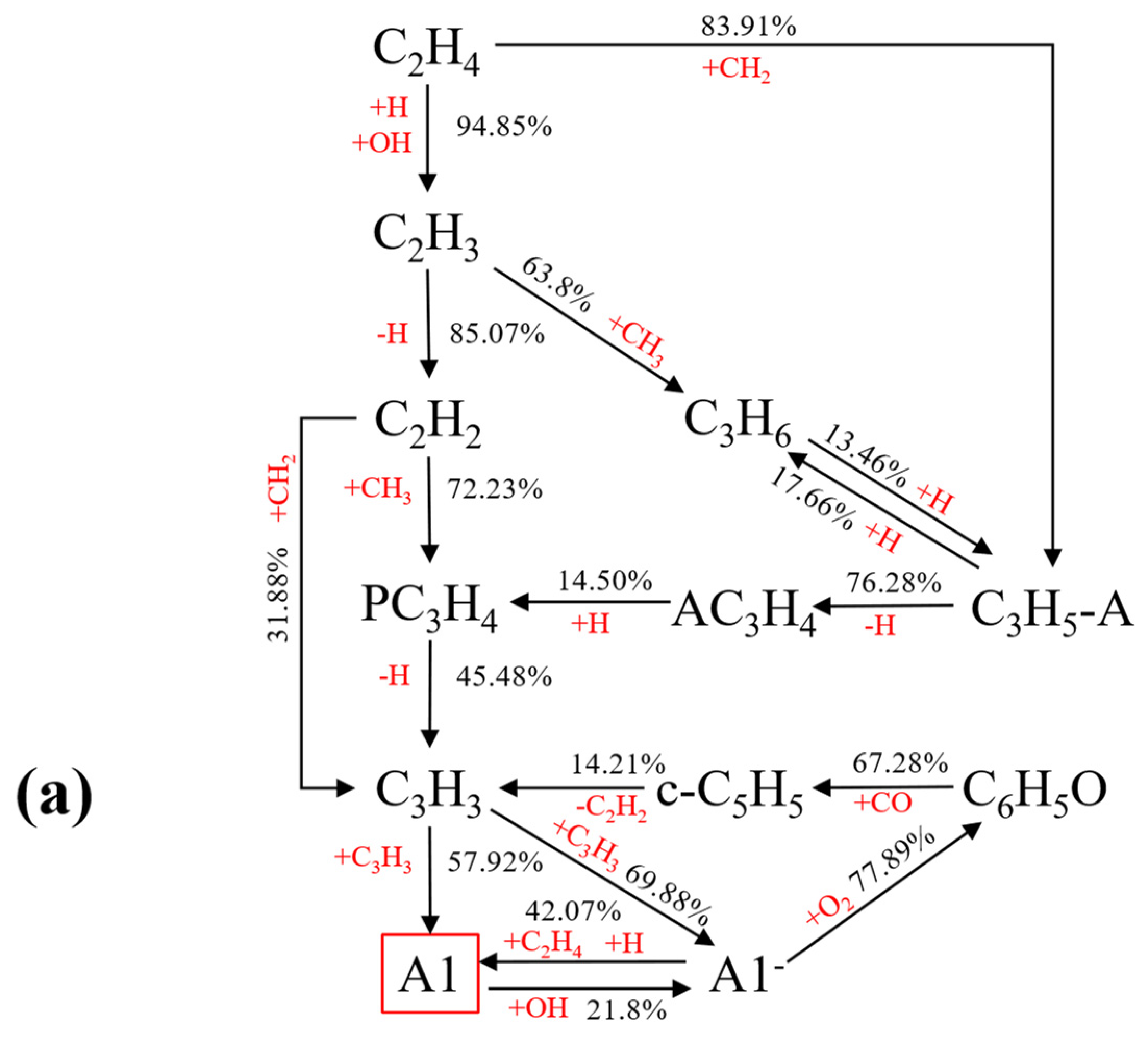
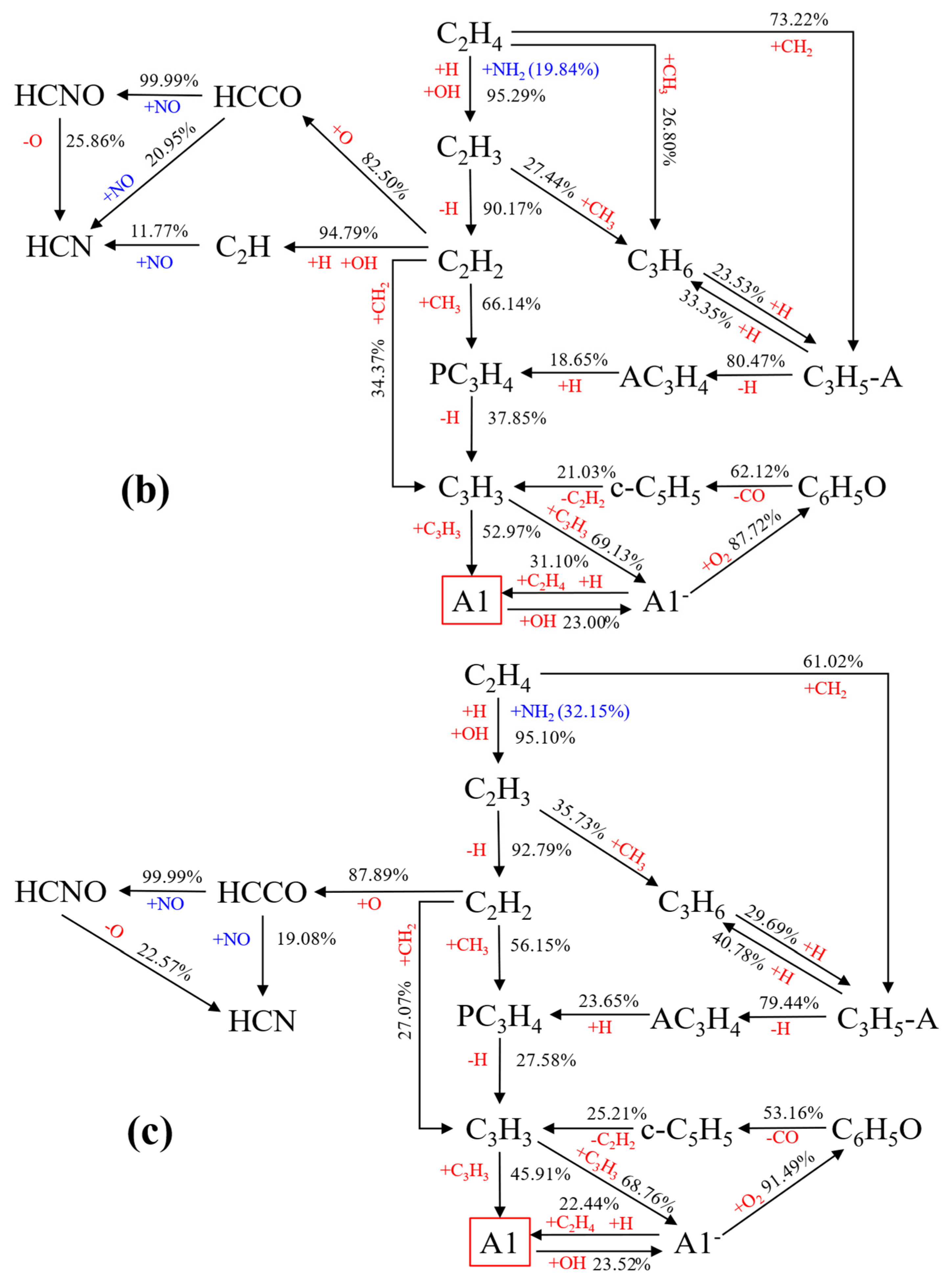
Reaction Path Analysis of A1
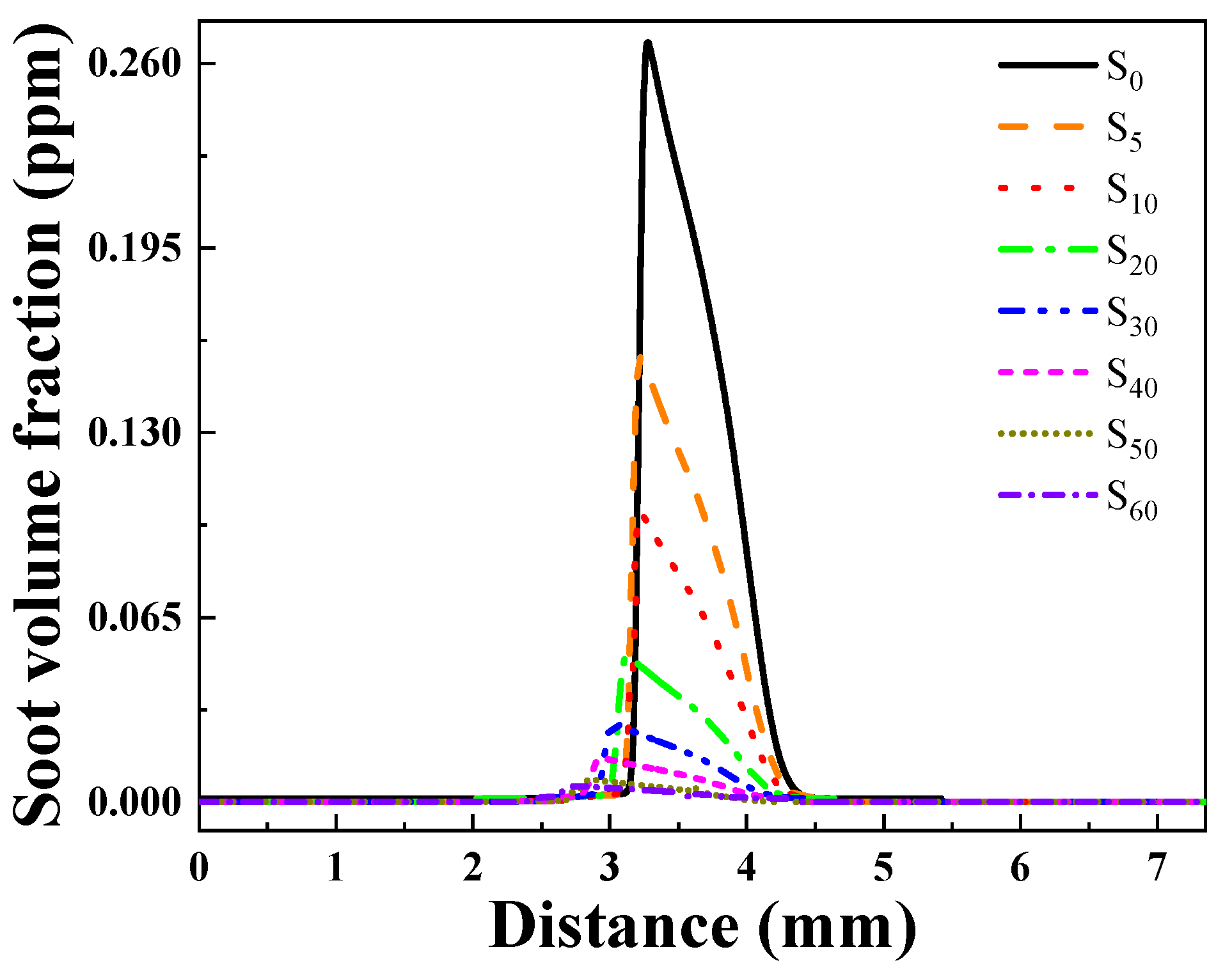
Soot Generation Concentration Analysis
2.2.2. The Effect of Ammonia on NOx Formation
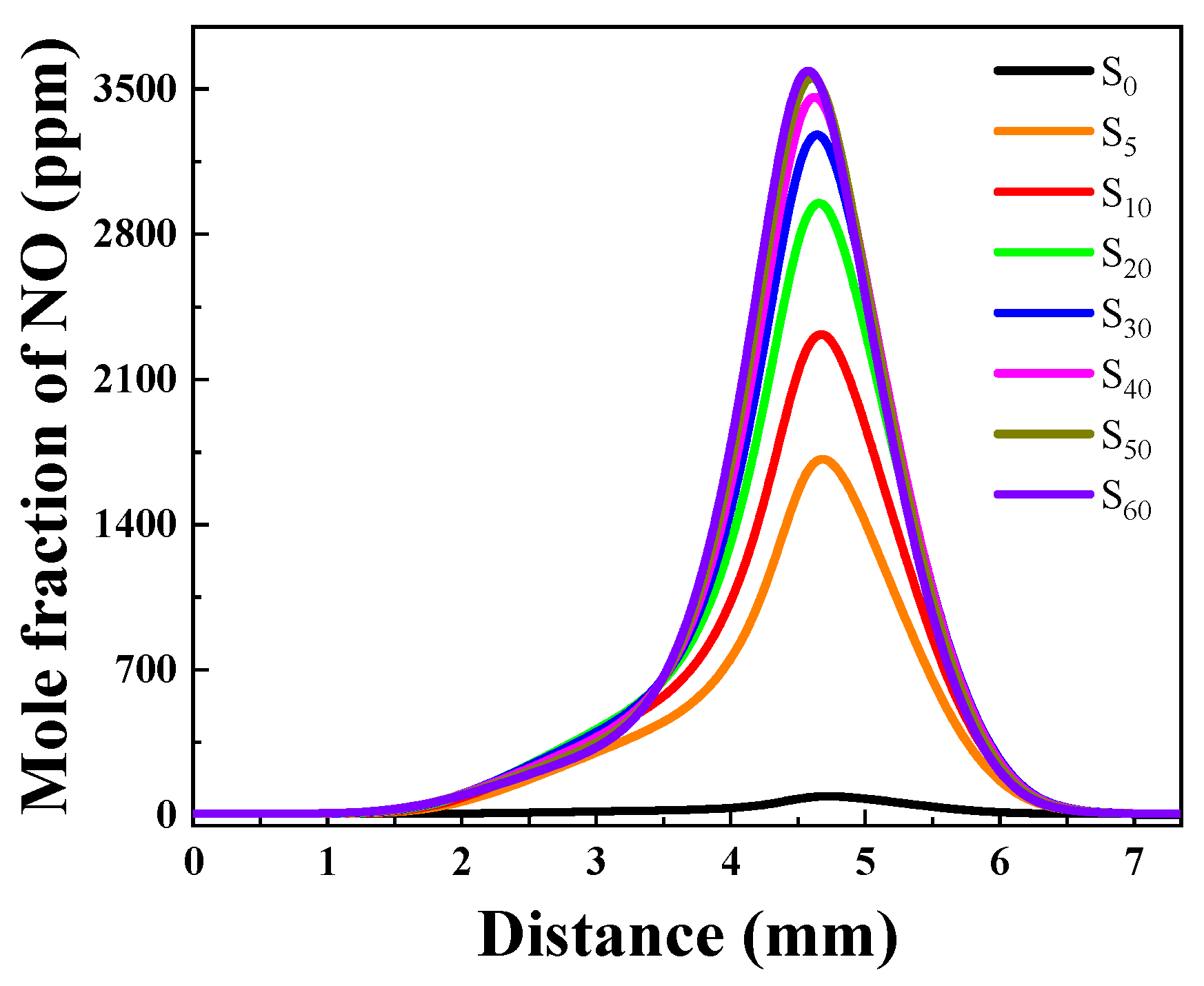
NOx Generation Concentration Analysis
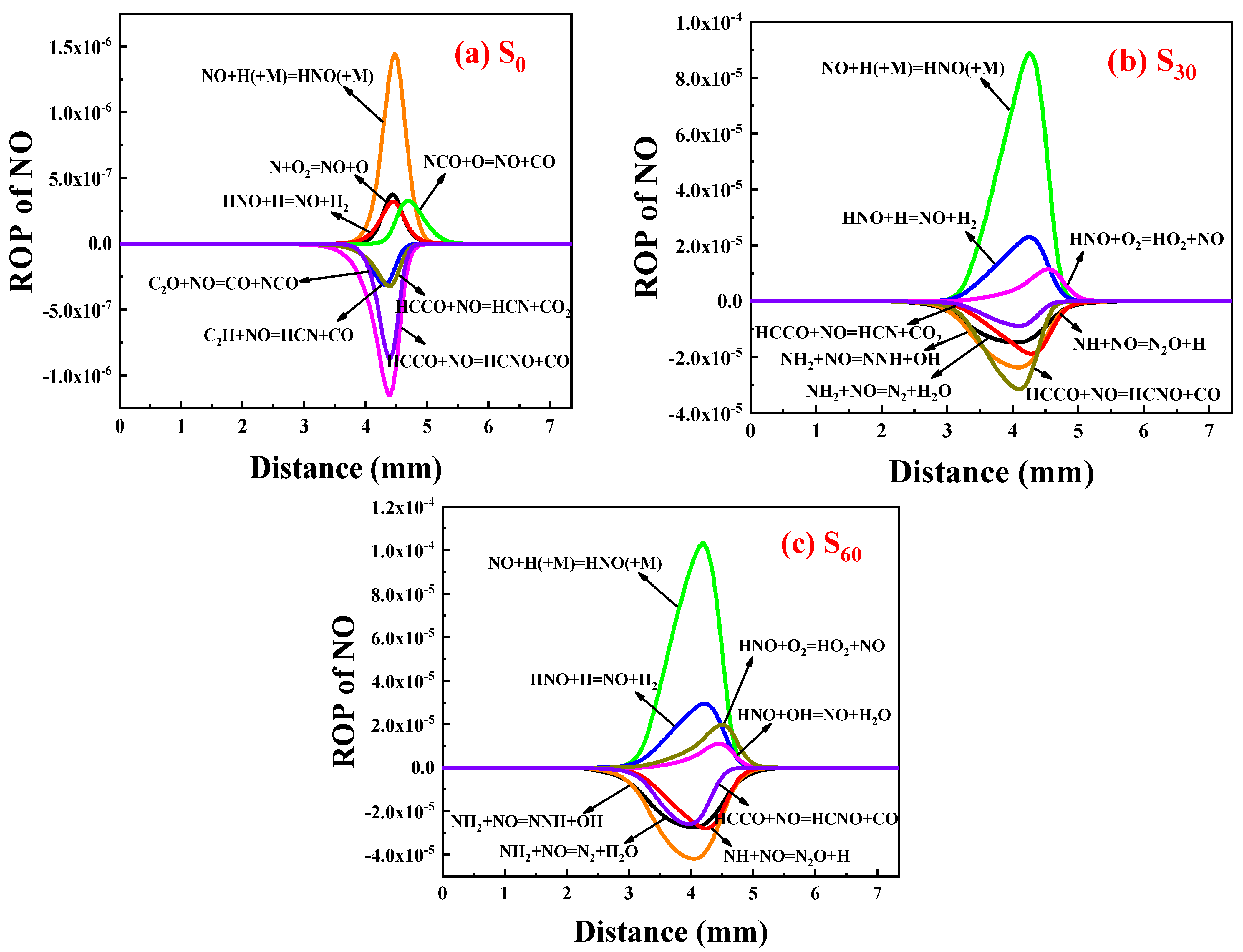
ROP Analysis of NO
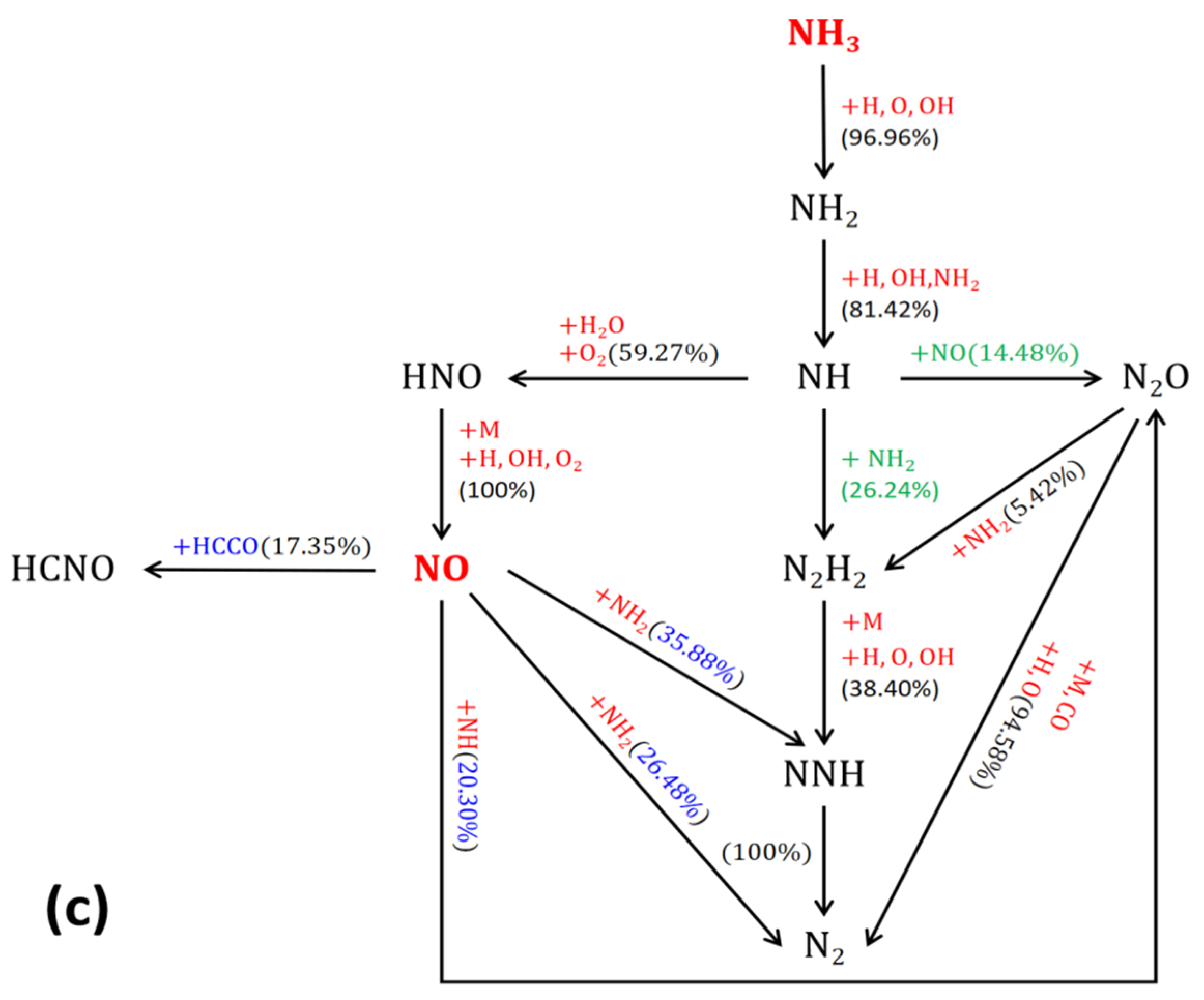
Reaction Path Analysis of NO
3. Materials and Methods
3.1. Experimental Setting
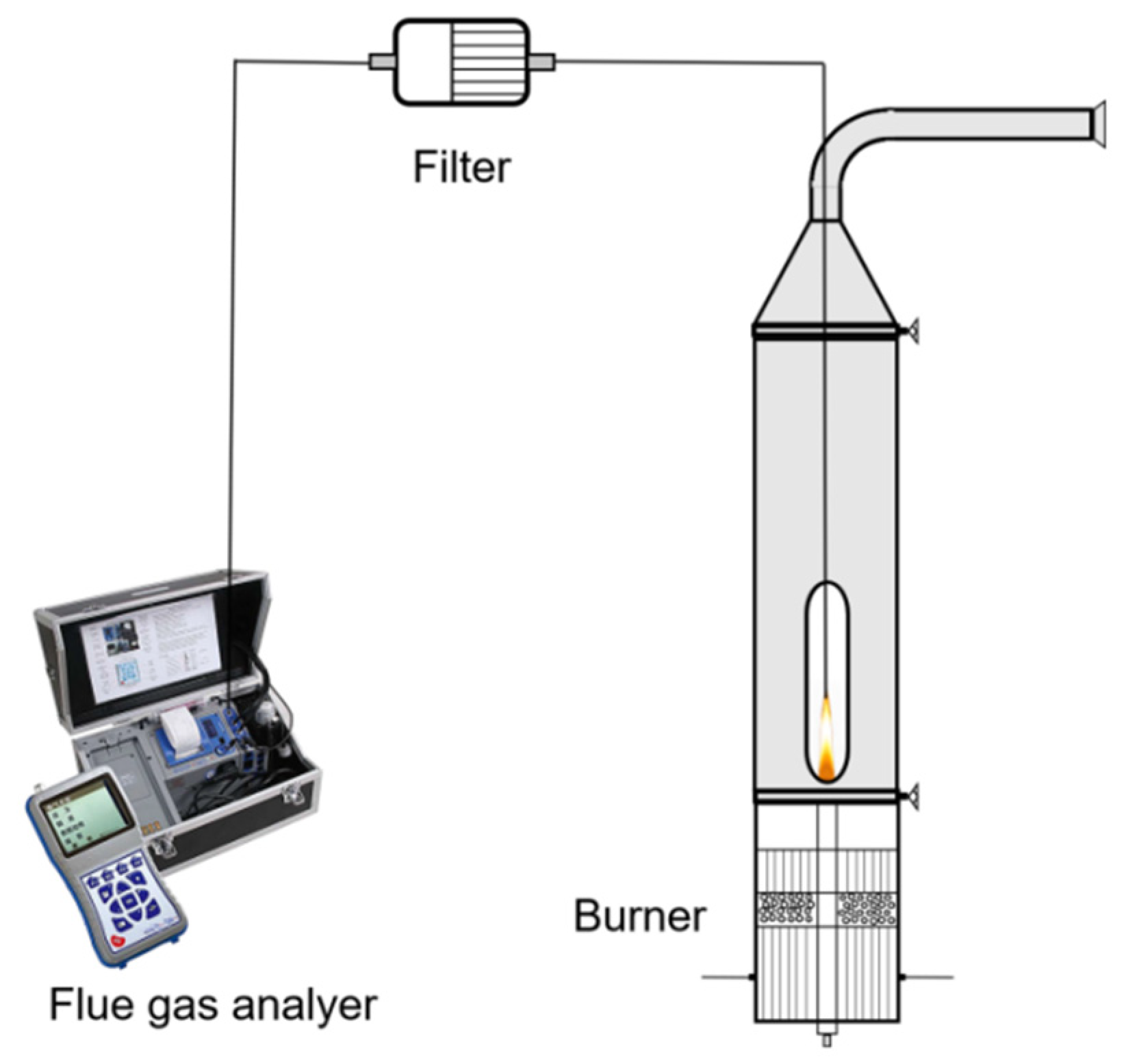
3.1.1. Experimental System and Setting
3.1.2. Measurement of Soot Volume Fraction
3.1.3. Measurement of NOx
3.2. Simulation Methods
4. Conclusions
- Ammonia doping reduces soot formation in ethylene flames. When the doping ratio of ammonia is below 20%, there is a strong inhibitory effect on the soot concentration in the flame, and the inhibitory effect on soot changes less for cases with more than 30% added ammonia.
- A higher concentration of NOx is generated in the initial part of the flame, and the lower the ammonia ratio, the higher the concentration of NOx. As the combustion process progresses, the concentration of NOx initially decreases and then subsequently increases rapidly. With higher ammonia ratios, there is a higher concentrations of NOx emissions.
- Ammonia doping reduces the mole fractions of C2H2, C3H3, PC3H4, and C4H4 responsible for the first aromatic ring formation and larger PAH growth, thus leading to the reduction of A1–A4 formation. In addition, sensitivity and reaction pathway analysis of A1 formation indicate that NH3 addition contributes to the nitrogen–hydrocarbon interaction, which hinders the HACA process and inhibits the generation of soot.
- The ammonia blending rate has little effect on the main pathway of NO generation, but the higher the amount of ammonia, the higher the chance of NH3 being oxidized to N2, and the more likely it is that the generated NO will be reduced to N2 again by the action of the free radicals NH2 and NH.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Outlook. Available online: https://www.irena.org/Energy-Transition/Outlook (accessed on 22 June 2023).
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Wang, S.; Guiberti, T.F.; Roberts, W.L. Review on the recent advances on ammonia combustion from the fundamentals to the applications. Fuel Commun. 2022, 10, 100053. [Google Scholar] [CrossRef]
- Ammonia Technology Roadmap; OECD: Paris, France, 2021. [CrossRef]
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; De Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on ammonia as a potential fuel: From synthesis to economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]
- Chai, W.S.; Bao, Y.; Jin, P.; Tang, G.; Zhou, L. A review on ammonia, ammonia-hydrogen and ammonia-methane fuels. Renew. Sustain. Energy Rev. 2021, 147, 111254. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Using ammonia as a sustainable fuel. J. Power Sources 2008, 185, 459–465. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, L.; Wei, H. Understanding the difference in combustion and flame propagation characteristics between ammonia and methane using an optical SI engine. Fuel 2022, 324, 124794. [Google Scholar] [CrossRef]
- Nozari, H.; Karabeyoğlu, A. Numerical study of combustion characteristics of ammonia as a renewable fuel and establishment of reduced reaction mechanisms. Fuel 2015, 159, 223–233. [Google Scholar] [CrossRef]
- Ariemma, G.B.; Sorrentino, G.; Ragucci, R.; de Joannon, M.; Sabia, P. Ammonia/Methane combustion: Stability and NOx emissions. Combust. Flame 2022, 241, 112071. [Google Scholar] [CrossRef]
- Ku, J.W.; Choi, S.; Kim, H.K.; Lee, S.; Kwon, O.C. Extinction limits and structure of counterflow nonpremixed methane-ammonia/air flames. Energy 2018, 165, 314–325. [Google Scholar] [CrossRef]
- Hashimoto, G.; Hadi, K.; Xia, Y.; Hamid, A.; Hashimoto, N.; Hayakawa, A.; Kobayashi, H.; Fujita, O. Turbulent flame propagation limits of ammonia/methane/air premixed mixture in a constant volume vessel. Proc. Combust. Inst. 2021, 38, 5181–5190. [Google Scholar] [CrossRef]
- Tang, R.; Xu, Q.; Pan, J. An experimental and modeling study of ammonia oxidation in a jet stirred reactor. Combust. Flame 2022, 240, 112007. [Google Scholar] [CrossRef]
- Awad, O.; Zhou, B.; Harrath, K.; Kadirgama, K. Characteristics of NH3/H2 blend as carbon-free fuels: A review. Int. J. Hydrogen Energy 2022, 48, 38077–38100. [Google Scholar] [CrossRef]
- Deng, Q.; Ying, Y.; Liu, D. Detailed chemical effects of ammonia as fuel additive in ethylene counterflow diffusion flames. Int. J. Hydrogen Energy 2022, 47, 33498–33516. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Yang, G.; Fuentes, A.; Han, D.; Huang, Z.; Lin, H. A comparative study on PAH characteristics of ethanol and ammonia as fuel additives in a premixed flame. J. Energy Inst. 2022, 101, 56–66. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, Y.; Li, Q.; Yan, Y.; Tian, Z.; Song, C.; Huang, Z. Study on effects of NH3 and/or H2 addition on the characteristics of soot formation and gas emissions in a laminar ethylene diffusion flame. Fuel Process. Technol. 2023, 242, 107633. [Google Scholar] [CrossRef]
- He, J.; Ying, Y.; Chen, M.; Liu, D. Soot formation characteristics in hybrid pyrolysis of zero-carbon fuel ammonia and ethylene mixtures. Front. Energy Res. 2022, 10, 1493. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, X.; Huang, C.; Yang, Y.; Yang, H.; Zhang, J.; Huang, T. High atmospheric wet nitrogen deposition and major sources in two cities of Yangtze River Delta: Combustion-related NH3 and non-fossil fuel NOx. Sci. Total Environ. 2022, 806, 150502. [Google Scholar] [CrossRef]
- Bennett, A.M.; Liu, P.; Li, Z.; Kharbatia, N.M.; Boyette, W.; Masri, A.R.; Roberts, W.L. Soot formation in laminar flames of ethylene/ammonia. Combust. Flame 2020, 220, 210–218. [Google Scholar] [CrossRef]
- Zhou, M.; Yan, F.; Ma, L.; Jiang, P.; Wang, Y.; Ho Chung, S. Chemical speciation and soot measurements in laminar counterflow diffusion flames of ethylene and ammonia mixtures. Fuel 2022, 308, 122003. [Google Scholar] [CrossRef]
- Steinmetz, S.A.; Ahmed, H.A.; Boyette, W.R.; Dunn, M.J.; Roberts, W.L.; Masri, A.R. Effects of ammonia and hydrogen on the sooting characteristics of laminar coflow flames of ethylene and methane. Fuel 2022, 307, 121914. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, X.; Li, Y.; Qiu, L.; Wang, X.; Xu, Y. Effects of ammonia addition on soot formation in ethylene laminar diffusion flames. Fuel 2021, 292, 120416. [Google Scholar] [CrossRef]
- Ren, F.; Cheng, X.; Gao, Z.; Huang, Z.; Zhu, L. Effects of NH3 addition on polycyclic aromatic hydrocarbon and soot formation in C2H4 co-flow diffusion flames. Combust. Flame 2022, 241, 111958. [Google Scholar] [CrossRef]
- Boyette, W.R.; Macfarlane, A.R.W.; Steinmetz, S.A.; Dunn, M.J.; Roberts, W.L.; Masri, A.R. On the combined effects of compositional inhomogeneity and ammonia addition to turbulent flames of ethylene. Proc. Combust. Inst. 2023, 39, 4355–4364. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Y.; Liu, Y.; Wang, H.; Liu, Y.; Cheng, X. Effects of ammonia addition on soot formation in ethylene laminar diffusion flames. Part 2. Further insights into soot inception, growth and oxidation. Fuel 2022, 331, 125623. [Google Scholar] [CrossRef]
- Da Rocha, R.C.; Costa, M.; Bai, X.-S. Chemical kinetic modelling of ammonia/hydrogen/air ignition, premixed flame propagation and NO emission. Fuel 2019, 246, 24–33. [Google Scholar] [CrossRef]
- Xiao, H.; Lai, S.; Valera-Medina, A.; Li, J.; Liu, J.; Fu, H. Study on counterflow premixed flames using high concentration ammonia mixed with methane. Fuel 2020, 275, 117902. [Google Scholar] [CrossRef]
- Abián, M.; Peribáñez, E.; Millera, Á.; Bilbao, R.; Alzueta, M.U. Impact of nitrogen oxides (NO, NO2, N2O) on the formation of soot. Combust. Flame 2014, 161, 280–287. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Z.; Zha, X.; Gao, G.; Mao, W.; Wu, F.; Li, X. Fuel-NO formation mechanism in MILD-oxy combustion of CH4/NH3 fuel blend. Fuel 2023, 331, 125817. [Google Scholar] [CrossRef]
- Shi, H.; Liu, S.; Zou, C.; Dai, L.; Li, J.; Xia, W.; Yang, J.; Luo, J.; Li, W. Experimental study and mechanism analysis of the NOx emissions in the NH3 MILD combustion by a novel burner. Fuel 2022, 310, 122417. [Google Scholar] [CrossRef]
- Montgomery, M.J.; Kwon, H.; Dreyer, J.A.H.; Xuan, Y.; McEnally, C.S.; Pfefferle, L.D. Effect of ammonia addition on suppressing soot formation in methane co-flow diffusion flames. Proc. Combust. Inst. 2021, 38, 2497–2505. [Google Scholar] [CrossRef]
- Guo, H.; Smallwood, G.J. The interaction between soot and NO formation in a laminar axisymmetric coflow ethylene/air diffusion flame. Combust. Flame 2007, 149, 225–233. [Google Scholar] [CrossRef]
- Wang, Q.; Legros, G.; Bonnety, J.; Morin, C. Experimental characterization of the different nitrogen dilution effects on soot formation in ethylene diffusion flames. Proc. Combust. Inst. 2017, 36, 3227–3235. [Google Scholar] [CrossRef]
- Gu, M.; Chu, H.; Liu, F. Effects of simultaneous hydrogen enrichment and carbon dioxide dilution of fuel on soot formation in an axisymmetric coflow laminar ethylene/air diffusion flame. Combust. Flame 2016, 166, 216–228. [Google Scholar] [CrossRef]
- Gu, M.; Liu, F.; Consalvi, J.L.; Gülder, Ö.L. Effects of pressure on soot formation in laminar coflow methane/air diffusion flames doped with n-heptane and toluene between 2 and 8 atm. Proc. Combust. Inst. 2021, 38, 1403–1412. [Google Scholar] [CrossRef]
- Daca, A.E.; Gülder, Ö.L. Soot formation characteristics of diffusion flames of methane doped with toluene and n-heptane at elevated pressures. Proc. Combust. Inst. 2017, 36, 737–744. [Google Scholar] [CrossRef]
- Suresh, R.; Kalvakala, K.C.; Aggarwal, S.K. A numerical study of NOx and soot emissions in ethylene-ammonia diffusion flames with oxygen enrichment. Fuel 2024, 362, 130834. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, S.; Sui, R.; Lu, Q. Impact of ammonia addition on soot and NO/N2O formation in methane/air co-flow diffusion flames. Combust. Flame 2023, 247, 112483. [Google Scholar] [CrossRef]
- Gülder, Ö.L.; Snelling, D.R.; Sawchuk, R.A. Influence of hydrogen addition to fuel on temperature field and soot formation in diffusion flames. Symp. Combust. 1996, 26, 2351–2358. [Google Scholar] [CrossRef]
- Zhang, F.; Li, S.; Liu, Q.; Sun, J.; Wei, X.; Gu, M.; Wang, Y.; Huang, X. Effect of ammonia on the soot surface characteristics in ammonia/ethylene co-flow diffusion flames. Fuel 2023, 341, 127633. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, B.; Chen, J.; Wu, J.; Lu, K.; Gu, M.; Chu, H. Study of soot functional groups and morphological characteristics in laminar coflow methane and ethylene diffusion flames with hydrogen addition. Fuel 2020, 279, 118474. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Zhu, B.; Wang, Y.; Gu, M. Experimental study on the effect of hydrogen addition on methane/ethylene diffusion flame soot formation based on light extinction measurement. Energy Rep. 2021, 7, 673–683. [Google Scholar] [CrossRef]
- Smyth, K.C.; Shaddix, C.R. The elusive history of m = 1.57 − 0.56i for the refractive index of soot. Combust. Flame 1996, 107, 314–320. [Google Scholar] [CrossRef]
- Snelling, D.R.; Thomson, K.A.; Smallwood, G.J.; Gülder, Ö.L. Two-dimensional imaging of soot volume fraction in laminar diffusion flames. Appl. Opt. 1999, 38, 2478. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, M.; Gao, Y.; Liu, X.; Lin, Y. An experimental and numerical study of soot formation of laminar coflow H2/C2H4 diffusion flames in O2-CO2 atmosphere. Combust. Flame 2020, 221, 50–63. [Google Scholar] [CrossRef]
- Ansys. Ansys Chemkin-Pro|Chemical Kinetics Simulation Software. Available online: https://www.ansys.com/products/fluids/ansys-chemkin-pro (accessed on 21 August 2024).
- Gao, Z.; Zhu, L.; Zou, X.; Liu, C.; Tian, B.; Huang, Z. Soot reduction effects of dibutyl ether (DBE) addition to a biodiesel surrogate in laminar coflow diffusion flames. Proc. Combust. Inst. 2019, 37, 1265–1272. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, L.; Thomson, M.J. Effects of n-propylbenzene addition to n-dodecane on soot formation and aggregate structure in a laminar coflow diffusion flame. Proc. Combust. Inst. 2017, 36, 1339–1347. [Google Scholar] [CrossRef]
- Wang, Y.; Raj, A.; Chung, S.H. A PAH growth mechanism and synergistic effect on PAH formation in counterflow diffusion flames. Combust. Flame 2013, 160, 1667–1676. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- University of California-Berkeley. GRI-Mech 3.0. Available online: http://combustion.berkeley.edu/gri-mech/version30/text30.html (accessed on 30 October 2002).
- Wang, Y.; Raj, A.; Chung, S.H. Soot modeling of counterflow diffusion flames of ethylene-based binary mixture fuels. Combust. Flame 2015, 162, 586–596. [Google Scholar] [CrossRef]







| Case | NH3 Ratio | NH3 Flow Rate (mL/min) | C2H4 Flow Rate (mL/min) | Ar Flow Rate (mL/min) | Air Flow Ate (L/min) |
|---|---|---|---|---|---|
| X0 | 0% | 0 | 180 | 270 | 100 |
| X5 | 5% | 22.5 | 180 | 247.5 | 100 |
| X10 | 10% | 45 | 180 | 225 | 100 |
| X20 | 20% | 90 | 180 | 180 | 100 |
| X30 | 30% | 135 | 180 | 135 | 100 |
| X40 | 40% | 180 | 180 | 90 | 100 |
| X50 | 50% | 225 | 180 | 50 | 100 |
| X60 | 60% | 270 | 180 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, Q.; Zhang, F.; Sun, J.; Wang, Y.; Gu, M. Study on Soot and NOx Formation Characteristics in Ammonia/Ethylene Laminar Co-Flow Diffusion Flame. Molecules 2024, 29, 4003. https://doi.org/10.3390/molecules29174003
Li S, Liu Q, Zhang F, Sun J, Wang Y, Gu M. Study on Soot and NOx Formation Characteristics in Ammonia/Ethylene Laminar Co-Flow Diffusion Flame. Molecules. 2024; 29(17):4003. https://doi.org/10.3390/molecules29174003
Chicago/Turabian StyleLi, Shuanglong, Qianqian Liu, Feng Zhang, Jingyun Sun, Yang Wang, and Mingyan Gu. 2024. "Study on Soot and NOx Formation Characteristics in Ammonia/Ethylene Laminar Co-Flow Diffusion Flame" Molecules 29, no. 17: 4003. https://doi.org/10.3390/molecules29174003
APA StyleLi, S., Liu, Q., Zhang, F., Sun, J., Wang, Y., & Gu, M. (2024). Study on Soot and NOx Formation Characteristics in Ammonia/Ethylene Laminar Co-Flow Diffusion Flame. Molecules, 29(17), 4003. https://doi.org/10.3390/molecules29174003








