Overcoming Cancer Drug Resistance with Nanoparticle Strategies for Key Protein Inhibition
Abstract
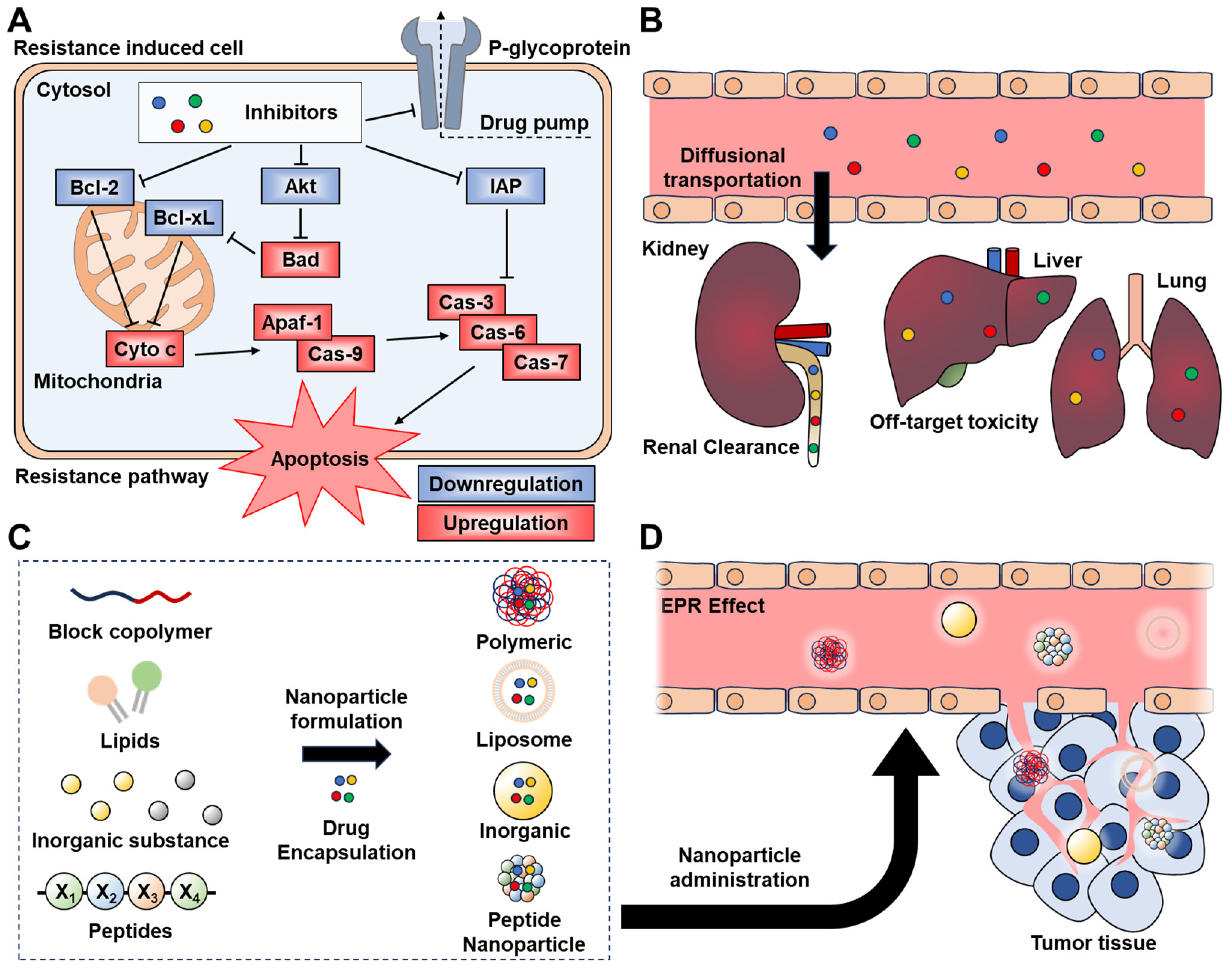
1. Introduction
2. Nano-Delivery of Bcl-2 Inhibitor
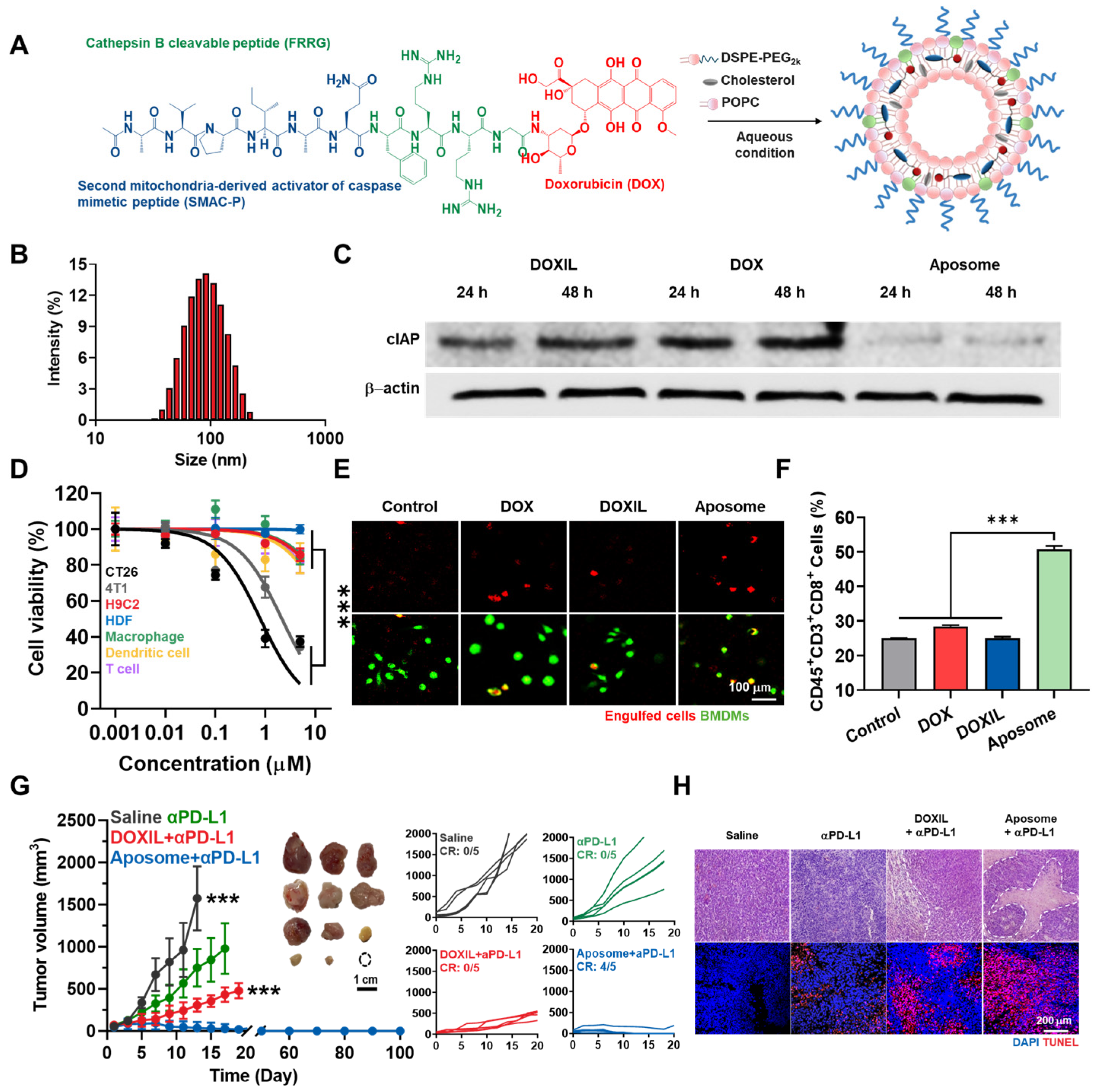
3. Nano-Delivery of IAPs Inhibitor
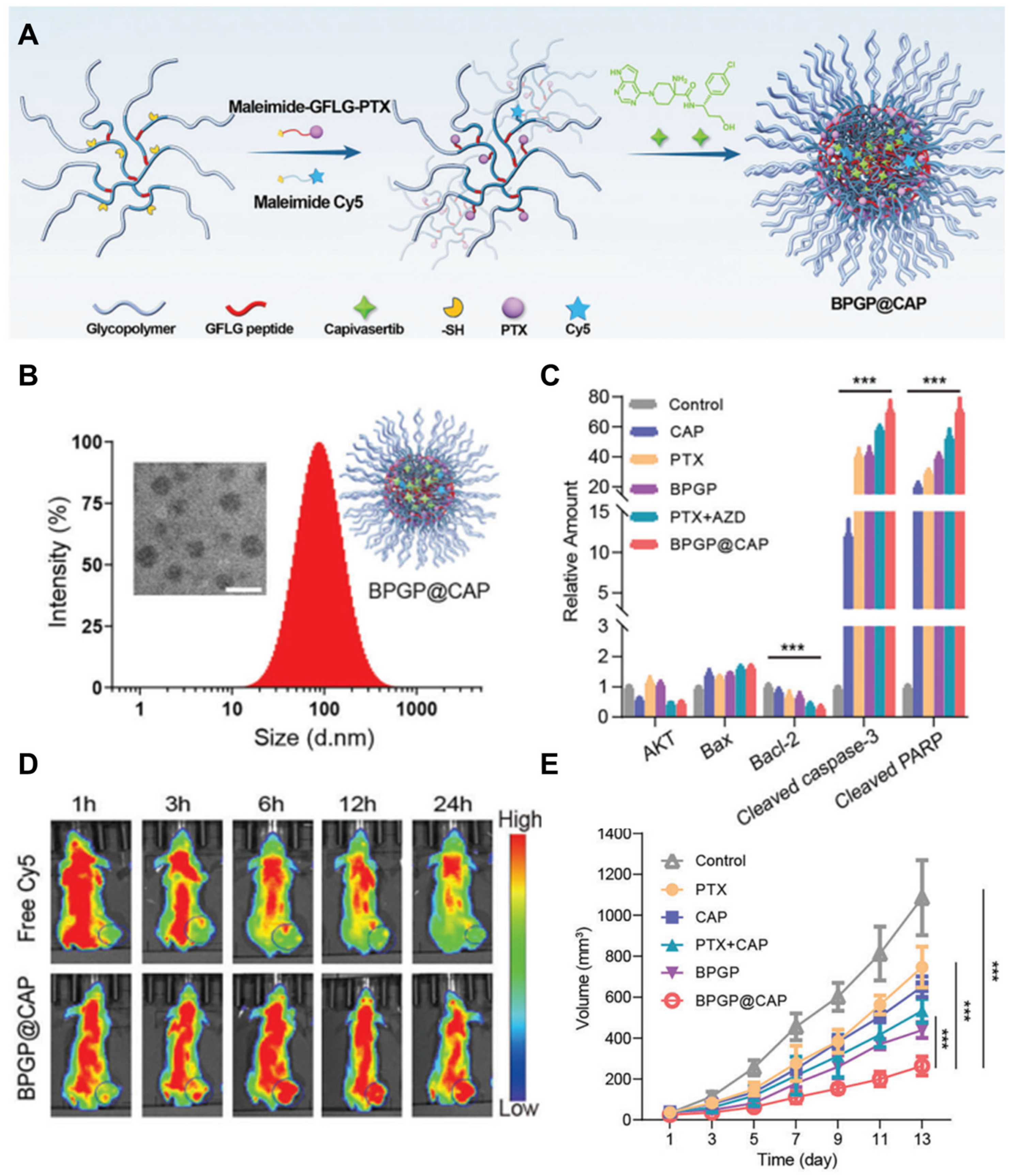
4. Nano-Delivery of Akt Inhibitor
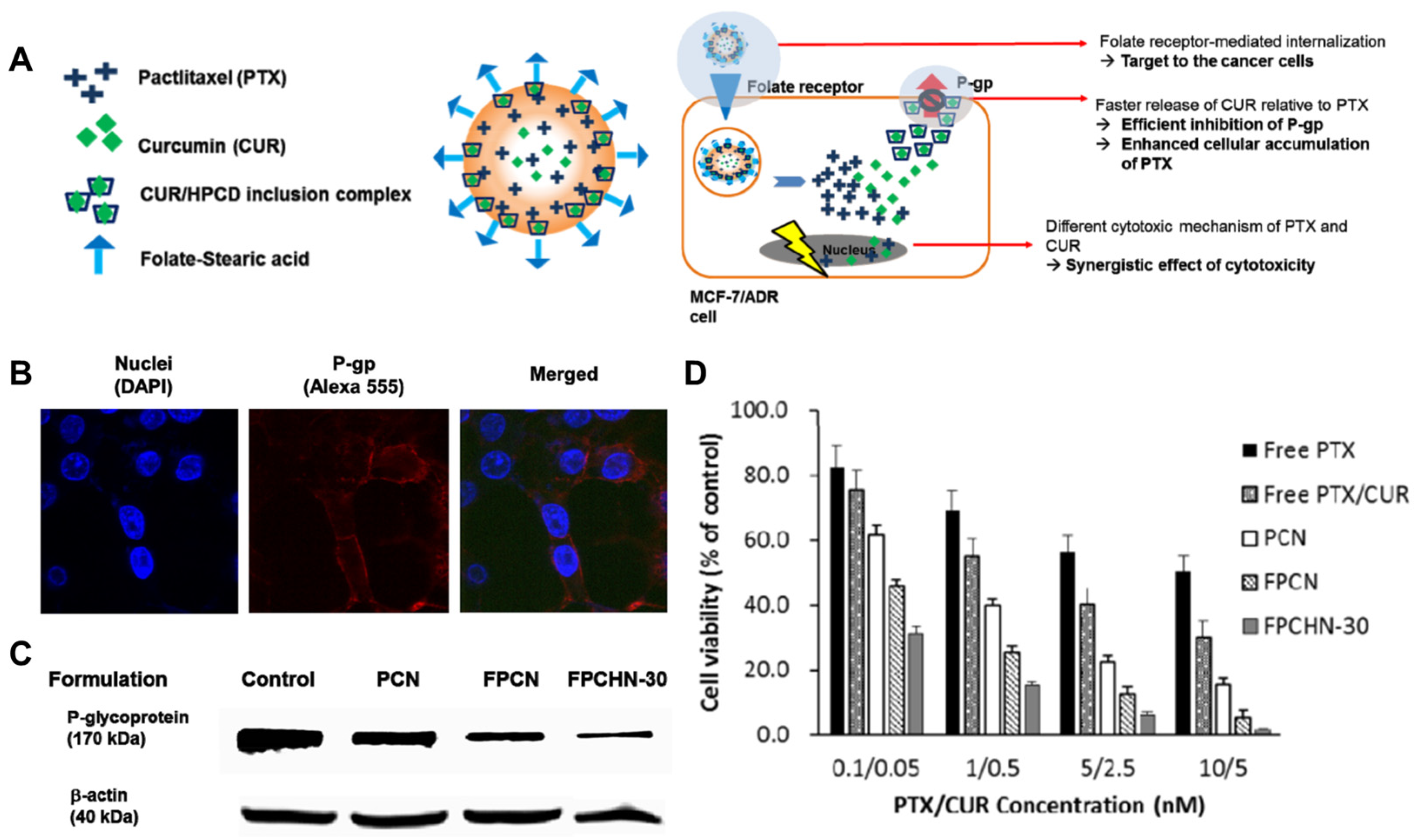
5. Nano-Delivery of P-Glycoprotein Inhibitor
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic cancer vaccines: Advancements, challenges, and prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Yuzhalin, A.E. Redefining cancer research for therapeutic breakthroughs. Br. J. Cancer 2024, 130, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Bhowmick, M.; Pal, R.; Khatoon, H.; Kumar, P.; Sharma, H.; Garg, A.; Kumar, S.; Das, U. Multi drug resistance in Colorectal Cancer- approaches to overcome, advancements and future success. Adv. Cancer Biol. Metastasis 2024, 10, 100114. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Zargar, A.; Chang, S.; Kothari, A.; Snijders, A.M.; Mao, J.H.; Wang, J.; Hernandez, A.C.; Keasling, J.D.; Bivona, T.G. Overcoming the challenges of cancer drug resistance through bacterial-mediated therapy. Chronic Dis. Transl. Med. 2019, 5, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, P.; Lin, J.; Chen, K.; Shen, J. Recent advances in access to overcome cancer drug resistance by nanocarrier drug delivery system. Cancer Drug Resist. 2023, 6, 390–415. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.N.; Tian, Q.; Teng, Q.X.; Wurpel, J.N.D.; Zeng, L.; Pan, Y.; Chen, Z.S. Understanding and targeting resistance mechanisms in cancer. MedComm 2023, 4, e265. [Google Scholar] [CrossRef]
- Qin, S.; Jiang, J.; Lu, Y.; Nice, E.C.; Huang, C.; Zhang, J.; He, W. Emerging role of tumor cell plasticity in modifying therapeutic response. Signal Transduct. Target. Ther. 2020, 5, 228. [Google Scholar] [CrossRef]
- Zhang, L.; Ye, B.; Chen, Z.; Chen, Z.S. Progress in the studies on the molecular mechanisms associated with multidrug resistance in cancers. Acta Pharm. Sin. B 2023, 13, 982–997. [Google Scholar] [CrossRef] [PubMed]
- Nussinov, R.; Tsai, C.J.; Jang, H. Anticancer drug resistance: An update and perspective. Drug Resist. Updat. 2021, 59, 100796. [Google Scholar] [CrossRef] [PubMed]
- Engle, K.; Kumar, G. Cancer multidrug-resistance reversal by ABCB1 inhibition: A recent update. Eur. J. Med. Chem. 2022, 239, 114542. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Trougakos, I.P.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci. Rep. 2018, 8, 967. [Google Scholar] [CrossRef]
- Karthika, C.; Sureshkumar, R.; Zehravi, M.; Akter, R.; Ali, F.; Ramproshad, S.; Mondal, B.; Tagde, P.; Ahmed, Z.; Khan, F.S.; et al. Multidrug Resistance of Cancer Cells and the Vital Role of P-Glycoprotein. Life 2022, 12, 897. [Google Scholar] [CrossRef]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.Y.; Lin, L.T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Telu, S.; Shukla, S.; Ambudkar, S.V.; Pike, V.W.; Halldin, C.; Gottesman, M.M.; Innis, R.B.; Hall, M.D. The “specific” P-glycoprotein inhibitor Tariquidar is also a substrate and an inhibitor for breast cancer resistance protein (BCRP/ABCG2). ACS Chem. Neurosci. 2011, 2, 82–89. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef]
- Nor Hisam, N.S.; Ugusman, A.; Rajab, N.F.; Ahmad, M.F.; Fenech, M.; Liew, S.L.; Mohamad Anuar, N.N. Combination Therapy of Navitoclax with Chemotherapeutic Agents in Solid Tumors and Blood Cancer: A Review of Current Evidence. Pharmaceutics 2021, 13, 1353. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Smith, D.C.; Wang, S. Small-molecule SMAC mimetics as new cancer therapeutics. Pharmacol. Ther. 2014, 144, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Chen, T.; Zhang, X.; Ma, X.L.; Shi, H.S. Small molecule inhibitors targeting the cancers. MedComm 2022, 3, e181. [Google Scholar] [CrossRef]
- Basak, D.; Arrighi, S.; Darwiche, Y.; Deb, S. Comparison of Anticancer Drug Toxicities: Paradigm Shift in Adverse Effect Profile. Life 2021, 12, 48. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Veselov, V.V.; Nosyrev, A.E.; Jicsinszky, L.; Alyautdin, R.N.; Cravotto, G. Targeted Delivery Methods for Anticancer Drugs. Cancers 2022, 14, 622. [Google Scholar] [CrossRef]
- Hong, L.; Li, W.; Li, Y.; Yin, S. Nanoparticle-based drug delivery systems targeting cancer cell surfaces. RSC Adv. 2023, 13, 21365–21382. [Google Scholar] [CrossRef] [PubMed]
- Shim, N.; Cho, H.; Jeon, S.I.; Kim, K. Recent developments in chemodrug-loaded nanomedicines and their application in combination cancer immunotherapy. J. Pharm. Investig. 2023, 54, 13–36. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Subhan, M.A.; Parveen, F.; Filipczak, N.; Yalamarty, S.S.K.; Torchilin, V.P. Approaches to Improve EPR-Based Drug Delivery for Cancer Therapy and Diagnosis. J. Pers. Med. 2023, 13, 389. [Google Scholar] [CrossRef]
- Zhou, S.; Shang, Q.; Wang, N.; Li, Q.; Song, A.; Luan, Y. Rational design of a minimalist nanoplatform to maximize immunotherapeutic efficacy: Four birds with one stone. J. Control. Release 2020, 328, 617–630. [Google Scholar] [CrossRef]
- Zhang, C.G.; Zhu, W.J.; Liu, Y.; Yuan, Z.Q.; Yang, S.D.; Chen, W.L.; Li, J.Z.; Zhou, X.F.; Liu, C.; Zhang, X.N. Novel polymer micelle mediated co-delivery of doxorubicin and P-glycoprotein siRNA for reversal of multidrug resistance and synergistic tumor therapy. Sci. Rep. 2016, 6, 23859. [Google Scholar] [CrossRef]
- Karmacharya, P.; Patil, B.R.; Kim, J.O. Recent advancements in lipid-mRNA nanoparticles as a treatment option for cancer immunotherapy. J. Pharm. Investig. 2022, 52, 415–426. [Google Scholar] [CrossRef]
- Zeb, A.; Gul, M.; Nguyen, T.-T.-L.; Maeng, H.-J. Recent progress and drug delivery applications of surface-functionalized inorganic nanoparticles in cancer therapy. J. Pharm. Investig. 2023, 53, 743–779. [Google Scholar] [CrossRef]
- Shrestha, S.; Shrestha, A.; Kim, J.; Thapa, R.K.; Kim, J.O. Recent advances in albumin nanoparticle-based cancer therapies. J. Pharm. Investig. 2024, 1–14. [Google Scholar] [CrossRef]
- Zeb, A.; Gul, M.; Nguyen, T.-T.-L.; Maeng, H.-J. Controlled release and targeted drug delivery with poly(lactic-co-glycolic acid) nanoparticles: Reviewing two decades of research. J. Pharm. Investig. 2022, 52, 683–724. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles horizontal line From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Moon, Y.; Cho, H.; Kim, K. Nano-Delivery of Immunogenic Cell Death Inducers and Immune Checkpoint Blockade Agents: Single-Nanostructure Strategies for Enhancing Immunotherapy. Pharmaceutics 2024, 16, 795. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K. Multi-functional nanomedicines for combinational cancer immunotherapy that transform cold tumors to hot tumors. Expert. Opin. Drug Deliv. 2024, 21, 627–638. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Izci, M.; Maksoudian, C.; Manshian, B.B.; Soenen, S.J. The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors. Chem. Rev. 2021, 121, 1746–1803. [Google Scholar] [CrossRef]
- Zhang, R.X.; Wong, H.L.; Xue, H.Y.; Eoh, J.Y.; Wu, X.Y. Nanomedicine of synergistic drug combinations for cancer therapy—Strategies and perspectives. J. Control. Release 2016, 240, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.A.; Wilkins, D.E.; Dang, M.N.; Hoover, E.C.; Aboeleneen, S.B.; Day, E.S. Cancer Cell Membrane Wrapped Nanoparticles for the Delivery of a Bcl-2 Inhibitor to Triple-Negative Breast Cancer. Mol. Pharm. 2023, 20, 3895–3913. [Google Scholar] [CrossRef]
- Tunc, C.U.; Aydin, O. Co-delivery of Bcl-2 siRNA and doxorubicin through gold nanoparticle-based delivery system for a combined cancer therapy approach. J. Drug Deliv. Sci. Tec. 2022, 74, 103603. [Google Scholar] [CrossRef]
- Mehrotra, N.; Anees, M.; Tiwari, S.; Kharbanda, S.; Singh, H. Polylactic acid based polymeric nanoparticle mediated co-delivery of navitoclax and decitabine for cancer therapy. Nanomed Nanotechnol. Biol. Med. 2023, 47, 102627. [Google Scholar] [CrossRef]
- Kim, J.; Shim, M.K.; Moon, Y.; Kim, J.; Cho, H.H.; Yun, W.S.; Shim, N.; Seong, J.K.; Lee, Y.H.Y.; Lim, D.K.; et al. Cancer cell-specific and pro-apoptotic SMAC peptide-doxorubicin conjugated prodrug encapsulated aposomes for synergistic cancer immunotherapy. J. Nanobiotechnol. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, Z.; Yuan, C.-S.; Deng, Z.-W.; Li, F.; Chen, X.-G.; Liu, Y. MMP-responsive transformation nanomaterials with IAP antagonist to boost immune checkpoint therapy. J. Control. Release 2022, 343, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Kao, W.C.; Yeh, C.A.; Chen, H.J.; Lin, S.Z.; Hsieh, H.H.; Sun, W.S.; Chang, C.H.; Hung, H.S. Hyaluronic acid-fabricated nanogold delivery of the inhibitor of apoptosis protein-2 siRNAs inhibits benzo[a]pyrene-induced oncogenic properties of lung cancer A549 cells. Nanotechnology 2015, 26, 105101. [Google Scholar] [CrossRef]
- Song, X.H.; Cai, H.; Shi, Z.C.; Li, Z.Q.; Zheng, X.L.; Yang, K.; Gong, Q.Y.; Gu, Z.W.; Hu, J.K.; Luo, K. Enzyme-Responsive Branched Glycopolymer-Based Nanoassembly for Co-Delivery of Paclitaxel and Akt Inhibitor toward Synergistic Therapy of Gastric Cancer. Adv. Sci. 2024, 11, e2306230. [Google Scholar] [CrossRef]
- Zuo, T.T.; Li, J.; Zhang, J.; Sun, L.; Liang, X.; Yang, J.; Shen, Q. Coadministration of chemotherapy and PI3K/Akt pathway treatment with multistage acidity/CathB enzyme-responsive nanocarriers for inhibiting the metastasis of breast cancer. Biomater. Sci. 2019, 7, 5054–5067. [Google Scholar] [CrossRef]
- Gonzalez-Valdivieso, J.; Garcia-Sampedro, A.; Hall, A.R.; Girotti, A.; Arias, F.J.; Pereira, S.P.; Acedo, P. Smart Nanoparticles as Advanced Anti-Akt Kinase Delivery Systems for Pancreatic Cancer Therapy. ACS Appl. Mater. Inter. 2021, 13, 55790–55805. [Google Scholar] [CrossRef]
- Baek, J.-S.; Cho, C.-W. A multifunctional lipid nanoparticle for co-delivery of paclitaxel and curcumin for targeted delivery and enhanced cytotoxicity in multidrug resistant breast cancer cells. Oncotarget 2017, 8, 30369–30382. [Google Scholar] [CrossRef]
- Esim, O.; Sarper, M.; Ozkan, C.K.; Oren, S.; Baykal, B.; Savaser, A.; Ozkan, Y. Effect simultaneous delivery with P-glycoprotein inhibitor and nanoparticle administration of doxorubicin on cellular uptake and in vitro anticancer activity. Saudi Pharm. J. 2020, 28, 465–472. [Google Scholar] [CrossRef]
- Eom, Y.H.; Kim, H.S.; Lee, A.; Song, B.J.; Chae, B.J. BCL2 as a Subtype-Specific Prognostic Marker for Breast Cancer. J. Breast Cancer 2016, 19, 252–260. [Google Scholar] [CrossRef]
- Iqbal, J.; Neppalli, V.T.; Wright, G.; Dave, B.J.; Horsman, D.E.; Rosenwald, A.; Lynch, J.; Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; et al. BCL2 expression is a prognostic marker for the activated B-cell-like type of diffuse large B-cell lymphoma. J. Clin. Oncol. 2006, 24, 961–968. [Google Scholar] [CrossRef]
- Martin, B.; Paesmans, M.; Berghmans, T.; Branle, F.; Ghisdal, L.; Mascaux, C.; Meert, A.P.; Steels, E.; Vallot, F.; Verdebout, J.M.; et al. Role of Bcl-2 as a prognostic factor for survival in lung cancer: A systematic review of the literature with meta-analysis. Br. J. Cancer 2003, 89, 55–64. [Google Scholar] [CrossRef]
- Pena-Blanco, A.; Garcia-Saez, A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Shimizu, S.; Narita, M.; Tsujimoto, Y. BcL-2 family proteins regulate the release of apoptogenic cytochrome by the mitochondrial channel VDAC. Nature 1999, 399, 483, Erratum in Nature 2000, 407, 767. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, J.; Nussinov, R.; Ma, B. Release of Cytochrome C from Bax Pores at the Mitochondrial Membrane. Sci. Rep. 2017, 7, 2635. [Google Scholar] [CrossRef]
- Xu, Y.; Ye, H. Progress in understanding the mechanisms of resistance to BCL-2 inhibitors. Exp. Hematol. Oncol. 2022, 11, 31. [Google Scholar] [CrossRef]
- Lopez, A.; Reyna, D.E.; Gitego, N.; Kopp, F.; Zhou, H.; Miranda-Roman, M.A.; Nordstrom, L.U.; Narayanagari, S.R.; Chi, P.; Vilar, E.; et al. Co-targeting of BAX and BCL-XL proteins broadly overcomes resistance to apoptosis in cancer. Nat. Commun. 2022, 13, 1199. [Google Scholar] [CrossRef]
- Mohamad Anuar, N.N.; Nor Hisam, N.S.; Liew, S.L.; Ugusman, A. Clinical Review: Navitoclax as a Pro-Apoptotic and Anti-Fibrotic Agent. Front. Pharmacol. 2020, 11, 564108. [Google Scholar] [CrossRef]
- Ploumaki, I.; Triantafyllou, E.; Koumprentziotis, I.A.; Karampinos, K.; Drougkas, K.; Karavolias, I.; Trontzas, I.; Kotteas, E.A. Bcl-2 pathway inhibition in solid tumors: A review of clinical trials. Clin. Transl. Oncol. 2023, 25, 1554–1578. [Google Scholar] [CrossRef]
- Roberts, A.W. Therapeutic development and current uses of BCL-2 inhibition. Hematol-Am. Soc. Hemat 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Xu, J.; Dong, X.; Huang, D.C.S.; Xu, P.; Zhao, Q.; Chen, B. Current Advances and Future Strategies for BCL-2 Inhibitors: Potent Weapons against Cancers. Cancers 2023, 15, 4957. [Google Scholar] [CrossRef]
- Tannan, N.B.; Manzari, M.T.; Herviou, L.; Ferreira, M.D.; Hagen, C.; Kiguchi, H.; Manova-Todorova, K.; Seshan, V.; de Stanchina, E.; Heller, D.A.; et al. Tumor-targeted nanoparticles improve the therapeutic index of BCL2 and MCL1 dual inhibition. Blood 2021, 137, 2057–2069. [Google Scholar] [CrossRef]
- Patterson, C.M.; Balachander, S.B.; Grant, I.; Pop-Damkov, P.; Kelly, B.; McCoull, W.; Parker, J.; Giannis, M.; Hill, K.J.; Gibbons, F.D.; et al. Design and optimisation of dendrimer-conjugated Bcl-2/x(L) inhibitor, AZD0466, with improved therapeutic index for cancer therapy. Commun. Biol. 2021, 4, 112. [Google Scholar] [CrossRef]
- Daoud, M.; Broxtermann, P.N.; Schorn, F.; Werthenbach, J.P.; Seeger, J.M.; Schiffmann, L.M.; Brinkmann, K.; Vucic, D.; Tuting, T.; Mauch, C.; et al. XIAP promotes melanoma growth by inducing tumour neutrophil infiltration. EMBO Rep. 2022, 23, e53608. [Google Scholar] [CrossRef]
- Lopes, R.B.; Gangeswaran, R.; McNeish, I.A.; Wang, Y.; Lemoine, N.R. Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. Int. J. Cancer 2007, 120, 2344–2352. [Google Scholar] [CrossRef]
- Fung, S.; Knoefel, W.T.; Krieg, A. Clinicopathological and Prognostic Significance of Inhibitor of Apoptosis Protein (IAP) Family Members in Lung Cancer: A Meta-Analysis. Cancers 2021, 13, 4098. [Google Scholar] [CrossRef]
- Kumar, S.; Fairmichael, C.; Longley, D.B.; Turkington, R.C. The Multiple Roles of the IAP Super-family in cancer. Pharmacol. Ther. 2020, 214, 107610. [Google Scholar] [CrossRef]
- Rathore, R.; McCallum, J.E.; Varghese, E.; Florea, A.M.; Busselberg, D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 2017, 22, 898–919. [Google Scholar] [CrossRef]
- Mace, P.D.; Shirley, S.; Day, C.L. Assembling the building blocks: Structure and function of inhibitor of apoptosis proteins. Cell Death Differ. 2010, 17, 46–53. [Google Scholar] [CrossRef]
- Lalaoui, N.; Merino, D.; Giner, G.; Vaillant, F.; Chau, D.; Liu, L.; Kratina, T.; Pal, B.; Whittle, J.R.; Etemadi, N.; et al. Targeting triple-negative breast cancers with the Smac-mimetic birinapant. Cell Death Differ. 2020, 27, 2768–2780. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Cheung, C.H.A. An Updated Review of Smac Mimetics, LCL161, Birinapant, and GDC-0152 in Cancer Treatment. Appl. Sci. 2020, 11, 335. [Google Scholar] [CrossRef]
- Cetraro, P.; Plaza-Diaz, J.; MacKenzie, A.; Abadia-Molina, F. A Review of the Current Impact of Inhibitors of Apoptosis Proteins and Their Repression in Cancer. Cancers 2022, 14, 1671. [Google Scholar] [CrossRef]
- Erickson, R.I.; Tarrant, J.; Cain, G.; Lewin-Koh, S.C.; Dybdal, N.; Wong, H.; Blackwood, E.; West, K.; Steigerwalt, R.; Mamounas, M.; et al. Toxicity profile of small-molecule IAP antagonist GDC-0152 is linked to TNF-alpha pharmacology. Toxicol. Sci. 2013, 131, 247–258. [Google Scholar] [CrossRef]
- Li, M.; Liu, P.; Gao, G.; Deng, J.; Pan, Z.; Wu, X.; Xie, G.; Yue, C.; Cho, C.H.; Ma, Y.; et al. Smac therapeutic Peptide nanoparticles inducing apoptosis of cancer cells for combination chemotherapy with Doxorubicin. ACS Appl. Mater. Interfaces 2015, 7, 8005–8012. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, Y.; Chan, H.F.; Kim, H.W.; Wang, Y.; Leong, K.W.; Chen, M. Nanoparticle-mediated inhibition of survivin to overcome drug resistance in cancer therapy. J. Control. Release 2016, 240, 454–464. [Google Scholar] [CrossRef]
- Shim, M.K.; Park, J.; Yoon, H.Y.; Lee, S.; Um, W.; Kim, J.H.; Kang, S.W.; Seo, J.W.; Hyun, S.W.; Park, J.H.; et al. Carrier-free nanoparticles of cathepsin B-cleavable peptide-conjugated doxorubicin prodrug for cancer targeting therapy. J. Control. Release 2019, 294, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef]
- Mundi, P.S.; Sachdev, J.; McCourt, C.; Kalinsky, K. AKT in cancer: New molecular insights and advances in drug development. Br. J. Clin. Pharmacol. 2016, 82, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Gradinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef]
- Parsons, C.M.; Muilenburg, D.; Bowles, T.L.; Virudachalam, S.; Bold, R.J. The Role of Akt Activation in the Response to Chemotherapy in Pancreatic Cancer. Anticancer Res. 2010, 30, 3279–3289. [Google Scholar]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.; Moyers, J.T.; Harbery, A.; Vivanco, I.; Yap, T.A. Clinical Development of AKT Inhibitors and Associated Predictive Biomarkers to Guide Patient Treatment in Cancer Medicine. Pharmgenomics Pers. Med. 2021, 14, 1517–1535. [Google Scholar] [CrossRef]
- Hirai, H.; Sootome, H.; Nakatsuru, Y.; Miyama, K.; Taguchi, S.; Tsujioka, K.; Ueno, Y.; Hatch, H.; Majumder, P.K.; Pan, B.S.; et al. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol. Cancer Ther. 2010, 9, 1956–1967. [Google Scholar] [CrossRef]
- Landel, I.; Quambusch, L.; Depta, L.; Rauh, D. Spotlight on AKT: Current Therapeutic Challenges. ACS Med. Chem. Lett. 2020, 11, 225–227. [Google Scholar] [CrossRef]
- Richardson, P.G.; Eng, C.; Kolesar, J.; Hideshima, T.; Anderson, K.C. Perifosine, an oral, anti-cancer agent and inhibitor of the Akt pathway: Mechanistic actions, pharmacodynamics, pharmacokinetics, and clinical activity. Expert. Opin. Drug Metab. Toxicol. 2012, 8, 623–633. [Google Scholar] [CrossRef]
- Yanes-Diaz, J.; Palao-Suay, R.; Aguilar, M.R.; Riestra-Ayora, J.I.; Ferruelo-Alonso, A.; Rojo Del Olmo, L.; Vazquez-Lasa, B.; Sanz-Fernandez, R.; Sanchez-Rodriguez, C. Antitumor Activity of Nanoparticles Loaded with PHT-427, a Novel AKT/PDK1 Inhibitor, for the Treatment of Head and Neck Squamous Cell Carcinoma. Pharmaceutics 2021, 13, 1242. [Google Scholar] [CrossRef]
- Chen, J.; Bu, X.; Shen, Q. Enhanced anti-cancer activity by co-delivery of docetaxel and perifosine with multifunctional nanoparticles via regulation of PI3K/Akt signalling pathway. Micro Nano Lett. 2015, 10, 253–257. [Google Scholar] [CrossRef]
- Lucero-Acuna, A.; Jeffery, J.J.; Abril, E.R.; Nagle, R.B.; Guzman, R.; Pagel, M.D.; Meuillet, E.J. Nanoparticle delivery of an AKT/PDK1 inhibitor improves the therapeutic effect in pancreatic cancer. Int. J. Nanomed. 2014, 9, 5653–5665. [Google Scholar] [CrossRef][Green Version]
- Dheer, D.; Nicolas, J.; Shankar, R. Cathepsin-sensitive nanoscale drug delivery systems for cancer therapy and other diseases. Adv. Drug Deliv. Rev. 2019, 151–152, 130–151. [Google Scholar] [CrossRef]
- Chandrawati, R. Enzyme-responsive polymer hydrogels for therapeutic delivery. Exp. Biol. Med. 2016, 241, 972–979. [Google Scholar] [CrossRef]
- Dong, J.; Yuan, L.; Hu, C.; Cheng, X.; Qin, J.J. Strategies to overcome cancer multidrug resistance (MDR) through targeting P-glycoprotein (ABCB1): An updated review. Pharmacol. Ther. 2023, 249, 108488. [Google Scholar] [CrossRef]
- Walsh, N.; Larkin, A.; Kennedy, S.; Connolly, L.; Ballot, J.; Ooi, W.; Gullo, G.; Crown, J.; Clynes, M.; O’Driscoll, L. Expression of multidrug resistance markers ABCB1 (MDR-1/P-gp) and ABCC1 (MRP-1) in renal cell carcinoma. BMC Urol. 2009, 9, 6. [Google Scholar] [CrossRef]
- Abd El-Aziz, Y.S.; Spillane, A.J.; Jansson, P.J.; Sahni, S. Role of ABCB1 in mediating chemoresistance of triple-negative breast cancers. Biosci. Rep. 2021, 41, BSR20204092. [Google Scholar] [CrossRef]
- Pilotto Heming, C.; Muriithi, W.; Wanjiku Macharia, L.; Niemeyer Filho, P.; Moura-Neto, V.; Aran, V. P-glycoprotein and cancer: What do we currently know? Heliyon 2022, 8, e11171. [Google Scholar] [CrossRef]
- Skinner, K.T.; Palkar, A.M.; Hong, A.L. Genetics of ABCB1 in Cancer. Cancers 2023, 15, 4236. [Google Scholar] [CrossRef]
- Dong, J.; Qin, Z.; Zhang, W.D.; Cheng, G.; Yehuda, A.G.; Ashby, C.R., Jr.; Chen, Z.S.; Cheng, X.D.; Qin, J.J. Medicinal chemistry strategies to discover P-glycoprotein inhibitors: An update. Drug Resist. Updat. 2020, 49, 100681. [Google Scholar] [CrossRef]
- Callaghan, R.; Luk, F.; Bebawy, M. Inhibition of the multidrug resistance P-glycoprotein: Time for a change of strategy? Drug Metab. Dispos. 2014, 42, 623–631. [Google Scholar] [CrossRef]
- Lai, J.I.; Tseng, Y.J.; Chen, M.H.; Huang, C.F.; Chang, P.M. Clinical Perspective of FDA Approved Drugs With P-Glycoprotein Inhibition Activities for Potential Cancer Therapeutics. Front. Oncol. 2020, 10, 561936. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Duong, V.A.; Maeng, H.J. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Halder, J.; Pradhan, D.; Kar, B.; Ghosh, G.; Rath, G. Nanotherapeutics approaches to overcome P-glycoprotein-mediated multi-drug resistance in cancer. Nanomedicine 2022, 40, 102494. [Google Scholar] [CrossRef]
- Niazi, M.; Zakeri-Milani, P.; Najafi Hajivar, S.; Soleymani Goloujeh, M.; Ghobakhlou, N.; Shahbazi Mojarrad, J.; Valizadeh, H. Nano-based strategies to overcome p-glycoprotein-mediated drug resistance. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 1021–1033. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, C.; Cai, Z.; Li, Y.; Liu, W.; Luan, Y.; Wang, C. Effective therapy of advanced breast cancer through synergistic anticancer by paclitaxel and P-glycoprotein inhibitor. Mater. Today Bio 2024, 26, 101029. [Google Scholar] [CrossRef]
- Tonbul, H.; Sahin, A.; Tavukcuoglu, E.; Esendagli, G.; Capan, Y. Combination drug delivery with actively-targeted PLGA nanoparticles to overcome multidrug resistance in breast cancer. J. Drug Deliv. Sci. Tec. 2019, 54, 101380. [Google Scholar] [CrossRef]

| Type of Resistance | Inhibitors | Type of Nanoparticle | Ref. |
|---|---|---|---|
| Bcl-2 | ABT-737 | PLGA | [52] |
| Bcl-2 siRNA | AuNP | [53] | |
| Navitoclax | PLA | [54] | |
| IAP | SMAC mimetic peptide (AVPIAQ) | Liposome | [55] |
| AZD5582 | PVA-peptide | [56] | |
| IAP-2 siRNA | AuNP | [57] | |
| Akt | Capivasertib | Poly(LAEMA) | [58] |
| GDC0941 | PLGA-p-PEI-DA | [59] | |
| Akt inhibitor peptide (AVTDHPDRLWAWERF) | ELR polypeptide | [60] | |
| P-glycoprotein | Curcumin | 2-hydroxypropyl-β-cyclodextrin (HPCD) | [61] |
| P-glycoprotein siRNA | N-succinyl chitosan–PLL–palmitic acid (NSC–PLL–PA). | [39] | |
| Verapamil | PLGA | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.; Kim, Y.; Kim, J.; Cho, H.; Kim, K. Overcoming Cancer Drug Resistance with Nanoparticle Strategies for Key Protein Inhibition. Molecules 2024, 29, 3994. https://doi.org/10.3390/molecules29173994
Yoo H, Kim Y, Kim J, Cho H, Kim K. Overcoming Cancer Drug Resistance with Nanoparticle Strategies for Key Protein Inhibition. Molecules. 2024; 29(17):3994. https://doi.org/10.3390/molecules29173994
Chicago/Turabian StyleYoo, Hyeonji, Yeonjin Kim, Jinseong Kim, Hanhee Cho, and Kwangmeyung Kim. 2024. "Overcoming Cancer Drug Resistance with Nanoparticle Strategies for Key Protein Inhibition" Molecules 29, no. 17: 3994. https://doi.org/10.3390/molecules29173994
APA StyleYoo, H., Kim, Y., Kim, J., Cho, H., & Kim, K. (2024). Overcoming Cancer Drug Resistance with Nanoparticle Strategies for Key Protein Inhibition. Molecules, 29(17), 3994. https://doi.org/10.3390/molecules29173994






