Enhanced Tribodegradation of a Tetracycline Antibiotic by Rare-Earth-Modified Zinc Oxide
Abstract
1. Introduction
2. Results and Discussion
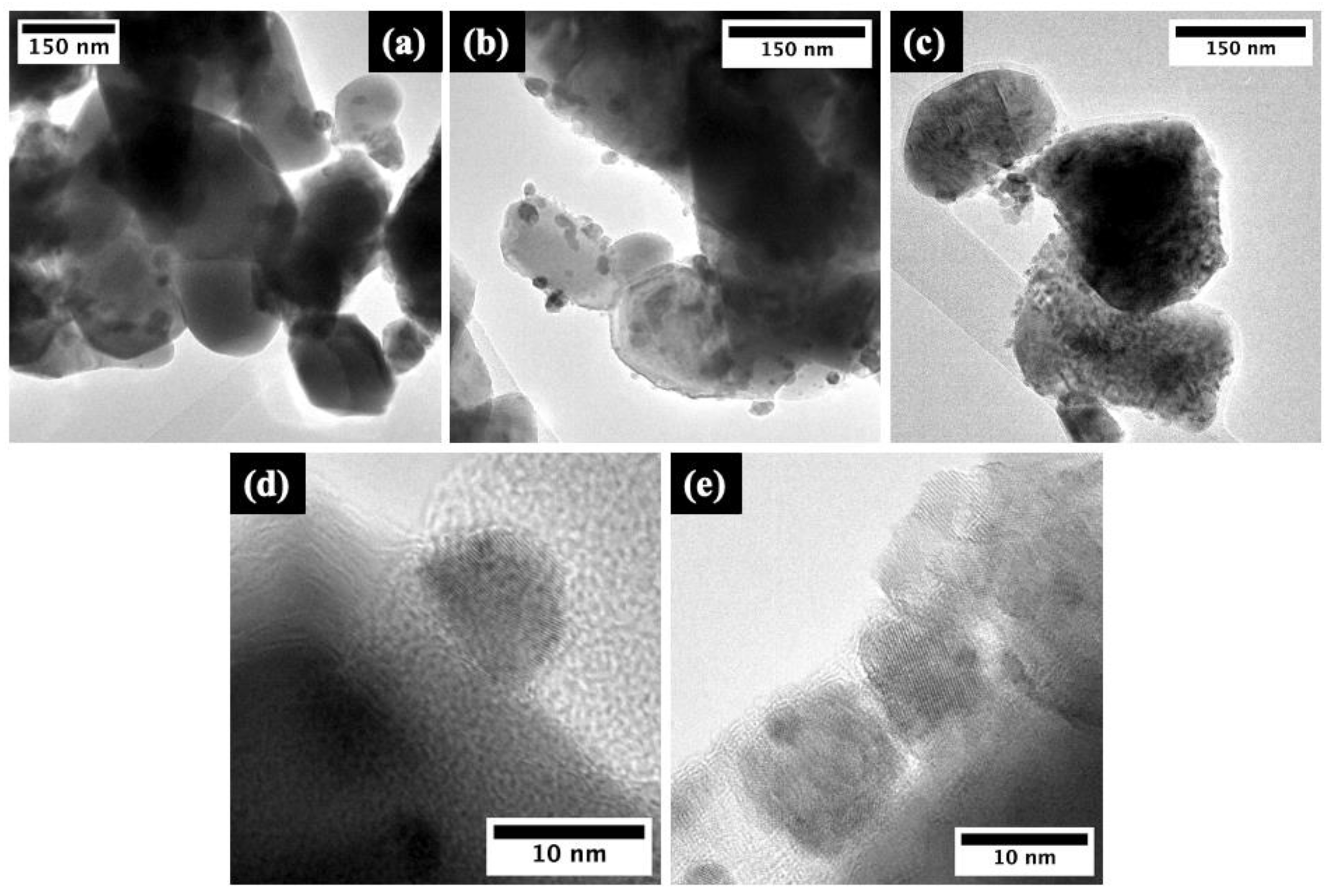
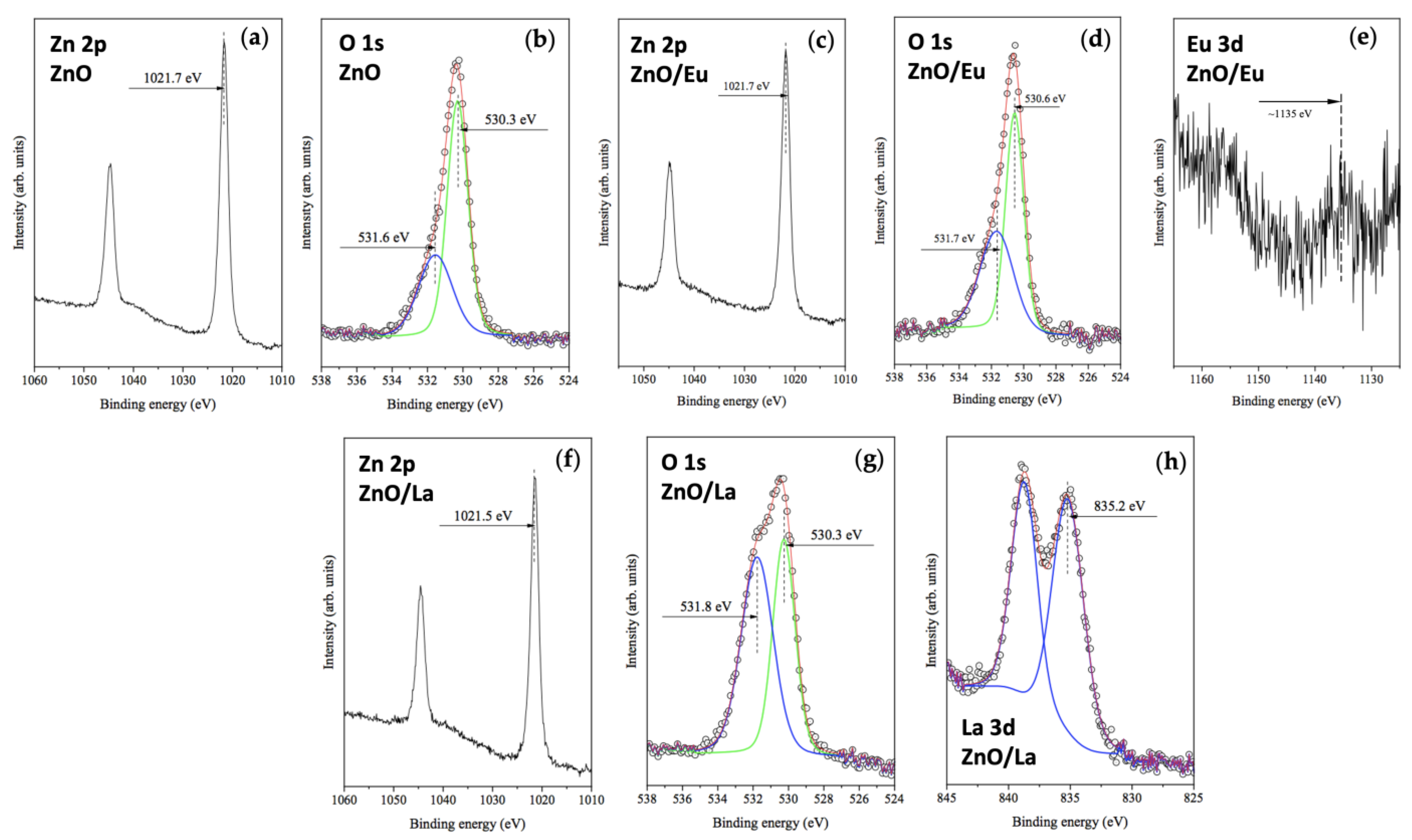
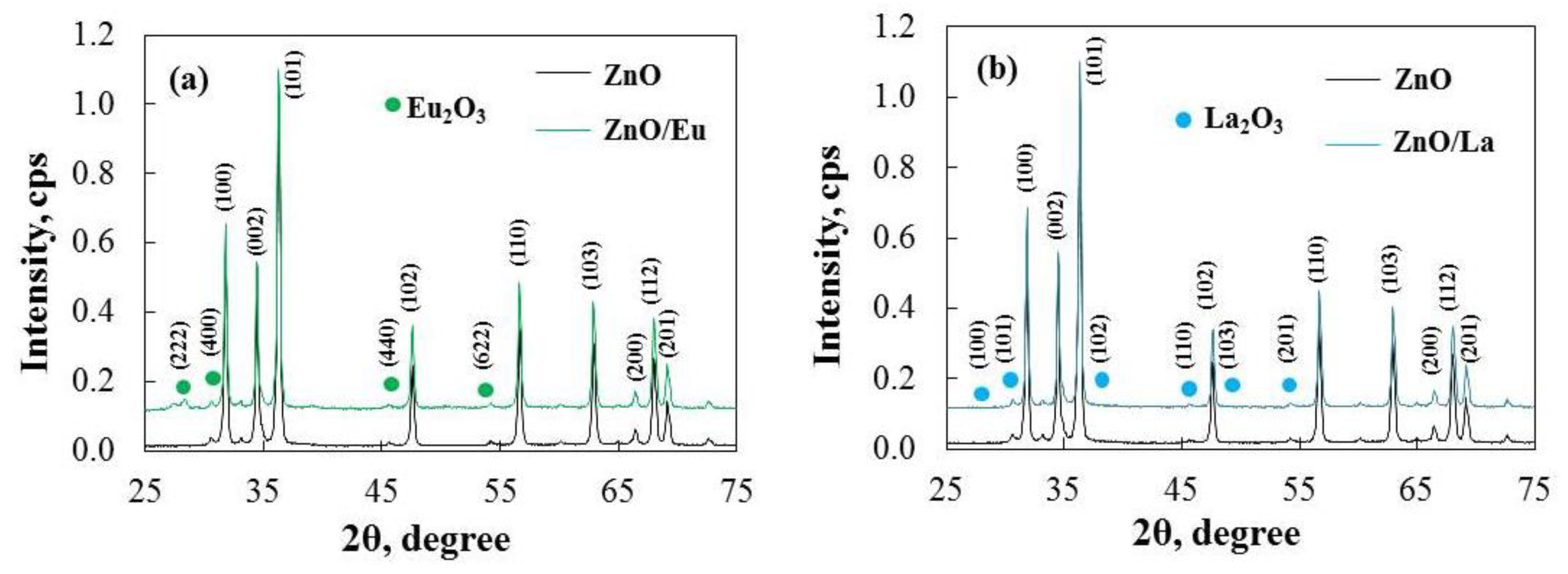
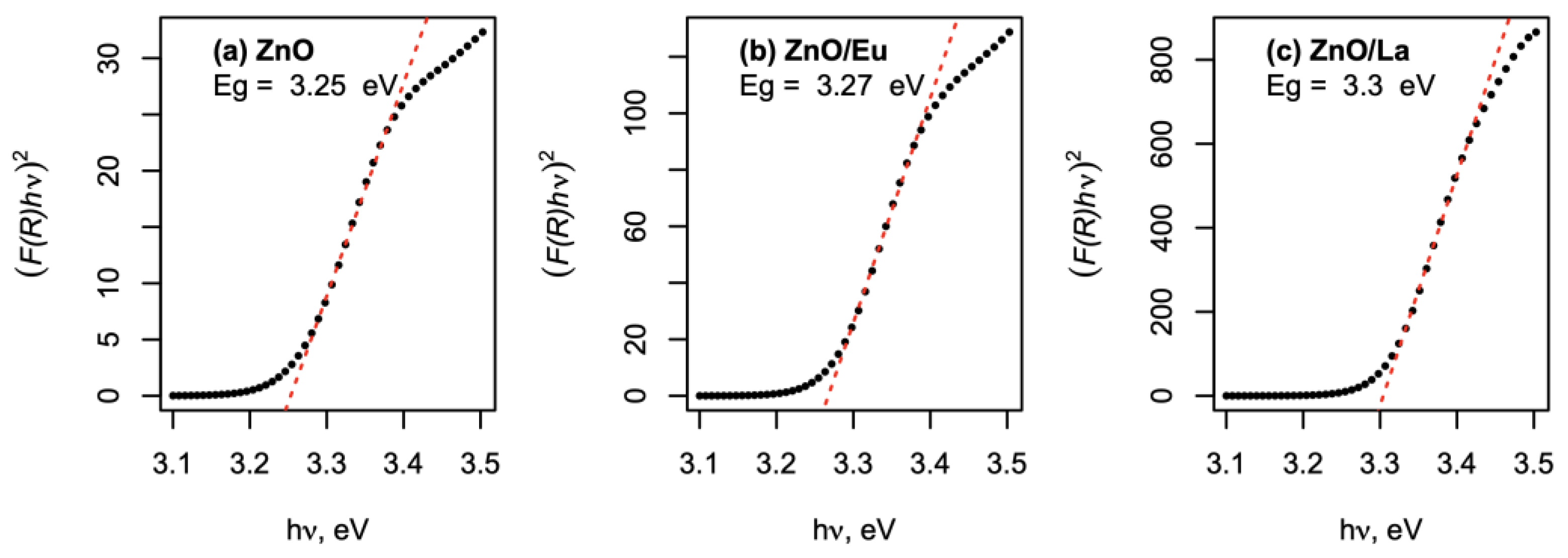
2.1. Structural and Morphological Characterization of the Pure and Rare-Earth-Modified ZnO Tribocatalysts
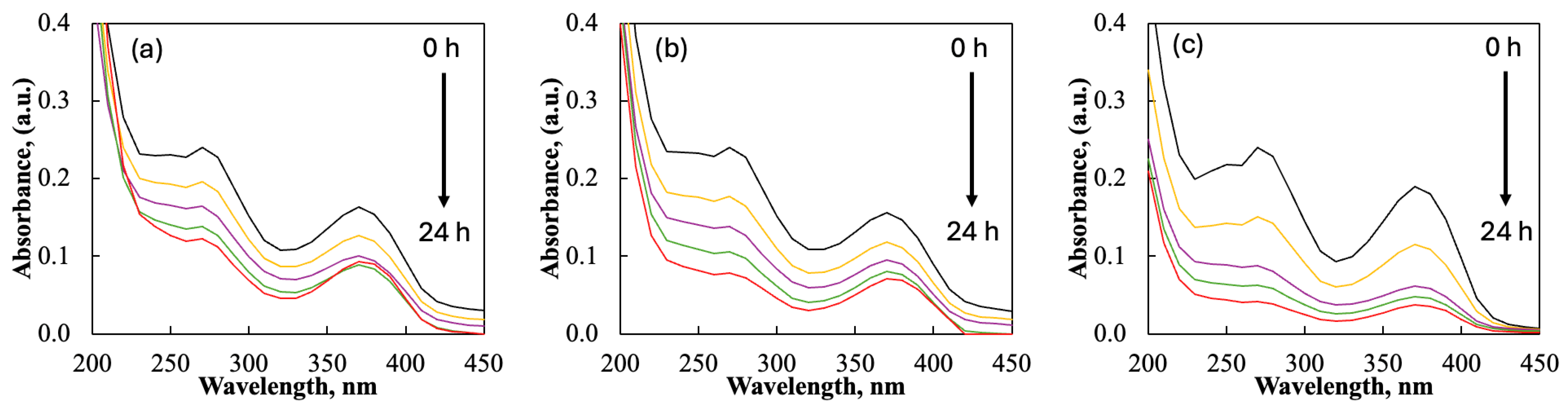
2.2. Tribocatalysis for Decomposition of Doxycycline—Effect of Rare-Earth Elements
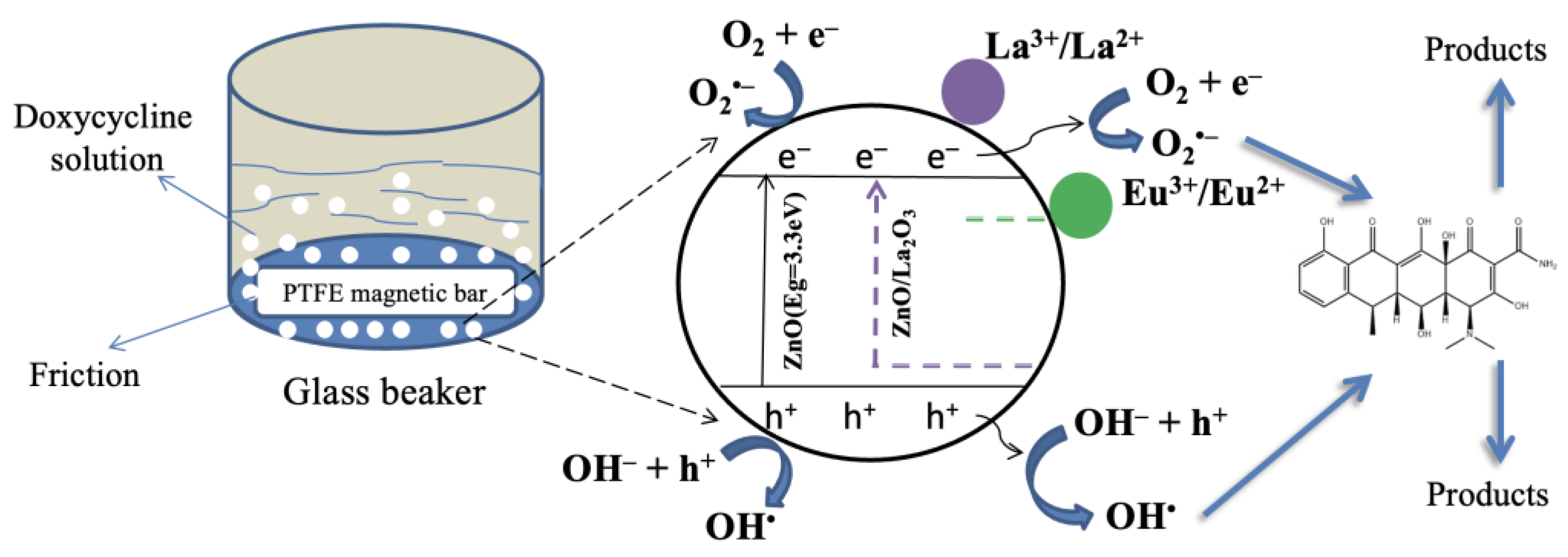
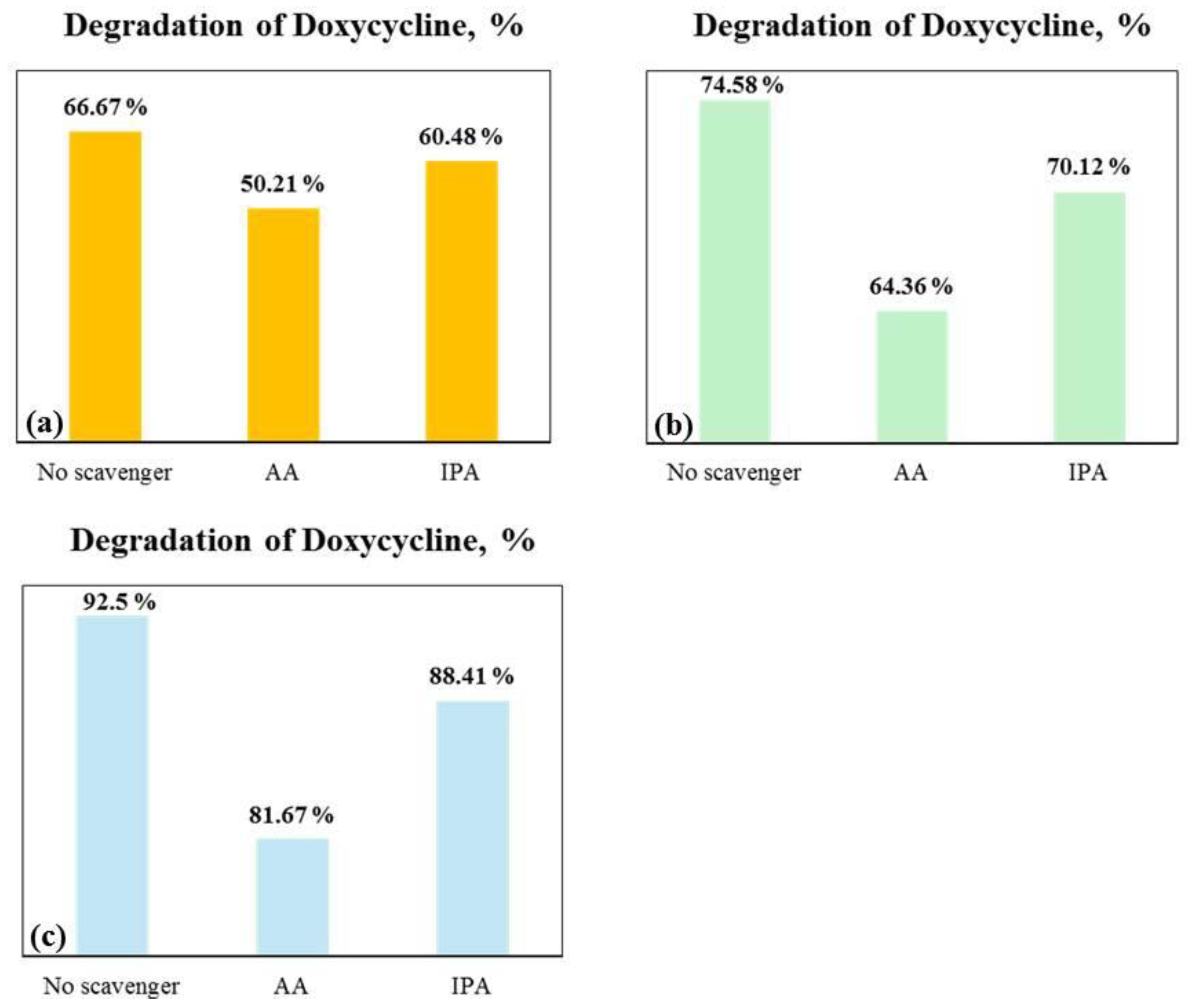
2.3. Tribocatalysis for Decomposition of Doxycycline—Plausible Mechanism
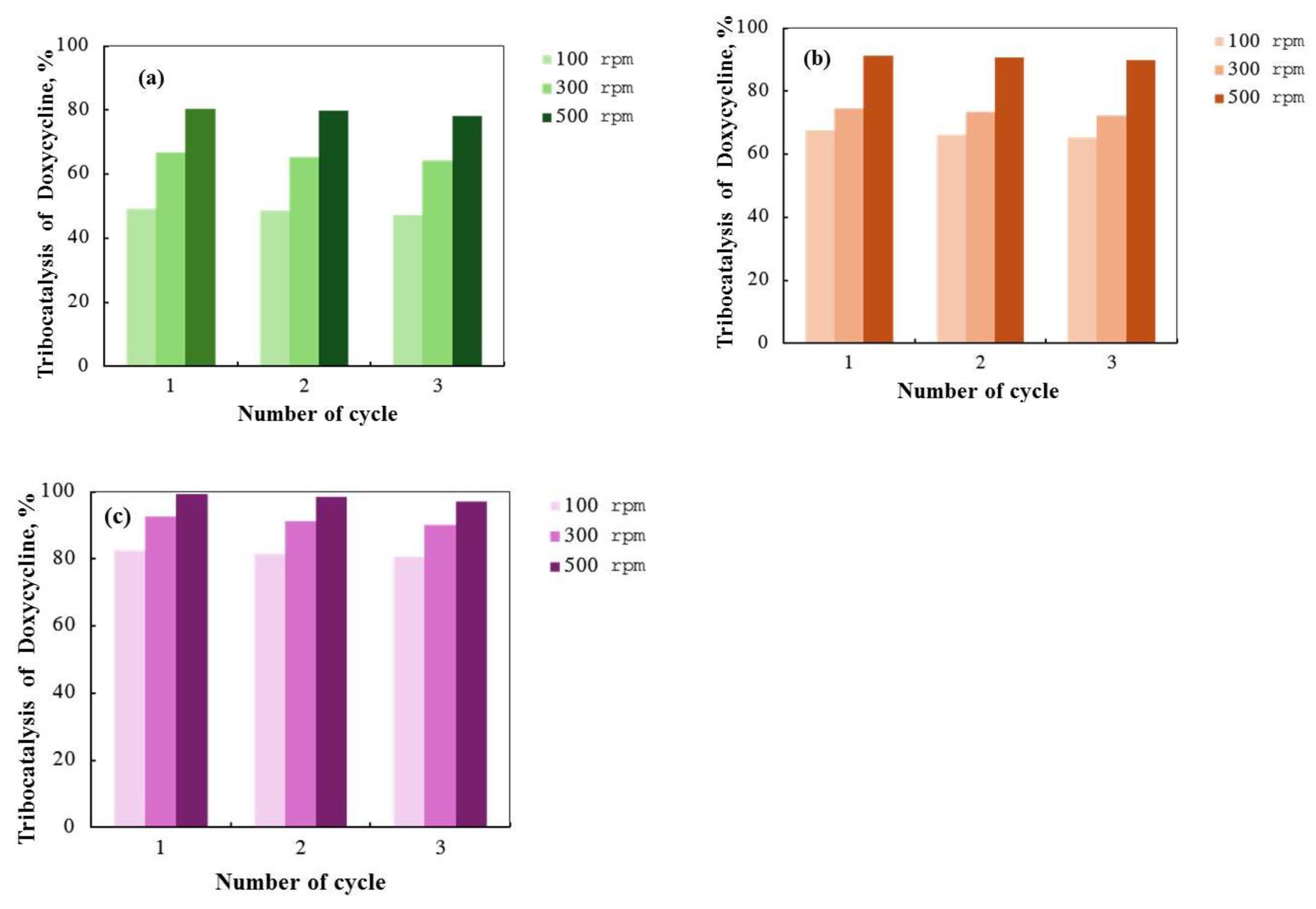
2.4. Tribocatalysis for Decomposition of Doxycycline—Effect of Magnetic Stirring and Catalyst Recycling
3. Materials and Methods
3.1. Reagents and Preparation of RE-Modified ZnO Powders
3.2. Instrumental Methods
3.3. Tribocatalytic Experiments and Radical Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amaechi, I.; Youssef, A.; Dorfler, A.; Gonzalez, Y.; Katoch, R.; Ruediger, A. Catalytic Applications of Non-Centrosymmetric Oxide Nanomaterials. Angew. Chem. Int. Ed. Engl. 2022, 61, e202207975. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Vecitis, C.; Schulze, A.; Cao, B.; Ismail, A.; Lu, X.; Chen, J.; Ramakrishna, S. Recent advances in nanomaterials for water protection and monitoring. Chem. Soc. Rev. 2017, 46, 6946–7020. [Google Scholar] [CrossRef]
- Jeon, I.; Ryberg, E.; Alvarez, P.; Kim, J. Technology assessment of solar disinfection for drinking water treatment. Nat. Sustain. 2022, 5, 801–808. [Google Scholar] [CrossRef]
- Alsharyani, A.; Muruganandam, L. Fabrication of zinc oxide nanorods for photocatalytic degradation of docosane, a petroleum pollutant, under solar light simulator. RSC Adv. 2024, 14, 9038–9049. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, H.; Jiang, W. Solar-driven Bi6O5(OH)3(NO3)5(H2O)3/Bi2WO6 heterojunction for efficient degradation of organic pollutants: Insights into adsorption mechanism, charge transfer and degradation pathway. Sep. Purif. Technol. 2024, 349, 127747. [Google Scholar] [CrossRef]
- Olasupo, A.; Corbin, D.; Shiflett, M. Trends in low temperature and non-thermal technologies for the degradation of persistent organic pollutants. J. Hazard. Mater. 2024, 468, 133830. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Wang, Z.; Xu, H.; Liu, C.; Huo, B.; Meng, F.; Wang, Y.; Sun, C. Low-frequency mechanical energy in the environment for energy production and piezocatalytic degradation of organic pollutants in water: A review. J. Water Process Eng. 2023, 56, 104312. [Google Scholar] [CrossRef]
- Verma, A.; Fu, Y. The prospect of CuxO-based catalysts in photocatalysis: From pollutant degradation, CO2 reduction, and H2 production to N2 fixation. Environ. Res. 2024, 241, 117656. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, S.; Sharma, G.; Dhiman, P.; Shekh, M.; Stadler, F. Recent advances in zeolitic imidazole frameworks based photocatalysts for organic pollutant degradation and clean energy production. J. Environ. Chem. Eng. 2023, 11, 110770. [Google Scholar] [CrossRef]
- Xu, H.; Yang, H.; Zhou, J.; Yin, Y. A Route Choice Model with Context-Dependent Value of Time. Transp. Sci. 2017, 51, 536–548. [Google Scholar] [CrossRef]
- Li, P.; Tang, C.; Xiao, X.; Jia, Y.; Chen, W. Flammable gases produced by TiO2 nanoparticles under magnetic stirring in water. Friction 2021, 10, 1127–1133. [Google Scholar] [CrossRef]
- Cui, X.; Li, P.; Lei, H.; Tu, C.; Wang, D.; Wang, Z.; Chen, W. Greatly enhanced tribocatalytic degradation of organic pollutants by TiO2 nanoparticles through efficiently harvesting mechanical energy. Sep. Purif. Technol. 2022, 289, 120814. [Google Scholar] [CrossRef]
- Hu, J.; Ma, W.; Pan, Y.; Chen, Z.; Zhang, Z.; Wan, C. Resolving the Tribocatalytic reaction mechanism for biochar regulated Zinc Oxide and its application in protein transformation. J. Colloid Interface Sci. 2022, 607, 1908–1918. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Xie, S.; Wang, G.; Tian, Z. Tribocatalysis: Challenges and perspectives. Sci. China Chem. 2021, 64, 1609–1613. [Google Scholar] [CrossRef]
- Geng, L.; Qian, Y.; Song, W.; Bao, L. Enhanced tribocatalytic pollutant degradation through tuning oxygen vacancy in BaTiO3 nanoparticles. Appl. Surf. Sci. 2023, 637, 157960. [Google Scholar] [CrossRef]
- Wu, J.; Qin, N.; Bao, D. Effective enhancement of piezocatalytic activity of BaTiO3 nanowires under ultrasonic vibration. Nano Energy 2018, 45, 44–51. [Google Scholar] [CrossRef]
- Feng, Y.; Ling, L.; Wang, Y.; Xu, Z.; Cao, F.; Li, H. Engineering spherical lead zirconate titanate to explore the essence of piezo-catalysis. Nano Energy 2017, 40, 481–486. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Guo, X.; Wang, L.; Xu, T.; Bian, J. Enhanced tribocatalytic degradation using piezoelectric CdS nanowires for efficient water remediation. J. Mater. Chem. C 2020, 8, 14845–14854. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Wu, Z.; Jia, Y.; Ma, J.; Chen, W.; Zhang, L.; Yang, J.; Liu, Y. Strong tribocatalytic dye decomposition through utilizing triboelectric energy of barium strontium titanate nanoparticles. Nano Energy 2019, 63, 103832. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, L.; Luo, W.; Li, H.; Wu, Z.; Xu, Z.; Zhang, Y.; Zhang, H.; Yuan, G.; Gao, J.; et al. Strong Tribo-Catalysis of Zinc Oxide Nanorods Via Triboelectrically-Harvesting Friction Energy. Ceram. Int. 2020, 46, 25293–25298. [Google Scholar] [CrossRef]
- Sun, S.; Sui, X.; Yu, H.; Zheng, Y.; Zhu, X.; Wu, X.; Li, Y.; Lin, Q.; Zhang, Y.; Ye, W.; et al. High Tribocatalytic Performance of FeOOH Nanorods for Degrading Organic Dyes and Antibiotics. Small Methods 2024, 2301784. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tong, W.; Song, W.; Shi, J.; Zhang, Y. Performance of tribocatalysis and tribo-photocatalysis of pyrite under agitation. J. Clean. Prod. 2023, 414, 137566. [Google Scholar] [CrossRef]
- Kaneva, N.; Bojinova, A.; Papazova, K.; Dimitrov, D. Photocatalytic purification of dye contaminated sea water by lanthanide (La3+, Ce3+, Eu3+) modified ZnO. Catal. Today 2015, 252, 113–119. [Google Scholar] [CrossRef]
- Qu, G.; Fan, G.; Zhou, M.; Rong, X.; Li, T.; Zhang, R.; Sun, J.; Chen, D. Graphene-Modified ZnO Nanostructures for Low-Temperature NO2 Sensing. ACS Omega 2019, 4, 4221–4232. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Li, A.; Chen, Y.; Gao, S.; Liu, W.; Wei, D. Effects of rare earth elements doping on gas sensing properties of ZnO nanowires. Ceram. Int. 2021, 47, 24218–24226. [Google Scholar] [CrossRef]
- Jia, T.; Wang, W.; Long, F.; Fu, Z.; Wang, H.; Zhang, Q. Fabrication, characterization and photocatalytic activity of La-doped ZnO nanowires. J. Alloys Compd. 2009, 484, 410–415. [Google Scholar] [CrossRef]
- Ada, K.; Gökgöz, M.; Önal, M.; Sarıkaya, Y. Preparation and characterization of a ZnO powder with the hexagonal plate particles. Powder Technol. 2008, 181, 285–291. [Google Scholar] [CrossRef]
- Kumar, S.; Kavitha, R. Lanthanide ions doped ZnO based photocatalysts. Sep. Purif. Technol. 2021, 274, 118853. [Google Scholar] [CrossRef]
- Tsuji, T.; Terai, Y.; Kamarudin, M.; Kawabata, M.; Fujiwara, Y. Photoluminescence properties of Sm-doped ZnO grown by sputtering-assisted metalorganic chemical vapor deposition. J. Non-Cryst. Solids 2012, 358, 2443–2445. [Google Scholar] [CrossRef]
- Khatamian, M.; Khandar, A.; Divband, B.; Haghighi, M.; Ebrahimiasl, S. Heterogeneous photocatalytic degradation of 4-nitrophenol in aqueous suspension by Ln (La3+, Nd3+ or Sm3+) doped ZnO nanoparticles. J. Mol. Catal. A Chem. 2012, 365, 120–127. [Google Scholar] [CrossRef]
- Peleš, A.; Pavlović, V.P.; Filipović, S.; Obradović, N.; Mančić, L.; Krstić, J.; Mitrić, M.; Vlahović, B.; Rašić, G.; Kosanović, D.; et al. Structural investigation of mechanically activated ZnO powder. J. Alloys Compd. 2015, 648, 971–979. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, R.; Zhang, Y.; Zhou, B.; Sun, P.; Dong, X. Unveiling the Mechanism of Frictional Catalysis in Water by Bi12TiO20: A Charge Transfer and Contaminant Decomposition Path Study. Langmuir 2022, 38, 14153–14161. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Gao, Y.; Wu, Z.; Ma, J.; Wang, Z.; Liu, C.; Jia, Y.; Wang, X. Review on tribocatalysis through harvesting friction energy for mechanically-driven dye decomposition. J. Alloys Compd. 2024, 1002, 175413. [Google Scholar] [CrossRef]
- Li, X.; Tong, W.; Shi, J.; Chen, Y.; Zhang, Y.; An, Q. Tribocatalysis mechanisms: Electron transfer and transition. J. Mater. Chem. A 2023, 11, 4458–4472. [Google Scholar] [CrossRef]
- Soares, A.; Araujo, F.; Osajima, J.; Guerra, Y.; Viana, B.; Peña-Garcia, R. Nanotubes/nanorods-like structures of La-doped ZnO for degradation of Methylene Blue and Ciprofloxacin. J. Photochem. Photobiol. A Chem. 2024, 447, 115235. [Google Scholar] [CrossRef]
- Campos, S.; Calzadilla, W.; Salazar-González, R.; Venegas-Yazigi, D.; León, J.; Fuentes, S. Photocatalytic activity of barium titanate composites with zinc oxide doped with lanthanide ions for sulfamethoxazole degradation. J. Environ. Chem. Eng. 2024, 12, 112938. [Google Scholar] [CrossRef]
- Duffy, J. Trends in energy gaps of binary compounds: An approach based upon electron transfer parameters from optical spectroscopy. J. Phys. C Solid State Phys. 1980, 13, 2979–2989. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, S.; Liu, M.; He, G.; Li, X. Enhanced tribocatalytic degradation performance of organic pollutants by Cu1.8S/CuCo2S4 p-n junction. J. Colloid Interface Sci. 2024, 655, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Anandan, S.; Vinu, A.; Lovely, K.; Gokulakrishnan, N.; Srinivasu, P.; Mori, T.; Murugesan, V.; Sivamurugan, V.; Ariga, K. Photocatalytic activity of La-doped ZnO for the degradation of monocrotophos in aqueous suspension. J. Mol. Catal. A Chem. 2007, 266, 149–157. [Google Scholar] [CrossRef]
- Korake, P.; Dhabbe, R.; Kadam, A.; Gaikwad, Y.; Garadkar, K. Highly active lanthanum doped ZnO nanorods for photodegradation of metasystox. J. Photochem. Photobiol. B Biol. 2014, 130, 11–19. [Google Scholar] [CrossRef]
- Zhao, J.; Dang, Z.; Muddassir, M.; Raza, S.; Zhong, A.; Wang, X.; Jin, J. A New Cd(II)-Based Coordination Polymer for Efficient Photocatalytic Removal of Organic Dyes. Molecules 2023, 28, 6848. [Google Scholar] [CrossRef] [PubMed]
- Xiang, R.; Zhou, C.; Liu, Y.; Qin, T.; Li, D.; Dong, X.; Muddassir, M.; Zhong, A. A new type Co(II)-based photocatalyst for the nitrofurantoin antibiotic degradation. J. Mol. Struct. 2024, 1312, 138501. [Google Scholar] [CrossRef]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Thomas, M.; Nałęcz-Jawecki, G.; Giebułtowicz, J.; Drzewicz, P. Degradation of oxytetracycline by ferrate (VI): Treatment optimization, UHPLC-MS/MS and toxicological studies of the degradation products, and impact of urea and creatinine on the removal. Chem. Eng. J. 2024, 485, 149802. [Google Scholar] [CrossRef]

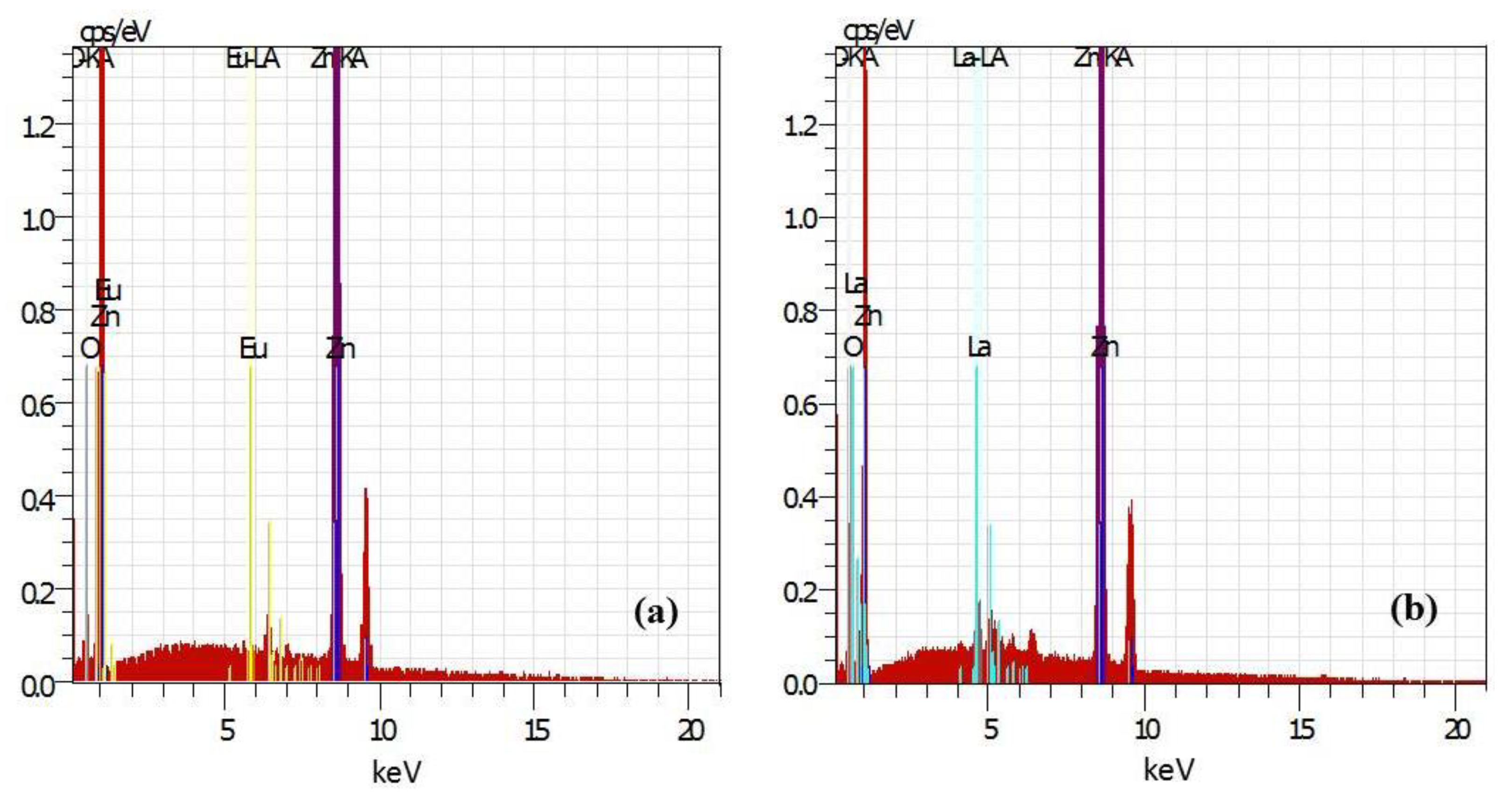
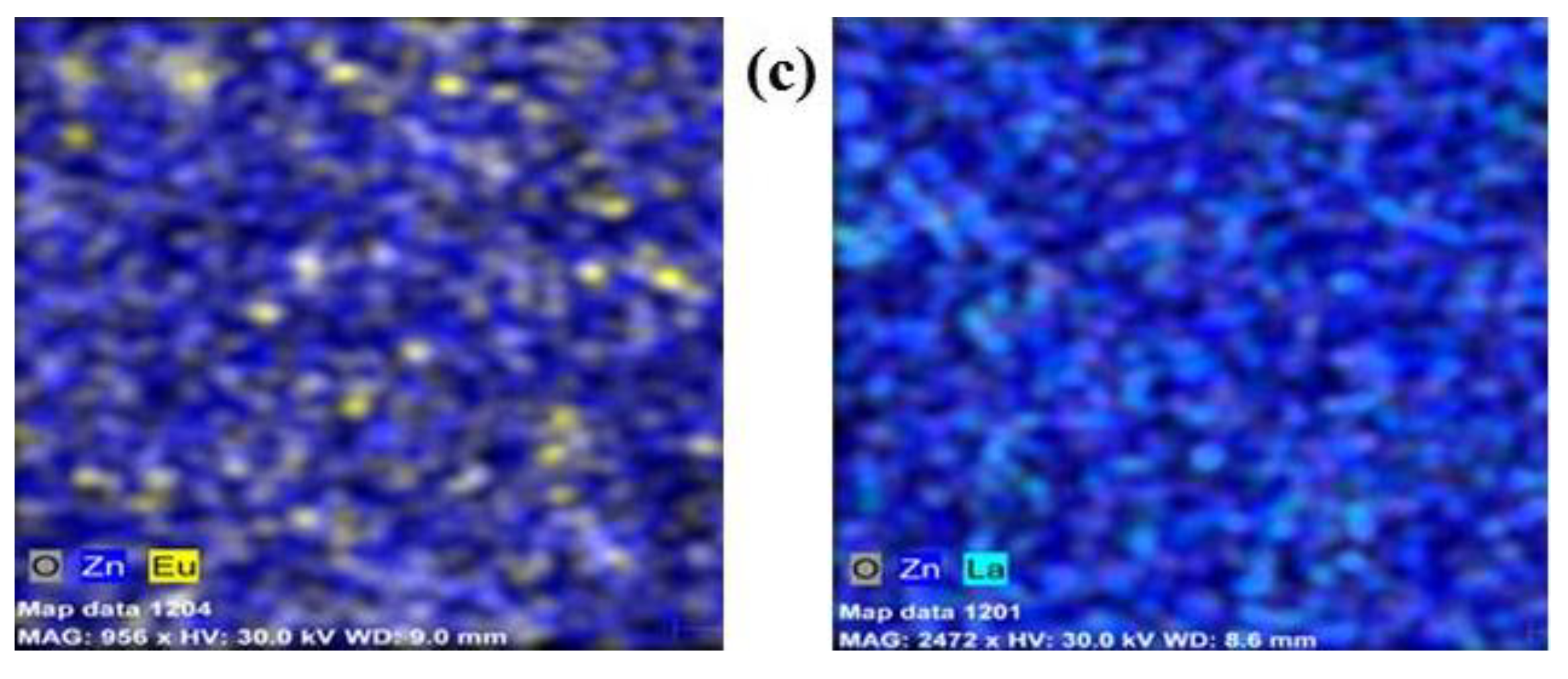



| Sample Powders | C Norm. [wt. %] | C Atom. [at. %] | C Error, [%] | |||
|---|---|---|---|---|---|---|
| ZnO/Eu | O | 41.66 | O | 64.90 | O | 4.9 |
| Zn | 45.84 | Zn | 34.26 | Zn | 1.6 | |
| Eu | 3.25 | Eu | 0.84 | Eu | 0.1 | |
| ZnO/La | O | 42.45 | O | 61.81 | O | 4.4 |
| Zn | 54.28 | Zn | 36.45 | Zn | 1.5 | |
| La | 3.27 | La | 1.74 | La | 0.2 | |
| Sample Powders | Crystallite Size, nm | Parameters of the Crystalline Lattice, Å | Microstrains, a.u. |
|---|---|---|---|
| ZnO | 37 | a, b: 3.2531 c: 5.2057 | 6 × 10−4 |
| ZnO/Eu | 41 | a, b: 3.2516 c: 5.1535 | 4 × 10−4 |
| ZnO/La | 42 | a, b: 3.2504 c: 5.1524 | 4 × 10−4 |
| Sample Powders | 100 rpm | 300 rpm | 500 rpm | |||
|---|---|---|---|---|---|---|
| k, h−1 | D, % | k, h−1 | D, % | k, h−1 | D, % | |
| ZnO | 0.0296 | 49 | 0.0483 | 67 | 0.0725 | 80 |
| ZnO/Eu | 0.0464 | 68 | 0.0609 | 75 | 0.1003 | 91 |
| ZnO/La | 0.0747 | 83 | 0.1015 | 93 | 0.2603 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, D.; Kolev, H.; Stefanov, B.I.; Kaneva, N. Enhanced Tribodegradation of a Tetracycline Antibiotic by Rare-Earth-Modified Zinc Oxide. Molecules 2024, 29, 3913. https://doi.org/10.3390/molecules29163913
Ivanova D, Kolev H, Stefanov BI, Kaneva N. Enhanced Tribodegradation of a Tetracycline Antibiotic by Rare-Earth-Modified Zinc Oxide. Molecules. 2024; 29(16):3913. https://doi.org/10.3390/molecules29163913
Chicago/Turabian StyleIvanova, Dobrina, Hristo Kolev, Bozhidar I. Stefanov, and Nina Kaneva. 2024. "Enhanced Tribodegradation of a Tetracycline Antibiotic by Rare-Earth-Modified Zinc Oxide" Molecules 29, no. 16: 3913. https://doi.org/10.3390/molecules29163913
APA StyleIvanova, D., Kolev, H., Stefanov, B. I., & Kaneva, N. (2024). Enhanced Tribodegradation of a Tetracycline Antibiotic by Rare-Earth-Modified Zinc Oxide. Molecules, 29(16), 3913. https://doi.org/10.3390/molecules29163913











