Complex Formation of Ag+ and Li+ with Host Molecules Modeled on Intercalation of Graphite
Abstract
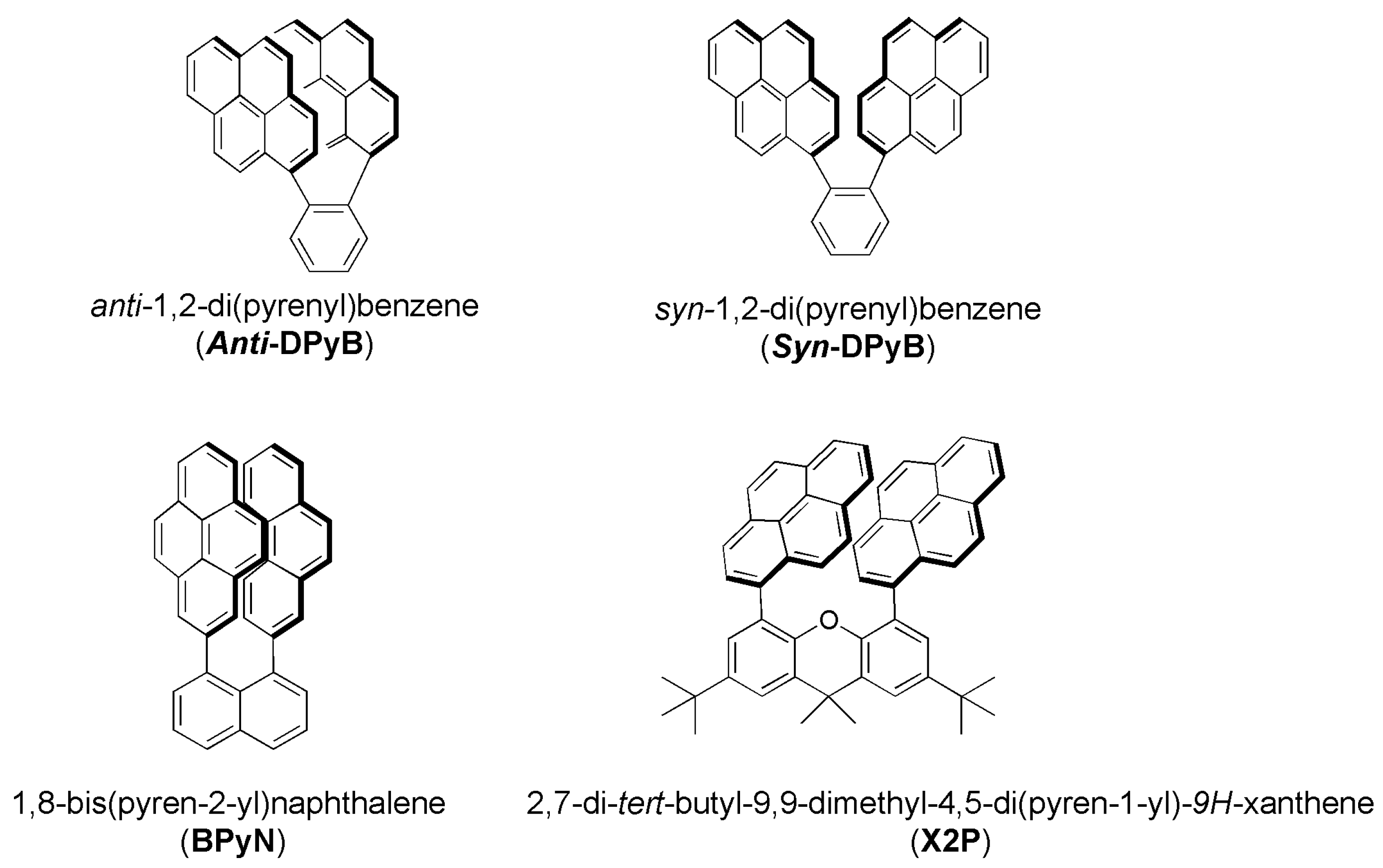
1. Introduction
2. Results and Discussion
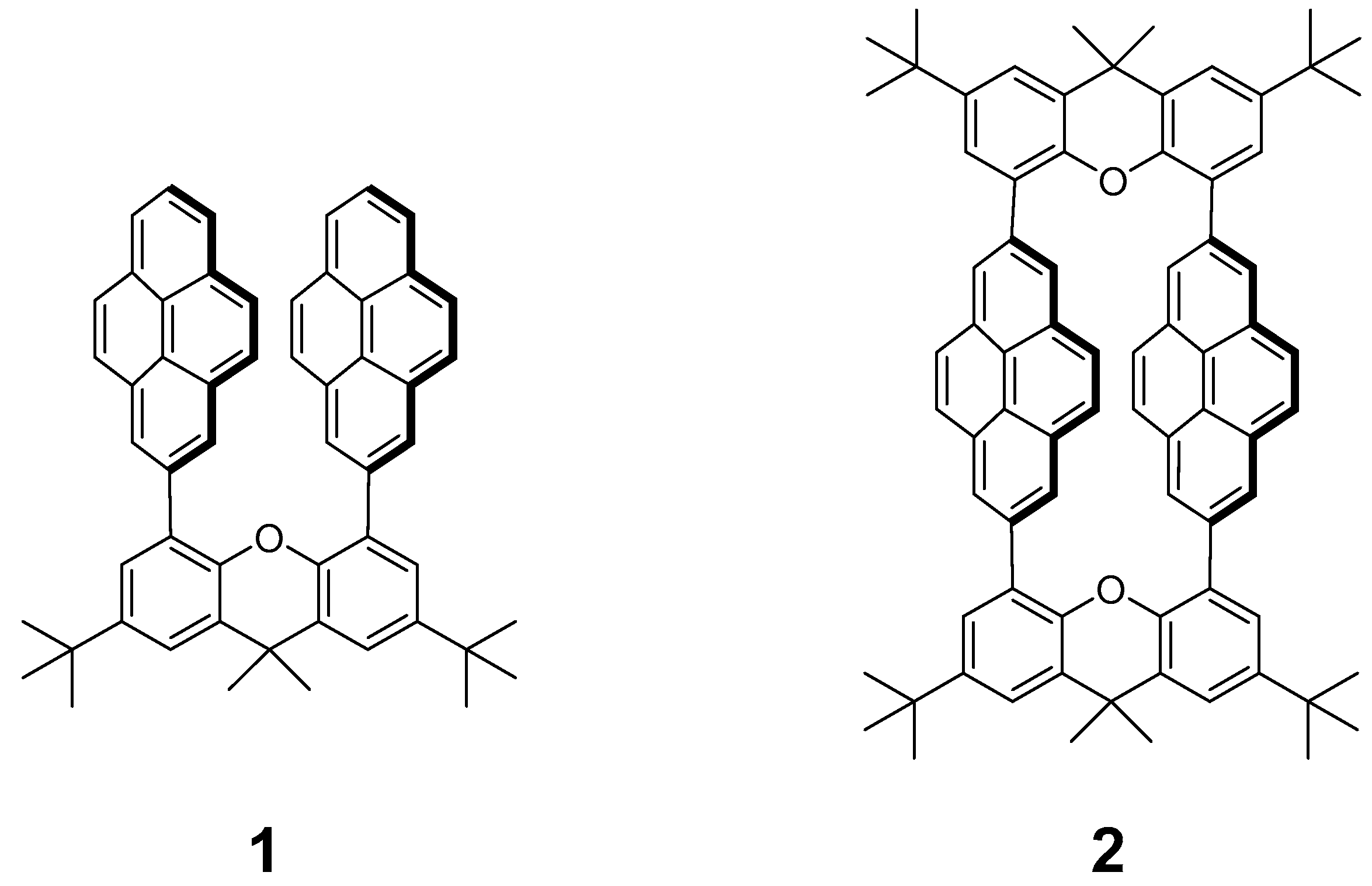
2.1. Preparation of Compounds
2.2. Optimized Structures by Theoretical Calculations
2.3. Complexation of Li+ and Ag+ Ions
3. Materials and Methods
3.1. Experimental Section
3.1.1. General Procedure
3.1.2. Synthesis of Compound 2 from 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrene
3.1.3. Silver Ion Complexation with 1: Preparation of Solutions for 1H NMR Titration
- Preparation of host solution (1): Dissolve 1 (0.72 mg, 2 × 10−3 mmol) in 1.0 mL of THF-d8 to make a solution of 2.0 × 10−3 mol/L.
- Preparation of guest (AgClO4) solution (2): AgClO4 (12.4 mg, 0.06 mmol) was dissolved in 0.30 mL of THF-d8 to make a 0.20 mol/L solution.
- Preparation of guest (AgClO4) solution (3): AgClO4 (0.25 g, 1.2 mmol) was dissolved in 0.60 mL THF-d8 to make a solution of 2.0 mol/L.
3.1.4. Job Plot
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woodin, R.L.; Beauchamp, J.L. Binding of Li+ to Lewis Bases in the Gas Phase. Reversals in Methyl Substituent Effects for Different Reference Acids. J. Am. Chem. Soc. 1978, 100, 501–508. [Google Scholar] [CrossRef]
- Sunner, J.; Nishizawa, K.; Kebarle, P. Ion-solvent molecule interactions in the gas phase. Potassium Ion Benzene. J. Phys. Chem. 1981, 85, 1814–1820. [Google Scholar] [CrossRef]
- Gal, J.-F.; Maria, P.-C.; Decouzon, M.; Mó, O.; Yáñez, M. Gas-phase lithium-cation basicities of some benzene derivatives: An experimental and theoretical study, Int. J. Mass Spectrom. 2002, 219, 445–456. [Google Scholar] [CrossRef]
- Hoyau, S.; Norrman, K.; McMahon, T.B.; Ohanessian, G.A. Quantitative Basis for a Scale of Na+ Affinities of Organic and Small Biological Molecules in the Gas Phase. J. Am. Chem. Soc. 1999, 121, 8864–8875. [Google Scholar] [CrossRef]
- Armentrout, P.B.; Rodgers, M.T. An Absolute Sodium Cation Affinity Scale: Threshold Collision-Induced Dissociation Experiments and ab Initio Theory. J. Phys. Chem. A 2000, 104, 2238–2247. [Google Scholar] [CrossRef]
- Mó, O.; Yáñez, M.; Gal, J.-F.; Maria, P.-C.; Decouzon, M. Enhanced Li+ Binding Energies in Alkylbenzene Derivatives: The Scorpion Effect. Chem. Eur. J. 2003, 9, 4330–4338. [Google Scholar] [CrossRef]
- Gross, J.; Harder, G.; Siepen, A.; Harren, J.; Vögtle, F.; Stephan, H.; Gloe, K.; Ahlers, B.; Cammann, K.; Rissanen, K. Concave Hydrocarbons. Chem. Eur. J. 1996, 2, 1585–1595. [Google Scholar] [CrossRef]
- Gokel, G.W.; Wall, S.L.; Meadows, E.S. Experimental Evidence for Alkali Metal Cation-π Interactions. Eur. J. Org. Chem. 2000, 17, 2967–22978. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, D.; Tarakeshwar, P.; Suh, S.B.; Kim, K.S. A New Type of Ionophore Family Utilizing the Cation-Olefinic π Interaction: Theoretical Study of [n]Beltenes. J. Org. Chem. 2002, 67, 1848–1851. [Google Scholar] [CrossRef]
- Takemura, H.; Nagaoka, M.; Kawasaki, C.; Tokumoto, K.; Tobita, N.; Takano, Y.; Iwanaga, T. Synthetic study and structure of cage-type cyclophane C36H36S6. Tetrahedron Lett. 2017, 58, 1066–1070. [Google Scholar] [CrossRef]
- Zaboli, A.; Raissi, H.; Farzad, F.; Hashemzadeh, H.; Fallahi, F. Cation-pi interaction: A strategy for enhancing the performance of graphene-based drug delivery systems. Inorg. Chem. Commun. 2022, 141, 109542. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Kheirabadi, N.; Shafiekhani, A. Graphene/Li-ion battery Crossmark: Check for Updates. J. Appl. Phys. 2012, 112, 124323. [Google Scholar] [CrossRef]
- Cai, X.; Lai, L.; Shen, Z.; Lin, J. Graphene and graphene-based composites as Li-ion battery electrode materials and their application in full cells. J. Mater. Chem. A 2017, 5, 15423–15446. [Google Scholar] [CrossRef]
- Sun, P.; Zheng, F.; Zhu, M.; Song, Z.; Wang, K.; Zhong, M.; Wu, D.; Little, R.B.; Xu, Z.H. Selective Trans-Membrane Transport of Alkali and Alkaline Earth Cations through Graphene Oxide Membranes Based on Cation-π Interactions. ACS Nano 2014, 8, 850–859. [Google Scholar] [CrossRef]
- Zhao, G.; Zhu, H. Cation–π Interactions in Graphene-Containing Systems for Water Treatment and Beyond. Adv. Mater. 2020, 32, 1905756. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Nakamichi, H.; Sako, K. Pyrene−azacrown ether hybrid: Cation−π interaction. Tetrahedron Lett. 2005, 46, 2063–2066. [Google Scholar] [CrossRef]
- Takemura, H. and Sako, K. Li+···π interaction in coronene−azacrown ether system. Tetrahedron Lett. 2005, 46, 8169–8172. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Adelhelm, P.; Titirici, M.-M.; Hu, Y.-S. Intercalation chemistry of graphite: Alkali metal ions and beyond. Chem Soc Rev. 2019, 48, 4655–4687. [Google Scholar] [CrossRef]
- Kim, Y.-O.; Park, S.-M. Intercalation Mechanism of Lithium Ions into Graphite Layers Studied by Nuclear Magnetic Resonance and Impedance Experiments. J. Electrochem. Soc. 2001, 148, A194–A199. [Google Scholar] [CrossRef]
- Maeda, Y.; Sugimori, D.; Inagaki, M. Electrochemical Intercalation of Alkali Metal Ions into Graphite. Tanso 1991, 149, 244–247. [Google Scholar] [CrossRef][Green Version]
- Choi, J.; Kim, S.; Ahn, M.; Kim, J.; Cho, D.; Kim, D.; Eom, S.; Im, D.; Kim, Y.; Kim, S.H.; et al. Singlet fission dynamics modulated by molecular configuration in covalently linked pyrene dimers, Anti- and Syn-1,2-di(pyrenyl)benzene. Communs. Chem. 2023, 6, 1–11. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Pu, Y.-J.; Nakata, G.; Kawata, S.; Sasabe, H.; Kido, J. A single-molecule excimer-emitting compound for highly efficient fluorescent organic light-emitting devices. Chem. Commun. 2012, 48, 8434–8436. [Google Scholar] [CrossRef]
- Wang, J.; Dang, Q.; Gong, Y.; Liao, Q.; Song, G.; Li, Q.; Li, Z. Precise Regulation of Distance between Associated Pyrene Units and Control of Emission Energy and Kinetics in Solid State. CCS Chem. 2021, 3, 274–286. [Google Scholar] [CrossRef]
- Li, G.-Q.; Yamamoto, Y.; Miyaura, N. Double-coupling of dibromo arenes with aryltriolborates for synthesis of diaryl-substituted planar frameworks. Tetrahedron 2011, 67, 6804–6811. [Google Scholar] [CrossRef]
- Coventry, D.N.; Batsanov, A.S.; Goeta, A.E.; Howard, J.A.K.; Marder, T.B.; Perutz, R.N. Selective Ir-catalysed borylation of polycyclic aromatic hydrocarbons: Structure of naphthalene-2,6-bis(boronate), pyrene-2,7-bis(boronate) and perylene-2,5,8,11-tetra(boronate) esters. Chem. Commun. 2005, 16, 2172–2174. [Google Scholar] [CrossRef] [PubMed]
- Akula, M.R.; Yao, M.-L.; Kabalka, G.W. Triolborates: Water-soluble complexes of arylboronic acids as precursors to iodoarenes. Tetrahedron Lett. 2010, 51, 1170–1171. [Google Scholar] [CrossRef]
- Kammermeier, S.; Jones, P.G.; Dix, I.; Herges, R. A Silver(I) Complex of a Tube-Shaped Hydrocarbon. Acta Cryst. 1998, C54, 1078–1081. [Google Scholar] [CrossRef]
- Seppälä, T.; Wegelius, E.; Rissanen, K. [2.2.2]m,p,p- and [2.2.2]m,m,p-Cyclophane-Ag-triflate: New p-prismand complexes. New J. Chem. 1998, 22, 789–791. [Google Scholar] [CrossRef]
- McMurry, J.E.; Haley, G.J.; Matz, J.R.; Clardy, J.C.; Mitchell, J. Pentacyclo[12.2.2.22,5.26,9.210,13]-1,5,9,13-tetracosatetraene and Its Reaction with AgOTf. Synthesis of a Square-Planar d10 Organometallic Complex. J. Am. Chem. Soc. 1986, 108, 515–516. [Google Scholar] [CrossRef]
- Lindeman, S.V.; Rathore, R.; Kochi, J.K. Silver(I) Complexation of (Poly)aromatic Ligands. Structural Criteria for Depth Penetration into cis-Stilbenoid Cavities. Inorg. Chem. 2000, 39, 5707–5716. [Google Scholar] [CrossRef] [PubMed]
- Experimental Methods in Biofunctional Chemistry, The Chemical Society of Japan, Division of Biofunctional Chemistry, ed.; The Chemical Society of Japan: Tokyo, Japan, 2003.
- Gal, J.-F.; Maria, P.-C.; Decouzon, M.; Mó, O.; Yáñez, M.; Abboud, J.L.M. Lithium-Cation/π Complexes of Aromatic Systems. The Effect of Increasing the Number of Fused Rings. J. Am. Chem. Soc. 2003, 125, 10394–10401. [Google Scholar] [CrossRef] [PubMed]


| Host · Guest | Ka (L·mol−1) |
|---|---|
| Pyrene·Ag+ | 4.19 ±1.6 |
| Pyrene·Li+ | 0.30 ± 0.02 |
| 1·Ag+ | 39.3 ± 9.7 |
| 1·Li+ | 57.6 ± 12.4 |
| 2·Ag+ | 108.1 ± 15.1 |
| 2·Li+ | 0.45 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uetake, Y.; Takemura, H. Complex Formation of Ag+ and Li+ with Host Molecules Modeled on Intercalation of Graphite. Molecules 2024, 29, 3987. https://doi.org/10.3390/molecules29173987
Uetake Y, Takemura H. Complex Formation of Ag+ and Li+ with Host Molecules Modeled on Intercalation of Graphite. Molecules. 2024; 29(17):3987. https://doi.org/10.3390/molecules29173987
Chicago/Turabian StyleUetake, Yuriko, and Hiroyuki Takemura. 2024. "Complex Formation of Ag+ and Li+ with Host Molecules Modeled on Intercalation of Graphite" Molecules 29, no. 17: 3987. https://doi.org/10.3390/molecules29173987
APA StyleUetake, Y., & Takemura, H. (2024). Complex Formation of Ag+ and Li+ with Host Molecules Modeled on Intercalation of Graphite. Molecules, 29(17), 3987. https://doi.org/10.3390/molecules29173987









