Protection of α-Tocopherol from UV-Induced Degradation by Encapsulation into Zein Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
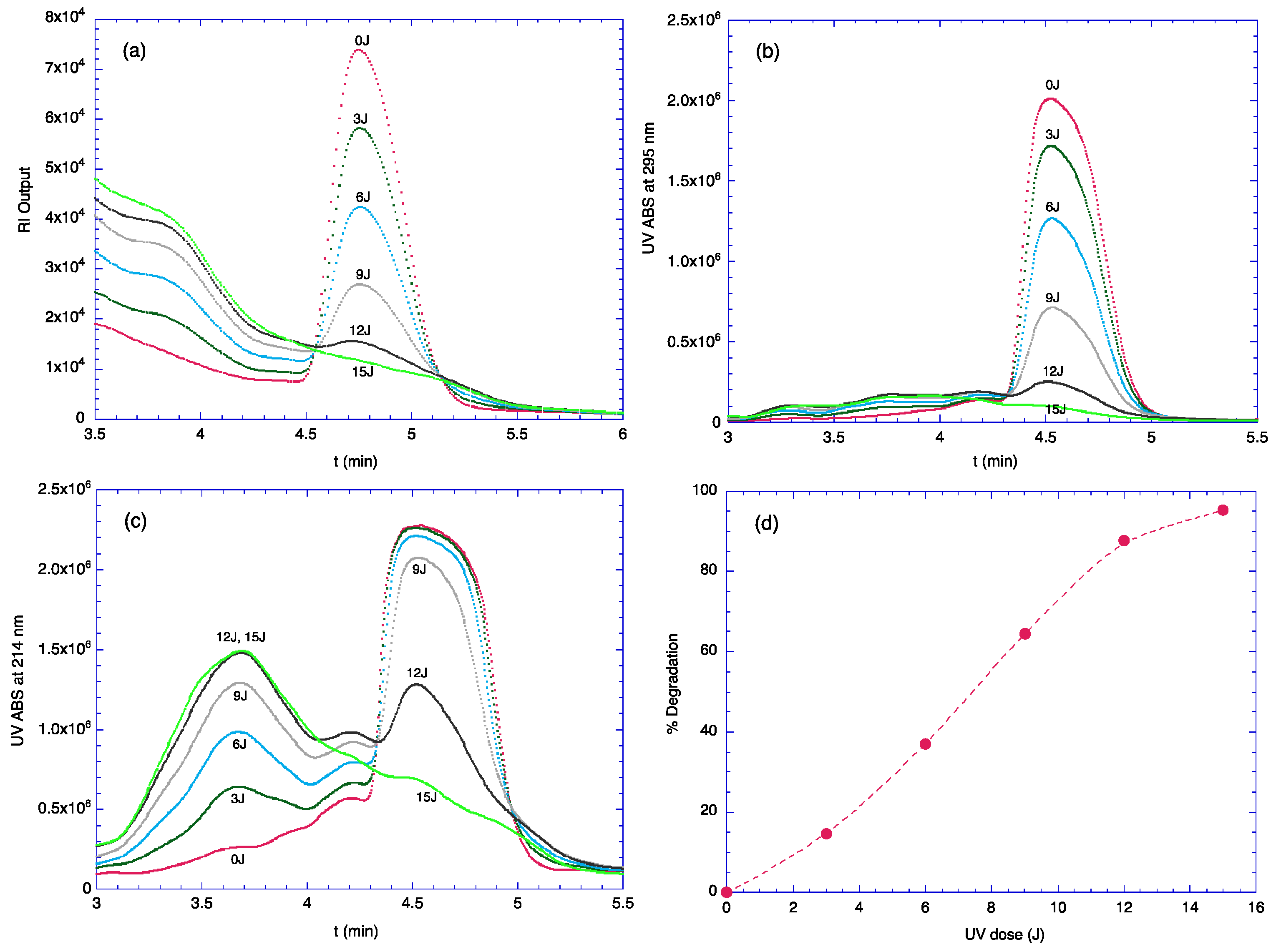
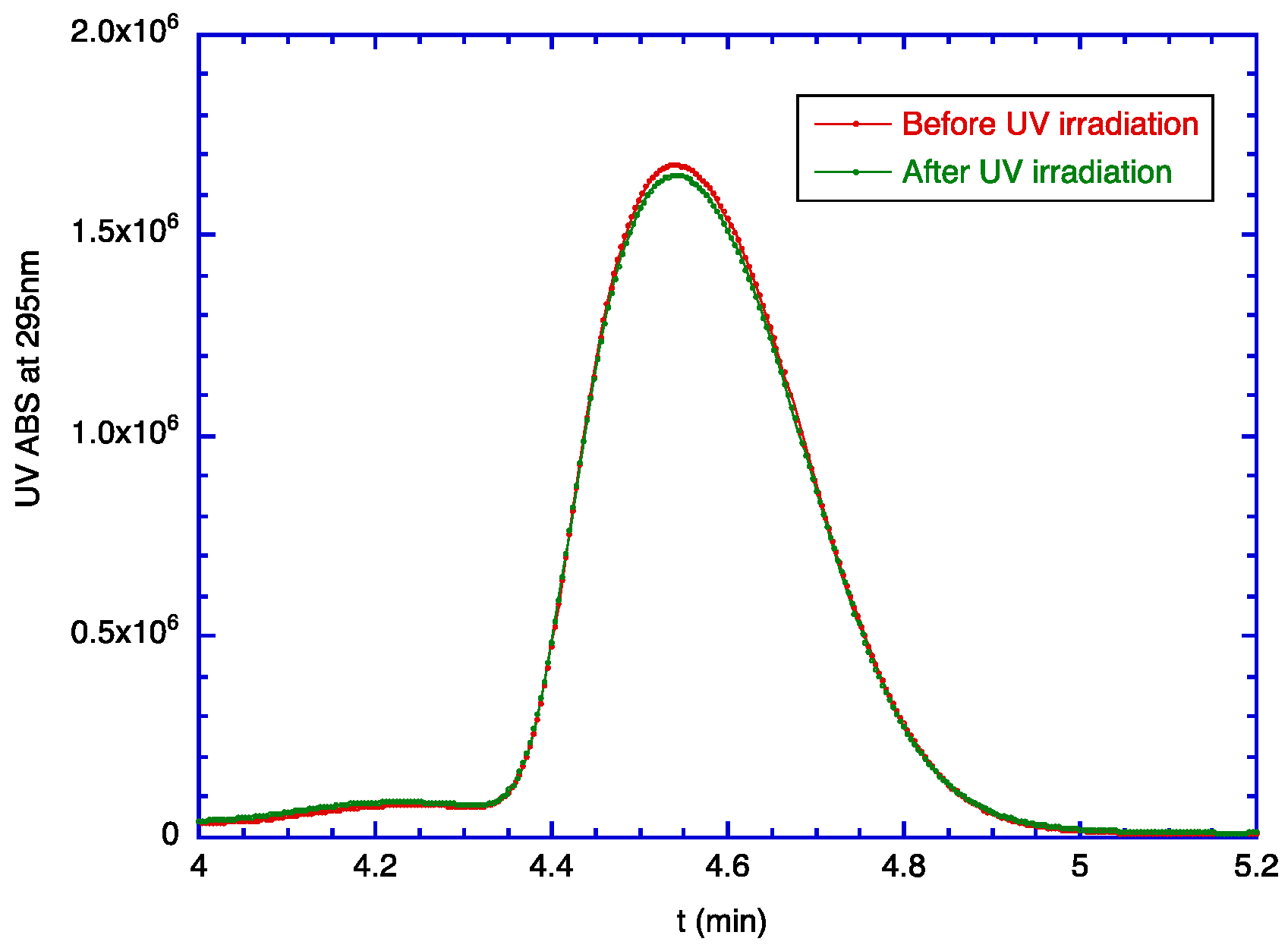
2.1. Degradation of TOC by UV Light
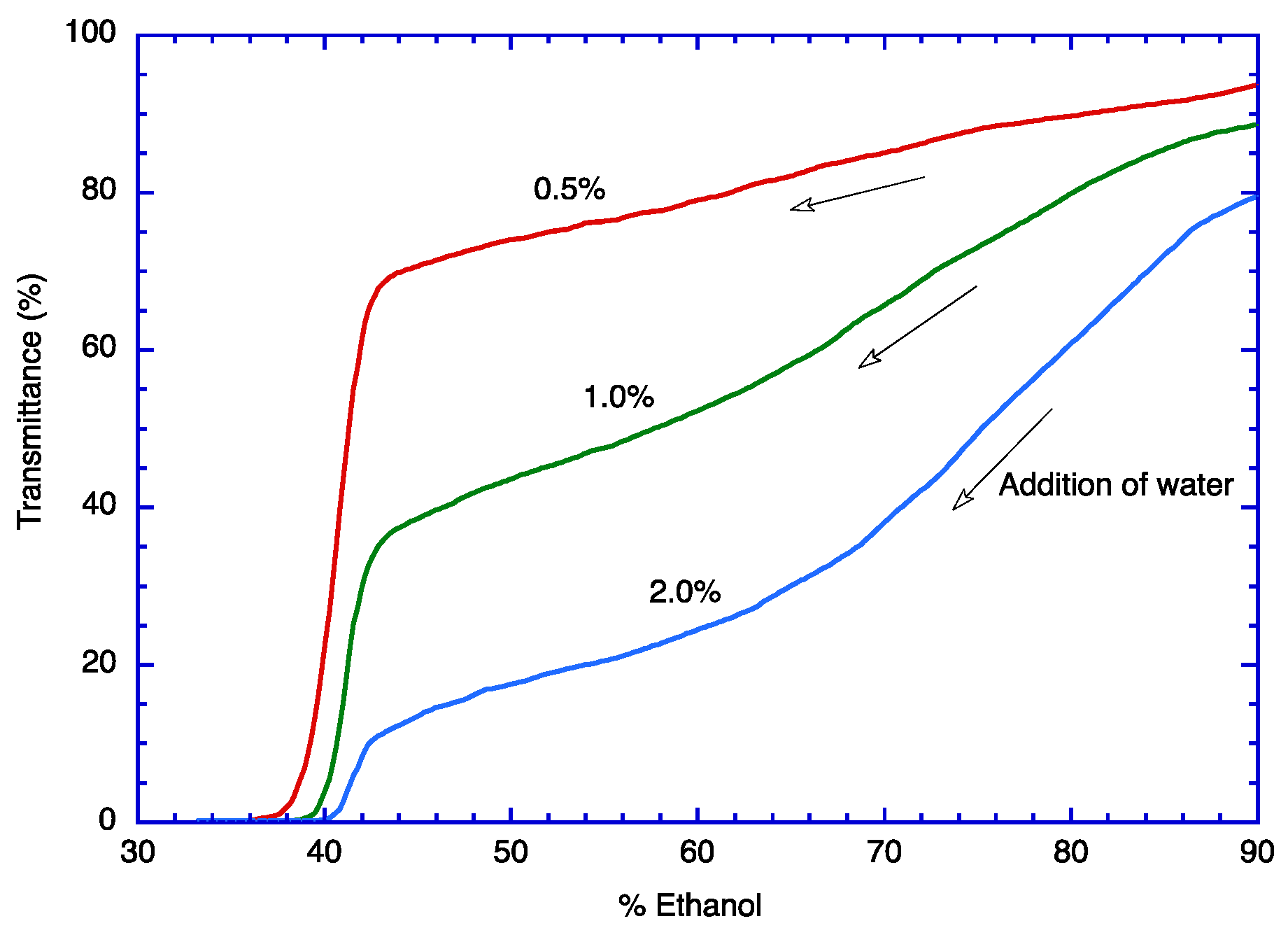
2.2. Conventional Encapsulation Process
2.3. New Encapsulation Process for TOC
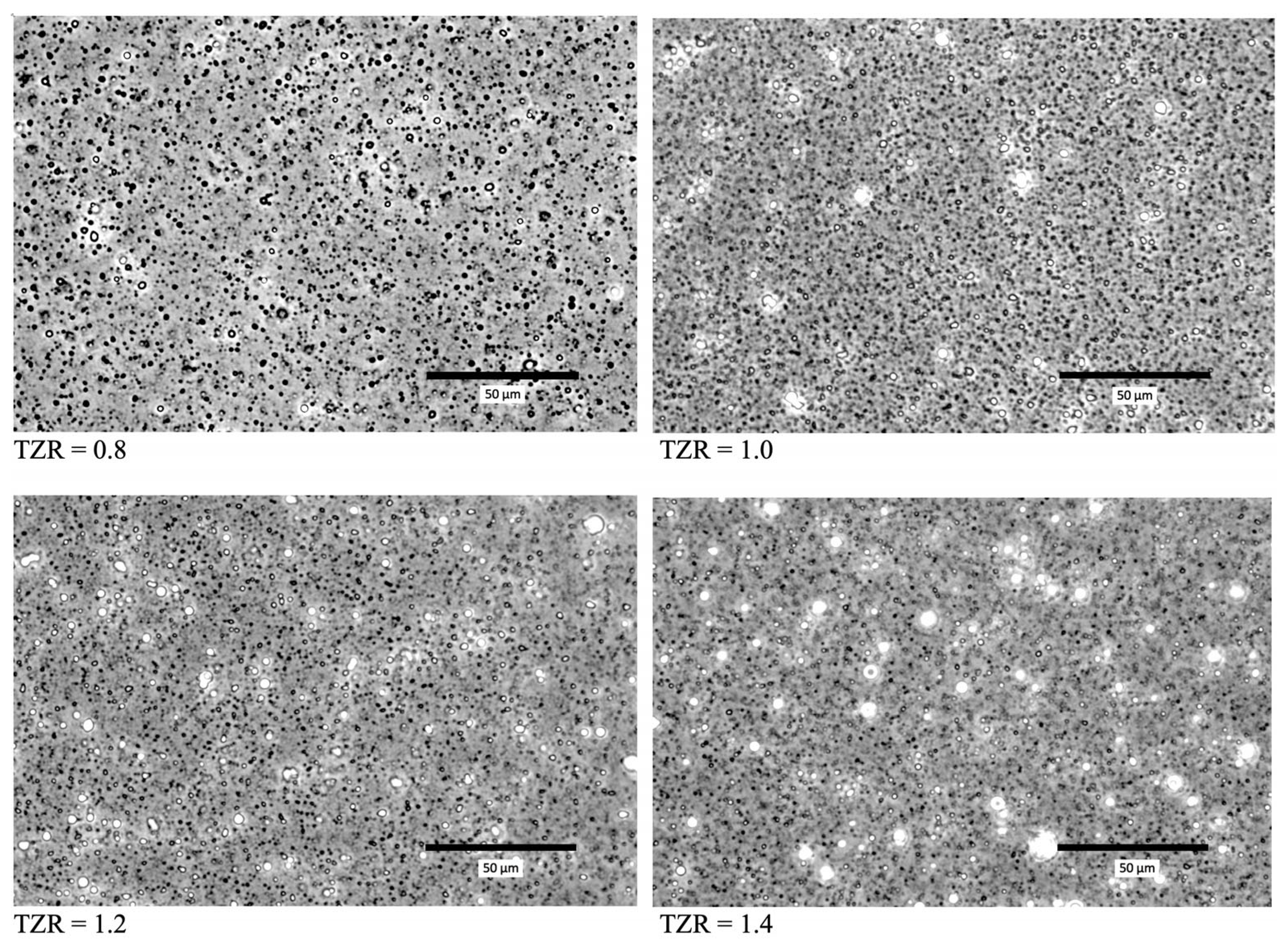
2.4. TOC-to-Zein Ratio (TZR) and Shell Thickness
2.5. Encapsulation Efficiency (EE)
2.6. Encapsulation Effect on the Degradation of TOC
3. Materials and Methods
3.1. Materials
3.2. Degradation of TOC by UV Light
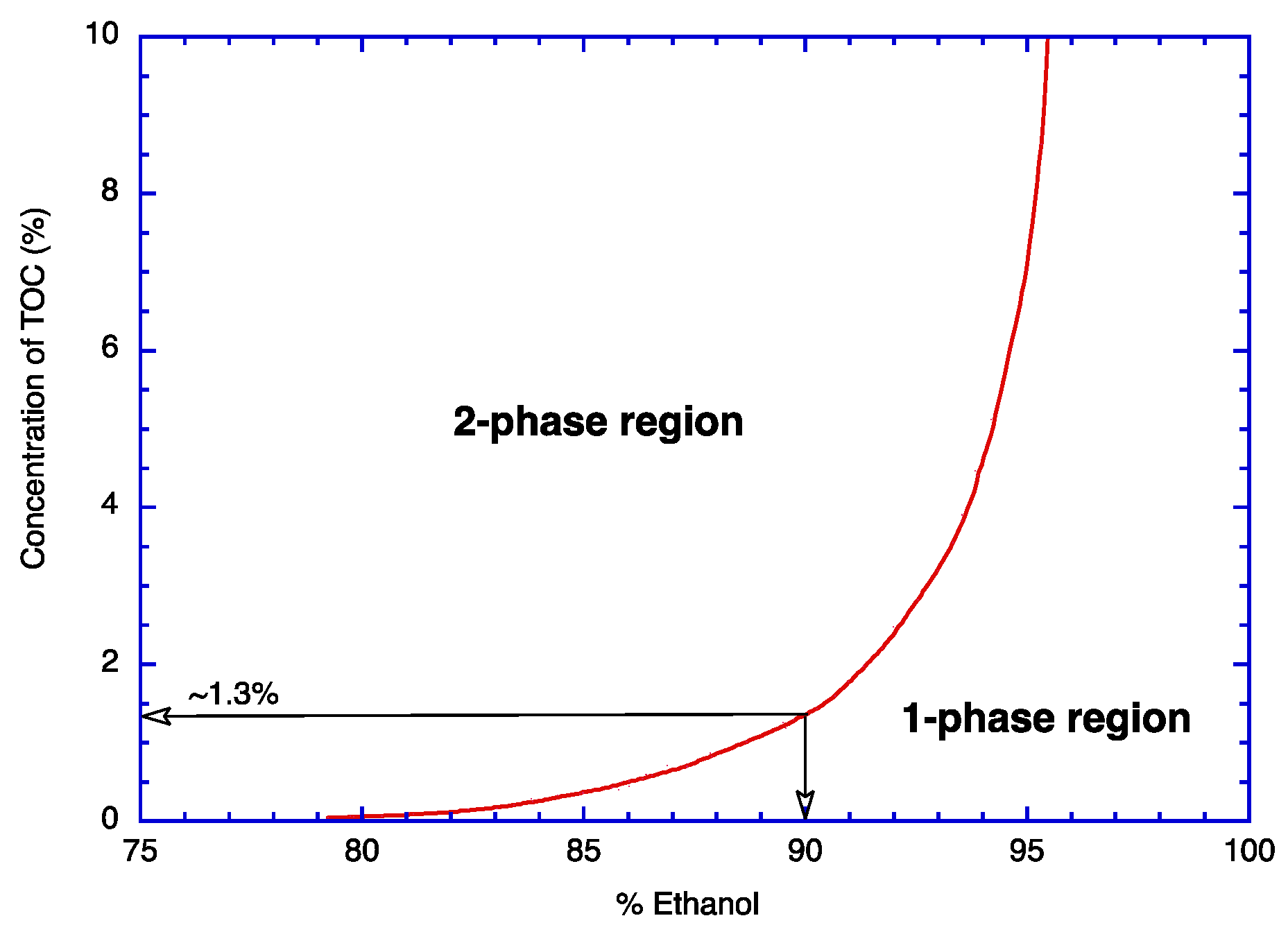
3.3. Phase Diagram of TOC in Aqueous Ethanol by Transmittance Measurement
3.4. Preparation of TOC-Encapsulated Zein Particles
3.5. Quantitative Analysis of TOC
3.6. Calculation of Encapsulation Efficiency (EE)
3.7. Hydrodynamic Diameter of Particles
3.8. Optical Microscopy of TOC-Encapsulated Particles
3.9. Scanning Electron Microscopy (SEM) of TOC-Encapsulated Particles
3.10. Statistical Analysis
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerdpol, K.; Nutho, B.; Krusong, K.; Poo-arporn, R.P.; Rungrotmongkol, T.; Hannongbua, S. Encapsulation of a-tocopherol in large-ring cyclodextrin containing 26 a-D-glucopyranose units: A molecular dynamics study. J. Mol. Liq. 2021, 339, 116802. [Google Scholar] [CrossRef]
- Indira, A.; Shahar, B.; Joshi, B. Assessment of bioactive compound variations and in-vitro and in-vivo antioxidant activity in edible fresh and processed Bambusa nutans shoot through FTIR, GC/MS and HPLC analyses. Food Chem. 2023, 452, 139552. [Google Scholar] [CrossRef]
- Combs, G.F.; McClung, J.P. The Vitamins, 6th ed.; Elsevier: London, UK, 2022; Chapter 7. [Google Scholar] [CrossRef]
- Dunn, B.K.; Richmond, E.; Anderson, D.E.; Greenwald, P. Testing the Ability of Selenium and Vitamin E to Prevent Prostate Cancer in a Large Randomized Phase III Clinical Trial: The Selenium and Vitamin E Cancer Prevention Trial. In Molecular Basis of Nutrition and Aging; Malavolta, M., Mocchegiani, E., Eds.; Elsevier: London, UK, 2016; pp. 567–582. [Google Scholar] [CrossRef]
- Francesco Violi, F.; Nocella, C.; Loffredo, L.; Carnevale, R.; Pignatelli, P. Interventional study with vitamin E in cardiovascular disease and meta-analysis. Free Radic. Biol. Med. 2022, 178, 26–41. [Google Scholar] [CrossRef]
- Niki, E.; Abe, K. Vitamin E: Structure, Properties and Functions. In Vitamin E: Chemistry and Nutritional Benefits; Etsuo, N., Ed.; The Royal Society of Chemistry: London, UK, 2019; pp. 1–11. [Google Scholar]
- Singh, P.; Wu, L.; Ren, X.; Zhang, W.; Tangb, Y.; Chen, Y.; Carrier, A.; Zhang, X.; Zhang, J. Hyaluronic-acid-based β-cyclodextrin grafted copolymers as biocompatible supramolecular hosts to enhance the water solubility of tocopherol. Int. J. Pharm. 2020, 586, 119542. [Google Scholar] [CrossRef]
- Cao, C.; Li Xu, L.; Xie, P.; Hu, J.; Qi, J.; Zhou, Y.; Cao, L. The characterization and evaluation of the synthesis of large-ring cyclodextrins (CD9–CD22) and a-tocopherol with enhanced thermal stability. R. Soc. Chem. Adv. 2020, 10, 6584–6591. [Google Scholar] [CrossRef]
- Xu, W.; Lv, K.; Mu, W.; Zhou, S.; Yang, Y. Encapsulation of α-tocopherol in whey protein isolate/chitosan particles using oil-in-water emulsion with optimal stability and bioaccessibility. LWT Food Sci. Technol. 2021, 148, 111724. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Chen, Z.; Wang, T.; Wang, L.; Zhong, Q. Biological macromolecule delivery system fabricated using zein and gum arabic to control the release rate of encapsulated tocopherol during in vitro digestion. Food Res. Int. 2018, 114, 251–257. [Google Scholar] [CrossRef]
- Zhang, F.; Khan, M.A.; Cheng, H.; Liang, L. Co-encapsulation of α-tocopherol and resveratrol within zein nanoparticles: Impact on antioxidant activity and stability. J. Food Eng. 2019, 247, 9–18. [Google Scholar] [CrossRef]
- Kasaai, M.R. Zein and zein-based nano-materials for food and nutrition applications: A review. Trends Food Sci. Technol. 2018, 79, 184–197. [Google Scholar] [CrossRef]
- Campos, L.A.A.; Neto, A.F.S.; Noronha, M.C.S.; Lima, M.F.; Cavalcanti, I.M.F.; Santos-Magalhaes, N.S. Zein nanoparticles for drug delivery: Preparation methods and biological applications. Int. J. Pharm. 2023, 635, 122754. [Google Scholar] [CrossRef]
- Kim, S.; Peterson, S.C. Optimal conditions for the encapsulation of menthol into zein nanoparticles. LWT Food Sci. Technol. 2021, 144, 111213. [Google Scholar] [CrossRef]
- Giteru, S.G.; Ali, M.A.; Oey, I. Recent progress in understanding fundamental interactions and applications of zein. Food Hydrocoll. 2021, 120, 106948. [Google Scholar] [CrossRef]
- Kramer, K.A.; Liebler, D.C. UVB induced photooxidation of vitamin E. Chem. Res. Toxicol. 1997, 10, 219–224. [Google Scholar] [CrossRef]
- Pirisi, F.M.; Angioni, A.; Bandino, G.; Cabras, P.; Guillou, C.; Maccioni, E.; Reniero, F. Photolysis of a-tocopherol in olive oils and model systems. J. Agric. Food Chem. 1998, 46, 4529–4533. [Google Scholar] [CrossRef]
- McVean, M.; Liebler, D.C. Inhibition of UVB induced DNA photodamage in mouse epidermis by topically applied a-tocopherol. Carcinogenesis 1997, 18, 1617–1622. [Google Scholar] [CrossRef]
- Kim, S.; Xu, J. Aggregate formation of zein and its structural inversion in aqueous ethanol. J. Cereal Sci. 2008, 47, 1–5. [Google Scholar] [CrossRef]
- Loureiro, J.; Miguel, S.P.; Seabra, I.J.; Ribeiro, M.P.; Coutinho, P. Single-Step Self-Assembly of Zein–Honey–Chitosan Nanoparticles for Hydrophilic Drug Incorporation by Flash Nanoprecipitation. Pharmaceutics 2022, 14, 920. [Google Scholar] [CrossRef] [PubMed]
- Shar, B.R.; Xu, W.; Mraz, J. Formulation and characterization of zein/chitosan complex particles stabilized Pickering emulsion with the encapsulation and delivery of vitamin D3. J. Sci. Food Agric. 2021, 101, 5419–5428. [Google Scholar] [CrossRef]
- de Melo, A.P.; da Rosa, C.G.; Noronha, C.M.; Machado, M.H.; Sganzerla, W.G.; da Cunha Bellinati, N.V.; Nunes, M.R.; Verruck, S.; Prudêncio, E.S.; Barreto, P.L. Nanoencapsulation of vitamin D3 and fortification in an experimental jelly model of Acca sellowiana: Bioaccessibility in a simulated gastrointestinal system. LWT 2021, 145, 111287. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. The progress and application of vitamin E encapsulation—A review. Food Hydrocol. 2021, 121, 106998. [Google Scholar] [CrossRef]
- Kim, S.; Biswas, A.; Boddu, V.; Hwang, H.-S.; Adkins, J. Solubilization of cashew gum from Anacardium occidentale in aqueous medium. Carbohydr. Polym. 2018, 199, 205–209. [Google Scholar] [CrossRef]
- de Folter, J.W.J.; van Ruijven, M.W.M.; Velikov, K.P. Oil-in-water Pickering emulsions stabilized by colloidal particles from the water-insoluble protein zein. Soft Matter 2012, 8, 6807–6815. [Google Scholar] [CrossRef]
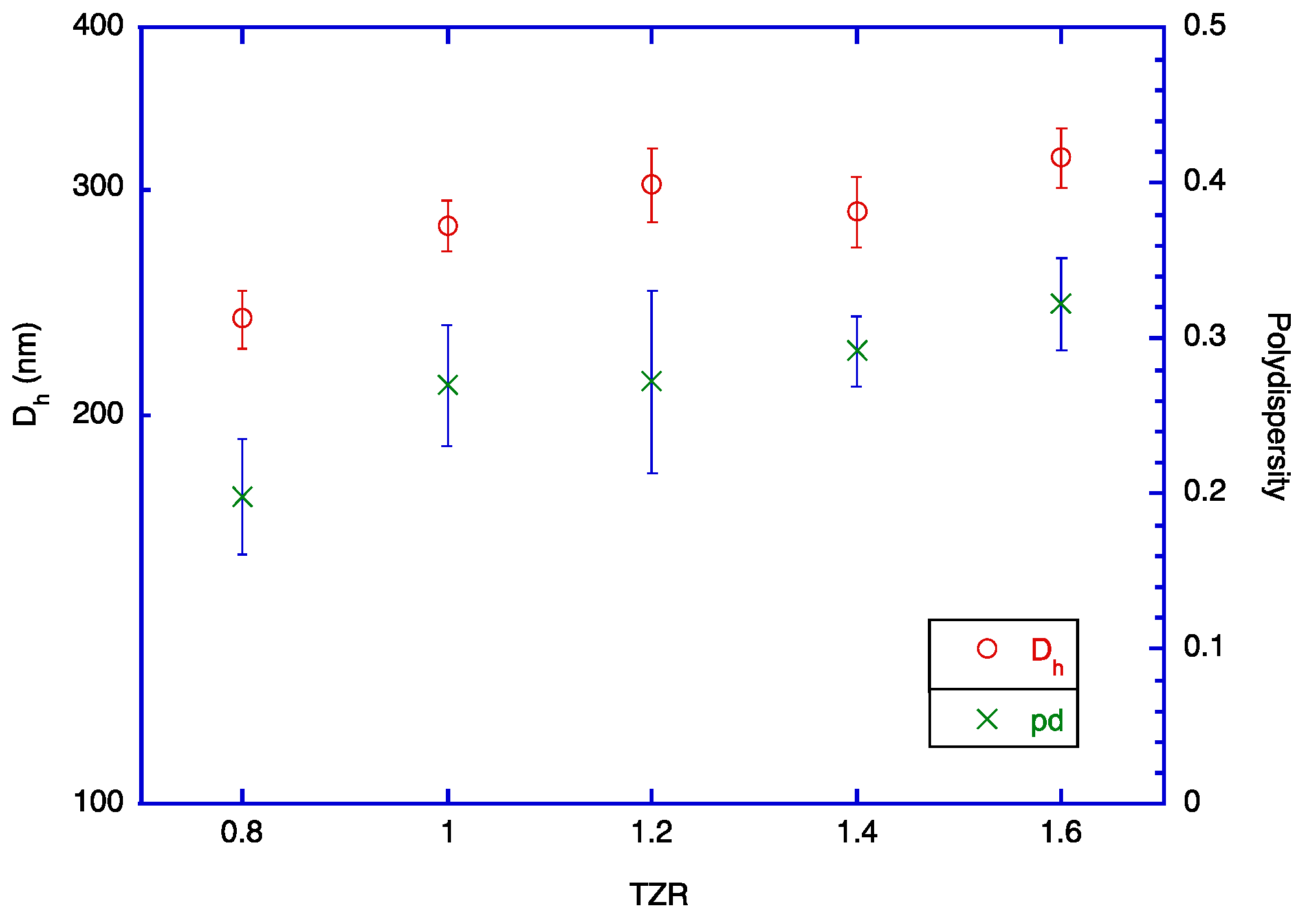

| Sample # | Solution A | Solution B | Solution C | Ethanol Content of the Mixture (%) | TZR * | Dh (nm) ** | Polydispersity | EE (%) |
|---|---|---|---|---|---|---|---|---|
| A1 | TOC 0.408 g + ethanol 6.0 g | zein 0.51 g + 90% ethanol 10.0 g | Water 34.0 g | 30 | 0.8 | 238 ± 6 d | 0.20 ± 0.04 b | 98.6 ± 0.2 c |
| A2 | TOC 0.51 g + ethanol 6.0 g | zein 0.51 g + 90% ethanol 10.0 g | Water 34.0 g | 30 | 1.0 | 281 ± 10 c | 0.27 ± 0.06 ab | 98.6 ± 0.2 c |
| A3 | TOC 0.612 g + ethanol 6.0 g | zein 0.51 g + 90% ethanol 10.0 g | Water 34.0 g | 30 | 1.2 | 303 ± 6 b | 0.27 ± 0.04 ab | 98.7 ± 0.2 bc |
| A4 | TOC 0.714 g + ethanol 6.0 g | zein 0.51 g + 90% ethanol 10.0 g | Water 34.0 g | 30 | 1.4 | 288 ± 6 bc | 0.29 ± 0.02 a | 99.2 ± 0.2 a |
| A5 | TOC 0.816 g + ethanol 6.0 g | zein 0.51 g + 90% ethanol 10.0 g | Water 34.0 g | 30 | 1.6 | 318 ± 9 a | 0.32 ± 0.03 a | 98.8 ± 0.2 b |
| Sample # | TZR | Wt. Ratio (Core/ Shell) | Volume Ratio a (Core/ Shell) | Volume Fraction of Core b | R c (nm) | r d (nm) | Shell Thickness e (nm) |
|---|---|---|---|---|---|---|---|
| A1 | 0.80 | 0.80 | 0.620 | 0.3827 | 119.0 ± 3.0 | 86.4 ± 2.2 | 32.6 ± 5.2 |
| A2 | 1.0 | 1.0 | 0.775 | 0.4366 | 140.5 ± 5.0 | 106.6 ± 3.8 | 33.9 ± 8.8 |
| A3 | 1.2 | 1.2 | 0.930 | 0.4819 | 151.5 ± 3.0 | 118.8 ± 2.4 | 32.7 ± 5.4 |
| A4 | 1.4 | 1.4 | 1.085 | 0.5204 | 144.0 ± 3.0 | 115.8 ± 2.4 | 28.2 ± 5.4 |
| A5 | 1.6 | 1.6 | 1.240 | 0.5536 | 159.0 ± 4.5 | 130.6 ± 3.7 | 28.5 ± 8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S. Protection of α-Tocopherol from UV-Induced Degradation by Encapsulation into Zein Nanoparticles. Molecules 2024, 29, 3911. https://doi.org/10.3390/molecules29163911
Kim S. Protection of α-Tocopherol from UV-Induced Degradation by Encapsulation into Zein Nanoparticles. Molecules. 2024; 29(16):3911. https://doi.org/10.3390/molecules29163911
Chicago/Turabian StyleKim, Sanghoon. 2024. "Protection of α-Tocopherol from UV-Induced Degradation by Encapsulation into Zein Nanoparticles" Molecules 29, no. 16: 3911. https://doi.org/10.3390/molecules29163911
APA StyleKim, S. (2024). Protection of α-Tocopherol from UV-Induced Degradation by Encapsulation into Zein Nanoparticles. Molecules, 29(16), 3911. https://doi.org/10.3390/molecules29163911






