1. Introduction
Phosphogypsum is a waste from the phosphorus chemical industry. The accumulation of phosphogypsum leads to the release of harmful substances that can pollute the air, soil, rivers, or groundwater [
1], accumulate in the food chain through enrichment, and cause public health problems [
2]. Although the main component of phosphogypsum is CaSO
4·2H
2O and other impurities, the impurities in phosphogypsum greatly limit its application. The current impurity removal methods include: (1) water washing can remove the acidic and soluble impurities of phosphogypsum, but it is difficult to remove insoluble impurities, and the water resource consumption is serious [
3]. (2) Flotation can only remove organics [
4]. (3) The calcination method can only remove eutectic phosphorus and organic matter, but it consumes high energy [
5]. In addition, acid leaching is a relatively effective purification technology; the acid is selective to impurities, and it is difficult to use a single acid to effectively purify phosphogypsum [
6,
7,
8]. The quicklime neutralization method also has the problem of difficulty in removing insoluble impurities [
4]. Although the purification effect is good, the process is very complicated. The anti-solvent method [
9] has a simple treatment process and mild reaction conditions. This method is environmentally friendly for extracting CaSO
4·2H
2O from phosphogypsum and can efficiently precipitate CaSO
4·2H
2O crystals from water because the anti-solvent makes CaSO
4·2H
2O solubility to produce a dramatic change [
10]. However, the morphological regulation of CaSO
4·2H
2O crystals has been difficult to realize due to the fast precipitation speed and elusive precipitation mechanism of the anti-solvent method.
The morphology of CaSO
4·2H
2O also has a great influence on the utilization of the materials engineer [
11,
12,
13]. In previous research, the morphology regulation of CaSO
4·2H
2O is mainly regulated by the addition of crystal form regulators. The common crystal form regulators include polymer additives [
14], metal ions [
15,
16,
17], calcium ion complexing agents [
18], hexadecyl pyridine chloride [
19], amino acids [
20], and oxalic acid [
21]. The addition of crystal form modifiers is mostly used in the morphology adjustment of pure CaSO
4·2H
2O, which is limited by the complex multi-component of phosphogypsum. The addition of a crystal form agent in phosphogypsum will also have more uncontrollable factors, such as inactivation in the acidic environment of phosphogypsum or adsorption by other components, which result in difficult purifying CaSO
4·2H
2O. In addition, the morphology regulation of CaSO
4·2H
2O crystal mainly focused on the third group of CaSO
4·2H
2O, the influence of solvent on the growth mechanism was mostly ignored in the research process [
22].
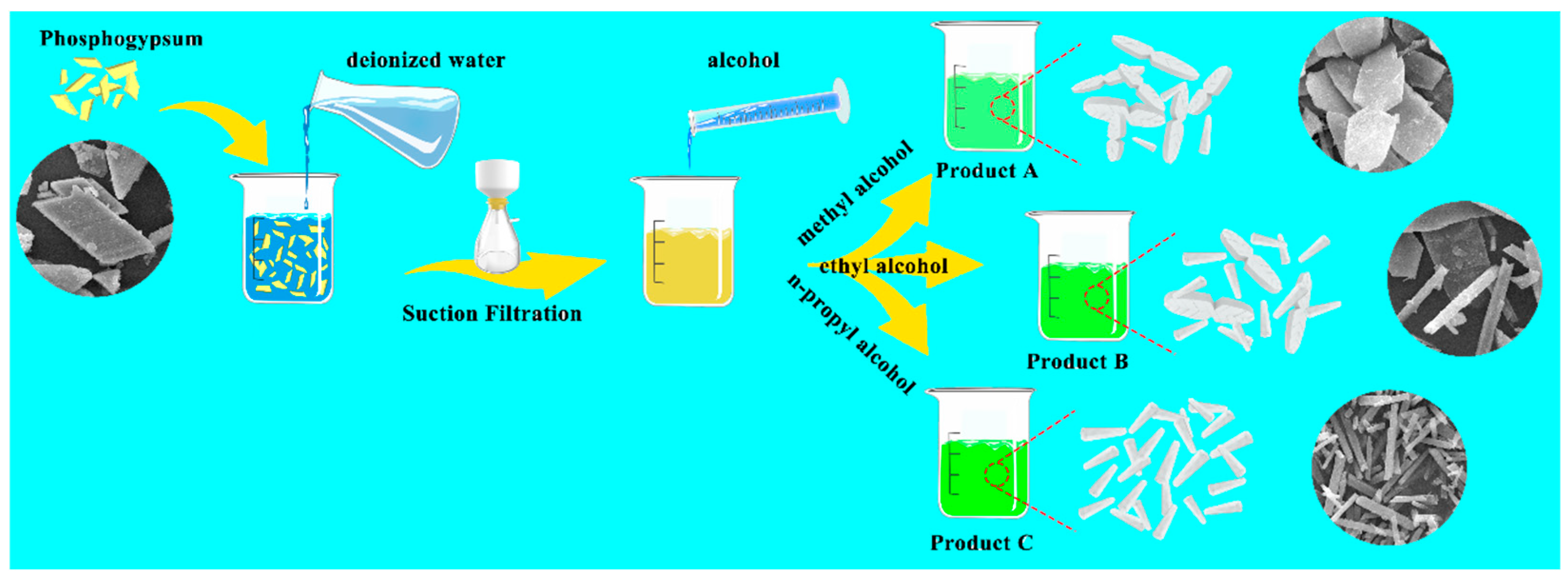
In this work, CaSO4·2H2O was directly purified from phosphogypsum by anti-solvent (method: ethanol and n-propanol) to regulate the morphology of CaSO4·2H2O crystals. The obtained butterfly-shaped, hexagonal prism-shaped, and mixed-shaped three-dimensional CaSO4·2H2O crystals have different morphologies and scales. The method has the advantages of excellent ability of morphology regulation, high purity efficiency, simple experimental operation, mild reaction conditioning, being environmentally friendly, and low energy consumption. Further studies on the mechanism of regulation showed that the anti-solvent adsorbed on the c-axis direction of the CaSO4·2H2O crystal through the van der Waals interaction, inhibiting the growth rate of the CaSO4·2H2O crystal in the c-axis direction to effectively regulate the morphology. Generally, the adsorption capacity of the CaSO4·2H2O crystal on the anti-solvent will increase with an increase in the polarity of the anti-solvent. The inhibitory effect of the anti-solvent on the CaSO4·2H2O crystal will increase with the addition of the anti-solvent’s chain length. Herein, the morphology of CaSO4·2H2O crystal can be controlled effectively by adjusting the polarity and chain length of the antisolvent molecules. When n-propanol was used as the anti-solvent, the morphology of the CaSO4·2H2O crystal showed a hexagonal prism with a specific surface area of 19.98 m2/g and a Cu2+ loading efficiency of 52.67%. In addition, the purified CaSO4·2H2O crystal from phosphogypsum has excellent biocompatibility and great application potential in the field of biomedicine, which provides new ideas for the application of phosphogypsum.
2. Results and Discussion
As shown in
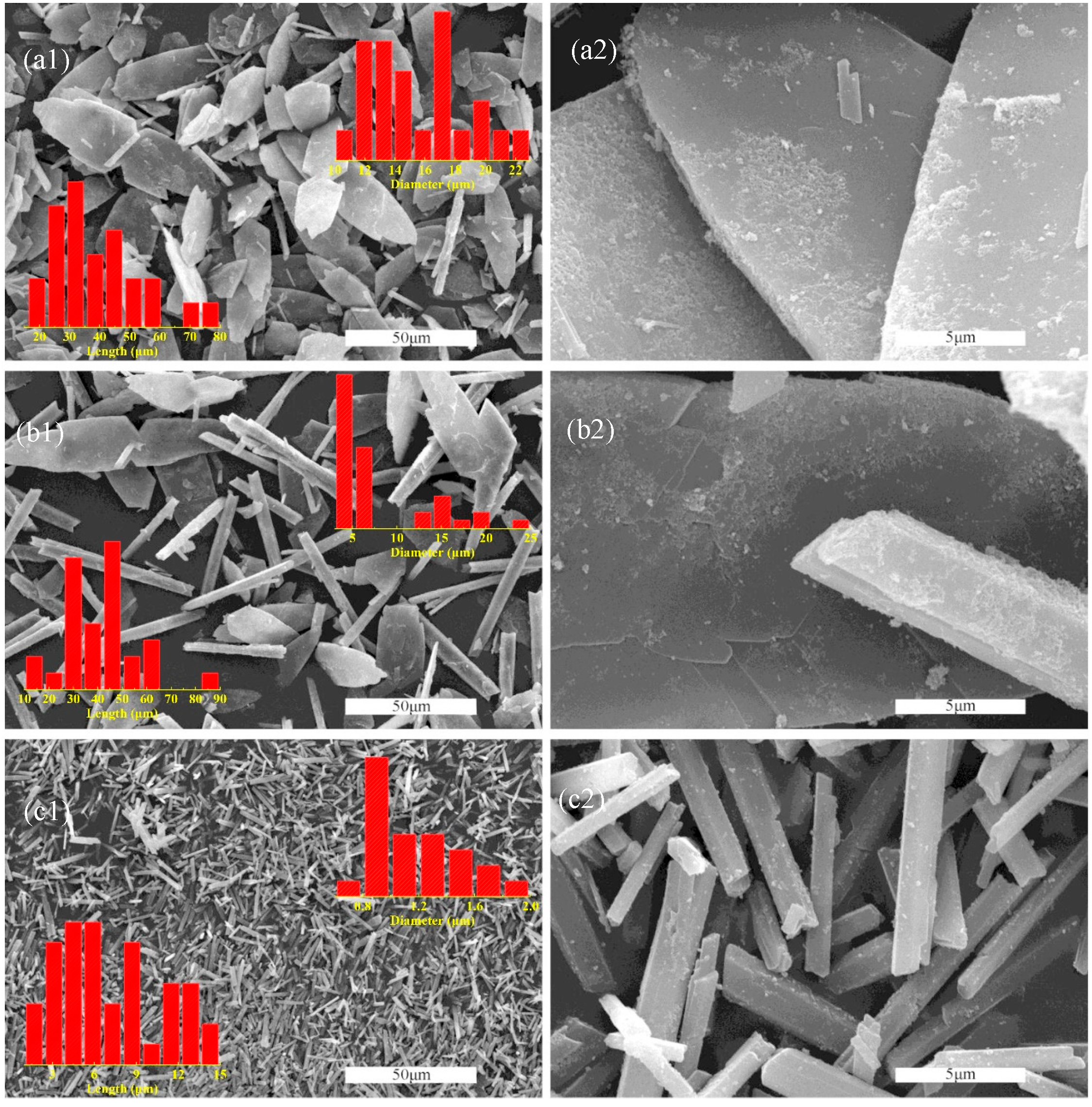
Supplementary Figure S1a, the phosphogypsum after natural drying is a gray solid powder. It can be seen from a SEM image (
Figure S1b, Supplementary Materials) that the particles of phosphogypsum show multi-scale distribution, including quadrilateral block crystals ranging from a dozen microns to tens of microns, short columnar crystals with a few microns in diameter and tens of microns in length, and regular particles with a large difference in scale. The PXRD pattern of phosphogypsum demonstrates that the main components of phosphogypsum are calcium sulfate dihydrate and silica (
Figure S1c, Supplementary Materials). In addition, the XPS shows that the constituent elements of phosphogypsum, which include P, F, and Si in addition to S, O, and Ca (
Figure S1d, Supplementary Materials). The utilization processes of phosphogypsum are generally complex and high energy consumptive. To solve the problems, an anti-solvent method with simple operation, environmental protection, and high product purity is selected to depurate the phosphogypsum and modulate the microstructure. The morphology of the product was successfully controlled by changing the chain length of the anti-solvent (the mixing ratio of methanol, ethanol, and n-propanol to solution is 3:10, respectively). The morphologies of the obtained products are characterized by SEM, as shown in
Figure 1. With the increase in anti-solvent chain length, the morphology of the product changes from butterfly-like flake crystal to hexagonal prism-like crystal. The morphology of Product A (methanol is used as an anti-solvent) is almost always butterfly-shaped flake crystals with a width of 10~20 μm and a length of 20~80 μm. Product B (ethanol is used as an anti-solvent) consists of two morphological crystals; one is a butterfly-shaped flake crystal with a width distribution of 10~25 μm, and the other is a hexagonal prism-shaped crystal with a width distribution of t 3~7 μm and a length distribution of 10~70 μm. The morphology of Product C (n-propanol is used as an anti-solvent) is not only almost a hexagonal prismatic crystal but also the width and length are reduced to 0.6~2 μm and 1~15 μm, respectively. The SEM micrographs of Products C1, C2, and C3, acquired at varying mixing ratios of anti-solvent (2:10, 4:10, and 5:10), reveal that the size of the products is influenced by the mixing ratio, while the geometric morphology remains consistent across all ratios (
Figure S2, Supplementary Materials).
The chain length change of the anti-solvent can regulate the morphology and micro-size of the corresponding product, which enhances the application potential of these products. To identify the safety and reliability of the product, the components and purity of these products are measured by XRD, FTIR, XPS, and DSC. The diffraction patterns of the products match the standard characteristic peaks of the (020), (-121), (040), and (-141) crystal faces of the CaSO
4·2H
2O, as shown in
Figure 2a and
Figure S3 (Supplementary Materials). This congruence confirms that the products are composed of CaSO
4·2H
2O and demonstrates the phase purity for Products A, B, and C.
Figure 2b shows multiple vibrational absorption bands of these products; these vibration peaks demonstrate that the main component of the product is CaSO
4·2H
2O. In gypsum molecules, the geometry of SO
4 deviates from the ideal tetrahedral configuration (Td symmetry group) to the lower molecular symmetry C
2 [
23,
24]. Therefore, a weak band at 1005 cm
−1 is observed in the FTIR spectrum of gypsum, which corresponds to vibrational absorption bands (S–O symmetric stretch) of the SO
4 tetrahedron in gypsum. This symmetric stretching mode is observed due to the lower symmetry of sulfate-ion in gypsum and is otherwise IR inactive in the free sulfate-ion [
25]. The polarity of n-propanol is quite different from that of water molecules, and it has the greatest influence on the structure of SO
4 during the crystallization process, and the ν
1 of Product C is the most obvious. In addition, there are four more distinctive peaks here. The peaks at 1138 cm
−1 and 1120 cm
−1 are assigned to the splitting of the triple-degenerate S–O asymmetric stretching vibration in the free sulfate ion. The peaks at 669 cm
−1 and 604 cm
−1 are assigned to antisymmetric bending vibration that is generated by splitting into double peaks for the triple degeneracy of sulfate tetrahedra in gypsum [
25]. The peak at 3546 cm
−1 and 3405 cm
−1 corresponded to vibrational absorption bands of hydroxyl (–OH), and the peak at 1685 cm
−1 and 1621 cm
−1 corresponded to bending vibration peaks of –OH in water. The XPS is used to further confirm the constituent elements of these products.
Figure 2c shows the constituent elements of these products (Ca, S, O, C, and F elements), where the Ca, S, and O elements correspond to the CaSO
4·2H
2O. in the product, and the F element is soluble F for precipitating together with the product during the crystallization process of the product in phosphogypsum (the C element comes from the test background). By integrating the correlative peak areas of each element, the approximate atomic percentage contents of the three main elements Ca, S, and O in the phosphogypsum product were obtained (
Table S1, Supplementary Materials). The proportion of Ca, S, and O elements is close to that of 1:1:4, indicating that three products are CaSO
4·2H
2O. In addition, the endothermic change during the heating process of the product is also used to indirectly corroborate the composition of the product. As shown in
Figure 2d, the DSC curve occurs two endothermic peaks overlapped together in the temperature range from 100 °C to 160 °C, which correspond to the two processes of the dehydration that the CaSO
4·2H
2O first loses 3/2H
2O to become CaSO
4·1/2H
2O (Equation (1)) and then loses 1/2H
2O to transform into γ-CaSO
4 (Equation (2)) [
25], which is consistent with the reported literature. Therefore, we believe that the main component of the product is CaSO
4·2H
2O, and the change in the anti-solvent chain length will not affect the composition of the product.
To further understand the mechanism of morphology regulation of CaSO
4·2H
2O, the XPS energy spectrum of the O element of these products is used to further investigate the impact of anti-solvent on the morphology of CaSO
4·2H
2O. Methanol, ethanol, and n-propanol all contain hydroxyl groups, and the change in the length of the carbon chain connected to the hydroxyl group changes the polarity of the molecule. The polarity of anti-solvent molecules decreases as the length of the carbon chain increases (methanol, 6.6 > ethanol, 4.3 > n-propanol, 4.0) [
26].
Figure 3a–c show two kinds of peaks of the O element (S–O and H–O–H) after peak fitting. The S–O and H–O–H peaks are the S–O and H
2O in CaSO
4·2H
2O, respectively. The positions of the characteristic peaks of the oxygen elements of each product are counted in
Table S2 (Supplementary Materials). It can be seen that there are differences in the positions of the S–O characteristic peaks of the products obtained under the action of different anti-solvents. The reason for this result is that the anti-solvent will selectively adsorb on the crystal surface during the crystallization process [
27]. As shown in
Figure 3d, the alcoholic hydroxyl group of the anti-solvent will form a hydrogen bond with the oxygen atom of the sulfate radical in CaSO
4·2H
2O, which will be adsorbed in a specific direction of the crystal. In addition, the PXRD peak of the product’s (020) crystal plane is selected to further confirm the impact of the anti-solvent on the crystal growth of CaSO
4·2H
2O, as shown in
Figure 3e. According to the Bragg equation, the (020) interplanar spacing is calculated and shown in
Figure 3f. This result indicates that the interplanar spacing of these products decreases from 3.77 Å to 3.74 Å as the polarity of the anti-solvent decreases because the anti-solvent of the lower polarity will decrease the van der Waals interaction between the anti-solvent and the CaSO
4·2H
2O.
Changes in the structure of the product will affect their thermal stability. To further investigate the impact of interplanar spacing on thermal stability, the TG experiment is used to characterize the thermal stability of these products and indirectly prove the change in the product structure. The thermogravimetric curve of these products is shown in
Figure 4a. The thermogravimetric curve of these products shows a single weight loss peak, and the temperature range of thermogravimetric loss is 100~160 °C, which is consistent with the DTG results (
Figure 4b,c). Furthermore, the maximum weight loss of Products A, B, and C are 20.63%, 20.63%, and 20.93%, respectively, which indicates the component of these products is CaSO
4·2H
2O and the loss is the crystal water in CaSO
4·2H
2O. However, the thermal stability of these products is slightly different (
Figure 4b). The T
5%, T
10%, and T
Max of these products are calculated and plotted in
Figure 4d (T
5%, T
10%, and T
Max represent the corresponding temperature at the weight loss of 5%, 10%, and maximum). It can be seen from the figure that T
5%, T
10%, and T
Max increase with the decrease of the interplanar spacing of the product. Product A with the largest interplanar spacing has T
5%, T
10%, and T
Max of 124.35 °C, 124.8 °C, and 131.12 °C, respectively, and the T
5%, T
10%, and T
Max of Product C with the smallest interplanar spacing are 136.74 °C, 139.79 °C, and 144.04 °C, respectively. The reason for this result is the reduction of the interplanar spacing of the product, which will enhance the confinement effect of the product on the water molecules. The change in the thermal stability of the product supports the change of the product structure from the side.
The above analysis indicates that the polarity of the anti-solvent clearly affects the crystal growth, structure, and thermal stability of the CaSO4·2H2O. To clearly understand the growth process of CaSO4·2H2O, the growth direction and rate of the crystal are discussed as two important factors. The CaSO4·2H2O crystal belongs to the monoclinic system and has three growth directions (a-, b-, and c-axis), of which the a-axis direction is enriched with Ca elements and oxygen enrichment in the c-axis direction.
It can be seen from
Figure 5 that the crystal growth of CaSO
4·2H
2O mainly grows along the
a-axis and the
c-axis without external interaction. The product morphology is quadrilateral block crystal if the growth rates in the two directions are similar. When methanol is used as the anti-solvent, the hydroxyl group of methanol interacts with the oxygen in the CaSO
4·2H
2O crystal and is enriched in the
c-axis direction during the crystallization of CaSO
4·2H
2O. However, the CaSO
4·2H
2O crystal growth will be subject to a relatively small inhibition effect due to the small volume of methanol molecular side chains, which results in a small difference in the growth rates of the
a-axis and
c-axis. Finally, the morphology of Product A appears as butterfly-like lamellar crystals. When n-propanol is used as the anti-solvent, the n-propanol is enriched in the
c-axis direction of the CaSO
4·2H
2O crystal during the growth of the CaSO
4·2H
2O crystal, which suppresses the growth of the crystal in the
c-axis direction. At the same time, the side chain of n-propanol is large, and n-propanol has a significant inhibitory effect on the growth of the CaSO
4·2H
2O crystal due to the long chain. Finally, the morphology of Product C appears as a hexagonal prismatic crystal. The difference in the morphology between Products A and C can be attributed to the difference in the inhibition effect of the anti-solvent during CaSO
4·2H
2O crystal formation, resulting in different growth rates of the
a- and
c-axes. Therefore, if the calcium ion chelating agent is adsorbed in the
a-axis direction to limit the growth rate in the
a-axis direction, it will lead to an increase in the precipitation induction time and change the morphology of the product [
28].
Diethylenetriaminepentaacetic acid (DTPA) (
Figure S4), which is an effective calcium chelator, is selected to control the growth rate in the
a-axis direction. DTPA was first dissolved in a phosphogypsum solution and chelated with calcium ions of CaSO
4·2H
2O. Subsequently, n-propanol is used as an anti-solvent to precipitate CaSO
4·2H
2O crystal (Product D). During the growth process, the DTPA will be enriched in the
a-axis direction of the CaSO
4·2H
2O crystal along with the calcium ions (
Figure 6a). The
a-axis of the crystal can inhibit the growth of the
a-axis direction and reduce the length of the product due to the large molecular size of DTPA.
Figure 6b,c shows the morphologies of the product before and after the addition of DTPA, respectively. When DTPA is not added, the product morphology is a hexagonal prism-like crystal. The product morphology becomes a hexagonal prism-thin crystal after adding DTPA, which is consistent with the theoretical prediction. From the structure of DTPA, it can be seen that DTPA contains a large number of carbon elements, and the enrichment of DTPA on the surface of Product D will inevitably cause a change in the element content on the surface of the product. The element content of the side and end faces of Product D was characterized by the energy spectrum, as shown in
Figure 6d,e. The carbon content of the end face and side in Product D is about 44.44% and 15.00%, respectively, which reveals that DTPA chelated with the calcium ion of the
a-axis direction. The result further confirms that organic molecules adhered to the surface of CaSO
4·2H
2O crystals will affect the growth rate of the product in different directions and further change their morphology.
Compared with phosphogypsum, the obtained CaSO
4·2H
2O product has no obvious toxicity to cells and no irritation to normal skin by the cell and animal experiments, indicating that the product can be effective in the field of biomedicine (
Figures S5 and S6, Supplementary Materials). Since Cu
2+ has excellent antibacterial properties, the Cu
2+ solution is used to load Cu
2+ in the product to expand its application field. The loading process mainly has two stages, as shown in
Figure 7a. First, the
and Ca
2+ entered the solution after the dissolution of the CaSO
4·2H
2O crystal on the surface product, and then a part of the Cu
2+ in the solution adhered to the surface of the product. This process was equivalent to the replacement of Ca
2+ with Cu
2+ on the product surface [
16]. Therefore, the specific surface area of the product has an important influence on the loading efficiency of the product Cu
2+.
Figure 7b is the adsorption and desorption curve of each product N
2. The specific surface area of each product is calculated by the BET algorithm as shown in
Figure 7c, the specific surface areas of products A, B, and C are 15.90 m
2/g, 17.54 m
2/g, and 19.98 m
2/g, respectively.
Figure 7d shows the Cu
2+ loading efficiency of the product, where the Cu
2+ loading efficiency of Products A, B, and C are 42.27%, 47.36%, and 52.67%, respectively. Product C with hexagonal prismatic crystal morphology has the largest specific surface area, and Product C also has the highest Cu
2+ loading efficiency.