Ionic Liquid Catalysis in Cyclic Carbonate Synthesis for the Development of Soybean Oil-Based Non-Isocyanate Polyurethane Foams
Abstract
1. Introduction
2. Results and Discussion
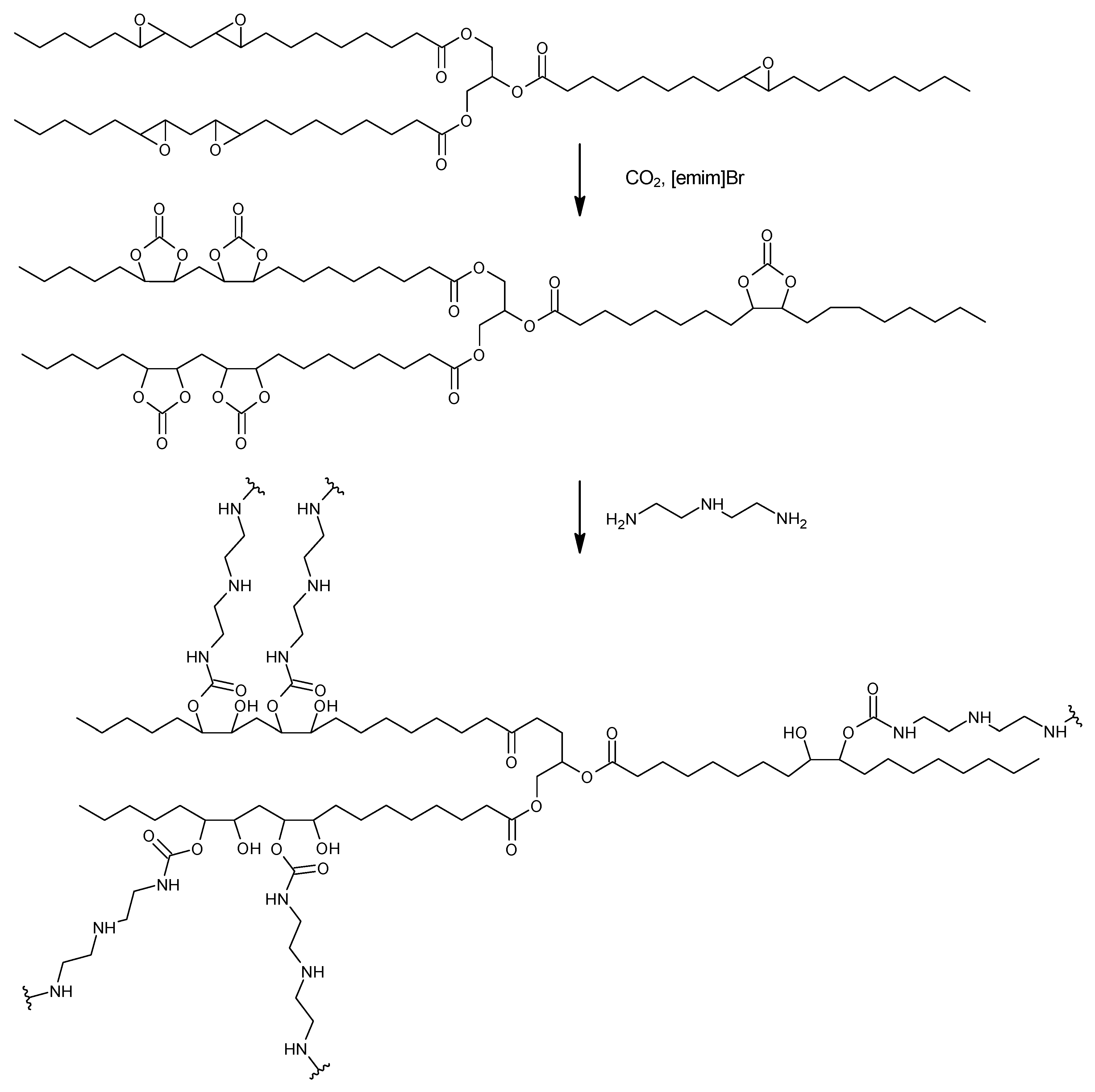
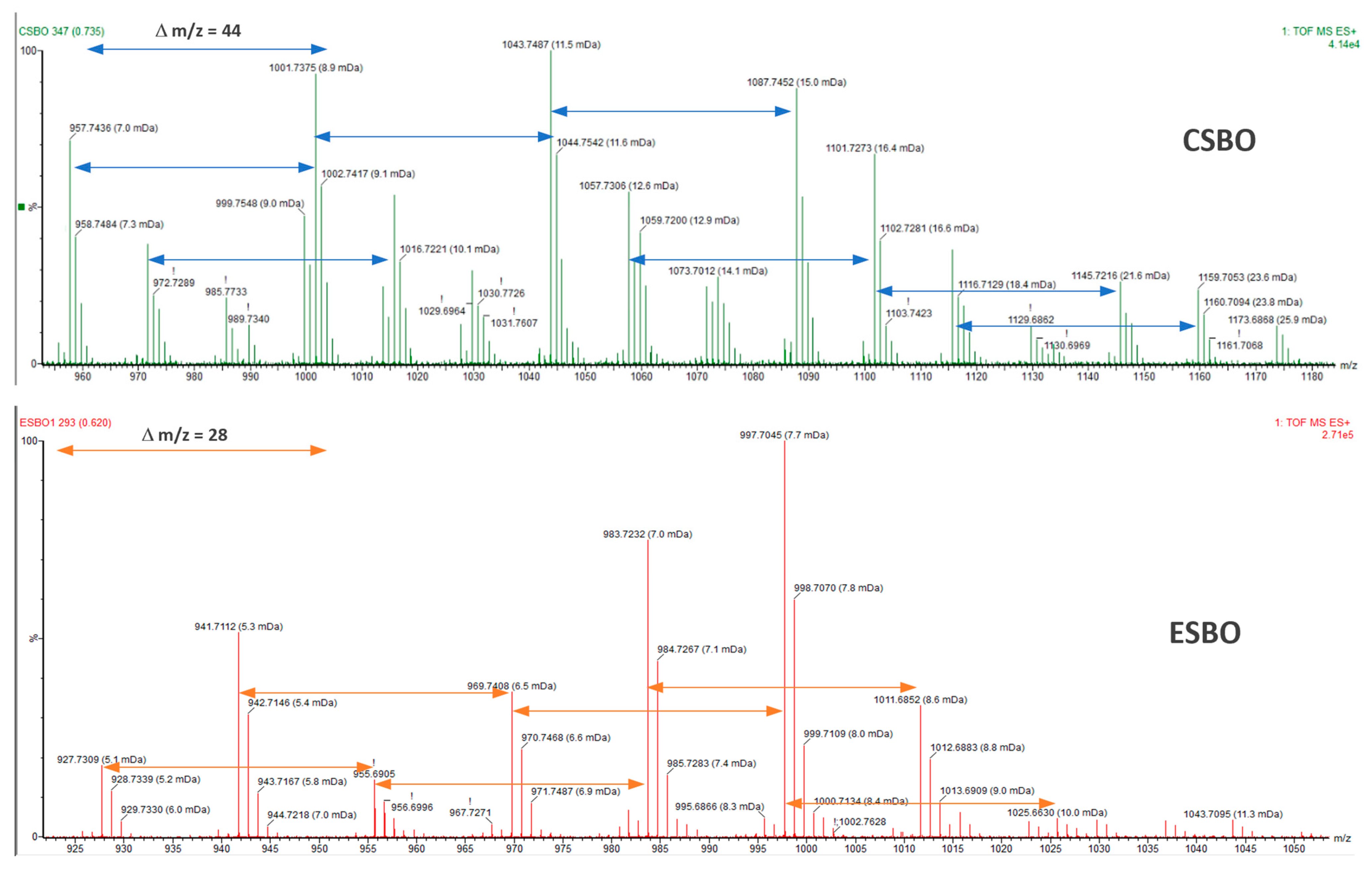
2.1. Synthesis of Cyclic Carbonates
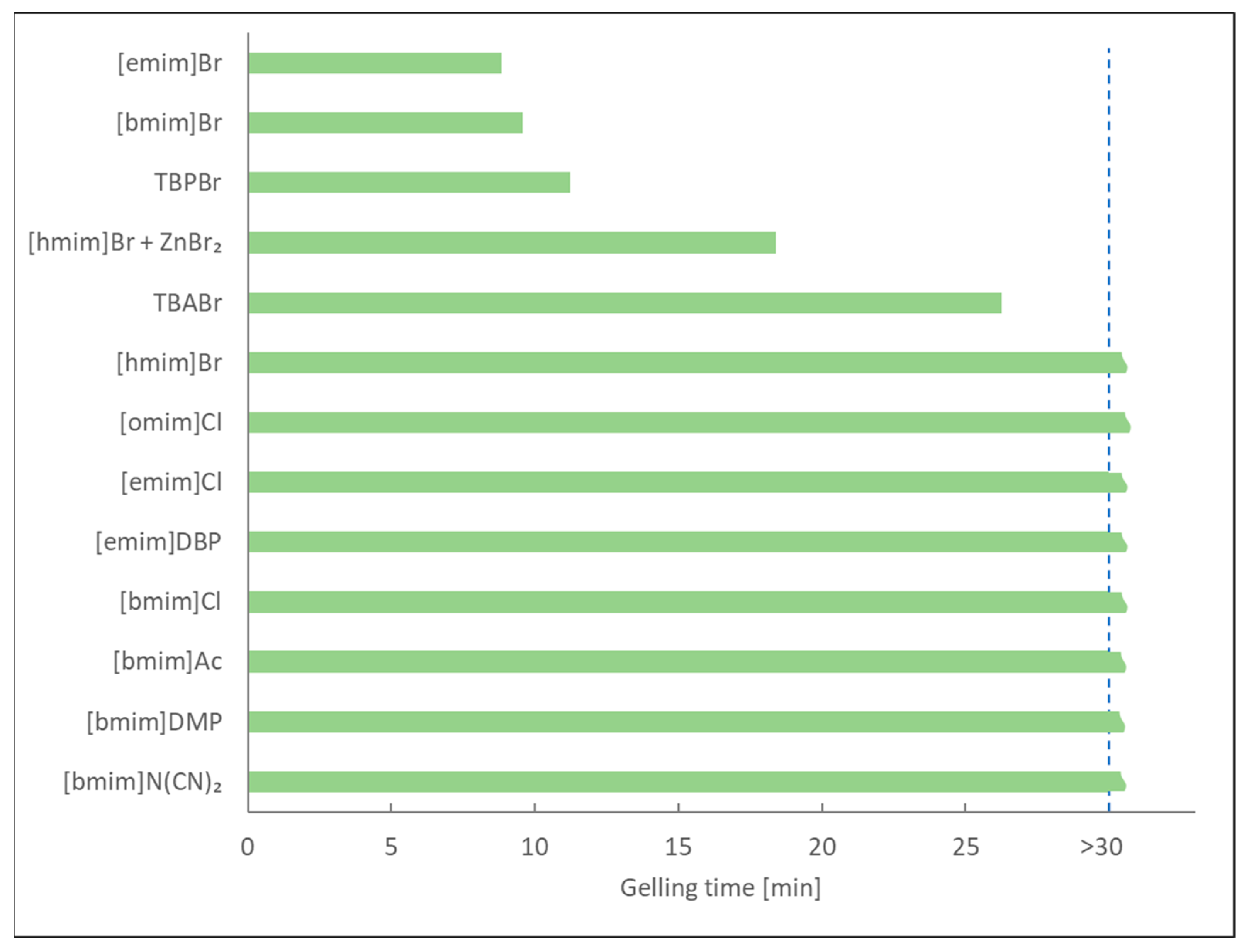
2.2. Synthesis of NIPU Foams
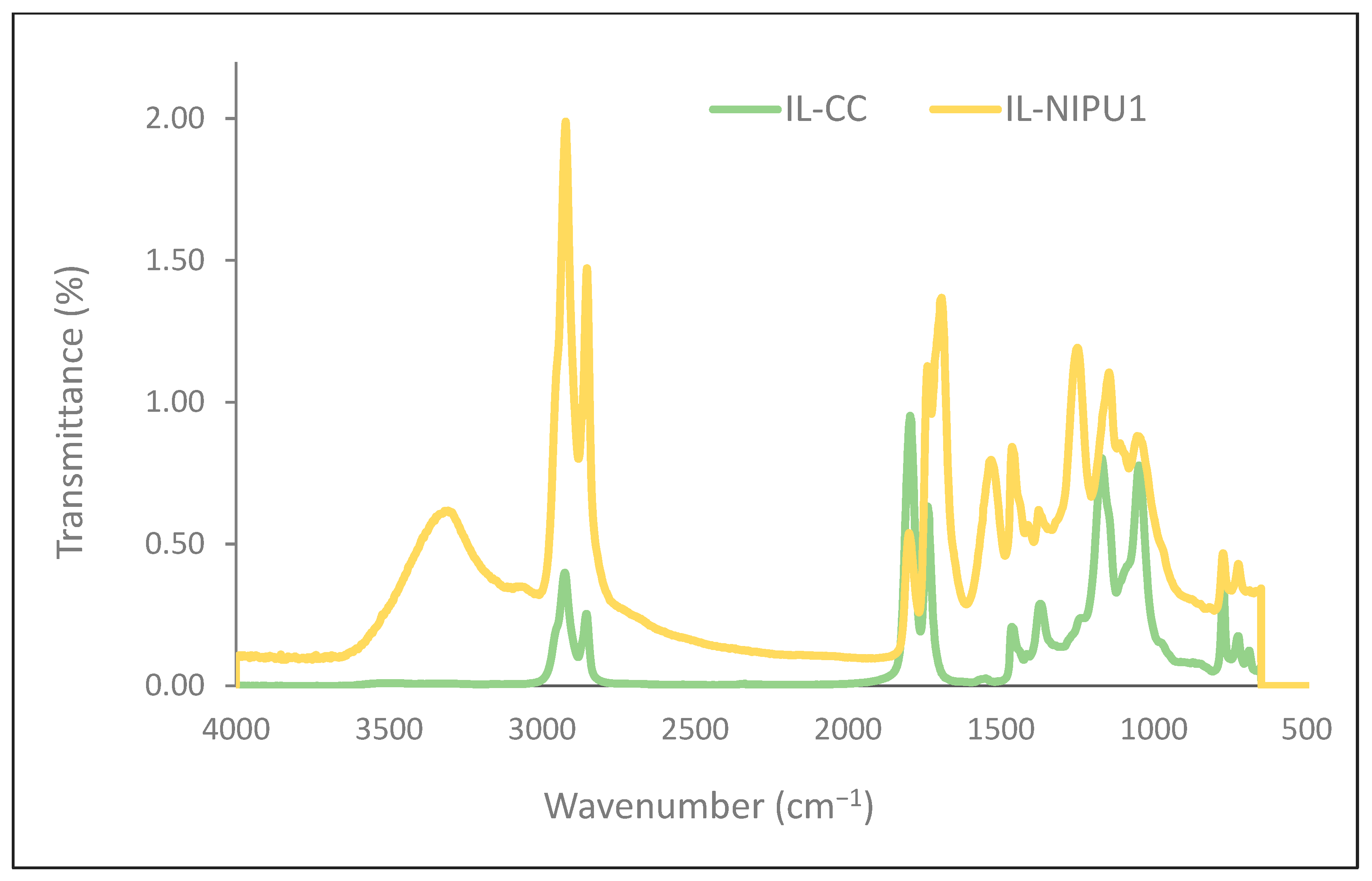
2.2.1. FT-IR Analysis
2.2.2. Amount of Blowing Agent
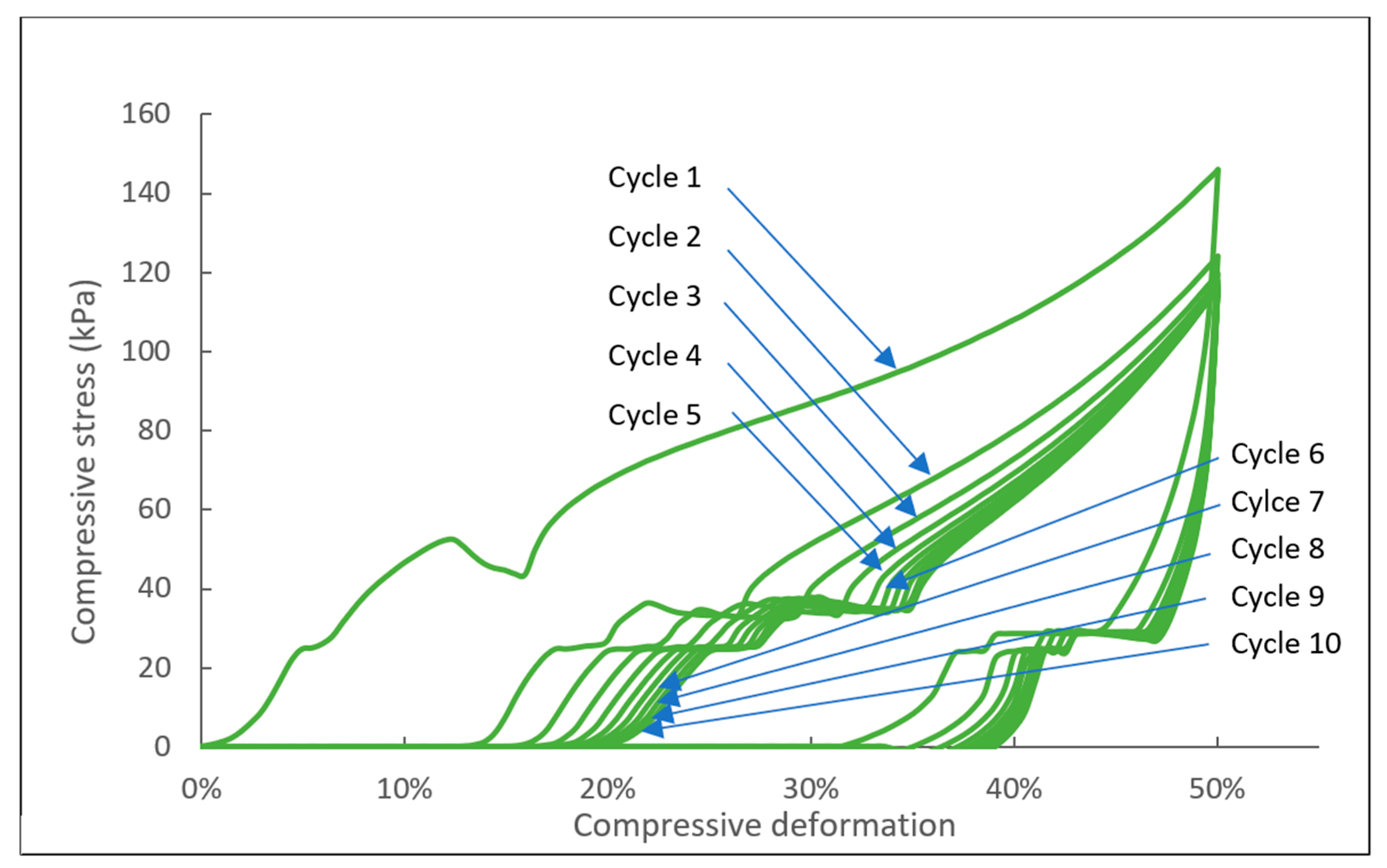
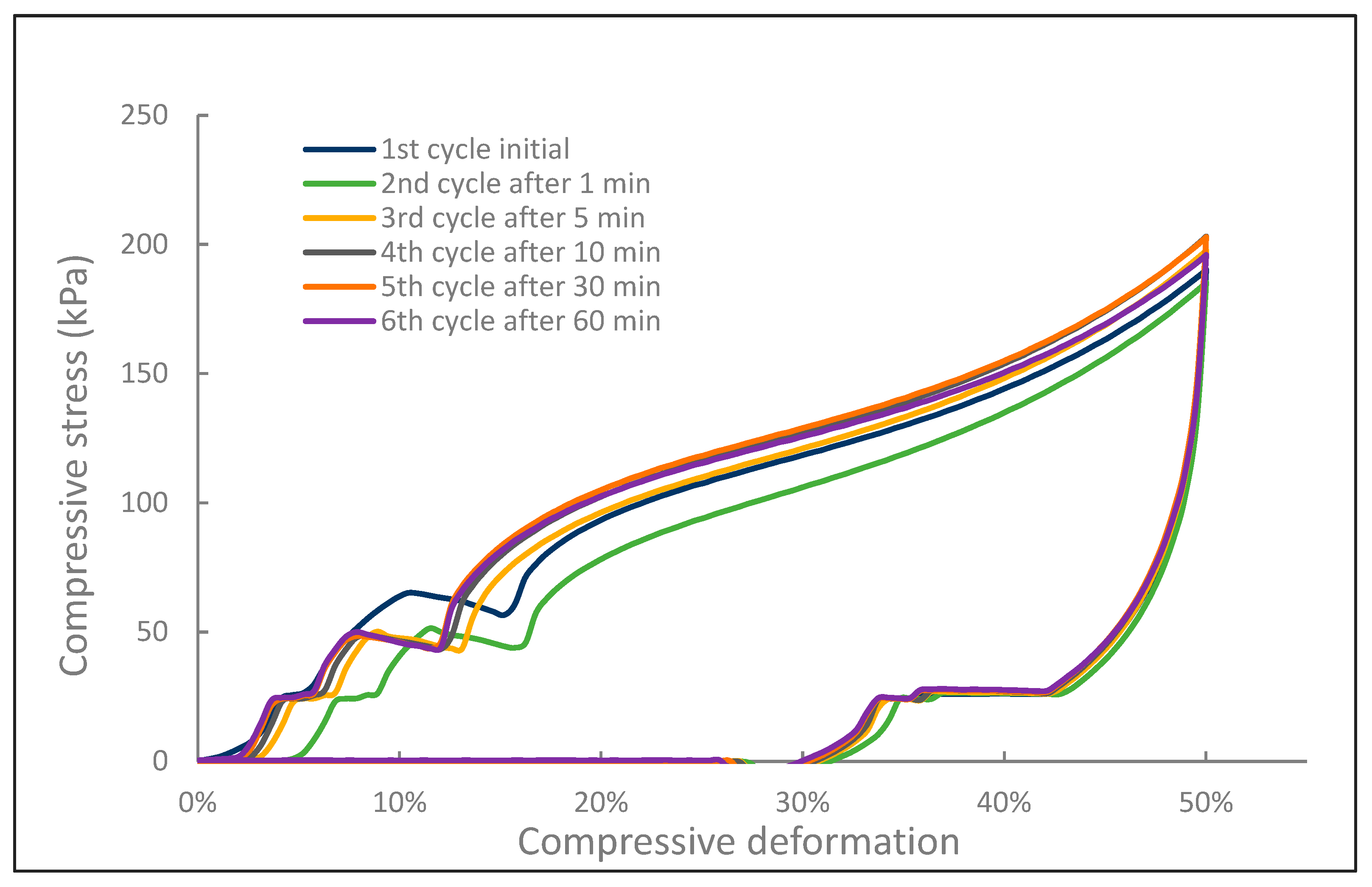
2.2.3. Mechanical Tests
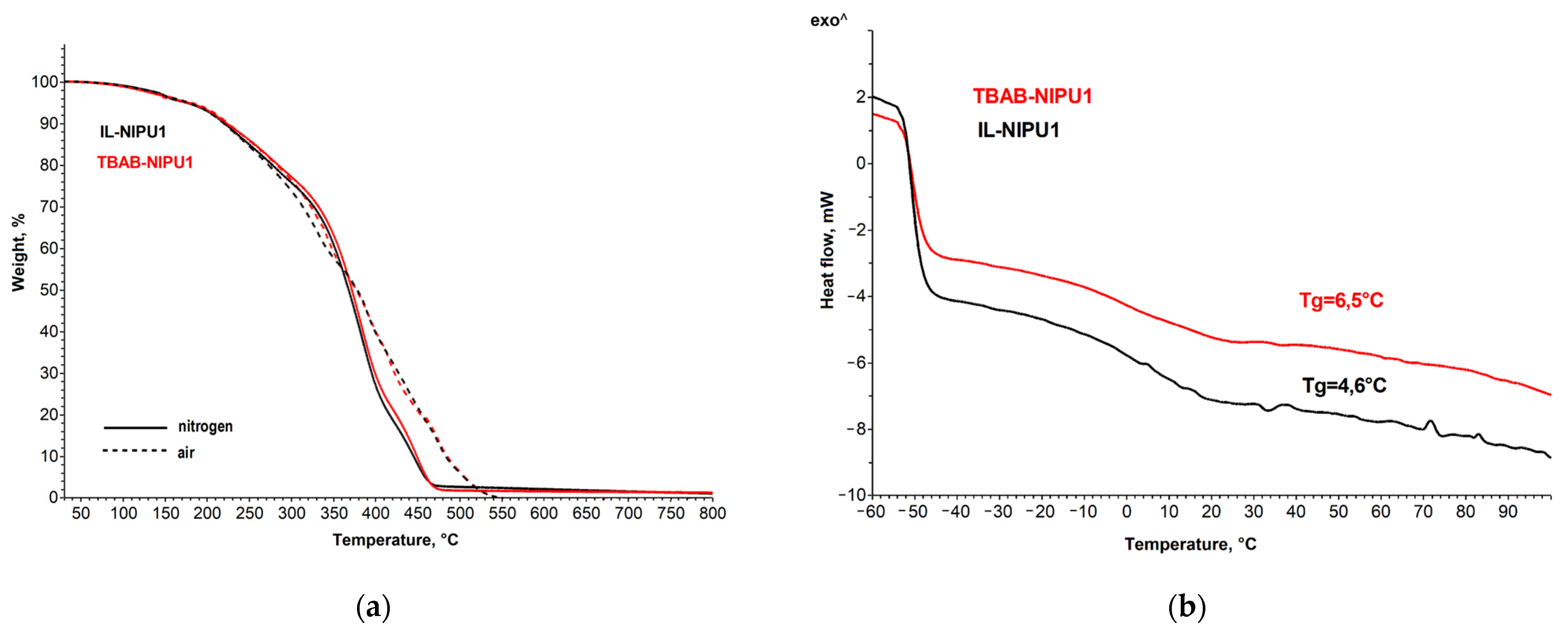
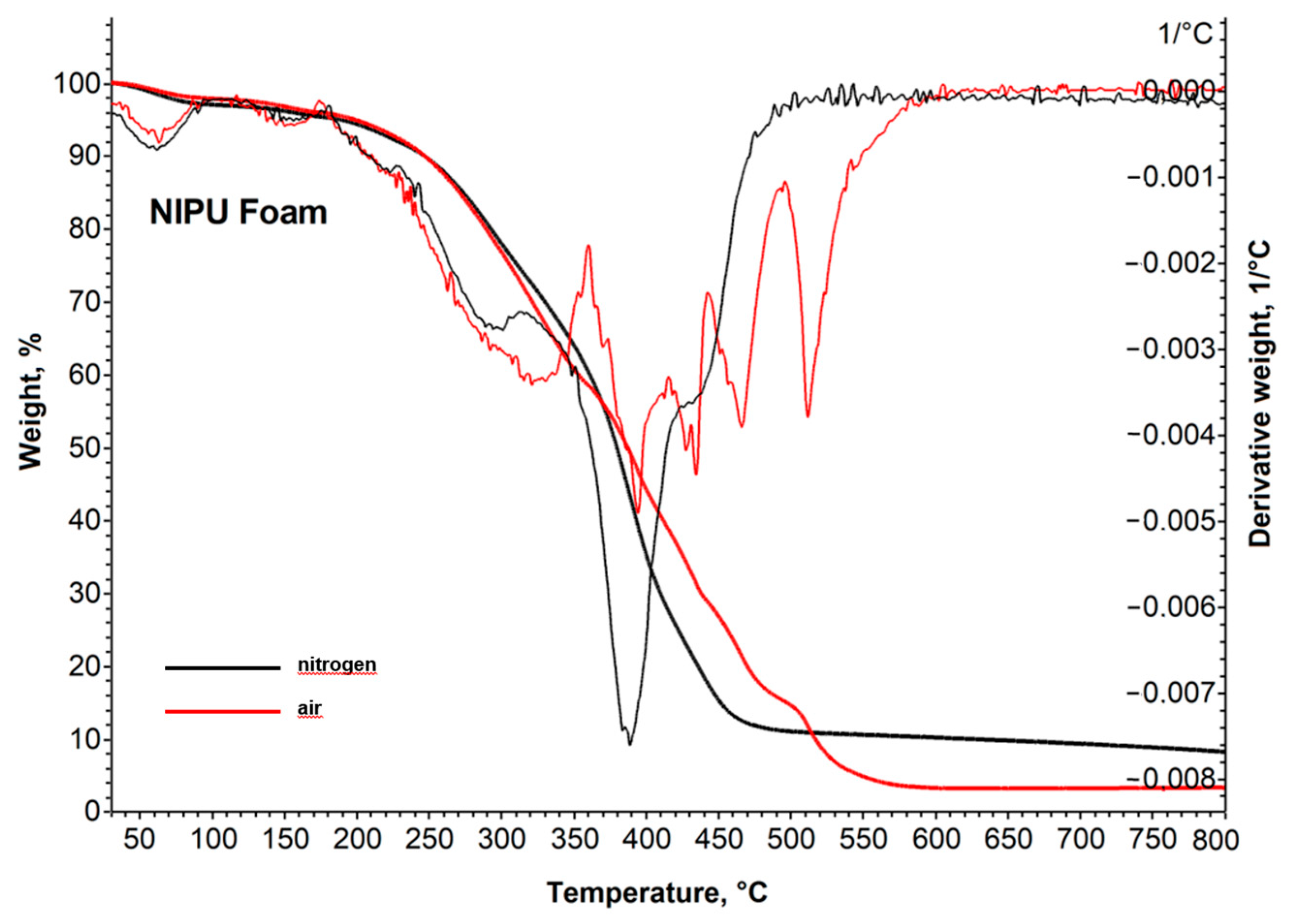
2.2.4. Thermal Stability
2.2.5. Influence of Solvents
2.2.6. Morphology Analysis
3. Materials and Methods
3.1. Materials
3.2. Analysis of Materials
3.3. Procedure for Cycloaddition of CO2 to Epoxidized Vegetable Oil
3.4. Procedure for Synthesis of NIPU Foams
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Islam, M.R.; Jeyaratnama, N.; Yuvara, A.R. Polyurethane types, synthesis and applications—A review. RSC Adv. 2016, 6, 14453–114482. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2021, 3, 93–101. [Google Scholar] [CrossRef]
- de Souza, F.M.; Gupta, R.K.; Kahol, P.K. Introduction to Polyurethane Chemistry. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; Gupta, R.K., Kahol, P.K., Eds.; American Chemical Society: Washington, DC, USA, 2021; Volume 1380, pp. 1–24. [Google Scholar]
- Rokicki, G.; Parzuchowski, P.; Mazurek, M. Non-isocyanate polyurethanes: Synthesis, properties, and applications. Polym. Adv. Technol. 2015, 26, 707–761. [Google Scholar] [CrossRef]
- Khatoon, H.; Iqbal, S.; Irfan, M.; Darda, A.; Rawat, N.K. A review on the production, properties and applications of non-isocyanate polyurethane: A greener perspective. Prog. Org. Coat. 2021, 154, 106124. [Google Scholar] [CrossRef]
- Rane, A.V.; Abitha, V.K.; Patil, S.; Jayaja, P. A greener and sustainable approach for converting polyurethane foam rejects into superior polyurethane coatings. Chem. Int. 2015, 1, 184–195. [Google Scholar]
- Guan, J.; Song, Y.; Lin, Y.; Yin, X.; Zuo, M.; Zhao, Y.; Tao, X.; Zheng, Q. Progress in study of non-isocyanate polyurethane. Ind. Eng. Chem. Res. 2011, 50, 6517–6527. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, L.; Dai, J.; Qu, J. Synthesis and properties of fluorinated nonisocyanate polyurethanes coatings with good hydrophobic and oleophobic properties. J. Coat. Technol. Res. 2019, 16, 1233–1241. [Google Scholar] [CrossRef]
- Rayung, M.; Ghani, N.A.; Hasanudin, N. A review on vegetable oil-based non isocyanate polyurethane: Towards a greener and sustainable production route. RSC Adv. 2024, 14, 9273–9299. [Google Scholar] [CrossRef] [PubMed]
- Turnaturi, R.; Zagni, C.; Floresta, G.; Patamia, V.; Barbera, V.; Rescifina, A. CO2-derived non-isocyanate polyurethanes (NIPUs) and their potential applications. Green Chem. 2023, 25, 9574–9602. [Google Scholar] [CrossRef]
- Błażek, K.; Datta, J. Renewable natural resources as green alternative substrates to obtain bio-based non-isocyanate polyurethanes-review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 173–211. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Sylvain Caillol, S. A Perspective Approach to Sustainable Routes for Non-Isocyanate Polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Delavarde, A.; Savin, G.; Derkenne, P.; Boursier, M.; Morales-Cerrada, R.; Nottelet, B.; Pinaud, J.; Caillol, S. Sustainable polyurethanes: Toward new cutting-edge opportunities. Prog. Polym. Sci. 2024, 151, 101. [Google Scholar] [CrossRef]
- Datta, J.; Włoch, M. Progress in non-isocyanate polyurethanes synthesized from cyclic carbonate intermediates and di- or polyamines in the context of structure–properties relationship and from an environmental point of view. Polym. Bull. 2016, 73, 1459–1469. [Google Scholar] [CrossRef]
- Theerathanagorn, T.; Kessaratikoon, T.; Rehman, H.; D’Elia, V.; Crespy, D. Polyhydroxyurethanes from Biobased Monomers and CO2: A Bridge between Sustainable Chemistry and CO2 Utilization. Chinese J. Chem. 2024, 42, 652–685. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Bio-based routes to synthesize cyclic carbonates and polyamines precursors of non-isocyanate polyurethanes: A review. Eur. Polym. J. 2019, 118, 668–684. [Google Scholar] [CrossRef]
- Haniffa, C.; Munawar, K.; Ching, Y.C.; Illias, H.A.; Chuah, C.H. Bio-based Poly(hydroxy urethane)s: Synthesis and Pre/Post-Functionalization. Chem. Asian J. 2021, 16, 1281–1297. [Google Scholar] [CrossRef]
- Poussard, L.; Mariage, J.; Grignard, B.; Detrembleur, C.; Jérôme, C.; Calberg, C.; Heinrichs, B.; De Winter, J.; Gerbaux, P.; Raquez, J.M.; et al. Non-Isocyanate Polyurethanes from Carbonated Soybean Oil Using Monomeric or Oligomeric Diamines to Achieve Thermosets or Thermoplastics. Macromolecules 2016, 49, 2162–2171. [Google Scholar] [CrossRef]
- Yang, W.; Qiu, S.; Zhang, J.; Cheng, Z.; Song, L.; Hu, Y. Innovative design and green synthesis of bio-based non-isocyanate polyurethanes: Efficient combination of cardanol and carbon dioxide with high fire safety and robust adhesion. Chem. Eng. J. 2024, 482, 148846. [Google Scholar] [CrossRef]
- Pfister, D.P.; Xia, Y.; Larock, R.C. Recent Advances in Vegetable Oil-Based Polyurethanes. ChemSusChem 2011, 4, 703–717. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Z.; Zhang, Y.; Chang, C.; Xu, C. Synthesis of Biobased Polyurethane Foams From Agricultural and Forestry Wastes. In Reactive and Functional Polymers; Gutiérrez, T.J., Ed.; Springer Nature: Cham, Switzerland, 2020; Volume 1, pp. 137–156. [Google Scholar]
- Mao, H.I.; Chen, C.W.; Yan, H.C.; Rwe, S.P. Synthesis and characteristics of nonisocyanate polyurethane composed of bio-based dimer diamine for supercritical CO2 foaming applications. J. Appl. Polym. Sci. 2022, 139, e52841. [Google Scholar] [CrossRef]
- Catalá, J.; Guerra, I.; García-Vargas, J.M.; Ramos, J.M.; García, M.T.; Rodríguez, J.F. Tailor-Made Bio-Based Non-Isocyanate Polyurethanes (NIPUs). Polymers 2023, 15, 1589. [Google Scholar] [CrossRef]
- Kotyrba, Ł.; Chrobok, A.; Siewniak, A. Synthesis of Propylene Carbonate by Urea Alcoholysis—Recent Advances. Catalysts 2022, 12, 309. [Google Scholar] [CrossRef]
- Rehman, A.; Saleem, F.; Javed, F.; Ikhlaq, A.; Ahmad, S.W.; Harvey, A. Recent advances in the synthesis of cyclic carbonates via CO2 cycloaddition to epoxides. J. Environ. Chem. Eng. 2021, 9, 105113. [Google Scholar] [CrossRef]
- Das, S.; D’Elia, V.; He, L.N.; Kleij, A.W.; Yamada, T. Carbon dioxide chemistry towards carbon-neutrality. Green Chem. Eng. 2022, 3, 93–95. [Google Scholar] [CrossRef]
- Carré, C.; Ecochard, Y.; Caillol, S.; Avérous, L. From the Synthesis of Biobased Cyclic Carbonate to Polyhydroxyurethanes: A Promising Route towards Renewable Non-Isocyanate Polyurethanes. ChemSusChem 2019, 12, 3410–3430. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Fujita, S.; Arai, M. Development in the green synthesis of cyclic carbonate from carbon dioxide using ionic liquids. J. Organomet. Chem. 2005, 690, 3490–3497. [Google Scholar] [CrossRef]
- Li, Z.J.; Sun, J.F.; Xu, Q.Q.; Yin, J.Z. Homogeneous and Heterogeneous Ionic Liquid System: Promising “Ideal Catalysts” for the Fixation of CO2 into Cyclic Carbonates. ChemCatChem 2021, 13, 1848–1866. [Google Scholar] [CrossRef]
- Long, G.; Wu, D.; Pan, H.; Zhao, T.; Hu, X. Imidazolium hydrogen carbonate ionic liquids: Versatile organocatalysts for chemical conversion of CO2 into valuable chemicals. J. CO2 Util. 2020, 39, 101155. [Google Scholar] [CrossRef]
- Siewniak, A.; Forajter, A.; Szymanska, K. Mesoporous Silica-Supported Ionic Liquids as Catalysts for Styrene Carbonate Synthesis from CO2. Catalysts 2020, 10, 1363. [Google Scholar] [CrossRef]
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514–1539. [Google Scholar] [CrossRef]
- He, Q.; O’Brien, J.W.; Kitselman, K.A.; Tompkins, L.E.; Curtis, G.C.T.; Kerton, F.M. Synthesis of cyclic carbonates from CO2 and epoxides using ionic liquids and related catalysts including choline chloride–metal halide mixtures. Catal. Sci. Technol. 2014, 4, 1513. [Google Scholar] [CrossRef]
- Xu, B.H.; Wang, J.Q.; Sun, J.; Huang, Y.; Zhang, J.P.; Zhang, X.P.; Zhang, S.J. Fixation of CO2 into cyclic carbonates catalyzed by ionic liquids: A multi-scale approach. Green Chem. 2015, 17, 108–122. [Google Scholar] [CrossRef]
- De Sarasa Buchaca, M.M.; De la Cruz-Martínez, F.; Francés-Poveda, E.; Fernández-Baeza, E.J.; Sánchez-Barba, L.F.; Garcés, A.; Castro-Osma, J.A.; Lara-Sánchez, A. Synthesis of Nonisocyanate Poly(hydroxy)urethanes from Bis(cyclic carbonates) and Polyamines. Polymers 2022, 14, 2719. [Google Scholar] [CrossRef]
- Cornille, A.; Dworakowska, S.; Bogdal, D.; Boutevin, B.; Caillol, S. A new way of creating cellular polyurethane materials: NIPU foams. Eur. Polym. J. 2015, 66, 129–138. [Google Scholar] [CrossRef]
- Cornille, A.; Guillet, C.; Benyahya, S.; Negrell, C.; Boutevin, B.; Caillol, S. Room temperature flexible isocyanate-free polyurethane foams. Eur. Polym. J. 2016, 84, 873–888. [Google Scholar] [CrossRef]
- Blattmann, H.; Lauth, M.; Mülhaupt, R. Flexible and Bio-Based Nonisocyanate Polyurethane (NIPU) Foams. Macromol. Mater. Eng. 2016, 301, 944–952. [Google Scholar] [CrossRef]
- Ahmad, Z.; Mahanwar, P. Synthesis and properties of foams from a blend of vegetable oil based polyhydroxyurethane and epoxy resin. Cell. Polym. 2022, 41, 163–186. [Google Scholar] [CrossRef]
- Lambeth, R.H.; Rizvi, A. New kinds of lignin/non-isocyanate polyurethane hybrid polymers: Facile synthesis, smart properties and adhesive applications. Polymer 2019, 183, 121881. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, S.; Ma, X.; Liang, S.; Jiang, T.; Han, B. Synthesis of cyclic carbonates from carbon dioxide and epoxides over betaine-based catalysts. J. Mol. Catal. A Chem. 2008, 284, 52–57. [Google Scholar] [CrossRef]
- Sun, J.; Ren, J.; Zhang, S.; Cheng, W. Water as an efficient medium for the synthesis of cyclic carbonate. Tetrahedron Lett. 2009, 50, 423–426. [Google Scholar] [CrossRef]
- ASTM D3574-05—Standard Test Methods for Flexible Cellular Materials—Slab, Bonded, and Molded Urethane Foams. Available online: https://www.astm.org/d3574-05.html (accessed on 27 July 2024).
- PN-EN ISO 3001:2002 PLASTICS—Epoxy Compounds—Determination of Epoxy Equivalent. Available online: https://sklep.pkn.pl/pn-iso-6286-1994p.html?options=cart (accessed on 27 July 2024).
- PN-86/C-89085/06; Epoxy Resins—Methods of Testing—Determination of Viscosity. Available online: https://sklep.pkn.pl/pn-iso-6286-1994p.html?options=cart (accessed on 27 July 2024).
- PN-ISO 6286:1994 Molecular absorption spectrometry—Vocabulary—General—Apparatus. Available online: https://sklep.pkn.pl/pn-iso-6286-1994p.html?options=cart (accessed on 27 July 2024).

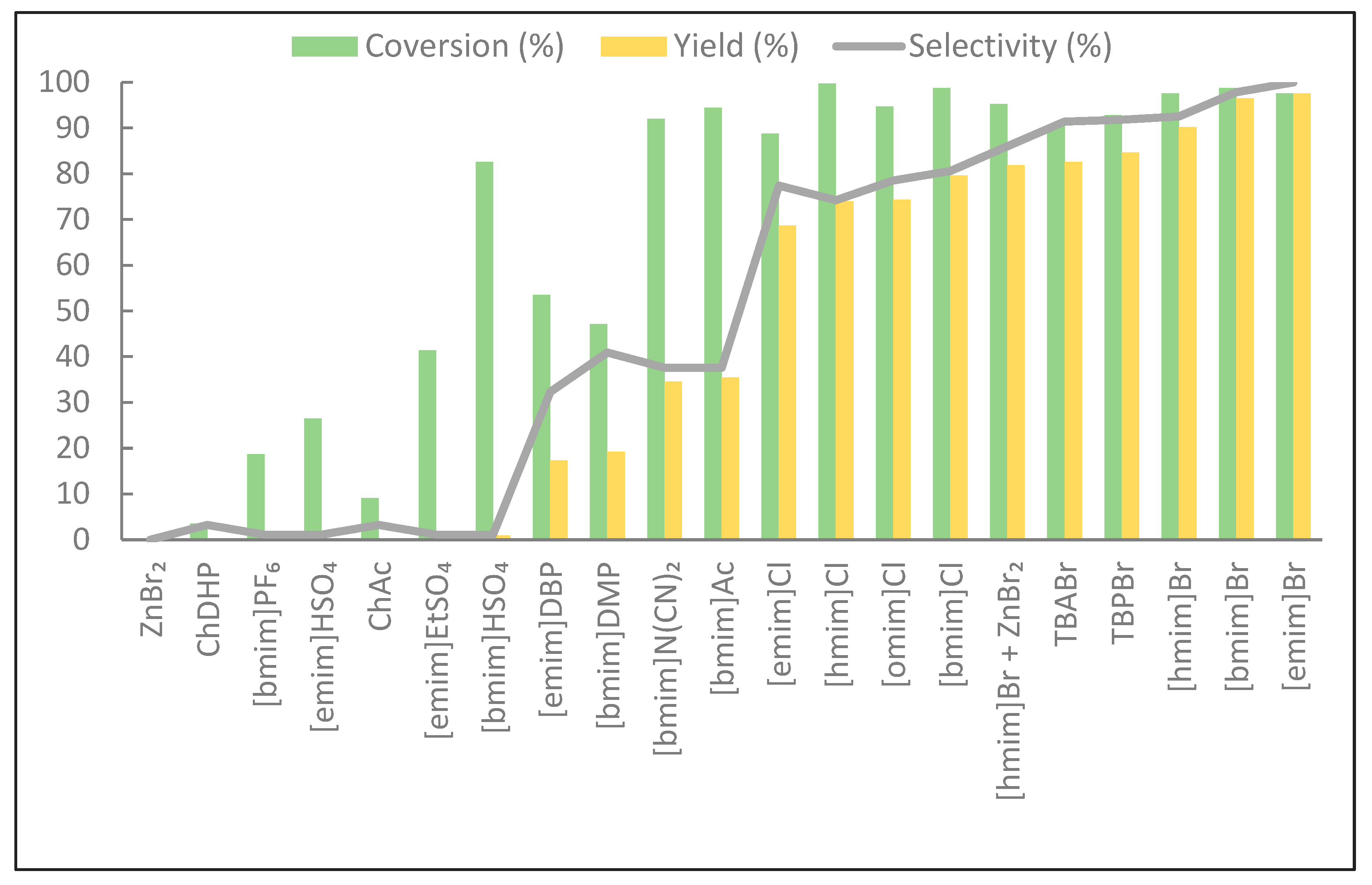





| Catalyst | % mol of Catalyst | Reaction Time/EV [h/(mol/100 g)] | Viscosity [mPa·s] | ||||
|---|---|---|---|---|---|---|---|
| 4.5 | 12 | 19 | 26 | 33 | |||
| [bmim]HSO4 | 12.37 | 0.336 | 0.283 | 0.189 | 0.105 | 0.065 | − |
| [bmim]PF6 | 10.28 | 0.318 | 0.316 | 0.313 | 0.306 | 0.304 | − |
| [bmim]N(CN)2 | 14.24 | 0.353 | 0.301 | 0.237 | 0.111 | 0.030 | 83,700 |
| [bmim]DMP | 11.06 | 0.355 | 0.312 | 0.269 | 0.238 | 0.198 | 6100 |
| [bmim]Ac | 14.74 | 0.338 | 0.134 | 0.071 | 0.021 | − | 28,800 |
| [bmim]Br | 13.34 | 0.307 | 0.237 | 0.107 | 0.005 | − | 54,500 |
| [bmim]Cl | 16.73 | 0.091 | 0.017 | 0.005 | − | − | 44,400 |
| [emim]Cl | 19.93 | 0.246 | 0.126 | 0.042 | 0.005 | − | 39,200 |
| [hmim]Cl | 14.41 | 0.124 | 0.076 | 0.001 | − | − | 37,200 |
| [omim]Cl | 12.66 | 0.212 | 0.080 | 0.020 | 0.009 | − | 38,900 |
| [emim]EtSO4 | 12.37 | 0.363 | 0.329 | 0.317 | 0.243 | 0.219 | − |
| [emim]DBP | 9.12 | 0.322 | 0.314 | 0.263 | 0.212 | 0.174 | 17,500 |
| [emim]HSO4 | 14.03 | 0.348 | 0.340 | 0.300 | 0.275 | 0.232 | 1460 |
| [emim]Br | 15.29 | 0.305 | 0.215 | 0.101 | 0.009 | − | 63,800 |
| [hmim]Br | 11.82 | 0.321 | 0.099 | 0.009 | − | − | 29,800 |
| [hmim]Br + ZnBr2 | 12.64 | 0.317 | 0.185 | 0.046 | 0.018 | − | 21,100 |
| ZnBr2 | 12.98 | 0.231 | 0.222 | 0.200 | − | − | gelled |
| ChDHP | 14.53 | 0.362 | 0.361 | 0.361 | − | − | − |
| ChAc | 17.90 | 0.340 | 0.340 | − | − | − | − |
| THPBr | 6.47 | 0.237 | 0.150 | 0.056 | 0.036 | − | 40,100 |
| TBABr | 9.06 | 0.301 | 0.198 | 0.054 | 0.027 | − | 30,800 |
| Cycle Number | CFD (kPa) | Elastic Recovery (%) |
|---|---|---|
| 1 | 146 | 100.0 |
| 2 | 124 | 75.5 |
| 3 | 120 | 70.9 |
| 4 | 118 | 68.1 |
| 5 | 117 | 66.9 |
| 6 | 116 | 66.0 |
| 7 | 116 | 65.0 |
| 8 | 116 | 64.1 |
| 9 | 116 | 64.0 |
| 10 | 116 | 63.1 |
| Component | IL-NIPU1 [g] | IL-NIPU1 [wt%] | IL-NIPU2 [g] | IL-NIPU2 [wt%] | IL-NIPU3 [g] | IL-NIPU3 [wt%] |
|---|---|---|---|---|---|---|
| IL-CSBO | 30.00 | 78.43 | 30.00 | 76.34 | 30.00 | 74.35 |
| Tegostab B8406 (surfactant) | 0.30 | 0.78 | 0.30 | 0.76 | 0.30 | 0.74 |
| AZO/ZnO (blowing agent) | 1.95 | 5.10 | 3.00 | 7.63 | 4,05 | 10.04 |
| DETA | 6.00 | 15.69 | 6.00 | 15.27 | 6.00 | 14.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiełkiewicz, D.; Siewniak, A.; Gaida, R.; Greif, M.; Chrobok, A. Ionic Liquid Catalysis in Cyclic Carbonate Synthesis for the Development of Soybean Oil-Based Non-Isocyanate Polyurethane Foams. Molecules 2024, 29, 3908. https://doi.org/10.3390/molecules29163908
Kiełkiewicz D, Siewniak A, Gaida R, Greif M, Chrobok A. Ionic Liquid Catalysis in Cyclic Carbonate Synthesis for the Development of Soybean Oil-Based Non-Isocyanate Polyurethane Foams. Molecules. 2024; 29(16):3908. https://doi.org/10.3390/molecules29163908
Chicago/Turabian StyleKiełkiewicz, Damian, Agnieszka Siewniak, Rafał Gaida, Małgorzata Greif, and Anna Chrobok. 2024. "Ionic Liquid Catalysis in Cyclic Carbonate Synthesis for the Development of Soybean Oil-Based Non-Isocyanate Polyurethane Foams" Molecules 29, no. 16: 3908. https://doi.org/10.3390/molecules29163908
APA StyleKiełkiewicz, D., Siewniak, A., Gaida, R., Greif, M., & Chrobok, A. (2024). Ionic Liquid Catalysis in Cyclic Carbonate Synthesis for the Development of Soybean Oil-Based Non-Isocyanate Polyurethane Foams. Molecules, 29(16), 3908. https://doi.org/10.3390/molecules29163908








