Novel Approach for the Preparation of a Highly Hydrophobic Coating Material Exhibiting Self-Healing Properties
Abstract
1. Introduction
2. Results and Discussion
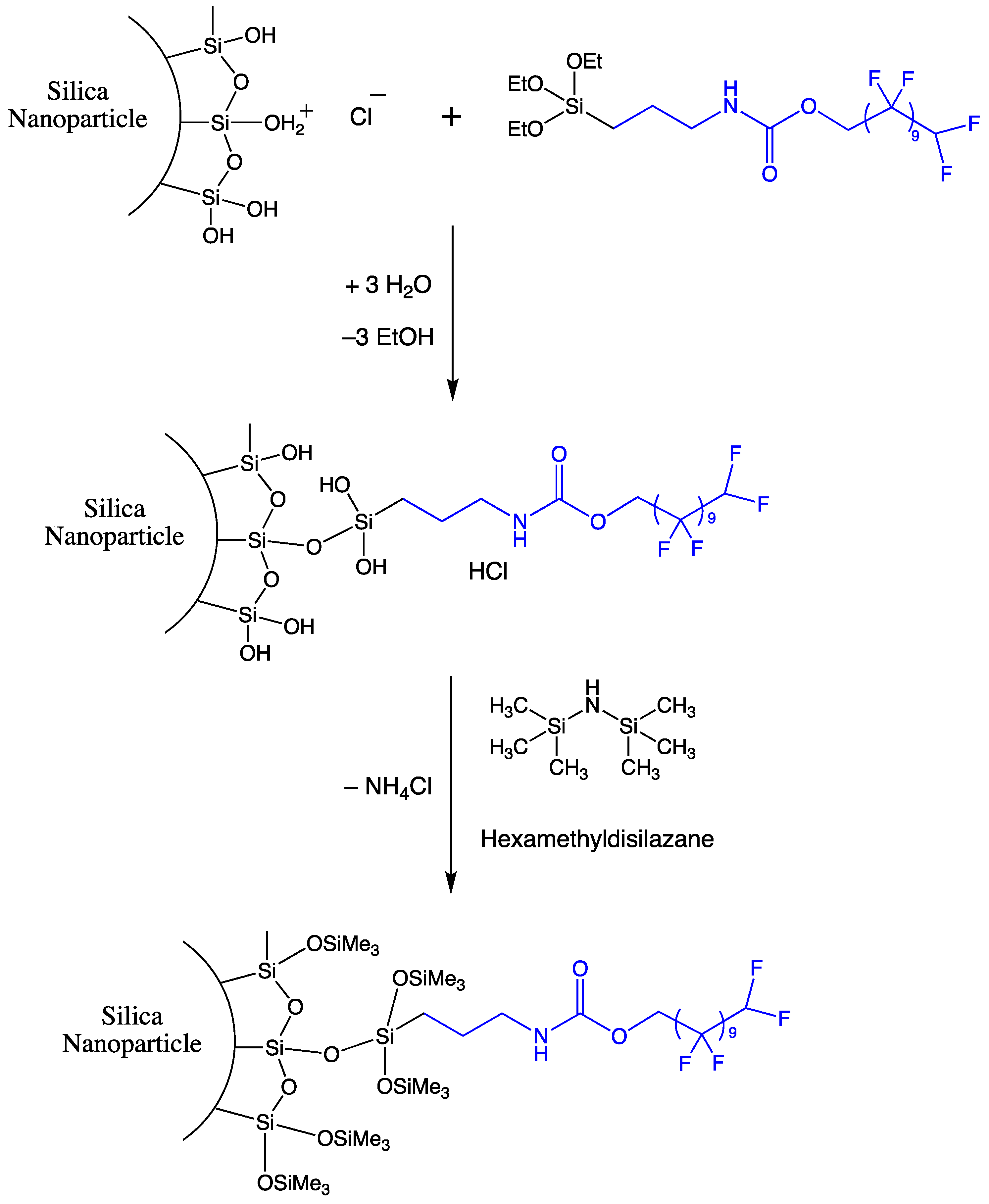
2.1. Preparation of Hydrophobic Nano Particles
2.2. Preparation of the Highly Repellent Wax
2.3. Preparation and Application of the Composite
2.4. Investigation of the Expected Inclusion of Wax Molecules
3. Materials and Methods
3.1. Materials
3.2. Analytic
3.3. Plasma Etching
3.4. Preparation of Stöber Particles
3.5. Preparation of the Hydrophobic Silane
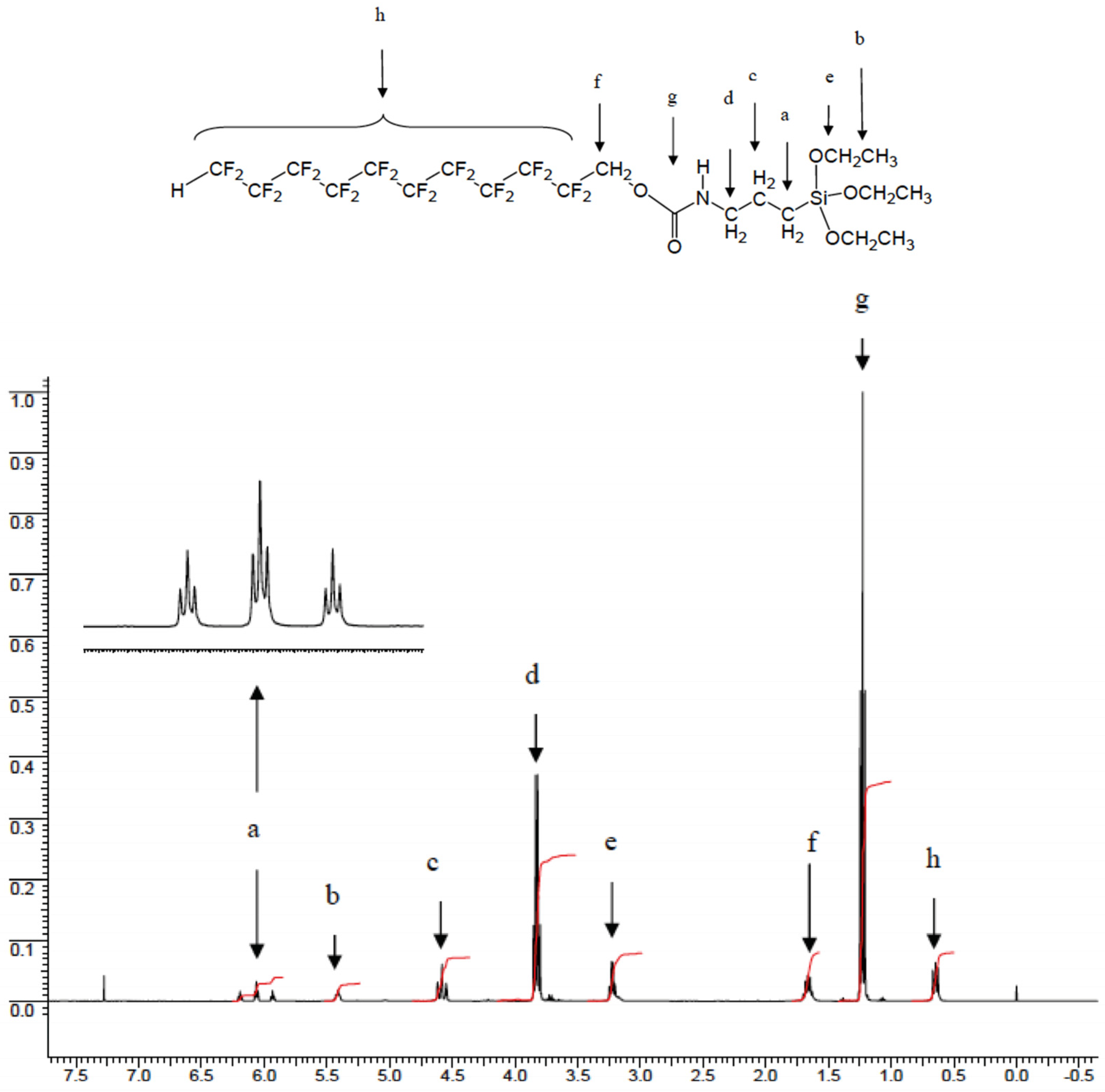
- 1H-NMR: dH (400 MHz; CDCl3; Me4Si): 6.06 (3t, 1H, H-CF2-, 2JH-CF2-CF2- = 51.8; 3J HCF2-CF2- = 5.04); 5.41 (b, 1H, -NH); 4.58 (m, 2H, -CF2-CH2-O-); 3.83 (m, 2H, -O-CH2-CH3); 3.22 (m, 2H, -NH-CH2-); 1.67 (m, 2H, -CH2-CH2-CH2-); 1.23 (t, 2H, -CH3); 0.64 (m, 2H, -CH2-Si).
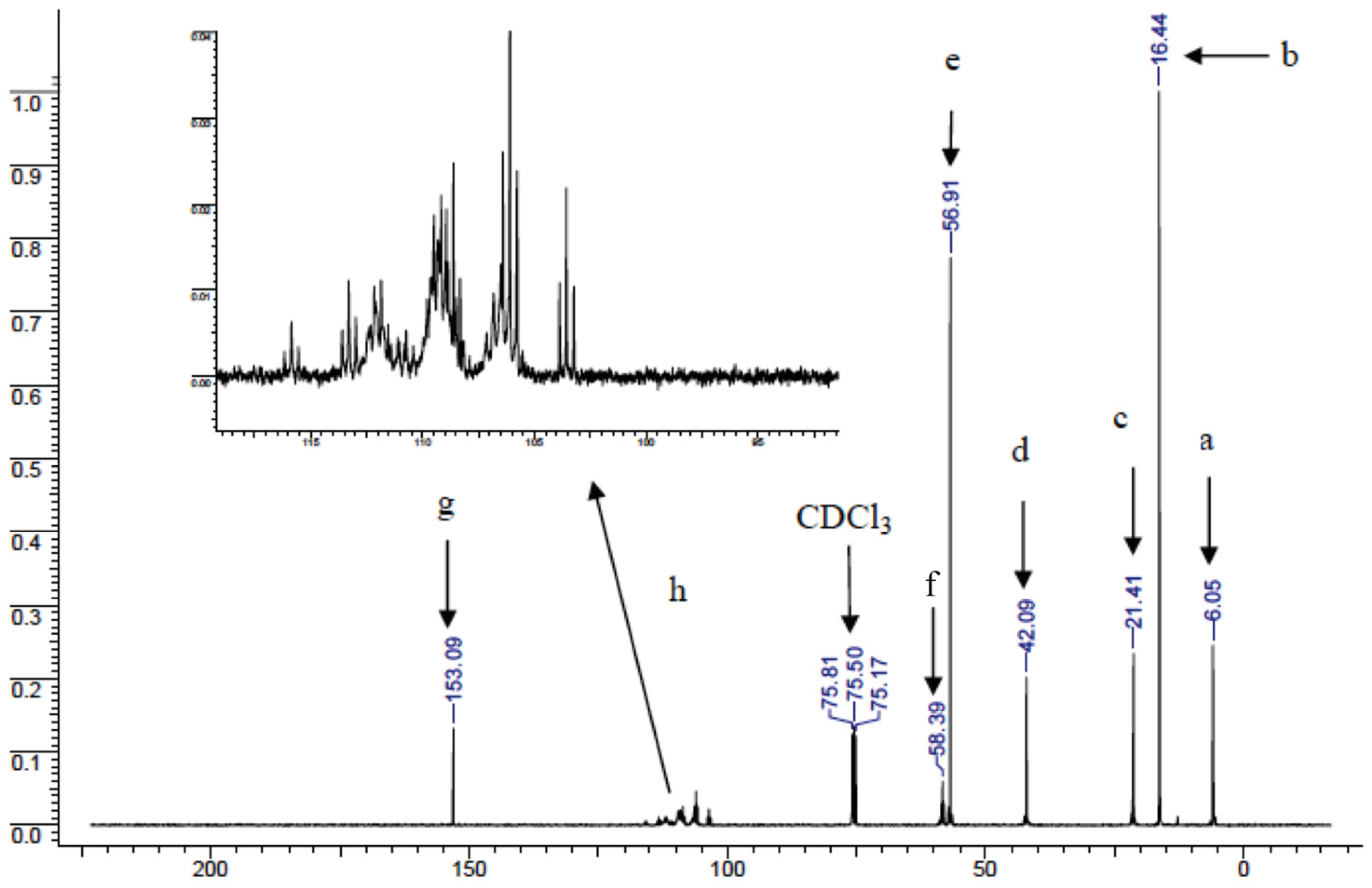
- 13C-NMR: dC (100 MHz, de-coupled, CDCl3, Me4Si): 6.05 (-CH2-Si-); 16.44 (-CH3); 21.41 (-NH-CH2-CH2-); 42.09 ((-NH-CH2-CH2-); 56.91 (Si-O-CH2); 58.39 (-CF2-CH2-O-) 2JFC-CH2- = 104); 103–116 not resolved (HCF2-C10F20-); 153.09 (C=O).
- IR (KBr molding film): νmax/cm−1 3348w (NH); 2978w (CH3), 2932w (-CH2-); 2889w (-CH2-); 1732w (-C=O); 1533w (-C-N); 1445w (-CH2-); 1380w (-CH3); 1206s (-C=O); 1145s (-CF2-); 1103s (-CF2-); 770w (-CF).
3.6. Surface Grafting of Stöber Particles with the Hydrophobic Silane
3.7. Preparation of a Wax Based on Luwax S and the Perfluoroethylene Alcohol (Fluowet EA 812 AC)
3.8. Preparation of an Alternative Wax Based on 2-Butyloctanoic Acid and the Perfluoroethylene Alcohol
- 1H-NMR (400 MHz; CDCl3; ppm): 4.37 (m, 2H, C6F13-CH2-CH2-; 3J = 8); 2.51 (m, 2H, C6F13-CH2-CH2-) 2.35 (2t, 1H, -CH-COOH); 1.49 (m, 2H, C3H7-CH2-); 1.45 (m, 2H, C5H11-CH2-); 1.29 (m, 12H, -CH2-); 0.88 (m, 6H, -CH3).
- 13C-NMR (100 MHz, decoupled, CDCl3; ppm): 12.49 (CH3-C3H6-); 12.65 (CH3-C5H10-); 21.21 (CH3-CH2-C4H8-); 21.24 (CH3-CH2-C2H4-); 26.03 (C4H9-CH2-CH2-); 27.85 (C5H11-CH2-CH-); 29.30 (C2H5-CH2-C2H4-); 28.25 (C3H7-CH2-CH-); 30.55 (C3H7-CH2-C2H4-); 31.03 (C2H5-CH2-C3H6-); 44.28 (CH Methine); 57.34 (Rf-CH2-CH2-); 106–118 (C6F13-); 174.87 (-C=O).
- IR (KBr liquid film; cm−1): 2959 (s) νas -CH3; 2932 (s) νas -CH2-; 2861 (s) νs -CH2-; 1740 (s) ν -C=O carboxylic acid esters; 1464 (m) νas -CH3; 1458; νs -CH2-; 1240 (s) ν -C-O- ester; ν -CF3; 1206 (s) ν -CF3; 1164 (w) ν -CF2-; 1145 (m) ν -CF2-; 733 (w) ν -CH2- rocking; 709 + 700 (w) ν -CF.
3.9. Preparation and Investigation of the Composites
3.10. XRD Measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wenzel, R.N. The Evaluation of Textile Waterproofing Agents. Am. Dyest. Rep. 1936, 25, 505. [Google Scholar]
- Wenzel, R.N. The Evaluation of Textile Waterproofing Agents. III. Measurement of Waterproofness. Am. Dyest. Rep. 1936, 25, 598–601. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Peters, A.M.; Pirat, C.; Sbragaglia, M.; Borkent, B.M.; Wessling, M.; Lohse, D.; Lammertink, R.G.H. Cassie-Baxter to Wenzel State Wetting Transition: Scaling of the Front Velocity. Eur. Phys. J. E 2009, 29, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Hipp, B.; Kunert, I.; Durr, M. Systematic Control of Hydrophobic and Superhydrophobic Properties Using Double-Rough Structures Based on Mixtures of Metal Oxide Nanoparticles. Langmuir 2010, 26, 6557–6560. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B. Biomimetics Inspired Surfaces for Drag Reduction and Oleophobicity/Philicity. Beilstein J. Nanotechnol. 2011, 2, 66–84. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, W.; Su, B.-L. Superhydrophobic Surfaces: From Natural to Biomimetic to Functional. J. Colloid Interface Sci. 2011, 353, 335–355. [Google Scholar] [CrossRef]
- Pilotek, S.; Schmidt, H.K. Wettability of Microstructured Hydrophobic Sol-Gel Coatings. J. Sol-Gel Sci. Technol. 2003, 26, 789–792. [Google Scholar] [CrossRef]
- Rodriguez-Hernandez, J.; Muñoz-Bonilla, A.; Bousquet, A.; Ibarboure, E.; Papon, E. Environmentally Responsive Particles: From Superhydrophobic Particle Films to Water-Dispersible Microspheres. Langmuir 2010, 26, 18617–18620. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Wang, X. Fabrication of Superhydrophobic Cellulose-Based Materials through a Solution-Immersion Process. Langmuir 2008, 24, 5585–5590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, D.; Gu, Z.-Z.; Möhwald, H. Fabrication of Superhydrophobic Surfaces from Binary Colloidal Assembly. Langmuir 2005, 21, 9143–9148. [Google Scholar] [CrossRef]
- Wagner, P.; Furstner, R.; Barthlott, W.; Neinhuis, C. Quantitative Assessment to the Structural Basis of Water Repellency in Natural and Technical Surfaces. J. Exp. Bot. 2003, 54, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shu, L.; Hu, Q.; Jiang, X.; Yang, H.; Wang, H.; Rao, L. Mechanism of Self-Recovery of Hydrophobicity after Surface Damage of Lotus Leaf. Plant Methods 2024, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y. Wear, Fatigue, Creep, and Stress-Relaxation Behavior of Polymer Nanocomposites; CRC Press: Boca Raton, FL, USA, 2010; p. 357. [Google Scholar]
- Burris, D.L.; Boesl, B.; Bourne, G.R.; Sawyer, W.G. Polymeric Nanocomposites for Tribological Applications. Macromol. Mater. Eng. 2007, 292, 387–402. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Ruan, W.H. Surface Modification of Nanoscale Fillers for Improving Properties of Polymer Nanocomposites: A Review. Mater. Sci. Technol. 2006, 22, 787–796. [Google Scholar] [CrossRef]
- Zimehl, R.; Textor, T.; Bahners, T.; Schollmeyer, E. From Colloids to Nanotechnology. Progr. Colloid Polym. Sci. 2004, 125, 49–53. [Google Scholar] [CrossRef]
- Tao, J.; Liu, Y.; Li, M.; Li, Z.; Zhang, Y.; Song, X.; Yang, Q.; Guan, F.; Guo, J. Robust Superhydrophobic Composite Fabric with Self-Healing and Chemical Durability. Small 2024, e2304894. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Zhang, W.; Ning, H.; Wang, H.; Liu, Z. Facile Preparation of Durable Superhydrophobic Coating with Microstructure Self-Repairing Function by Spraying Method. Prog. Org. Coat. 2024, 187, 108166. [Google Scholar] [CrossRef]
- Zhou, S.; Jia, X.; Li, S.; Li, Y.; Tian, R.; Song, H. Epoxy/Polytetrafluoro-Wax Composite Coatings Demonstrate Remarkable Wear Resistance, Offering Rapid and Durable Cyclic Self-Healing Capabilities Facilitated by the “Sweating” Behavior of Polytetrafluoro-Wax. Prog. Org. Coat. 2024, 188, 108227. [Google Scholar] [CrossRef]
- Celik, N.; Sahin, F.; Ozel, S.S.; Sezer, G.; Gunaltay, N.; Ruzi, M.; Onses, M.S. Self-Healing of Biocompatible Superhydrophobic Coatings: The Interplay of the Size and Loading of Particles. Langmuir 2023, 39, 3194–3203. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yang, S.; Xiao, X.; Wang, J. Hydrophobic Solid Photothermal Slippery Surfaces with Rapid Self-Repairing, Dual Anti-Icing/Deicing, and Excellent Stability Based on Paraffin and Etching. Langmuir 2024, 40, 7747–7759. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhang, G.; Zhang, Q.; Zhan, X.; Chen, F. Silicone Oil-Infused Slippery Surfaces Based on Sol–Gel Process-Induced Nanocomposite Coatings: A Facile Approach to Highly Stable Bioinspired Surface for Biofouling Resistance. ACS Appl. Mater. Interfaces 2016, 8, 34810–34819. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Guo, Z. Robust Multi-Functional Slippery Surface with Hollow ZnO Nanotube Structures. New J. Chem. 2020, 44, 15483–15491. [Google Scholar] [CrossRef]
- Lorusso, E.; Ali, W.; Hildebrandt, M.; Mayer-Gall, T.; Gutmann, J.S. Hydrogel Functionalized Polyester Fabrics by UV-Induced Photopolymerization. Polymers 2019, 11, 1329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Jia, X.; Tian, R.; Song, H. Self-Lubricating, Self-Healing and Anti-Corrosion Coating with Ultra-Short Run-in Period. Tribol. Int. 2024, 196, 109706. [Google Scholar] [CrossRef]
- Trinh, B.; Owen, P.; vanderHeide, A.; Gupta, A.; Mekonnen, T.H. Recyclable and Self-Healing Natural Rubber Vitrimers from Anhydride-Epoxy Exchangeable Covalent Bonds. ACS Appl. Polym. Mater. 2023, 5, 8890–8906. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.-J.; Luo, Z.-H.; Zhou, Y.-N. Fast Room-temperature Self-healing Vitrimers Enabled by Accelerated Associative Exchange Kinetics. Chem. Eng. J. 2023, 452, 139452. [Google Scholar] [CrossRef]
- Jiang, L.; Tian, Y.; Wang, X.; Zhang, J.; Cheng, J.; Gao, F. A Fully Bio-Based Schiff Base Vitrimer with Self-Healing Ability at Room Temperature. Polym. Chem. 2023, 14, 862–871. [Google Scholar] [CrossRef]
- Matthies, L. Natural Montan Wax and Its Raffinates. Eur. J. Lipid Sci. Technol. 2001, 103, 239–248. [Google Scholar] [CrossRef]
- Presting, W.; Kreuter, T. Zur Kenntnis Der Säuren Des Rohen Montanwachses Und Seiner Raffinate. Fette Seifen Anstrichm. 1962, 64, 695–701. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Döhring, A. A Convenient Protocol for the Esterification of Carboxylic Acids with Alcohols in the Presence of Di-t-Butyl Dicarbonate. Synlett 2004, 35, 0263–0266. [Google Scholar] [CrossRef]
- Hassner, A.; Alexanian, V. Direct Room Temperature Esterification of Carboxylic Acids. Tetrahedron Lett. 1978, 19, 4475–4478. [Google Scholar] [CrossRef]
- Seebach, D.; Hungerbühler, E.; Naef, R.; Schnurrenberger, P.; Weidmann, B.; Züger, M. Titanate-Mediated Transesterifications with Functionalized Substrates. Synthesis 1982, 1982, 138–141. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Gorlov, Y.I.; Nesterenko, A.M.; Chuiko, A.A. The Chemisorption of Acid Chlorides on the Silica Surface. Colloids Surf. A Physicochem. Eng. Asp. 1996, 106, 83–88. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Schöniger, W. Eine Mikroanalytische Schnellbestimmung von Halogenen und Schwefel in Organischen Substanzen. Mikrochimica Acta 1956, 869–876. Available online: https://amuseum.de/chemie/Schoeniger/Publikationen/1956SchnellbestVonHalogenenUndSchwefelinOrgVerb.pdf (accessed on 5 August 2024). [CrossRef]
- Lorenz, K.; Frey, H.; Stühn, B.; Mülhaupt, R. Carbosilane Dendrimers with Perfluoroalkyl End Groups. Core−Shell Macromolecules with Generation-Dependent Order. Macromolecules 1997, 30, 6860–6868. [Google Scholar] [CrossRef]




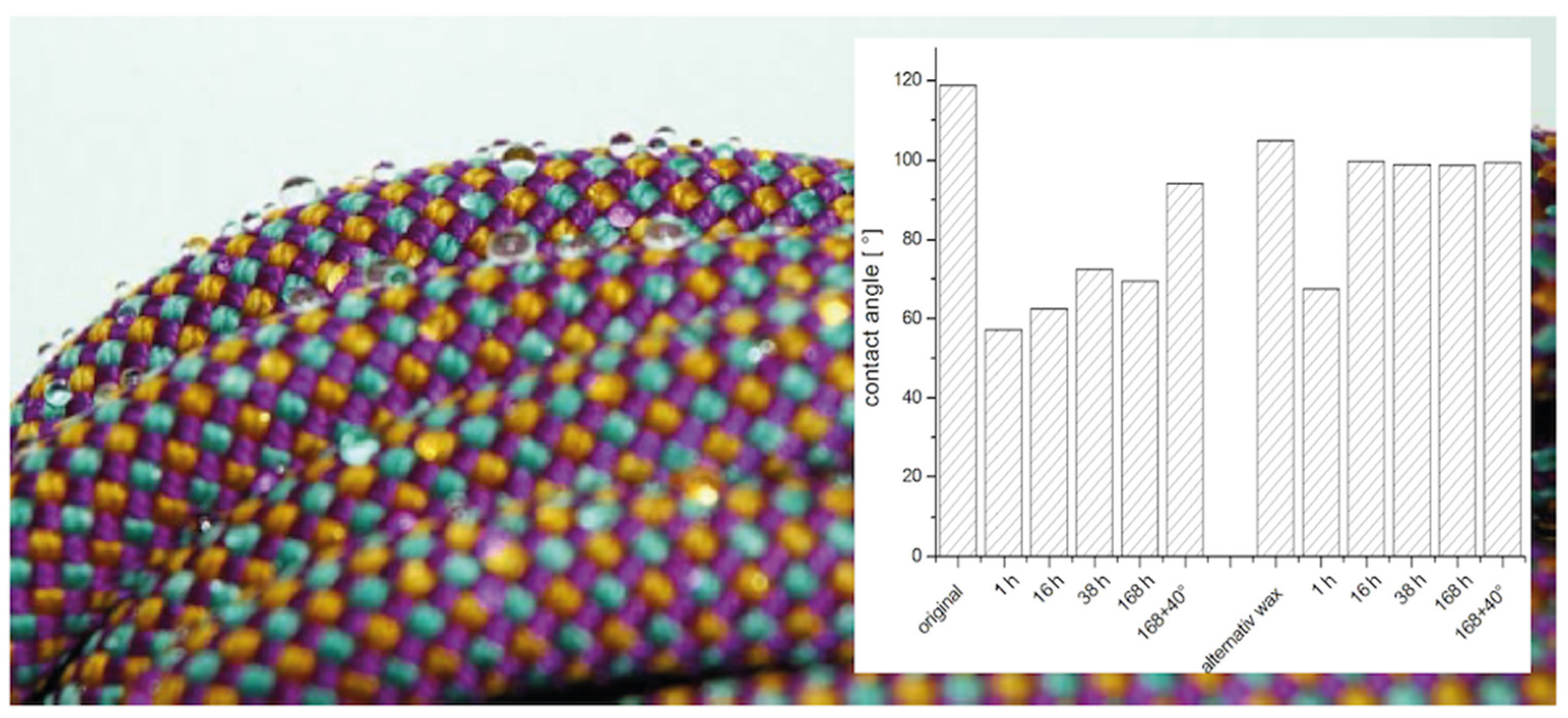

| As Prepared | 1 h | 16 h | 38 h | 168 h | 168 h + Storage at 40 °C | |
|---|---|---|---|---|---|---|
| original (high crystallin) | 119 | 57 | 62 | 73 | 70 | 94 |
| alternative wax (low crystallin) | 105 | 68 | 100 | 100 | 99 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holzdörfer, U.; Ali, W.; Schollmeyer, E.; Gutmann, J.S.; Mayer-Gall, T.; Textor, T. Novel Approach for the Preparation of a Highly Hydrophobic Coating Material Exhibiting Self-Healing Properties. Molecules 2024, 29, 3766. https://doi.org/10.3390/molecules29163766
Holzdörfer U, Ali W, Schollmeyer E, Gutmann JS, Mayer-Gall T, Textor T. Novel Approach for the Preparation of a Highly Hydrophobic Coating Material Exhibiting Self-Healing Properties. Molecules. 2024; 29(16):3766. https://doi.org/10.3390/molecules29163766
Chicago/Turabian StyleHolzdörfer, Uwe, Wael Ali, Eckhard Schollmeyer, Jochen S. Gutmann, Thomas Mayer-Gall, and Torsten Textor. 2024. "Novel Approach for the Preparation of a Highly Hydrophobic Coating Material Exhibiting Self-Healing Properties" Molecules 29, no. 16: 3766. https://doi.org/10.3390/molecules29163766
APA StyleHolzdörfer, U., Ali, W., Schollmeyer, E., Gutmann, J. S., Mayer-Gall, T., & Textor, T. (2024). Novel Approach for the Preparation of a Highly Hydrophobic Coating Material Exhibiting Self-Healing Properties. Molecules, 29(16), 3766. https://doi.org/10.3390/molecules29163766









