Exploring the Mutated Kinases for Chemoenzymatic Synthesis of N4-Modified Cytidine Monophosphates
Abstract
1. Introduction
2. Results and Discussion
2.1. Substrates Exploited in the Study
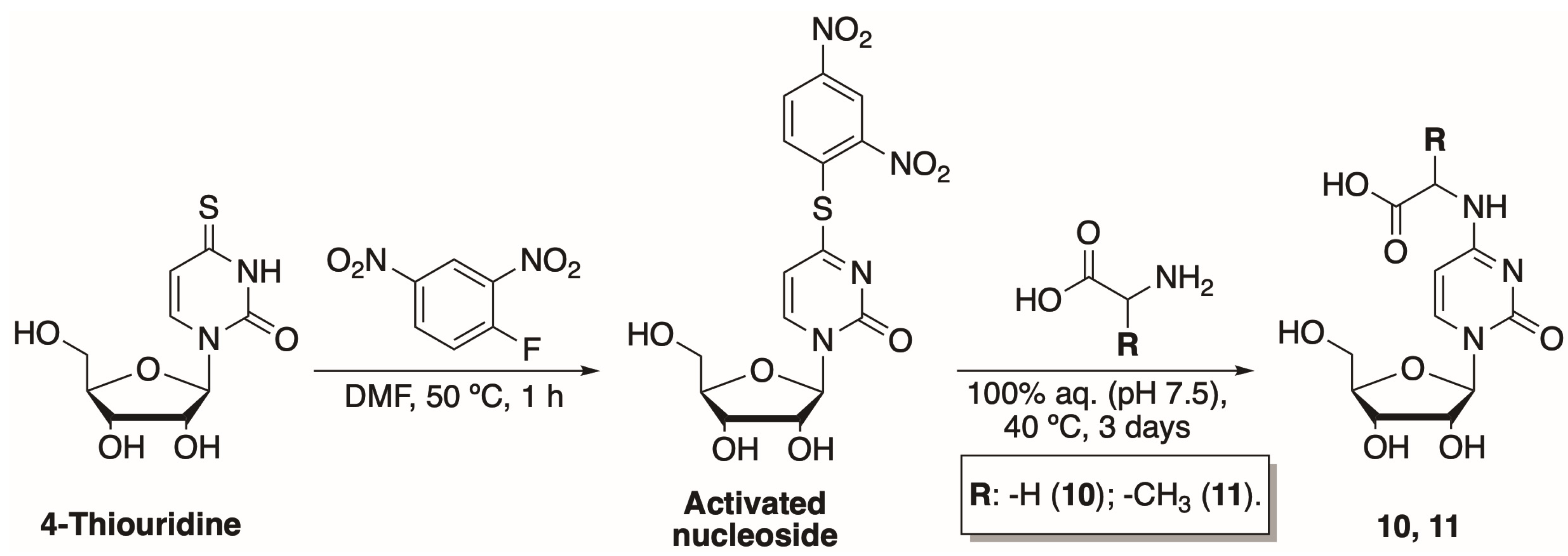
2.1.1. Synthesis of N-[1-(β-D-Ribofuranosyl)-2-oxo-4-pyrimidinyl]-amino Acids
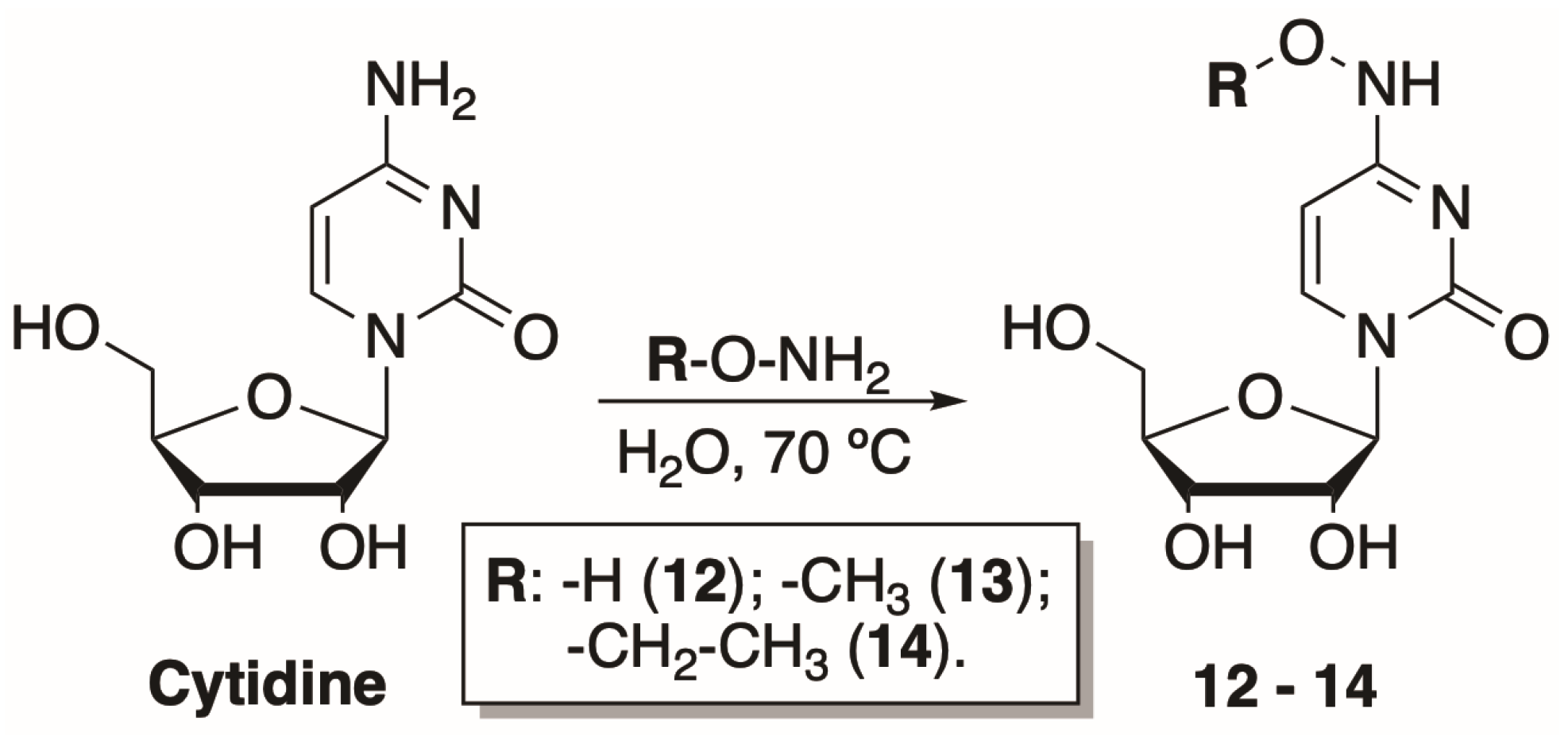
2.1.2. Synthesis of N4-Hydroxy- and N4-Alkoxycytidines
2.2. Synthesis and Application of the Mutant Kinases for the Phosphorylation of Canonical and N4-Modified Cytidine Nucleosides
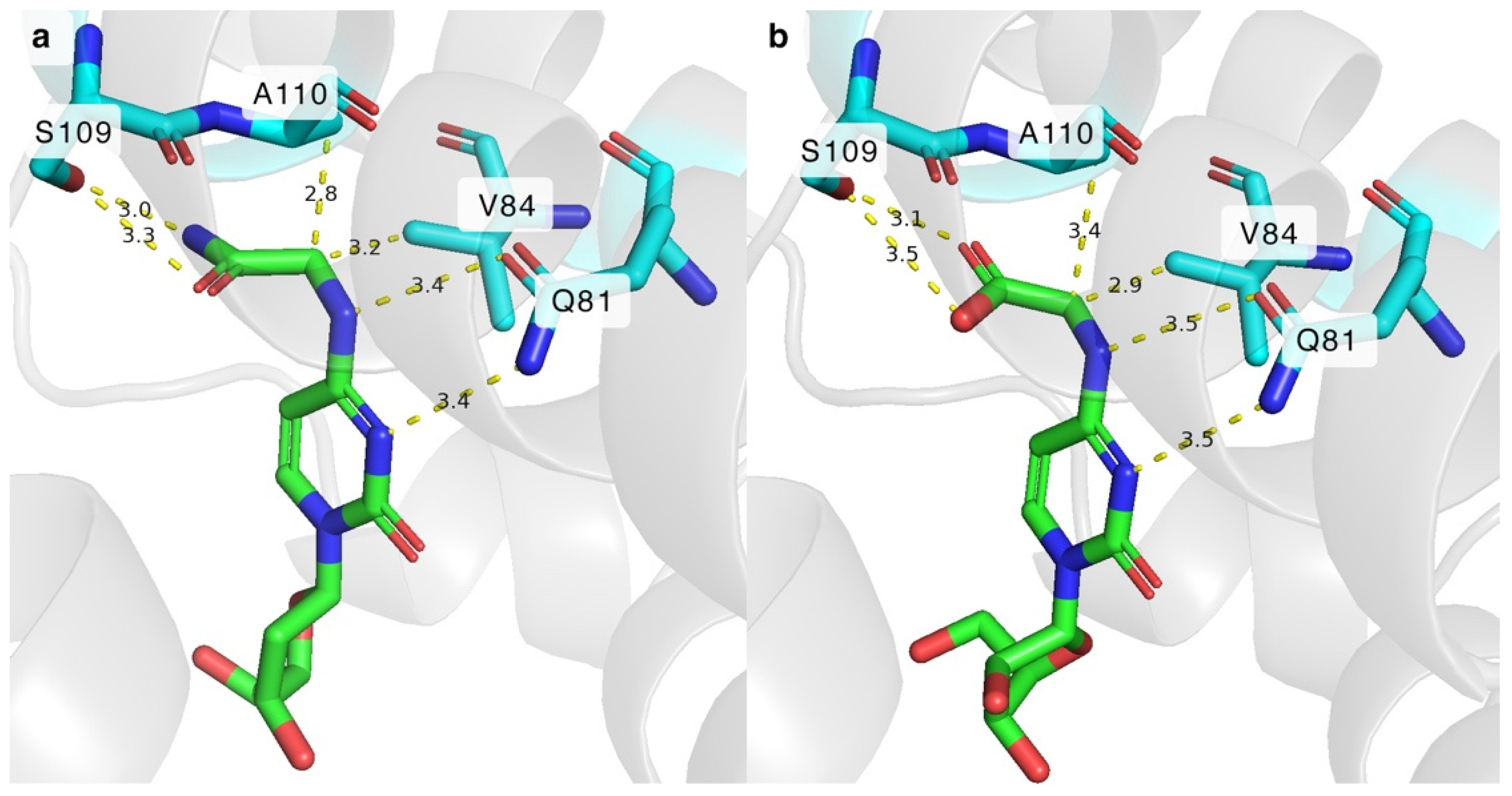
2.3. Mutations in the Hydrophobic Cavity of DmdNK Active Site
2.4. Significance of the Q81 Residue in the Active Site of DmdNK
2.5. Significance of the W57 Residue in the Active Site of DmdNK
2.6. Enhancing BsdCK Substrate Diversity via Single-Site Mutagenesis
2.7. Differences in Phosphorylating N4-Amino Acid Modified Nucleosides Bearing Amide and Carboxyl Functional Groups
3. Materials and Methods
3.1. General Information
3.2. Synthesis of N-[1-(β-D-Ribofuranosyl)-2-oxo-4-pyrimidinyl]-amino Acids
3.3. Synthesis of N4-Hydroxy- and N4-Alkoxycytidines
3.4. Bacterial Strains, Plasmids, and Reagents
3.5. Site-Directed Mutagenesis
3.6. Expression of Target Genes and Purification of Proteins
3.7. Substrate Specificity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saito, Y.; Hudson, R.H.E. Base-Modified Fluorescent Purine Nucleosides and Nucleotides for Use in Oligonucleotide Probes. J. Photochem. Photobiol. C Photochem. Rev. 2018, 36, 48–73. [Google Scholar] [CrossRef]
- Urbelienė, N.; Tiškus, M.; Tamulaitienė, G.; Gasparavičiūtė, R.; Lapinskaitė, R.; Jauniškis, V.; Sūdžius, J.; Meškienė, R.; Tauraitė, D.; Skrodenytė, E.; et al. Cytidine Deaminases Catalyze the Conversion of N(S,O)4-Substituted Pyrimidine Nucleosides. Sci. Adv. 2023, 9, eade4361. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.-G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A Multivalent Nucleoside-Modified MRNA Vaccine against All Known Influenza Virus Subtypes. Science 2022, 378, 899–904. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Kleiner, R.E. Cell- and Polymerase-Selective Metabolic Labeling of Cellular RNA with 2′-Azidocytidine. J. Am. Chem. Soc. 2020, 142, 14417–14421. [Google Scholar] [CrossRef] [PubMed]
- Nainar, S.; Cuthbert, B.J.; Lim, N.M.; England, W.E.; Ke, K.; Sophal, K.; Quechol, R.; Mobley, D.L.; Goulding, C.W.; Spitale, R.C. An Optimized Chemical-Genetic Method for Cell-Specific Metabolic Labeling of RNA. Nat. Methods 2020, 17, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Zenchenko, A.A.; Drenichev, M.S.; Il’icheva, I.A.; Mikhailov, S.N. Antiviral and Antimicrobial Nucleoside Derivatives: Structural Features and Mechanisms of Action. Mol. Biol. 2021, 55, 786–812. [Google Scholar] [CrossRef] [PubMed]
- Guinan, M.; Benckendorff, C.; Smith, M.; Miller, G.J. Recent Advances in the Chemical Synthesis and Evaluation of Anticancer Nucleoside Analogues. Molecules 2020, 25, 2050. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Depaix, A.; Périgaud, C.; Peyrottes, S. Recent Trends in Nucleotide Synthesis. Chem. Rev. 2016, 116, 7854–7897. [Google Scholar] [CrossRef]
- Slot Christiansen, L.; Munch-Petersen, B.; Knecht, W. Non-Viral Deoxyribonucleoside Kinases—Diversity and Practical Use. J. Genet. Genom. 2015, 42, 235–248. [Google Scholar] [CrossRef]
- Sandrini, M.P.B.; Piškur, J. Deoxyribonucleoside Kinases: Two Enzyme Families Catalyze the Same Reaction. Trends Biochem. Sci. 2005, 30, 225–228. [Google Scholar] [CrossRef]
- Deville-Bonne, D.; El Amri, C.; Meyer, P.; Chen, Y.; Agrofoglio, L.A.; Janin, J. Human and Viral Nucleoside/Nucleotide Kinases Involved in Antiviral Drug Activation: Structural and Catalytic Properties. Antivir. Res. 2010, 86, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M.F.; Winiger, C.B.; Shaw, R.W.; Kim, M.-J.; Kim, M.-S.; Daugherty, A.B.; Chen, F.; Moussatche, P.; Moses, J.D.; Lutz, S.; et al. A Single Deoxynucleoside Kinase Variant from Drosophila Melanogaster Synthesizes Monophosphates of Nucleosides That Are Components of an Expanded Genetic System. ACS Synth. Biol. 2017, 6, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Abraham, C.; Suslov, O.; Yaren, O.; Shaw, R.W.; Kim, M.-J.; Wan, S.; Marliere, P.; Benner, S.A. Synthetic Biology Pathway to Nucleoside Triphosphates for Expanded Genetic Alphabets. ACS Synth. Biol. 2023, 12, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Van Rompay, A.R.; Norda, A.; Lindén, K.; Johansson, M.; Karlsson, A. Phosphorylation of Uridine and Cytidine Nucleoside Analogs by Two Human Uridine-Cytidine Kinases. Mol. Pharmacol. 2001, 59, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Boison, D. Adenosine Kinase: Exploitation for Therapeutic Gain. Pharmacol. Rev. 2013, 65, 906–943. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Iida, A.; Teshiba, S.; Fujio, T. Cloning of a Guanosine-Inosine Kinase Gene of Escherichia Coli and Characterization of the Purified Gene Product. J. Bacteriol. 1995, 177, 4921–4926. [Google Scholar] [CrossRef] [PubMed]
- Hellendahl, K.F.; Kamel, S.; Wetterwald, A.; Neubauer, P.; Wagner, A. Human Deoxycytidine Kinase Is a Valuable Biocatalyst for the Synthesis of Nucleotide Analogues. Catalysts 2019, 9, 997. [Google Scholar] [CrossRef]
- Wild, K.; Bohner, T.; Folkers, G.; Schulz, G.E. The Structures of Thymidine Kinase from Herpes Simplex Virus Type 1 in Complex with Substrates and a Substrate Analogue. Protein Sci. 1997, 6, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Munch-Petersen, B.; Knecht, W.; Lenz, C.; Søndergaard, L.; Piškur, J. Functional Expression of a Multisubstrate Deoxyribonucleoside Kinase from Drosophila Melanogaster and Its C-Terminal Deletion Mutants. J. Biol. Chem. 2000, 275, 6673–6679. [Google Scholar] [CrossRef]
- Andersen, R.B.; Neuhard, J. Deoxynucleoside Kinases Encoded by the YaaG AndyaaF Genes of Bacillus Subtilis. J. Biol. Chem. 2001, 276, 5518–5524. [Google Scholar] [CrossRef]
- Johansson, M.; Van Rompay, A.R.; Degrève, B.; Balzarini, J.; Karlsson, A. Cloning and Characterization of the Multisubstrate Deoxyribonucleoside Kinase of Drosophila Melanogaster. J. Biol. Chem. 1999, 274, 23814–23819. [Google Scholar] [CrossRef]
- Wu, Y.; Fa, M.; Tae, E.L.; Schultz, P.G.; Romesberg, F.E. Enzymatic Phosphorylation of Unnatural Nucleosides. J. Am. Chem. Soc. 2002, 124, 14626–14630. [Google Scholar] [CrossRef]
- Koplūnaitė, M.; Butkutė, K.; Špelveris, D.; Urbelienė, N.; Meškys, R. Enzymatic Synthesis of Modified Nucleoside 5′-Monophosphates. Catalysts 2022, 12, 1401. [Google Scholar] [CrossRef]
- Serra, I.; Conti, S.; Piškur, J.; Clausen, A.R.; Munch-Petersen, B.; Terreni, M.; Ubiali, D. Immobilized Drosophila Melanogaster Deoxyribonucleoside Kinase (Dm DNK) as a High Performing Biocatalyst for the Synthesis of Purine Arabinonucleotides. Adv. Synth. Catal. 2014, 356, 563–570. [Google Scholar] [CrossRef]
- Johansson, K.; Ramaswamy, S.; Ljungcrantz, C.; Knecht, W.; Piskur, J.; Munch-Petersen, B.; Eriksson, S.; Eklund, H. Structural Basis for Substrate Specificities of Cellular Deoxyribonucleoside Kinases. Nat. Struct. Biol. 2001, 8, 616–620. [Google Scholar] [CrossRef]
- Mikkelsen, N.E.; Johansson, K.; Karlsson, A.; Knecht, W.; Andersen, G.; Piškur, J.; Munch-Petersen, B.; Eklund, H. Structural Basis for Feedback Inhibition of the Deoxyribonucleoside Salvage Pathway: Studies of the Drosophila Deoxyribonucleoside Kinase. Biochemistry 2003, 42, 5706–5712. [Google Scholar] [CrossRef]
- Mikkelsen, N.E.; Munch-Petersen, B.; Eklund, H. Structural Studies of Nucleoside Analog and Feedback Inhibitor Binding to Drosophila Melanogaster Multisubstrate Deoxyribonucleoside Kinase. FEBS J. 2008, 275, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Møllgaard, H. Deoxyadenosine/Deoxycytidine Kinase from Bacillus Subtilis. Purification, Characterization, and Physiological Function. J. Biol. Chem. 1980, 255, 8216–8220. [Google Scholar] [CrossRef] [PubMed]
- Koplūnaitė, M.; Butkutė, K.; Meškys, R.; Tauraitė, D. Synthesis of Pyrimidine Nucleoside and Amino Acid Conjugates. Tetrahedron Lett. 2020, 61, 152598. [Google Scholar] [CrossRef]
- Ohashi, S.; Nakamura, M.; Acharyya, S.; Inagaki, M.; Abe, N.; Kimura, Y.; Hashiya, F.; Abe, H. Development and Comparison of 4-Thiouridine to Cytidine Base Conversion Reaction. ACS Omega 2024, 9, 9300–9308. [Google Scholar] [CrossRef]
- Paymode, D.J.; Vasudevan, N.; Ahmad, S.; Kadam, A.L.; Cardoso, F.S.P.; Burns, J.M.; Cook, D.W.; Stringham, R.W.; Snead, D.R. Toward a Practical, Two-Step Process for Molnupiravir: Direct Hydroxamination of Cytidine Followed by Selective Esterification. Org. Process Res. Dev. 2021, 25, 1822–1830. [Google Scholar] [CrossRef]
- Knecht, W.; Munch-Petersen, B.; Piškur, J. Identification of Residues Involved in the Specificity and Regulation of the Highly Efficient Multisubstrate Deoxyribonucleoside Kinase from Drosophila Melanogaster. J. Mol. Biol. 2000, 301, 827–837. [Google Scholar] [CrossRef]
- Knecht, W. A Few Amino Acid Substitutions Can Convert Deoxyribonucleoside Kinase Specificity from Pyrimidines to Purines. EMBO J. 2002, 21, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Solaroli, N.; Johansson, M.; Balzarini, J.; Karlsson, A. Enhanced Toxicity of Purine Nucleoside Analogs in Cells Expressing Drosophila Melanogaster Nucleoside Kinase Mutants. Gene Ther. 2007, 14, 86–92. [Google Scholar] [CrossRef][Green Version]
- Chen, F.; Zhang, Y.; Daugherty, A.B.; Yang, Z.; Shaw, R.; Dong, M.; Lutz, S.; Benner, S.A. Biological Phosphorylation of an Unnatural Base Pair (UBP) Using a Drosophila Melanogaster Deoxynucleoside Kinase (DmdNK) Mutant. PLoS ONE 2017, 12, e0174163. [Google Scholar] [CrossRef] [PubMed]
- Solaroli, N.; Bjerke, M.; Amiri, M.H.; Johansson, M.; Karlsson, A. Active Site Mutants of Drosophila Melanogaster Multisubstrate Deoxyribonucleoside Kinase. Eur. J. Biochem. 2003, 270, 2879–2884. [Google Scholar] [CrossRef]
- Knecht, W.; Mikkelsen, N.E.; Clausen, A.R.; Willer, M.; Eklund, H.; Gojković, Z.; Piškur, J. Drosophila Melanogaster Deoxyribonucleoside Kinase Activates Gemcitabine. Biochem. Biophys. Res. Commun. 2009, 382, 430–433. [Google Scholar] [CrossRef]
- Egeblad-Welin, L.; Sonntag, Y.; Eklund, H.; Munch-Petersen, B. Functional Studies of Active-site Mutants from Drosophila Melanogaster Deoxyribonucleoside Kinase. FEBS J. 2007, 274, 1542–1551. [Google Scholar] [CrossRef]
- Larsen, P.O. Physical and Chemical Properties of Amino Acids. In Amino Acids and Derivatives; Elsevier: Amsterdam, The Netherlands, 1980; pp. 225–269. [Google Scholar]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making Protein Folding Accessible to All. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Stärk, H.; Jing, B.; Barzilay, R.; Jaakkola, T. DiffDock: Diffusion Steps, Twists, and Turns for Molecular Docking. arXiv 2022, arXiv:2210.01776. [Google Scholar]
- Huang, Y.; Zhang, L. An In Vitro Single-Primer Site-Directed Mutagenesis Method for Use in Biotechnology. Methods Mol. Biol. 2017, 1498, 375–383. [Google Scholar] [PubMed]
- Fehlau, M.; Kaspar, F.; Hellendahl, K.F.; Schollmeyer, J.; Neubauer, P.; Wagner, A. Modular Enzymatic Cascade Synthesis of Nucleotides Using a (d)ATP Regeneration System. Front. Bioeng. Biotechnol. 2020, 8, 854. [Google Scholar] [CrossRef]
- Zou, Z.; Ding, Q.; Ou, L.; Yan, B. Efficient Production of Deoxynucleoside-5′-Monophosphates Using Deoxynucleoside Kinase Coupled with a GTP-Regeneration System. Appl. Microbiol. Biotechnol. 2013, 97, 9389–9395. [Google Scholar] [CrossRef] [PubMed]
- Van Rompay, A.R.; Johansson, M.; Karlsson, A. Phosphorylation of Nucleosides and Nucleoside Analogs by Mammalian Nucleoside Monophosphate Kinases. Pharmacol. Ther. 2000, 87, 189–198. [Google Scholar] [CrossRef]
- Lascu, I.; Gonin, P. The Catalytic Mechanism of Nucleoside Diphosphate Kinases. J. Bioenerg. Biomembr. 2000, 32, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Chen, Y.; Feng, J.Y.; Borroto-Esoda, K.; Deville-Bonne, D.; Janin, J.; Moréra, S. Nucleoside Diphosphate Kinase and the Activation of Antiviral Phosphonate Analogs of Nucleotides: Binding Mode and Phosphorylation of Tenofovir Derivatives. Nucleosides Nucleotides Nucleic Acids 2009, 28, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, M.; Zhang, Y.; Zhong, L.; Zheng, X. Novel Combination Oncolytic Adenoviral Gene Therapy Armed with Dm-DNK and CD40L for Breast Cancer. Curr. Gene Ther. 2019, 19, 54–65. [Google Scholar] [CrossRef]
- Fillat, C.; Carrio, M.; Cascante, A.; Sangro, B. Suicide Gene Therapy Mediated by the Herpes Simplex Virus Thymidine Kinase Gene/Ganciclovir System: Fifteen Years of Application. Curr. Gene Ther. 2003, 3, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Zhao, L.; Zhu, Z.; Liu, Q.; Xu, H.; Johansson, M.; Karlsson, A.; Zheng, X. The Multisubstrate Deoxyribonucleoside Kinase of Drosophila Melanogaster as a Therapeutic Suicide Gene of Breast Cancer Cells. J. Gene Med. 2011, 13, 305–311. [Google Scholar] [CrossRef]
- Knecht, W.; Rozpedowska, E.; Le Breton, C.; Willer, M.; Gojkovic, Z.; Sandrini, M.P.B.; Joergensen, T.; Hasholt, L.; Munch-Petersen, B.; Piskur, J. Drosophila Deoxyribonucleoside Kinase Mutants with Enhanced Ability to Phosphorylate Purine Analogs. Gene Ther. 2007, 14, 1278–1286. [Google Scholar] [CrossRef]

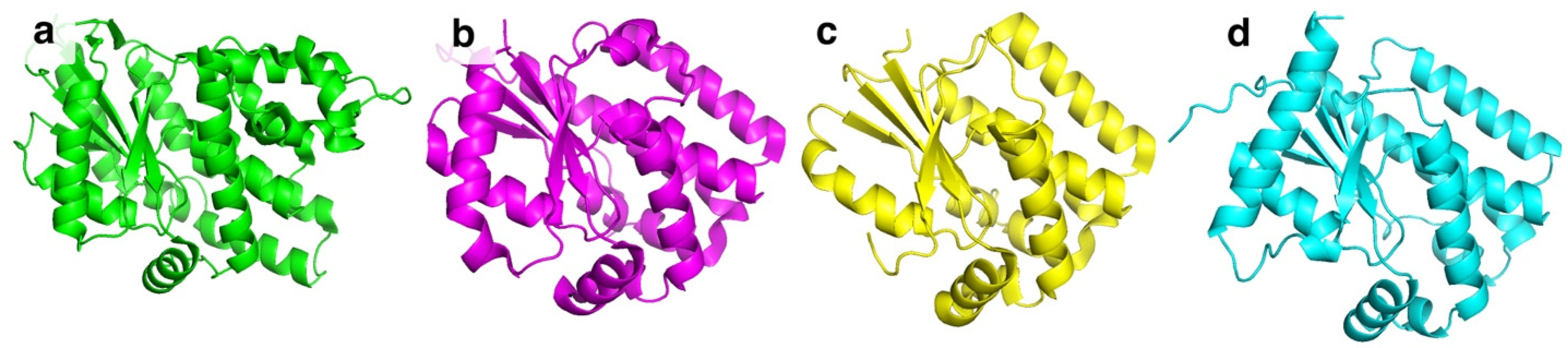

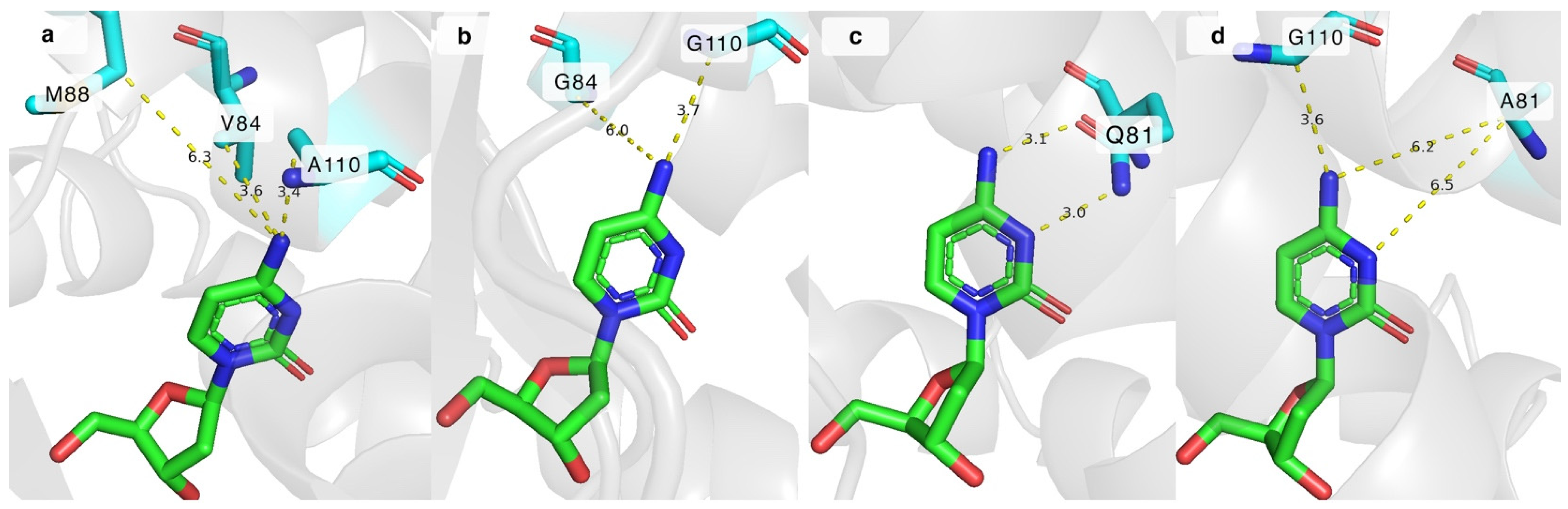


| Substrate | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DmdNK | |||||||||||||||
| WT | 98 | 78 | 31 | 0 | 3 | 0 | 0 | 100 | 0 | 0 | 0 | 46 | 5 | 0 | |
| W57F | 89 | 51 | 1 | 0 | 0 | 0 | 0 | 27 | 1 | 0 | 0 | 6 | 0 | 0 | |
| W57V | 89 | 6 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Q81A | 88 | 14 | 20 | 0 | 0 | 0 | 0 | 100 | 9 | 0 | 0 | 2 | 11 | 4 | |
| Q81A+V84G | 92 | 2 | 82 | 0 | 31 | 0 | 0 | 88 | 61 | 0 | 0 | 0 | 4 | 3 | |
| Q81A+M88G | 94 | 4 | 7 | 0 | 1 | 0 | 0 | 93 | 3 | 0 | 0 | 0 | 6 | 1 | |
| Q81A+A110G | 93 | 13 | 98 | 0 | 55 | 0 | 0 | 100 | 87 | 0 | 0 | 11 | 30 | 30 | |
| V84A | 97 | 75 | 95 | 0 | 75 | 0 | 0 | 100 | 68 | 0 | 0 | 24 | 8 | 2 | |
| V84A+M88A | 98 | 71 | 60 | 0 | 56 | 0 | 0 | 100 | 0 | 0 | 0 | 1 | 3 | 2 | |
| V84A+A110D | 22 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| V84G | 88 | 41 | 98 | 0 | 84 | 0 | 0 | 100 | 94 | 0 | 0 | 4 | 6 | 2 | |
| M88A | 79 | 47 | 14 | 0 | 1 | 0 | 0 | 54 | 4 | 0 | 0 | 9 | 0 | 0 | |
| M88G | 87 | 30 | 5 | 0 | 1 | 0 | 0 | 29 | 2 | 0 | 0 | 6 | 0 | 0 | |
| M88R | 34 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| M88R+A110G | 91 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| A110D | 96 | 10 | 11 | 0 | 0 | 0 | 0 | 27 | 0 | 0 | 0 | 0 | 0 | 0 | |
| A110G | 89 | 65 | 98 | 0 | 33 | 0 | 0 | 100 | 30 | 0 | 0 | 8 | 4 | 0 | |
| Substrate | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BsdCK | |||||||||||||||
| WT | 100 | 93 | 11 | 0 | 0 | 0 | 0 | 95 | 2 | 0 | 0 | 18 | 1 | 7 | |
| R70M | 7 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| R70M+D93A | 100 | 55 | 98 | 0 | 37 | 0 | 0 | 99 | 98 | 0 | 0 | 9 | 0 | 1 | |
| D93A | 100 | 5 | 20 | 0 | 16 | 0 | 0 | 97 | 79 | 0 | 0 | 2 | 0 | 0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koplūnaitė, M.; Butkutė, K.; Stankevičiūtė, J.; Meškys, R. Exploring the Mutated Kinases for Chemoenzymatic Synthesis of N4-Modified Cytidine Monophosphates. Molecules 2024, 29, 3767. https://doi.org/10.3390/molecules29163767
Koplūnaitė M, Butkutė K, Stankevičiūtė J, Meškys R. Exploring the Mutated Kinases for Chemoenzymatic Synthesis of N4-Modified Cytidine Monophosphates. Molecules. 2024; 29(16):3767. https://doi.org/10.3390/molecules29163767
Chicago/Turabian StyleKoplūnaitė, Martyna, Kamilė Butkutė, Jonita Stankevičiūtė, and Rolandas Meškys. 2024. "Exploring the Mutated Kinases for Chemoenzymatic Synthesis of N4-Modified Cytidine Monophosphates" Molecules 29, no. 16: 3767. https://doi.org/10.3390/molecules29163767
APA StyleKoplūnaitė, M., Butkutė, K., Stankevičiūtė, J., & Meškys, R. (2024). Exploring the Mutated Kinases for Chemoenzymatic Synthesis of N4-Modified Cytidine Monophosphates. Molecules, 29(16), 3767. https://doi.org/10.3390/molecules29163767






