Mapping Photogenerated Electron–Hole Behavior of Graphene Oxide: Insight into a New Mechanism of Photosensitive Pollutant Degradation
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization
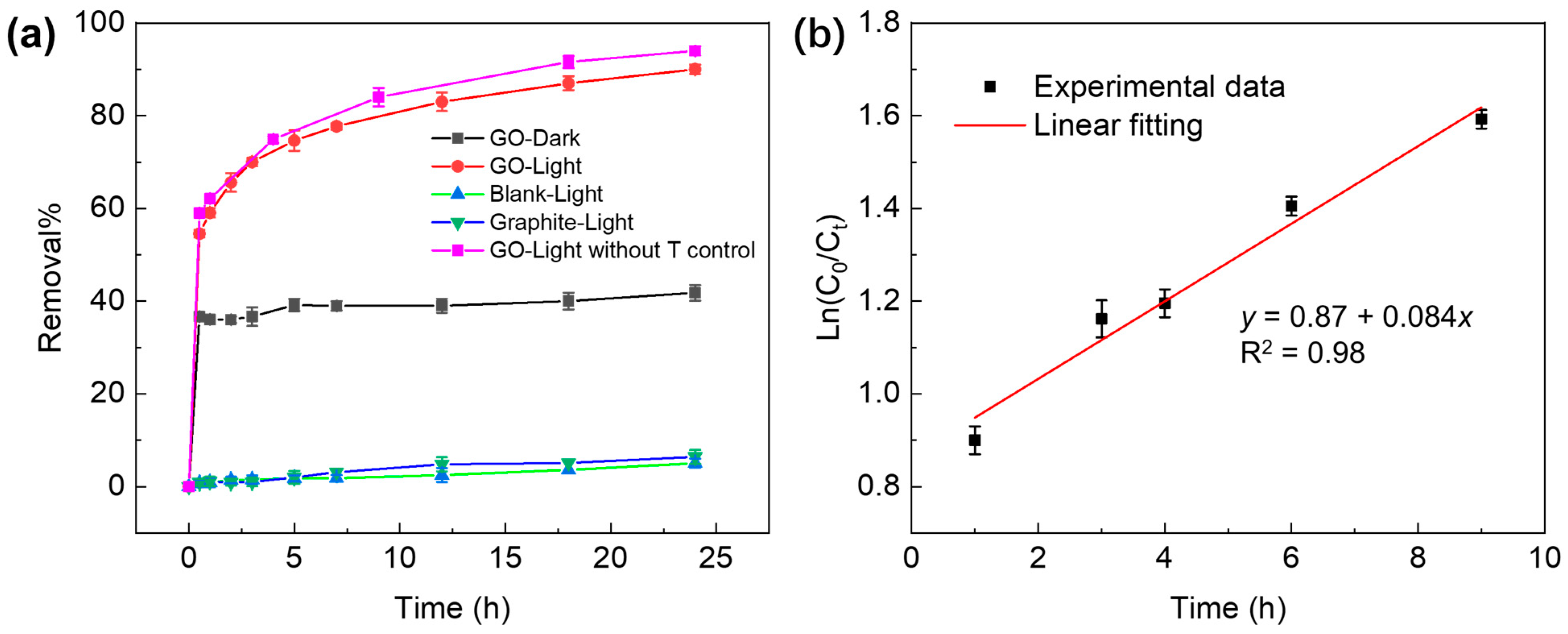
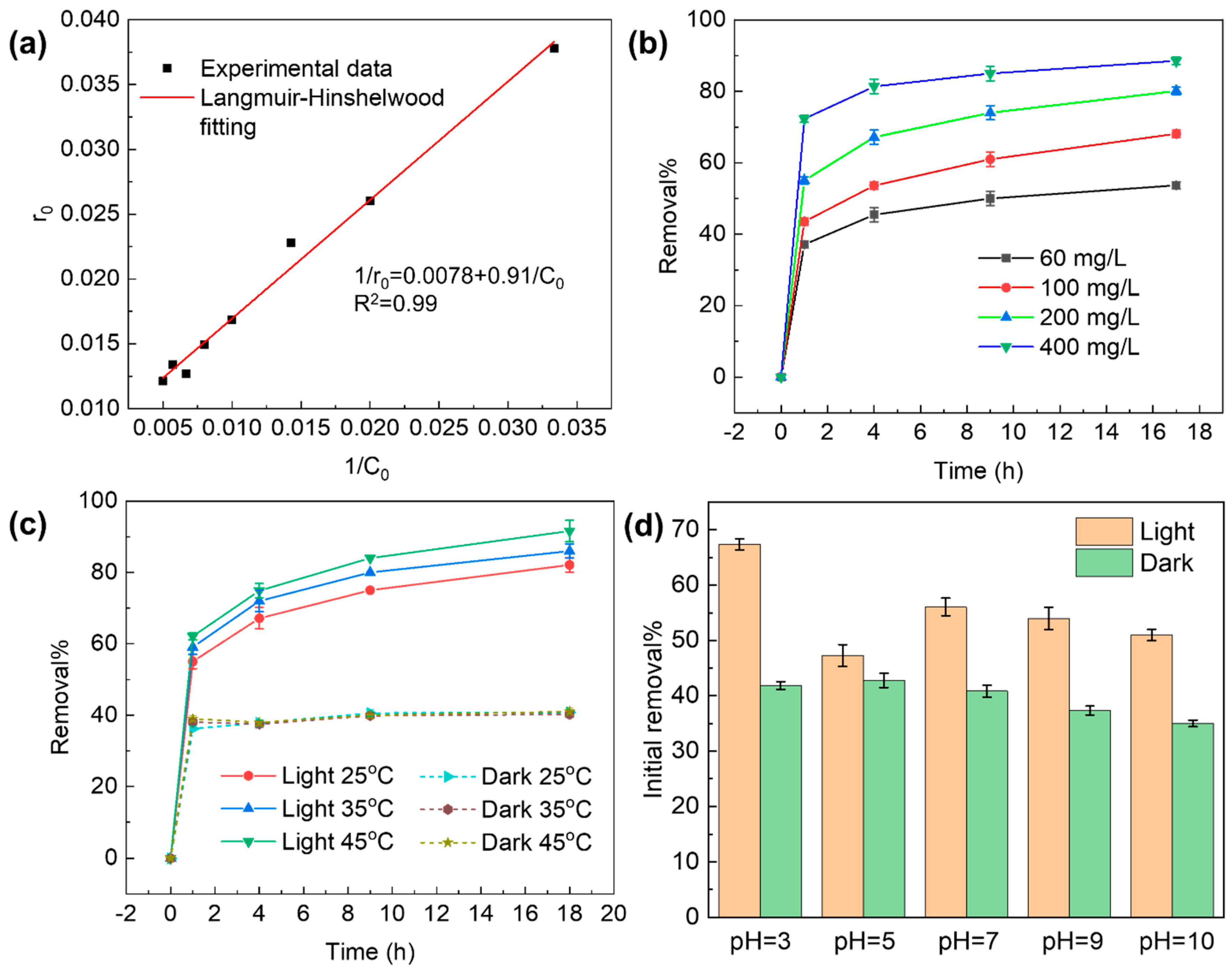
2.2. Removal of TC Promoted by GO under Light
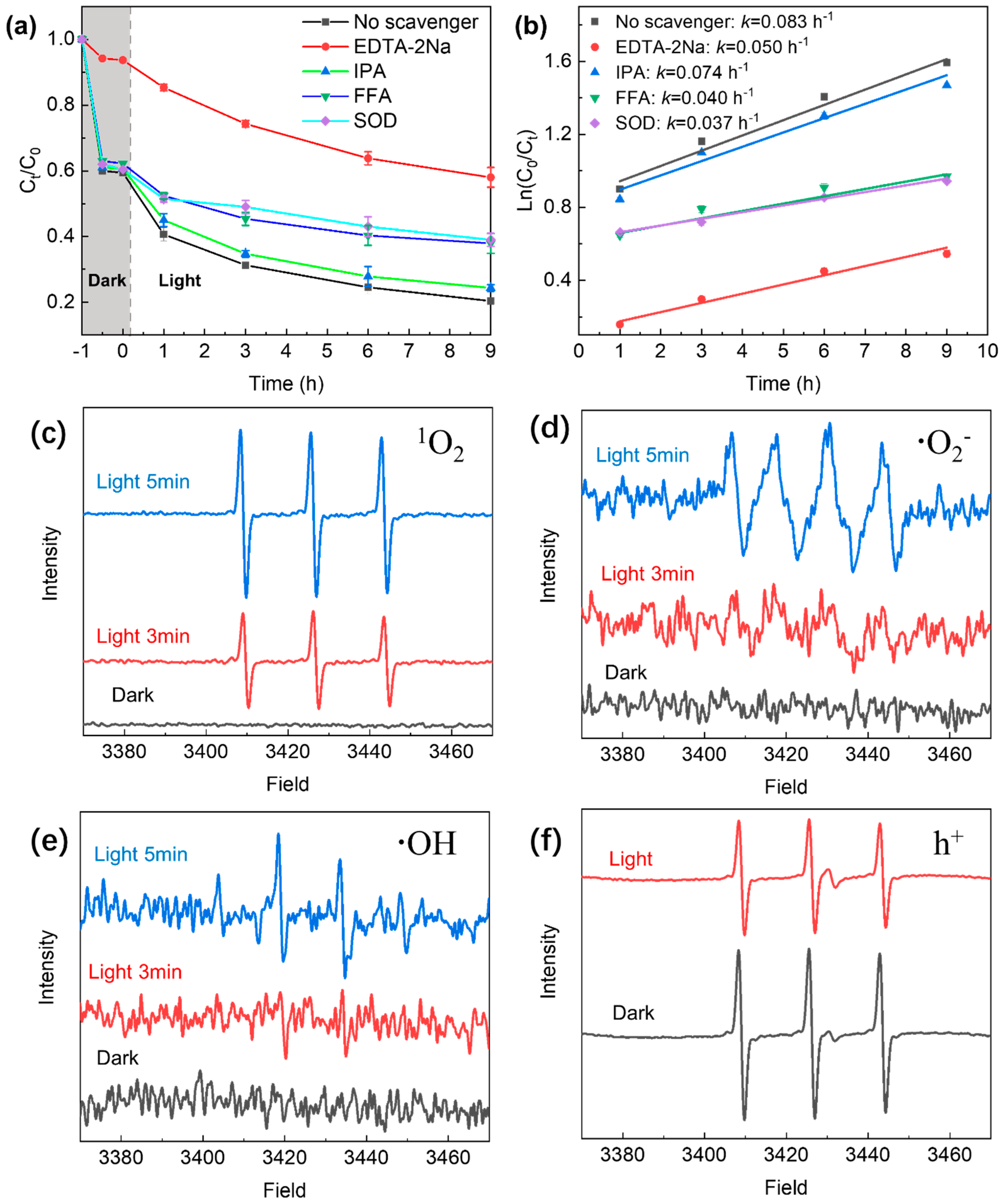
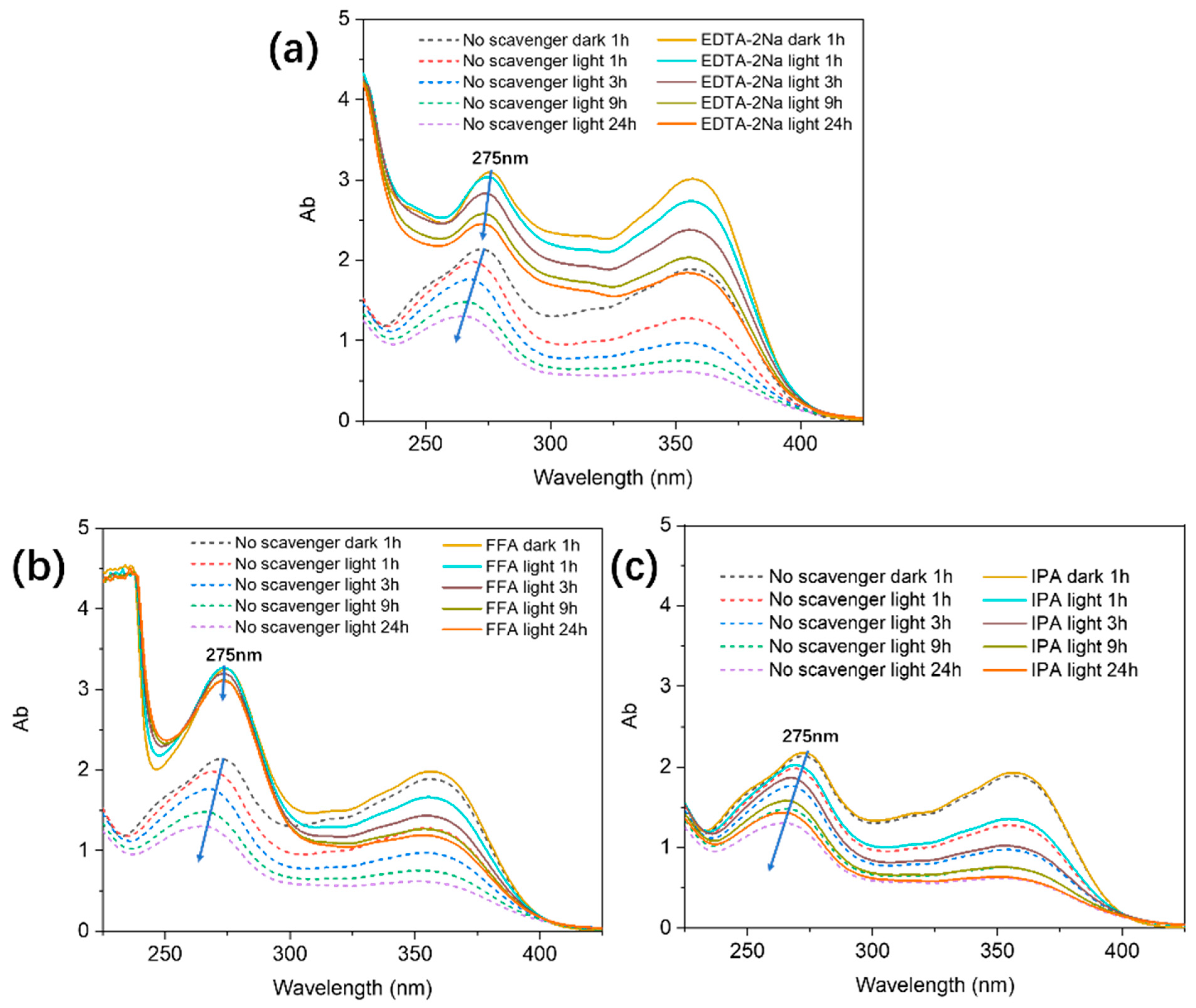
2.3. Effects of Radical and Hole Inhibitors on TC Removal Efficiency and ESR Analysis
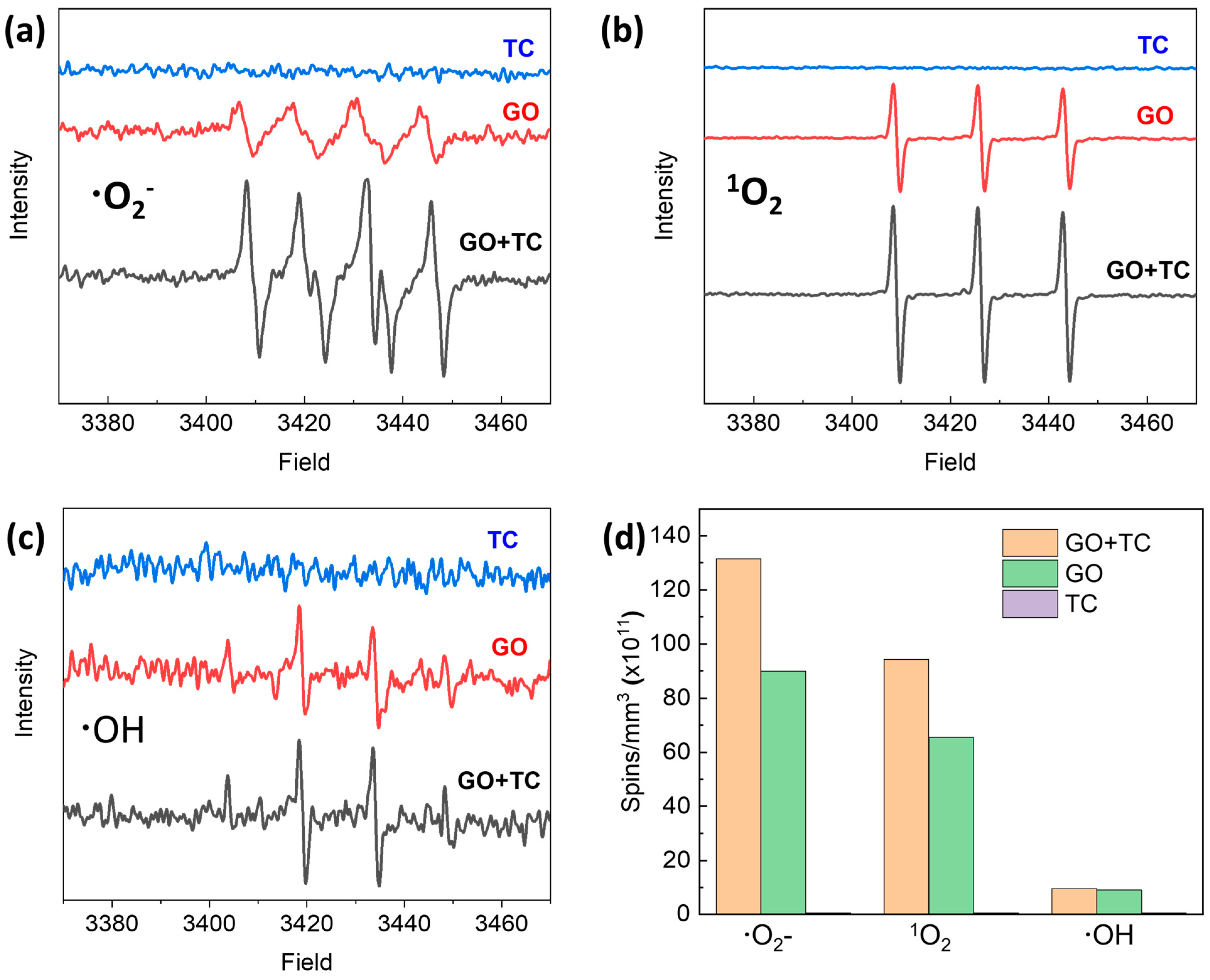
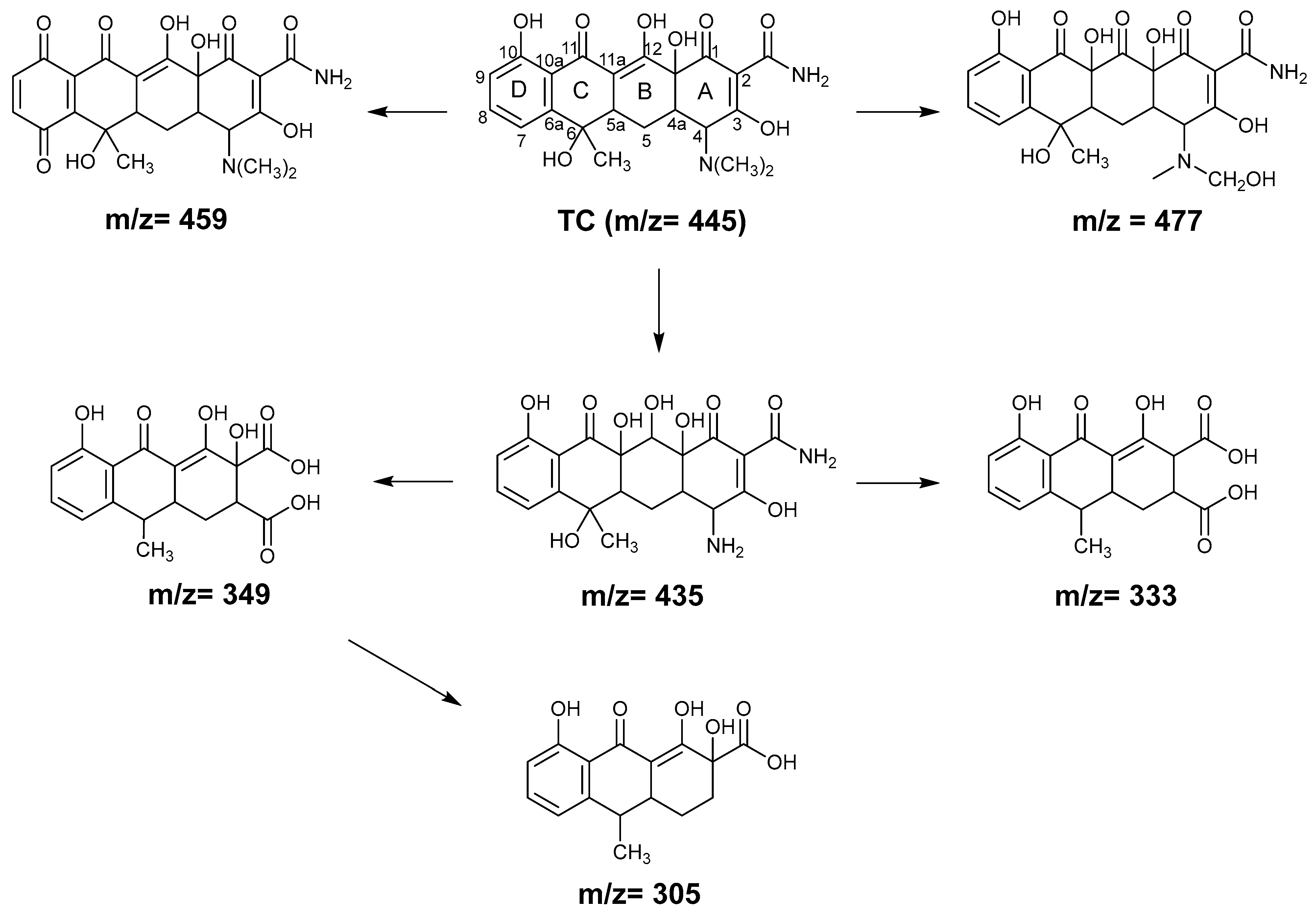
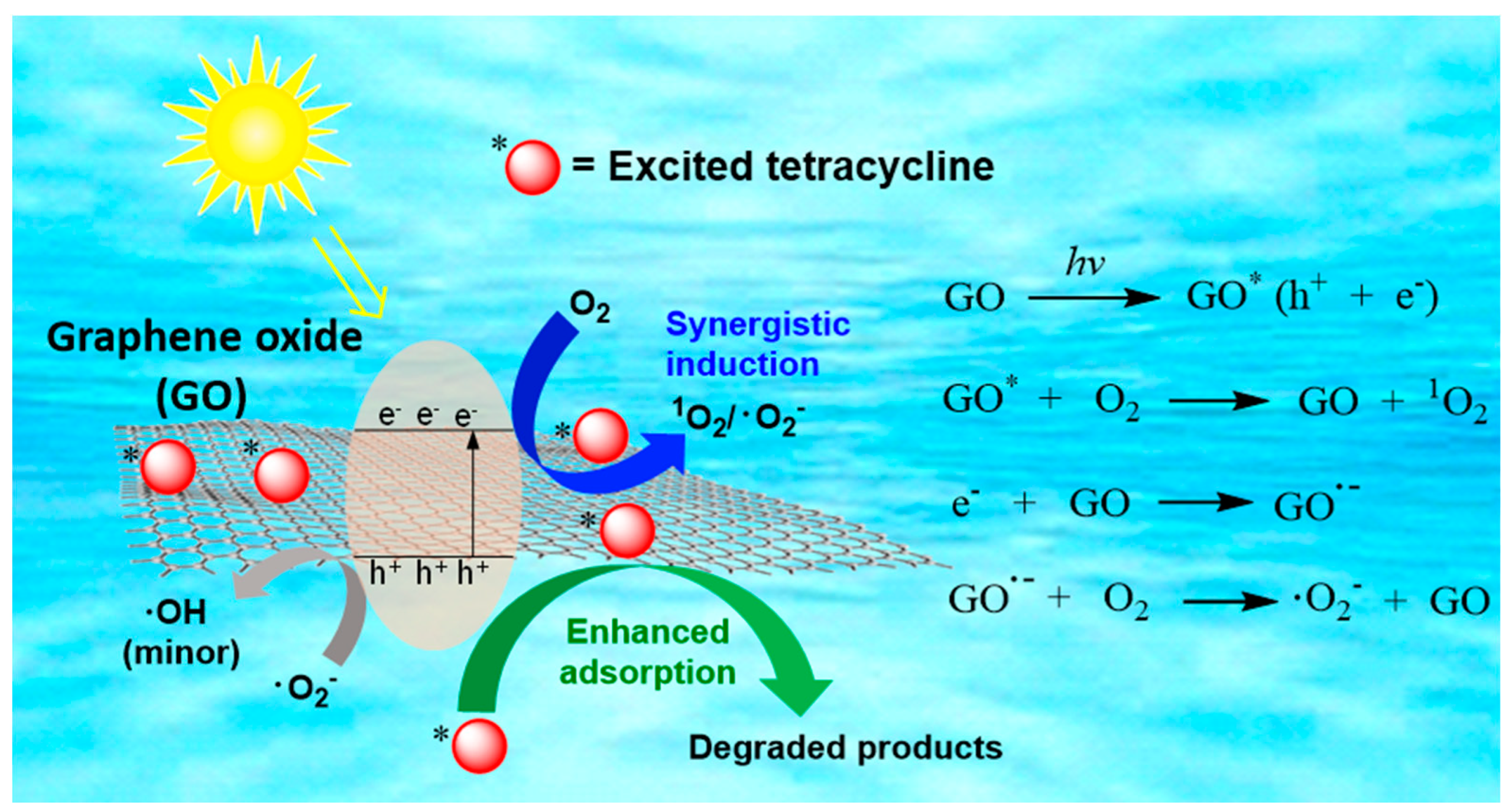
2.4. TC Degradation Mechanism in GO–Light System
3. Materials and Methods
3.1. Chemical Reagents
3.2. Preparation of GO
3.3. Removal of TC under Light Condition
3.4. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P.; et al. The Chemistry and Promising Applications of Graphene and Porous Graphene Materials. Adv. Funct. Mater. 2020, 30, 1909035. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, K.; Liu, G.; Chen, Y.; Wang, M.; Li, S.; Li, R. Recent advances on graphene: Synthesis, properties and applications. Compos. Part A Appl. Sci. Manuf. 2022, 160, 107051. [Google Scholar] [CrossRef]
- Wu, J.; Jia, L.; Zhang, Y.; Qu, Y.; Jia, B.; Moss, D.J. Graphene Oxide for Integrated Photonics and Flat Optics. Adv. Mater. 2021, 33, 2006415. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ma, S.; Ma, J.; Guiraud, P.; Guo, X.; Zhang, Y.; Jiao, T. In-situ desorption of acetaminophen from the surface of graphene oxide driven by an electric field: A study by molecular dynamics simulation. Chem. Eng. J. 2021, 418, 129391. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Valentini, C.; Montes-García, V.; Livio, P.A.; Chudziak, T.; Raya, J.; Ciesielski, A.; Samorì, P. Tuning the electrical properties of graphene oxide through low-temperature thermal annealing. Nanoscale 2023, 15, 5743–5755. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wu, H.; Chen, H.; Xu, G.; Li, C. Graphene oxide in aqueous and nonaqueous media: Dispersion behaviour and solution chemistry. Carbon 2020, 158, 568–579. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Y.H. Effect of Oxygen Content on Structures of Graphite Oxides. Ind. Eng. Chem. Res. 2011, 50, 6132–6137. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Kalashgrani, M.Y.; Gholami, A.; Binazadeh, M.; Chiang, W.-H.; Rahman, M.M. Recent advances in energy storage with graphene oxide for supercapacitor technology. Sustain. Energy Fuels 2023, 7, 5176–5197. [Google Scholar] [CrossRef]
- Ha, S.H.; Jeong, Y.S.; Lee, Y.J. Free Standing Reduced Graphene Oxide Film Cathodes for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2013, 5, 12295–12303. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fu, Q.; Pan, N.; Yu, X.; Liu, J.; Luo, Y.; Wang, X.; Yang, J.; Hou, J. Direct writing of electronic devices on graphene oxide by catalytic scanning probe lithography. Nat. Commun. 2012, 3, 1194. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Putri, L.K.; Tan, L.-L.; Ong, W.-J.; Chang, W.S.; Chai, S.-P. Graphene oxide: Exploiting its unique properties toward visible-light-driven photocatalysis. Appl. Mater. Today 2016, 4, 9–16. [Google Scholar] [CrossRef]
- Cruz, M.; Gomez, C.; Duran-Valle, C.J.; Pastrana-Martínez, L.M.; Faria, J.L.; Silva, A.M.T.; Faraldos, M.; Bahamonde, A. Bare TiO2 and graphene oxide TiO2 photocatalysts on the degradation of selected pesticides and influence of the water matrix. Appl. Surf. Sci. 2017, 416, 1013–1021. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Kontos, A.G.; Moustakas, N.G.; Faria, J.L.; Doña-Rodríguez, J.M.; Falaras, P.; Silva, A.M.T. TiO2, surface modified TiO2 and graphene oxide-TiO2 photocatalysts for degradation of water pollutants under near-UV/Vis and visible light. Chem. Eng. J. 2013, 224, 17–23. [Google Scholar] [CrossRef]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Figueiredo, J.L.; Faria, J.L.; Falaras, P.; Silva, A.M.T. Advanced nanostructured photocatalysts based on reduced graphene oxide–TiO2 composites for degradation of diphenhydramine pharmaceutical and methyl orange dye. Appl. Catal. B Environ. 2012, 123–124, 241–256. [Google Scholar] [CrossRef]
- Li, B.; Liu, T.; Wang, Y.; Wang, Z. ZnO/graphene-oxide nanocomposite with remarkably enhanced visible-light-driven photocatalytic performance. J. Colloid Interface Sci. 2012, 377, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Martínez, L.M.; Morales-Torres, S.; Likodimos, V.; Falaras, P.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Role of oxygen functionalities on the synthesis of photocatalytically active graphene–TiO2 composites. Appl. Catal. B Environ. 2014, 158–159, 329–340. [Google Scholar] [CrossRef]
- Linley, S.; Liu, Y.; Ptacek, C.J.; Blowes, D.W.; Gu, F.X. Recyclable Graphene Oxide-Supported Titanium Dioxide Photocatalysts with Tunable Properties. ACS Appl. Mater. Interfaces 2014, 6, 4658–4668. [Google Scholar] [CrossRef]
- Liu, J.; Ke, J.; Li, D.; Sun, H.; Liang, P.; Duan, X.; Tian, W.; Tadé, M.O.; Liu, S.; Wang, S. Oxygen Vacancies in Shape Controlled Cu2O/Reduced Graphene Oxide/In2O3 Hybrid for Promoted Photocatalytic Water Oxidation and Degradation of Environmental Pollutants. ACS Appl. Mater. Interfaces 2017, 9, 11678–11688. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J. Enhanced photocatalytic activity of three-dimensional TiO2/reduced graphene oxide aerogel by efficient interfacial charge transfer. Appl. Surf. Sci. 2023, 612, 155849. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Shi, J.; Nan, Y.; Sun, Y.; Jiang, Z. Three-Dimensional Porous Aerogel Constructed by g-C3N4 and Graphene Oxide Nanosheets with Excellent Visible-Light Photocatalytic Performance. ACS Appl. Mater. Interfaces 2015, 7, 25693–25701. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.; Pastrana-Martínez, L.M.; Pereira, M.F.R.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T. N/S-doped graphene derivatives and TiO2 for catalytic ozonation and photocatalysis of water pollutants. Chem. Eng. J. 2018, 348, 888–897. [Google Scholar] [CrossRef]
- Xing, M.; Fang, W.; Yang, X.; Tian, B.; Zhang, J. Highly-dispersed boron-doped graphene nanoribbons with enhanced conductibility and photocatalysis. Chem. Commun. 2014, 50, 6637–6640. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-R.; Zhang, Y.; Zhang, N.; Xu, Y.-J. New insight into the enhanced visible light photocatalytic activity over boron-doped reduced graphene oxide. Nanoscale 2015, 7, 7030–7034. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, X. Synthesis of a sulfur-graphene composite as an enhanced metal-free photocatalyst. Nano Res. 2013, 6, 286–292. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, L.; Wang, J.; Zhu, Y.; Pu, Y.; Dai, W. Photocatalytic degradation of tetracycline antibiotics using three-dimensional network structure perylene diimide supramolecular organic photocatalyst under visible-light irradiation. Appl. Catal. B Environ. 2020, 277, 119122. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Wang, X.; Wang, F.; Hao, R.; Song, W.; Li, Y. Photoreactivity of graphene oxide in aqueous system: Reactive oxygen species formation and bisphenol A degradation. Chemosphere 2018, 195, 344–350. [Google Scholar] [CrossRef]
- Oh, J.; Chang, Y.H.; Kim, Y.-H.; Park, S. Thickness-dependent photocatalytic performance of graphite oxide for degrading organic pollutants under visible light. Phys. Chem. Chem. Phys. 2016, 18, 10882–10886. [Google Scholar] [CrossRef]
- Li, C.; Xu, Q.; Xu, S.; Zhang, X.; Hou, X.; Wu, P.J.R.a. Synergy of adsorption and photosensitization of graphene oxide for improved removal of organic pollutants. RSC Adv. 2017, 7, 16204–16209. [Google Scholar] [CrossRef]
- Bustos-Ramírez, K.; Barrera-Díaz, C.E.; De Icaza-Herrera, M.; Martínez-Hernández, A.L.; Natividad-Rangel, R.; Velasco-Santos, C. 4-chlorophenol removal from water using graphite and graphene oxides as photocatalysts. J. Environ. Health Sci. Eng. 2015, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.-C.; Chowdhury, I.; Goodwin, D.G., Jr.; Henderson, W.M.; Fairbrother, D.H.; Bouchard, D.; Zepp, R.G. Photochemical Transformation of Graphene Oxide in Sunlight. Environ. Sci. Technol. 2015, 49, 3435–3443. [Google Scholar] [CrossRef] [PubMed]
- Shams, M.; Guiney, L.M.; Huang, L.; Ramesh, M.; Yang, X.; Hersam, M.C.; Chowdhury, I. Influence of functional groups on the degradation of graphene oxide nanomaterials. Environ. Sci. Nano 2019, 6, 2203–2214. [Google Scholar] [CrossRef]
- Pedrosa, M.; Da Silva, E.S.; Pastrana-Martínez, L.M.; Drazic, G.; Falaras, P.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T. Hummers’ and Brodie’s graphene oxides as photocatalysts for phenol degradation. J. Colloid Interface Sci. 2020, 567, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, W.; Wang, H.; Pan, C.; Xu, J.; Pozdnyakov, I.P.; Wu, F.; Li, J. Interaction between graphene oxide and acetaminophen in water under simulated sunlight: Implications for environmental photochemistry of PPCPs. Water Res. 2023, 228, 119364. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Ahmad, S.; Liu, L.; Wang, L.; Tang, J. A review of antibiotics and antibiotic resistance genes (ARGs) adsorption by biochar and modified biochar in water. Sci. Total Environ. 2023, 858, 159815. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Zhang, Y.; Liu, S.; Wang, C.; Chen, W.; Liu, C.; Chen, Z.; Zhang, Y. ZnCl2 modified biochar derived from aerobic granular sludge for developed microporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2020, 297, 122381. [Google Scholar] [CrossRef]
- Song, Z.; Ma, Y.-L.; Li, C.-E. The residual tetracycline in pharmaceutical wastewater was effectively removed by using MnO2/graphene nanocomposite. Sci. Total Environ. 2019, 651, 580–590. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Minale, M.; Gu, Z.; Guadie, A.; Kabtamu, D.M.; Li, Y.; Wang, X. Application of graphene-based materials for removal of tetracyclines using adsorption and photocatalytic-degradation: A review. J. Environ. Manag. 2020, 276, 111310. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Gomaa, A.; Taher, F.A.; El-Fass, M.M.; Kashyout, A.E.-H.B. Optimizing the preparation parameters of GO and rGO for large-scale production. J. Mater. Sci. 2016, 51, 5664–5675. [Google Scholar] [CrossRef]
- Sharma, N.; Arif, M.; Monga, S.; Shkir, M.; Mishra, Y.K.; Singh, A. Investigation of bandgap alteration in graphene oxide with different reduction routes. Appl. Surf. Sci. 2020, 513, 145396. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Rhoden, C.R.B.; Bruckmann, F.d.S.; Salles, T.d.R.; Kaufmann Junior, C.G.; Mortari, S.R. Study from the influence of magnetite onto removal of hydrochlorothiazide from aqueous solutions applying magnetic graphene oxide. J. Water Process Eng. 2021, 43, 102262. [Google Scholar] [CrossRef]
- Yuan, J.; Li, H.; Wang, G.; Zhang, C.; Wang, Y.; Yang, L.; Li, M.; Lu, J. Adsorption, isolated electron/hole transport, and confined catalysis coupling to enhance the photocatalytic degradation performance. Appl. Catal. B Environ. 2022, 303, 120892. [Google Scholar] [CrossRef]
- Gottfried, V.; Kimel, S. Temperature effects on photosensitized processes. J. Photochem. Photobiol. B Biol. 1991, 8, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Banci, L.; Luchinat, C.; Bielski, B.H.J.; Cabelli, D.E.; Mullenbach, G.T.; Hallewell, R.A. An investigation of a human erythrocyte SOD modified at position 137. J. Am. Chem. Soc. 1989, 111, 714–719. [Google Scholar] [CrossRef]
- Palominos, R.A.; Mondaca, M.A.; Giraldo, A.; Peñuela, G.; Pérez-Moya, M.; Mansilla, H.D. Photocatalytic oxidation of the antibiotic tetracycline on TiO2 and ZnO suspensions. Catal. Today 2009, 144, 100–105. [Google Scholar] [CrossRef]
- Yang, Y.; Bian, Z. Oxygen doping through oxidation causes the main active substance in g-C3N4 photocatalysis to change from holes to singlet oxygen. Sci. Total Environ. 2021, 753, 141908. [Google Scholar] [CrossRef]
- Li, L.; Niu, C.-G.; Guo, H.; Wang, J.; Ruan, M.; Zhang, L.; Liang, C.; Liu, H.-Y.; Yang, Y.-Y. Efficient degradation of Levofloxacin with magnetically separable ZnFe2O4/NCDs/Ag2CO3 Z-scheme heterojunction photocatalyst: Vis-NIR light response ability and mechanism insight. Chem. Eng. J. 2020, 383, 123192. [Google Scholar] [CrossRef]
- He, W.; Kim, H.-K.; Wamer, W.G.; Melka, D.; Callahan, J.H.; Yin, J.-J. Photogenerated Charge Carriers and Reactive Oxygen Species in ZnO/Au Hybrid Nanostructures with Enhanced Photocatalytic and Antibacterial Activity. J. Am. Chem. Soc. 2014, 136, 750–757. [Google Scholar] [CrossRef]
- Beliakova, M.M.; Bessonov, S.I.; Sergeyev, B.M.; Smirnova, I.G.; Dobrov, E.N.; Kopylov, A.M. Rate of Tetracycline Photolysis during Irradiation at 365 nm. Biochemistry 2003, 68, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Verma, B.; Headley, J.V.; Robarts, R.D. Behaviour and fate of tetracycline in river and wetland waters on the Canadian Northern Great Plains. J. Environ. Sci. Health Part A 2007, 42, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hu, H.; Lin, Y.; Zhang, J.; Hu, Y.H. Visible light photocatalytic degradation of tetracycline over TiO2. Chem. Eng. J. 2020, 382, 122842. [Google Scholar] [CrossRef]
- Gao, B.; Iftekhar, S.; Srivastava, V.; Doshi, B.; Sillanpää, M. Insights into the generation of reactive oxygen species (ROS) over polythiophene/ZnIn2S4 based on different modification processing. Catal. Sci. Technol. 2018, 8, 2186–2194. [Google Scholar] [CrossRef]
- Nguyen, H.V.-M.; Lee, D.-H.; Lee, H.-S.; Shin, H.-S. Investigating the different transformations of tetracycline using birnessite under different reaction conditions and various humic acids. Environ. Pollut. 2023, 339, 122763. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Wang, Z.; Yu, Q.; Cheng, Y.; Liu, Z.; Zhang, T.; Zhou, S. Removal of tetracycline from an aqueous solution using manganese dioxide modified biochar derived from Chinese herbal medicine residues. Environ. Res. 2020, 183, 109195. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Huang, L.; Wu, X.; Wang, C.; Liu, Y.; Liu, G.; Wang, L.; Liu, X.; Xia, H. Effect and mechanism analysis of MnO2 on permeable reactive barrier (PRB) system for the removal of tetracycline. Chemosphere 2018, 193, 702–710. [Google Scholar] [CrossRef]
- Xie, Z.; Feng, Y.; Wang, F.; Chen, D.; Zhang, Q.; Zeng, Y.; Lv, W.; Liu, G. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline. Appl. Catal. B Environ. 2018, 229, 96–104. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, R.; Zeng, L.; Guo, W.; Zhu, M. Insight into the effects of hydroxyl groups on the rates and pathways of tetracycline antibiotics degradation in the carbon black activated peroxydisulfate oxidation process. J. Hazard. Mater. 2021, 412, 125256. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zheng, A.; Li, J.; Han, Y.; Xue, M.; Zhang, L.; Yin, Z.; Xie, C.; Chen, Z.; Ji, L.; et al. Integrated adsorption and photodegradation of tetracycline by bismuth oxycarbonate/biochar nanocomposites. Chem. Eng. J. 2023, 457, 141228. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, L.; Liang, J.; Xu, W.; Yu, H.; Zhang, J.; Ye, S.; Xing, W.; Yuan, X. Photocatalytic degradation of tetracycline antibiotics using delafossite silver ferrite-based Z-scheme photocatalyst: Pathways and mechanism insight. Chemosphere 2021, 270, 128651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Ma, Y.-L.; Yang, J.; Wang, L.-Q.; Lv, J.-M.; Ren, C.-J. Aqueous tetracycline degradation by H2O2 alone: Removal and transformation pathway. Chem. Eng. J. 2017, 307, 15–23. [Google Scholar] [CrossRef]
- Chong, Y.; Ge, C.; Fang, G.; Wu, R.; Zhang, H.; Chai, Z.; Chen, C.; Yin, J.-J. Light-Enhanced Antibacterial Activity of Graphene Oxide, Mainly via Accelerated Electron Transfer. Environ. Sci. Technol. 2017, 51, 10154–10161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-F.; Wang, S.-C.; Zhu, Z.-L.; Wang, S.-G.; Liu, F.-F.; Liu, G.-Z. Effects of oxidation degree on photo-transformation and the resulting toxicity of graphene oxide in aqueous environment. Environ. Pollut. 2019, 249, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Qutob, M.; Rafatullah, M.; Qamar, M.; Alorfi, H.S.; Al-Romaizan, A.N.; Hussein, M.A. A review on heterogeneous oxidation of acetaminophen based on micro and nanoparticles catalyzed by different activators. Nanotechnol. Rev. 2022, 11, 497–525. [Google Scholar] [CrossRef]
- Hou, W.-C.; BeigzadehMilani, S.; Jafvert, C.T.; Zepp, R.G. Photoreactivity of Unfunctionalized Single-Wall Carbon Nanotubes Involving Hydroxyl Radical: Chiral Dependency and Surface Coating Effect. Environ. Sci. Technol. 2014, 48, 3875–3882. [Google Scholar] [CrossRef]
- Jiménez-Marín, E.; Moreno-Valenzuela, J.; Trejo-Valdez, M.; Martinez-Rivas, A.; Vargas-García, J.R.; Torres-Torres, C. Laser-induced electrical signal filtering by multilayer reduced graphene oxide decorated with Au nanoparticles. Opt. Express 2019, 27, 7330–7343. [Google Scholar] [CrossRef]
- Lavin-Lopez, M.d.P.; Romero, A.; Garrido, J.; Sanchez-Silva, L.; Valverde, J.L. Influence of Different Improved Hummers Method Modifications on the Characteristics of Graphite Oxide in Order to Make a More Easily Scalable Method. Ind. Eng. Chem. Res. 2016, 55, 12836–12847. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Tian Lu, Molclus Program, Version 1.9.9.2. Available online: http://www.keinsci.com/research/molclus.html (accessed on 5 August 2024).
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended tight-binding quantum chemistry methods. WIREs Comput. Mol. Sci. 2021, 11, e1493. [Google Scholar] [CrossRef]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A Robust and Accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-block elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, K.; Chen, Y.; Xu, R.; Zhao, Y.; Guo, M. Mapping Photogenerated Electron–Hole Behavior of Graphene Oxide: Insight into a New Mechanism of Photosensitive Pollutant Degradation. Molecules 2024, 29, 3765. https://doi.org/10.3390/molecules29163765
Ni K, Chen Y, Xu R, Zhao Y, Guo M. Mapping Photogenerated Electron–Hole Behavior of Graphene Oxide: Insight into a New Mechanism of Photosensitive Pollutant Degradation. Molecules. 2024; 29(16):3765. https://doi.org/10.3390/molecules29163765
Chicago/Turabian StyleNi, Kaijie, Yanlong Chen, Ruiqi Xu, Yuming Zhao, and Ming Guo. 2024. "Mapping Photogenerated Electron–Hole Behavior of Graphene Oxide: Insight into a New Mechanism of Photosensitive Pollutant Degradation" Molecules 29, no. 16: 3765. https://doi.org/10.3390/molecules29163765
APA StyleNi, K., Chen, Y., Xu, R., Zhao, Y., & Guo, M. (2024). Mapping Photogenerated Electron–Hole Behavior of Graphene Oxide: Insight into a New Mechanism of Photosensitive Pollutant Degradation. Molecules, 29(16), 3765. https://doi.org/10.3390/molecules29163765




