Establishing the Thermodynamic Cards of Dipine Models’ Oxidative Metabolism on 21 Potential Elementary Steps
Abstract
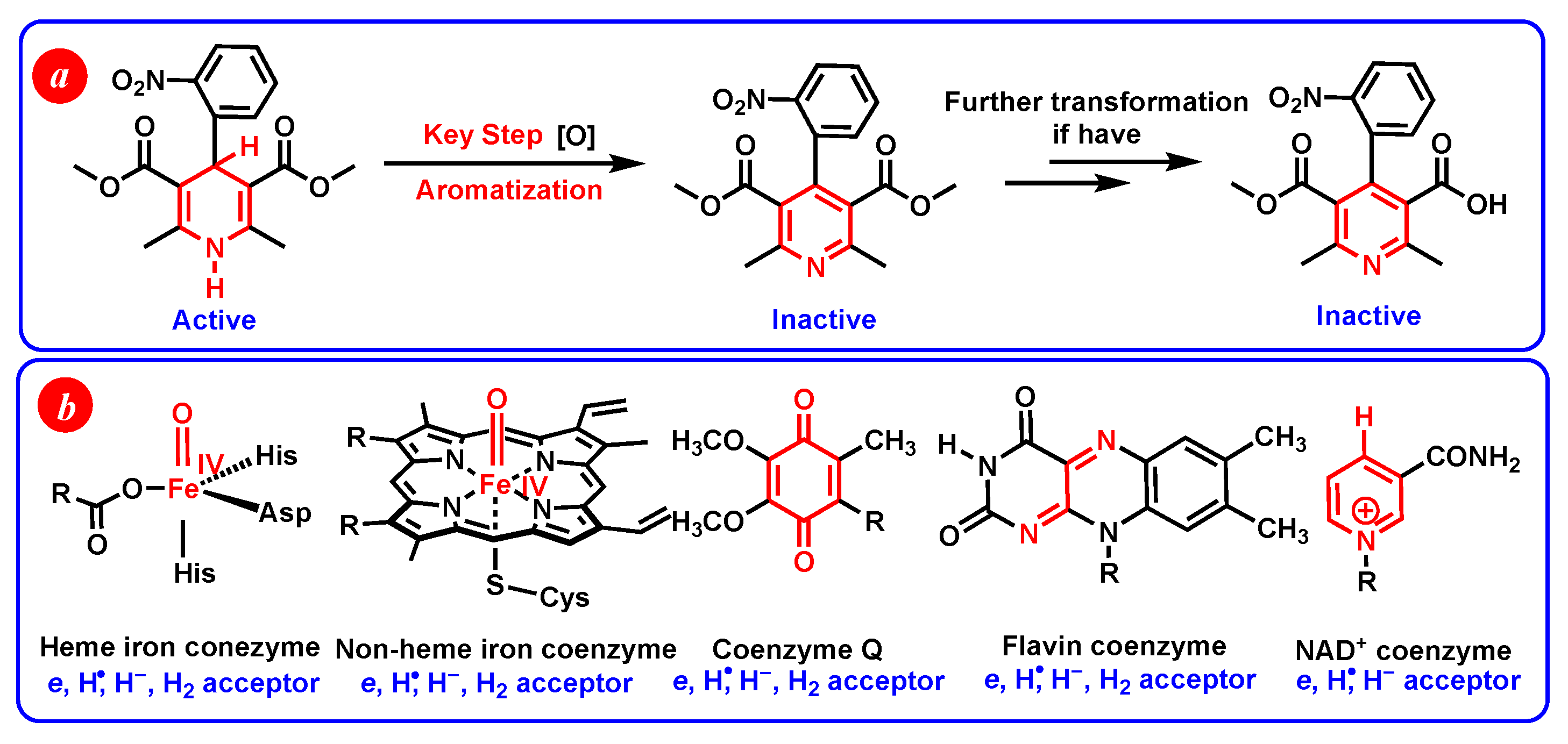
1. Introduction
2. Results and Discussion
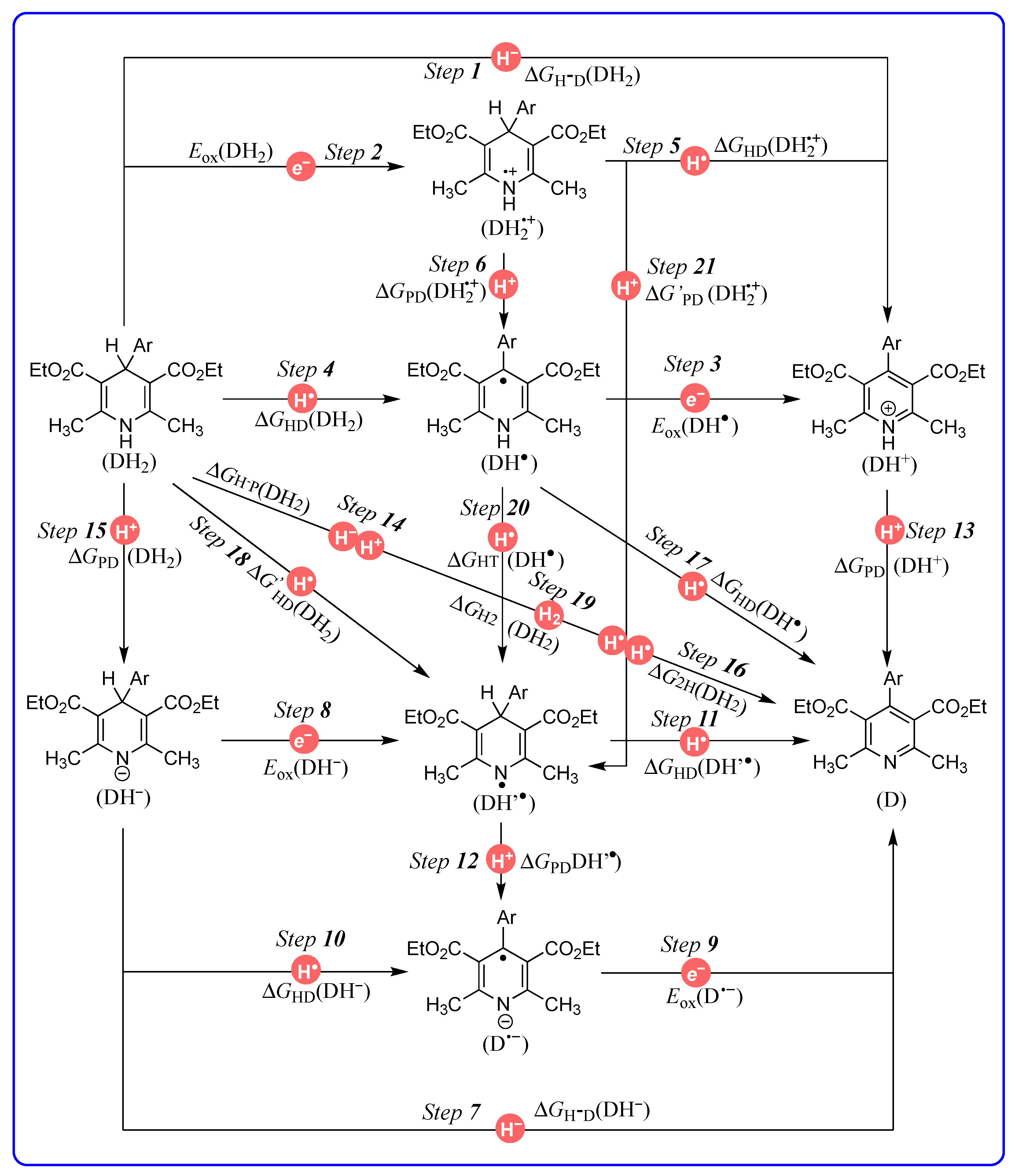
2.1. Thermodynamic Parameters and Results
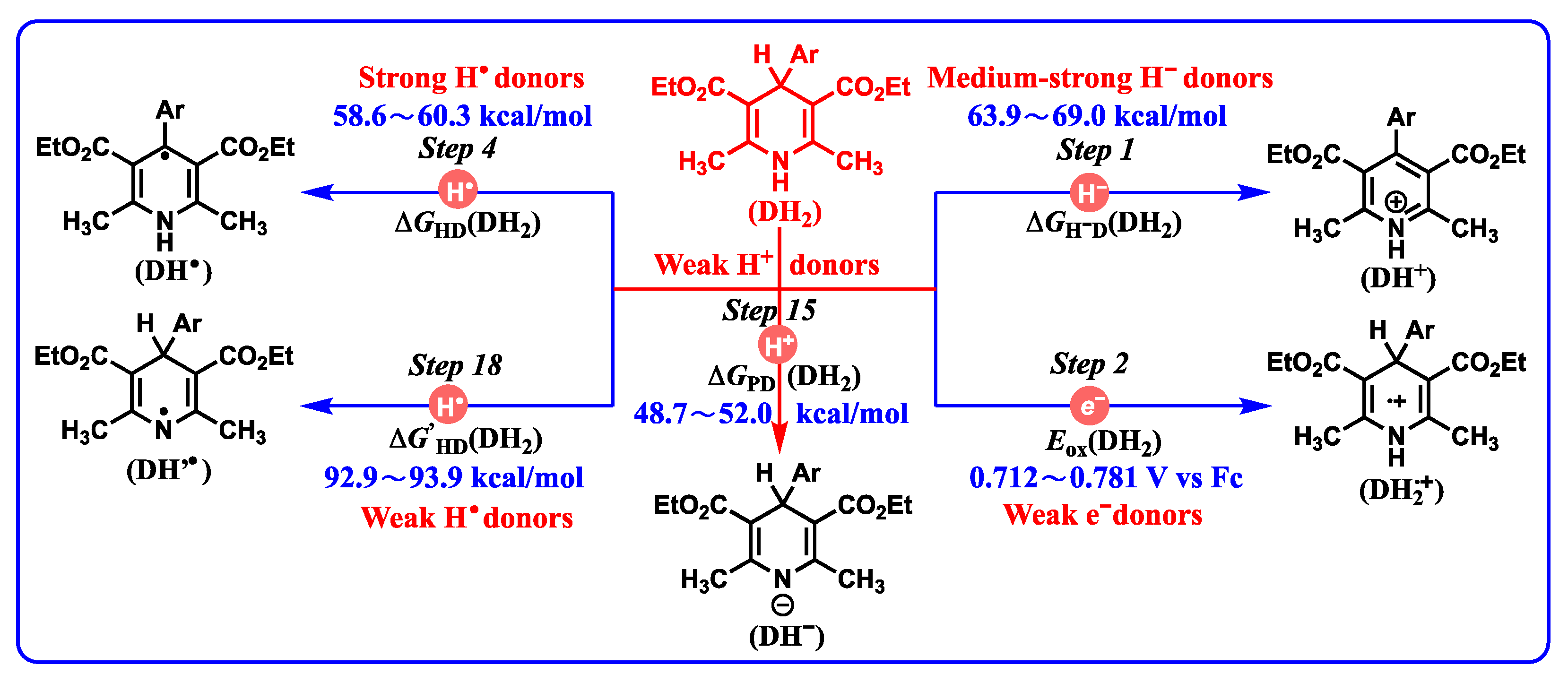
| Step X | Chemical Process | Potentials | Computed Equations or Data Sources | Equation |
|---|---|---|---|---|
| Step 1 | DH2 → DH+ + H− | ΔGH−D(DH2) | ΔGH−D(DH2) = −ΔHH−D(DH2) − 4.9 | (1) |
| Step 2 | DH2 → DH2•+ + e− | Eox(DH2) | [38] | - |
| Step 3 | DH• → DH+ + e− | Eox(DH•) | [38] | - |
| Step 4 | DH2 → DH• + H• | ΔGHD(DH2) | ΔGHD(DH2) = ΔGH−D(DH2) − F[Ered(DH+) − Eox(H−)] | (2) |
| Step 5 | DH2•+ → DH+ + H• | ΔGHD(DH2•+) | ΔGHD(DH2•+) = ΔGH−D(DH2) − F[Eox(DH2) − Ered(H•)] | (3) |
| Step 6 | DH2•+ → DH• + H+ | ΔGPD(DH2•+) | ΔGPD(DH2•+) = ΔGHD(DH2) − F[Eox(DH2) − Ered(H+)] | (4) |
| Step 7 | DH− → D + H− | ΔGH−D(DH−) | ΔGH−D(DH−) = ΔHH−D(DH−) − 4.9 | (5) |
| Step 8 | DH− → DH• + e− | Eox(DH−) | [38] | - |
| Step 9 | D•− → D + e− | Eox(D•−) | [38] | - |
| Step 10 | DH− → D•− + H• | ΔGHD(DH−) | ΔGHD(DH−) = ΔGH-D(DH−) − F[Ered(D) − Eox(H−)] | (6) |
| Step 11 | DH’• → D + H• | ΔGHD(DH’•) | ΔGHD(DH’•) = ΔGH-D(DH−) − F[Eox(DH−) − Ered(H•)] | (7) |
| Step 12 | DH’• → D− + H+ | ΔGPD(DH’•) | ΔGPD(DH’•) = ΔGHD(DH−) − F[Eox(DH−) − Ered(H+)] | (8) |
| Step 13 | DH+ → D + H+ | ΔGPD(DH+) | ΔGPD(DH+) = 1.37 pKa(DH+) | (9) |
| Step 14 | DH2 → D + H− + H+ | ΔGH−P(DH2) | ΔGH−P(DH2) = ΔGH-D(DH2) + ΔGPD(DH+) | (10) |
| Step 15 | DH2 → DH− + H+ | ΔGPD(DH2) | ΔGPD(DH2) = ΔGH−P(DH2) − ΔGH−D(DH−) | (11) |
| Step 16 | DH2 → D + H• + H• | ΔG2H(DH2) | ΔG2H(DH2) = ΔGH−P(DH2) + F[Ered(H•) − Eox(H•)] | (12) |
| Step 17 | DH• → D + H• | ΔGHD(DH•) | ΔGHD(DH•) = ΔG2H(DH2) − ΔGHD(DH2) | (13) |
| Step 18 | DH2 → DH’• + H• | ΔG’HD(DH2) | ΔGHD(DH2) = ΔG2H(DH2) − ΔGHD(DH’•) | (14) |
| Step 19 | DH2 → D + H2 | ΔGH2(DH2) | ΔGH2(DH2) = ΔG2H(DH2) − ΔGH−D(H2) | (15) |
| Step 20 | DH• → DH’• | ΔGHT(DH•) | ΔGHT(DH•) = ΔGHD(DH•) − ΔGHD(DH’•) | (16) |
| Step 21 | DH2•+ → DH’• + H+ | ΔG’PD(DH2•+) | ΔG’PD(DH2•+) = ΔGPD(DH2•+) + ΔGHT(DH•) | (17) |
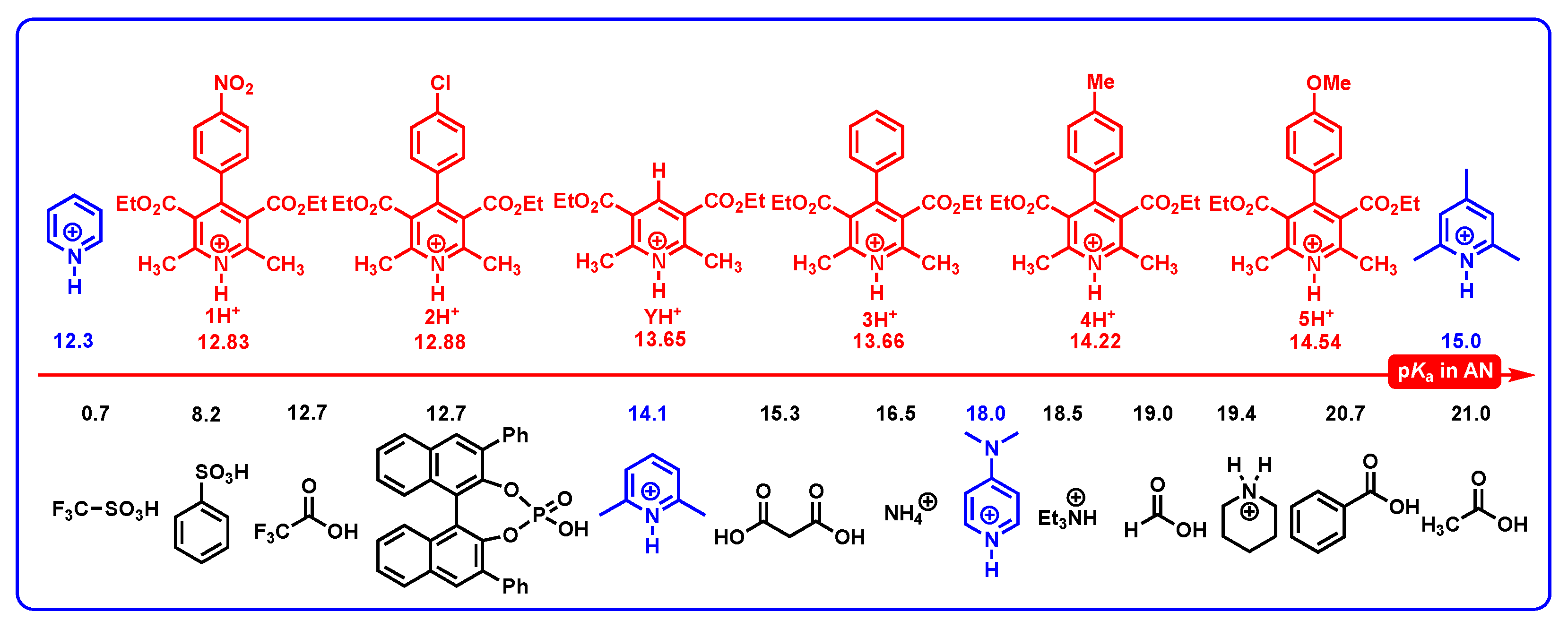
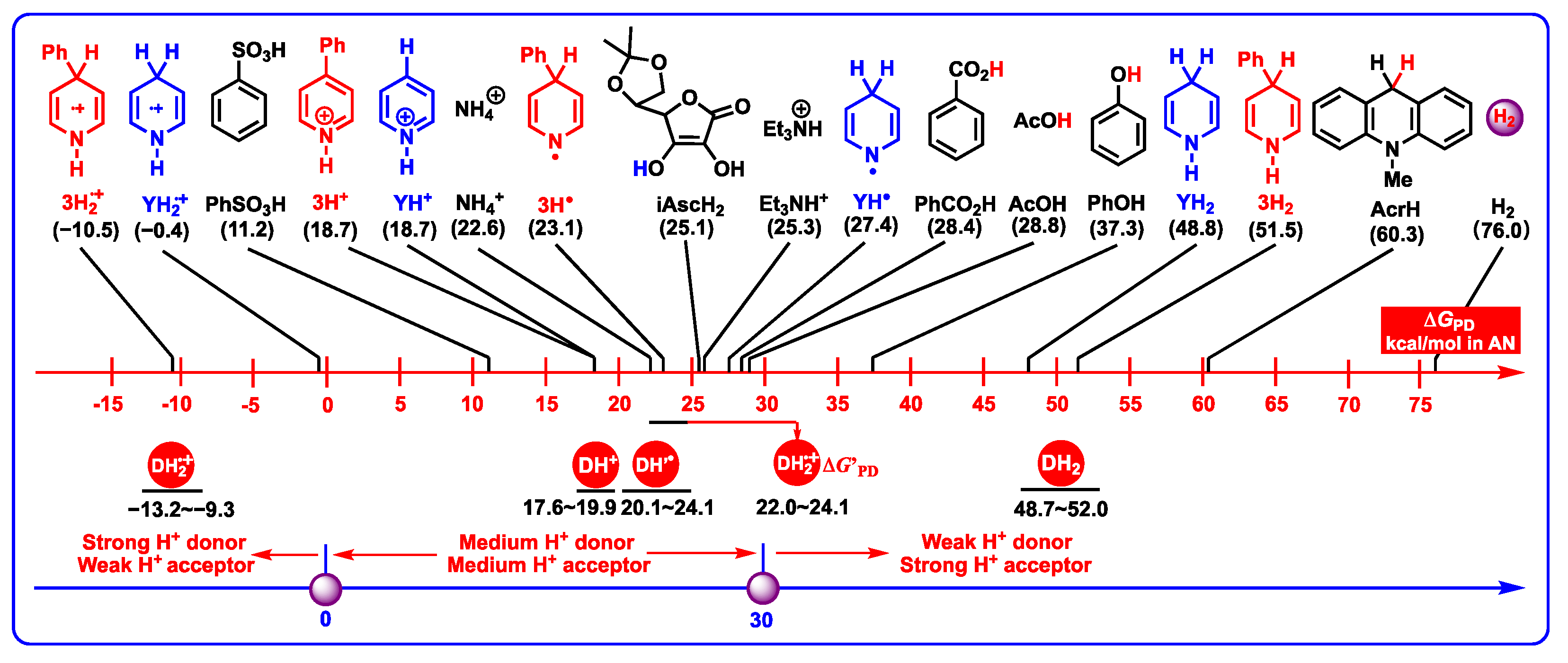
2.2. The Acidities of DH+ in Acetonitrile
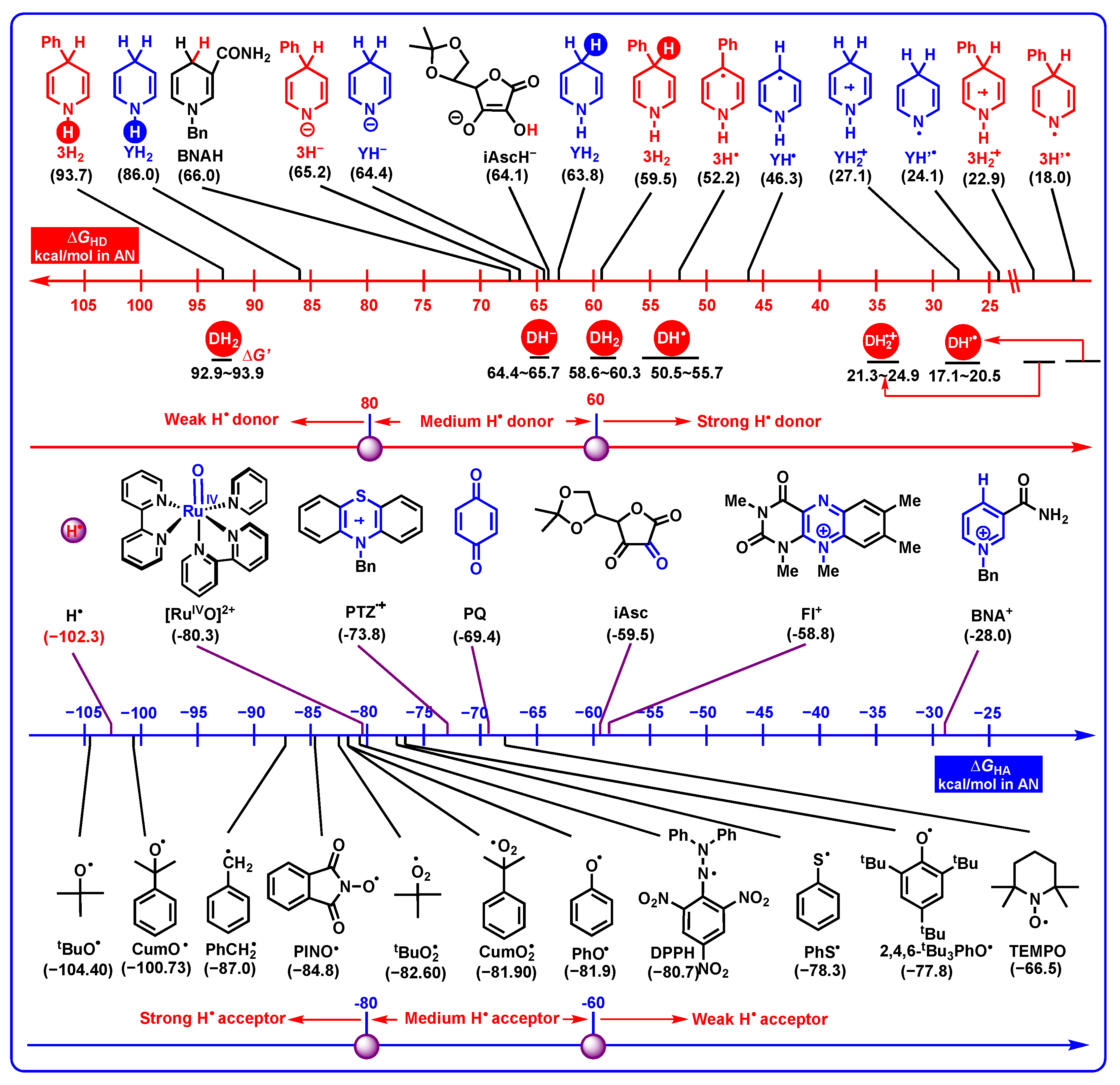
2.3. Thermodynamic Properties of DH2 and Related Intermediates Acting as Electrons, Hydrides, Hydrogen Atoms, Protons, and Two Hydrogen Ions (Atoms) Donors in Acetonitrile
2.3.1. Electron-Donating Properties
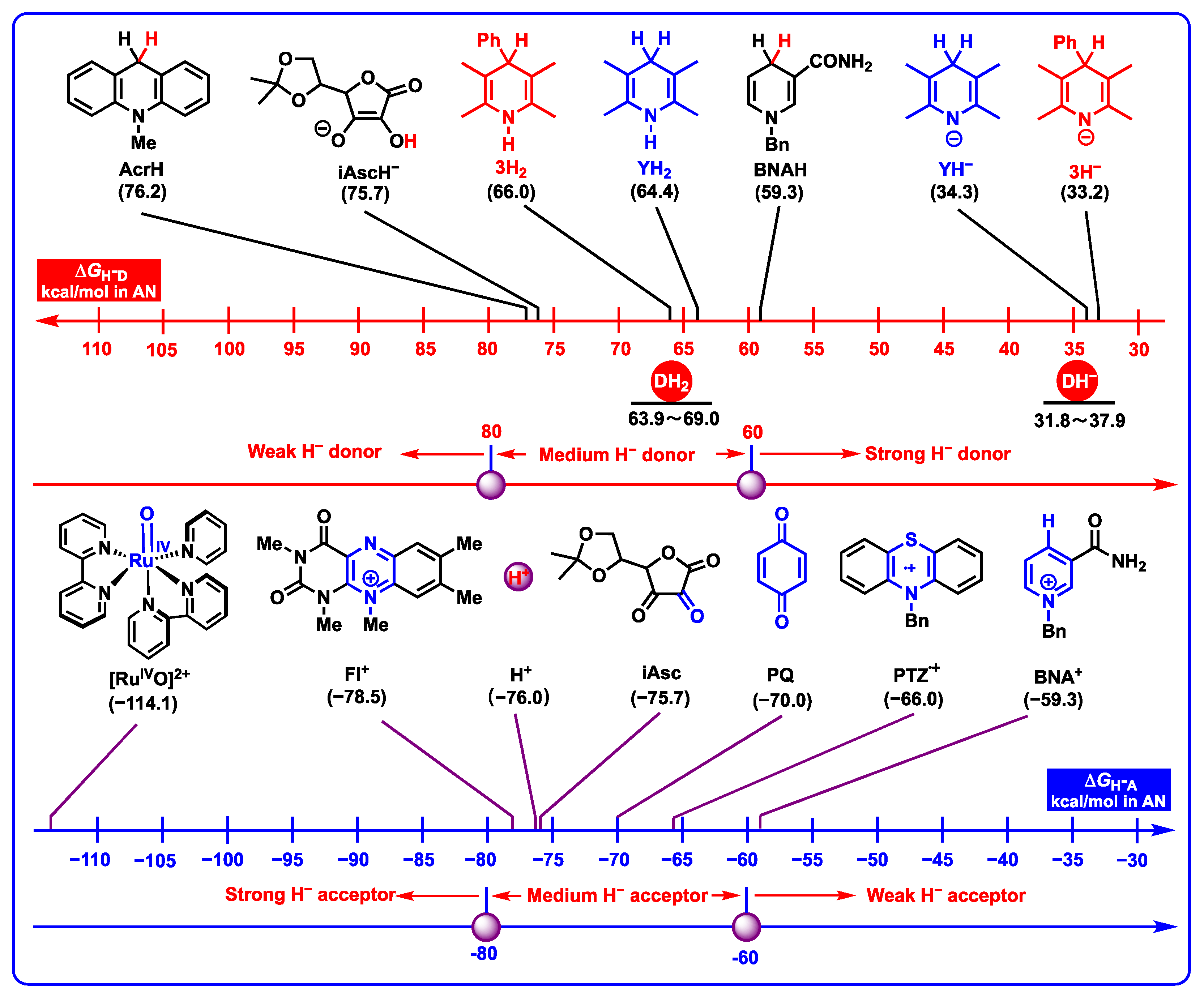
2.3.2. Hydride-Donating Properties
2.3.3. Hydrogen-Atom-Donating Properties
2.3.4. Proton-Donating Properties
- (1)
- DH2•+ (C4-H, −13.2–−9.3 kcal/mol) are the strongest organic acids among them. After the single-electron oxidation of DH2 (0.712–0.781 V vs. Fc), DH2•+ are extremely unstable intermediates that spontaneously release protons from C4-H bonds with significant thermodynamic potentials (−13.2–−9.3 kcal/mol).
- (2)
- DH2 (48.7–52.0 kcal/mol) are the weakest organic acids in acetonitrile among them, indicating that the N1-H bond in DH2 is the weak polar bond. Since the proton affinity of hydride ions (H−) is determined as −76.0 kcal/mol in acetonitrile [41,42], the proton-abstracting reaction from N1-H bonds in DH2 molecules to H− (DH2 + H− → DH− + H2) is thermodynamically favorable and extremely exothermic with −27.3 ≤ ΔGPT(DH2/H−) ≤ 24.0 kcal/mol.
- (3)
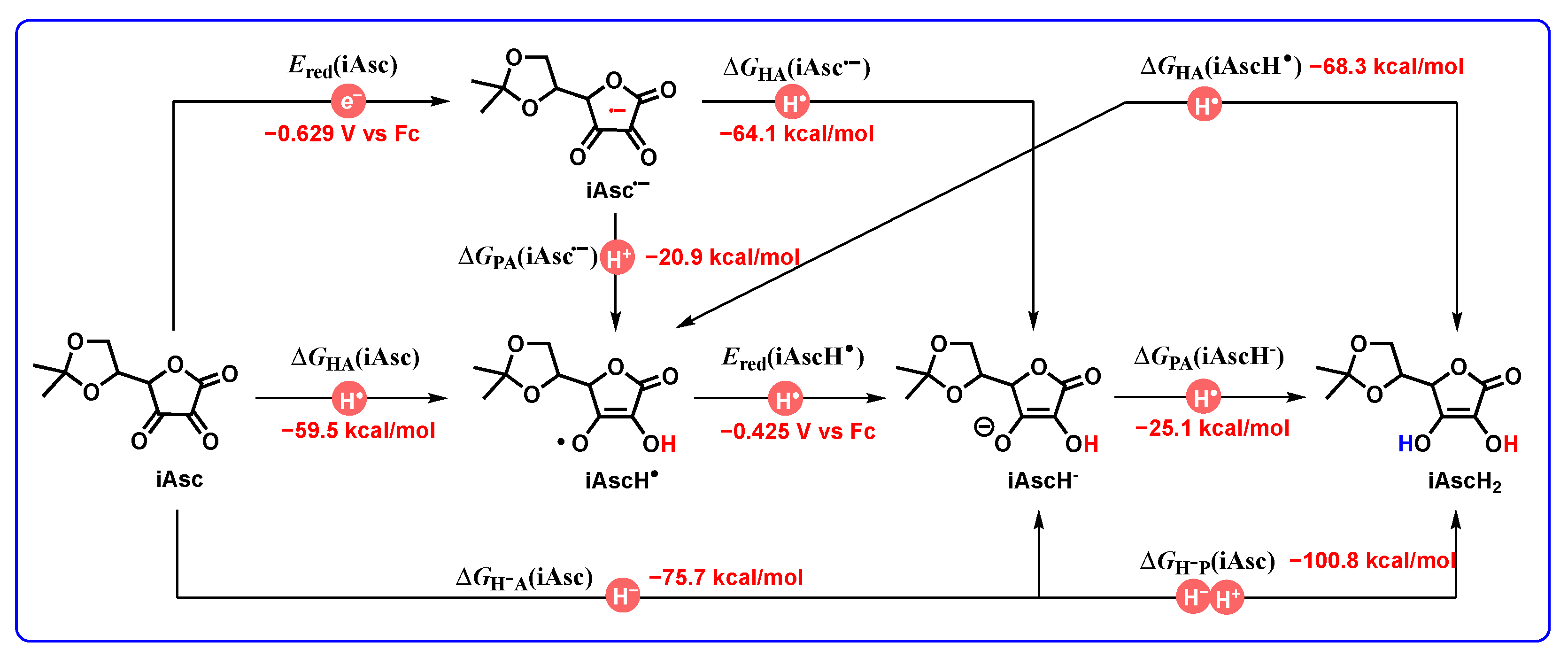
- From Figure 8, the thermodynamic proton-abstracting abilities of common organic bases [43,44,45], consisting of iAscH− (−25.1 kcal/mol), Et3N (−25.3 kcal/mol), NH3 (−22.6 kcal/mol), PhCO2− (−28.4 kcal/mol), AcO− (−28.8 kcal/mol), and PhO− (−37.3 kcal/mol), are generally smaller than −40 kcal/mol. Although the proton abstraction reactions from DH2 to common organic bases are thermodynamically unfeasible (ΔGPT > 0), the resulting DH− (31.8–37.9 kcal/mol) are very excellent hydride donors, and the hydride transfers process from DH− to hydride acceptors is extremely exothermic with ΔGH−T(DH−/H−-acceptor) << 0 kcal/mol. Therefore, it is reasonable to suppose that the concerted or successive proton and hydride (H+ + H−) transfer mechanism is thermodynamically feasible for the oxidation of dipines by hydride-acceptor/base pair in vivo.
- (4)
- The common bases, such Et3N (−25.3 kcal/mol), NH3 (−22.6 kcal/mol) or amino acids, iAscH− (−25.1 kcal/mol), PhCO2− (−28.4 kcal/mol), AcO− (−28.8 kcal/mol), and PhO− (−37.3 kcal/mol) in vivo could easily absorb protons from the related intermediates of DH2, i.e., DH2•+ (C4-H, −13.2–−9.3 kcal/mol), DH2•+ (N1-H, 22.0–24.1 kcal/mol), DH’• (20.1–24.1 kcal/mol), and DH+ (17.6–19.9 kcal/mol).
- (5)
- According to structural features, DH2•+ (N1-H, 22.0–24.1 kcal/mol) are typical aromatic radical cations, while DH’• (C4-H, 20.1–24.1 kcal/mol) are N-radical structures. However, they have thermodynamically similar proton-donating abilities for DH2•+ releasing protons from N1H bonds and DH’• releasing protons from C4-H bonds. Interestingly, DH2•+ (C4-H, −13.2–−9.3 kcal/mol) are much stronger C-acids than DH’• (C4-H, 20.1–24.1 kcal/mol) in acetonitrile.
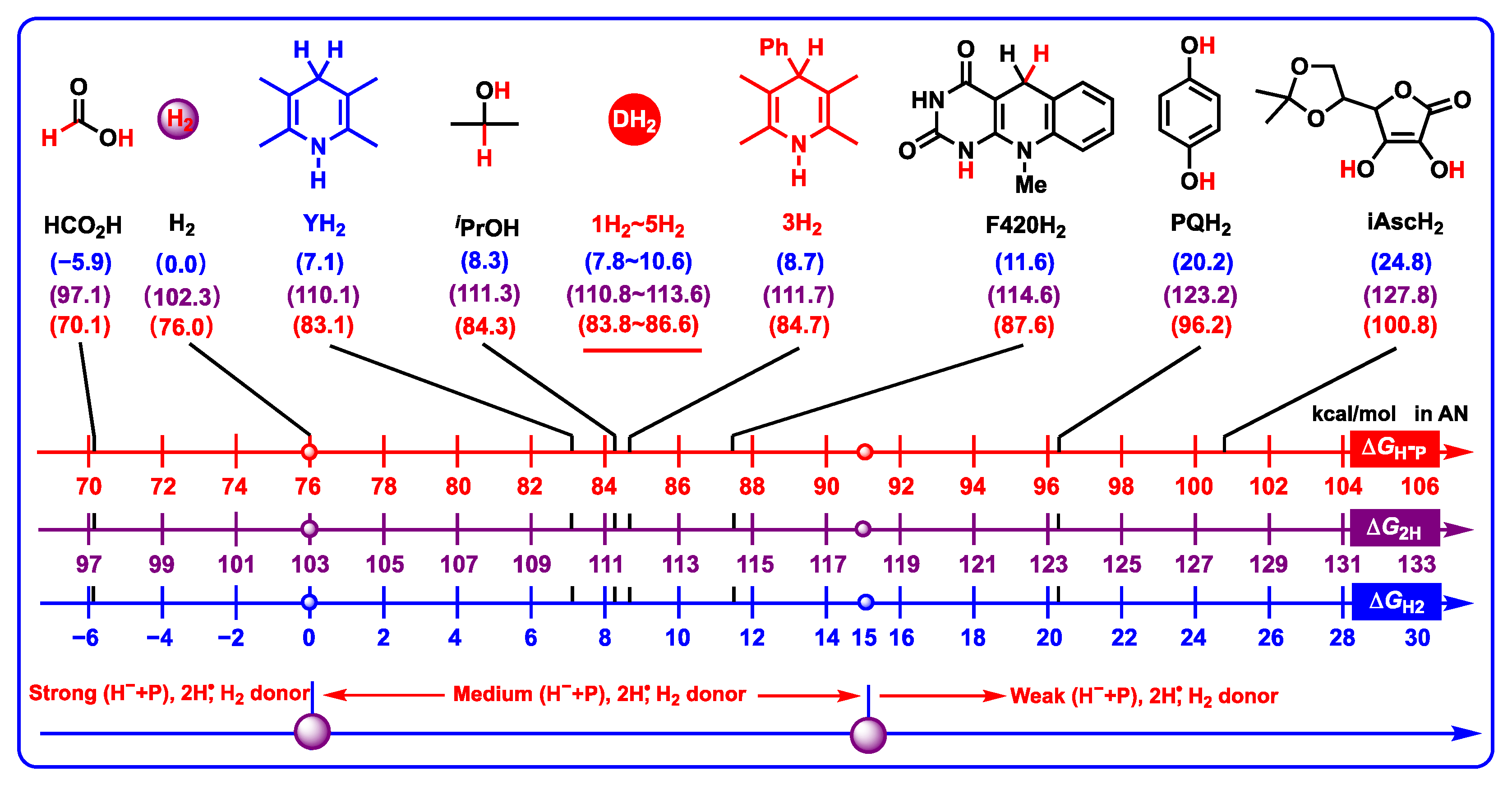
2.3.5. Two Hydrogen Ions (Atoms) Donating Properties
2.4. Application of Thermodynamic Data to Evaluate the Redox Properties of DH2
2.5. Application of Thermodynamic Data of Important Intermediates to Possible Oxidative Mechanism Judgement
2.6. Application of Thermodynamic Data of DH2 to Oxidative Mechanism Diagnosis between DH2 and iAsc
3. Materials and Methods
4. Conclusions
- (1)
- Electron-donating properties. The electron-donating abilities increase in the order of DH2 < DH− < DH• < D•−. DH2, DH−, DH•, and D•− are recognized as the weak electron donors, medium–strong electron donors, strong electron donors, and very strong electron donors, respectively.
- (2)
- Hydride-donating properties. DH2 and DH− belong to thermodynamically medium–strong and strong hydride donors, respectively. It can be inferred that dipines may be oxidated by heme enzyme, cytochrome P450, flavin coenzyme, coenzyme Q, and H+ under suitable oxidoreductase by hydride oxidation in vivo.
- (3)
- Hydrogen-atom-donating properties. The thermodynamic hydrogen-atom-donating abilities increase in the order of DH2 (N1-H) < DH− < DH2 (C4-H) < DH•< DH2•+ < DH’•. Dipines may be oxidated by heme enzyme, cytochrome P450, coenzyme Q (Co Q), and H• under suitable oxidoreductase, by hydrogen atom oxidation in vivo.
- (4)
- Proton-donating properties. DH2 belong to weak proton donors, DH2•+ (N1-H), DH’•, and DH+ belong to medium–strong proton donors, and DH2•+ (C4-H) belong to strong proton donors. After DH2 are oxidized by hydride acceptors, the acidities of resulting DH+ should be considered when the organic bases are involved in the reaction system.
- (5)
- Two hydrogen ions (atoms) donating properties. DH2 belong to medium–strong two hydrogen ion donors. H2 could hydrogenate D to regenerate DH2 in solution under suitable catalysts. DH2 maybe oxidated by Co Q via two hydrogen ions (atoms) transfer in vivo.
- (1)
- Application in evaluating the redox properties of DH2: DH2 generally release hydrogen atoms from the C4-H bond instead of the N1-H bond in the radical oxidation process. Generally, DH2 undergoing hydride or radical oxidations is thermodynamically favorable. Since DH2 are thermodynamically very weak proton donors, the concerted or successive proton and hydride (H+ + H−) transfer mechanism is thermodynamically practicable. The single-electron oxidation of DH2 is thermodynamically disadvantageous, and a high single-electron oxidant is needed.
- (2)
- Application in judging the possible oxidative mechanism for important intermediates: DH2•+ may act as proton donors or hydrogen atom donors, which depends on the properties of substrates. If the substrates are radicals, DH2•+, act as hydrogen atom donors. If the substrates are bases, DH2•+, act as proton donors. Most importantly, with a combination of the oxidation potentials of DH2 and the properties of resulting DH2•+ releasing hydrogen ions (atoms), the initial slight energy barrier (10–20 kcal/mol) of electron oxidation could be overcome by the subsequent protons transfer or hydrogen atoms transfer owning extreme thermodynamic driving forces (ΔG << 0).
- (3)
- The application of the thermodynamic data of DH2 to oxidative mechanism diagnosis between DH2 and iAsc: Because of the ortho dicarbonyl feature, Asc has the property of o-benzoquinone. Therefore, the oxidative mechanism between 3H2 and iAsc is taken as an example to represent the application of the thermodynamic cards of DH2 to oxidative mechanism diagnosis. Based on thermodynamic analyses, the oxidation of 3H2 by iAsc may experience three possible pathways, that is, H− + H+, H• + e + H+, and H• + H•.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, S.; Yan, N. Structural Basis of the Modulation of the Voltage-gated Calcium Ion Channel Cav1.1 by Dihydropyridine Compounds. Angew. Chem. Int. Ed. 2021, 60, 3131–3137. [Google Scholar] [CrossRef] [PubMed]
- Peri, R.; Padmanabhan, S.; Rutledge, A.; Singh, S.; Triggle, D.J. Permanently Charged Chiral 1,4-Dihydropyridines: Molecular Probes of L-Type Calcium Channels. Synthesis and Pharmacological Characterization of Methyl (ω-Trimethylalkylammonium) 1,4-Dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5- pyridinedicarboxylate Iodide, Calcium Channel Antagonists. J. Med. Chem. 2000, 43, 2906–2914. [Google Scholar]
- Längle, D.; Werner, T.R.; Wesseler, F.; Reckzeh, E.; Schaumann, N.; Drowley, L.; Polla, M.; Plowright, A.T.; Hirt, M.N.; Eschenhagen, T.; et al. Towards Second Generation Cardiomyogenic and Anticardiofibrotic 1,4-Dihydropyridine-Class of TGFβ Inhibitors. ChemMedChem 2019, 14, 810–822. [Google Scholar] [CrossRef]
- Knapik-Kowalczuk, J.; Tu, W.; Chmiel, K.; Rams-Baron, M.; Paluch, M. Co-Stabilization of Amorphous Pharmaceuticals—The Case of Nifedipine and Nimodipine. Mol. Pharm. 2018, 15, 2455–2465. [Google Scholar] [CrossRef]
- Pan, X.; Li, R.; Guo, H.; Zhang, W.; Xu, X.; Chen, X.; Ding, L. Dihydropyridine Calcium Channel Blockers Suppress the Transcription of PD-L1 by Inhibiting the Activation of STAT1. Front. Pharmacol. 2021, 11, 539261. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Liu, H.; Zhang, R.; Liu, M.; Hu, Z.; Panaye, A.; Doucet, J.P.; Fan, B. QSAR and Classification Study of 1,4-Dihydropyridine Calcium Channel Antagonists Based on Least Squares Support Vector Machines. Mol. Pharm. 2005, 2, 348–356. [Google Scholar] [CrossRef]
- Christen, D.P. Dihydropyridine Calcium Channel Blockers for Hypertension. Art Drug Synth. 2007, 159–167. [Google Scholar]
- Takahata, Y.; Costa, M.C.A.; Gaudio, A.C. Comparison between Neural Networks (NN) and Principal Component Analysis (PCA): Structure Activity Relationships of 1,4-Dihydropyridine Calcium Channel Antagonists (Nifedipine Analogues). J. Chem. Inf. Com. Sci. 2003, 43, 540–544. [Google Scholar] [CrossRef]
- Mulder, P.; Litwinienko, G.; Lin, S.; MacLean, P.D.; Barclay, L.R.C.; Ingold, K.U. The L-type Calcium Channel Blockers, Hantzsch 1,4-Dihydropyridines, Are Not Peroxyl Radical-Trapping, Chain Breaking Antioxidants. Chem. Res. Toxicol. 2006, 19, 79–85. [Google Scholar] [CrossRef]
- Shen, G.-B.; Xie, L.; Wang, Y.-X.; Gong, T.-Y.; Wang, B.-Y.; Hu, Y.-H.; Fu, Y.-H.; Yan, M. Quantitative Estimation of the Hydrogen-Atom-Donating Ability of 4-Substituted Hantzsch Ester Radical Cations. ACS Omega 2021, 6, 23621–23629. [Google Scholar] [CrossRef]
- Valikhani, D.; Bolivar, J.M.; Pelletier, J.N. An Overview of Cytochrome P450 Immobilization Strategies for Drug Metabolism Studies, Biosensing, and Biocatalytic Applications: Challenges and Opportunities. ACS Catal. 2021, 11, 9418–9434. [Google Scholar] [CrossRef]
- Mittra, K.; Green, M.T. Reduction Potentials of P450 Compounds I and II: Insight into the Thermodynamics of C-H Bond Activation. J. Am. Chem. Soc. 2019, 141, 5504–5510. [Google Scholar] [CrossRef]
- Li, M.; Yuan, Y.; Harrison, W.; Zhang, Z.; Zhao, H. Asymmetric Photoenzymatic Incorporation of Fluorinated Motifs into Olefins. Science 2024, 385, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Hyster, T.K. From Ground-State to Excited-State Activation Modes: Flavin-Dependent “Ene”-Reductases Catalyzed Non-natural Radical Reactions. Acc. Chem. Res. 2024, 57, 1446–1457. [Google Scholar] [CrossRef]
- Deng, W.-H.; Zhou, T.-P.; Liao, R.-Z. Computational Exploration of Enzyme Promiscuity: Mechanisms of O2 and NO Reduction Activities of the Desulfovibrio gigas Flavodiiron Protein. ACS Catal. 2023, 13, 16318–16336. [Google Scholar] [CrossRef]
- Guo, M.; Corona, T.; Ray, K.; Nam, W. Heme and Nonheme High-Valent Iron and Manganese Oxo Cores in Biological and Abiological Oxidation Reactions. ACS Cent. Sci. 2019, 5, 13–28. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, P.; Larson, V.A.; Kumar, A.; Li, X.-X.; Seo, M.S.; Lee, Y.-M.; Wang, B.; Lehnert, N.; Nam, W. Electronic Structure and Reactivity of Mononuclear Nonheme Iron–Peroxo Complexes as a Biomimetic Model of Rieske Oxygenases: Ring Size Effects of Macrocyclic Ligands. J. Am. Chem. Soc. 2024, 146, 250–262. [Google Scholar] [CrossRef]
- Ray, K.; Pfaff, F.F.; Wang, B.; Nam, W. Status of Reactive Non-Heme Metal-Oxygen Intermediates in Chemical and Enzymatic Reactions. J. Am. Chem. Soc. 2014, 136, 13942–13958. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Paine, T.K. Dioxygen Reduction and Bioinspired Oxidations by Non-heme Iron(II)−α-Hydroxy Acid Complexes. Acc. Chem. Res. 2023, 56, 3175–3187. [Google Scholar] [CrossRef]
- Bogeski, I.; Gulaboski, R.; Kappl, R.; Mirceski, V.; Stefova, M.; Petreska, J.; Hoth, M. Calcium Binding and Transport by Coenzyme Q. J. Am. Chem. Soc. 2011, 133, 9293–9303. [Google Scholar] [CrossRef]
- Wada, O.Z.; Rashid, N.; Wijten, P.; Thornalley, P.; Mckay, G.; Mackey, H.R. Evaluation of Cell Disruption Methods for Protein and Coenzyme Q10 Quantification in Purple Non-Sulfur Bacteria. Front. Microbiol. 2024, 15, 1324099. [Google Scholar] [CrossRef] [PubMed]
- Suenobu, T.; Shibata, S.; Fukuzumi, S. Catalytic Formation of Hydrogen Peroxide from Coenzyme NADH and Dioxygen with a Water-Soluble Iridium Complex and a Ubiquinone Coenzyme Analogue. Inorg. Chem. 2016, 55, 7747–7754. [Google Scholar] [CrossRef] [PubMed]
- Meka, A.K.; Gopalakrishna, A.; Iriarte-Mesa, C.; Rewatkar, P.; Qu, Z.; Wu, X.; Cao, Y.; Prasadam, I.; Janjua, T.I.; Kleitz, F.; et al. Influence of Pore Size and Surface Functionalization of Mesoporous Silica Nanoparticles on the Solubility and Antioxidant Activity of Confined Coenzyme Q10. Mol. Pharm. 2023, 20, 2966–2977. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, J.; Wu, Y.; Guo, Z.; Li, S.; Li, W.; Wu, Z.; Wang, H.; Jiang, H.; Jiang, Z. NADH Photosynthesis System with Affordable Electron Supply and Inhibited NADH Oxidation. Angew. Chem. Int. Ed. 2023, 62, e202310238. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shi, W.; Tian, S.; Zhou, Y.; Feng, C.; Ding, C.; Li, C. Synergy Between Metal and Molecular Catalysts for (Photo)electrocatalytic 1,4-NADH Regeneration. J. Phys. Chem. C 2024, 128, 5927–5933. [Google Scholar] [CrossRef]
- Yekta, R.; Xiong, X.; Li, J.; Heater, B.S.; Lee, M.M.; Chan, M.K. Mechanoresponsive Protein Crystals for NADH Recycling in Multicycle Enzyme Reactions. J. Am. Chem. Soc. 2024, 146, 18817–18822. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.-B.; Qian, B.-C.; Fu, Y.-H.; Zhu, X.-Q. Thermodynamics of the Elementary Steps of Organic Hydride Chemistry Determined in Acetonitrile and their Applications. Org. Chem. Front. 2022, 9, 6001–6062. [Google Scholar] [CrossRef]
- Agarwal, R.G.; Coste, S.C.; Groff, B.D.; Heuer, A.M.; Noh, H.; Parada, G.A.; Wise, C.F.; Nichols, E.M.; Warren, J.J.; Mayer, J.M. Free Energies of Proton-Coupled Electron Transfer Reagents and Their Applications. Chem. Rev. 2022, 122, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.J.; Tronic, T.A.; Mayer, J.M. Thermochemistry of Proton-Coupled Electron Transfer Reagents and its Implications. Chem. Rev. 2010, 110, 6961–7001. [Google Scholar] [CrossRef]
- Böckert, R.H.; Guengerich, F.P. Oxidation of 4-Aryl- and 4-Alkyl-Substituted 2,6-Dimethyl-3,5-bis(alkoxycarbony1)-1,4-dihydropyridines by Human Liver Microsomes and Immunochemical Evidence for the Involvement of a Form of Cytochrome P-450. J. Med. Chem. 1986, 29, 1596–1603. [Google Scholar] [CrossRef]
- Shen, G.-B.; Fu, Y.-H.; Zhu, X.-Q. Thermodynamic Network Cards of Hantzsch Ester, Benzothiazoline, and Dihydrophenanthridine Releasing Two Hydrogen Atoms or Ions on 20 Elementary Steps. J. Org. Chem. 2020, 85, 12535–12543. [Google Scholar] [CrossRef] [PubMed]
- Ilic, S.; Alherz, A.; Musgrave, C.B.; Glusac, K.D. Thermodynamic and kinetic hydricities of metal-free hydrides. Chem. Soc. Rev. 2018, 47, 2809–2836. [Google Scholar] [PubMed]
- Ilic, S.; Kadel, U.P.; Basdogan, Y.; Keith, J.A.; Glusac, K.D. Thermodynamic Hydricities of Biomimetic Organic Hydride Donors. J. Am. Chem. Soc. 2018, 140, 4569–4579. [Google Scholar] [CrossRef] [PubMed]
- Tsay, C.; Livesay, B.N.; Ruelas, S.; Yang, J.Y. Solvation Effects on Transition Metal Hydricity. J. Am. Chem. Soc. 2015, 137, 14114–14121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-M.; Bruno, J.W.; Enyinnaya, E. Hydride Affinities of Arylcarbenium Ions and Iminium Ions in Dimethyl Sulfoxide and Acetonitrile. J. Org. Chem. 1998, 63, 4671–4678. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Deng, F.-H.; Yang, J.-D.; Li, X.-T.; Chen, Q.; Lei, N.-P.; Meng, F.-K.; Zhao, X.-P.; Han, S.-H.; Hao, E.-J.; et al. A Classical but New Kinetic Equation for Hydride Transfer Reactions. Org. Biomol. Chem. 2013, 11, 6071–6089. [Google Scholar] [CrossRef]
- Shen, G.-B.; Qian, B.-C.; Fu, Y.-H.; Zhu, X.-Q. Discovering and Evaluating the Reducing Abilities of Polar Alkanes and Related Family Members as Organic Reductants Using Thermodynamics. J. Org. Chem. 2022, 87, 9357–9374. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Liu, Q.-Y.; Chen, Q.; Mei, L.-R. Hydride, Hydrogen, Proton, and Electron Affinities of Imines and Their Reaction Intermediates in Acetonitrile and Construction of Thermodynamic Characteristic Graphs (TCGs) of Imines as a “Molecule ID Card.”. J. Org. Chem. 2010, 75, 789–808. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.-B.; Qian, B.-C.; Luo, G.-Z.; Fu, Y.-H.; Zhu, X.-Q. Thermodynamic Evaluations of Amines as Hydrides or Two Hydrogen Ions Reductants, and Imines as Protons or Two Hydrogen Ions Acceptors, as well as Their Application in Hydrogenation Reactions. ACS Omega 2023, 8, 31984–31997. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Yang, J.-D.; Liu, Y.; Zhang, L.; Luo, S.; Cheng, J.-P. Holistic prediction of pKa in diverse solvents based on machine learning approach. Angew. Chem. Int. Ed. 2020, 59, 19282–19291. [Google Scholar] [CrossRef]
- Wiedner, E.S.; Chambers, M.B.; Pitman, C.L.; Bullock, R.M.; Miller, A.J.M.; Appel, A.M. Thermodynamic Hydricity of Transition Metal Hydrides. Chem. Rev. 2016, 116, 8655–8692. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-C.; Wang, X.; Wang, Q.; Zhu, X.-Q.; Shen, G.-B. Thermodynamic Evaluations on the Acceptorless Dehydrogenation and Hydrogenation of Pre-Aromatic and Aromatic N-Heterocycles in Acetonitrile. RSC Adv. 2024, 14, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.-B.; Qian, B.-C.; Zhang, G.-S.; Luo, G.-Z.; Fu, Y.-H.; Zhu, X.-Q. Thermodynamics Regulated Organic Hydride/Acid Pairs as Novel Organic Hydrogen Reductants. Org. Chem. Front. 2022, 9, 6833–6848. [Google Scholar] [CrossRef]
- Kütt, A.; Tshepelevitsh, S.; Saame, J.; Lõkov, M.; Kaljurand, I.; Selberg, S.; Leito, I. Strengths of Acids in Acetonitrile. Eur. J. Org. Chem. 2021, 2021, 1407–1419. [Google Scholar] [CrossRef]
- Tshepelevitsh, S.; Kütt, A.; Lõkov, M.; Kaljurand, I.; Saame, J.; Heering, A.; Plieger, P.G.; Vianello, R.; Leito, I. On the Basicity of Organic Bases in Different Media. Eur. J. Org. Chem. 2019, 2019, 6735–6748. [Google Scholar] [CrossRef]
- Peng, B.; Ma, J.; Guo, J.; Gong, Y.; Wang, R.; Zhang, Y.; Zeng, J.; Chen, W.-W.; Ding, K.; Zhao, B. A Powerful Chiral Super Brønsted C-H Acid for Asymmetric Catalysis. J. Am. Chem. Soc. 2022, 144, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- Liles, J.P.; Rouget-Virbel, C.; Wahlman, J.L.H.; Rahimoff, R.; Crawford, J.M.; Medlin, A.; O’Connor, V.S.; Li, J.; Roytman, V.A.; Toste, F.D.; et al. Data Science Enables the Development of a New Class of Chiral Phosphoric Acid Catalysts. Chem 2023, 9, 1518–1537. [Google Scholar] [CrossRef] [PubMed]
- Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev. 2014, 114, 9047–9153. [Google Scholar] [CrossRef] [PubMed]
- Kaupmees, K.; Tolstoluzhsky, N.; Raja, S.; Rueping, M.; Leito, I. On the Acidity and Reactivity of Highly Effective Chiral Brønsted Acid Catalysts: Establishment of an Acidity Scale. Angew. Chem. Int. Ed. 2013, 52, 11569–11572. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Zhang, J.-Y.; Cheng, J.-P. Negative Kinetic Temperature Effect on the Hydride Transfer from NADH Analogue BNAH to the Radical Cation of N-Benzylphenothiazine in Acetonitrile. J. Org. Chem. 2006, 71, 7007–7015. [Google Scholar] [CrossRef]
- Chen, B.-L.; Yan, S.-Y.; Zhu, X.-Q. A Mechanism Study of Redox Reactions of the Ruthenium-oxo-polypyridyl Complex. Molecules 2023, 28, 4401. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.-Q.; Mu, Y.-Y.; Li, X.-T. What Are the Differences between Ascorbic Acid and NADH as Hydride and Electron Sources in Vivo on Thermodynamics, Kinetics, and Mechanism? J. Phys. Chem. B 2011, 115, 14794–14811. [Google Scholar] [CrossRef]
- Fu, Y.-H.; Wang, F.; Zhao, L.; Zhang, Y.; Shen, G.-B.; Zhu, X.-Q. Thermodynamic and Kinetic Studies of the Activities of Aldehydic C-H Bonds toward Their H-Atom Transfer Reactions. ChemistrySelect 2023, 8, e202204789. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, Y.-L.; Qian, B.-C.; Shen, G.-B. Thermodynamic H-Abstraction Abilities of Nitrogen Centered Radical Cations as Potential HAT Catalysts in Y-H Bond Functionalization. ACS Omega 2024, 9, 26708–26718. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.-B.; Xie, L.; Yu, H.-Y.; Liu, J.; Fu, Y.-H.; Yan, M. Theoretical Investigation on the Nature of 4-Substituted Hantzsch Esters as Alkylation Agents. RSC Adv. 2020, 10, 31425–31434. [Google Scholar] [CrossRef]
- Zheng, C.; You, S.-L. Transfer Hydrogenation with Hantzsch Esters and Related Organic Hydride Donors. Chem. Soc. Rev. 2012, 41, 2498–2518. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, S.G.; Walji, A.M.; Macmillan, D.W.C. Enantioselective Organocatalytic Transfer Hydrogenation Reactions Using Hantzsch Esters. Acc. Chem. Res. 2007, 40, 1327–1339. [Google Scholar] [CrossRef]
- Rueping, M.; Dufour, J.; Schoepke, F.R. Advances in Catalytic Metal-Free Reductions: From Bioinspired Concepts to Applications in the Organocatalytic Synthesis of Pharmaceuticals and Natural Products. Green Chem. 2011, 13, 1084–1105. [Google Scholar] [CrossRef]
- Cordell, G.A.; Daley, S. Pyrroloquinoline Quinone Chemistry, Biology, and Biosynthesis. Chem. Res. Toxicol. 2022, 35, 355–377. [Google Scholar] [CrossRef]




| Step X | Parameters | 1H2 | 2H2 | 3H2 | 4H2 | 5H2 | YH2 |
|---|---|---|---|---|---|---|---|
| Step 1 | ΔGH−D(DH2) | 63.9 | 64.7 | 66.0 | 66.9 | 69.0 | 64.4 |
| Step 2 | Eox(DH2) | 0.712 | 0.721 | 0.731 | 0.745 | 0.781 | 0.479 |
| Step 3 | Eox(DH•) | −0.980 | −0.932 | −0.856 | −0.775 | −0.655 | −1.112 |
| Step 4 | ΔGHD(DH2) | 60.3 | 60.0 | 59.5 | 58.6 | 57.9 | 63.8 |
| Step 5 | ΔGHD(DH2•+) | 21.3 | 21.9 | 22.9 | 23.5 | 24.9 | 27.1 |
| Step 6 | ΔGPD(DH2•+) | −9.3 | −9.9 | −10.5 | −11.8 | −13.2 | −0.4 |
| Step 7 | ΔGH−D(DH−) | 31.8 | 32.2 | 33.2 | 34.6 | 37.9 | 34.3 |
| Step 8 | Eox(DH−) | −0.497 | −0.491 | −0.479 | −0.444 | −0.382 | −0.695 |
| Step 9 | Eox(D•−) | −2.611 | −2.580 | −2.528 | −2.453 | −2.289 | −2.446 |
| Step 10 | ΔGHD(DH−) | 65.7 | 65.4 | 65.2 | 64.9 | 64.4 | 64.4 |
| Step 11 | ΔGHD(DH’•) | 17.1 | 17.3 | 18.0 | 18.6 | 20.5 | 24.1 |
| Step 12 | ΔGPD(DH’•) | 24.1 | 23.6 | 23.1 | 22.0 | 20.1 | 27.4 |
| Step 13 | ΔGPD(DH+) | 19.9 | 19.5 | 18.7 | 17.6 | 17.6 | 18.7 |
| Step 14 | ΔGH−P(DH2) | 83.8 | 84.2 | 84.7 | 84.5 | 86.6 | 83.1 |
| Step 15 | ΔGPD(DH2) | 52.0 | 52.0 | 51.5 | 49.9 | 48.7 | 48.8 |
| Step 16 | ΔG2H(DH2) | 110.8 | 111.2 | 111.7 | 111.5 | 113.6 | 110.1 |
| Step 17 | ΔGHD(DH•) | 50.5 | 51.2 | 52.2 | 52.9 | 55.7 | 46.3 |
| Step 18 | ΔG’HD(DH2) | 93.7 | 93.9 | 93.7 | 92.9 | 93.1 | 86.0 |
| Step 19 | ΔGH2(DH2) | 7.8 | 8.2 | 8.7 | 8.5 | 10.6 | 7.1 |
| Step 20 | ΔGHT(DH•) | 33.4 | 33.9 | 34.2 | 34.3 | 35.2 | 22.2 |
| Step 21 | ΔG’PD(DH2•+) | 24.1 | 24.0 | 23.7 | 22.5 | 22.0 | 21.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, G.-B.; Gao, S.-H.; Jia, Y.-W.; Zhu, X.-Q.; Qian, B.-C. Establishing the Thermodynamic Cards of Dipine Models’ Oxidative Metabolism on 21 Potential Elementary Steps. Molecules 2024, 29, 3706. https://doi.org/10.3390/molecules29153706
Shen G-B, Gao S-H, Jia Y-W, Zhu X-Q, Qian B-C. Establishing the Thermodynamic Cards of Dipine Models’ Oxidative Metabolism on 21 Potential Elementary Steps. Molecules. 2024; 29(15):3706. https://doi.org/10.3390/molecules29153706
Chicago/Turabian StyleShen, Guang-Bin, Shun-Hang Gao, Yan-Wei Jia, Xiao-Qing Zhu, and Bao-Chen Qian. 2024. "Establishing the Thermodynamic Cards of Dipine Models’ Oxidative Metabolism on 21 Potential Elementary Steps" Molecules 29, no. 15: 3706. https://doi.org/10.3390/molecules29153706
APA StyleShen, G.-B., Gao, S.-H., Jia, Y.-W., Zhu, X.-Q., & Qian, B.-C. (2024). Establishing the Thermodynamic Cards of Dipine Models’ Oxidative Metabolism on 21 Potential Elementary Steps. Molecules, 29(15), 3706. https://doi.org/10.3390/molecules29153706







