Analytical and Antimicrobial Characterization of Zn-Modified Clays Embedding Thymol or Carvacrol
Abstract
1. Introduction
2. Results and Discussion
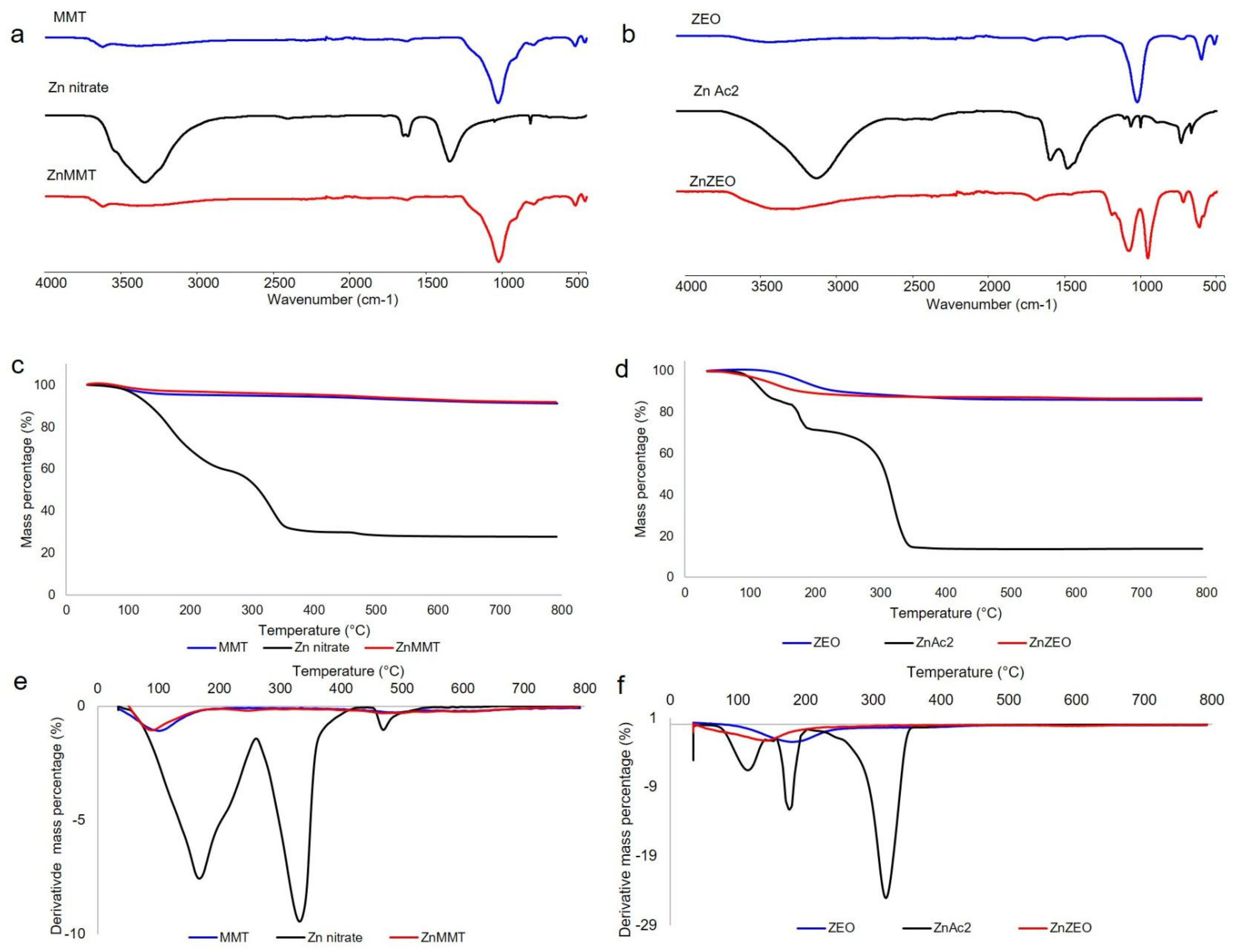
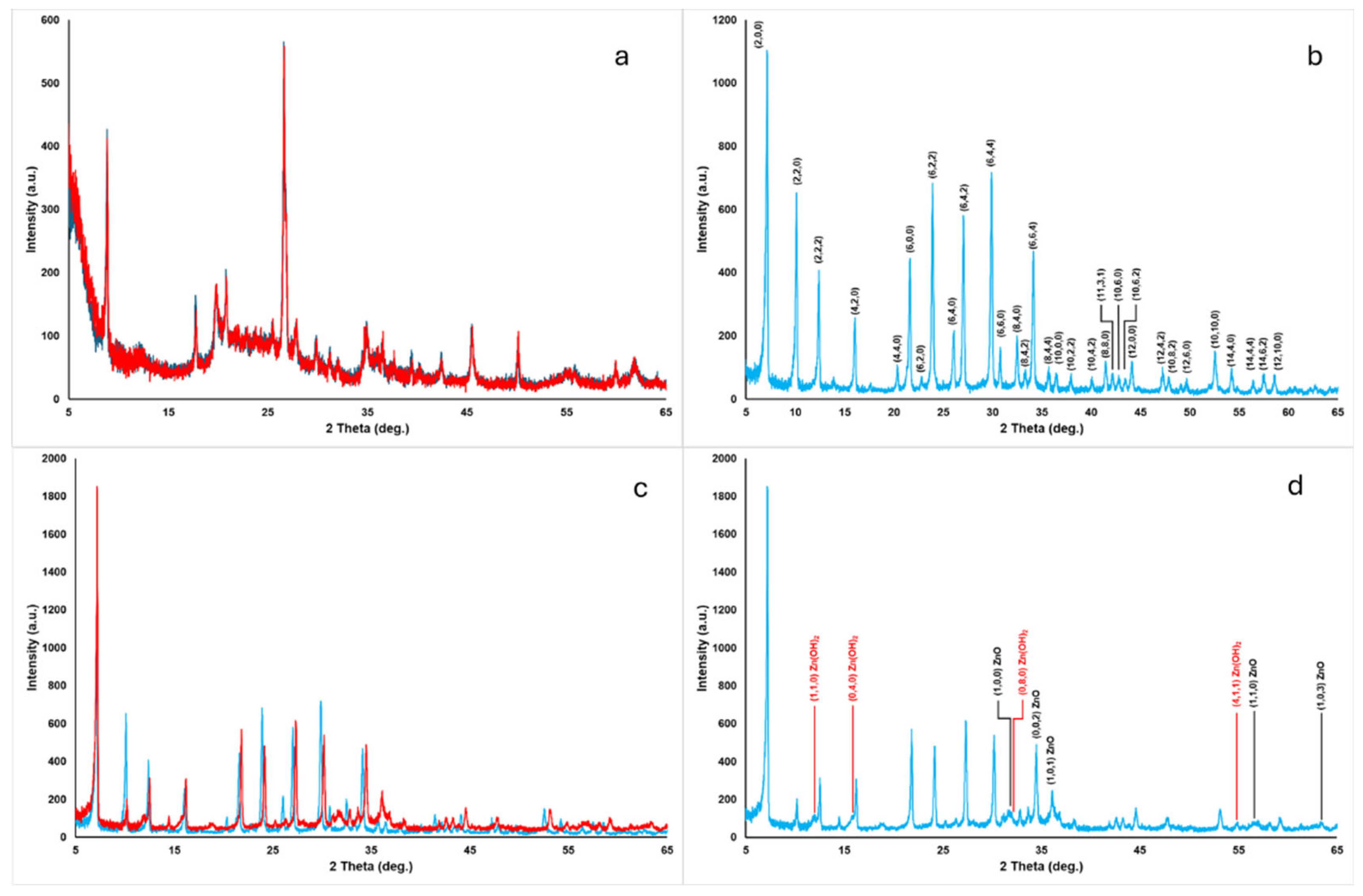
2.1. Chemical–Physical Characterization of the Zn-Modified Clays
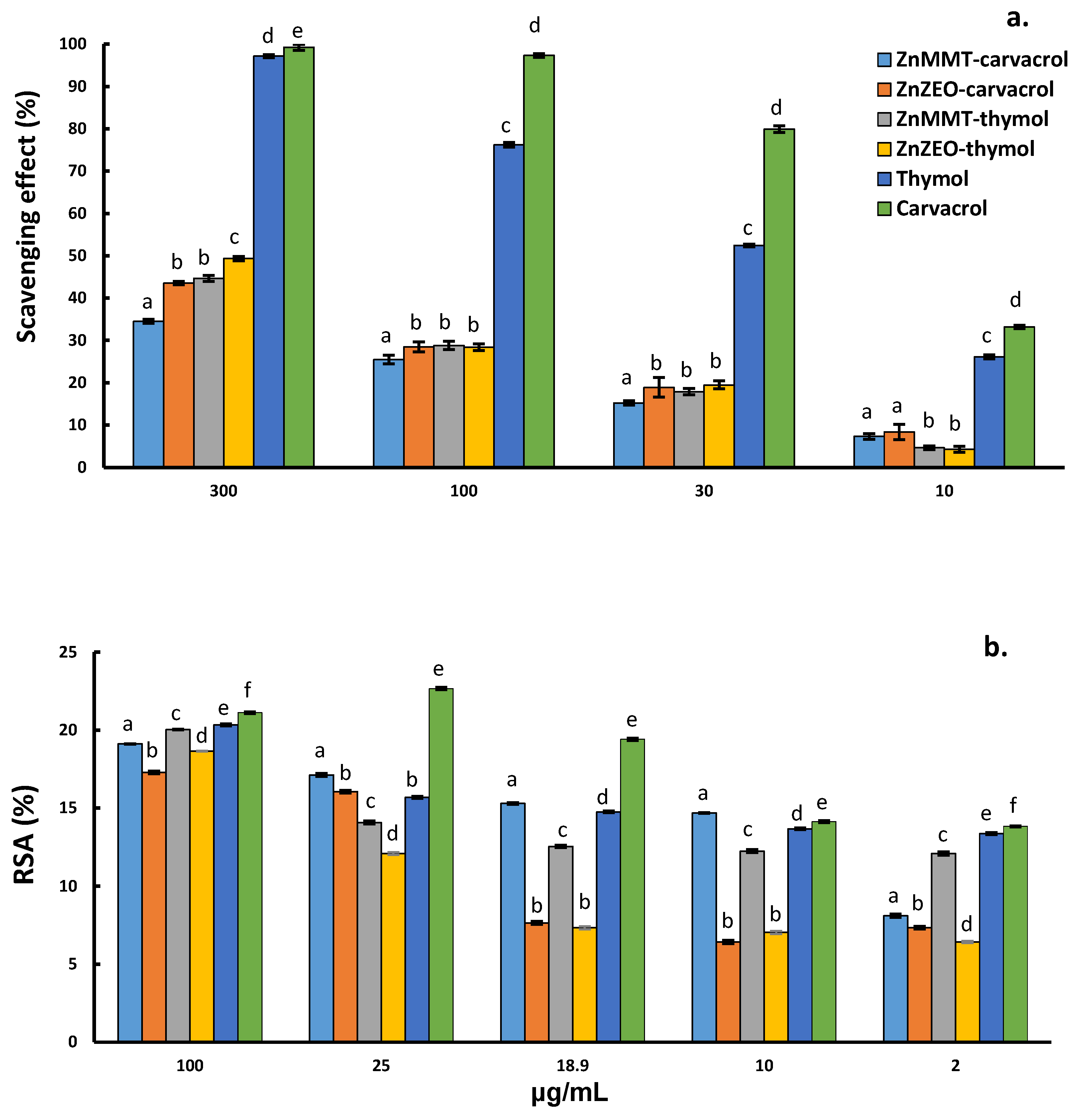
2.2. Antioxidant Activity Evaluation of Thymol and Carvacrol
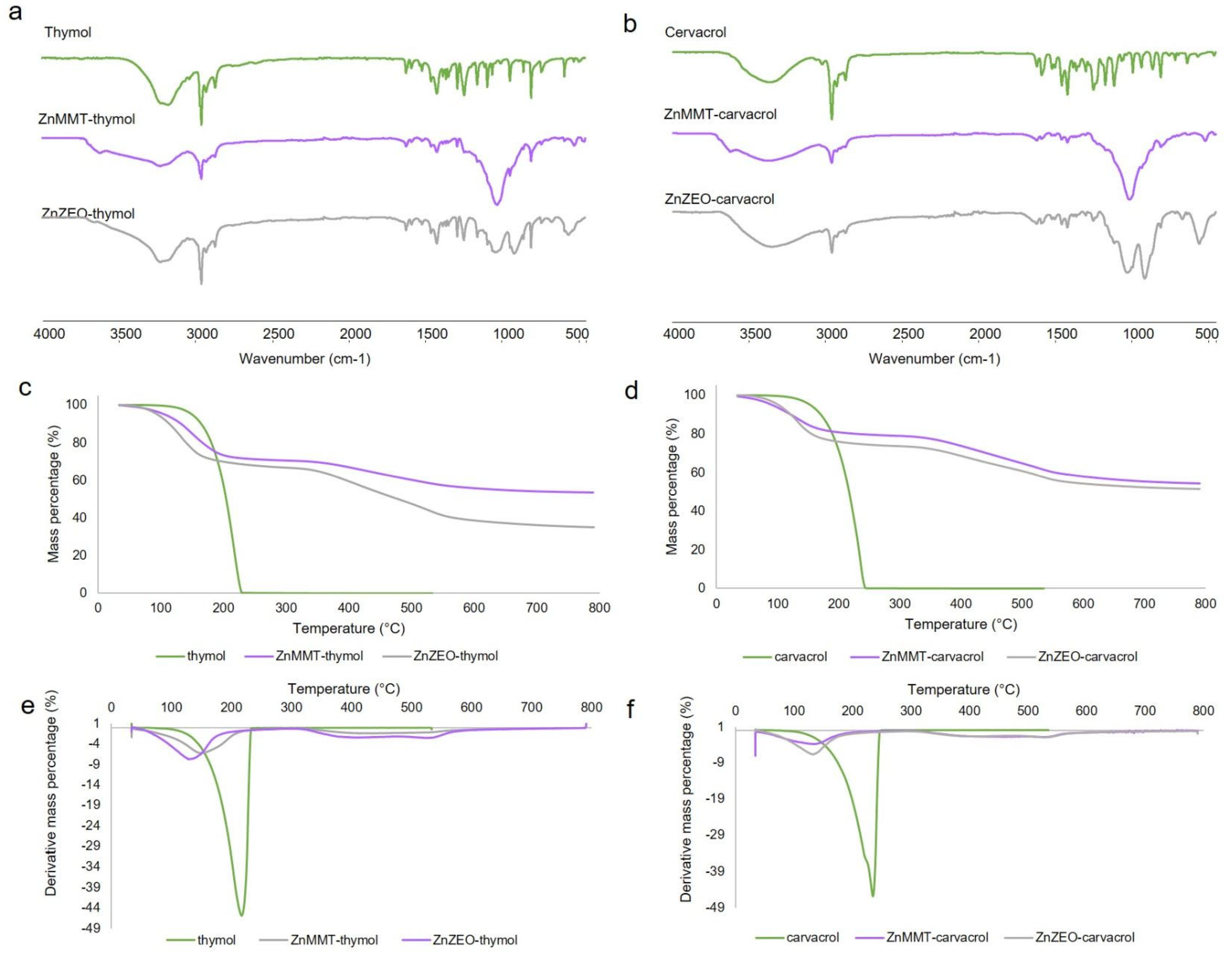
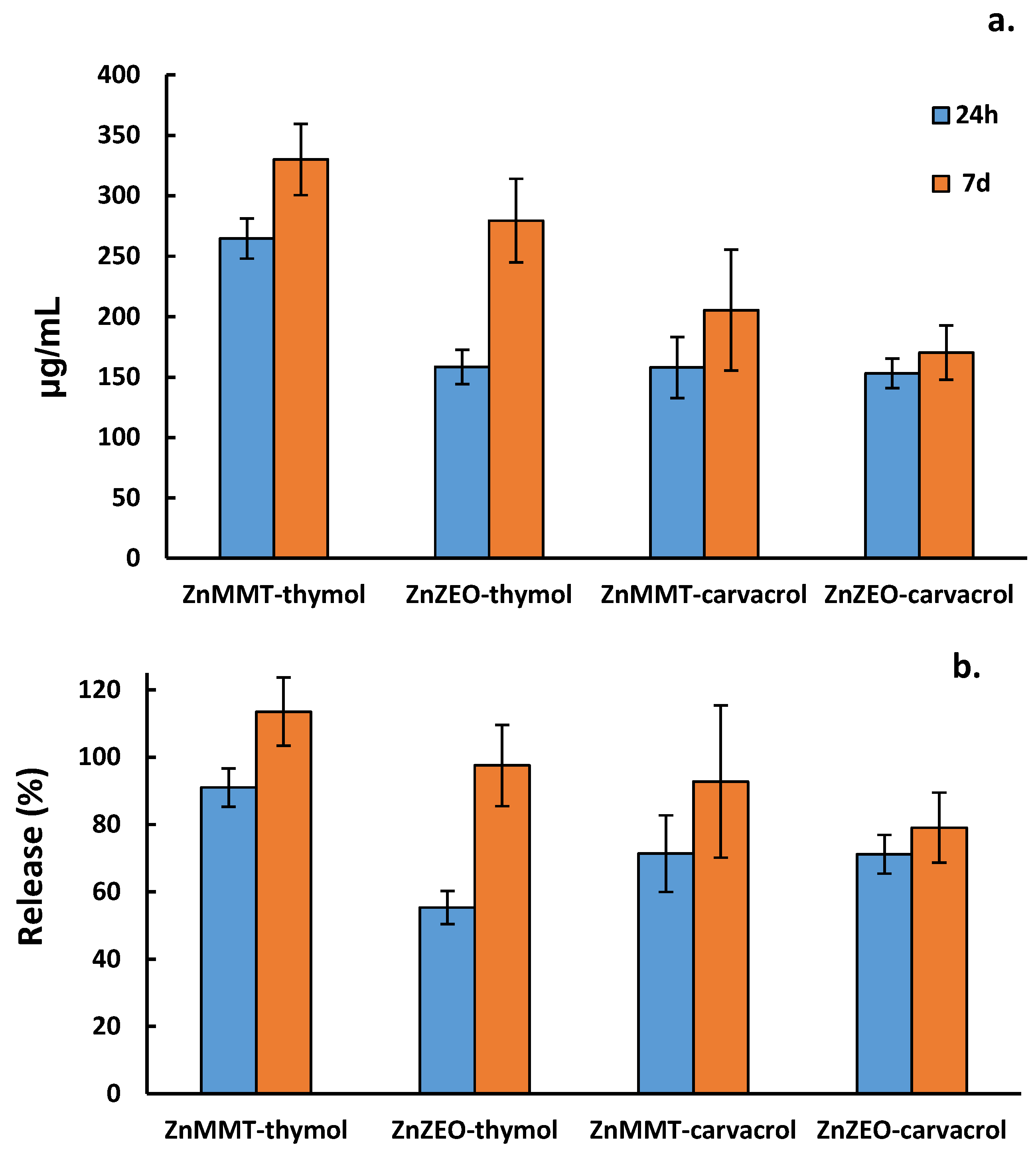
2.3. Loading Capacity and Loading Efficiency of Thymol and Carvacrol by GC-MS Analysis
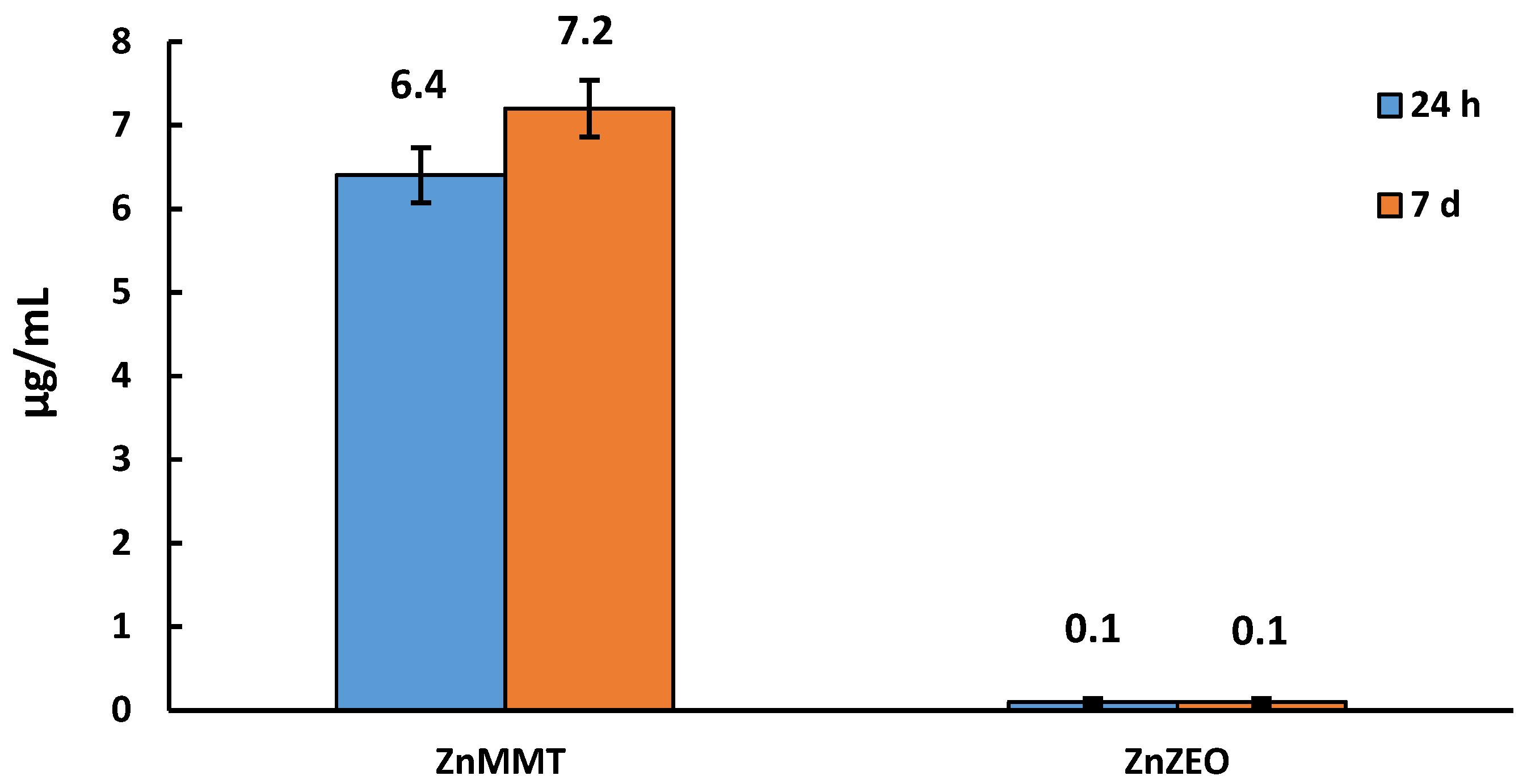
2.4. Quantification of Zinc by Spectrophotometric Analysis
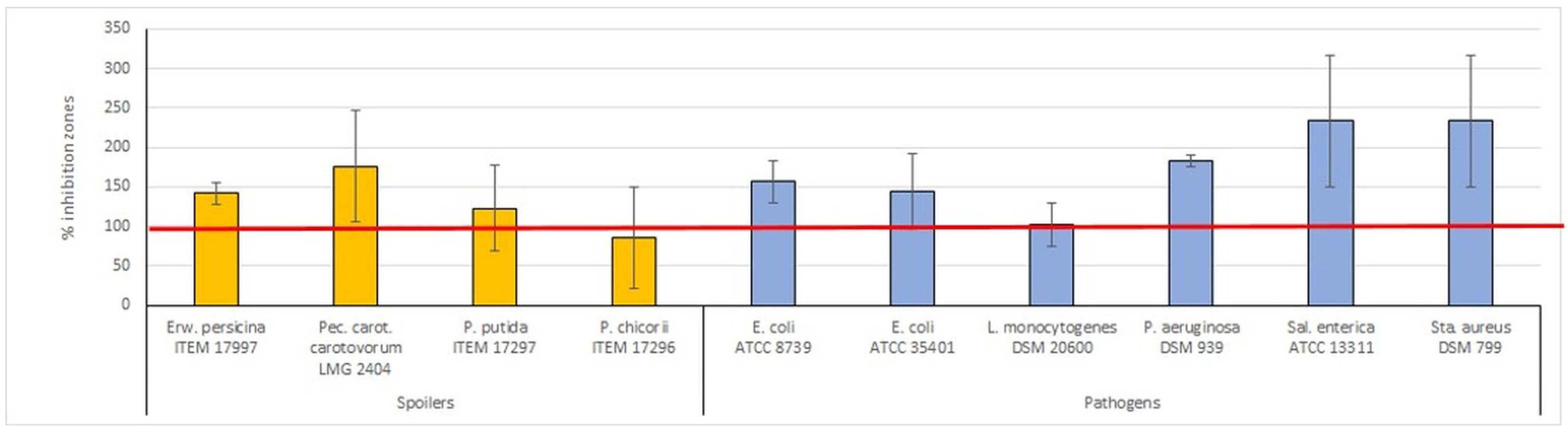
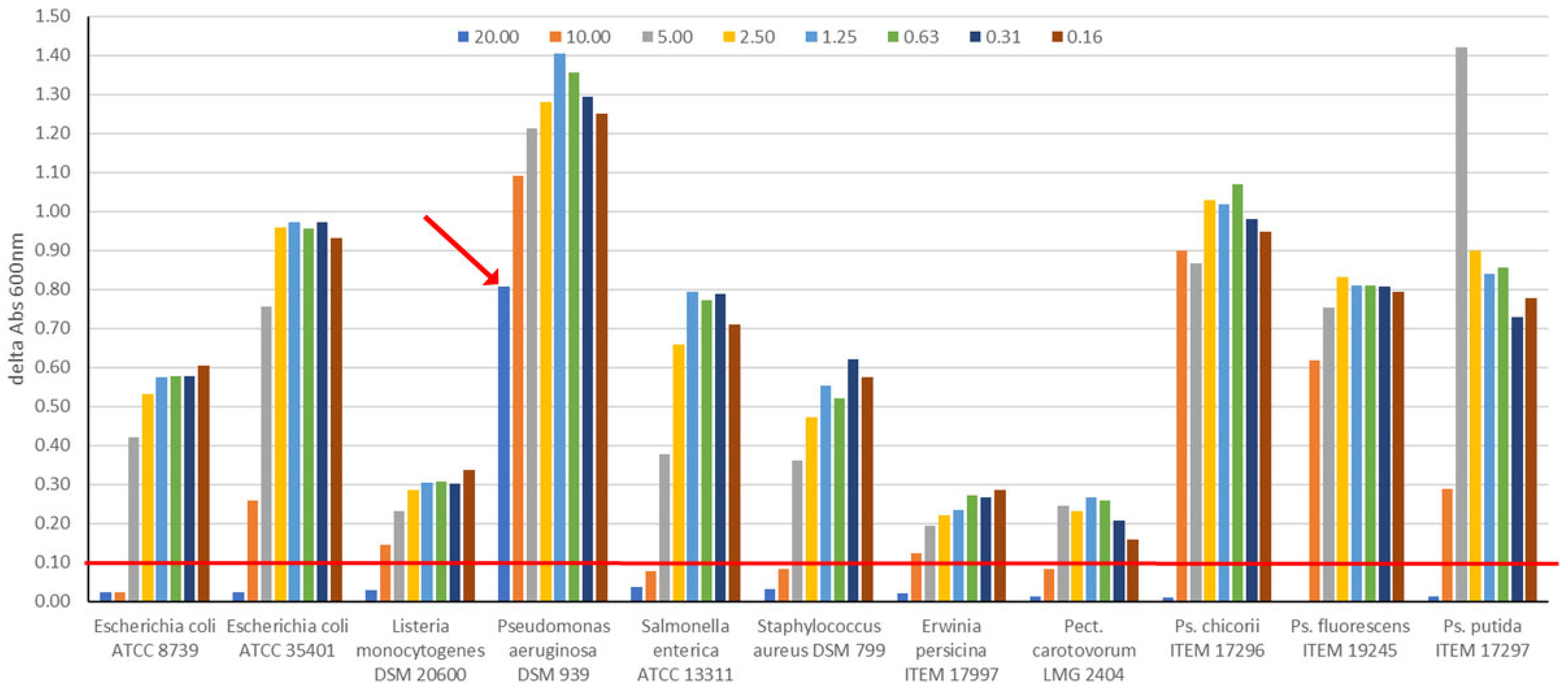
2.5. Antimicrobial Activity
3. Materials and Methods
3.1. Materials
3.2. Development of Zn-Modified Clays
3.3. Loading of the Zn-Modified Clays with Thymol or Carvacrol
3.4. Chemical–Physical Characterization of the Zn-Modified Clays
3.5. Quantification of Thymol and Carvacrol by GC-MS
3.6. Quantification of Zinc by Spectrophotometric Analysis
3.7. Antioxidant Activity Evaluation of Thymol and Carvacrol
3.8. Bacterial Strains and Growth Conditions
3.9. Evaluation of Antibacterial Activity
3.10. Statistical Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villalobos-Delgado, L.H.; Nevárez-Moorillon, G.V.; Caro, I.; Quinto, E.J.; Mateo, J. Natural Antimicrobial Agents to Improve Foods Shelf Life. In Food Quality and Shelf Life; Elsevier: Amsterdam, The Netherlands, 2019; pp. 125–157. ISBN 978-0-12-817190-5. [Google Scholar]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef] [PubMed]
- Najafloo, R.; Behyari, M.; Imani, R.; Nour, S. A Mini-Review of Thymol Incorporated Materials: Applications in Antibacterial Wound Dressing. J. Drug Deliv. Sci. Technol. 2020, 60, 101904. [Google Scholar] [CrossRef]
- Rathod, N.B.; Kulawik, P.; Ozogul, F.; Regenstein, J.M.; Ozogul, Y. Biological Activity of Plant-Based Carvacrol and Thymol and Their Impact on Human Health and Food Quality. Trends Food Sci. Technol. 2021, 116, 733–748. [Google Scholar] [CrossRef]
- Michalska-Sionkowska, M.; Walczak, M.; Sionkowska, A. Antimicrobial Activity of Collagen Material with Thymol Addition for Potential Application as Wound Dressing. Polym. Test. 2017, 63, 360–366. [Google Scholar] [CrossRef]
- Luna, M.; Beltran, O.; Encinas-Basurto, D.A.; Ballesteros-Monrreal, M.G.; Topete, A.; Hassan, N.; López-Mata, M.A.; Reyes-Márquez, V.; Valdez, M.A.; Juarez, J. High Antibacterial Performance of Hydrophobic Chitosan-Based Nanoparticles Loaded with Carvacrol. Colloids Surf. B Biointerfaces 2022, 209, 112191. [Google Scholar] [CrossRef] [PubMed]
- Hajibonabi, A.; Yekani, M.; Sharifi, S.; Nahad, J.S.; Dizaj, S.M.; Memar, M.Y. Antimicrobial Activity of Nanoformulations of Carvacrol and Thymol: New Trend and Applications. OpenNano 2023, 13, 100170. [Google Scholar] [CrossRef]
- Kinninmonth, M.A.; Liauw, C.M.; Verran, J.; Taylor, R.; Edwards-Jones, V.; Shaw, D.; Webb, M. Investigation into the Suitability of Layered Silicates as Adsorption Media for Essential Oils Using FTIR and GC–MS. Appl. Clay Sci. 2013, 83–84, 415–425. [Google Scholar] [CrossRef]
- Wu, H.; Lu, J.; Xiao, D.; Yan, Z.; Li, S.; Li, T.; Wan, X.; Zhang, Z.; Liu, Y.; Shen, G.; et al. Development and Characterization of Antimicrobial Protein Films Based on Soybean Protein Isolate Incorporating Diatomite/Thymol Complex. Food Hydrocoll. 2021, 110, 106138. [Google Scholar] [CrossRef]
- Zhong, H.; Mu, B.; Zhang, M.; Hui, A.; Kang, Y.; Wang, A. Preparation of Effective Carvacrol/Attapulgite Hybrid Antibacterial Materials by Mechanical Milling. J. Porous Mater. 2020, 27, 843–853. [Google Scholar] [CrossRef]
- Dikić, J.; Lukić, I.; Pajnik, J.; Pavlović, J.; Hrenović, J.; Rajić, N. Antibacterial Activity of Thymol/Carvacrol and Clinoptilolite Composites Prepared by Supercritical Solvent Impregnation. J. Porous Mater. 2021, 28, 1577–1584. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Bellissimo, A.; Pinto, L.; Petrella, A.; De Vietro, N.; Iannaccone, G.; Baruzzi, F.; De Giglio, E. A Green Approach to Develop Zeolite-Thymol Antimicrobial Composites: Analytical Characterization and Antimicrobial Activity Evaluation. Heliyon 2022, 8, e09551. [Google Scholar] [CrossRef] [PubMed]
- Essifi, K.; Hammani, A.; Berraaouan, D.; El Bachiri, A.; Fauconnier, M.-L.; Tahani, A. Montmorillonite Nanoclay Based Formulation for Controlled and Selective Release of Volatile Essential Oil Compounds. Mater. Chem. Phys. 2022, 277, 125569. [Google Scholar] [CrossRef]
- De Oliveira, L.H.; Trigueiro, P.; Souza, J.S.N.; De Carvalho, M.S.; Osajima, J.A.; Da Silva-Filho, E.C.; Fonseca, M.G. Montmorillonite with Essential Oils as Antimicrobial Agents, Packaging, Repellents, and Insecticides: An Overview. Colloids Surf. B Biointerfaces 2022, 209, 112186. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Carriles, C.; Macías-Rodríguez, M.E.; Ramírez-Alvarado, O.; Corona-González, R.I.; Macías-Lamas, A.; García-Vera, I.; Cavazos-Garduño, A.; Villagrán, Z.; Silva-Jara, J.M. Nanohybrid of Thymol and 2D Simonkolleite Enhances Inhibition of Bacterial Growth, Biofilm Formation, and Free Radicals. Molecules 2022, 27, 6161. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Zaharioudakis, K.; Georgopoulos, S.; Asimakopoulos, G.; Aktypis, A.; Proestos, C.; Karakassides, A.; Avgeropoulos, A.; et al. The Increase of Soft Cheese Shelf-Life Packaged with Edible Films Based on Novel Hybrid Nanostructures. Gels 2022, 8, 539. [Google Scholar] [CrossRef]
- Salmas, C.E.; Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Karabagias, I.K.; Gioti, C.; Georgopoulos, S.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Development and Evaluation of a Novel-Thymol@Natural-Zeolite/Low-Density-Polyethylene Active Packaging Film: Applications for Pork Fillets Preservation. Antioxidants 2023, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Salmas, C.E.; Kollia, E.; Avdylaj, L.; Kopsacheili, A.; Zaharioudakis, K.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Kehayias, G.; Karakassides, A.; et al. Thymol@Natural Zeolite Nanohybrids for Chitosan/Polyvinyl-Alcohol-Based Hydrogels Applied as Active Pads. Gels 2023, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to Combat Antimicrobial Resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial Properties of ZnO Nanomaterials: A Review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Jiao, L.F.; Ke, Y.L.; Xiao, K.; Song, Z.H.; Lu, J.J.; Hu, C.H. Effects of Zinc-Exchanged Montmorillonite with Different Zinc Loading Capacities on Growth Performance, Intestinal Microbiota, Morphology and Permeability in Weaned Piglets. Appl. Clay Sci. 2015, 112–113, 40–43. [Google Scholar] [CrossRef]
- Tanchuling, M.A.; Khan, M.R.A.; Kusakabe, O. Sorption of Zinc in Bentonite, Illite and Kaolin Clay Using Column Leaching Tests. In Contaminated Soils, Sediments and Water; Calabrese, E.J., Kostecki, P.T., Dragun, J., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2005; pp. 251–263. ISBN 978-0-387-23036-8. [Google Scholar]
- Jiao, L.; Lin, F.; Cao, S.; Wang, C.; Wu, H.; Shu, M.; Hu, C. Preparation, Characterization, Antimicrobial and Cytotoxicity Studies of Copper/Zinc- Loaded Montmorillonite. J. Anim. Sci. Biotechnol. 2017, 8, 27. [Google Scholar] [CrossRef]
- Nunes Dos Santos, A.; Brito França, D.; Humberto De Oliveira, L.; Sá De Lima, I.; Anteveli Osajima, J.; Cavalcanti Silva-Filho, E.; Rigout, B.; Jaber, M.; Gardênnia Fonseca, M. Zn(II) Loaded Silylated Bentonites as Antibacterial Materials: Influence of the Surface Functionalization. Appl. Surf. Sci. 2024, 659, 159878. [Google Scholar] [CrossRef]
- Niu, B.; Yan, Z.; Shao, P.; Kang, J.; Chen, H. Encapsulation of Cinnamon Essential Oil for Active Food Packaging Film with Synergistic Antimicrobial Activity. Nanomaterials 2018, 8, 598. [Google Scholar] [CrossRef]
- Zhan, J.; Chen, H.; Zhou, H.; Hao, L.; Xu, H.; Zhou, X. Essential Oil-Loaded Chitosan/Zinc (II) Montmorillonite Synergistic Sustained-Release System as Antibacterial Material. J. Dispers. Sci. Technol. 2023, 44, 288–298. [Google Scholar] [CrossRef]
- Bergaya, F.; Theng, B.K.G.; Lagaly, G. (Eds.) Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–1224. [Google Scholar]
- Juliusz, W.; Tylus, W.; Katarzyna, W.; Szczygieł, I.; Bogdan, S. XPS and FT-IR Characterization of Selected Synthetic Corrosion Products of Zinc Expected in Neutral Environment Containing Chloride Ions. J. Spectrosc. 2018, 2018, 14. [Google Scholar] [CrossRef]
- Horzum, N.; Hilal, M.E.; Isık, T. Enhanced Bactericidal and Photocatalytic Activities of ZnO Nanostructures by Changing the Cooling Route. New J. Chem. 2018, 42, 11831–11838. [Google Scholar] [CrossRef]
- Akbar, S.; Dad, K.; Shah, T.H.; Shahnaz, R. Reina Thermal Studies of NaX Zeolite with Different Degrees of Cadmium Exchange. Chem. Soc. Pak. 2005, 27, 456–461. [Google Scholar]
- Castaldi, P.; Santona, L.; Cozza, C.; Giuliano, V.; Abbruzzese, C.; Nastro, V.; Melis, P. Thermal and Spectroscopic Studies of Zeolites Exchanged with Metal Cations. J. Mol. Struct. 2005, 734, 99–105. [Google Scholar] [CrossRef]
- Alswata, A.A.; Ahmad, M.B.; Al-Hada, N.M.; Kamari, H.M.; Hussein, M.Z.B.; Ibrahim, N.A. Preparation of Zeolite/Zinc Oxide Nanocomposites for Toxic Metals Removal from Water. Results Phys. 2017, 7, 723–731. [Google Scholar] [CrossRef]
- Rukmani, A.; Sundrarajan, M. Inclusion of Antibacterial Agent Thymol on β-Cyclodextrin-Grafted Organic Cotton. J. Ind. Text. 2012, 42, 132–144. [Google Scholar] [CrossRef]
- Yildiz, Z.I.; Celebioglu, A.; Kilic, M.E.; Durgun, E.; Uyar, T. Fast-Dissolving Carvacrol/Cyclodextrin Inclusion Complex Electrospun Fibers with Enhanced Thermal Stability, Water Solubility, and Antioxidant Activity. J. Mater. Sci. 2018, 53, 15837–15849. [Google Scholar] [CrossRef]
- Valderrama, A.C.S.; Rojas De, G.C. Traceability of Active Compounds of Essential Oils in Antimicrobial Food Packaging Using a Chemometric Method by ATR-FTIR. AJAC 2017, 8, 726–741. [Google Scholar] [CrossRef]
- Canales, D.; Montoille, L.; Rivas, L.M.; Ortiz, J.A.; Yañez-S, M.; Rabagliati, F.M.; Ulloa, M.T.; Alvarez, E.; Zapata, P.A. Fungicides Films of Low-Density Polyethylene (LDPE)/Inclusion Complexes (Carvacrol and Cinnamaldehyde) Against Botrytis Cinerea. Coatings 2019, 9, 795. [Google Scholar] [CrossRef]
- Al-Mansori, B.; El-Ageeli, W.H.; Alsagheer, S.H.; Ben-Khayal, F.A.F. Antioxidant Activity- Synergistic Effects of Thymol and Carvacrol. MJSc 2020, 35, 185–194. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. In Vitro Pro-Oxidant/Antioxidant Role of Carvacrol, Thymol and Their Mixture in the Intestinal Caco-2 Cell Line. Toxicol. Vitr. 2015, 29, 647–656. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Catalkaya, G.; Capanoglu, E.; Karbancioglu-Guler, F. Antioxidant and Antimicrobial Activities of Fennel, Ginger, Oregano and Thyme Essential Oils. Food Front. 2021, 2, 508–518. [Google Scholar] [CrossRef]
- Bernardos, A.; Bozik, M.; Alvarez, S.; Saskova, M.; Perez-Esteve, E.; Kloucek, P.; Lhotka, M.; Frankova, A.; Martinez-Manez, R. The Efficacy of Essential Oil Components Loaded into Montmorillonite against Aspergillus niger and Staphylococcus aureus. Flavour. Fragr. J. 2019, 34, 151–162. [Google Scholar] [CrossRef]
- Zhong, H.; Mu, B.; Yan, P.; Jing, Y.; Hui, A.; Wang, A. A Comparative Study on Surface/Interface Mechanism and Antibacterial Properties of Different Hybrid Materials Prepared with Essential Oils Active Ingredients and Palygorskite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126455. [Google Scholar] [CrossRef]
- Hui, A.; Yang, F.; Yan, R.; Kang, Y.; Wang, A. Palygorskite-Based Organic–Inorganic Hybrid Nanocomposite for Enhanced Antibacterial Activities. Nanomaterials 2021, 11, 3230. [Google Scholar] [CrossRef]
- Mokhtar, A.; Ahmed, A.B.; Asli, B.; Boukoussa, B.; Hachemaoui, M.; Sassi, M.; Abboud, M. Recent Advances in Antibacterial Metallic Species Supported on Montmorillonite Clay Mineral: A Review. Minerals 2023, 13, 1268. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Shi, C.; Hu, W.; Cui, H.; Lin, L. Characterization of Controlled-Release Eucalyptus Citriodora Oil/Zinc Ions Nanoparticles with Enhanced Antibacterial Properties against E. coli O157:H7 in Fruit Juice. Food Res. Int. 2022, 162, 112138. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, L.; Chou, K.C.; Lu, X. Campylobacter Jejuni Antimicrobial Resistance Profiles and Mechanisms Determined Using a Raman Spectroscopy-Based Metabolomic Approach. Appl. Environ. Microbiol. 2021, 87, e00388-21. [Google Scholar] [CrossRef] [PubMed]
- Daković, A.; Kragović, M.; Rottinghaus, G.E.; Ledoux, D.R.; Butkeraitis, P.; Vojislavljević, D.Z.; Zarić, S.D.; Stamenić, L. Preparation and Characterization of Zinc-Exchanged Montmorillonite and Its Effectiveness as Aflatoxin B1 Adsorbent. Mater. Chem. Phys. 2012, 137, 213–220. [Google Scholar] [CrossRef]
- Nyankson, E.; Efavi, J.K.; Yaya, A.; Manu, G.; Asare, K.; Daafuor, J.; Abrokwah, R.Y. Synthesis and Characterisation of Zeolite-A and Zn-Exchanged Zeolite-A Based on Natural Aluminosilicates and Their Potential Applications. Cogent Eng. 2018, 5, 1440480. [Google Scholar] [CrossRef]
- Wang, J.; Niu, Y.; Zhang, C.; Chen, Y. A Micro-Plate Colorimetric Assay for Rapid Determination of Trace Zinc in Animal Feed, Pet Food and Drinking Water by Ion Masking and Statistical Partitioning Correction. Food Chem. 2018, 245, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Luo, A.; Fan, Y. Antioxidant Activities of Berberine Hydrochloride. J. Med. Plants Res. 2011, 5, 3702–3707. [Google Scholar]
- Baruzzi, F.; Cefola, M.; Carito, A.; Vanadia, S.; Calabrese, N. Changes in Bacterial Composition of Zucchini Flowers Exposed to Refrigeration Temperatures. Sci. World J. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Baruzzi, F.; Pinto, L.; Quintieri, L.; Carito, A.; Calabrese, N.; Caputo, L. Efficacy of Lactoferricin B in Controlling Ready-to-Eat Vegetable Spoilage Caused by Pseudomonas Spp. Int. J. Food Microbiol. 2015, 215, 179–186. [Google Scholar] [CrossRef]
- M02-A11; Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard—Eleventh Edition. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- Pinto, L.; Cefola, M.; Bonifacio, M.A.; Cometa, S.; Bocchino, C.; Pace, B.; De Giglio, E.; Palumbo, M.; Sada, A.; Logrieco, A.F.; et al. Effect of Red Thyme Oil (Thymus vulgaris L.) Vapours on Fungal Decay, Quality Parameters and Shelf-Life of Oranges during Cold Storage. Food Chem. 2021, 336, 127590. [Google Scholar] [CrossRef]

| Clay | Atomic Percentage (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C1s | O1s | Al2p | Si2p | Mg1s | Ca2p | Na1s | N1s | Zn2p3/2 | |
| MMT | 3.9 | 67.3 | 4.0 | 22.8 | 1.5 | 0.5 | -- | -- | -- |
| ZnMMT | 4.1 | 67.4 | 4.1 | 22.7 | 1.1 | -- | -- | -- | 0.6 |
| ZEO | 21.9 | 45.7 | 9.9 | 9.2 | -- | -- | 13.3 | -- | -- |
| ZnZEO | 15.4 | 54.4 | 8.4 | 9.3 | -- | -- | -- | -- | 12.4 |
| Hybrid Material | μg/mL | LC% | LE% |
|---|---|---|---|
| MMT–carvacrol | 272 ± 12 | 27.2% | 136% |
| ZEO–carvacrol | 258 ± 1 | 25.8% | 129% |
| ZnMMT–carvacrol | 249 ± 23 | 24.9% | 125% |
| ZnZEO–carvacrol | 246 ± 11 | 24.6% | 123% |
| MMT–thymol | 331 ± 10 | 33.1% | 99% |
| ZEO–thymol | 306 ± 7 | 30.6% | 92% |
| ZnMMT–thymol | 347 ± 12 | 34.7% | 104% |
| ZnZEO–thymol | 363 ± 21 | 36.3% | 109% |
| Carvacrol | Thymol | MMT–Carvacrol | MMT–Thymol | ZnMMT | ZnMMT–Carvacrol | ZnMMT–Thymol | ||
|---|---|---|---|---|---|---|---|---|
| Spoilers | Erw. persicina ITEM 17997 | 117.40 c ± 10.60 | 165.10 b ± 14.80 | 93.60 c ± 8.50 | 220.70 a ± 12.80 | 46.42 d ± 16.92 | 234.60 a ± 20.60 | 218.70 a ± 8.30 |
| Pec. carot. carot. LMG 2404 | 369.30 b ± 15.70 | 256.60 d ± 16.60 | 122.00 e ± 25.00 | 379.30 b ± 38.30 | 69.67 f ± 15.67 | 304.74 c ± 13.74 | 648.44 a ± 22.56 | |
| P. putida ITEM 17297 | 91.20 c ± 20.80 | 82.02 c ± 8.98 | 48.50 d ± 11.50 | 128.60 b ± 10.60 | 64.91 cd ± 3.91 | 249.80 a ± 24.20 | 275.80 a ± 15.80 | |
| P. chicorii ITEM 17296 | 47.20 f ± 8.20 | 60.20 e ± 5.50 | 93.60 d ± 4.50 | 120.70 c ± 3.20 | 41.29 ef ± 8.21 | 208.70 b ± 14.20 | 504.30 a ± 12.50 | |
| Pathogens | E. coli ATCC 8739 | 543.28 b ± 11.02 | 432.43 c ± 39.07 | 315.13 d ± 14.03 | 417.96 c ± 17.16 | 61.89 e ± 5.01 | 416.44 c ± 13.74 | 631.31 a ± 29.81 |
| E. coli ATCC 35401 | 238.7 d ± 20.8 | 205.34 e ± 13.84 | 412.75 c ± 13.45 | 541.84 a ± 13.43 | 92.89 f ± 2.79 | 491.67 b ± 6.77 | 391.68 c ± 10.48 | |
| L. monocytogenes DSM 20600 | 267.74 c ± 38.44 | 133.77 d ± 18.87 | 299.87 c ± 14.43 | 161.87 b ± 10.57 | 296.17 c ± 18.73 | 468.68 d ± 18.58 | 825.16 a ± 63.84 | |
| P. aeruginosa DSM 939 | 10.08 c ± 1.92 | 58.1 b ± 3.4 | 7.05 c ± 3.05 | 51.31 b ± 3.31 | 0 c ± 0 | 73.75 a ± 10.85 | 61.26 ab ± 18.54 | |
| Sal. enterica ATCC 13311 | 554.76 a ± 13.26 | 374.8 d ± 13.6 | 471.54 b ± 26.74 | 393.54 d ± 6.44 | 91.43 e ± 9.57 | 430.59 c ± 10.41 | 541.08 a ± 13.12 | |
| Sta. aureus DSM 799 | 3877.67 a ± 76.83 | 2418.31 d ± 197.09 | 3308.81 c ± 117.71 | 3547.65 b ± 160.55 | 53.62 e ± 15.38 | 4022.11 a ± 18.89 | 2240.16 d ± 114.04 |
| Carvacrol | Thymol | ZEO–Carvacrol | ZEO–Thymol | ZnZEO | ZnZEO–Carvacrol | ZnZEO–Thymol | ||
|---|---|---|---|---|---|---|---|---|
| Spoilers | Erw. persicina ITEM 17997 | 117.40 b ± 10.60 | 165.10 a ± 14.80 | 82.60 c ± 4.60 | 101.00 bc ± 40.50 | 0.00 d ± 0.00 | 79.60 c ± 8.60 | 154.00 a ± 13.50 |
| Pec. carot. carot. LMG 2404 | 369.30 a ± 15.70 | 256.60 bc ± 16.60 | 284.70 b ± 22.30 | 270.20 b ± 29.00 | 0.00 d ± 0.00 | 218.50 c ± 11.00 | 245.71 bc ± 38.31 | |
| P. putida ITEM 17297 | 91.20 b ± 20.80 | 82.02 bc ± 8.98 | 134.50 a ± 17.20 | 61.80 c ± 9.80 | 0.00 d ± 0.00 | 80.30 bc ± 7.80 | 93.30 b ± 6.00 | |
| P. chicorii ITEM 17296 | 47.20 c ± 8.20 | 60.20 bc ± 5.50 | 82.60 ab ± 11.50 | 101.00 a ± 36.50 | 0.00 d ± 0.00 | 45.30 c ± 7.60 | 57.00 bc ± 7.20 | |
| Pathogens | E. coli ATCC 8739 | 543.28 a ± 11.02 | 432.43 b ± 39.07 | 478.10 b ± 31.10 | 281.13 d ± 51.23 | 0.00 e ± 0.00 | 364.39 c ± 10.51 | 292.05 d ± 11.85 |
| E. coli ATCC 35401 | 238.7 a ± 20.8 | 205.34 b ± 13.84 | 116.96 d ± 7.96 | 162.22 c ± 10.32 | 0.00 e ± 0.00 | 169.91 c ± 18.57 | 232.81 a ± 10.11 | |
| L. monocytogenes DSM 20600 | 267.74 b ± 38.44 | 133.77 d ± 18.87 | 238.29 b ± 16.39 | 62.87 e ± 11.37 | 0.00 f ± 0.00 | 191.63 c ± 17.87 | 389.48 a ± 10.28 | |
| P. aeruginosa DSM 939 | 10.08 d ± 1.92 | 58.1 b ± 3.4 | 46.78 c ± 9.42 | 121.02 a ± 9.82 | 0.00 d ± 0.00 | 5.02 d ± 0.98 | 49.60 bc ± 5.20 | |
| Sal. enterica ATCC 13311 | 554.76 b ± 13.26 | 374.8 c ± 13.6 | 604.69 a ± 40.11 | 392.41 c ± 35.31 | 0.00 f ± 0.00 | 203.30 e ± 2.30 | 302.90 d ± 31.30 | |
| Sta. aureus DSM 799 | 3877.67 c ± 76.83 | 2418.31 d ± 197.09 | 2427.07 d ± 135.97 | 5236.85 a ± 79.75 | 0.00 e ± 0.00 | 4024.46 c ± 266.54 | 4681.65 b ± 227.45 |
| Spoilers | Pathogens | Average | |
|---|---|---|---|
| Zn–MMT–carvacrol/carvacrol | 254.83 ± 8.59 | 234.34 ± 37.75 | 244.58 ± 14.49 |
| Zn–MMT–thymol/thymol | 392.20 ± 2.84 | 218.56 ± 28.84 | 305.38 ± 122.78 |
| Zn–ZEO–carvacrol/carvacrol | 79.17 ± 5.96 | 66.92 ± 3.64 | 73.04 ± 8.66 |
| Zn–ZEO–thymol/thymol | 99.84 ± 12.20 | 139.58 ± 3.75 | 119.71 ± 28.11 |
| Control | 0.5xMIC | % Mortality | |||
|---|---|---|---|---|---|
| Spoilres | Erw. persicina ITEM 17997 | 8.18 ± 0.12 | nd | 100.00 | |
| Pec. carotovorum LMG 2404 | 7.33 ± 0.11 | nd | 100.00 | ||
| P. chicorii ITEM 17296 | 7.81 ± 0.08 | nd | 100.00 | ||
| P. fluorescens ITEM 19245 | 9.00 ± 0.36 | nd | 100.00 | ||
| P. putida ITEM 17297 | 8.48 ± 0.61 | nd | 100.00 | ||
| Pathogens | E. coli ATCC 8739 | 8.00 ± 0.37 | 1.97 ± 0.04 | 99.99 | |
| E. coli ATCC 35401 | 9.54 ± 0.56 | 3.96 ± 0.14 | 99.98 | ||
| L. monocytogenes DSM 20600 | 8.39 ± 0.11 | nd | 100.00 | ||
| P. aeruginosa DSM 939 | 10.10 ± 0.09 | nd | 100.00 | ||
| Sal. enterica ATCC 13311 | 9.29 ± 0.38 | nd | 100.00 | ||
| Sta. aureus DSM 799 | 8.70 ± 0.15 | nd | 100.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, L.; Baruzzi, F.; Terzano, R.; Busto, F.; Marzulli, A.; Magno, C.; Cometa, S.; De Giglio, E. Analytical and Antimicrobial Characterization of Zn-Modified Clays Embedding Thymol or Carvacrol. Molecules 2024, 29, 3607. https://doi.org/10.3390/molecules29153607
Pinto L, Baruzzi F, Terzano R, Busto F, Marzulli A, Magno C, Cometa S, De Giglio E. Analytical and Antimicrobial Characterization of Zn-Modified Clays Embedding Thymol or Carvacrol. Molecules. 2024; 29(15):3607. https://doi.org/10.3390/molecules29153607
Chicago/Turabian StylePinto, Loris, Federico Baruzzi, Roberto Terzano, Francesco Busto, Alessia Marzulli, Carmela Magno, Stefania Cometa, and Elvira De Giglio. 2024. "Analytical and Antimicrobial Characterization of Zn-Modified Clays Embedding Thymol or Carvacrol" Molecules 29, no. 15: 3607. https://doi.org/10.3390/molecules29153607
APA StylePinto, L., Baruzzi, F., Terzano, R., Busto, F., Marzulli, A., Magno, C., Cometa, S., & De Giglio, E. (2024). Analytical and Antimicrobial Characterization of Zn-Modified Clays Embedding Thymol or Carvacrol. Molecules, 29(15), 3607. https://doi.org/10.3390/molecules29153607








