Improvement of Echinacea purpurea and Ganoderma lucidum Extracts with Cell Model on Influenza A/B Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line and Viruses
2.2. Testing Samples
2.3. Determination of Total Phenolics and Polysaccharide
2.4. HPLC Analysis of Phenolic Compounds
2.5. Calibration Curve and Quantification
2.6. Cytotoxicity Test
2.7. 50% Tissue Culture Infectious Dose, TCID50
2.8. Cell Survival Assay
2.9. Hemagglutination Assay (HA Assay)
2.10. Analysis of Mitochondria Membrane Potential by JC-1 Staining
3. Results
3.1. Virus
3.2. HPLC Chromatograms of Caffeic Acid Derivatives
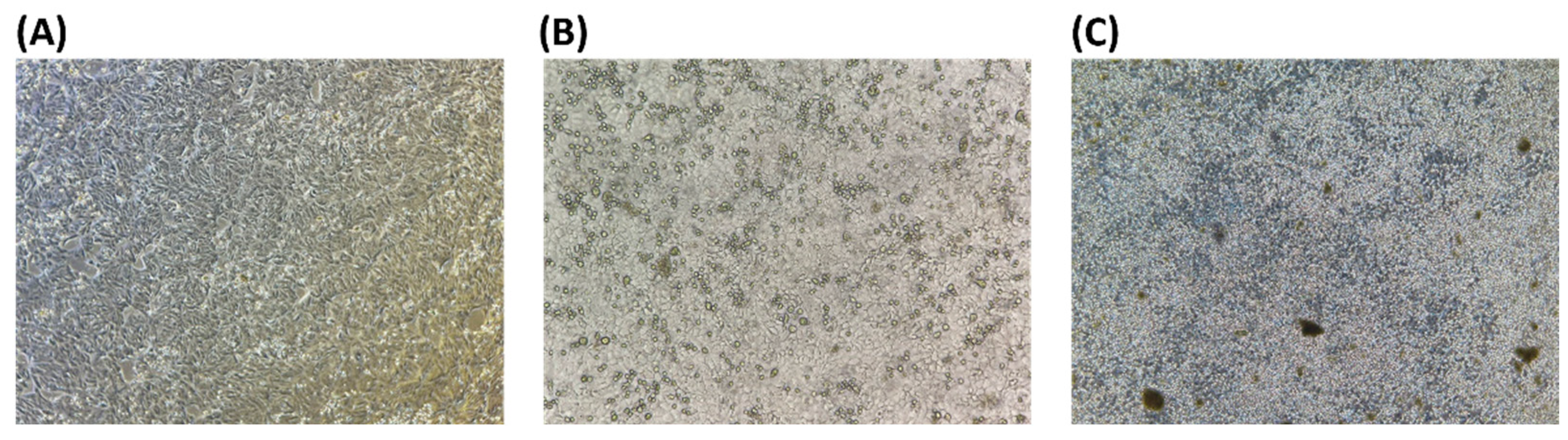
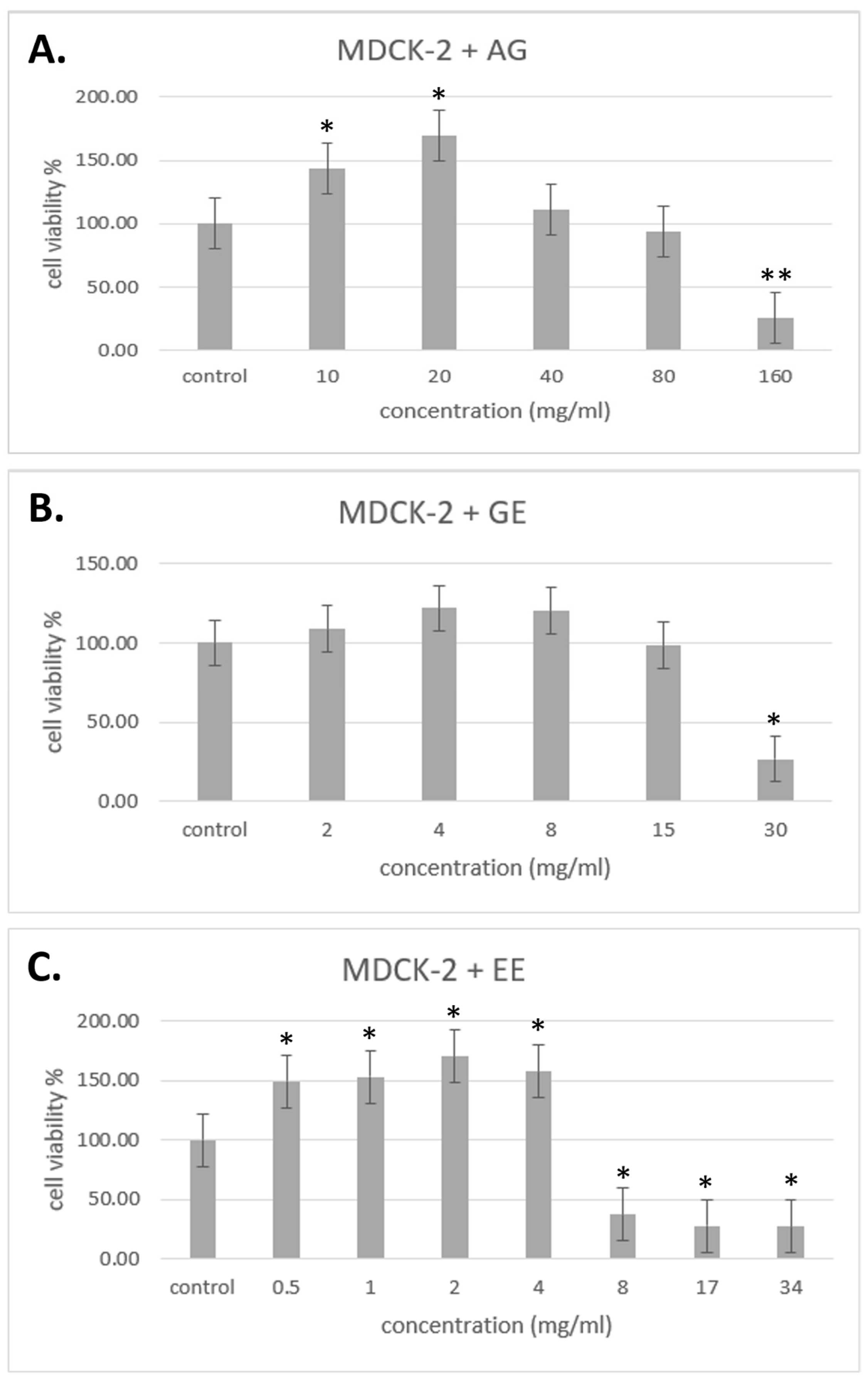
3.3. Cytotoxicity Test
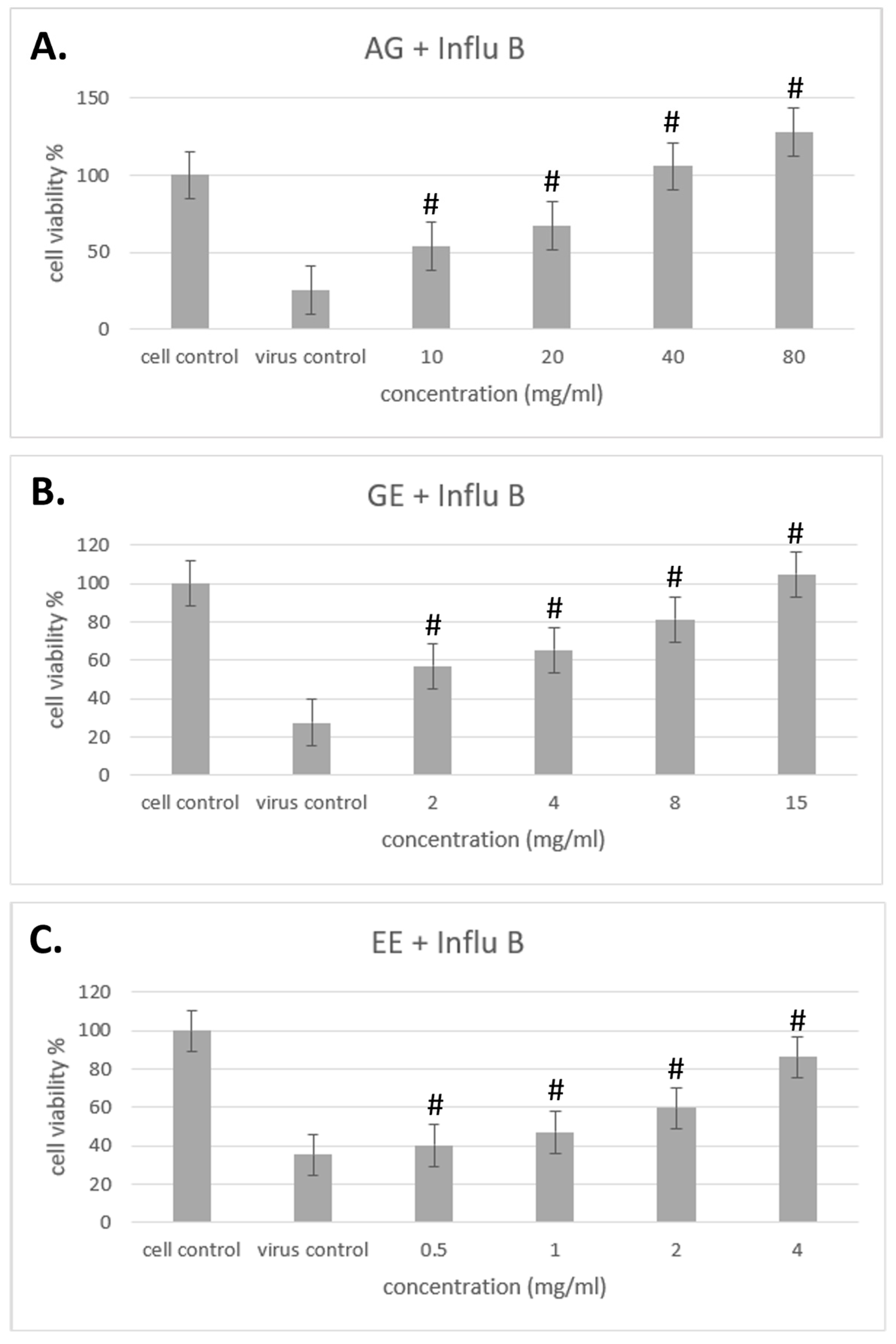
3.4. Improvement of Different Samples on Cell Viability with Influenza Virus
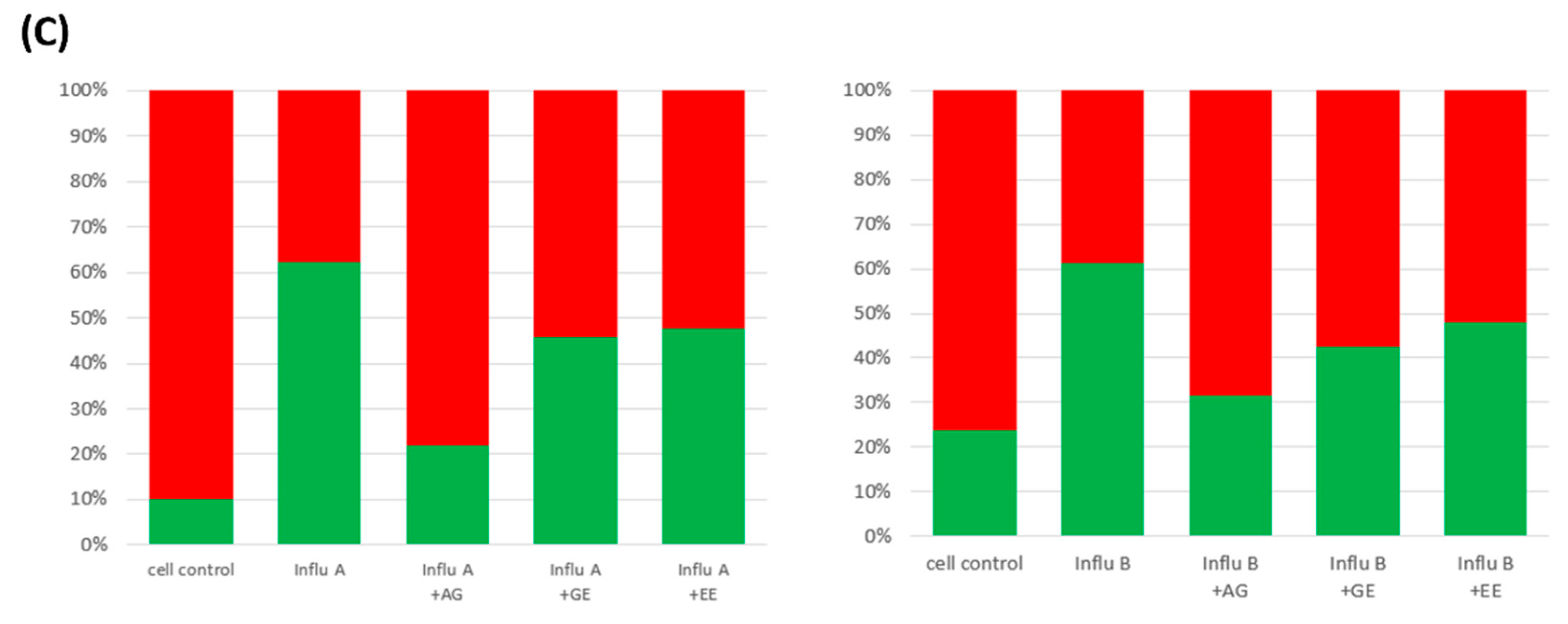
3.5. Hemagglutination Assay (HA Assay)
3.6. Improvement of Mitochondria Dysfunction by Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hay, A.J.; Lomniczi, B.; Bellamy, A.R.; Skehel, J.J. Transcription of the influenza virus genome. Virology 1977, 83, 337–355. [Google Scholar] [CrossRef]
- Keilman, L.J. Seasonal influenza (flu). Nurs. Clin. 2019, 54, 227–243. [Google Scholar] [CrossRef]
- Brown, E.G. Influenza virus genetics. Biomed. Pharmacother. 2000, 54, 196–209. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Reid, A.H.; Fanning, T.G. The 1918 influenza virus: A killer comes into view. Virology 2000, 274, 241–245. [Google Scholar] [CrossRef]
- Fu, A.; Wang, Y.; Wu, Y.; Chen, H.; Zheng, S.; Li, Y.; Xu, X.; Li, W. Echinacea purpurea extract polarizes M1 macrophages in murine bone marrow-derived macrophages through the activation of JNK. J. Cell. Biochem. 2017, 118, 2664–2671. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Vazirian, M. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacogn. Rev. 2015, 9, 63–72. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef]
- Zhang, X.; Rizshsky, L.; Hauck, C.; Qu, L.; Widrlechner, M.P.; Nikolau, B.J.; Murphy, P.A.; Birt, D.F. Bauer ketones 23 and 24 from Echinacea paradoxa var. paradoxa inhibit lipopolysaccharide-induced nitric oxide, prostaglandin E2 and cytokines in RAW264. 7 mouse macrophages. Phytochemistry 2012, 74, 146–158. [Google Scholar] [CrossRef]
- Aarland, R.C.; Bañuelos-Hernández, A.E.; Fragoso-Serrano, M.; Sierra-Palacios, E.D.C.; Díaz de León-Sánchez, F.; Pérez-Flores, L.J.; Rivera-Cabrera, F.; Mendoza-Espinoza, J.A. Studies on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and antiproliferative activities of Echinacea purpurea and Echinacea angustifolia extracts. Pharm. Biol. 2017, 55, 649–656. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Magro, L.; Melegari, M.; Soragni, F. Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. J. Pharm. Biomed. Anal. 2004, 35, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Sohretoglu, D.; Huang, S. Ganoderma lucidum polysaccharides as an anti-cancer agent. Anti-Cancer Agents Med. Chem. 2018, 18, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Wachtel-Galor, S. Herbal Medicine: Biomolecular and Clinical Aspects; Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from Ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.F. Ganoderma lucidum: A rational pharmacological approach to surmount cancer. J. Ethnopharmacol. 2020, 260, 113047. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.Z.; Cheng, P.G.; Abdulrahman, A.Y.; Teoh, T.C. The identification of active compounds in Ganoderma lucidum var. antler extract inhibiting dengue virus serine protease and its computational studies. J. Biomol. Struct. Dyn. 2020, 38, 4273–4288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, H.; Tao, Z.; Yuan, C.; Jiang, Z.; Liu, J.; Kurihara, H.; Xu, W. Ganoderma lucidum spore oil (GLSO), a novel antioxidant, extends the average life span in Drosophila melanogaster. Food Sci. Hum. Wellness 2021, 10, 38–44. [Google Scholar] [CrossRef]
- Sang, T.; Guo, C.; Guo, D.; Wu, J.; Wang, Y.; Wang, Y.; Chen, J.; Chen, C.; Wu, K.; Na, K.; et al. Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr. Polym. 2021, 256, 117594. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lai, G.; Guo, Y.; Tang, X.; Shuai, O.; Xie, Y.; Wu, Q.; Chen, D.; Yuan, X. Protective effect of Ganoderma lucidum spore extract in trimethylamine-N-oxide-induced cardiac dysfunction in rats. J. Food Sci. 2021, 86, 546–562. [Google Scholar] [CrossRef] [PubMed]
- Savin, S.; Craciunescu, O.; Oancea, A.; Ilie, D.; Ciucan, T.; Antohi, L.S.; Toma, A.; Nicolescu, A.; Deleanu, C.; Oancea, F. Antioxidant, cytotoxic and antimicrobial activity of chitosan preparations extracted from Ganoderma lucidum mushroom. Chem. Biodivers. 2020, 17, e2000175. [Google Scholar] [CrossRef]
- González, A.; Atienza, V.; Montoro, A.; Soriano, J.M. Use of Ganoderma lucidum (Ganodermataceae, Basidiomycota) as radioprotector. Nutrients 2020, 12, 1143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Y.; Wang, W.; Jia, L.; Zhang, J. Characterization and hepatoprotections of Ganoderma lucidum Polysaccharides against multiple organ dysfunction syndrome in mice. Oxidative Med. Cell. Longev. 2021, 1, 9703682. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Sadiq, N.B.; Kim, J.C.; Lee, B.; Hamayun, M.; Lee, T.S.; Kim, H.S.; Park, S.H.; Nho, C.W.; Kim, H.Y. Optimization of antioxidant, anti-diabetic, and anti-inflammatory activities and ganoderic acid content of differentially dried Ganoderma lucidum using response surface methodology. Food Chem. 2021, 335, 127645. [Google Scholar]
- Jiang, L.; Huang, J.; Lu, J.; Hu, S.; Pei, S.; Ouyang, Y.; Ding, Y.; Hu, Y.; Kang, L.; Huang, L.; et al. Ganoderma lucidum polysaccharide reduces melanogenesis by inhibiting the paracrine effects of keratinocytes and fibroblasts via IL-6/STAT3/FGF2 pathway. J. Cell. Physiol. 2019, 234, 22799–22808. [Google Scholar] [CrossRef] [PubMed]
- Fujita, R.; Liu, J.; Shimizu, K.; Konishi, F.; Noda, K.; Kumamoto, S.; Ueda, C.; Tajiri, H.; Kaneko, S.; Suimi, Y.; et al. Anti-androgenic activities of Ganoderma lucidum. J. Ethnopharmacol. 2005, 102, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Fast, D.J.; Balles, J.A.; Scholten, J.D.; Mulder, T.; Rana, J. Echinacea purpurea root extract inhibits TNF release in response to Pam3Csk4 in a phosphatidylinositol-3-kinase dependent manner. Cell. Immunol. 2015, 297, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Xu, T.; Li, Q.; Yang, F.; Wang, C.; Huang, T.; Hao, Z. Polysaccharide from Echinacea purpurea reduce the oxidant stress in vitro and in vivo. Int. J. Biol. Macromol. 2020, 149, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Kindscher, K. The medicinal chemistry of Echinacea species. Echinacea Herb. Med. A Wild Hist. 2016, 127–145. [Google Scholar]
- De Silva, D.D.; Rapior, S.; Sudarman, E.; Stadler, M.; Xu, J.; Aisyah Alias, S.; Hyde, K.D. Bioactive metabolites from macrofungi: Ethnopharmacology, biological activities and chemistry. Fungal Divers. 2013, 62, 1–40. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Panda, B.P.; Azad, Z.; Ahmad, A. Simultaneous bioprospecting of Ganoderma lucidum OE 52 with ganoderic acid B and C2 by submerged fermentation process. Adv. Sci. Focus 2013, 1, 258–261. [Google Scholar] [CrossRef]
- Reeves, M.B.; Davies, A.A.; McSharry, B.P.; Wilkinson, G.W.; Sinclair, J.H. Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science 2007, 316, 1345–1348. [Google Scholar] [CrossRef]
- Pal, A.D.; Basak, N.P.; Banerjee, A.S.; Banerjee, S. Epstein–Barr virus latent membrane protein-2A alters mitochondrial dynamics promoting cellular migration mediated by Notch signaling pathway. Carcinogenesis 2014, 35, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Kersting, U.; Schwab, A.; Treidtel, M.; Pfaller, W.; Gstraunthaler, G.; Steigner, W.; Oberleithner, H. Differentiation of Madin-Darby canine kidney cells depends on cell culture conditions. Cell. Physiol. Biochem. 1993, 3, 42–55. [Google Scholar] [CrossRef]
- Nielsen, S.S. Phenol-sulfuric acid method for total carbohydrates. In Food Analysis Laboratory Manual; Food Science Texts Series; Springer: Boston, MA, USA, 2010; pp. 47–53. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, 11.11–11.18. [Google Scholar]
- Bauer, R.; Remiger, P. TLC and HPLC analysis of alkamides in Echinacea drugs1, 2. Planta Medica 1989, 55, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.X.; Hoult, J.R.S.; Raman, A. Sulphorhodamine B assay for measuring proliferation of a pigmented melanocyte cell line and its application to the evaluation of crude drugs used in the treatment of vitiligo. J. Ethnopharmacol. 1999, 66, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Liu, M.; Wang, C.; Xu, W.; Lan, Q.; Feng, S.; Qi, F.; Bao, L.; Du, L.; Liu, S.; et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020, 30, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, M.; Miyazono, Y.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta 1997, 44, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Hirst, G.K. The quantitative determination of influenza virus and antibodies by means of red cell agglutination. J. Exp. Med. 1942, 75, 49. [Google Scholar] [CrossRef]
- Woollacott, A.J.; Simpson, P.B. High throughput fluorescence assays for the measurement of mitochondrial activity in intact human neuroblastoma cells. SLAS Discov. 2001, 6, 413–420. [Google Scholar] [CrossRef]
- Sharma, M.; Anderson, S.A.; Schoop, R.; Hudson, J.B. Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antivir. Res. 2009, 83, 165–170. [Google Scholar] [CrossRef]
- Chang, Y.C.; Hsiao, Y.M.; Hung, S.C.; Chen, Y.W.; Ou, C.C.; Chang, W.T.; Lue, K.H.; Ko, J.L. Alleviation of Dermatophagoides microceras-induced allergy by an immunomodulatory protein, FIP-fve, from Flammulina velutipes in mice. Biosci. Biotechnol. Biochem. 2015, 79, 88–96. [Google Scholar] [CrossRef]
- Zhu, Q.; Bang, T.H.; Ohnuki, K.; Sawai, T.; Sawai, K.; Shimizu, K. Inhibition of neuraminidase by Ganoderma triterpenoids and implications for neuraminidase inhibitor design. Sci. Rep. 2015, 5, 13194. [Google Scholar] [CrossRef]
- Pleschka, S.; Stein, M.; Schoop, R.; Hudson, J.B. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol. J. 2009, 6, 197. [Google Scholar] [CrossRef]
- Sharma, M.; Schoop, R.; Hudson, J.B. Echinacea as an antiinflammatory agent: The influence of physiologically relevant parameters. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 863–867. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, S.; Kumar, R.; Tripathi, B.N.; Barua, S.; Ly, H.; Rouse, B.T. Host-directed antiviral therapy. Clin. Microbiol. Rev. 2020, 33, e00168-19. [Google Scholar] [CrossRef]
- Signer, J.; Jonsdottir, H.R.; Albrich, W.C.; Strasser, M.; Züst, R.; Ryter, S.; Ackermann-Gäumann, R.; Lenz, N.; Siegrist, D.; Suter, A.; et al. In vitro virucidal activity of Echinaforce®, an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2. Virol. J. 2020, 17, 1–11. [Google Scholar] [CrossRef]
- Artika, I.M.; Wiyatno, A.; Ma’roef, C.N. Pathogenic viruses: Molecular detection and characterization. Infect. Genet. Evol. 2020, 81, 104215. [Google Scholar] [CrossRef]
- Bauer, R.; Remiger, P.; Jurcic, K.; Wagner, H. Influence of Echinacea extracts on phagocytotic activity. Z Phytother. 1989, 10, 43–48. [Google Scholar]
- Facino, R.M.; Carini, M.; Aldini, G.; Marinello, C.; Arlandini, E.; Franzoi, L.; Colombo, M.; Pietta, P.; Mauri, P. Direct characterization of caffeoyl esters with antihyaluronidase activity in crude extracts from Echinacea angustifolia roots by fast atom bombardment tandem mass spectrometry. Farmaco 1993, 48, 1447–1461. [Google Scholar]
- Cheminat, A.; Zawatzky, R.; Becker, H.; Brouillard, R. Caffeoyl conjugates from Echinacea species: Structures and biological activity. Phytochemistry 1988, 27, 2787–2794. [Google Scholar] [CrossRef]
- Lin, Z.; Neamati, N.; Zhao, H.; Kiryu, Y.; Turpin, J.A.; Aberham, C.; Strebel, K.; Kohn, K.; Witvrouw, M.; Pannecouque, C.; et al. Chicoric acid analogues as HIV-1 integrase inhibitors. J. Med. Chem. 1999, 42, 1401–1414. [Google Scholar] [CrossRef]
- Facino, R.M.; Carini, M.; Aldini, G.; Saibene, L.; Pietta, P.; Mauri, P. Echinacoside and caffeoyl conjugates protect collagen from free radical-induced degradation: A potential use of Echinacea extracts in the prevention of skin photodamage. Planta Medica 1995, 61, 510–514. [Google Scholar] [CrossRef]
- Cervellati, R.; Renzulli, C.; Guerra, M.C.; Speroni, E. Evaluation of antioxidant activity of some natural polyphenolic compounds using the Briggs− Rauscher reaction method. J. Agric. Food Chem. 2002, 50, 7504–7509. [Google Scholar] [CrossRef]
- Binns, S.E.; Hudson, J.; Merali, S.; Arnason, J.T. Antiviral activity of characterized extracts from Echinacea spp. (Heliantheae: Asteraceae) against herpes simplex virus (HSV-I). Planta Medica 2002, 68, 780–783. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Ichinose, M.; Ikeda, K.; Uozaki, M.; Morishita, J.; Kuwahara, T.; Koyama, A.H.; Yamasaki, H. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int. J. Mol. Med. 2014, 34, 1020–1024. [Google Scholar] [CrossRef]
- Montini, M.; Levoni, P.; Ongaro, A.; Pagani, G. Controlled application of cynarin in the treatment of hyperlipemic syndrome. Observations in 60 cases. Arzneim.-Forsch. 1975, 25, 1311–1314. [Google Scholar]
- Adzet, T.; Camarasa, J.; Laguna, J.C. Hepatoprotective activity of polyphenolic compounds from Cynara scolymus against CCl4 toxicity in isolated rat hepatocytes. J. Nat. Prod. 1987, 50, 612–617. [Google Scholar] [CrossRef]
- Raheem, K.S.; Botting, N.P.; Williamson, G.; Barron, D. Total synthesis of 3, 5-O-dicaffeoylquinic acid and its derivatives. Tetrahedron Lett. 2011, 52, 7175–7177. [Google Scholar] [CrossRef]
- Topal, M.; Gocer, H.; Topal, F.; Kalin, P.; Köse, L.P.; Gülçin, İ.; Çakmak, K.C.; Küçük, M.; Durmaz, L.; Gören, A.C.; et al. Antioxidant, antiradical, and anticholinergic properties of cynarin purified from the Illyrian thistle (Onopordum illyricum L.). J. Enzym. Inhib. Med. Chem. 2016, 31, 266–275. [Google Scholar] [CrossRef] [PubMed]





| Samples | Content (mg/g Dry Weight) | |
|---|---|---|
| Polysaccharide | Total Phenolic | |
| AG | 30.23 ± 1.51 a | 0.28 ± 0.01 |
| GE | 145.16 ± 7.258 | - |
| EE | - | 386.98 ± 19.35 |
| AG | EE | |
|---|---|---|
| mg/g Dry Weight | ||
| Chlorogenic acid | 0.28 ± 0.01 | 131.15 ± 6.56 |
| Echinacoside | 0.30 ± 0.01 | 37.03 ± 1.85 |
| Cichoric acid | 0.13 ± 0.06 | 40.26 ± 2.01 |
| Caffeic acid | 0.53 ± 0.03 | 261.48 ± 13.07 |
| Cynarin | 0.04 ± 0.002 | 14.50 ± 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.-K.; Chan, C.-H.; Tsao, A.; Wang, C.-K. Improvement of Echinacea purpurea and Ganoderma lucidum Extracts with Cell Model on Influenza A/B Infection. Molecules 2024, 29, 3609. https://doi.org/10.3390/molecules29153609
Chen B-K, Chan C-H, Tsao A, Wang C-K. Improvement of Echinacea purpurea and Ganoderma lucidum Extracts with Cell Model on Influenza A/B Infection. Molecules. 2024; 29(15):3609. https://doi.org/10.3390/molecules29153609
Chicago/Turabian StyleChen, Bo-Kai, Chi-Ho Chan, Arthur Tsao, and Chin-Kun Wang. 2024. "Improvement of Echinacea purpurea and Ganoderma lucidum Extracts with Cell Model on Influenza A/B Infection" Molecules 29, no. 15: 3609. https://doi.org/10.3390/molecules29153609
APA StyleChen, B.-K., Chan, C.-H., Tsao, A., & Wang, C.-K. (2024). Improvement of Echinacea purpurea and Ganoderma lucidum Extracts with Cell Model on Influenza A/B Infection. Molecules, 29(15), 3609. https://doi.org/10.3390/molecules29153609







