Advantages of Spray Drying over Freeze Drying: A Comparative Analysis of Lonicera caerulea L. Juice Powders—Matrix Diversity and Bioactive Response
Abstract
1. Introduction
2. Results and Discussion
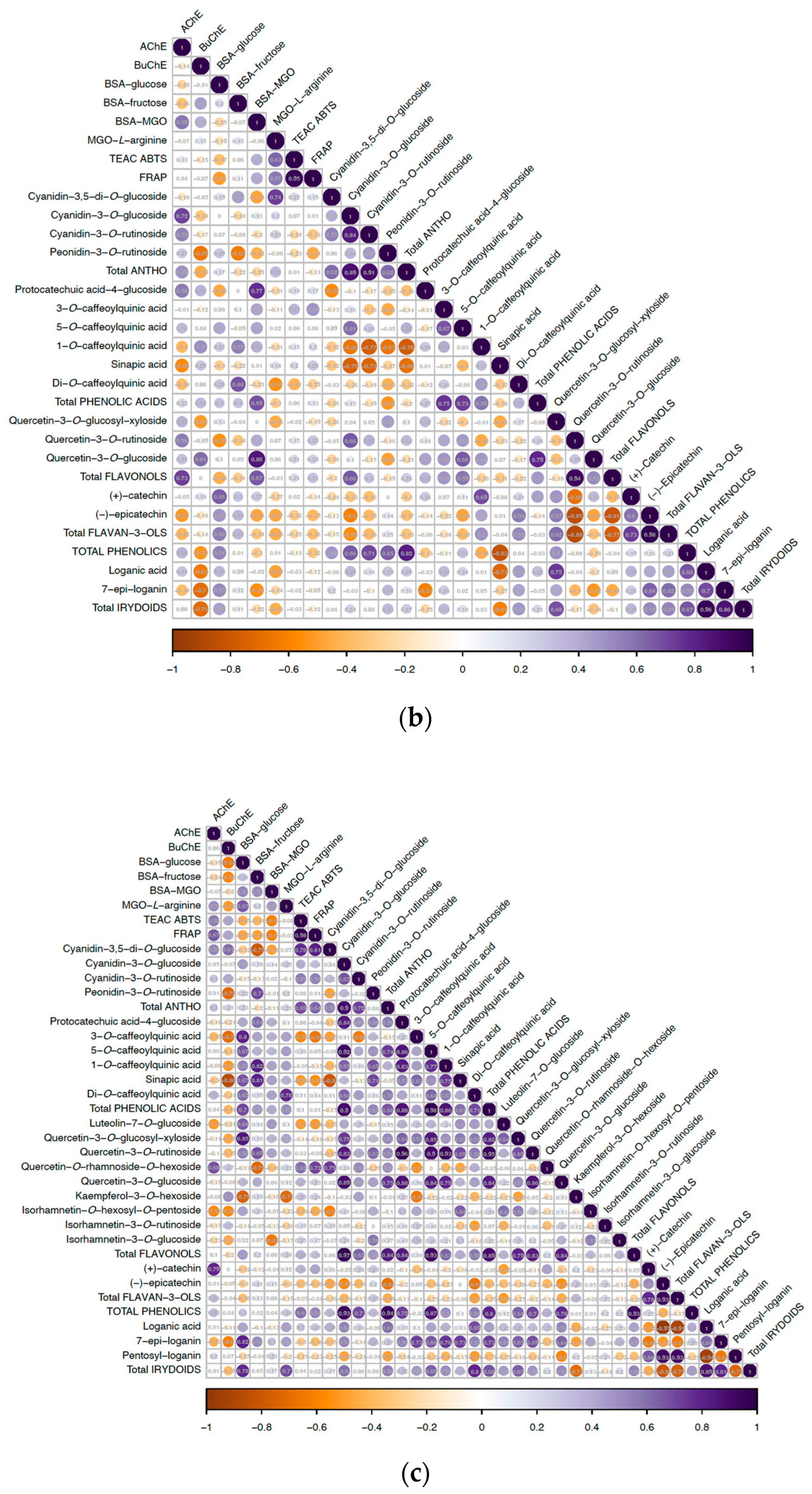
2.1. Phenolics
2.2. Iridoids Content
2.3. Antioxidant Capacity
2.4. Antiglycation Capacity
2.5. Anticholinesterase Capacity
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Juicing and Preparation of Sugar Free XAD Extracts
Basic Chemical Properties of Lonicera caerulea L. Fruit and Its Fractions
3.2.2. Drying of Juice and XAD Extracts
3.2.3. Chemical Composition and in Vitro Biological Properties of Powders
Quantification of Phenolics and Iridoids
Antioxidant Capacity
Antiglycation Assays in BSA–Glucose/Fructose, BSA–Methylglyoxal and L-Arginine-Methylglyoxal Model
Inhibition of Acetylcholinesterase (AChE) and Butylcholinesterase (BuChE)
3.2.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wojdyło, A.; Jáuregui, P.N.N.; Carbonell-Barrachina, Á.A.; Oszmiański, J.; Golis, T. Variability of Phytochemical Properties and Content of Bioactive Compounds in Lonicera caerulea L. Var. Kamtschatica Berries. J. Agric. Food Chem. 2013, 61, 12072–12084. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The Potential Health Benefits of Haskap (Lonicera caerulea L.): Role of Cyanidin-3-O-Glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- De Silva, A.B.K.H.; Rupasinghe, H.P.V. Polyphenols Composition and Anti-Diabetic Properties in Vitro of Haskap (Lonicera caerulea L.) Berries in Relation to Cultivar and Harvesting Date. J. Food Compos. Anal. 2020, 88, 103402. [Google Scholar] [CrossRef]
- Sivapragasam, N.; Neelakandan, N.; Rupasinghe, H.P.V. Potential Health Benefits of Fermented Blueberry: A Review of Current Scientific Evidence. Trends Food Sci. Technol. 2023, 132, 103–120. [Google Scholar] [CrossRef]
- Bei, X.; Yu, X.; Zhou, C.; Yagoub, A.E.A. Improvement of the Drying Quality of Blueberries by Catalytic Infrared Blanching Combined with Ultrasound Pretreatment. Food Chem. 2024, 447, 138983. [Google Scholar] [CrossRef]
- Wang, Z.; Svyantek, A.; Miller, Z.; Jarrett, B.; Kapus, A. Haskap Juicing Method Effects on Haskap Juice Quality. Appl. Sci. 2023, 13, 10784. [Google Scholar] [CrossRef]
- Senica, M.; Stampar, F.; Petkovsek, M. Different Extraction Processes Affect the Metabolites in Blue Honeysuckle (Lonicera caerulea L. Subsp. edulis) Food Products. Turk. J. Agric. For. 2019, 43, 576–585. [Google Scholar] [CrossRef]
- Molina, A.K.; Vega, E.N.; Pereira, C.; Dias, M.I.; Heleno, S.A.; Rodrigues, P.; Fernandes, I.P.; Barreiro, M.F.; Kostić, M.; Soković, M.; et al. Promising Antioxidant and Antimicrobial Food Colourants from Lonicera caerulea L. Var. Kamtschatica. Antioxidants 2019, 8, 394. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A.; Lachowicz, S. Effect of Dried Powder Preparation Process on Polyphenolic Content and Antioxidant Activity of Blue Honeysuckle Berries (Lonicera caerulea L. Var. Kamtschatica). LWT-Food Sci. Technol. 2016, 67, 214–222. [Google Scholar] [CrossRef]
- Ueda, J.M.; Morales, P.; Fernández-Ruiz, V.; Ferreira, A.; Barros, L.; Carocho, M.; Heleno, S.A. Powdered Foods: Structure, Processing, and Challenges: A Review. Appl. Sci. 2023, 13, 12496. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Hu, L. High Efficient Freeze-Drying Technology in Food Industry. Crit. Rev. Food Sci. Nutr. 2022, 62, 3370–3388. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chiu, H.T.; Feng, Z.; Maes, E.; Serventi, L. Effect of Spray-Drying and Freeze-Drying on the Composition, Physical Properties, and Sensory Quality of Pea Processing Water (Liluva). Foods 2021, 10, 1401. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Ciechanowska, A.; Brzezowska, J.; Wojdyło, A.; Gajewicz-Skretna, A.; Ciska, E.; Majerska, J. Chemometric Contribution for Deeper Understanding of Thermally-Induced Changes of Polyphenolics and the Formation of Hydroxymethyl-L-Furfural in Chokeberry Powders. Food Chem. 2021, 342, 128335. [Google Scholar] [CrossRef] [PubMed]
- Brzezowska, J.; Martinez-Rodriguez, A.J.; Silvan, J.M.; Łysiak, G.P.; Wojdyło, A.; Lech, K.; Michalska-Ciechanowska, A. Matrix Changes Driven by Cultivar Diversity, Inulin Addition and Drying Techniques-Shaping the Antioxidant, Antimicrobial and Anti-Inflammatory Properties of Blueberry Powders. Innov. Food Sci. Emerg. Technol. 2023, 89, 103481. [Google Scholar] [CrossRef]
- Brzezowska, J.; Skrzypczak, K.; Radzki, W.; Turkiewicz, I.P.; Ziaja-Sołtys, M.; Bogucka-Kocka, A.; Wojdyło, A.; Michalska-Ciechanowska, A. Comparative Study of Antioxidant, Antiglycation and Chemoprotective Potential of Beetroot Juice Powder Formulations with Functional Carriers. Food Biosci. 2023, 55, 103049. [Google Scholar] [CrossRef]
- Shahidi, F.; Pan, Y. Influence of Food Matrix and Food Processing on the Chemical Interaction and Bioaccessibility of Dietary Phytochemicals: A Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6421–6445. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Lin, L.; Zhang, Y.; Li, S.; Tang, Z. Component Characteristics and Reactive Oxygen Species Scavenging Activity of Anthocyanins from Fruits of Lonicera caerulea L. Food Chem. 2023, 403, 134391. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea var. Kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.; Gajdos Kljusuric, J.; Carle, R.; Schieber, A. Recovery of Anthocyanins from Grape Pomace Extracts (Vitis vinifera L. cv. Cabernet Mitos) Using a Polymeric Adsorber Resin. Eur. Food Res. Technol. 2005, 220, 431–437. [Google Scholar] [CrossRef]
- Auzanneau, N.; Weber, P.; Kosińska-Cagnazzo, A.; Andlauer, W. Bioactive Compounds and Antioxidant Capacity of Lonicera caerulea Berries: Comparison of Seven Cultivars over Three Harvesting Years. J. Food Compos. Anal. 2018, 66, 81–89. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Meng, X.; Liu, S.; Mu, J.; Ning, C. Comparison of Polyphenol, Anthocyanin and Antioxidant Capacity in Four Varieties of Lonicera caerulea Berry Extracts. Food Chem. 2016, 197, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Brzezowska, J.; Hendrysiak, A.; Wojdyło, A.; Michalska-Ciechanowska, A. Extraction-Depended and Thermally-Modulated Physical and Chemical Properties of Powders Produced from Cranberry Pomace Extracts. Curr. Res. Food Sci. 2024, 8, 100664. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-Dependent Studies on the Total Phenolics, Flavonoids, Antioxidant Activities, and Sugar Content in Six Onion Varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Kucharska, A.Z. Effect of Pre-Treatment of Blue Honeysuckle Berries on Bioactive Iridoid Content. Food Chem. 2018, 240, 1087–1091. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Łysiak, G.P.; Figiel, A. Chemical Composition and Antioxidant Properties of Powders Obtained from Different Plum Juice Formulations. Int. J. Mol. Sci. 2017, 18, 176. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Wojdyło, A.; Honke, J.; Ciska, E.; Andlauer, W. Drying-Induced Physico-Chemical Changes in Cranberry Products. Food Chem. 2018, 240, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Gugliucci, A. Formation of Fructose-Mediated Advanced Glycation End Products and Their Roles in Metabolic and Inflammatory Diseases. Adv. Nutr. 2017, 8, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, P.G.; Greenspan, P. Inhibition of Nonenzymatic Protein Glycation by Pomegranate and Other Fruit Juices. J. Med. Food 2014, 17, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Sun, Y.; Li, L.; Zhou, Q.; Wang, M. The Antiglycative Effect of Apple Flowers in Fructose/Glucose-BSA Models and Cookies. Food Chem. 2020, 330, 127170. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, S.; Wang, J. Trehalose: Current Use and Future Applications. J. Pharm. Sci. 2011, 100, 2020–2053. [Google Scholar] [CrossRef]
- Ramkissoon, J.S.; Mahomoodally, M.F.; Subratty, A.H.; Ahmed, N. Inhibition of Glucose- and Fructose-Mediated Protein Glycation by Infusions and Ethanolic Extracts of Ten Culinary Herbs and Spices. Asian Pac. J. Trop. Biomed. 2016, 6, 492–500. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Z.; Wang, Y.; Wang, Y.; Fu, L.; Su, L. Chinese Bayberry (Myrica rubra) Phenolics Mitigated Protein Glycoxidation and Formation of Advanced Glycation End-Products: A Mechanistic Investigation. Food Chem. 2021, 361, 130102. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J.; Siraki, A.G.; Shangari, N. Aldehyde Sources, Metabolism, Molecular Toxicity Mechanisms, and Possible Effects on Human Health. Crit. Rev. Toxicol. 2005, 35, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, F.; Varidi, M.; Koocheki, A.; Hadizadeh, F. Effect of Microwave and Conventional Heating on Structural, Functional and Antioxidant Properties of Bovine Serum Albumin-Maltodextrin Conjugates through Maillard Reaction. Food Res. Int. 2017, 100, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Wang, T.; Managi, H.; Yoshikawa, M. Structural Requirements of Flavonoids for Inhibition of Protein Glycation and Radical Scavenging Activities. Bioorg. Med. Chem. 2003, 11, 5317–5323. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yagiz, Y.; Buran, T.J.; do Nunes, C.N.; Gu, L. Phytochemicals from Berries and Grapes Inhibited the Formation of Advanced Glycation End-products by Scavenging Reactive Carbonyls. Food Res. Int. 2011, 44, 2666–2673. [Google Scholar] [CrossRef]
- Liu, J.; He, Y.; Wang, S.; He, Y.; Wang, W.; Li, Q.; Cao, X. Ferulic Acid Inhibits Advanced Glycation End Products (AGEs) Formation and Mitigates the AGEs-Induced Inflammatory Response in HUVEC Cells. J. Funct. Foods 2018, 48, 19–26. [Google Scholar] [CrossRef]
- Anis, M.A.; Sreerama, Y.N. Inhibition of Protein Glycoxidation and Advanced Glycation End-Product Formation by Barnyard Millet (Echinochloa frumentacea) Phenolics. Food Chem. 2020, 315, 126265. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, S.; Chen, Y.; Zhang, R.; Zhou, M.; Wang, C.; Feng, N.; Wu, Q. Structure-Activity Relationship of Procyanidins on Advanced Glycation End Products Formation and Corresponding Mechanisms. Food Chem. 2019, 272, 679–687. [Google Scholar] [CrossRef]
- West, B.J.; Deng, S.; Uwaya, A.; Isami, F.; Abe, Y.; Yamagishi, S.; Jensen, C.J. Iridoids Are Natural Glycation Inhibitors. Glycoconj. J. 2016, 33, 671–681. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Haque, M.A.; Adhikari, B. Encapsulation in the Food Industry: A Brief Historical Overview to Recent Developments. Food Nutr. Sci. 2020, 11, 481–508. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Vongsvivut, J.; Tobin, M.J.; Adhikari, R.; Barrow, C.; Adhikari, B. Investigation of Oil Distribution in Spray-Dried Chia Seed Oil Microcapsules Using Synchrotron-FTIR Microspectroscopy. Food Chem. 2019, 275, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Fadaeinasab, M.; Basiri, A.; Kia, Y.; Karimian, H.; Ali, H.M.; Murugaiyah, V. New Indole Alkaloids from the Bark of Rauvolfia reflexa and Their Cholinesterase Inhibitory Activity. Cell. Physiol. Biochem. 2015, 37, 1997–2011. [Google Scholar] [CrossRef] [PubMed]
- Somjai, C.; Siriwoharn, T.; Kulprachakarn, K.; Chaipoot, S.; Phongphisutthinant, R.; Chaiyana, W.; Srinuanpan, S.; Wiriyacharee, P. Effect of Drying Process and Long-Term Storage on Characterization of Longan Pulps and Their Biological Aspects: Antioxidant and Cholinesterase Inhibition Activities. LWT-Food Sci. Technol. 2022, 154, 112692. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Tkacz, K.; Nowicka, P.; Michalska-Ciechanowska, A.; Lech, K.; Wojdyło, A. Physicochemical Characterization and Biological Potential of Japanese Quince Polyphenol Extract Treated by Different Drying Techniques. LWT-Food Sci. Technol. 2021, 152, 112247. [Google Scholar] [CrossRef]
- Gruskiene, R.; Lavelli, V.; Sereikaite, J. Application of Inulin for the Formulation and Delivery of Bioactive Molecules and Live Cells. Carbohydr. Polym. 2024, 327, 121670. [Google Scholar] [CrossRef] [PubMed]
- Amat-ur-Rasool, H.; Symes, F.; Tooth, D.; Schaffert, L.-N.; Elmorsy, E.; Ahmed, M.; Hasnain, S.; Carter, W.G. Potential Nutraceutical Properties of Leaves from Several Commonly Cultivated Plants. Biomolecules 2020, 10, 1556. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Iridoids and Other Monoterpenes in the Alzheimer’s Brain: Recent Development and Future Prospects. Molecules 2018, 23, 117. [Google Scholar] [CrossRef] [PubMed]
- Mahran, E.; Morlock, G.E.; Keusgen, M. Guided Isolation of New Iridoid Glucosides from Anarrhinum pubescens by High-Performance Thin-Layer Chromatography-Acetylcholinesterase Assay. J. Chromatogr. A 2020, 1609, 460438. [Google Scholar] [CrossRef]
- Abubakar, S.; Khor, B.-K.; Khaw, K.-Y.; Murugaiyah, V.; Chan, K.-L. Cholinesterase Inhibitory Potential of Dillenia suffruticosa Chemical Constituents and Protective Effect against Aβ-induced Toxicity in Transgenic Caenorhabditis elegans Model. Phytomed. Plus 2021, 1, 100022. [Google Scholar] [CrossRef]
- Szwajgier, D. Anticholinesterase Activities of Selected Polyphenols—A Short Report. Pol. J. Food Nutr. Sci. 2014, 64, 59–64. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Ferreres, F.; Moreno, D.A.; Nowicka, P. UPLC-PDA-Q/TOF-MS Profiling of Phenolic and Carotenoid Compounds and Their Influence on Anticholinergic Potential for AChE and BuChE Inhibition and on-Line Antioxidant Activity of Selected Hippophaë rhamnoides L. Cultivars. Food Chem. 2020, 309, 125766. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, M.; Wang, F.; Cai, J.; Luo, Q.; Li, S.; Zhu, J.; Tang, Z.; Fang, Z.; Wang, C.; et al. The Inhibition Mechanism of Polyphenols from Phyllanthus emblica Linn. Fruit on Acetylcholinesterase: A Interaction, Kinetic, Spectroscopic, and Molecular Simulation Study. Food Res. Int. 2022, 158, 111497. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huang, G. The Biological Activities of Butyrylcholinesterase Inhibitors. Biomed. Pharmacother. 2022, 146, 112556. [Google Scholar] [CrossRef] [PubMed]
- Capuano, E.; Oliviero, T.; van Boekel, M.A.J.S. Modeling Food Matrix Effects on Chemical Reactivity: Challenges and Perspectives. Crit. Rev. Food Sci. Nutr. 2018, 58, 2814–2828. [Google Scholar] [CrossRef] [PubMed]
- Figiel, A. Drying Kinetics and Quality of Beetroots Dehydrated by Combination of Convective and Vacuum-Microwave Methods. J. Food Eng. 2010, 98, 461–470. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Bobak, Ł.; Nowicka, P. Anti-Oxidant and Anti-Enzymatic Activities of Sea Buckthorn (Hippophaë rhamnoides L.) Fruits Modulated by Chemical Components. Antioxidants 2019, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Bąbelewski, P. Phenolic and Carotenoid Profile of New Goji Cultivars and Their Anti-Hyperglycemic, Anti-Aging and Antioxidant Properties. J. Funct. Foods 2018, 48, 632–642. [Google Scholar] [CrossRef]
- Wang, S.; Konkol, E.; Langrish, T.A.G. Spray Drying of Fruit Juice Using Proteins as Additives. Dry. Technol. 2011, 29, 1868–1875. [Google Scholar] [CrossRef]


| Sample | Drying Technique | Carrier | Anthocyanins | Phenolic Acids | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyanidin-3-O-Glucoside | Cyanidin-3,5-Di-O-Glucoside | Cyanidin-3-O-Rutinoside | Peonidin-3-O-Rutinoside | 5-O-Caffeoyl-Quinic Acid | Di-O-Caffeoylquinic Acid | Protocatechuic Acid-4-Glucoside | 1-O-Caffeoyl-Qunic Acid | 3-O-Caffeoyl-Quinic Acid | Sinapic Acid | |||
| J | FD | - | 1442.39 ± 128.26 A | 221.59 ± 14.32 A | 50.12 ± 6.98 A | 47.92 ± 6.89 A | 290.50 ± 14.33 A | 60.56 ± 4.60 A | 52.00 ± 2.42 A | 48.14 ± 2.79 A | 10.28 ± 0.95 A | 4.42 ± 0.44 A |
| XE | FD | - | 16837.80 ± 1689.78 B | 2480.74 ± 189.93 B | 534.63 ± 109.58 B | 509.84 ± 31.05 B | 3478.01 ± 5.18 B | 691.03 ± 1.36 B | 592.28 ± 25.64 B | 314.18 ± 33.79 B | 128.94 ± 8.17 B | 48.74 ± 0.39 B |
| XE | SD | - | 15783.86 ± 30.84 B | 2535.96 ± 66.95 B | 504.21 ± 39.80 B | 543.71 ± 10.41 B | 3297.20 ± 106.05 B | 680.08 ± 33.62 B | 580.68 ± 32.36 B | 370.32 ± 9.04 B | 121.44 ± 1.88 B | 49.64 ± 1.37 B |
| J | FD | M | 303.81 ± 8.37 b | 42.46 ± 4.51 a | 11.30 ± 0.34 a | 12.01 ± 1.69 a | 56.68 ± 1.50 a | 11.43 ± 0.45 a | 9.12 ± 1.51 a | 7.33 ± 0.02 a | 2.77 ± 0.10 a | 0.60 ± 0.02 a |
| I | 284.35 ± 4.56 ab | 48.38 ± 2.99 a | 9.15 ± 2.13 a | 9.74 ± 0.58 a | 54.75 ± 0.77 a | 10.84 ± 0.76 a | 7.53 ± 0.14 a | 7.41 ± 0.77 a | 2.59 ± 0.07 a | 0.82 ± 0.05 a | ||
| T | 307.65 ± 6.27 b | 52.55 ± 14.10 a | 13.18 ± 0.13 a | 12.79 ± 2.35 a | 56.42 ± 1.58 a | 9.59 ± 1.86 a | 9.17 ± 2.66 a | 6.77 ± 0.49 a | 2.42 ± 0.26 a | 0.69 ± 0.02 a | ||
| P | 298.41 ± 5.65 ab | 36.99 ± 3.63 a | 10.61 ± 1.30 a | 13.37 ± 0.64 a | 54.71 ± 2.11 a | 9.73 ± 0.36 a | 9.52 ± 1.07 a | 6.47 ± 0.73 a | 2.53 ± 0.23 a | 0.79 ± 0.29 a | ||
| SD | M | 291.11 ± 9.82 ab | 38.56 ± 7.38 a | 11.28 ± 1.12 a | 11.83 ± 0.36 a | 55.56 ± 1.61 a | 10.85 ± 0.41 a | 9.34 ± 1.03 a | 7.31 ± 0.07 a | 2.33 ± 0.43 a | 0.73 ± 0.05 a | |
| I | 278.73 ± 2.75 a | 39.80 ± 4.42 a | 8.23 ± 0.16 a | <LOD | 52.94 ± 2.07 a | 11.01 ± 0.54 a | 10.45 ± 1.15 a | 8.15 ± 0.24 a | 2.47 ± 0.27 a | 0.81 ± 0.03 a | ||
| T | 294.15 ± 5.69 ab | 38.55 ± 5.98 a | 8.61 ± 0.83 a | <LOD | 58.52 ± 1.26 a | 10.48 ± 1.76 a | 9.48 ± 0.83 a | 8.11 ± 1.04 a | 2.99 ± 0.88 a | 0.79 ± 0.02 a | ||
| P | 302.55 ± 0.15 ab | 49.17 ± 7.62 a | 12.31 ± 3.42 a | <LOD | 56.08 ± 0.83 a | 11.38 ± 1.43 a | 8.85 ± 0.52 a | 6.99 ± 0.38 a | 2.66 ± 0.37 a | 0.68 ± 0.20 a | ||
| XE | FD | M | 2154.08 ± 43.47 ab | 382.10 ± 17.10 ab | 75.94 ± 3.34 a | 72.62 ± 10.12 a | 503.64 ± 15.38 ab | 98.37 ± 1.86 ab | 89.44 ± 1.51 ab | 64.44 ± 0.90 a | 19.60 ± 0.12 a | 7.46 ± 0.06 a |
| I | 2074.61 ± 30.09 a | 375.65 ± 0.44 ab | 69.10 ± 4.19 a | 77.62 ± 0.18 a | 490.00 ± 5.92 a | 93.72 ± 1.46 ab | 85.90 ± 0.87 a | 62.75 ± 4.06 a | 20.29 ± 0.77 a | 7.59 ± 1.22 a | ||
| T | 2256.54 ± 25.87 b | 410.92 ± 23.51 b | 77.81 ± 4.09 a | 78.91 ± 7.01 a | 525.74 ± 0.43 b | 95.72 ± 0.97 ab | 93.01 ± 0.46 ab | 66.51 ± 1.70 a | 19.83 ± 0.11 a | 7.43 ± 0.25 a | ||
| P | 2138.28 ± 25.17 ab | 409.71 ± 8.56 b | 78.96 ± 2.82 a | 75.71 ± 4.64 a | 488.79 ± 12.87 a | 93.91 ± 3.92 ab | 88.86 ± 0.56 ab | 63.47 ± 0.71 a | 17.10 ± 1.30 a | 7.15 ± 0.16 a | ||
| SD | M | 2195.29 ± 43.20 ab | 297.84 ± 42.89 a | 75.20 ± 6.68 a | 81.04 ± 2.56 a | 513.77 ± 6.54 ab | 95.51 ± 2.62 ab | 93.93 ± 2.61 b | 69.19 ± 5.59 a | 20.47 ± 1.75 a | 8.24 ± 0.96 a | |
| I | 2104.11 ± 13.60 a | 375.77 ± 17.13 ab | 70.40 ± 4.49 a | 73.41 ± 0.86 a | 491.11 ± 10.38 a | 89.02 ± 1.96 a | 88.74 ± 0.89 ab | 63.08 ± 0.66 a | 20.14 ± 0.18 a | 7.24 ± 0.03 a | ||
| T | 2245.84 ± 28.76 b | 405.81 ± 32.85 b | 76.65 ± 5.52 a | 75.52 ± 3.89 a | 516.83 ± 2.38 ab | 99.75 ± 0.56 b | 91.29 ± 0.34 ab | 67.26 ± 0.88 a | 19.97 ± 0.36 a | 7.67 ± 0.10 a | ||
| P | 2160.28 ± 35.23 ab | 387.86 ± 32.76 ab | 77.80 ± 5.53 a | 73.17 ± 0.59 a | 495.37 ± 0.07 ab | 92.78 ± 3.44 ab | 88.60 ± 4.47 ab | 60.11 ± 0.80 a | 18.68 ± 0.66 a | 7.31 ± 0.36 a | ||
| Sample | Drying Technique | Carrier | Flavonols | Flavan-3-Ols | Iridoids | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quercetin- 3-O-Rutinoside | Quercetin- 3-O-Glucoside | Quercetin- 3-O-Glucosyl-Xyloside | Isorhamnetin-3-O-Glucoside | Quercetin-O-Rhamnoside-O-Hexoside | Kaempferol- -3-O-Hexoside | Isorhamnetin-O-Hexosyl-O-Pentoside | Luteolin-7-O-Glucoside | Isorhamne-Tin 3-O-Rutinoside | Quercetin | Kaempferol-Rhamnosyl-Di-O-Glucoside | Quercetin-3-O-Vicianoside | (+)-Catechin | (−)-Epicatechin | Loganic Acid | 7-Epi-Loganin | Pentosyl-Loganin | |||
| J | FD | - | 209.43 ± 17.90 A | 37.77 ± 2.95 A | 34.04 ± 1.13 A | 9.39 ± 2.05 A | <LOD | <LOD | 11.68 ± 0.97 A | 5.47 ± 0.38 A | <LOD | <LOD | <LOD | <LOD | 83.17 ± 0.43 A | 46.63 ± 7.44 A | 13.96 ± 1.68 A | 12.42 ± 0.96 A | 10.09 ± 1.93 A |
| XE | FD | - | 2576.72 ± 48.70 B | 478.98 ± 23.76 B | 432.07 ± 23.45 B | 145.29 ± 2.63 B | 136.89 ± 11.17 A | 132.23 ± 10.12 A | 116.15 ± 12.36 B | 68.02 ± 4.49 B | 51.16 ± 2.17 A | 31.85 ± 5.12 A | 33.35 ± 2.62 A | 22.57 ± 2.85 A | 380.50 ± 16.11 B | 408.05 ± 10.59 B | 131.58 ± 3.91 B | 51.76 ± 1.86 A | 154.17 ± 4.35 C |
| XE | SD | - | 2561.42 ± 12.40 B | 469.03 ± 20.14 B | 403.15 ± 26.51 B | 159.24 ± 7.07 B | 222.50 ± 18.94 B | 150.57 ± 12.44 B | 117.96 ± 7.60 B | 65.26 ± 2.31 B | 46.70 ± 0.98 A | 29.42 ± 3.73 A | 30.58 ± 4.61 A | 42.75 ± 6.60 B | 350.28 ± 21.46 B | 371.92 ± 31.01 B | 121.21 ± 9.89 B | 103.07 ± 17.17 B | 107.90 ± 6.35 B |
| J | FD | M | 41.95 ± 1.20 a | 6.89 ± 0.44 ab | 7.92 ± 1.46 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 29.32 ± 2.17 a | 19.42 ± 0.55 a | 11.03 ± 1.74 | 4.66 ± 2.16 a | <LOD |
| I | 39.05 ± 3.33 a | 6.22 ± 1.39 a | 6.23 ± 1.12 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 26.61 ± 0.96 a | 20.32 ± 3.59 a | <LOD | 4.20 ± 0.41 a | <LOD | ||
| T | 42.13 ± 0.16 a | 7.61 ± 0.38 ab | 6.65 ± 0.42 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 27.15 ± 2.76 a | <LOD | <LOD | <LOD | <LOD | ||
| P | 45.09 ± 6.10 a | 6.76 ± 0.78 ab | 6.77 ± 0.01 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 22.91 ± 2.62 a | <LOD | <LOD | <LOD | <LOD | ||
| SD | M | 40.25 ± 1.35 a | 7.50 ± 0.70 ab | 6.76 ± 0.58 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 28.68 ± 1.92 a | 16.06 ± 3.22 a | <LOD | <LOD | <LOD | |
| I | 39.54 ± 5.84 a | 7.30 ± 0.46 ab | 7.17 ± 1.53 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 29.13 ± 2.76 a | 18.95 ± 0.37 a | <LOD | <LOD | <LOD | ||
| T | 43.86 ± 0.26 a | 9.32 ± 0.89 b | 5.89 ± 1.49 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 28.61 ± 1.59 a | <LOD | <LOD | <LOD | <LOD | ||
| P | 45.45 ± 3.13 a | 7.19 ± 0.19 ab | 5.84 ± 1.02 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 22.41 ± 2.57 a | <LOD | <LOD | <LOD | <LOD | ||
| XE | FD | M | 359.72 ± 8.15 a | 64.31 ± 0.79 a | 59.68 ± 2.19 a | 16.21 ± 0.64 a | 16.56 ± 3.35 a | 16.85 ± 1.59 ab | 15.72 ± 3.15 a | 9.38 ± 0.55 a | <LOD | <LOD | <LOD | <LOD | 72.46 ± 1.69 bc | 80.84 ± 6.06 b | 26.99 ± 1.15 a | 14.49 ± 1.44 ab | 25.71 ± 1.98 a |
| I | 347.39 ± 5.71 a | 62.93 ± 0.84 a | 57.55 ± 1.06 a | 21.38 ± 3.23 a | 16.63 ± 0.89 a | 15.41 ± 0.46 a | 17.98 ± 2.53 a | 7.49 ± 1.26 a | <LOD | <LOD | <LOD | <LOD | 76.43 ± 1.01 c | 93.97 ± 7.73 b | 25.26 ± 2.24 a | 14.31 ± 0.15 ab | 24.57 ± 3.49 a | ||
| T | 371.99 ± 4.32 a | 67.65 ± 0.32 a | 62.33 ± 0.05 a | 21.35 ± 1.39 a | 18.69 ± 0.21 ab | 18.35 ± 1.30 ab | 17.17 ± 1.38 a | 7.68 ± 0.54 a | 7.04 ± 0.81 a | <LOD | <LOD | <LOD | 81.21 ± 5.51 c | 82.82 ± 2.55 b | 27.34 ± 4.75 a | 15.08 ± 3.74 ab | 24.20 ± 2.78 a | ||
| P | 353.86 ± 12.17 a | 65.22 ± 6.62 a | 53.51 ± 8.16 a | 19.17 ± 2.70 a | 20.08 ± 1.89 ab | 22.43 ± 2.77 b | 15.31 ± 1.58 a | 7.20 ± 0.69 a | <LOD | <LOD | <LOD | <LOD | 77.17 ± 0.27 c | 77.40 ± 6.96 b | 26.38 ± 2.11 a | 9.32 ± 1.31 a | 18.84 ± 0.03 a | ||
| SD | M | 377.66 ± 13.03 a | 66.86 ± 6.13 a | 61.29 ± 1.57 a | 20.86 ± 0.88 a | <LOD | 19.91 ± 1.92 ab | 20.48 ± 1.47 a | 9.65 ± 1.78 a | <LOD | <LOD | <LOD | <LOD | 68.04 ± 5.22 a-c | 93.52 ± 0.63 b | 21.52 ± 1.49 a | 19.55 ± 2.08 b | 21.64 ± 1.02 a | |
| I | 351.95 ± 8.17 a | 65.13 ± 4.73 a | 56.08 ± 0.96 a | 20.18 ± 3.05 a | <LOD | 19.25 ± 1.88 ab | 16.96 ± 1.40 a | 7.94 ± 0.47 a | 8.02 ± 0.85 a | <LOD | <LOD | <LOD | 58.66 ± 4.31 ab | 91.96 ± 1.44 b | 20.46 ± 2.01 a | 14.84 ± 0.84 ab | 22.26 ± 2.50 a | ||
| T | 369.00 ± 2.78 a | 67.97 ± 0.14 a | 61.54 ± 1.30 a | 21.39 ± 3.66 a | 18.40 ± 0.58 ab | 16.00 ± 1.51 ab | 18.21 ± 0.09 a | 9.69 ± 1.19 a | <LOD | <LOD | <LOD | <LOD | 55.07 ± 2.91 a | 30.39 ± 1.58 a | 60.42 ± 1.51 b | 22.43 ± 1.52 c | <LOD | ||
| P | 350.51 ± 3.07 a | 64.12 ± 4.38 a | 60.28 ± 0.71 a | 20.35 ± 3.73 a | 22.96 ± 1.51 b | 18.71 ± 1.29 ab | 20.10 ± 0.29 a | 9.74 ± 1.74 a | <LOD | <LOD | <LOD | <LOD | 71.38 ± 5.05 bc | 81.81 ± 9.21 b | 22.78 ± 2.04 a | 11.69 ± 0.83 a | 24.78 ± 0.25 a |
| Antioxidant Assay | Antiglycation Assay | Anti-Ageing Assay | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TEAC ABTS | FRAP | BSA–Glucose | BSA–Fructose | BSA–MGO | MGO–L-Arginine | Acetylcholinesterase | Butylcholinesterase | |||
| (mmol Trolox/100 g dm) | (% of Fluorescent AGE Inhibition) | IC50 (mg/mL) | ||||||||
| J | FD | - | 21.22 ± 0.15 b | 23.58 ± 0.73 b | 62.99 ± 0.70 d | 89.51 ± 0.21 d | 36.69 ± 2.89 c | 40.68 ± 4.72 e | 11.66 ± 0.99 a | 12.78 ± 1.08 a |
| XE | FD | - | 266.03 ± 2.89 e | 261.71 ± 8.44 d | 79.31 ± 0.93 e | 95.02 ± 0.06 d | 89.73 ± 0.03 f | 95.38 ± 0.47 g | 19.91 ± 1.69 b–e | 22.08 ± 1.87 a |
| XE | SD | - | 252.65 ± 1.95 d | 238.38 ± 5.96 c | 79.07 ± 0.76 e | 94.57 ± 0.22 d | 88.16 ± 0.43 f | 94.72 ± 0.23 g | 20.07 ± 1.71 b–e | 25.91 ± 2.20 a |
| J | FD | M | 3.83 ± 0.02 a | 4.71 ± 0.12 a | 50.27 ± 0.13 c | 66.18 ± 3.86 bc | 18.43 ± 0.44 ab | −2.80 ± 0.97 a | 14.40 ± 1.22 ab | 24.06 ± 2.04 a |
| I | 3.85 ± 0.10 a | 4.81 ± 0.08 a | 50.18 ± 0.98 c | 65.91 ± 0.73 bc | 15.31 ± 0.41 a | 4.41 ± 0.42 c | 21.17 ± 1.80 c–f | 22.42 ± 1.90 a | ||
| T | 4.13 ± 0.19 a | 4.97 ± 0.08 a | 48.99 ± 0.34 bc | 64.89 ± 1.35 bc | 18.78 ± 0.46 ab | 10.05 ± 0.32 d | 13.92 ± 1.18 ab | 21.03 ± 1.78 a | ||
| P | 3.92 ± 0.17 a | 4.92 ± 0.06 a | 45.27 ± 0.92 a | 54.26 ± 0.43 a | 18.73 ± 0.96 ab | 1.16 ± 0.15 a-c | 14.26 ± 1.21 ab | 23.30 ± 1.98 a | ||
| SD | M | 3.25 ± 0.18 a | 4.07 ± 0.38 a | 50.68 ± 0.41 c | 63.17 ± 0.39 b | 18.02 ± 0.62 ab | −2.33 ± 0.91 ab | 16.18 ± 1.37 a–c | 19.51 ± 1.67 a | |
| I | 4.05 ± 0.20 a | 5.05 ± 0.01 a | 46.74 ± 1.13 ab | 71.01 ± 0.64 c | 19.49 ± 0.74 ab | 2.20 ± 0.72 bc | 17.10 ± 1.45 a–d | 19.57 ± 1.61 a | ||
| T | 4.06 ± 0.04 a | 5.12 ± 0.08 a | 49.16 ± 0.28 bc | 64.17 ± 0.48 bc | 21.50 ± 2.09 b | 2.24 ± 0.27 bc | 16.04 ± 1.36 a–c | 19.58 ± 1.66 a | ||
| P | 3.71 ± 0.14 a | 4.82 ± 0.02 a | 46.89 ± 0.79 ab | 70.46 ± 5.64 c | 17.88 ± 1.71 ab | 3.47 ± 0.23 c | 16.41 ± 1.39 a–c | 20.08 ± 1.70 a | ||
| XE | FD | M | 23.99 ± 0.19 bc | 29.35 ± 0.64 b | 63.28 ± 0.81 d | 90.79 ± 0.18 d | 51.43 ± 0.63 e | 61.10 ± 0.06 f | 18.80 ± 1.60 b–e | 60.06 ± 5.10 b |
| I | 24.42 ± 0.62 bc | 30.35 ± 0.91 b | 62.87 ± 0.71 d | 90.44 ± 0.03 d | 45.88 ± 0.14 d | 60.68 ± 0.64 f | 18.51 ± 1.57 b–d | 68.76 ± 5.83 b | ||
| T | 26.01 ± 0.01 c | 32.98 ± 0.94 b | 62.97 ± 0.22 d | 90.62 ± 0.03 d | 46.86 ± 0.93 d | 60.27 ± 1.10 f | 17.21 ± 1.46 a–d | 61.57 ± 5.22 b | ||
| P | 26.02 ± 0.37 c | 33.48 ± 0.26 b | 61.29 ± 0.25 d | 90.51 ± 0.19 d | 45.50 ± 1.06 d | 59.03 ± 0.08 f | 17.56 ± 1.49 a–d | 56.05 ± 4.76 b | ||
| SD | M | 23.72 ± 0.51 bc | 28.13 ± 0.62 b | 63.27 ± 0.52 d | 94.57 ± 0.22 d | 49.43 ± 0.32 de | 59.67 ± 0.48 f | 25.21 ± 2.14 ef | 69.01 ± 5.85 b | |
| I | 24.23 ± 0.67 bc | 29.11 ± 1.63 b | 62.34 ± 0.27 d | 90.71 ± 0.04 d | 46.81 ± 0.45 d | 58.62 ± 0.28 f | 26.90 ± 2.28 f | 57.55 ± 4.88 b | ||
| T | 25.21 ± 0.48 c | 31.43 ± 0.97 b | 63.55 ± 0.43 d | 90.57 ± 0.01 d | 46.51 ± 1.84 d | 60.64 ± 0.01 f | 23.03 ± 1.95 d–f | 63.55 ± 5.39 b | ||
| P | 24.71 ± 0.47 c | 30.74 ± 1.61 b | 62.56 ± 0.25 d | 88.27 ± 3.07 d | 45.73 ± 0.01 d | 58.53 ± 0.82 f | 23.51 ± 1.99 d–f | 58.68 ± 4.98 b | ||
| Fruit | Juice | XAD Extract | Pomace | |
|---|---|---|---|---|
| Dry matter (%) | 14.03 ± 0.42 b | 12.93 ± 0.10 b | 6.02 ± 0.06 a | 38.29 ± 1.08 c |
| Acidity (mg malic acid/100 g) | 1.92 ± 0.01 c | 2.30 ± 0.07 d | 1.07 ± 0.02 a | 1.75 ± 0.01 b |
| Vitamin C (mg ascorbic acid/100 g) | 19.19 ± 0.19 c | 3.57 ± 0.16 b | 3.71 ± 0.03 b | 2.32 ± 0.02 a |
| Ash (%) | 0.38 ± 0.04 b | 0.43 ± 0.02 b | 0.07 ± 0.02 a | 1.05 ± 0.07 c |
| Pectins (g/100 g) | 1.01 ± 0.08 b | ND | ND | 0.23 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalska-Ciechanowska, A.; Brzezowska, J.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P.; Hendrysiak, A.; Oszmiański, J.; Andlauer, W. Advantages of Spray Drying over Freeze Drying: A Comparative Analysis of Lonicera caerulea L. Juice Powders—Matrix Diversity and Bioactive Response. Molecules 2024, 29, 3586. https://doi.org/10.3390/molecules29153586
Michalska-Ciechanowska A, Brzezowska J, Nowicka P, Tkacz K, Turkiewicz IP, Hendrysiak A, Oszmiański J, Andlauer W. Advantages of Spray Drying over Freeze Drying: A Comparative Analysis of Lonicera caerulea L. Juice Powders—Matrix Diversity and Bioactive Response. Molecules. 2024; 29(15):3586. https://doi.org/10.3390/molecules29153586
Chicago/Turabian StyleMichalska-Ciechanowska, Anna, Jessica Brzezowska, Paulina Nowicka, Karolina Tkacz, Igor Piotr Turkiewicz, Aleksandra Hendrysiak, Jan Oszmiański, and Wilfried Andlauer. 2024. "Advantages of Spray Drying over Freeze Drying: A Comparative Analysis of Lonicera caerulea L. Juice Powders—Matrix Diversity and Bioactive Response" Molecules 29, no. 15: 3586. https://doi.org/10.3390/molecules29153586
APA StyleMichalska-Ciechanowska, A., Brzezowska, J., Nowicka, P., Tkacz, K., Turkiewicz, I. P., Hendrysiak, A., Oszmiański, J., & Andlauer, W. (2024). Advantages of Spray Drying over Freeze Drying: A Comparative Analysis of Lonicera caerulea L. Juice Powders—Matrix Diversity and Bioactive Response. Molecules, 29(15), 3586. https://doi.org/10.3390/molecules29153586











