2.1. Wettability and Surface Parameters of Starch before the Adsorption Process
Starch is a polar biopolymer, so it can be expected to have a higher ability to interact with polar substances, such as water and formamide, due to the interactions of dipoles and/or polar groups. Apolar diiodomethane CH2I2, due to the lowest affinity for starch and the lack of polar groups in the structure, should show the fastest penetration time. However, the matter is not that simple. The course of the wetting process is influenced by a number of other parameters; in addition to the porous structure and the size of the specific surface area, the size of the pores, their shape, the viscosity of the system, and surface tension may be decisive. This makes the process very complicated and multi-stage. However, it is certain that such a deep analysis of the wetting process is the starting point when discussing the adsorption process and can be very helpful in determining the adsorption mechanism in systems using native and modified starch.
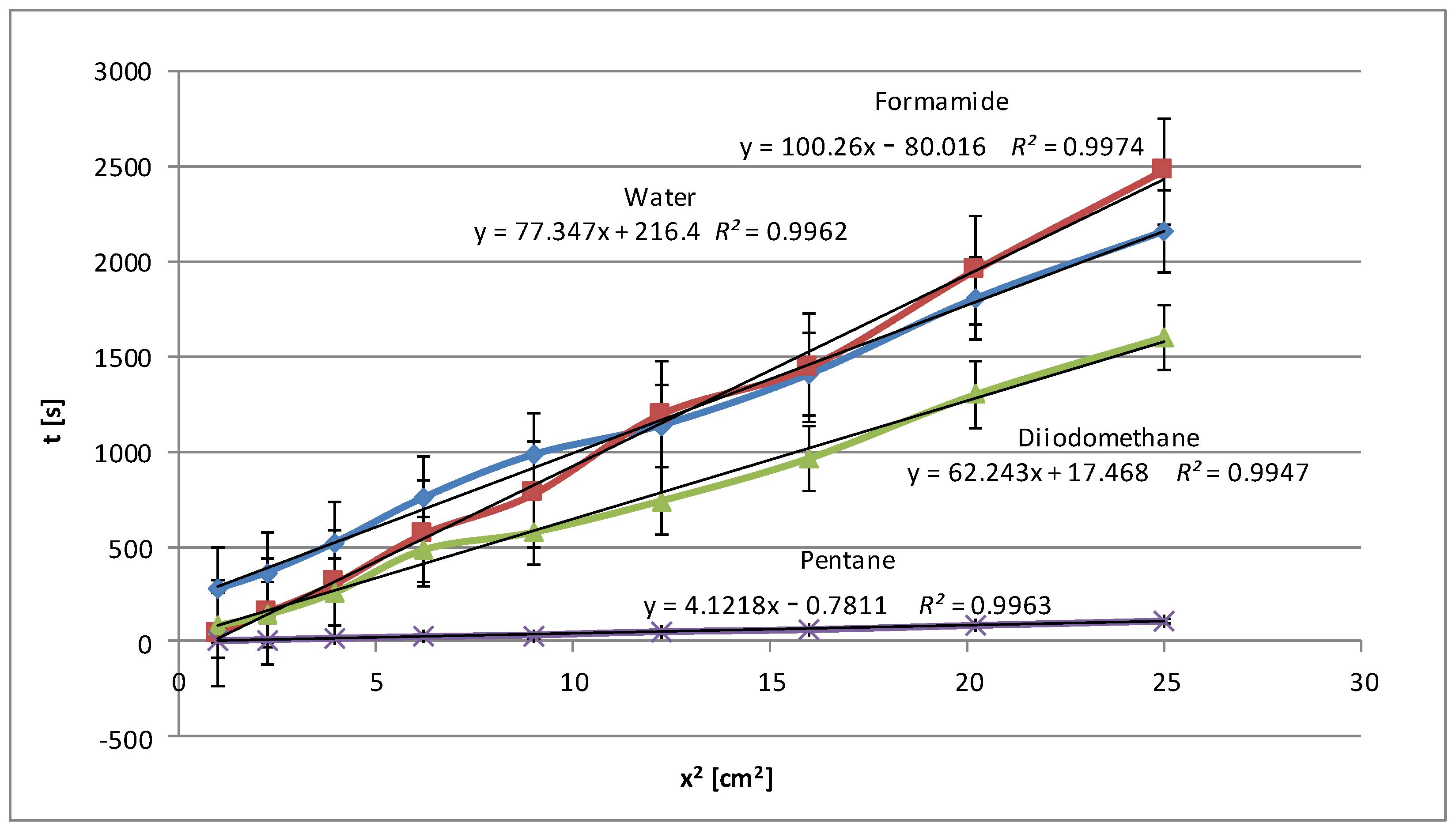
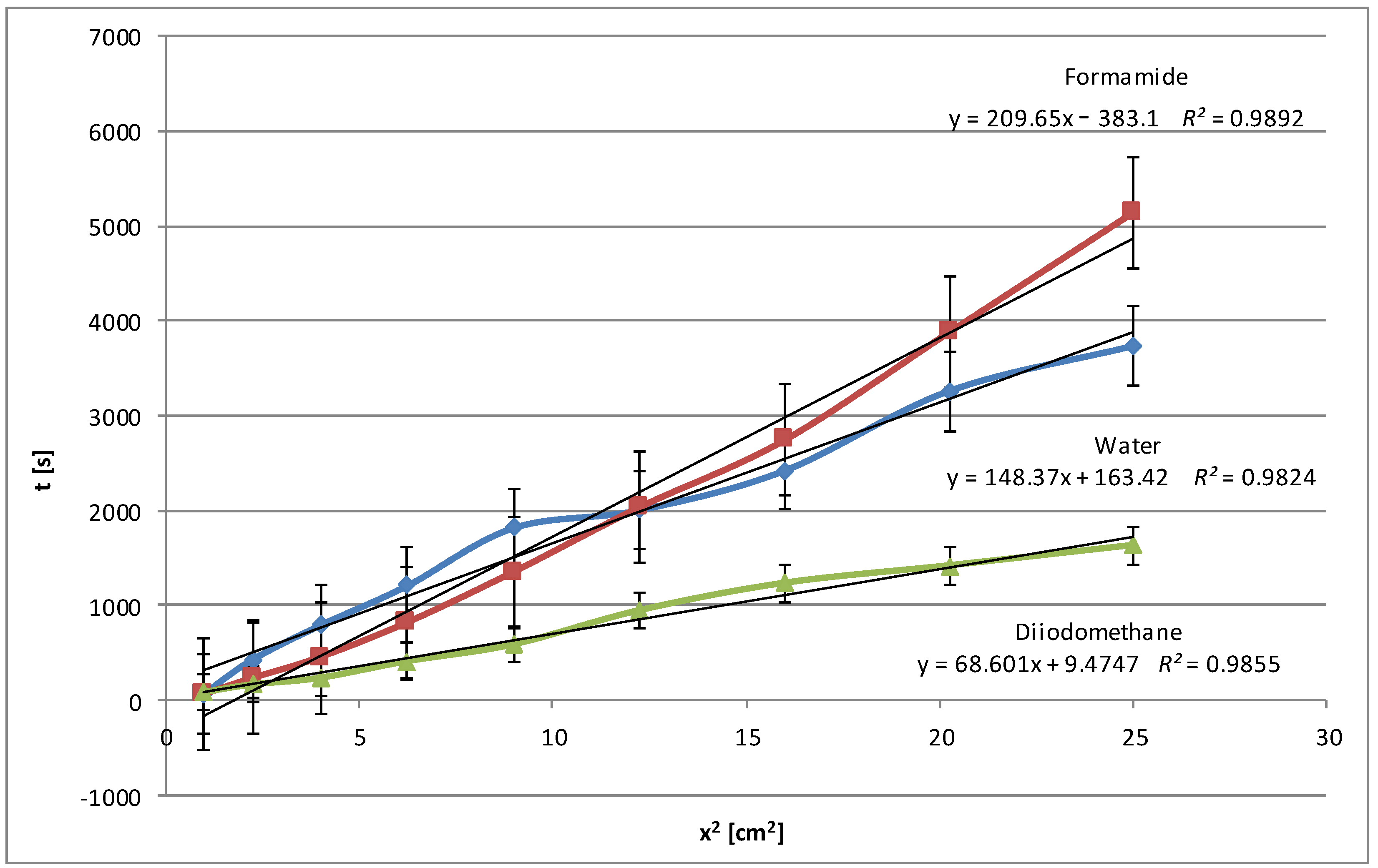
Figure 1 shows the relationships obtained for native potato starch. In addition to the three test liquids (water, formamide, diiodomethane), the wetting process was also performed with pentane, as these data are needed to determine the effective capillary radius (details are described in the description of the method; see
Section 3.2.1 and
Section 3.2.2). Pentane, as an
n-alkane, is used for these calculations due to its simple molecular structure and physicochemical properties, which define the surface properties well. The apolarity of pentane means its ability to wet polar substances such as starch is minimal or nil. This is reflected in the very low slope of its regression line.
For this type of starch, the best matches were found both before and after modification above R2 = 0.99. Each graph contains standard deviations for individual measurements. High values of the determination coefficient R2, exceeding 0.99 for each of the tested test liquids, confirm that the linear regression model describes the relationship between the wetting time and the square of the penetration distance very well, indicating the linear nature of the Washburn equation.
In fact, the wetting time with polar water is shorter than the wetting time with less polar formamide, which may suggest that the interactions of the polar groups of HCONH2 with starch are more effective than water dipoles. This may also be due to the difference in the surface tension values of both liquids and different micropore penetration efficiency. It is also worth emphasizing that such a situation did not occur during the entire process. Approximately halfway through the process, the water showed longer penetration times, and then the situation reversed. It is possible that molecules can effectively fill small micropores. This process is supported by strong intermolecular interactions and the ability of molecules to form hydrogen bonds. In addition to the degree of polarity, other types of interactions, such as van der Waals forces or hydrophobic interactions, may be important. On the other hand, apolar diiodomethane shows fast penetration, which was proven to be accurate according to the graph presented. Only the results for pentane, which is understandable for an alkane compound with a very low surface tension (15.49 mN/m at 25 °C), were lower.
However, the correctness of the methodology was confirmed even though the plates were covered with a starch dispersion in laboratory conditions, which did not guarantee high homogeneity, unlike commercial plates. The presented linear relationships describe the relationship between the wetting time and the length of the starch penetration section quite well, demonstrating a high degree of fit of the model to the data according to the Washburn equation (Equation (1)). A fit of
R2 value of 0.95 and above is considered sufficient in this method. Comparing
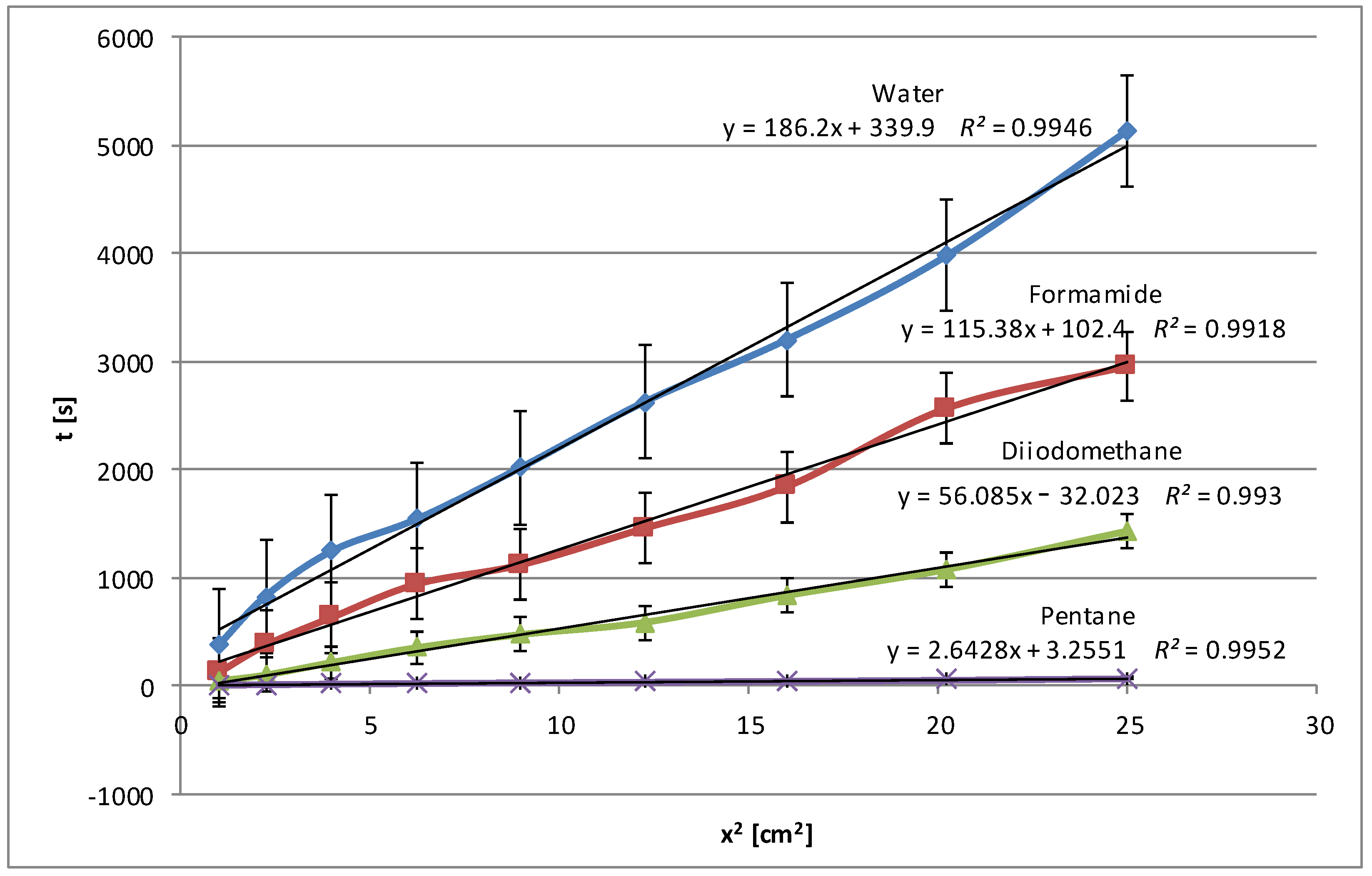
Figure 1 and
Figure 2, what is immediately noticeable is the doubling of the penetration times for water. Therefore, the range of the scale is double. In this case, penetration times also increased, but not so dramatically. The curves are clearly separated from each other, which may confirm the homogeneity of the examined layers. The higher results obtained for polar liquids and lower results for non-polar liquids (diiodomethane and pentane) confirm the increase in polarity in the case of modified potato starch. Water and formamide, due to their high polarity, are able to form hydrogen bonds with starch, which results in an increase in its penetration time.
In contrast to this process, diiodomethane and pentane, as apolar liquids, have weaker or no interactions with polar starch molecules; they do not penetrate deep into the starch but move along the surface, which is manifested by the highest wetting (in this case displacement) speed and the lowest times.
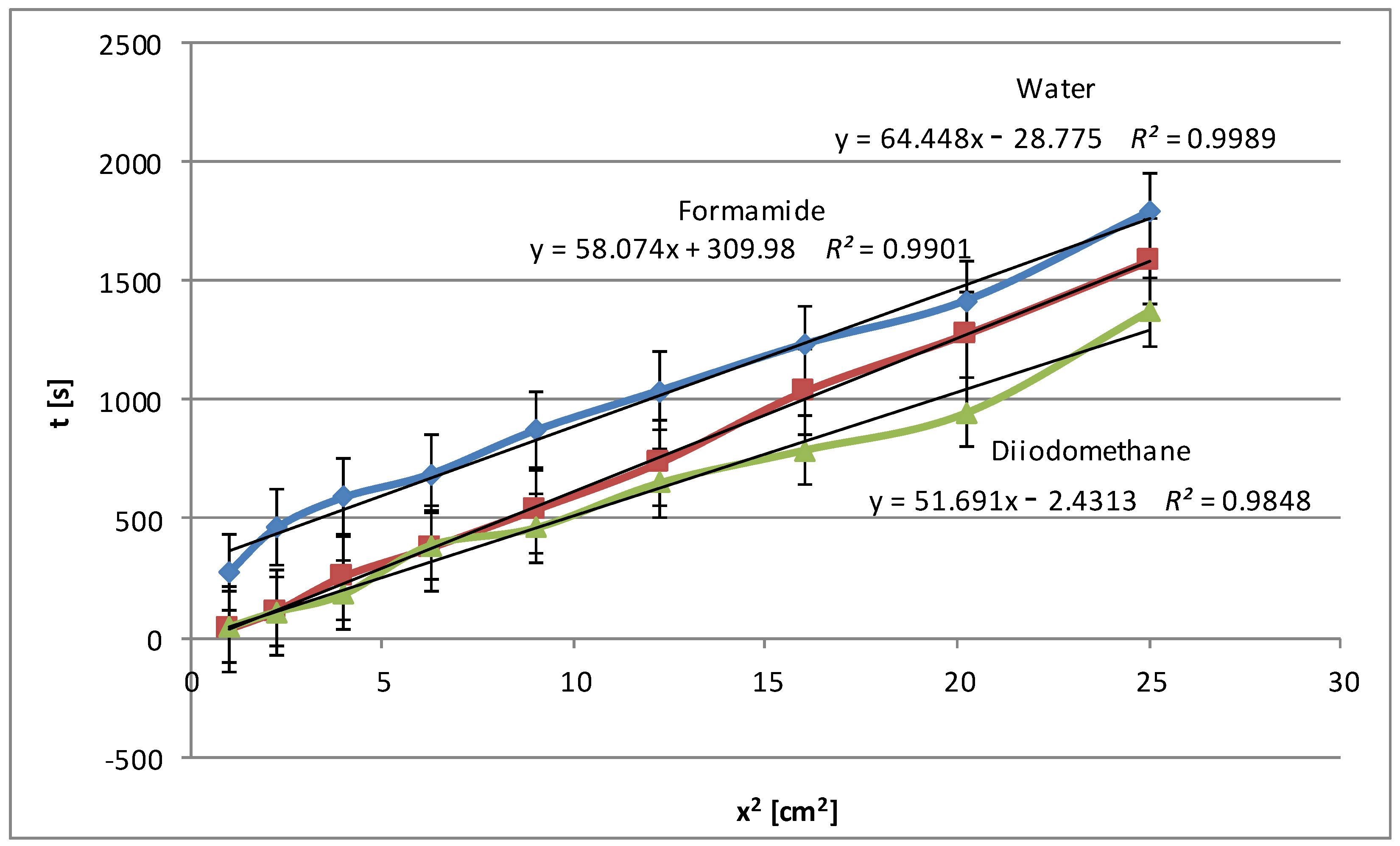
The next figure (
Figure 3) shows the wetting time dependencies obtained for pea starch. The low values of wetting times (below 2000 s) were obtained, and, what is more, the curves are close to each other for polar and non-polar liquids. This may suggest the weak polar nature of this kind of starch. This conclusion is also confirmed by the low zeta potential values described in detail in Part I [
24], which indicate a small charge accumulated on the surface of the granules of this type of starch. As expected, water achieves the greatest penetration time, as seen from the trend line’s highest slope. Formamide is in the middle, while diiodomethane has the shortest penetration time. This is because the most polar water interacts strongly with polar starch, extending the time it takes to wet the starch surface. Apolar diiodomethane has the lowest degree of interaction with the starch. Formamide, which is less polar than water, falls between the two, presenting a medium rate of starch wetting. The obtained results are consistent with the adsorption data discussed in detail in Part I [
24], i.e., for example, with the smallest specific surface areas. Again, high values of the coefficient of determination
R2 for all three liquids (water: 0.999, formamide: 0.990, diiodomethane: 0.985) indicate a very good fit of the linear model to the collected data.
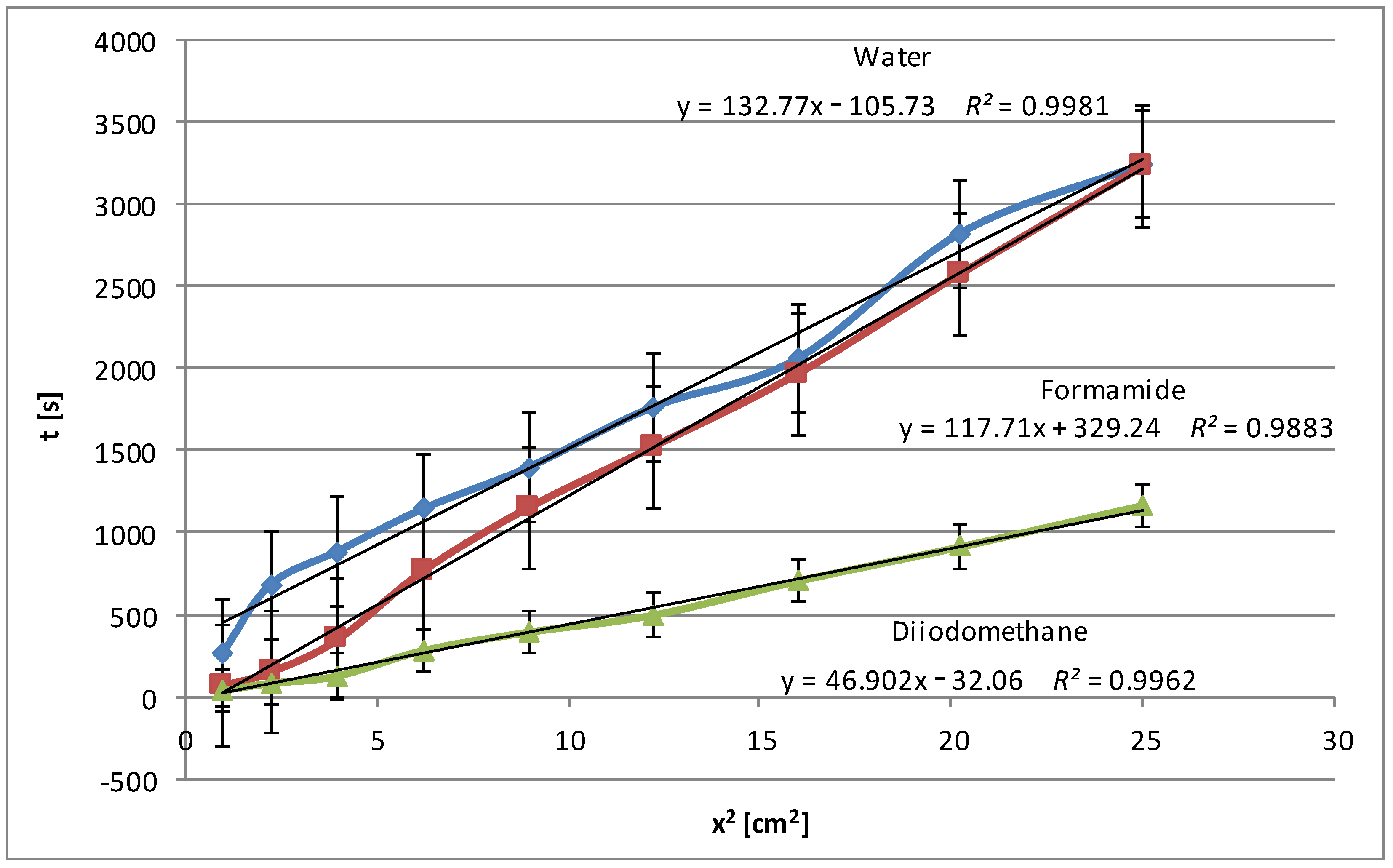
The modification of pea starch slightly increases its polarity, which causes water and formamide to penetrate the modified starch deeper than the native pea starch and therefore the wetting process is slower. Therefore, additional time is needed to create a network of hydrogen bonds (
Figure 4). Diiodomethane, as an apolar liquid, does not form hydrogen bonds with starch. The increased polarity of the modified pea starch makes diiodomethane penetrate it faster, but only on the surface, because there is no resistance associated with the interaction of polar groups of starch with non-polar diiodomethane. In this case, bigger deviations from linearity are visible, especially for water. Such deviations in the penetration process may result from the manual coating of the plates, which may lead to surface inhomogeneities.
Figure 5 shows the average values of the wetting rate of corn starch using water and diiodomethane. As mentioned above, the type and number of the functional groups in the structure of test liquids are essential, as they are responsible for the interactions with the polar groups of starch. In this case (
Figure 5), similarly to potato starch (
Figure 1), the relationships obtained for water and formamide are unclear over the entire penetration length. In the beginning, wetting times are longer for water, which translates into a slower rate, and the opposite for formamide. Near the halfway point, the situation reverses. However, such behavior of polar liquids and long values of wetting times indicate the polar nature of corn starch.
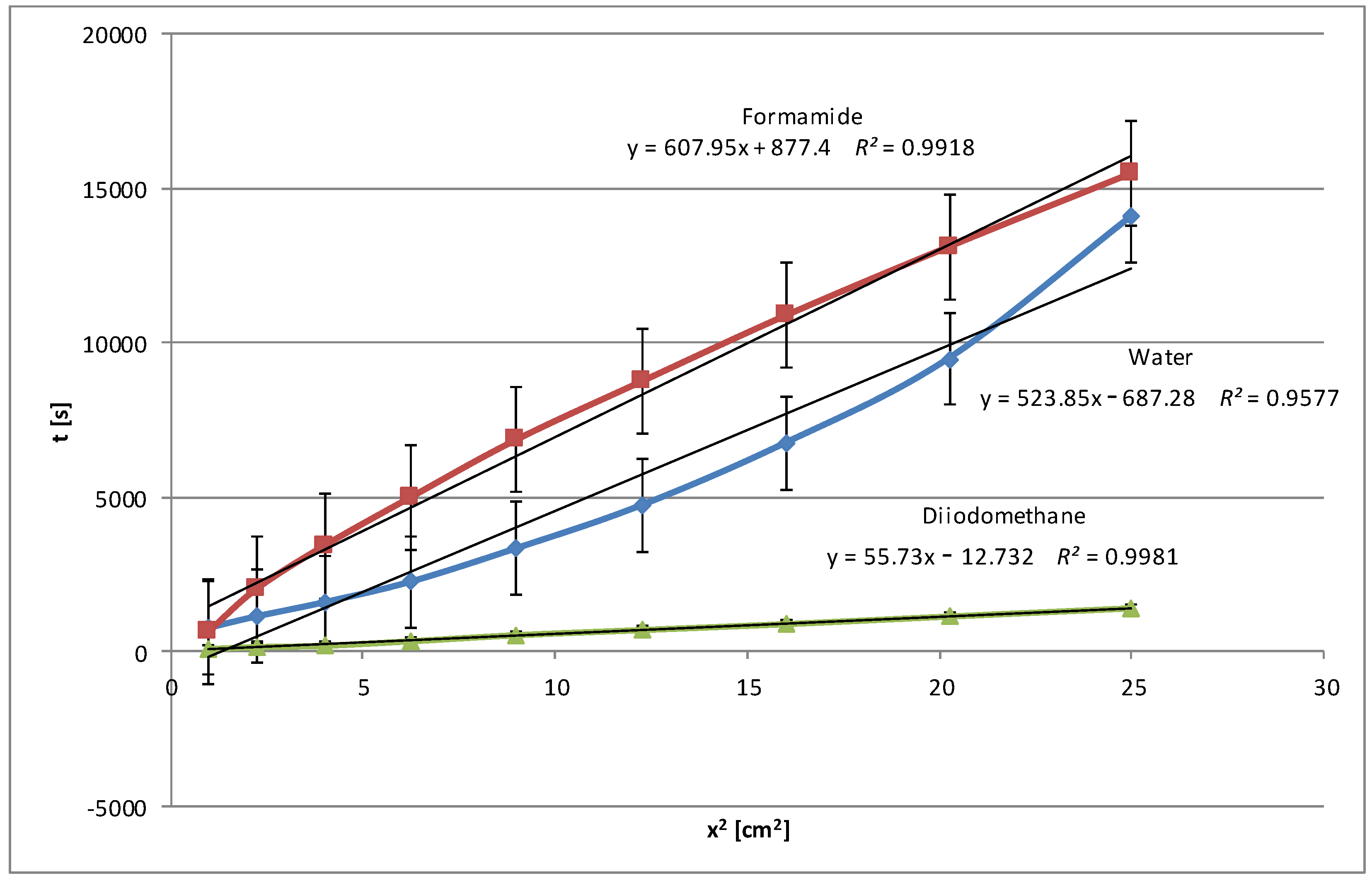
An analysis of all the wettability graphs shows that the liquid’s polarity directly impacts the wetting efficiency of native and modified starch. More polar liquids, such as water and formamide, effectively wet the starch by penetrating its structure with the possibility of forming polar bonds, while non-polar liquids, such as diiodomethane and pentane, move on the starch surface quickly or very quickly because the process takes place only on the surface and uses weak dispersion interactions. To confirm these conclusions, we present below the relationships obtained for modified corn starch (
Figure 6). In this case, a significant increase in wetting times, almost 4-fold for water and 3-fold for formamide, was observed. This is understandable because the highest degree of hydrolysis, over 48%, was achieved for this starch, and the most significant changes in the specific surface area and electrokinetic zeta potential were noted, as described in Part I [
24].
After the three-stage modification, the water wetting times of the plates covered with the modified starch dispersions were noticeably extended, sometimes even several times. This was the result of the presence of additional polar groups on the surface of the starch film due to hydrolysis, larger pore volume, and specific surface area. To obtain the full picture, similar tests were performed with polar formamide, non-polar diiodomethane, and pentane. In general, the wetting process of the modified starches with polar water and formamide was slower compared to the native starches. However, diiodomethane and pentane, which are non-polar liquids, penetrate modified starch faster than native starch. These changes suggest that the modification increases the polarity of all starches but to different degrees, increasing their interactions with polar liquids and decreasing them with apolar liquids. The relationships were expressed using linear equations with coefficients of determination R2, which in each case were very close to 1, which indicated an excellent fit of the linear model to the collected data.
The selection of liquids of different chemical natures (strongly polar, less polar, or apolar) gives an overview of the various types of interactions and enables a full description of the phenomenon of the wetting process. On this basis, the hydrophilic/hydrophobic nature of different kinds of starch can be determined, which consequently helps to select specific applications, e.g., adsorbents for food application [
23], and will be helpful for a description of the gallic acid adsorption mechanism presented in the next
Section 2.3.
2.2. Energetic Parameters of Starch before the Adsorption Process
It is well known that, apart from surface parameters, the energetic parameters of substances are also very important and influence the course of the adsorption process. The specific adsorption properties of starch are determined by the number of functional hydroxyl groups on the surface connected to the skeleton by covalent bonds, as well as the type of adsorption centers they create. These centers are considered as strong adsorption centers (unequal in terms of adsorption capacity) due to specific interactions with adsorbate molecules by the formation of hydrogen bonds or, more generally, donor–acceptor (acid–base) bonds.
Knowing the number of surface groups allows the determination of the adsorption activity of starch. On the other hand, the concentration of surface polar groups is reflected in the surface free-energy components, which result from the type and size of intermolecular interactions. It is assumed that the amount of adsorption increases with an increase in the number of double bonds and polar functional groups present in the molecules of the substances penetrating the starch. The movement of the substances on the starch surface is primarily the result of the formation of hydrogen bonds between the functional groups of the substance and the active sites of starch. Depending on the type and structure of the test liquid, the adsorption process occurs or does not. Additionally, the adsorption mechanism itself may change as the concentration of the substance changes, so the interpretation of the results may be complicated.
In the case of modified starches, the situation may be completely different. The presence of free hydroxyl groups in native starch allows the introduction of a maximum of three substituents to each glucose unit. A distinguishing feature of this process is the concept of the degree of substitution (DS), which is defined as the number of functional groups introduced per one glucose unit. If the modifying factor is a multifunctional compound, one of its molecules may take part in the reactions with glucose units located in various starch chains. This is the so-called cross-linking reaction.
During modification, native starch hydrolysis and oxidation processes may occur simultaneously, producing carboxyl and aldehyde ketone groups. The hydrolysis process may result in the substitution of hydroxyl groups, which changes or even reduces the hydrophilic properties, or the formation of new polar groups, e.g., carboxyl groups, as a result of which the hydrophilic character can increase.
Below is a table (
Table 1) presenting the adhesion tension values,
(change in free energy (enthalpy) accompanying the replacement of the unit area of the solid–gas interface with the solid–liquid interface during the movement of the liquid in the porous layer) determined for all three types of starch before and after modification in contact with three test liquids of different polar natures (two polar and one apolar). In the test liquid–solid system, there may be not only a change in the surface tension of the solution but also changes in the solid–liquid interfacial tension.
As a result of adsorption, the hydrophilic surface of a solid may change its character to become less hydrophilic or even hydrophobic. The relationship between the amount of adsorbed substance at the phase boundary and the wetting parameters (e.g., contact angle) can be determined by changes in adhesive tension. The lower the value of the adhesive tension, the easier the wetting process takes place and, consequently, the adsorption process is facilitated. In the case of the adhesive tension for polar substances, this applies to the adsorption of hydrophilic substances, while in the case of apolar substances, this applies to hydrophobic substances. In the adsorption process of gallic acid, the relationships obtained for polar substances will be crucial.
The values calculated for water and formamide confirmed the polar nature of all types of starch: weak polar for pea starch and potato starch (medium values of adhesion tension) and the most polar for corn starch (the lowest value of adhesion tension). After modification, the polar character increased even more, especially in the case of corn starch, for which a more than 3-fold decrease in the value of adhesive tension for water and formamide was noted. In the case of the other two starches (potato and pea), this tension was about two times lower for water. The very high values obtained for diiodomethane (
Table 1) can be extended to all hydrophobic substances. According to these values, it can be stated that adsorption of such substances is practically impossible on the starch surface, and after modification, these values increased even more, which further confirms this tendency. These considerations turned out to be helpful for description of the gallic acid adsorption mechanism on three types of native and modified starch presented in the next
Section 2.3.
2.3. Adsorption of Gallic Acid on Native and Modified Starch
Figure 7 shows the effect of pH, temperature, and phase contact time on the adsorption of gallic acid on native starch. In this particular case, we wanted to optimize the most important parameters affecting the adsorption of gallic acid on starch. Thus, we performed optimization only for the native starch, and then we adsorbed gallic acid on the modified starch, under identical conditions previously established for a given starch. In this way, we obtained a comparison of how the modification affected the sorption properties of starch. The highest amount of GA was adsorbed on corn starch for each parameter. In the pH range of 5–9, an increase in adsorption was observed with an increase in the pH of the reaction medium (
Figure 7a). According to Ertan et al. [
25] gallic acid interacts more intensely with biopolymers at a pH of 9 rather than at a pH of 7, as this compound converts to quinone, and crosslinking may occur. In our case, this process was also confirmed for all three starches. Between pH 7 and pH 9, a jump in the course of the relationship is visible. The largest increase in the trend line was noted in the case of potato starch (
Figure 7a).
Similar experiments and results confirming the occurrence of these processes were described by Zhao and co-workers for cassava starch/chitosan/gallic acid systems obtained via pressurized fluid technology combined with ultrasonication [
26]. The crosslinking between -COOH groups of gallic acid and -CH
2OH groups of starch by ester bonds, and electrostatic interactions between COO
− and NH
3+ were observed. These phenomena promoted film hydrophobicity by reducing the availability of -OH in starch, as observed by the decrease of the wettability parameter and as an effect of the decrease of water vapor permeability. These conclusions also confirm our considerations presented in
Section 2.1 and
Section 2.2 and data discussed in Part I [
24]. Wettability and energy parameters are very sensitive to any changes occurring on the surface of starch granules (charge, number of hydroxyl groups, degree of substitution, etc.). Therefore, even a slight change may lead to a completely different course of the adsorption process, in this case of gallic acid.
The influence of temperature on GA adsorption was tested in the range of 10–40 °C (below the onset temperature of starch gelatinization; the range of T
0 for potato, corn, and pea starches is 51–64 °C [
27,
28]). For each starch, an increase in temperature of 30 °C caused an approximately 1.3–1.5-fold increase in the amount of adsorbed GA (
Figure 7b). Adsorption reached equilibrium after 120 min for corn starch, 150 min for potato starch, and 180 min for pea starch (
Figure 7c). After these time periods, the highest amounts of adsorbed gallic acid were noted for corn starch at 3.10 mg/g, then for pea starch at 2.49 mg/g, and the lowest adsorption was recorded for potato starch at 2.05 mg/g. These results correlate well with the values of S
BET for modified starches (1.66 m
2/g for corn, 0.40 m
2/g for pea, and 0.22 m
2/g for potato starch) obtained in Part I [
24].
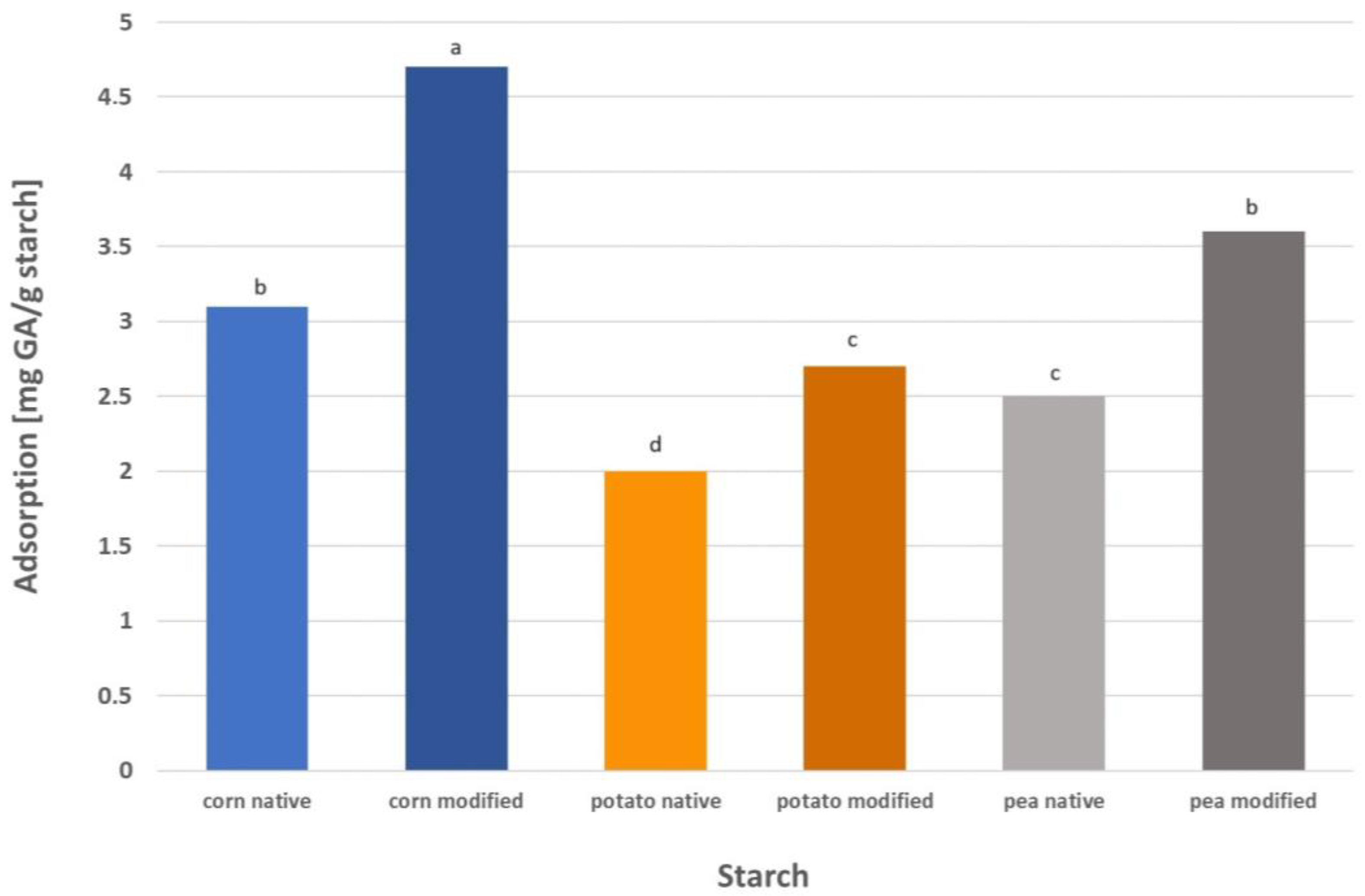
GA adsorption on modified starch was performed under the optimized conditions for native starch (pH 9, and 40 °C for all starches, and the phase contact time appropriate for a given starch), and the results are presented in
Figure 8. The adsorption of GA on modified corn starch was 51% higher than on native starch. In the case of pea starch, the adsorption of this compound was 45% higher, and for potato starch it was 38% higher. After three-stage modification, the starch granules retained their structure and shape, but irregularities and pores were visible on the surface, especially in the case of corn starch [
24]. It is well known that the surface morphology of the adsorbent affects its adsorption performance. The presence of irregularities and numerous pores on the surface of the modified starches influences their potential as adsorbents. Starch granules with pores demonstrate enhanced adsorption capacity compared with native starch granules as the content of hydroxyl groups increases. The adsorption performance of modified starch mainly depends on pore characteristics and, more precisely, on the specific surface area. The highest specific area was noted for corn starch (1.66 m
2/g), which also showed the highest adsorption capacity toward gallic acid. Next in order were pea (0.40 m
2/g) and potato (0.22 m
2/g) starches, which correlates well with the results obtained for GA adsorption on these starches presented in
Figure 8 [
24]. Wang et al. [
17] carried out the adsorption of grape seed proanthocyanidins on porous corn starch prepared by the enzymatic method using α-amylase and amyloglucosidase. The adsorption kinetics curve presented in these studies shows that after approximately 120 min, the adsorption curve flattened and equilibrium was achieved. Despite a slightly different modification methodology than in our case and a different adsorbed substance, the time to reach equilibrium for corn starch is identical, 120 min. This is a good future prognosis for the use of the starches tested by us for the adsorption and/or encapsulation of not only gallic acid but other substances with a similar structure or a similar adsorption mechanism. Wang et al. [
17] reported that the amount of proanthocyanidins adsorbed on modified starch increased by approximately 10% compared to native starch. Similar results were obtained by Jiang et al. [
15], who achieved adsorption equilibrium after approximately 100 min and the adsorption rate of procyanidins on porous rice starch increased by almost 45% compared to native starch.
2.4. ATR-FTIR Spectra
As was expected, the positions of the characteristic absorption bands for modified starches and modified starches complexed with gallic acid in some cases differed from the positions of the bands for native starches, as shown in
Figure 9. The intensity of the characteristic absorption bands of porous corn starch decreased compared to native starch. Scientists, in their studies, provide various explanations for this phenomenon. Jiang et al. [
15] found that all characteristic absorption bands of native starch were present in the spectrum of porous starch, suggesting that the use of enzymatic hydrolysis did not change the chemical structure of obtained modified granules. However, according to Zhang et al. [
29], the change in the intensity of the characteristic bands results from the fact that the formation of pores on the starch surface reduces the density of starch granules. We would be more inclined to accept the latter explanation, all the more so because slightly different results were observed in the other two starches. In the case of potato starch, a lower intensity of the characteristic modified starch bands was observed compared to native starch. In contrast, the intensity of the complexed starch bands increased compared to the modified ones. A similar relationship in the change in the intensity of the characteristic bands was observed in the case of pea starch.
After the adsorption process, the intensity of the bands of starch complexed with gallic acid decreased even more compared to the modified starch. The most considerable shift in the band position was observed in the case of pea starch for the band of starch complexed with gallic acid 3278 cm
−1 compared to the native starch band of 3249 cm
−1. Regarding another study, researchers performed the adsorption of gallic acid on porous starch and showed that the characteristic absorption bands of porous starch were not significantly changed after the adsorption of gallic acid, which was due to the fact that the adsorption process did not cause any changes in the molecular structure of the starch. Therefore, no new kind of chemical bonds were formed between gallic acid and the porous starch. The results suggested that gallic acid was mainly adsorbed by forming hydrogen bonds with porous starch [
30]. Similar results were presented by Hu and Du [
14], who reported no changes in the characteristic bands before and after adsorption of tea polyphenols on porous starch. They also found that adsorption occurred mainly through the formation of hydrogen bonds with functional groups on the microporous starch surface. In our research, we also consider this process as a main mechanism, e.g., the creation of new hydrogen bonds or replacing the old ones on the surface of the starch, in connection with the creation of a microporous structure. Since this microporous structure is most developed in the case of corn starch, the described mechanism most closely correlates with the results we obtained for this particular starch. Slightly different results were obtained for native pea and potato starch and also, after modification, again confirmed the lower polarity and lower porosity of these starches, which we have already discussed in this manuscript in Part I [
24].