The Mass Spectrometric Ortho Effect for Distinguishing the Coeluting Isomers of Polychlorinated Biphenyls and the Coeluting Isomers of Polybrominated Biphenyls: Qualitative and Quantitative Aspects
Abstract
1. Introduction
2. Results and Discussion
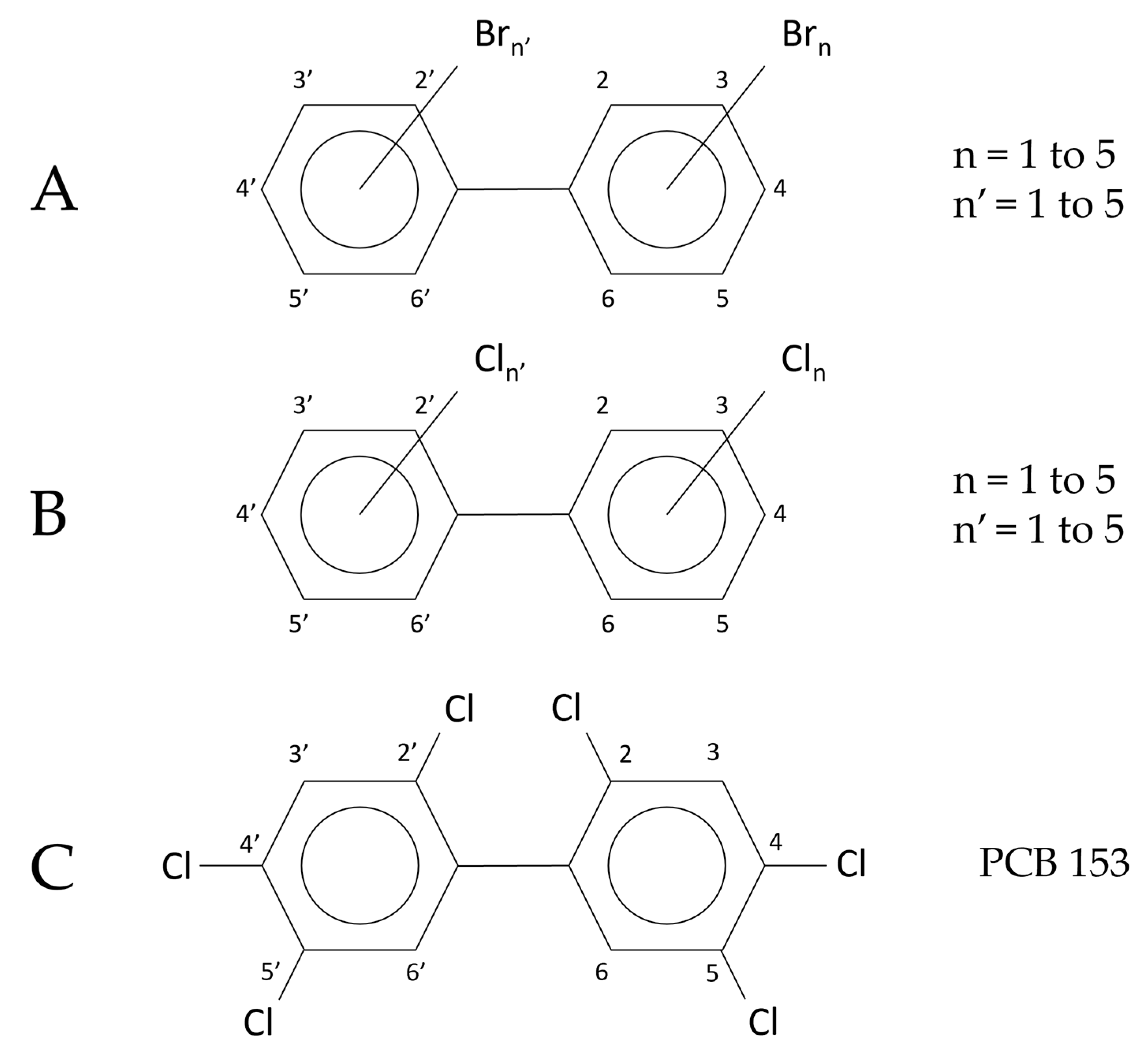
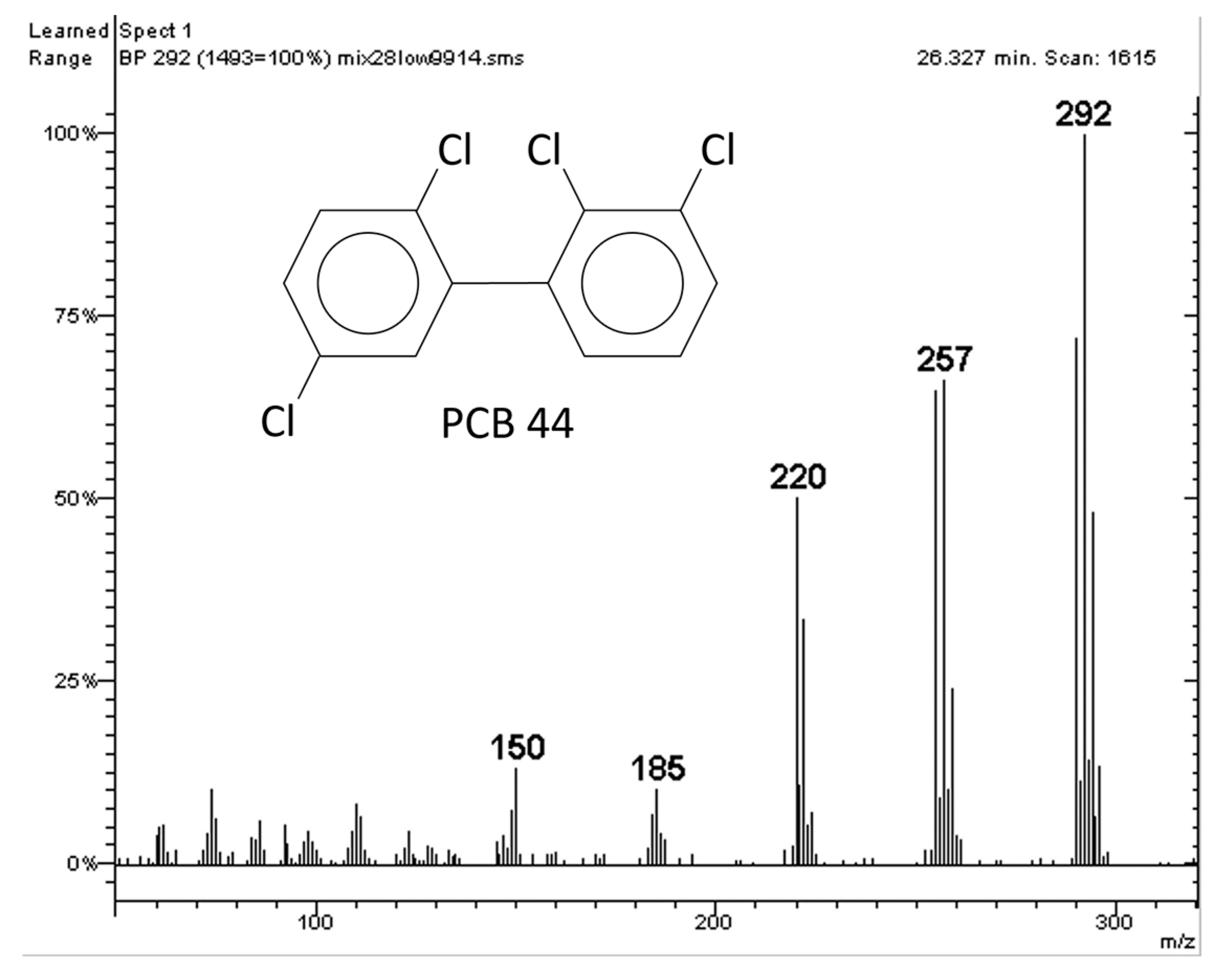
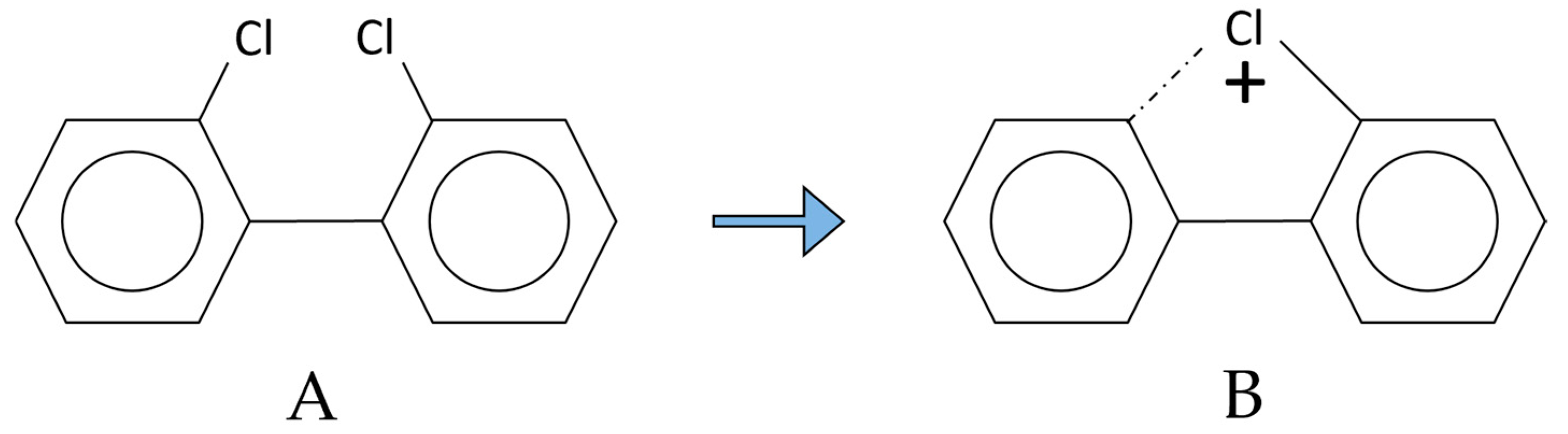
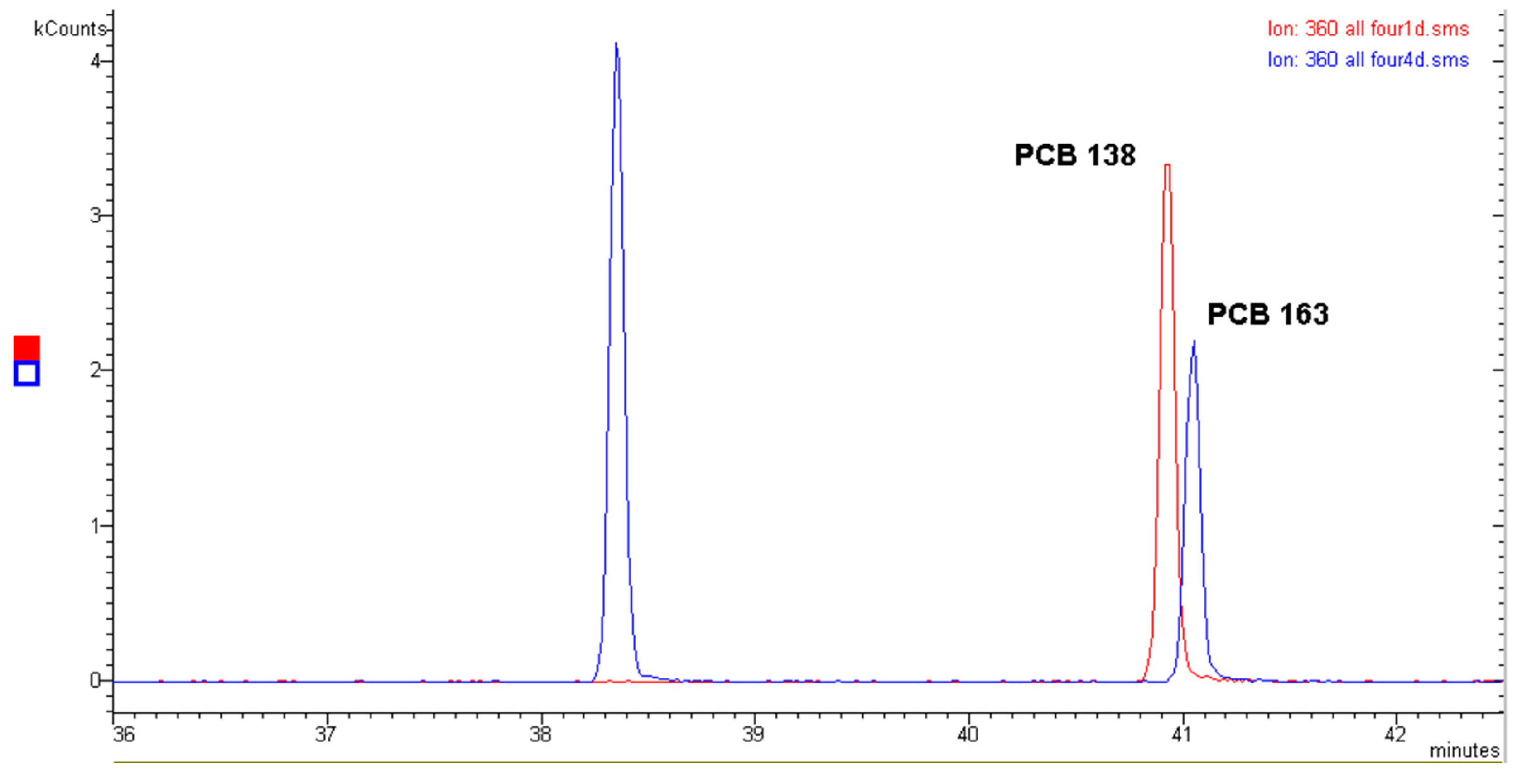
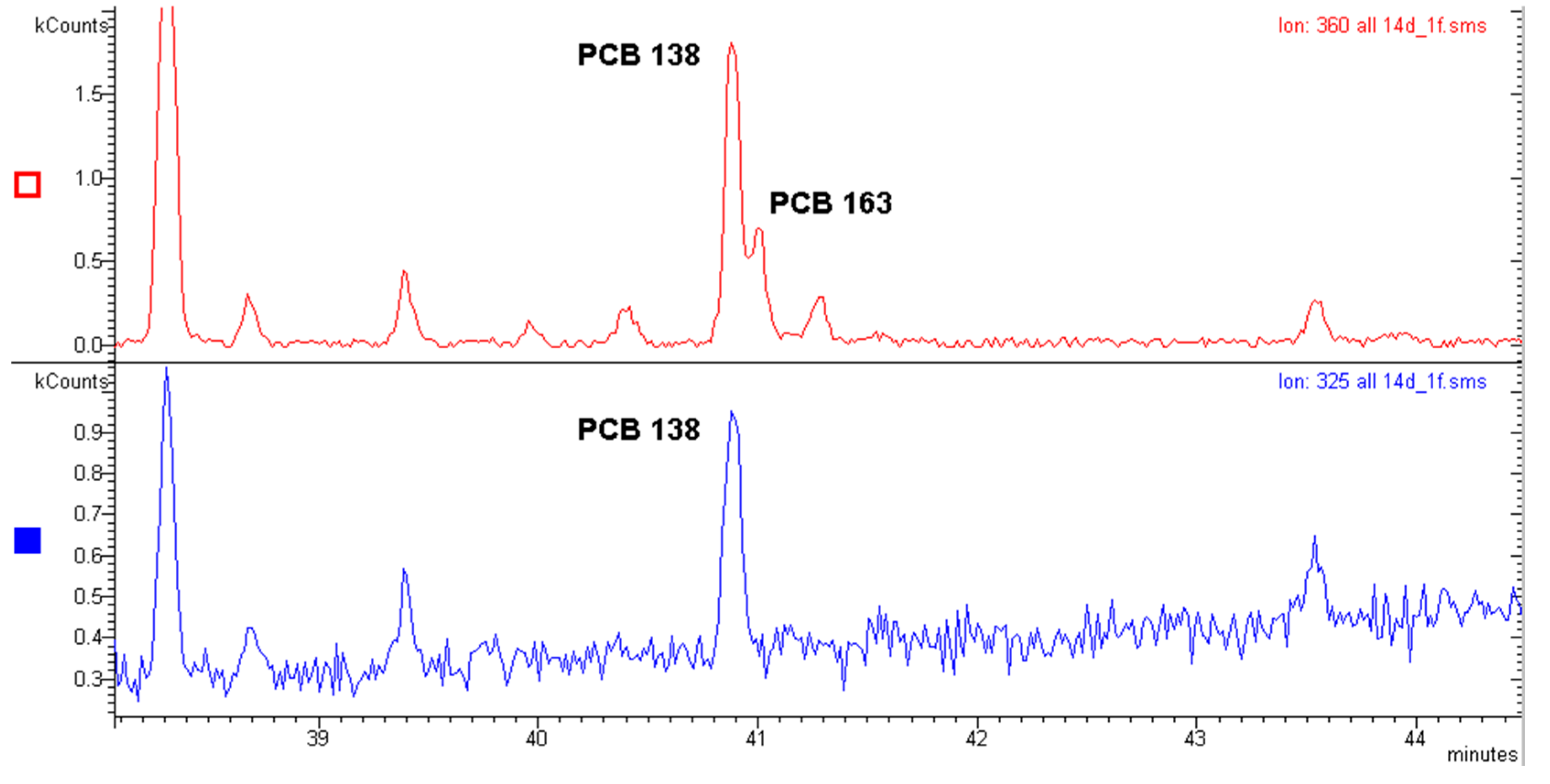
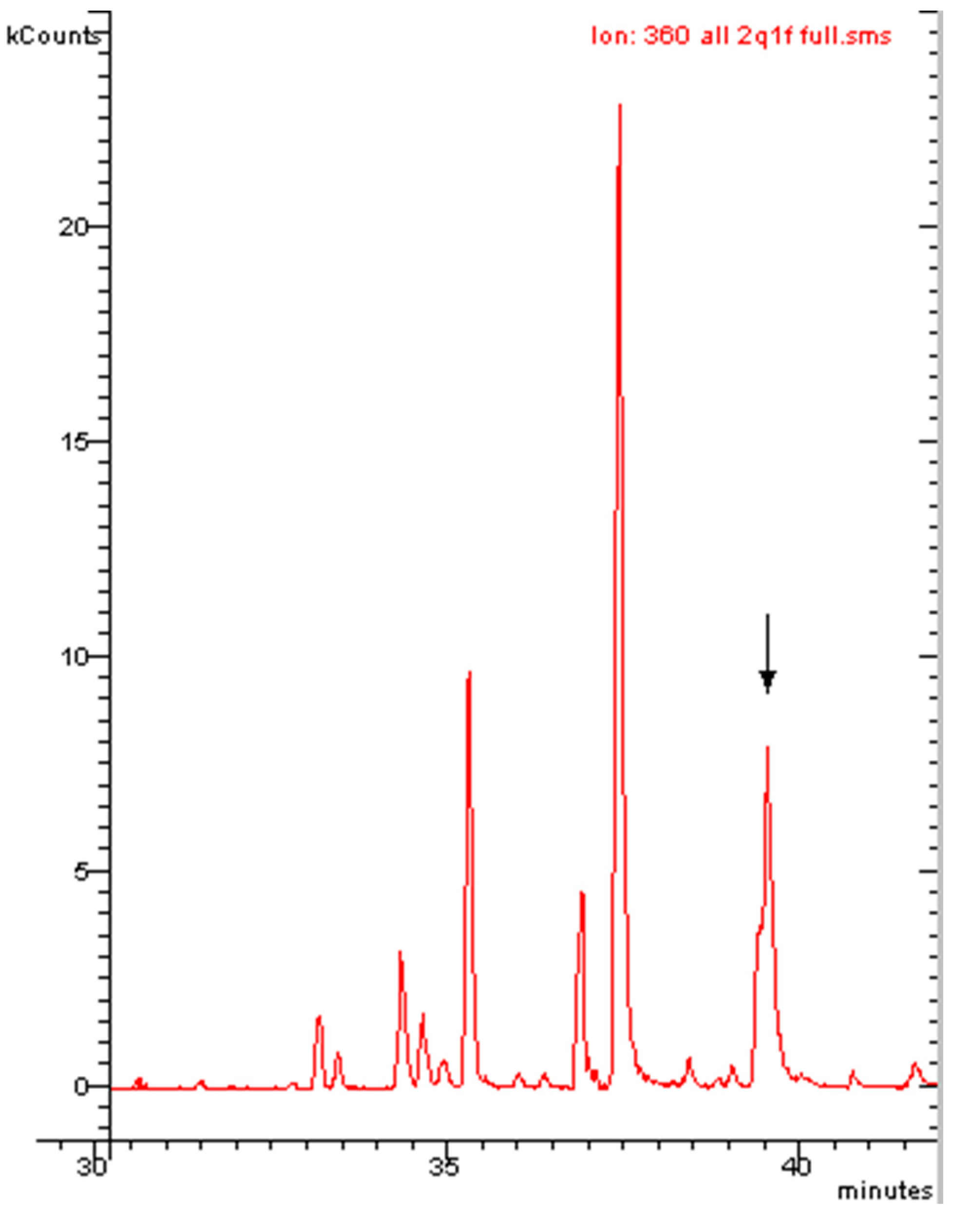
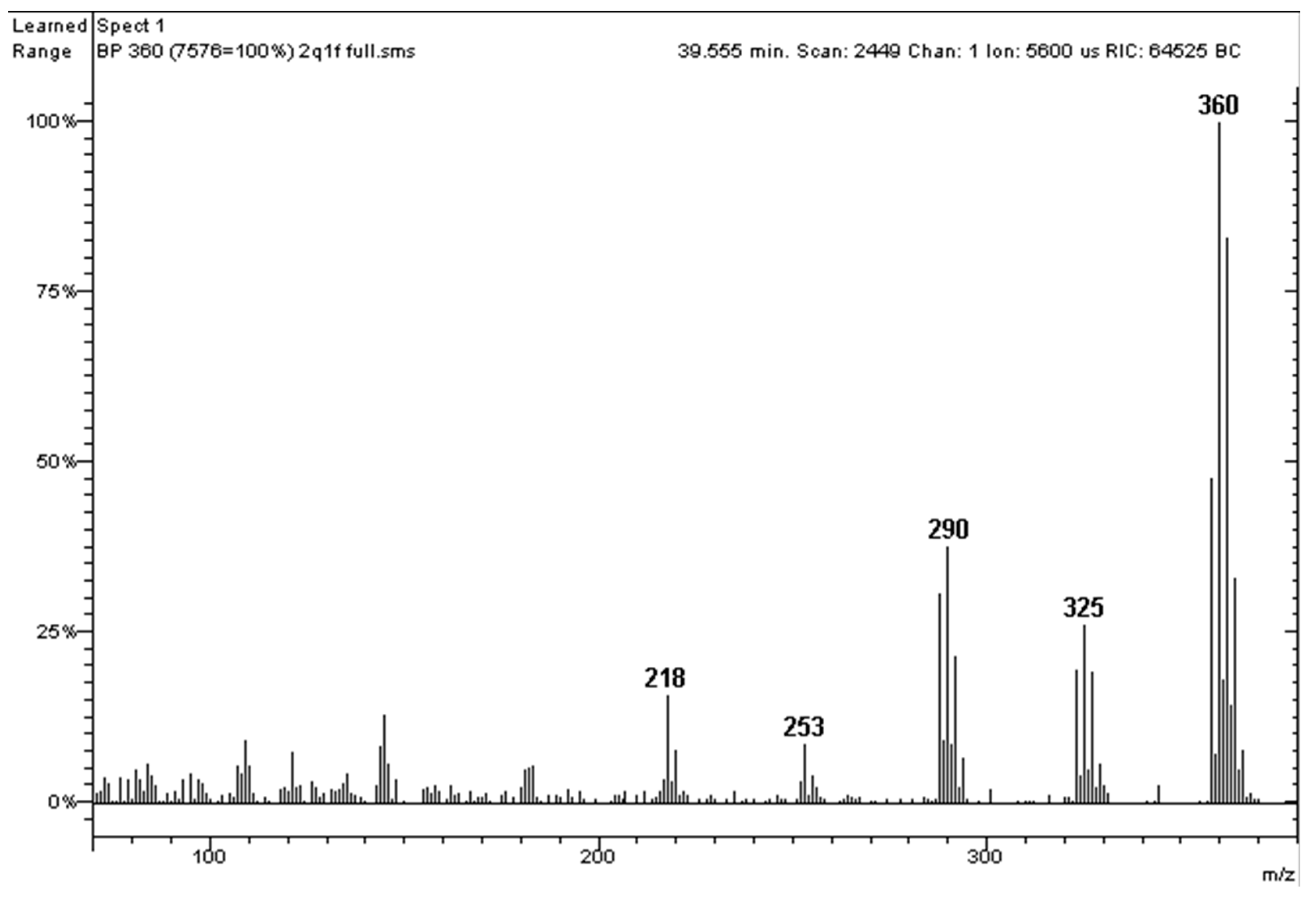
2.1. Polychlorinated Biphenyls (PCBs)
- (a)
- The quantitative analysis performed with the molecular ion [M●+];
- (b)
- The quantitative analysis performed with the first dechlorination ion [M-Cl]+ (ortho effect);
- (c)
- The quantitative analysis performed with an electron capture detector (GC-ECD).
Recent Application to a Real Case
2.2. Polybrominated Biphenyls (PBBs)
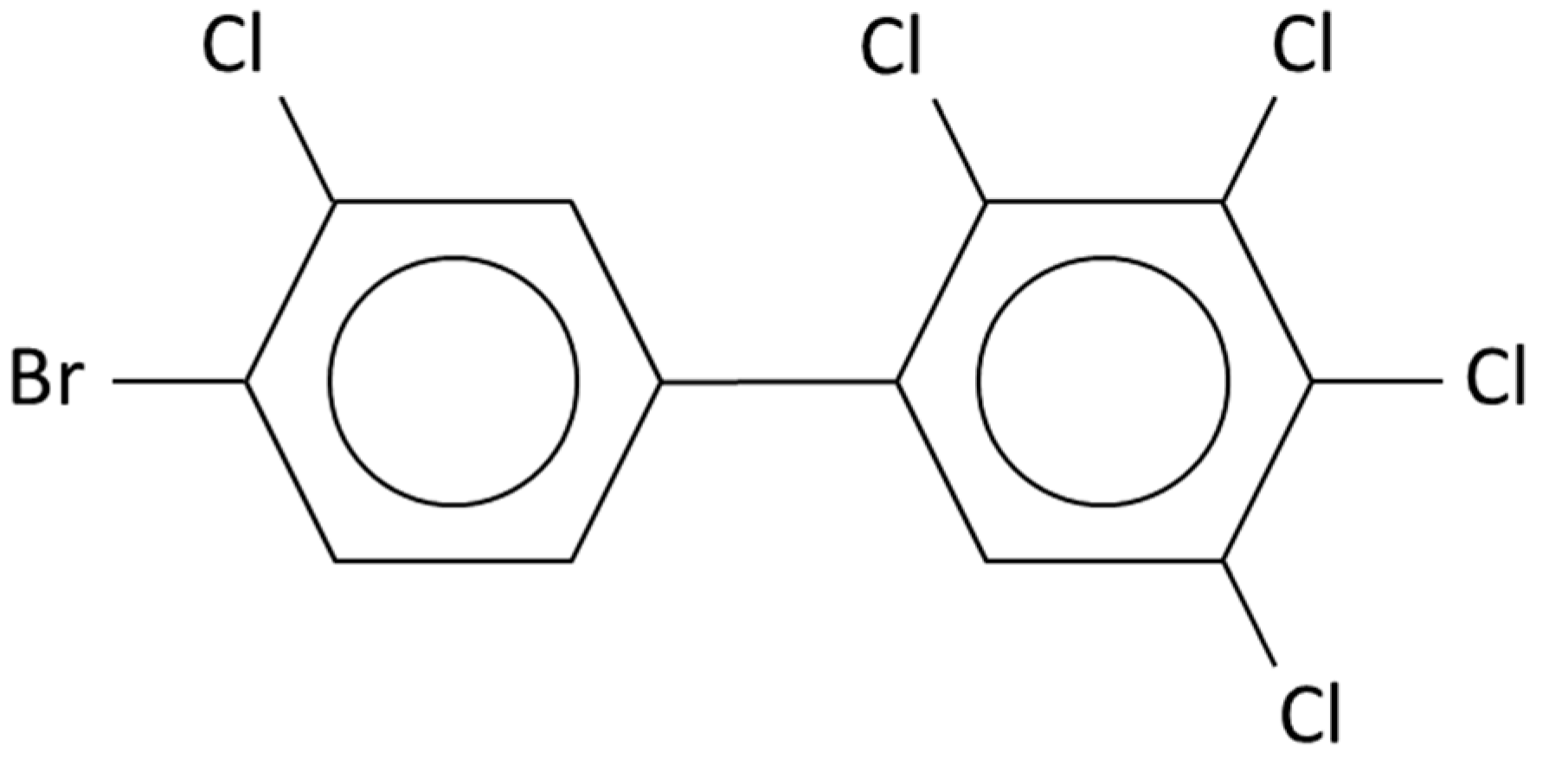
2.3. Mixed Poly-Brominated/Chlorinated Biphenyls (PXBs)
3. Materials and Methods
3.1. Reagents
3.2. Food Samples
3.3. Sample Preparation
3.4. Instrumental
3.4.1. GC-ECD Analysis
3.4.2. GC-MS Analysis: Experimental Conditions A
3.4.3. GC-MS Analysis: Experimental Conditions B
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polychlorinated Biphenyls and Polybrominated Biphenyls. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2015, Volume 107. Available online: https://publications.iarc.fr/131 (accessed on 21 June 2024).
- Hopf, N.B.; Ruder, A.M.; Succop, P. Background levels of polychlorinated biphenyls in the U.S. population. Sci. Total Environ. 2009, 407, 6109–6119. [Google Scholar] [CrossRef]
- Wang, S.; Jin, J.; Guo, C.; Li, Z.; Wang, Y.; Wei, Y.; Jin, J. Previously identified and unidentified polybrominated biphenyl congeners in serum from people living in an electronic waste dismantling area in China. Chemosphere 2021, 279, 130478. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Darrow, L.A.; Boyd Barr, D.; Howards, P.P.; Lyles, R.H.; Terrell, M.L.; Smith, A.K.; Conneely, K.N.; Marder, M.E.; Marcus, M. Serum polybrominated biphenyls (PBBs) and polychlorinated biphenyls (PCBs) and thyroid function among Michigan adults several decades after the 1973–1974 PBB contamination of livestock feed. Environ. Health Persp. 2017, 125, 097020. [Google Scholar] [CrossRef]
- Stockholm Convention, Alternatives to POPs, Chemicals Listed in Annex A, Hexabromobiphenyl. Available online: https://chm.pops.int/Implementation/Alternatives/AlternativestoPOPs/ChemicalslistedinAnnexA/HBB/tabid/5860/Default.aspx (accessed on 26 June 2024).
- Marder, M.E.; Panuwet, P.; Hunter, R.E.; Ryan, P.B.; Marcus, M.; Boyd Barr, D. Quantification of Polybrominated and Polychlorinated Biphenyls in human matrices by isotope-dilution gas chromatography—Tandem mass spectrometry. J. Anal. Toxicol. 2016, 40, 511–518. [Google Scholar] [CrossRef]
- Ballschmiter, K.; Zell, M. Analysis of polychlorinated biphenyls (PCB) by glass capillary gas chromatography—Composition of technical aroclor- and Clophen-PCB mixtures. Fresen. Z. Anal. Chem. 1980, 302, 20–31. [Google Scholar] [CrossRef]
- Ballschmiter, K.; Bacher, R.; Mennel, A.; Fischer, R.; Riehle, U.; Swerev, M. The determination of chlorinated biphenyls, chlorinated dibenzodioxins, and chlorinated dibenzofurans by GC-MS. J. High Res. Chromatog. 1992, 15, 260–270. [Google Scholar] [CrossRef]
- Safe, S.; Hutzinger, O. Chlorine randomization between phenyl groups in the electron impact-induced fragmentation of polychlorinated biphenyls. J. Chem. Soc. Chem. Commun. 1971, 446–448. [Google Scholar] [CrossRef]
- Zitko, V.; Choi, P.M.K. PCB and Other Industrial Halogenated Hydrocarbons in the Environment; Technical Report No. 272; Fisheries Research Board of Canada: St. Andrews Biological Station, NB, Canada, 1971. [Google Scholar]
- Frame, G.M. Comprehensive, quantitative, congener-specific PCB analysis: When is it required and what is necessary to achieve it? In Proceedings of the 13th Annual Waste Testing & Quality Assurance Symposium (WTQA ’97), Arlington, VA, USA, 6–9 July 1997; pp. 125–130. [Google Scholar]
- Hayakawa, S.; Taguchi, K.; Kotani, R.; Arakawa, K.; Morishita, N. Discrimination of isomers of dichlorobenzene using charge inversion mass spectrometry. J. Mass Spectrom. Soc. Jpn. 2001, 49, 219–223. [Google Scholar] [CrossRef]
- Zeng, E.Y.; Chou, C.C.; Yu, C. Potential application of gas chromatography/tandem mass spectrometry in the measurement of coeluting isomers. Anal. Chem. 2002, 74, 4513–4518. [Google Scholar] [CrossRef] [PubMed]
- Cochran, J.W. Fast gas chromatography-time-of-flight mass spectrometry of polychlorinated biphenyls and other environmental contaminants. J. Chromatogr. Sci. 2002, 40, 254–268. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Santos, F.J.; Galceran, M.T. Modern developments in gas chromatography-mass spectrometry-based environmental analysis. J. Chromatogr. A 2003, 1000, 125–151. [Google Scholar] [CrossRef]
- Marriott, P.J.; Haglund, P.; Ong, R.C.Y. A review of environmental toxicant analysis by using multidimensional gas chromatography and comprehensive GC. Clin. Chim. Acta 2003, 328, 1–19. [Google Scholar] [CrossRef]
- Oswald, E.O.; Levy, L.; Corbett, B.J.; Walker, M.P. Differentiation and characterization of isomeric polychlorinated biphenyls by gas-liquid chromatography coupled with electron impact and chemical ionization mass spectrometry. J. Chromatogr. A 1974, 93, 63–90. [Google Scholar] [CrossRef]
- Levy, L.A.; Oswald, E.O. The effect of ortho substitution on the mass spectral fragmentation of polychlorinated biphenyls. Biol. Mass Spectrom. 1976, 3, 88–90. [Google Scholar] [CrossRef]
- Sovocool, G.W.; Mitchum, R.K.; Donnelly, J.R. Use of the ‘ortho effect’ for chlorinated biphenyl and brominated biphenyl isomer identification. Biomed. Environ. Mass. 1987, 14, 579–582. [Google Scholar] [CrossRef]
- Osemwengie, L.I.; Sovocool, G.W. The mass spectrometric ortho effect studied for all 209 PCB congeners. Int. J. Mass Spectrom. 2013, 352, 51–64. [Google Scholar] [CrossRef]
- Masci, M.; Orban, E.; Nevigato, T. Fish contamination by polychlorobiphenyls: The mass spectrometric ortho effect in a new and easy gas chromatography-mass spectrometry method for the analysis of the seven indicators. The case of Bluefin Tuna. J. Chromatogr. A 2015, 1375, 110–122. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32023R0915 (accessed on 26 June 2024).
- Masci, M.; Turrini, A.; Caproni, R.; Nevigato, T. Organochlorines as contaminants in butter and other shortenings: Occurrence and exposure assessment. Food Addit. Contam. A 2023, 40, 1333–1344. [Google Scholar] [CrossRef]
- Masci, M.; Nevigato, T. Non-dioxin-like PCBs: A survey on fishery and aquaculture from the Mediterranean area. Food Addit. Contam. B 2016, 9, 159–169. [Google Scholar] [CrossRef]
- Hombrecher, K.; Quass, U.; Leisner, J.; Wichert, M. Significant release of unintentionally produced non-Aroclor polychlorinated biphenyl (PCB) congeners PCB 47, PCB 51 and PCB 68 from a silicone rubber production site in North Rhine-Westphalia, Germany. Chemosphere 2021, 285, 131449. [Google Scholar] [CrossRef] [PubMed]
- Hombrecher, K.; Quass, U.; Sievering, S.; Schöppe, A.; Rauchfuss, K. Contamination of food crops by unintentionally released PCB 47, PCB 51 and PCB 68 in the vicinity of silicone production sites and their relevance for human health assessment. Chemosphere 2022, 308, 136392. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Smith, F.; Fernandes, A.R. Dioxin-like polybrominated biphenyls (PBBs) and ortho-substituted PBBs in edible cod (Gadus morhua) liver oils and canned cod livers. Chemosphere 2020, 248, 126109. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.; Denison, M.S.; Birnbaum, L.S.; DeVito, M.J.; Fiedler, H.; Falandysz, J.; Rose, M.; Schrenk, D.; Safe, S.; Tohyama, C.; et al. Polybrominated dibenzo-p-dioxins, dibenzofurans, and biphenyls: Inclusion in the toxicity equivalency factor concept for dioxin-like compounds. Toxicol. Sci. 2013, 133, 197–208. [Google Scholar] [CrossRef]
- Rose, M.; Fernandes, A.; Mortimer, D.; Baskaran, C. Contamination of fish in UK fresh water systems: Risk assessment for human consumption. Chemosphere 2015, 122, 183–189. [Google Scholar] [CrossRef]
- Fernandes, A.R.; Mortimer, D.; Holmes, M.; Rose, M.; Zhihua, L.; Huang, X.; Smith, F.; Panton, S.; Marshall, L. Occurrence and spatial distribution of chemical contaminants in edible fish species collected from UK and proximate marine waters. Environ. Int. 2018, 114, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.R.; Lake, I.R.; Dowding, A.; Rose, M.; Jones, N.R.; Petch, R.; Smith, F.; Panton, S. The potential of recycled materials used in agriculture to contaminate food through uptake by livestock. Sci. Total Environ. 2019, 667, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Small, C.M.; DeCaro, J.J.; Terrell, M.L.; Dominguez, C.; Cameron, L.L.; Wirth, J.; Marcus, M. Maternal exposure to a brominated flame retardant and genitourinary conditions in male offspring. Environ. Health Persp. 2009, 117, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.R.; Rose, M.; Mortimer, D.; Carr, M.; Panton, S.; Smiyh, F. Mixed brominated/chlorinated dibenzo-p-dioxins, dibenzofurans and biphenyls: Simultaneous congener-selective determination in food. J. Chromatogr. A 2011, 1218, 9279–9287. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Rose, M.; Fernandes, A.R. Mixed poly-brominated/chlorinated biphenyls (PXBs): Widespread food and environmental contaminants. Environ. Int. 2012, 44, 118–127. [Google Scholar] [CrossRef]
- Ohta, S.; Tokusawa, H.; Nakao, t.; Aozasa, O.; Miyata, H.; Alaee, M. Global contamination of coplanar polybrominated/chlorinated biphenyls (Co-PXBs) in the market fishes from Japan. Chemosphere 2008, 73, S31–S38. [Google Scholar] [CrossRef]
- Gómara, B.; Herrero, L.; Pacepavicius, G.; Ohta, S.; Alaee, M.; González, M.J. Occurrence of co-planar polybrominated/chlorinated biphenyls (PXBs), polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk of women from Spain. Chemosphere 2011, 83, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.R.; Mortimer, D.; Wall, R.J.; Bell, D.R.; Rose, M.; Carr, M.; Panton, S.; Smith, F. Mixed halogenated dioxins/furans (PXDD/Fs) and biphenyls (PXBs) in food: Occurrence and toxic equivalent exposure using specific relative potencies. Environ. Int. 2014, 73, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, H.; Aozasa, O.; Mizuno, A.; Akiyama, E.; Nakao, T.; Ohta, S. In vitro and in vivo induction of cytochrome P450 by coplanar polychlorinated/brominated biphenyls (Co-PXBs) providing high TEQ in mother’s milk in Japan. Toxicology 2014, 324, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Rebryk, A.; Gallampois, C.; Haglund, P. A time-trend guided non-target screening study of organic contaminants in Baltic Sea harbor porpoise (1988–2019), guillemot (1986–2019), and white-tailed sea eagle (1965–2017) using gas chromatography–high-resolution mass spectrometry. Sci. Total Environ. 2022, 829, 154620. [Google Scholar] [CrossRef] [PubMed]
- Zacs, D.; Rjabova, J.; Fernandes, A.R.; Bartkevics, V. Brominated, chlorinated and mixed brominated/chlorinated persistent organic pollutants in European eels (Anquilla anquilla) from Latvian lakes. Food Addit. Contam. A 2016, 33, 460–472. [Google Scholar] [CrossRef]
- Masci, M.; Orban, E.; Nevigato, T. Organochlorine pesticide residues: An extensive monitoring of Italian fishery and aquaculture. Chemosphere 2014, 94, 190–198. [Google Scholar] [CrossRef]
- UNEP. United Nations Environmental Program-Stockholm Convention on Persistent Organic Pollutants: “Guidance on the Global Monitoring Plan for Persistent Organic Pollutants”. UNEP/POPS/COP.7/INF/39. 26 February 2015. Available online: https://www.unitar.org/sites/default/files/media/file/15._guidance_on_the_global_monitoring_plan_for_pops.pdf (accessed on 22 July 2024).


| PCB | Intensity of Ortho Effect | Quantitative Analysis with GC-MS, [M●+] | Quantitative Analysis with GC-MS, [M-Cl]+ | Quantitative Analysis with GC-ECD |
|---|---|---|---|---|
| 49 | 39% | 2.6 | <LOQ | 2.7 |
| 52 | 49% | 7.8 | 7.6 | 8.5 |
| 87 | 41% | 2.8 | 3.8 | 2.9 |
| 97 | 52% | 2.1 | 2.8 | 2.0 |
| 132 | 48% | 3.9 | 3.6 | 3.0 |
| 151 | 57% | 4.1 | 4.2 | 4.3 |
| 153 | 20% | 42.9 | 39.9 | 39.9 |
| 180 | 35% | 19.5 | 18.0 | 19.0 |
| 187 | 47% | 11.0 | 10.4 | 10.0 |
| PCB | Quantitative Analysis with GC-MS, [M●+] | Quantitative Analysis with GC-MS, [M-Cl]+ | Quantitative Analysis with GC-ECD | |||
|---|---|---|---|---|---|---|
| LOD | LOQ | LOD | LOQ | LOD | LOQ | |
| 49 | 0.7 | 1.0 | 3.0 | 4.0 | 0.09 | 0.14 |
| 52 | 0.4 | 0.6 | 1.9 | 2.9 | 0.14 | 0.21 |
| 87 | 0.6 | 0.8 | 1.1 | 1.6 | 0.08 | 0.12 |
| 97 | 1.4 | 2.1 | 1.9 | 2.8 | 0.09 | 0.14 |
| 132 | 0.8 | 1.2 | 2.4 | 3.6 | 0.09 | 0.14 |
| 151 | 0.5 | 0.8 | 2.1 | 3.2 | 0.09 | 0.13 |
| 153 | 1.1 | 1.6 | 7.3 | 10.9 | 0.16 | 0.23 |
| 180 | 1.4 | 2.2 | 4.0 | 6.0 | 0.13 | 0.19 |
| 187 | 0.9 | 1.4 | 2.3 | 3.5 | 0.11 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masci, M. The Mass Spectrometric Ortho Effect for Distinguishing the Coeluting Isomers of Polychlorinated Biphenyls and the Coeluting Isomers of Polybrominated Biphenyls: Qualitative and Quantitative Aspects. Molecules 2024, 29, 3484. https://doi.org/10.3390/molecules29153484
Masci M. The Mass Spectrometric Ortho Effect for Distinguishing the Coeluting Isomers of Polychlorinated Biphenyls and the Coeluting Isomers of Polybrominated Biphenyls: Qualitative and Quantitative Aspects. Molecules. 2024; 29(15):3484. https://doi.org/10.3390/molecules29153484
Chicago/Turabian StyleMasci, Maurizio. 2024. "The Mass Spectrometric Ortho Effect for Distinguishing the Coeluting Isomers of Polychlorinated Biphenyls and the Coeluting Isomers of Polybrominated Biphenyls: Qualitative and Quantitative Aspects" Molecules 29, no. 15: 3484. https://doi.org/10.3390/molecules29153484
APA StyleMasci, M. (2024). The Mass Spectrometric Ortho Effect for Distinguishing the Coeluting Isomers of Polychlorinated Biphenyls and the Coeluting Isomers of Polybrominated Biphenyls: Qualitative and Quantitative Aspects. Molecules, 29(15), 3484. https://doi.org/10.3390/molecules29153484







