A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity
Abstract
1. Introduction
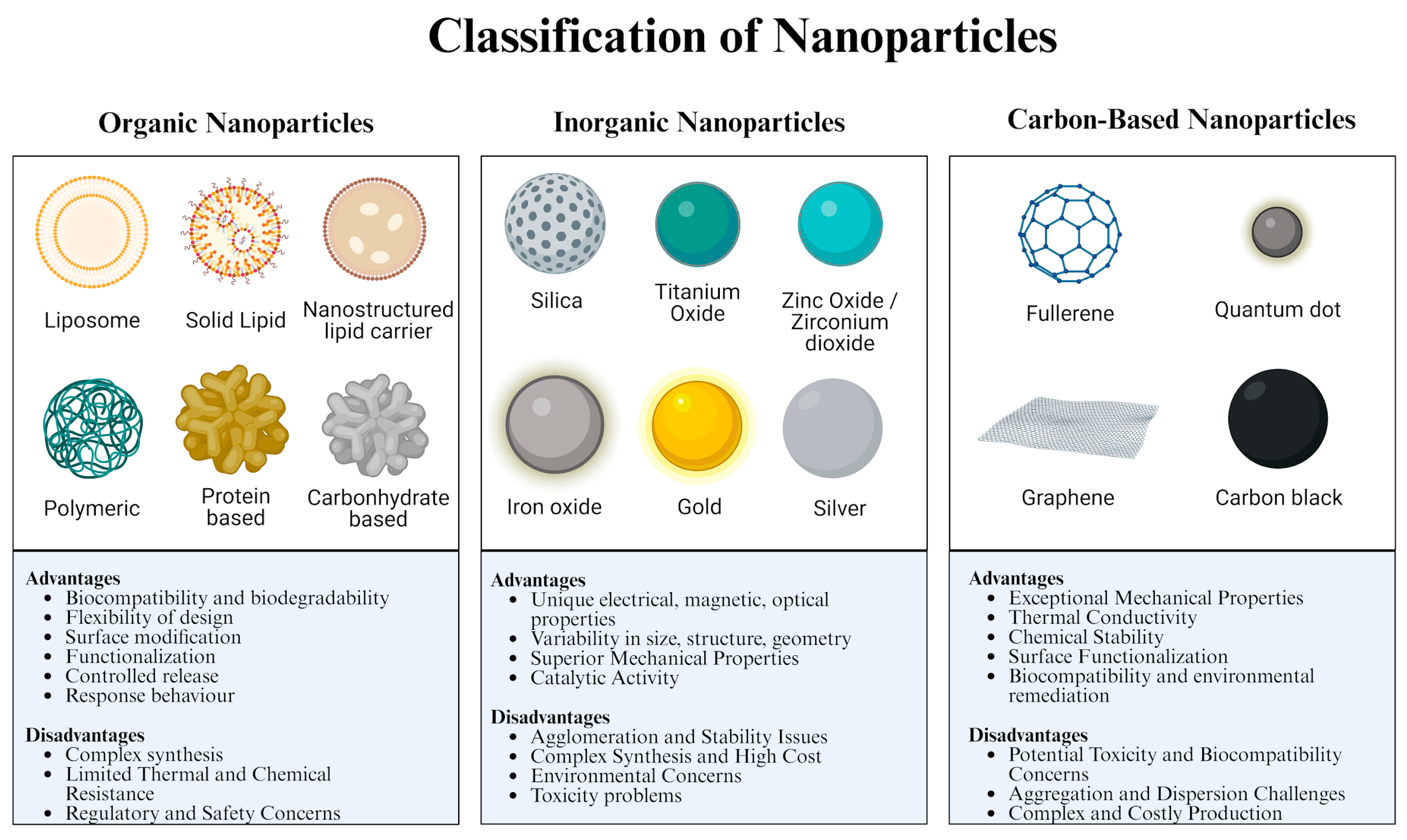
2. Classification of NPs
2.1. Inorganic NPs

2.1.1. Magnetic NPs
2.1.2. Ceramic NPs
2.1.3. Semiconductor NPs
2.2. Carbon-Based NPs
2.2.1. Graphene
2.2.2. Fullerenes
2.2.3. Carbon Black NPs
2.2.4. Carbon Quantum Dots
2.3. Organic NPs
2.3.1. Polymeric NPs
Chitosan NPs
Alginate NPs
Polylactic Acid and Polyglycolic Acid NPs
2.3.2. Lipid-Based NPs
Liposomes
Solid Lipid NPs
Nanostructured Lipid Carriers
2.3.3. Carbohydrate NPs
Starch NPs
Dextran NPs
Cyclodextrin NPs
Protein NPs
Collagen NPs
Albumin NPs
Gelatin NPs
Milk Protein NPs
Casein NPs
Whey Protein-Based NPs
Plant Protein-Based NPs
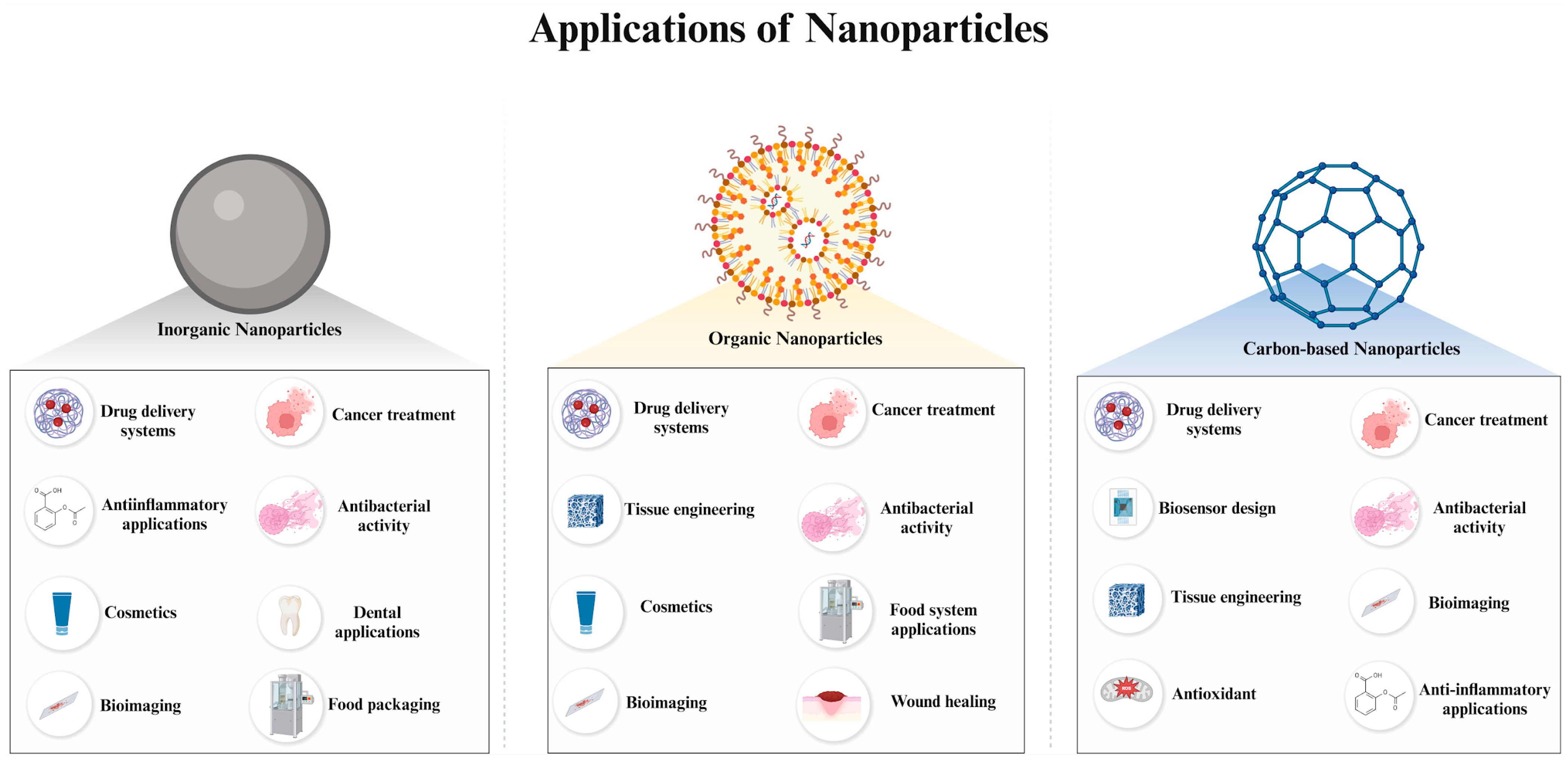
3. Applications of Nanoparticles Based on Their Classification
3.1. Inorganic NP Applications
3.1.1. Antibacterial Application of Inorganic Nanoparticles
3.1.2. Inorganic Nanoparticles in Drug Delivery Systems
| Type of the iNP | Application Area | Highlighted Results | References |
|---|---|---|---|
| Iron oxide | Drug delivery |
| [349,350,351,352] |
| Iron oxide | Bioimaging |
| [353,354,355] |
| Iron oxide | Cancer treatment |
| [356,357,358] |
| Iron oxide | Antibacterial |
| [359,360,361] |
| Iron oxide | Drug delivery |
| [362,363] |
| Silver | Antibacterial activity |
| [364,365,366] |
| Silver | Drug delivery |
| [367,368] |
| Silver | Anti-inflammatory activity |
| [369,370] |
| Silver | Anticancer activity |
| [371,372] |
| Silver | Food packaging |
| [373,374,375] |
| Gold | Antibacterial |
| [376,377,378] |
| Gold | Drug delivery |
| [379,380] |
| SiO2 | Industrial |
| [381,382,383] |
| ZrO2 | Dental applications |
| [384,385,386] |
| ZrO2 | Antibacterial |
| [387,388,389] |
| ZnO | Food packaging |
| [390,391,392] |
| ZnO | Antibacterial |
| [393,394] |
| ZnO | Cosmetics |
| [395] |
| ZnO | Cancer |
| [396,397,398,399] |
3.1.3. Inorganic Nanoparticles in Food Packaging and Preservation
3.1.4. Inorganic Nanoparticles in Cosmetics
3.2. Carbon-Based NP Applications
3.2.1. Carbon-Based NPs in Tissue Engineering
3.2.2. Carbon-Based NPs in Antitumor and Cancer Research
| Type of the Carbon-Based NP | Application Area | Highlighted Results | References |
|---|---|---|---|
| Fullerene | Antioxidant |
| [432,433] |
| Fullerene | Diabetes |
| [434] |
| Fullerene | Anti-inflammatory |
| [435,436] |
| Fullerene | Drug delivery |
| [437] |
| Fullerene | Cancer |
| [438] |
| Graphene | Antibacterial |
| [439,440] |
| Graphene | Drug delivery |
| [441,442,443] |
| Graphene | Anticancer |
| [444,445] |
| Graphene | Industrial |
| [446,447,448,449,450] |
| Graphene | Tissue engineering |
| [451,452,453] |
| Carbon black | Electrochemical sensor design |
| [454,455] |
| Carbon black | Reinforcement of cement-based materials |
| [456,457] |
| Carbon black | Energy storage |
| [458,459] |
| Carbon quantum dot | Bioimaging |
| [460,461,462] |
| Carbon quantum dot | Biosensor design |
| [463,464,465,466] |
| Carbon quantum dot | Antibacterial |
| [467,468,469] |
| Carbon quantum dot | Drug delivery |
| [470,471,472] |
| Carbon quantum dot | Tissue engineering |
| [473,474] |
| Carbon quantum dot | Wound healing |
| [475,476,477] |
3.2.3. Carbon-Based NPs’ Antioxidant Activity and Cosmetic Applications
3.2.4. Carbon-Based NPs’ Anti-Inflammatory Activity
3.2.5. Carbon-Based NPs in Diabetes
3.2.6. Carbon-Based NPs in Drug Delivery Systems
3.2.7. Carbon-Based NPs in Antibacterial Research
3.2.8. Carbon-Based NPs in Industrial Applications
3.3. Organic NP Applications
3.3.1. Organic NPs in Wound Healing Applications
3.3.2. Organic NPs in Diabetic Research
| Type of Organic NP | Application Area | Highlighted Results | References |
|---|---|---|---|
| Chitosan | Wound healing |
| [521,522,523] |
| Chitosan | Diabetes |
| [524,525,526] |
| Chitosan | Drug delivery |
| [527,528,529,530] |
| Chitosan | Food preservation |
| [531,532,533,534] |
| Chitosan | Food packaging |
| [535,536] |
| Alginate | Diabetes |
| [537,538] |
| Alginate | Drug delivery |
| [539,540,541] |
| Alginate | Food preservation |
| [542,543] |
| PLGA | Drug delivery |
| [544,545,546,547] |
| PLGA | Tissue engineering |
| [548,549,550] |
| PLGA | Wound healing |
| [551,552] |
| PLGA | Diabetes |
| [553,554,555] |
| PLGA | Imaging |
| [556,557] |
| Liposome | Drug delivery |
| [558,559,560] |
| Liposome | Cosmetics |
| [561,562] |
| Liposome | Wound healing |
| [563,564,565] |
| Liposome | Food preservation |
| [566,567,568] |
| Liposome | Gene delivery |
| [569,570] |
| Solid lipid | Food preservation |
| [571,572,573] |
| Solid lipid | Drug delivery |
| [574,575,576,577] |
| Solid lipid | Cancer |
| [578,579,580] |
| Solid lipid | Cosmetics |
| [581,582,583,584] |
| Nanostructured lipid carriers | Drug delivery |
| [585,586,587,588,589] |
| Nanostructured lipid carriers | Cancer |
| [590,591,592,593,594] |
| Nanostructured lipid carriers | Cosmetics |
| [595,596,597] |
| Nanostructured lipid carriers | Food preservation |
| [598,599,600,601] |
| Cyclodextrin | Cancer |
| [136,138] |
| Starch | Drug delivery |
| [163,602] |
| Starch | Antimicrobial agent |
| [603] |
| Lactoferrin | Drug delivery |
| [604,605] |
| Lactoferrin | Antimicrobial activity |
| [606] |
3.3.3. Organic NPs in Drug Delivery Systems
3.3.4. Organic NPs in Food Packaging and Food Preservation Applications
3.3.5. Organic NPs in Cosmetics
4. Toxicity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? Int. J. Mol. Sci. 2021, 22, 385. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Hasan, A.; Primavera, R.; Wilson, R.J.; Thakor, A.S.; Kevadiya, B.D. Cellular Uptake and Retention of Nanoparticles: Insights on Particle Properties and Interaction with Cellular Components. Mater. Today Commun. 2020, 25, 101692. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial Applications of Nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed]
- Mallia, J.D.O.; Galea, R.; Nag, R.; Cummins, E.; Gatt, R.; Valdramidis, V. Nanoparticle Food Applications and Their Toxicity: Current Trends and Needs in Risk Assessment Strategies. J. Food Prot. 2022, 85, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Varier, K.M.; Gudeppu, M.; Chinnasamy, A.; Thangarajan, S.; Balasubramanian, J.; Li, Y.; Gajendran, B. Nanoparticles: Antimicrobial Applications and Its Prospects. Adv. Nanostructured Mater. Environ. Remediat. 2019, 25, 321. [Google Scholar] [CrossRef]
- Algar, W.R.; Massey, M.; Rees, K.; Higgins, R.; Krause, K.D.; Darwish, G.H.; Peveler, W.J.; Xiao, Z.; Tsai, H.Y.; Gupta, R.; et al. Photoluminescent Nanoparticles for Chemical and Biological Analysis and Imaging. Chem. Rev. 2021, 121, 9243–9358. [Google Scholar] [CrossRef] [PubMed]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail Review on Chemical, Physical and Green Synthesis, Classification, Characterizations and Applications of Nanoparticles. Green Chem. Lett. Rev. 2020, 13, 59–81. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Ealias, A.M.; Saravanakumar, M.P. A Review on the Classification, Characterisation, Synthesis of Nanoparticles and Their Application. In Proceedings of the IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: London, UK, 2017; Volume 263. [Google Scholar]
- Kim, T.; Hyeon, T. Applications of Inorganic Nanoparticles as Therapeutic Agents. Nanotechnology 2014, 25, 012001. [Google Scholar] [CrossRef] [PubMed]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Onyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of Nanoparticles in Biomedical Application: Nanotoxicology. J. Toxicol. 2021, 2021, 9954443. [Google Scholar] [CrossRef] [PubMed]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, R.; Shakeel, H.; Malik, K.; Qasim, M.; Khan, M.A.; Ahmed, N.; Jabeen, S. Inorganic Nanoparticles: Toxic Effects, Mechanisms of Cytotoxicity and Phytochemical Interactions. Adv. Pharm. Bull. 2022, 12, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed]
- Shubayev, V.I.; Pisanic, T.R.; Jin, S. Magnetic Nanoparticles for Theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, I.M.; Issa, B.; Haik, Y. Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials 2014, 5, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem.-Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological Applications of Magnetic Nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, J.; Purayil, S.K.; Ponnaiah, P. A Review on Synthesis, Characterization and Potential Biological Applications of Superparamagnetic Iron Oxide Nanoparticles. Curr. Res. Green Sustain. Chem. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Ahmed, M.; Douek, M. The Role of Magnetic Nanoparticles in the Localization and Treatment of Breast Cancer. BioMed Res. Int. 2013, 2013, 281230. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef]
- Dobson, J. Gene Therapy Progress and Prospects: Magnetic Nanoparticle-Based Gene Delivery. Gene Ther. 2006, 13, 283–287. [Google Scholar] [CrossRef]
- Dobson, J. Magnetic Nanoparticles for Drug Delivery. Drug Dev. Res. 2006, 67, 55–60. [Google Scholar] [CrossRef]
- Tuçek, J.; Kemp, K.C.; Kim, K.S.; Zboŗil, R. Iron-Oxide-Supported Nanocarbon in Lithium-Ion Batteries, Medical, Catalytic, and Environmental Applications. ACS Nano 2014, 8, 7571–7612. [Google Scholar] [CrossRef]
- Wu, C.; Zreiqat, H. Porous Bioactive Diopside (CaMgSi2O6) Ceramic Microspheres for Drug Delivery. Acta Biomater. 2010, 6, 820–829. [Google Scholar] [CrossRef]
- Singh, D.; Singh, S.; Sahu, J.; Srivastava, S.; Singh, M.R. Ceramic Nanoparticles: Recompense, Cellular Uptake and Toxicity Concerns. Artif. Cells Nanomed. Biotechnol. 2016, 44, 401–409. [Google Scholar] [CrossRef]
- Gurumurthy, B.; Balasubramanian, A.; Balasubramanian, S.; Gurumurthy, B. Biomedical Applications of Ceramic Nanomaterials: A Review. Int. J. Pharm. Sci. Res. 2017, 8, 4950–4959. [Google Scholar] [CrossRef]
- Armatas, G.S.; Kanatzidis, M.G. Mesostructured Germanium with Cubic Pore Symmetry. Nature 2006, 441, 1122–1125. [Google Scholar] [CrossRef]
- Singh, D.; Dubey, P.; Pradhan, M.; Singh, M.R. Ceramic Nanocarriers: Versatile Nanosystem for Protein and Peptide Delivery. Expert Opin. Drug Deliv. 2013, 10, 241–259. [Google Scholar] [CrossRef]
- Liu, S.; Jin, L.; Chronakis, I.S.; Li, X.; Ge, M. Hyperbranched Polyether Hybrid Nanospheres with CdSe Quantum Dots Incorporated for Selective Detection of Nitric Oxide. Mater. Lett. 2014, 123, 104–106. [Google Scholar] [CrossRef]
- Fadeel, B.; Garcia-Bennett, A.E. Better Safe than Sorry: Understanding the Toxicological Properties of Inorganic Nanoparticles Manufactured for Biomedical Applications. Adv. Drug Deliv. Rev. 2010, 62, 362–374. [Google Scholar] [CrossRef]
- Wang, N.; Thameem Dheen, S.; Fuh, J.Y.H.; Senthil Kumar, A. A Review of Multi-Functional Ceramic Nanoparticles in 3D Printed Bone Tissue Engineering. Bioprinting 2021, 23, e00146. [Google Scholar] [CrossRef]
- Terna, A.D.; Elemike, E.E.; Mbonu, J.I.; Osafile, O.E.; Ezeani, R.O. The Future of Semiconductors Nanoparticles: Synthesis, Properties and Applications. Mater. Sci. Eng. B 2021, 272, 115363. [Google Scholar] [CrossRef]
- Hossain, N.; Mobarak, M.H.; Mimona, M.A.; Islam, M.A.; Hossain, A.; Zohura, F.T.; Chowdhury, M.A. Advances and Significances of Nanoparticles in Semiconductor Applications—A Review. Results Eng. 2023, 19, 101347. [Google Scholar] [CrossRef]
- Hossain, N.; Islam, M.A.; Chowdhury, M.A.; Alam, A. Advances of Nanoparticles Employment in Dental Implant Applications. Appl. Surf. Sci. Adv. 2022, 12, 100341. [Google Scholar] [CrossRef]
- Patil, N.A.; Kandasubramanian, B. Biological and Mechanical Enhancement of Zirconium Dioxide for Medical Applications. Ceram. Int. 2020, 46, 4041–4057. [Google Scholar] [CrossRef]
- Hossain, N.; Mobarak, M.H.; Hossain, A.; Khan, F.; Mim, J.J.; Chowdhury, M.A. Advances of Plant and Biomass Extracted Zirconium Nanoparticles in Dental Implant Application. Heliyon 2023, 9, e15973. [Google Scholar] [CrossRef]
- Chitoria, A.K.; Mir, A.; Shah, M.A. A Review of ZrO2 Nanoparticles Applications and Recent Advancements. Ceram. Int. 2023, 49, 32343–32358. [Google Scholar] [CrossRef]
- Tabassum, N.; Kumar, D.; Verma, D.; Bohara, R.A.; Singh, M.P. Zirconium Oxide (ZrO2) Nanoparticles from Antibacterial Activity to Cytotoxicity: A next-Generation of Multifunctional Nanoparticles. Mater. Today Commun. 2021, 26, 102156. [Google Scholar] [CrossRef]
- Rasmidi, R.; Duinong, M.; Chee, F.P. Radiation Damage Effects on Zinc Oxide (ZnO) Based Semiconductor Devices—A Review. Radiat. Phys. Chem. 2021, 184, 109455. [Google Scholar] [CrossRef]
- Thambidurai, S.; Gowthaman, P.; Venkatachalam, M.; Suresh, S. Enhanced Bactericidal Performance of Nickel Oxide-Zinc Oxide Nanocomposites Synthesized by Facile Chemical Co-Precipitation Method. J. Alloys Compd. 2020, 830, 154642. [Google Scholar] [CrossRef]
- Subhan, M.A.; Neogi, N.; Choudhury, K.P. Industrial Manufacturing Applications of Zinc Oxide Nanomaterials: A Comprehensive Study. Nanomanufacturing 2022, 2, 265–291. [Google Scholar] [CrossRef]
- Jain, D.; Shivani; Bhojiya, A.A.; Singh, H.; Daima, H.K.; Singh, M.; Mohanty, S.R.; Stephen, B.J.; Singh, A. Microbial Fabrication of Zinc Oxide Nanoparticles and Evaluation of Their Antimicrobial and Photocatalytic Properties. Front. Chem. 2020, 8, 778. [Google Scholar] [CrossRef]
- Cleetus, C.M.; Primo, F.A.; Fregoso, G.; Raveendran, N.L.; Noveron, J.C.; Spencer, C.T.; Ramana, C.V.; Joddar, B. Alginate Hydrogels with Embedded Zno Nanoparticles for Wound Healing Therapy. Int. J. Nanomed. 2020, 15, 5097–5111. [Google Scholar] [CrossRef]
- Islam, F.; Shohag, S.; Uddin, M.J.; Islam, M.R.; Nafady, M.H.; Akter, A.; Mitra, S.; Roy, A.; Bin Emran, T.; Cavalu, S. Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials 2022, 15, 2160. [Google Scholar] [CrossRef]
- Kokorina, A.A.; Ermakov, A.V.; Abramova, A.M.; Goryacheva, I.Y.; Sukhorukov, G.B. Carbon Nanoparticles and Materials on Their Basis. Colloids Interfaces 2020, 4, 42. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, K.R.B. Nanobiotechnology in Animal Production and Health. In Advances in Animal Genomics; Academic Press: Cambridge, MA, USA, 2021; pp. 185–198. [Google Scholar] [CrossRef]
- Mukherjee, D.; Sil, M.; Goswami, A.; Lahiri, D.; Nag, M. Antibiofilm Activities of Carbon-Based Nanoparticles and Nanocomposites: A Comparative Review. J. Inorg. Organomet. Polym. Mater. 2023, 33, 3961–3983. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and Graphene Oxide as Nanomaterials for Medicine and Biology Application. J. Nanostructure Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef]
- Patel, K.D.; Singh, R.K.; Kim, H.W. Carbon-Based Nanomaterials as an Emerging Platform for Theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the Functional Modification of Graphene/Graphene Oxide: A Review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.B. Design, Synthesis, and Characterization of Graphene-Nanoparticle Hybrid Materials for Bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Shi, X.; Liu, Z. Nano-Graphene in Biomedicine: Theranostic Applications. Chem. Soc. Rev. 2013, 42, 530–547. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and Graphene Oxide as New Nanocarriers for Drug Delivery Applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Kumar, S.; Chatterjee, K. Comprehensive Review on the Use of Graphene-Based Substrates for Regenerative Medicine and Biomedical Devices. ACS Appl. Mater. Interfaces 2016, 8, 26431–26457. [Google Scholar] [CrossRef]
- Shin, S.R.; Li, Y.C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-Based Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef]
- Bai, R.G.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-Based 3D Scaffolds in Tissue Engineering: Fabrication, Applications, and Future Scope in Liver Tissue Engineering. Int. J. Nanomedicine 2019, 14, 5753–5783. [Google Scholar]
- Xu, T.; Shen, W.; Huang, W.; Lu, X. Fullerene Micro/Nanostructures: Controlled Synthesis and Energy Applications. Mater. Today Nano 2020, 11, 100081. [Google Scholar] [CrossRef]
- Dhall, S.; Nathawat, R.; Sood, K. Carbon-Based Nanomaterials. Carbon Nanomaterials and Their Nanocomposite-Based Chemiresistive Gas Sensors: Applications, Fabrication and Commercialization; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–39. [Google Scholar] [CrossRef]
- Shetti, N.P.; Mishra, A.; Basu, S.; Aminabhavi, T.M. Versatile Fullerenes as Sensor Materials. Mater. Today Chem. 2021, 20, 100454. [Google Scholar] [CrossRef]
- Dugan, L.L.; Lovett, E.G.; Quick, K.L.; Lotharius, J.; Lin, T.T.; O’Malley, K.L. Fullerene-Based Antioxidants and Neurodegenerative Disorders. Park. Relat. Disord. 2001, 7, 243–246. [Google Scholar] [CrossRef]
- Lai, Y.Y.; Cheng, Y.J.; Hsu, C.S. Applications of Functional Fullerene Materials in Polymer Solar Cells. Energy Environ. Sci. 2014, 7, 1866–1883. [Google Scholar] [CrossRef]
- Dellinger, A.; Zhou, Z.; Connor, J.; Madhankumar, A.; Pamujula, S.; Sayes, C.M.; Kepley, C.L. Application of Fullerenes in Nanomedicine: An Update. Nanomedicine 2013, 8, 1191–1208. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Zhang, W.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; He, Q.; Yuan, X.; Huang, D.; et al. Advances in Photocatalysis Based on Fullerene C60 and Its Derivatives: Properties, Mechanism, Synthesis, and Applications. Appl. Catal. B 2020, 265, 118579. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Hong, L.; Zhang, L.; Ji, Q.; Wan, J.; Yang, C. Fullerene Nanorings as Nitric Oxide Radical Scavengers for Ultraviolet-Induced Cellular Injury. ACS Appl. Nano Mater. 2024, 7, 5689–5697. [Google Scholar] [CrossRef]
- Markovic, Z.; Trajkovic, V. Biomedical Potential of the Reactive Oxygen Species Generation and Quenching by Fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef]
- Mousavi, S.Z.; Nafisi, S.; Maibach, H.I. Fullerene Nanoparticle in Dermatological and Cosmetic Applications. Nanomedicine 2017, 13, 1071–1087. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef]
- Kazemzadeh, H.; Mozafari, M. Fullerene-Based Delivery Systems. Drug Discov. Today 2019, 24, 898–905. [Google Scholar] [CrossRef]
- Chen, Z.; Mao, R.; Liu, Y. Fullerenes for Cancer Diagnosis and Therapy: Preparation, Biological and Clinical Perspectives. Curr. Drug Metab. 2012, 13, 1035–1045. [Google Scholar] [CrossRef]
- Lindner, K.; Ströbele, M.; Schlick, S.; Webering, S.; Jenckel, A.; Kopf, J.; Danov, O.; Sewald, K.; Buj, C.; Creutzenberg, O.; et al. Biological Effects of Carbon Black Nanoparticles Are Changed by Surface Coating with Polycyclic Aromatic Hydrocarbons. Part. Fibre Toxicol. 2017, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Nalon, G.H.; Ribeiro, J.C.L.; Araújo, E.N.D.D.; Pedroti, L.G.; Carvalho, J.M.F.D.; Santos, R.F.; Aparecido-Ferreira, A. Effects of Different Kinds of Carbon Black Nanoparticles on the Piezoresistive and Mechanical Properties of Cement-Based Composites. J. Build. Eng. 2020, 32, 101724. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Mazzaracchio, V.; Scognamiglio, V.; Amine, A.; Moscone, D. Carbon Black as an Outstanding and Affordable Nanomaterial for Electrochemical (Bio)Sensor Design. Biosens. Bioelectron. 2020, 156, 112033. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zheng, F.; Xu, J.; Zhang, S.; Bhosale, S.S.; Gu, J.; Hong, R. Surface Modification of Nano-Sized Carbon Black for Reinforcement of Rubber. Nanotechnol. Rev. 2019, 8, 405–414. [Google Scholar] [CrossRef]
- Silva, T.A.; Moraes, F.C.; Janegitz, B.C.; Fatibello-Filho, O.; Ganta, D. Electrochemical Biosensors Based on Nanostructured Carbon Black: A Review. J. Nanomater. 2017, 2017, 4571614. [Google Scholar] [CrossRef]
- Sun, Z.; Xiao, M.; Wang, S.; Han, D.; Song, S.; Chen, G.; Meng, Y. Specially Designed Carbon Black Nanoparticle-Sulfur Composite Cathode Materials with a Novel Structure for Lithium-Sulfur Battery Application. J. Power Sources 2015, 285, 478–484. [Google Scholar] [CrossRef]
- Huang, J.C. Carbon Black Filled Conducting Polymers and Polymer Blends. Adv. Polym. Technol. 2002, 21, 299–313. [Google Scholar] [CrossRef]
- Olorundare, F.O.G.; Sipuka, D.S.; Sebokolodi, T.I.; Kodama, T.; Arotiba, O.A.; Nkosi, D. An Electrochemical Immunosensor for an Alpha-Fetoprotein Cancer Biomarker on a Carbon Black/Palladium Hybrid Nanoparticles Platform. Anal. Methods 2023, 15, 3577–3585. [Google Scholar] [CrossRef]
- Mohamed, R.M.K.; Mohamed, S.H.; Asran, A.M.; Alsohaimi, I.H.; Hassan, H.M.A.; Ibrahim, H.; El-Wekil, M.M. Synergistic Effect of Gold Nanoparticles Anchored on Conductive Carbon Black as an Efficient Electrochemical Sensor for Sensitive Detection of Anti-COVID-19 Drug Favipiravir in Absence and Presence of Co-Administered Drug Paracetamol. Microchem. J. 2023, 190, 108696. [Google Scholar] [CrossRef]
- Koike, E.; Kobayashi, T. Chemical and Biological Oxidative Effects of Carbon Black Nanoparticles. Chemosphere 2006, 65, 946–951. [Google Scholar] [CrossRef]
- Das, R.; Bandyopadhyay, R.; Pramanik, P. Carbon Quantum Dots from Natural Resource: A Review. Mater. Today Chem. 2018, 8, 96–109. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, A. Carbon Quantum Dots: Synthesis, Properties and Applications. J. Mater. Chem. C Mater. 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Samimi, S.; Ardestani, M.S.; Dorkoosh, F.A. Preparation of Carbon Quantum Dots- Quinic Acid for Drug Delivery of Gemcitabine to Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2021, 61, 102287. [Google Scholar] [CrossRef]
- Shi, C.; Qi, H.; Ma, R.; Sun, Z.; Xiao, L.; Wei, G.; Huang, Z.; Liu, S.; Li, J.; Dong, M.; et al. N,S-Self-Doped Carbon Quantum Dots from Fungus Fibers for Sensing Tetracyclines and for Bioimaging Cancer Cells. Mater. Sci. Eng. C 2019, 105, 110132. [Google Scholar] [CrossRef]
- Loo, A.H.; Sofer, Z.; Bouša, D.; Ulbrich, P.; Bonanni, A.; Pumera, M. Carboxylic Carbon Quantum Dots as a Fluorescent Sensing Platform for DNA Detection. ACS Appl. Mater. Interfaces 2016, 8, 1951–1957. [Google Scholar] [CrossRef]
- John, V.L.; Nair, Y.; Vinod, T.P. Doping and Surface Modification of Carbon Quantum Dots for Enhanced Functionalities and Related Applications. Part. Part. Syst. Charact. 2021, 38, 2100170. [Google Scholar] [CrossRef]
- Yang, S.T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Meziani, M.J.; Liu, J.H.; Liu, Y.; Chen, M.; et al. Carbon Dots as Nontoxic and High-Performance Fluorescence Imaging Agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, R.; Li, H.; Shao, J.; Chi, Y.; Lin, X.; Chen, G. Polyamine-Functionalized Carbon Quantum Dots for Chemical Sensing. Carbon 2012, 50, 2810–2815. [Google Scholar] [CrossRef]
- Huang, C.; Dong, H.; Su, Y.; Wu, Y.; Narron, R.; Yong, Q. Synthesis of Carbon Quantum Dot Nanoparticles Derived from Byproducts in Bio-Refinery Process for Cell Imaging and in Vivo Bioimaging. Nanomaterials 2019, 9, 387. [Google Scholar] [CrossRef]
- Alavi, M.; Jabari, E.; Jabbari, E. Functionalized Carbon-Based Nanomaterials and Quantum Dots with Antibacterial Activity: A Review. Expert Rev. Anti Infect. Ther. 2021, 19, 35–44. [Google Scholar] [CrossRef]
- Hao, X.; Huang, L.; Zhao, C.; Chen, S.; Lin, W.; Lin, Y.; Zhang, L.; Sun, A.; Miao, C.; Lin, X.; et al. Antibacterial Activity of Positively Charged Carbon Quantum Dots without Detectable Resistance for Wound Healing with Mixed Bacteria Infection. Mater. Sci. Eng. C 2021, 123, 111971. [Google Scholar] [CrossRef]
- Li, P.; Han, F.; Cao, W.; Zhang, G.; Li, J.; Zhou, J.; Gong, X.; Turnbull, G.; Shu, W.; Xia, L.; et al. Carbon Quantum Dots Derived from Lysine and Arginine Simultaneously Scavenge Bacteria and Promote Tissue Repair. Appl. Mater. Today 2020, 19, 100601. [Google Scholar] [CrossRef]
- Qu, X.; Gao, C.; Fu, L.; Chu, Y.; Wang, J.H.; Qiu, H.; Chen, J. Positively Charged Carbon Dots with Antibacterial and Antioxidant Dual Activities for Promoting Infected Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 18608–18619. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, E. Magnetic Nanoparticles in Targeted Drug Delivery: A Review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.P.; Gómez-Pastora, J.; et al. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small 2024, 20, e2304848. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, M.S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic Nanoparticles for Hyperthermia in Cancer Treatment: An Emerging Tool. Environ. Sci. Pollut. Res. 2020, 27, 19214–19225. [Google Scholar] [CrossRef]
- Joshi, N.C.; Chaudhary, N.; Rai, N. Medicinal Plant Leaves Extract Based Synthesis, Characterisations and Antimicrobial Activities of ZrO2 Nanoparticles (ZrO2 NPs). Bionanoscience 2021, 11, 497–505. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, L.; Wen, S.; Liu, L. Graphene Oxide-Supported Zinc Oxide Nanoparticles for Chloroprene Rubber with Improved Crosslinking Network and Mechanical Properties. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105492. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Ilyas, S.; Sultana, A.; Hezam, A.; Sunil, L.; Surmeneva, M.A.; Surmenev, R.A.; Nayan, M.B.; Ramakrishna, S.; et al. Emerging Trends for ZnO Nanoparticles and Their Applications in Food Packaging. ACS Food Sci. Technol. 2022, 2, 763–781. [Google Scholar] [CrossRef]
- Yusaf, T.; Mahamude, A.S.F.; Farhana, K.; Harun, W.S.W.; Kadirgama, K.; Ramasamy, D.; Kamarulzaman, M.K.; Subramonian, S.; Hall, S.; Dhahad, H.A. A Comprehensive Review on Graphene Nanoparticles: Preparation, Properties, and Applications. Sustainability 2022, 14, 12336. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef]
- Markovic, Z.M.; Harhaji-Trajkovic, L.M.; Todorovic-Markovic, B.M.; Kepić, D.P.; Arsikin, K.M.; Jovanović, S.P.; Pantovic, A.C.; Dramićanin, M.D.; Trajkovic, V.S. In Vitro Comparison of the Photothermal Anticancer Activity of Graphene Nanoparticles and Carbon Nanotubes. Biomaterials 2011, 32, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Aslan, A. Protective Effect of Pristine C60 Fullerene Nanoparticle in Combination with Curcumin against Hyperglycemia-Induced Kidney Damage in Diabetes Caused by Streptozotocin. J. Food Biochem. 2020, 44, e13470. [Google Scholar] [CrossRef]
- Demir, E.; Nedzvetsky, V.S.; Ağca, C.A.; Kirici, M. Pristine C60 Fullerene Nanoparticles Ameliorate Hyperglycemia-Induced Disturbances via Modulation of Apoptosis and Autophagy Flux. Neurochem. Res. 2020, 45, 2385–2397. [Google Scholar] [CrossRef]
- Ye, L.; Kollie, L.; Liu, X.; Guo, W.; Ying, X.; Zhu, J.; Yang, S.; Yu, M. Antitumor Activity and Potential Mechanism of Novel Fullerene Derivative Nanoparticles. Molecules 2021, 26, 3252. [Google Scholar] [CrossRef]
- Öner, G.A. Flexural Strength and Thermal Properties of Carbon Black Nanoparticle Reinforced Epoxy Composites Obtained from Waste Tires. Open Chem. 2022, 20, 863–872. [Google Scholar] [CrossRef]
- Singh, M.; Vander Wal, R. Nanostructure Quantification of Carbon Blacks. C 2018, 5, 2. [Google Scholar] [CrossRef]
- Molaei, M.J. A Review on Nanostructured Carbon Quantum Dots and Their Applications in Biotechnology, Sensors, and Chemiluminescence. Talanta 2019, 196, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-Based Nanoparticles as Effective Drug Delivery Systems—A Review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Lotfy, T.M.R.; Shawir, S.M.S. Preparation and Antibacterial Activity of Chitosan-Silver Nanoparticles for Application in Preservation of Minced Meat. Bull. Natl. Res. Cent. 2019, 43, 83. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, Characterization, and Potential Application of Chitosan, Chitosan Derivatives, and Chitosan Metal Nanoparticles in Pharmaceutical Drug Delivery. Drug Des. Devel. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed]
- Loo, H.L.; Goh, B.H.; Lee, L.H.; Chuah, L.H. Application of Chitosan-Based Nanoparticles in Skin Wound Healing. Asian J. Pharm. Sci. 2022, 17, 299–332. [Google Scholar] [CrossRef] [PubMed]
- Taheriazam, A.; Entezari, M.; Firouz, Z.M.; Hajimazdarany, S.; Hossein Heydargoy, M.; Amin Moghadassi, A.H.; Moghadaci, A.; Sadrani, A.; Motahhary, M.; Harif Nashtifani, A.; et al. Eco-Friendly Chitosan-Based Nanostructures in Diabetes Mellitus Therapy: Promising Bioplatforms with Versatile Therapeutic Perspectives. Environ. Res. 2023, 228, 115912. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Abdalhalim, L.R.; Atalla, K.M.; Mohdaly, A.A.A.; Ramadan, M.F.; Abdelaliem, Y.F. Chitosan and Sodium Alginate Nanoparticles Synthesis and Its Application in Food Preservation. Rendiconti Lincei 2023, 34, 415–425. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles—An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.S.; Nayak, A.K.; Kurakula, M.; Hoda, M.N. Alginate Nanoparticles in Drug Delivery. In Alginates in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2020; pp. 129–152. ISBN 9780128176405. [Google Scholar]
- Zeb, A.; Gul, M.; Nguyen, T.T.L.; Maeng, H.J. Controlled Release and Targeted Drug Delivery with Poly(Lactic-Co-Glycolic Acid) Nanoparticles: Reviewing Two Decades of Research. J. Pharm. Investig. 2022, 52, 683–724. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(Lactic-Co-Glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, K.K.; Vandermeulen, G.; Préat, V. PLGA Based Drug Delivery Systems: Promising Carriers for Wound Healing Activity. Wound Repair Regen. 2016, 24, 223–236. [Google Scholar] [CrossRef]
- Pang, H.; Huang, X.; Xu, Z.P.; Chen, C.; Han, F.Y. Progress in Oral Insulin Delivery by PLGA Nanoparticles for the Management of Diabetes. Drug Discov. Today 2023, 28, 103393. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A Perspective on Polylactic Acid-Based Polymers Use for Nanoparticles Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Qiu, L.; Chen, J. Liposome: Composition, Characterisation, Preparation, and Recent Innovation in Clinical Applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef] [PubMed]
- Ajeeshkumar, K.K.; Aneesh, P.A.; Raju, N.; Suseela, M.; Ravishankar, C.N.; Benjakul, S. Advancements in Liposome Technology: Preparation Techniques and Applications in Food, Functional Foods, and Bioactive Delivery: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1280–1306. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, Y.; Hamishehkar, H. Liposomes in Cosmeceutics. Expert Opin. Drug Deliv. 2012, 9, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetti, S.; Vijayan, V.; Rangasamy, J.; Bardhan, R.; Uthaman, S.; Park, I.K. A Multi-Stimuli Responsive Alginate Nanogel for Anticancer Chemo-Photodynamic Therapy. J. Ind. Eng. Chem. 2023, 123, 361–370. [Google Scholar] [CrossRef]
- Bayón-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Netto MPharm, G.; Jose, J. Development, Characterization, and Evaluation of Sunscreen Cream Containing Solid Lipid Nanoparticles of Silymarin. J. Cosmet. Dermatol. 2018, 17, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Decker, E.A.; McClements, D.J.; Kristbergsson, K.; Helgason, T.; Awad, T. Solid Lipid Nanoparticles as Delivery Systems for Bioactive Food Components. Food Biophys. 2008, 3, 146–154. [Google Scholar] [CrossRef]
- Salvi, V.R.; Pawar, P. Nanostructured Lipid Carriers (NLC) System: A Novel Drug Targeting Carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- Piran, P.; Kafil, H.S.; Ghanbarzadeh, S.; Safdari, R.; Hamishehkar, H. Formulation of Menthol-Loaded Nanostructured Lipid Carriers to Enhance Its Antimicrobial Activity for Food Preservation. Adv. Pharm. Bull. 2017, 7, 261–268. [Google Scholar] [CrossRef]
- Rizwanullah, M.; Ahmad, M.Z.; Garg, A.; Ahmad, J. Advancement in Design of Nanostructured Lipid Carriers for Cancer Targeting and Theranostic Application. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129936. [Google Scholar] [CrossRef]
- Prasertpol, T.; Tiyaboonchai, W. Nanostructured Lipid Carriers: A Novel Hair Protective Product Preventing Hair Damage and Discoloration from UV Radiation and Thermal Treatment. J. Photochem. Photobiol. B 2020, 204, 111769. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, O.P.; Torres, F.G. Non-conventional Starch Nanoparticles for Drug Delivery Applications. Med. Devices Sens. 2020, 3, e10111. [Google Scholar] [CrossRef]
- Le Corre, D.; Angellier-Coussy, H. Preparation and Application of Starch Nanoparticles for Nanocomposites: A Review. React. Funct. Polym. 2014, 85, 97–120. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, C.; Hua, Y.; Wei, T.; Zhang, L. Multifunctional Nanoparticles of Paclitaxel and Cyclodextrin–Polypeptide Conjugates with in Vitro Anticancer Activity. Pharm. Dev. Technol. 2020, 25, 1071–1080. [Google Scholar] [CrossRef]
- Khatua, T.N.; Dey, S.; Abbasi, Y.F.; Bera, H.; Suresh, S. Casein-Based Nanomaterials in Drug Delivery and Biomedical Applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Academic Press: Cambridge, MA, USA, 2021; pp. 519–534. [Google Scholar] [CrossRef]
- El Fakharany, E.M.; Abu Serie, M.M.; Ibrahim, A.; Eltarahony, M. Anticancer Activity of Lactoferrin-Coated Biosynthesized Selenium Nanoparticles for Combating Different Human Cancer Cells via Mediating Apoptotic Effects. Sci. Rep. 2023, 13, 9579. [Google Scholar] [CrossRef]
- Attri, K.; Chudasama, B.; Mahajan, R.L.; Choudhury, D. Therapeutic Potential of Lactoferrin-Coated Iron Oxide Nanospheres for Targeted Hyperthermia in Gastric Cancer. Sci. Rep. 2023, 13, 17875. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Lal, S. Synthesis of Organic Nanoparticles and Their Applications in Drug Delivery and Food Nanotechnology: A Review. J. Nanomater. Mol. Nanotechnol. 2014, 3. [Google Scholar] [CrossRef]
- Mitragotri, S.; Stayton, P. Organic Nanoparticles for Drug Delivery and Imaging. MRS Bull. 2014, 39, 219–223. [Google Scholar] [CrossRef]
- Dilliard, S.A.; Siegwart, D.J. Passive, Active and Endogenous Organ-Targeted Lipid and Polymer Nanoparticles for Delivery of Genetic Drugs. Nat. Rev. Mater. 2023, 8, 282–300. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric Nanoparticles: The Future of Nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan Nanoparticles Preparation and Applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Othman, S.H.; Othman, N.F.L.; Shapi’i, R.A.; Ariffin, S.H.; Yunos, K.F.M. Corn Starch/Chitosan Nanoparticles/Thymol Bio-Nanocomposite Films for Potential Food Packaging Applications. Polymers 2021, 13, 390. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Shi, Z.; Neoh, K.G.; Kang, E.T. Antioxidant and Antibacterial Activities of Eugenol and Carvacrol-Grafted Chitosan Nanoparticles. Biotechnol. Bioeng. 2009, 104, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, M.; Liang, S.; Li, Y. Enhanced Antioxidant and Antibacterial Activities of Chitosan/Zein Nanoparticle Pickering Emulsion-Incorporated Chitosan Coatings in the Presence of Cinnamaldehyde and Tea Polyphenol. Int. J. Biol. Macromol. 2024, 266, 131181. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Gupta, A.; Bharadwaj, R.; Ranganath, P.; Silverman, N.; Agrawal, G. Biodegradable Disulfide Crosslinked Chitosan/Stearic Acid Nanoparticles for Dual Drug Delivery for Colorectal Cancer. Carbohydr. Polym. 2022, 294, 119833. [Google Scholar] [CrossRef] [PubMed]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.U. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan Nanoparticles as a Promising Tool in Nanomedicine with Particular Emphasis on Oncological Treatment. Cancer Cell Int. 2021, 21, 318. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial Activity of Chitosan Nanoparticles: A Review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Kumar-Krishnan, S.; Prokhorov, E.; Hernández-Iturriaga, M.; Mota-Morales, J.D.; Vázquez-Lepe, M.; Kovalenko, Y.; Sanchez, I.C.; Luna-Bárcenas, G. Chitosan/Silver Nanocomposites: Synergistic Antibacterial Action of Silver Nanoparticles and Silver Ions. Eur. Polym. J. 2015, 67, 242–251. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional Silver Nanoparticles Based on Chitosan: Antibacterial, Antibiofilm, Antifungal, Antioxidant, and Wound-Healing Activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Eker, F.; Akdaşçi, E.; Duman, H.; Yalçıntaş, Y.M.; Canbolat, A.A.; Kalkan, A.E.; Karav, S.; Šamec, D. Antimicrobial Properties of Colostrum and Milk. Antibiotics 2024, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wu, C.; Fu, S.; Wang, L.; Yuan, C.; Chen, S.; Hu, Y. Integration of Lysozyme into Chitosan Nanoparticles for Improving Antibacterial Activity. Carbohydr. Polym. 2017, 155, 192–200. [Google Scholar] [CrossRef]
- Yang, J.S.; Xie, Y.J.; He, W. Research Progress on Chemical Modification of Alginate: A Review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Dodero, A.; Alberti, S.; Gaggero, G.; Ferretti, M.; Botter, R.; Vicini, S.; Castellano, M. An Up-to-Date Review on Alginate Nanoparticles and Nanofibers for Biomedical and Pharmaceutical Applications. Adv. Mater. Interfaces 2021, 8, 2100809. [Google Scholar] [CrossRef]
- Spadari, C.C.; Lanser, D.M.; Araújo, M.V.; De Jesus, D.F.F.; Lopes, L.B.; Gelli, A.; Ishida, K. Oral Delivery of Brain-Targeted Miltefosine-Loaded Alginate Nanoparticles Functionalized with Polysorbate 80 for the Treatment of Cryptococcal Meningitis. J. Antimicrob. Chemother. 2023, 78, 1092–1101. [Google Scholar] [CrossRef]
- Thomas, D.; Mathew, N.; Nath, M.S. Starch Modified Alginate Nanoparticles for Drug Delivery Application. Int. J. Biol. Macromol. 2021, 173, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Sahatsapan, N.; Ngawhirunpat, T.; Rojanarata, T.; Opanasopit, P.; Patrojanasophon, P. Catechol-Functionalized Alginate Nanoparticles as Mucoadhesive Carriers for Intravesical Chemotherapy. AAPS PharmSciTech 2020, 21, 212. [Google Scholar] [CrossRef]
- Alallam, B.; Altahhan, S.; Taher, M.; Mohd Nasir, M.H.; Doolaanea, A.A. Electrosprayed Alginate Nanoparticles as Crispr Plasmid Dna Delivery Carrier: Preparation, Optimization, and Characterization. Pharmaceuticals 2020, 13, 158. [Google Scholar] [CrossRef]
- Liu, Q.; Jing, Y.; Han, C.; Zhang, H.; Tian, Y. Encapsulation of Curcumin in Zein/ Caseinate/Sodium Alginate Nanoparticles with Improved Physicochemical and Controlled Release Properties. Food Hydrocoll. 2019, 93, 432–442. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Ma, C.; Hua, Y.; Zhang, L.; Shen, J. Design and Investigation of Penetrating Mechanism of Octaarginine-Modified Alginate Nanoparticles for Improving Intestinal Insulin Delivery. J. Pharm. Sci. 2021, 110, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan Oligosaccharide/Alginate Nanoparticles as an Effective Carrier for Astaxanthin with Improving Stability, in Vitro Oral Bioaccessibility, and Bioavailability. Food Hydrocoll. 2022, 124, 107246. [Google Scholar] [CrossRef]
- Sohail, R.; Abbas, S.R. Evaluation of Amygdalin-Loaded Alginate-Chitosan Nanoparticles as Biocompatible Drug Delivery Carriers for Anticancerous Efficacy. Int. J. Biol. Macromol. 2020, 153, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, H.; Chen, K.; Jin, M.; Vu, S.H.; Jung, S.; He, N.; Zheng, Z.; Lee, M.S. Application of Chitosan/Alginate Nanoparticle in Oral Drug Delivery Systems: Prospects and Challenges. Drug Deliv. 2022, 29, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Caruso, G.M.; Carroccio, S.C.; Boninelli, S.; Curcuruto, G.; Zimbone, M.; Allegra, M.; Torrisi, B.; Ferlito, F.; Miritello, M. Smart Nanocomposites of Chitosan/Alginate Nanoparticles Loaded with Copper Oxide as Alternative Nanofertilizers. Environ. Sci. Nano 2021, 8, 174–187. [Google Scholar] [CrossRef]
- AbdelAllah, N.H.; Gaber, Y.; Rashed, M.E.; Azmy, A.F.; Abou-Taleb, H.A.; AbdelGhani, S. Alginate-Coated Chitosan Nanoparticles Act as Effective Adjuvant for Hepatitis A Vaccine in Mice. Int. J. Biol. Macromol. 2020, 152, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Taghiloo, S.; Ghajari, G.; Zand, Z.; Kabiri-Samani, S.; Kabiri, H.; Rajaei, N.; Piri-Gharaghie, T. Designing Alginate/Chitosan Nanoparticles Containing Echinacea Angustifolia: A Novel Candidate for Combating Multidrug-Resistant Staphylococcus Aureus. Chem. Biodivers 2023, 20, e202201008. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, V.; Nivetha, R.P.; Tamilanban, T.; Narayanan, J.; Vetriselvan, S.; Fuloria, N.K.; Chinni, S.V.; Sekar, M.; Fuloria, S.; Wong, L.S.; et al. Nanogels as Novel Drug Nanocarriers for CNS Drug Delivery. Front. Mol. Biosci. 2023, 10, 1232109. [Google Scholar] [CrossRef]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A Versatile Nano-Delivery System for Biomedical Applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef]
- Suhail, M.; Fang, C.W.; Chiu, I.H.; Khan, A.; Wu, Y.C.; Lin, I.L.; Tsai, M.J.; Wu, P.C. Synthesis and Evaluation of Alginate-Based Nanogels as Sustained Drug Carriers for Caffeine. ACS Omega 2023, 8, 23991–24002. [Google Scholar] [CrossRef]
- Chen, Y.B.; Zhang, Y.B.; Wang, Y.L.; Kaur, P.; Yang, B.G.; Zhu, Y.; Ye, L.; Cui, Y.L. A Novel Inhalable Quercetin-Alginate Nanogel as a Promising Therapy for Acute Lung Injury. J. Nanobiotechnol. 2022, 20, 272. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef] [PubMed]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(Lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties-from Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef]
- Teixeira, S.; Eblagon, K.M.; Miranda, F.; Pereira, M.F.R.; Figueiredo, J.L. Towards Controlled Degradation of Poly(Lactic) Acid in Technical Applications. C 2021, 7, 42. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The Development and Challenges of Poly (Lactic Acid) and Poly (Glycolic Acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Antonio, E.; dos Reis Antunes Junior, O.; Marcano, R.G.D.J.V.; Diedrich, C.; da Silva Santos, J.; Machado, C.S.; Khalil, N.M.; Mainardes, R.M. Chitosan Modified Poly (Lactic Acid) Nanoparticles Increased the Ursolic Acid Oral Bioavailability. Int. J. Biol. Macromol. 2021, 172, 133–142. [Google Scholar] [CrossRef]
- Niza, E.; Božik, M.; Bravo, I.; Clemente-Casares, P.; Lara-Sanchez, A.; Juan, A.; Klouček, P.; Alonso-Moreno, C. PEI-Coated PLA Nanoparticles to Enhance the Antimicrobial Activity of Carvacrol. Food Chem. 2020, 328, 127131. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, R.; Sepehri, N.; Manoochehri, S.; Rouhani, H.; Atyabi, F. Polylactide-Co-Glycolide Nanoparticles for Controlled Delivery of Anticancer Agents. Int. J. Nanomed. 2011, 6, 877–895. [Google Scholar] [CrossRef]
- Mahar, R.; Chakraborty, A.; Nainwal, N.; Bahuguna, R.; Sajwan, M.; Jakhmola, V. Application of PLGA as a Biodegradable and Biocompatible Polymer for Pulmonary Delivery of Drugs. AAPS PharmSciTech 2023, 24, 39. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Motsoene, F.; Abrahamse, H.; Dhilip Kumar, S.S. Multifunctional Lipid-Based Nanoparticles for Wound Healing and Antibacterial Applications: A Review. Adv. Colloid Interface Sci. 2023, 321, 103002. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of Liposomes in Medicine and Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Haldorai, Y.; Hwang, S.K.; Bajpai, V.K.; Huh, Y.S.; Han, Y.K. Current Demands for Food-Approved Liposome Nanoparticles in Food and Safety Sector. Front. Microbiol. 2017, 8, 2398. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles from Liposomes to MRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Sfar, S.; Charcosset, C.; Fessi, H. Preparation, Characterization and Applications of Liposomes: State of the Art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Jesorka, A.; Orwar, O. Liposomes: Technologies and Analytical Applications. Annu. Rev. Anal. Chem. 2008, 1, 801–832. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Shibata, A. Chapter 2: Surface Properties of Liposomes Depending on Their Composition. Adv. Planar Lipid Bilayers Liposomes 2006, 4, 49–77. [Google Scholar]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Betageri, G.V.; Parsons, D.L. Drug Encapsulation and Release from Multilamellar and Unilamellar Liposomes. Int. J. Pharm. 1992, 81, 235–241. [Google Scholar] [CrossRef]
- Sułkowski, W.W.; Pentak, D.; Nowak, K.; Sułkowska, A. The Influence of Temperature, Cholesterol Content and PH on Liposome Stability. J. Mol. Struct. 2005, 744, 737–747. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated Liposomes: Immunological Responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-Da-silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef] [PubMed]
- Rommasi, F.; Esfandiari, N. Liposomal Nanomedicine: Applications for Drug Delivery in Cancer Therapy. Nanoscale Res. Lett. 2021, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Balazs, D.A.; Godbey, W.T. Liposomes for Use in Gene Delivery. J. Drug Deliv. 2011, 2011, 326497. [Google Scholar] [CrossRef] [PubMed]
- Tretiakova, D.S.; Vodovozova, E.L. Liposomes as Adjuvants and Vaccine Delivery Systems. Biochem. Mosc. Suppl. Ser. A Membr. Cell Biol. 2022, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Reyes, E.D.; Perea-Flores, M.d.J.; Davila-Ortiz, G.; Lee, Y.; de Mejia, E.G. Development, Characterization and Use of Liposomes as Amphipathic Transporters of Bioactive Compounds for Melanoma Treatment and Reduction of Skin Inflammation: A Review. Int. J. Nanomed. 2020, 15, 7627–7650. [Google Scholar] [CrossRef] [PubMed]
- Lingayat, V.J.; Zarekar, N.S.; Shendge, R.S. Solid Lipid Nanoparticles: A Review. Nanosci. Nanotechnol. Res. 2017, 4, 67–72. [Google Scholar]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid Lipid Nanoparticles: A Modern Formulation Approach in Drug Delivery System. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef]
- Almeida, A.J.; Souto, E. Solid Lipid Nanoparticles as a Drug Delivery System for Peptides and Proteins. Adv. Drug Deliv. Rev. 2007, 59, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.; Getti, G.; Nandi, U.; Mithu, M.S.; Douroumis, D. Bioconjugated Solid Lipid Nanoparticles (SLNs) for Targeted Prostate Cancer Therapy. Int. J. Pharm. 2021, 599, 120416. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.A.; Müller, R.H. Cosmetic Applications for Solid Lipid Nanoparticles (SLN). Int. J. Pharm. 2003, 254, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [PubMed]
- Nasirizadeh, S.; Malaekeh-Nikouei, B. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers in Oral Cancer Drug Delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101458. [Google Scholar] [CrossRef]
- Müller, R.H.; Petersen, R.D.; Hommoss, A.; Pardeike, J. Nanostructured Lipid Carriers (NLC) in Cosmetic Dermal Products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, Fine Structure and Architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Luchese, C.L.; Spada, J.C.; Tessaro, I.C. Starch Content Affects Physicochemical Properties of Corn and Cassava Starch-Based Films. Ind. Crop. Prod. 2017, 109, 619–626. [Google Scholar] [CrossRef]
- Le Corre, D.; Bras, J.; Dufresne, A. Starch Nanoparticles: A Review. Biomacromolecules 2010, 11, 1139–1153. [Google Scholar] [CrossRef]
- Ashogbon, A.O.; Akintayo, E.T. Recent Trend in the Physical and Chemical Modification of Starches from Different Botanical Sources: A Review. Starch/Staerke 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Guessan, N.; Amani, G.; Kamenan, A.; Rolland-Sabaté, A.; Colonna, P. Stability of Yam Starch Gels during Processing. Afr. J. Biotechnol. 2005, 4, 94–101. [Google Scholar]
- Suma, P.F.; Urooj, A. Isolation and Characterization of Starch from Pearl Millet (Pennisetum typhoidium) Flours. Int. J. Food Prop. 2015, 18, 2675–2687. [Google Scholar] [CrossRef]
- Chavan, P.; Sinhmar, A.; Nehra, M.; Thory, R.; Pathera, A.K.; Sundarraj, A.A.; Nain, V. Impact on Various Properties of Native Starch after Synthesis of Starch Nanoparticles: A Review. Food Chem. 2021, 364, 130416. [Google Scholar] [CrossRef] [PubMed]
- Campelo, P.H.; Sant’Ana, A.S.; Pedrosa Silva Clerici, M.T. Starch Nanoparticles: Production Methods, Structure, and Properties for Food Applications. Curr. Opin. Food Sci. 2020, 33, 136–140. [Google Scholar] [CrossRef]
- García-Gurrola, A.; Rincón, S.; Escobar-Puentes, A.A.; Zepeda, A.; Pérez-Robles, J.F.; Martínez-Bustos, F. Synthesis and Succinylation of Starch Nanoparticles by Means of a Single Step Using Sonochemical Energy. Ultrason Sonochem. 2019, 56, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Xue, L.; Hu, Y.; Qiu, C.; Jin, Z.; Xu, X.; Wang, J. Green Fabrication and Characterization of Debranched Starch Nanoparticles via Ultrasonication Combined with Recrystallization. Ultrason Sonochem. 2020, 66, 105074. [Google Scholar] [CrossRef] [PubMed]
- Angellier, H.; Molina-Boisseau, S.; Lebrun, L.; Dufresne, A. Processing and Structural Properties of Waxy Maize Starch Nanocrystals Reinforced Natural Rubber. Macromolecules 2005, 38, 3783–3792. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park, S.S.; Lim, S.T. Preparation, Characterization and Utilization of Starch Nanoparticles. Colloids Surf. B Biointerfaces 2015, 126, 607–620. [Google Scholar] [CrossRef]
- Wongpanit, P.; Sanchavanakit, N.; Pavasant, P.; Bunaprasert, T.; Tabata, Y.; Rujiravanit, R. Preparation and Characterization of Chitin Whisker-Reinforced Silk Fibroin Nanocomposite Sponges. Eur. Polym. J. 2007, 43, 4123–4135. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, L.; Yu, J. Effect of Polysaccharide Nanocrystals on Structure, Properties, and Drug Release Kinetics of Alginate-Based Microspheres. Colloids Surf. B Biointerfaces 2011, 85, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bandopadhyay, R. Use of Dextran Nanoparticle: A Paradigm Shift in Bacterial Exopolysaccharide Based Biomedical Applications. Int. J. Biol. Macromol. 2016, 87, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Wasiak, I.; Kulikowska, A.; Janczewska, M.; Michalak, M.; Cymerman, I.A.; Nagalski, A.; Kallinger, P.; Szymanski, W.W.; Ciach, T. Dextran Nanoparticle Synthesis and Properties. PLoS ONE 2016, 11, e0146237. [Google Scholar] [CrossRef] [PubMed]
- Alhareth, K.; Vauthier, C.; Bourasset, F.; Gueutin, C.; Ponchel, G.; Moussa, F. Conformation of Surface-Decorating Dextran Chains Affects the Pharmacokinetics and Biodistribution of Doxorubicin-Loaded Nanoparticles. Eur. J. Pharm. Biopharm. 2012, 81, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Casadei, M.A.; Cerreto, F.; Cesa, S.; Giannuzzo, M.; Feeney, M.; Marianecci, C.; Paolicelli, P. Solid Lipid Nanoparticles Incorporated in Dextran Hydrogels: A New Drug Delivery System for Oral Formulations. Int. J. Pharm. 2006, 325, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Geng, Y.; Wu, F.; Liu, Y.; Guo, M.; Zhao, H.; Jin, T. Preparation of Polysaccharide Glassy Microparticles with Stabilization of Proteins. Int. J. Pharm. 2009, 366, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, Z.; Su, J.; Wei, L.; Yuan, W.; Jin, T. Development of Dextran Nanoparticles for Stabilizing Delicate Proteins. Nanoscale Res. Lett. 2013, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Mehvar, R. Dextrans for Targeted and Sustained Delivery of Therapeutic and Imaging Agents. J. Control. Release 2000, 69, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Fenyvesi, É.; Szente, L. Outstanding Contribution of Professor József Szejtli to Cyclodextrin Applications in Foods, Cosmetics, Drugs, Chromatography and Biotechnology: A Review. Environ. Chem. Lett. 2021, 19, 2619–2641. [Google Scholar] [CrossRef]
- Petitjean, M.; García-Zubiri, I.X.; Isasi, J.R. History of Cyclodextrin-Based Polymers in Food and Pharmacy: A Review. Environ. Chem. Lett. 2021, 19, 3465–3476. [Google Scholar] [CrossRef]
- Lysik, M.A.; Wu-Pong, S. Innovations in Oligonucleotide Drug Delivery. J. Pharm. Sci. 2003, 92, 1559–1573. [Google Scholar] [CrossRef] [PubMed]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in Drug Delivery: An Updated Review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Saneja, A.; Jaglan, S. Cyclodextrin-Based Delivery Systems for Dietary Pharmaceuticals. Environ. Chem. Lett. 2019, 17, 1263–1270. [Google Scholar] [CrossRef]
- Lakkakula, J.R.; Maçedo Krause, R.W. A Vision for Cyclodextrin Nanoparticles in Drug Delivery Systems and Pharmaceutical Applications. Nanomedicine 2014, 9, 877–894. [Google Scholar] [CrossRef] [PubMed]
- Bilensoy, E. Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications. In Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from Molecules to Applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Weber, C.; Coester, C.; Kreuter, J.; Langer, K. Desolvation Process and Surface Characterisation of Protein Nanoparticles. Int. J. Pharm. 2000, 194, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Lohcharoenkal, W.; Wang, L.; Chen, Y.C.; Rojanasakul, Y. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy. Biomed. Res. Int. 2014, 2014, 180549. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, M.; Zhang, Z.; Lyddiatt, A. Subtractive Chromatography for Purification and Recovery of Nano-Bioproducts. IEE Proc. Nanobiotechnol. 2005, 152, 121–126. [Google Scholar] [CrossRef]
- MaHam, A.; Tang, Z.; Wu, H.; Wang, J.; Lin, Y. Protein-Based Nanomedicine Platforms for Drug Delivery. Small 2009, 5, 1706–1721. [Google Scholar] [CrossRef]
- Jin, S.; Li, S.; Wang, C.; Liu, J.; Yang, X.; Wang, P.C.; Zhang, X.; Liang, X.J. Biosafe Nanoscale Pharmaceutical Adjuvant Materials. J. Biomed. Nanotechnol. 2014, 10, 2393–2419. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current Development of Biodegradable Polymeric Materials for Biomedical Applications. Drug Des. Devel. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Uzel, S.G.M.; Buehler, M.J. Molecular Structure, Mechanical Behavior and Failure Mechanism of the C-Terminal Cross-Link Domain in Type I Collagen. J. Mech. Behav. Biomed. Mater. 2011, 4, 153–161. [Google Scholar] [CrossRef]
- Aditya, A.; Kim, B.; Koyani, R.D.; Oropeza, B.; Furth, M.; Kim, J.; Kim, N.P. Kinetics of Collagen Microneedle Drug Delivery System. J. Drug Deliv. Sci. Technol. 2019, 52, 618–623. [Google Scholar] [CrossRef]
- Chak, V.; Kumar, D.; Visht, S. A Review on Collagen Based Drug Delivery Systems. Int. J. Pharm. Teach. Pract. 2013, 4, 811–820. [Google Scholar]
- Lee, J.H. Injectable Hydrogels Delivering Therapeutic Agents for Disease Treatment and Tissue Engineering. Biomater. Res. 2018, 22, s40824-018-0138-6. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The Role of Collagen in Cancer: From Bench to Bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Shekhter, A.B.; Fayzullin, A.L.; Vukolova, M.N.; Rudenko, T.G.; Osipycheva, V.D.; Litvitsky, P.F. Medical Applications of Collagen and Collagen-Based Materials. Curr. Med. Chem. 2017, 26, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, E.I.; Udekwu, K.; Skog, M.; Pacioni, N.L.; Stamplecoskie, K.G.; González-Béjar, M.; Polisetti, N.; Wickham, A.; Richter-Dahlfors, A.; Griffith, M.; et al. The Biocompatibility and Antibacterial Properties of Collagen-Stabilized, Photochemically Prepared Silver Nanoparticles. Biomaterials 2012, 33, 4947–4956. [Google Scholar] [CrossRef]
- Li, S.; Peng, Z.; Leblanc, R.M. Method to Determine Protein Concentration in the Protein-Nanoparticle Conjugates Aqueous Solution Using Circular Dichroism Spectroscopy. Anal. Chem. 2015, 87, 6455–6459. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Pan, J.; Jiang, X.; Ji, Y.; Li, Y.; Qu, Y.; Zhao, Y.; Wu, X.; Chen, C. Revealing the Binding Structure of the Protein Corona on Gold Nanorods Using Synchrotron Radiation-Based Techniques: Understanding the Reduced Damage in Cell Membranes. J. Am. Chem. Soc. 2013, 135, 17359–17368. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.J.; Liu, Y.; Sun, T.Q.; Bai, A.M.; Lü, J.Q.; Pi, Z.B. Binding of Anti-Inflammatory Drug Cromolyn Sodium to Bovine Serum Albumin. Int. J. Biol. Macromol. 2006, 39, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Tantra, R.; Tompkins, J.; Quincey, P. Characterisation of the De-Agglomeration Effects of Bovine Serum Albumin on Nanoparticles in Aqueous Suspension. Colloids Surf. B Biointerfaces 2010, 75, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. Albumin as a Drug Carrier: Design of Prodrugs, Drug Conjugates and Nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.V. Biopolymer Albumin for Diagnosis and in Drug Delivery. Drug Dev. Res. 2003, 58, 219–247. [Google Scholar] [CrossRef]
- Weber, C.; Kreuter, J.; Langer, K. Desolvation Process and Surface Characteristics of HSA-Nanoparticles. Int. J. Pharm. 2000, 196, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Irache, J.M.; Merodio, M.; Arnedo, A.; Camapanero, M.A.; Mirshahi, M.; Espuelas, S. Albumin Nanoparticles for the Intravitreal Delivery of Anticytomegaloviral Drugs. Mini Rev. Med. Chem. 2005, 5, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-Based Nanoparticles as Potential Controlled Release Drug Delivery Systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Hekmatara, T.; Herbert, E.; Kreuter, J. Transferrin- and Transferrin-Receptor-Antibody-Modified Nanoparticles Enable Drug Delivery across the Blood-Brain Barrier (BBB). Eur. J. Pharm. Biopharm. 2009, 71, 251–256. [Google Scholar] [CrossRef]
- Geny, B.; Mettauer, B.; Muan, B.; Bischoff, P.; Epailly, E.; Piquard, F.; Eisenmann, B.; Haberey, P. Safety and Efficacy of a New Transpulmonary Echo Contrast Agent in Echocardiographic Studies in Patients. J. Am. Coll. Cardiol. 1993, 22, 1193–1198. [Google Scholar] [CrossRef]
- Ibrahim, N.K.; Desai, N.; Legha, S.; Soon-Shiong, P.; Theriault, R.L.; Rivera, E.; Esmaeli, B.; Ring, S.E.; Bedikian, A.; Hortobagyi, G.N.; et al. Phase I and Pharmacokinetic Study of ABI-007, a Cremophor-Free, Protein-Stabilized, Nanoparticle Formulation of Paclitaxel 1. Clin. Cancer Res. 2002, 8, 1038–1044. [Google Scholar] [PubMed]
- Simões, S.; Slepushkin, V.; Pires, P.; Gaspar, R.; Pedroso De Lima, M.C.; Düzgüneş, N. Human Serum Albumin Enhances DNA Transfection by Lipoplexes and Confers Resistance to Inhibition by Serum. Biochim. Biophys. Acta 2000, 1463, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Brzoska, M.; Langer, K.; Coester, C.; Loitsch, S.; Wagner, T.O.F.; Mallinckrodt, C.V. Incorporation of Biodegradable Nanoparticles into Human Airway Epithelium Cells—In Vitro Study of the Suitability as a Vehicle for Drug or Gene Delivery in Pulmonary Diseases. Biochem. Biophys. Res. Commun. 2004, 318, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J. Albumin-Bound Paclitaxel: A next-Generation Taxane. Expert Opin. Pharmacother. 2006, 7, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein Nanoparticles as Drug Carriers in Clinical Medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Coester, C.; Nayyar, P.; Samuel, J. In Vitro Uptake of Gelatin Nanoparticles by Murine Dendritic Cells and Their Intracellular Localisation. Eur. J. Pharm. Biopharm. 2006, 62, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Raymond, G.; Degennaro, M.; Mikeal, R. Preparation of Gelatin: Phenytoin Sodium Microsphers: An IN VITRO and IN VIVO Evaluation. Drug Dev. Ind. Pharm. 1990, 16, 1025–1051. [Google Scholar] [CrossRef]
- Carvalho, J.A.; Abreu, A.S.; Ferreira, V.T.P.; Gonçalves, E.P.; Tedesco, A.C.; Pinto, J.G.; Ferreira-Strixino, J.; Beltrame Junior, M.; Simioni, A.R. Preparation of Gelatin Nanoparticles by Two Step Desolvation Method for Application in Photodynamic Therapy. J. Biomater. Sci. Polym. Ed. 2018, 29, 1287–1301. [Google Scholar] [CrossRef]
- Varnamkhasti, B.S.; Hosseinzadeh, H.; Azhdarzadeh, M.; Vafaei, S.Y.; Esfandyari-Manesh, M.; Mirzaie, Z.H.; Amini, M.; Ostad, S.N.; Atyabi, F.; Dinarvand, R. Protein Corona Hampers Targeting Potential of MUC1 Aptamer Functionalized SN-38 Core-Shell Nanoparticles. Int. J. Pharm. 2015, 494, 430–444. [Google Scholar] [CrossRef]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-Functionalized Nanoparticles Lose Their Targeting Capabilities When a Biomolecule Corona Adsorbs on the Surface. Nat. Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef]
- Lu, Z.; Yeh, T.K.; Tsai, M.; Au, J.L.S.; Wientjes, M.G. Paclitaxel-Loaded Gelatin Nanoparticles for Intravesical Bladder Cancer Therapy. Clin. Cancer Res. 2004, 10, 7677–7684. [Google Scholar] [CrossRef]
- Narayanan, D.; Geena, M.G.; Lakshmi, H.; Koyakutty, M.; Nair, S.; Menon, D. Poly-(Ethylene Glycol) Modified Gelatin Nanoparticles for Sustained Delivery of the Anti-Inflammatory Drug Ibuprofen-Sodium: An in Vitro and in Vivo Analysis. Nanomedicine 2013, 9, 818–828. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Choubey, J. Design of Gelatin Nanoparticles as Swelling Controlled Delivery System for Chloroquine Phosphate. J. Mater. Sci. Mater. Med. 2006, 17, 345–358. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Zhang, F.; Yu, S. Solid-Film Sampling Method for the Determination of Protein Secondary Structure by Fourier Transform Infrared Spectroscopy. Anal. Bioanal. Chem. 2017, 409, 4459–4465. [Google Scholar] [CrossRef] [PubMed]
- Pederzoli, F.; Tosi, G.; Vandelli, M.A.; Belletti, D.; Forni, F.; Ruozi, B. Protein Corona and Nanoparticles: How Can We Investigate On? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1467. [Google Scholar] [CrossRef]
- Yang, H.; Wang, M.; Zhang, Y.; Liu, X.; Yu, S.; Guo, Y.; Yang, S.; Yang, L. Detailed Insight into the Formation of Protein Corona: Conformational Change, Stability and Aggregation. Int. J. Biol. Macromol. 2019, 135, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Amenabar, I.; Poly, S.; Nuansing, W.; Hubrich, E.H.; Govyadinov, A.A.; Huth, F.; Krutokhvostov, R.; Zhang, L.; Knez, M.; Heberle, J.; et al. Structural Analysis and Mapping of Individual Protein Complexes by Infrared Nanospectroscopy. Nat. Commun. 2013, 4, 2890. [Google Scholar] [CrossRef] [PubMed]
- Głąb, T.K.; Boratyński, J. Potential of Casein as a Carrier for Biologically Active Agents. Top. Curr. Chem. 2017, 375, 71. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Abo El-Fotoh, W.S.; Elgindy, N.A. Casein-Based Formulations as Promising Controlled Release Drug Delivery Systems. J. Control. Release 2011, 153, 206–216. [Google Scholar] [CrossRef]
- Singh, H.; Ye, A.; Thompson, A. Nanoencapsulation Systems Based on Milk Proteins and Phospholipids. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2009; Volume 1007, pp. 131–142. [Google Scholar]
- Nakagawa, K.; Kagemoto, M. Characterization of Casein-Based Nanoparticles Formed upon Freezing by in Situ SAXS Measurement. Colloids Surf. B Biointerfaces 2013, 103, 366–374. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural Biodegradable Polymers Based Nano-Formulations for Drug Delivery: A Review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Elgohary, M.M.; Kamel, N.M. Implications of Protein- and Peptide-Based Nanoparticles as Potential Vehicles for Anticancer Drugs. In Advances in Protein Chemistry and Structural Biology; Academic Press Inc.: Cambridge, MA, USA, 2015; Volume 98, pp. 169–221. [Google Scholar]
- Shapira, A.; Markman, G.; Assaraf, Y.G.; Livney, Y.D. β-Casein-Based Nanovehicles for Oral Delivery of Chemotherapeutic Drugs: Drug-Protein Interactions and Mitoxantrone Loading Capacity. Nanomedicine 2010, 6, 547–555. [Google Scholar] [CrossRef]
- Ma, J.; Xu, Q.; Zhou, J.; Zhang, J.; Zhang, L.; Tang, H.; Chen, L. Synthesis and Biological Response of Casein-Based Silica Nano-Composite Film for Drug Delivery System. Colloids Surf. B Biointerfaces 2013, 111, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Zimet, P.; Rosenberg, D.; Livney, Y.D. Re-Assembled Casein Micelles and Casein Nanoparticles as Nano-Vehicles for ω-3 Polyunsaturated Fatty Acids. Food Hydrocoll. 2011, 25, 1270–1276. [Google Scholar] [CrossRef]
- Gülseren, I.; Fang, Y.; Corredig, M. Whey Protein Nanoparticles Prepared with Desolvation with Ethanol: Characterization, Thermal Stability and Interfacial Behavior. Food Hydrocoll. 2012, 29, 258–264. [Google Scholar] [CrossRef]
- Karav, S.; German, J.B.; Rouquié, C.; Le Parc, A.; Barile, D. Studying Lactoferrin N-Glycosylation. Int. J. Mol. Sci. 2017, 18, 870. [Google Scholar] [CrossRef] [PubMed]
- Bolat, E.; Eker, F.; Kaplan, M.; Duman, H.; Arslan, A.; Saritaş, S.; Şahutoğlu, A.S.; Karav, S. Lactoferrin for COVID-19 Prevention, Treatment, and Recovery. Front. Nutr. 2022, 9, 992733. [Google Scholar] [CrossRef] [PubMed]
- Duman, H.; Karav, S. Bovine Colostrum and Its Potential Contributions for Treatment and Prevention of COVID-19. Front. Immunol. 2023, 14, 1214514. [Google Scholar] [CrossRef] [PubMed]
- Eker, F.; Bolat, E.; Pekdemir, B.; Duman, H.; Karav, S. Lactoferrin: Neuroprotection against Parkinson’s Disease and Secondary Molecule for Potential Treatment. Front. Aging Neurosci. 2023, 15, 1204149. [Google Scholar] [CrossRef]
- Karav, S. Selective Deglycosylation of Lactoferrin to Understand Glycans’ Contribution to Antimicrobial Activity of Lactoferrin. Cell. Mol. Biol. 2018, 64, 52–57. [Google Scholar] [CrossRef]
- Kondapi, A.K. Targeting Cancer with Lactoferrin Nanoparticles: Recent Advances. Nanomedicine 2020, 15, 2071–2083. [Google Scholar] [CrossRef]
- Duarte, L.G.R.; Alencar, W.M.P.; Iacuzio, R.; Silva, N.C.C.; Picone, C.S.F. Synthesis, Characterization and Application of Antibacterial Lactoferrin Nanoparticles. Curr. Res. Food Sci. 2022, 5, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, C.; Liu, X.; Lin, Z.; Yang, N.; Yu, S. Probing the Mechanism of Plasma Protein Adsorption on Au and Ag Nanoparticles with FT-IR Spectroscopy. Nanoscale 2015, 7, 15191–15196. [Google Scholar] [CrossRef]
- Ghalandari, B.; Divsalar, A.; Saboury, A.A.; Parivar, K. β-Lactoglobulin Nanoparticle as a Chemotherapy Agent Carrier for Oral Drug Delivery System. J. Iran. Chem. Soc. 2015, 12, 613–619. [Google Scholar] [CrossRef]
- Arroyo-Maya, I.J.; Rodiles-López, J.O.; Cornejo-Mazón, M.; Gutiérrez-López, G.F.; Hernández-Arana, A.; Toledo-Núñez, C.; Barbosa-Cánovas, G.V.; Flores-Flores, J.O.; Hernández-Sánchez, H. Effect of Different Treatments on the Ability of α-Lactalbumin to Form Nanoparticles. J. Dairy Sci. 2012, 95, 6204–6214. [Google Scholar] [CrossRef] [PubMed]
- Serpooshan, V.; Mahmoudi, M.; Zhao, M.; Wei, K.; Sivanesan, S.; Motamedchaboki, K.; Malkovskiy, A.V.; Goldstone, A.B.; Cohen, J.E.; Yang, P.C.; et al. Protein Corona Influences Cell-Biomaterial Interactions in Nanostructured Tissue Engineering Scaffolds. Adv. Funct. Mater. 2015, 25, 4379–4389. [Google Scholar] [CrossRef] [PubMed]
- Durowoju, I.B.; Bhandal, K.S.; Hu, J.; Carpick, B.; Kirkitadze, M. Differential Scanning Calorimetry—A Method for Assessing the Thermal Stability and Conformation of Protein Antigen. J. Vis. Exp. 2017, 2017, e55262. [Google Scholar] [CrossRef]
- Goy-López, S.; Juárez, J.; Alatorre-Meda, M.; Casals, E.; Puntes, V.F.; Taboada, P.; Mosquera, V. Physicochemical Characteristics of Protein-NP Bioconjugates: The Role of Particle Curvature and Solution Conditions on Human Serum Albumin Conformation and Fibrillogenesis Inhibition. Langmuir 2012, 28, 9113–9126. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Potential of Plant Proteins for Medical Applications. Trends Biotechnol. 2011, 29, 490–498. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking Biopolymers for Biomedical Applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Kianfar, E. Protein Nanoparticles in Drug Delivery: Animal Protein, Plant Proteins and Protein Cages, Albumin Nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Xu, X.; Tang, W.; Xiong, L.; Sun, Q. Formation of Protein Corona on Nanoparticles with Digestive Enzymes in Simulated Gastrointestinal Fluids. J. Agric. Food Chem. 2019, 67, 2296–2306. [Google Scholar] [CrossRef] [PubMed]
- Clemments, A.M.; Botella, P.; Landry, C.C. Protein Adsorption from Biofluids on Silica Nanoparticles: Corona Analysis as a Function of Particle Diameter and Porosity. ACS Appl. Mater. Interfaces 2015, 7, 21682–21689. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Dasari, M.; Priyamvada, S.; Kotcherlakota, R.; Bollu, V.S.; Patra, C.R. A Green Chemistry Approach for the Synthesis of Gold Nanoconjugates That Induce the Inhibition of Cancer Cell Proliferation through Induction of Oxidative Stress and Their in Vivo Toxicity Study. J. Mater. Chem. B 2015, 3, 3820–3830. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Huang, M.; Wang, J.; Sun, B. Biopolyelectrolyte Complex (BioPEC)-Based Carriers for Anthocyanin Delivery. Food Hydrocoll. Health 2021, 1, 100037. [Google Scholar] [CrossRef]
- Yuan, D.; Zhou, F.; Shen, P.; Zhang, Y.; Lin, L.; Zhao, M. Self-Assembled Soy Protein Nanoparticles by Partial Enzymatic Hydrolysis for PH-Driven Encapsulation and Delivery of Hydrophobic Cargo Curcumin. Food Hydrocoll. 2021, 120, 106759. [Google Scholar] [CrossRef]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green Biopolymers from By-Products as Wall Materials for Spray Drying Microencapsulation of Phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Hadidi, M.; Boostani, S.; Jafari, S.M. Pea Proteins as Emerging Biopolymers for the Emulsification and Encapsulation of Food Bioactives. Food Hydrocoll. 2022, 126, 107474. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Du, F. Potential Use of Peanut By-Products in Food Processing: A Review. J. Food Sci. Technol. 2012, 49, 521–529. [Google Scholar] [CrossRef]
- Ning, F.; Ge, Z.; Qiu, L.; Wang, X.; Luo, L.; Xiong, H.; Huang, Q. Double-Induced Se-Enriched Peanut Protein Nanoparticles Preparation, Characterization and Stabilized Food-Grade Pickering Emulsions. Food Hydrocoll. 2020, 99, 105308. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.X.; Zhang, C.; Dai, C.; Ju, X.; He, R. Fabrication of Stable and Self-Assembling Rapeseed Protein Nanogel for Hydrophobic Curcumin Delivery. J. Agric. Food Chem. 2019, 67, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.K.; Banerjee, P.; Chatterjee, R.; Dhar, P. Designing of ω-3 PUFA Enriched Biocompatible Nanoemulsion with Sesame Protein Isolate as a Natural Surfactant: Focus on Enhanced Shelf-Life Stability and Biocompatibility. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 36–44. [Google Scholar] [CrossRef]
- Azizi, S.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Microencapsulation of Lactobacillus rhamnosus Using Sesame Protein Isolate: Effect of Encapsulation Method and Transglutaminase. Food Biosci. 2021, 41, 101012. [Google Scholar] [CrossRef]
- Berndtsson, E.; Andersson, R.; Johansson, E.; Olsson, M.E. Side Streams of Broccoli Leaves: A Climate Smart and Healthy Food Ingredient. Int. J. Environ. Res. Public Health 2020, 17, 2406. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zha, X.Q.; Li, Q.M.; Pan, L.H.; Luo, J.P. Hydrophobic Interaction and Hydrogen Bonding Driving the Self-Assembling of Quinoa Protein and Flavonoids. Food Hydrocoll. 2021, 118, 106807. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, H.L.; Pan, L.H.; Li, Q.M.; Luo, J.P.; Zha, X.Q. The Nanomicelles Consisting of Lotus Root Amylopectin and Quinoa Protein: Construction and Encapsulation for Quercetin. Food Chem. 2022, 387, 132924. [Google Scholar] [CrossRef] [PubMed]
- Waglay, A.; Karboune, S.; Alli, I. Potato Protein Isolates: Recovery and Characterization of Their Properties. Food Chem. 2014, 142, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Edelman, R.; Engelberg, S.; Fahoum, L.; Meyron-Holtz, E.G.; Livney, Y.D. Potato Protein- Based Carriers for Enhancing Bioavailability of Astaxanthin. Food Hydrocoll. 2019, 96, 72–80. [Google Scholar] [CrossRef]
- Altammar, K.A. A Review on Nanoparticles: Characteristics, Synthesis, Applications, and Challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K. Potential Applications of Engineered Nanoparticles in Medicine and Biology: An Update. J. Biol. Inorg. Chem. 2018, 23, 1185–1204. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic Nanoparticles and Composite Films for Antimicrobial Therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial Activity of Silver Nanoparticles: A Surface Science Insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Silver Nanoparticles as an Antimicrobial Agent: A Case Study on Staphylococcus Aureus and Escherichia Coli as Models for Gram-Positive and Gram-Negative Bacteria. J. Gen. Appl. Microbiol. 2017, 63, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shen, C.; Ji, Q.; An, H.; Wang, J.; Liu, Q.; Zhang, Z. Food Storage Material Silver Nanoparticles Interfere with DNA Replication Fidelity and Bind with DNA. Nanotechnology 2009, 20, 085102. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, J.Y.; Kim, J.; Lee, J.H.; Hahn, J.S.; Gu, M.B.; Yoon, J. Silver-Ion-Mediated Reactive Oxygen Species Generation Affecting Bactericidal Activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y. Ben Antibacterial Activity and Mechanism of Silver Nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Vu, X.H.; Duong, T.T.T.; Pham, T.T.H.; Trinh, D.K.; Nguyen, X.H.; Dang, V.S. Synthesis and Study of Silver Nanoparticles for Antibacterial Activity against Escherichia Coli and Staphylococcus Aureus. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025019. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Yin, J.; Yang, J.; Li, Q.; Zheng, W.; Liu, S.; Jiang, X. The Density of Surface Coating Can Contribute to Different Antibacterial Activities of Gold Nanoparticles. Nano Lett. 2020, 20, 5036–5042. [Google Scholar] [CrossRef]
- Sathiyaraj, S.; Suriyakala, G.; Dhanesh Gandhi, A.; Babujanarthanam, R.; Almaary, K.S.; Chen, T.W.; Kaviyarasu, K. Biosynthesis, Characterization, and Antibacterial Activity of Gold Nanoparticles. J. Infect. Public Health 2021, 14, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Shah, Z.H.; Riaz, S.; Ahmad, N.; Islam, S.; Raza, M.A.; Naseem, S. Antimicrobial Activity of Citric Acid Functionalized Iron Oxide Nanoparticles—Superparamagnetic Effect. Ceram. Int. 2020, 46, 10942–10951. [Google Scholar] [CrossRef]
- Jangra, S.L.; Stalin, K.; Dilbaghi, N.; Kumar, S.; Tawale, J.; Singh, S.P.; Pasricha, R. Antimicrobial Activity of Zirconia (ZrO2) Nanoparticles and Zirconium Complexes. J. Nanosci. Nanotechnol. 2012, 12, 7105–7112. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Shaik, M.R.; Khan, S.T.; Adil, S.F.; Kuniyil, M.; Khan, M.; Al-Warthan, A.A.; Siddiqui, M.R.H.; Nawaz Tahir, M. Enhanced Antimicrobial Activity of Biofunctionalized Zirconia Nanoparticles. ACS Omega 2020, 5, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.P.; Kandasamy, S.; Chinnathambi, A.; Alahmadi, T.A.; Brindhadevi, K. Synthesis of Zirconia Nanoparticles Using Laurus Nobilis for Use as an Antimicrobial Agent. Appl. Nanosci. 2023, 13, 1337–1344. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. Inorganic Nanoparticles for Targeted Drug Delivery. In Biointegration of Medical Implant Materials; Woodhead Publishing: Cambridge, UK, 2020; pp. 333–373. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent Biomedical Applications of Gold Nanoparticles: A Review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Nasrolahi Shirazi, A.; Mandal, D.; Tiwari, R.K.; Guo, L.; Lu, W.; Parang, K. Cyclic Peptide-Capped Gold Nanoparticles as Drug Delivery Systems. Mol. Pharm. 2013, 10, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Ocampo, S.M.; Simões, B.M.; Josefa Rodríguez, M.; Cadenas, J.F.; Couleaud, P.; Spence, K.; Latorre, A.; Miranda, R.; Somoza, Á.; et al. Multifunctionalized Iron Oxide Nanoparticles for Selective Drug Delivery to CD44-Positive Cancer Cells. Nanotechnology 2016, 27, 065103. [Google Scholar] [CrossRef] [PubMed]
- Nigam, A.; Pawar, S.J. Synthesis and Characterization of ZnO Nanoparticles to Optimize Drug Loading and Release Profile for Drug Delivery Applications. Mater. Today Proc. 2019, 26, 2625–2628. [Google Scholar] [CrossRef]
- Din, I.U.; Khan, I.S.; Gul, I.H.; Hussain, Z.; Miran, W.; Javaid, F.; Liaqat, U. Novel Cytotoxicity Study of Strontium (Sr) Doped Iron Oxide (Fe3O4) Nanoparticles Aided with Ibuprofen for Drug Delivery Applications. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 189–205. [Google Scholar] [CrossRef]
- Ebadi, M.; Rifqi Md Zain, A.; Tengku Abdul Aziz, T.H.; Mohammadi, H.; Tee, C.A.T.; Rahimi Yusop, M. Formulation and Characterization of Fe3O4@PEG Nanoparticles Loaded Sorafenib; Molecular Studies and Evaluation of Cytotoxicity in Liver Cancer Cell Lines. Polymers 2023, 15, 971. [Google Scholar] [CrossRef]
- Ehteshamzadeh, T.; Kakaei, S.; Ghaffari, M.; Khanchi, A.R. Doxorubicin Embedded Polyvinylpyrrolidone-Coated Fe3O4 Nanoparticles for Targeted Drug Delivery System. J. Supercond. Nov. Magn. 2021, 34, 3345–3360. [Google Scholar] [CrossRef]
- Dutta, B.; Checker, S.; Barick, K.C.; Salunke, H.G.; Gota, V.; Hassan, P.A. Malic Acid Grafted Fe3O4 Nanoparticles for Controlled Drug Delivery and Efficient Heating Source for Hyperthermia Therapy. J. Alloys Compd. 2021, 883, 160950. [Google Scholar] [CrossRef]
- Danafar, H.; Baghdadchi, Y.; Barsbay, M.; Ghaffarlou, M.; Mousazadeh, N.; Mohammadi, A. Synthesis of Fe3O4-Gold Hybrid Nanoparticles Coated by Bovine Serum Albumin as a Contrast Agent in MR Imaging. Heliyon 2023, 9, e13874. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Wang, C.; An, L.; Lin, J.; Tian, Q.; Yang, S. Ultrasmall Fe@Fe3O4 Nanoparticles as T1–T2 Dual-Mode MRI Contrast Agents for Targeted Tumor Imaging. Nanomedicine 2021, 32, 102335. [Google Scholar] [CrossRef] [PubMed]
- Das, R.S.; Maiti, D.; Kar, S.; Bera, T.; Mukherjee, A.; Saha, P.C.; Mondal, A.; Guha, S. Design of Water-Soluble Rotaxane-Capped Superparamagnetic, Ultrasmall Fe3O4 Nanoparticles for Targeted NIR Fluorescence Imaging in Combination with Magnetic Resonance Imaging. J. Am. Chem. Soc. 2023, 145, 20451–20461. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Jin, H.; Huang, X.; Shi, Y.; Liu, Z.; Ben, S. PPy@Fe3O4 Nanoparticles Inhibit Tumor Growth and Metastasis Through Chemodynamic and Photothermal Therapy in Non-Small Cell Lung Cancer. Front. Chem. 2021, 9, 789934. [Google Scholar] [CrossRef] [PubMed]
- Pazouki, N.; Irani, S.; Olov, N.; Atyabi, S.M.; Bagheri-Khoulenjani, S. Fe3O4 Nanoparticles Coated with Carboxymethyl Chitosan Containing Curcumin in Combination with Hyperthermia Induced Apoptosis in Breast Cancer Cells. Prog. Biomater. 2022, 11, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Vahabi, L.; Rashidi Ranjbar, P.; Davar, F. Cladosporium Protease/Doxorubicin Decorated Fe3O4@SiO2 Nanocomposite: An Efficient Nanoparticle for Drug Delivery and Combating Breast Cancer. J. Drug Deliv. Sci. Technol. 2023, 80, 104144. [Google Scholar] [CrossRef]
- Kirdat, P.N.; Dandge, P.B.; Hagwane, R.M.; Nikam, A.S.; Mahadik, S.P.; Jirange, S.T. Synthesis and Characterization of Ginger (z. Officinale) Extract Mediated Iron Oxide Nanoparticles and Its Antibacterial Activity. Mater. Today Proc. 2020, 43, 2826–2831. [Google Scholar] [CrossRef]
- Zakariya, N.A.; Majeed, S.; Jusof, W.H.W. Investigation of Antioxidant and Antibacterial Activity of Iron Oxide Nanoparticles (IONPS) Synthesized from the Aqueous Extract of Penicillium spp. Sens. Int. 2022, 3, 100164. [Google Scholar] [CrossRef]
- Nie, L.; Chang, P.; Ji, C.; Zhang, F.; Zhou, Q.; Sun, M.; Sun, Y.; Politis, C.; Shavandi, A. Poly(Acrylic Acid) Capped Iron Oxide Nanoparticles via Ligand Exchange with Antibacterial Properties for Biofilm Applications. Colloids Surf. B Biointerfaces 2021, 197, 111385. [Google Scholar] [CrossRef] [PubMed]
- Ramezani Farani, M.; Azarian, M.; Heydari Sheikh Hossein, H.; Abdolvahabi, Z.; Mohammadi Abgarmi, Z.; Moradi, A.; Mousavi, S.M.; Ashrafizadeh, M.; Makvandi, P.; Saeb, M.R.; et al. Folic Acid-Adorned Curcumin-Loaded Iron Oxide Nanoparticles for Cervical Cancer. ACS Appl. Bio Mater. 2022, 5, 1305–1318. [Google Scholar] [CrossRef]
- Ebadi, M.; Buskaran, K.; Bullo, S.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Drug Delivery System Based on Magnetic Iron Oxide Nanoparticles Coated with (Polyvinyl Alcohol-Zinc/Aluminium-Layered Double Hydroxide-Sorafenib). Alex. Eng. J. 2021, 60, 733–747. [Google Scholar] [CrossRef]
- Vasiliev, G.; Kubo, A.L.; Vija, H.; Kahru, A.; Bondar, D.; Karpichev, Y.; Bondarenko, O. Synergistic Antibacterial Effect of Copper and Silver Nanoparticles and Their Mechanism of Action. Sci. Rep. 2023, 13, 9202. [Google Scholar] [CrossRef] [PubMed]
- Bhuyar, P.; Rahim, M.H.A.; Sundararaju, S.; Ramaraj, R.; Maniam, G.P.; Govindan, N. Synthesis of Silver Nanoparticles Using Marine Macroalgae Padina sp. and Its Antibacterial Activity towards Pathogenic Bacteria. Beni Suef Univ. J. Basic Appl. Sci. 2020, 9, 3. [Google Scholar] [CrossRef]
- Balachandar, R.; Navaneethan, R.; Biruntha, M.; Ashok Kumar, K.K.; Govarthanan, M.; Karmegam, N. Antibacterial Activity of Silver Nanoparticles Phytosynthesized from Glochidion candolleanum Leaves. Mater. Lett. 2022, 311, 131572. [Google Scholar] [CrossRef]
- Romdoni, Y.; Kadja, G.T.M.; Kitamoto, Y.; Khalil, M. Synthesis of Multifunctional Fe3O4@SiO2-Ag Nanocomposite for Antibacterial and Anticancer Drug Delivery. Appl. Surf. Sci. 2023, 610, 155610. [Google Scholar] [CrossRef]
- Nikolova, S.; Milusheva, M.; Gledacheva, V.; Feizi-Dehnayebi, M.; Kaynarova, L.; Georgieva, D.; Delchev, V.; Stefanova, I.; Tumbarski, Y.; Mihaylova, R.; et al. Drug-Delivery Silver Nanoparticles: A New Perspective for Phenindione as an Anticoagulant. Biomedicines 2023, 11, 2201. [Google Scholar] [CrossRef]
- Khashan, A.A.; Dawood, Y.; Khalaf, Y.H. Green Chemistry and Anti-Inflammatory Activity of Silver Nanoparticles Using an Aqueous Curcumin Extract. Results Chem. 2023, 5, 100913. [Google Scholar] [CrossRef]
- Chirumamilla, P.; Vankudoth, S.; Dharavath, S.B.; Dasari, R.; Taduri, S. In Vitro Anti-Inflammatory Activity of Green Synthesized Silver Nanoparticles and Leaf Methanolic Extract of Solanum Khasianum Clarke. Proc. Natl. Acad. Sci. India Sect. B—Biol. Sci. 2022, 92, 301–307. [Google Scholar] [CrossRef]
- Gomathi, A.C.; Xavier Rajarathinam, S.R.; Mohammed Sadiq, A.; Rajeshkumar, S. Anticancer Activity of Silver Nanoparticles Synthesized Using Aqueous Fruit Shell Extract of Tamarindus Indica on MCF-7 Human Breast Cancer Cell Line. J. Drug Deliv. Sci. Technol. 2020, 55, 101376. [Google Scholar] [CrossRef]
- Shyamalagowri, S.; Charles, P.; Manjunathan, J.; Kamaraj, M.; Anitha, R.; Pugazhendhi, A. In Vitro Anticancer Activity of Silver Nanoparticles Phyto-Fabricated by Hylocereus Undatus Peel Extracts on Human Liver Carcinoma (HepG2) Cell Lines. Process Biochem. 2022, 116, 17–25. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, N.; Kaushal, N. Utilization of Novel Bacteriocin Synthesized Silver Nanoparticles (AgNPs) for Their Application in Antimicrobial Packaging for Preservation of Tomato Fruit. Front. Sustain. Food Syst. 2023, 7, 1072738. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Starch/Agar-Based Functional Films Integrated with Enoki Mushroom-Mediated Silver Nanoparticles for Active Packaging Applications. Food Biosci. 2022, 49, 101867. [Google Scholar] [CrossRef]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennise, T.T.; Wacławek, S.; Cerník, M.; Padil, V.V.T. Chitosan/Gelatin/Silver Nanoparticles Composites Films for Biodegradable Food Packaging Applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.U.; Khan, H.; Liaqat, W.; Zeb, M.A. Phytochemical Screening, Green Synthesis of Gold Nanoparticles, and Antibacterial Activity Using Seeds Extract of Ricinus communis L. Microsc. Res. Tech. 2022, 85, 202–208. [Google Scholar] [CrossRef]
- Vinayagam, R.; Santhoshkumar, M.; Lee, K.E.; David, E.; Kang, S.G. Bioengineered Gold Nanoparticles Using Cynodon Dactylon Extract and Its Cytotoxicity and Antibacterial Activities. Bioprocess Biosyst. Eng. 2021, 44, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.A.M.; Grinholc, M.; Dena, A.S.A.; El-Sherbiny, I.M.; Megahed, M. Boosting the Antibacterial Activity of Chitosan–Gold Nanoparticles against Antibiotic–Resistant Bacteria by Punicagranatum L. Extract. Carbohydr. Polym. 2021, 256, 117498. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ardjmand, M.; Rad, A.S.; Esfahani, M.R. Gelatin-Coated Gold Nanoparticles as an Effective PH-Sensitive Methotrexate Drug Delivery System for Breast Cancer Treatment. Mater. Today Chem. 2021, 20, 100474. [Google Scholar] [CrossRef]
- Hassanen, E.I.; Korany, R.M.S.; Bakeer, A.M. Cisplatin-Conjugated Gold Nanoparticles-Based Drug Delivery System for Targeting Hepatic Tumors. J. Biochem. Mol. Toxicol. 2021, 35, e22722. [Google Scholar] [CrossRef] [PubMed]
- Căprărescu, S.; Modrogan, C.; Purcar, V.; Dăncilă, A.M.; Orbuleț, O.D. Study of Polyvinyl Alcohol-SiO2 Nanoparticles Polymeric Membrane in Wastewater Treatment Containing Zinc Ions. Polymers 2021, 13, 1875. [Google Scholar] [CrossRef] [PubMed]
- Al-Masoud, M.A.; Khalaf, M.M.; Mohamed, I.M.A.; Shalabi, K.; Abd El-Lateef, H.M. Computational, Kinetic, and Electrochemical Studies of Polyaniline Functionalized ZnO and ZnO-SiO2 Nanoparticles as Corrosion Protection Films on Carbon Steel in Acidic Sodium Chloride Solutions. J. Ind. Eng. Chem. 2022, 112, 398–422. [Google Scholar] [CrossRef]
- Salkhi Khasraghi, S.; Momenilandi, M.; Shojaei, A. Tire Tread Performance of Silica-Filled SBR/BR Rubber Composites Incorporated with Nanodiamond and Nanodiamond/Nano-SiO2 Hybrid Nanoparticle. Diam. Relat. Mater. 2022, 126, 109068. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Hossain, N.; Mostofa, M.G.; Mia, M.R.; Tushar, M.; Rana, M.M.; Hossain, M.H. Green Synthesis and Characterization of Zirconium Nanoparticlefor Dental Implant Applications. Heliyon 2023, 9, e12711. [Google Scholar] [CrossRef] [PubMed]
- Aati, S.; Akram, Z.; Ngo, H.; Fawzy, A.S. Development of 3D Printed Resin Reinforced with Modified ZrO2 Nanoparticles for Long-Term Provisional Dental Restorations. Dent. Mater. 2021, 37, e360–e374. [Google Scholar] [CrossRef]
- Kim, H.S.; Jang, W.; Im, Y.G.; Lim, H.P. Antibacterial Effect of Zirconia Nanoparticles on Polyethyl Methacrylate Resin for Provisional Crowns. Int. J. Nanomed. 2022, 17, 6551–6560. [Google Scholar] [CrossRef] [PubMed]
- Aati, S.; Shrestha, B.; Fawzy, A. Cytotoxicity and Antimicrobial Efficiency of ZrO2 Nanoparticles Reinforced 3D Printed Resins. Dent. Mater. 2022, 38, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez B, J.M.; Hincapié C, W.S.; de Andrade, V.M.; Conceição, K.; Trava-Airoldi, V.J.; Capote, G. Diamond-like Carbon Films Doped with ZrO2 Nanoparticles: Improving Antimicrobial Properties. Diam. Relat. Mater. 2023, 140, 110500. [Google Scholar] [CrossRef]
- Zhang, X.; Saravanakumar, K.; Sathiyaseelan, A.; Park, S.; Wang, M.H. Synthesis, Characterization, and Comparative Analysis of Antibiotics (Ampicillin and Erythromycin) Loaded ZrO2 Nanoparticles for Enhanced Antibacterial Activity. J. Drug Deliv. Sci. Technol. 2023, 82, 104293. [Google Scholar] [CrossRef]
- Hoque, M.; Sarkar, P.; Ahmed, J. Preparation and Characterization of Tamarind Kernel Powder/ZnO Nanoparticle-Based Food Packaging Films. Ind. Crop. Prod. 2022, 178, 114670. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Dutta, P.K. Chitosan Based ZnO Nanoparticles Loaded Gallic-Acid Films for Active Food Packaging. Food Chem. 2021, 334, 127605. [Google Scholar] [CrossRef] [PubMed]
- Gasti, T.; Dixit, S.; Hiremani, V.D.; Chougale, R.B.; Masti, S.P.; Vootla, S.K.; Mudigoudra, B.S. Chitosan/Pullulan Based Films Incorporated with Clove Essential Oil Loaded Chitosan-ZnO Hybrid Nanoparticles for Active Food Packaging. Carbohydr. Polym. 2022, 277, 118866. [Google Scholar] [CrossRef] [PubMed]
- El-Khawaga, A.M.; Elsayed, M.A.; Gobara, M.; Soliman, A.A.; Hashem, A.H.; Zaher, A.A.; Mohsen, M.; Salem, S.S. Green Synthesized ZnO Nanoparticles by Saccharomyces Cerevisiae and Their Antibacterial Activity and Photocatalytic Degradation. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- MuthuKathija, M.; Sheik Muhideen Badhusha, M.; Rama, V. Green Synthesis of Zinc Oxide Nanoparticles Using Pisonia Alba Leaf Extract and Its Antibacterial Activity. Appl. Surf. Sci. Adv. 2023, 15, 100400. [Google Scholar] [CrossRef]
- Porrawatkul, P.; Nuengmatcha, P.; Kuyyogsuy, A.; Pimsen, R.; Rattanaburi, P. Effect of Na and Al Doping on ZnO Nanoparticles for Potential Application in Sunscreens. J. Photochem. Photobiol. B 2023, 240, 112668. [Google Scholar] [CrossRef] [PubMed]
- Aljabali, A.A.A.; Obeid, M.A.; Bakshi, H.A.; Alshaer, W.; Ennab, R.M.; Al-Trad, B.; Khateeb, W.A.; Al-Batayneh, K.M.; Al-Kadash, A.; Alsotari, S.; et al. Synthesis, Characterization, and Assessment of Anti-Cancer Potential of ZnO Nanoparticles in an In Vitro Model of Breast Cancer. Molecules 2022, 27, 1827. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Shivyari, A.; Tafvizi, F.; Noorbazargan, H. Anti-Cancer Effects of Biosynthesized Zinc Oxide Nanoparticles Using Artemisia Scoparia in Huh-7 Liver Cancer Cells. Inorg. Nano-Met. Chem. 2022, 52, 375–386. [Google Scholar]
- Thomas, S.; Gunasangkaran, G.; Arumugam, V.A.; Muthukrishnan, S. Synthesis and Characterization of Zinc Oxide Nanoparticles of Solanum Nigrum and Its Anticancer Activity via the Induction of Apoptosis in Cervical Cancer. Biol. Trace Elem. Res. 2022, 200, 2684–2697. [Google Scholar] [CrossRef]
- Efati, Z.; Shahangian, S.S.; Darroudi, M.; Amiri, H.; Hashemy, S.I.; Aghamaali, M.R. Green Chemistry Synthesized Zinc Oxide Nanoparticles in Lepidium Sativum L. Seed Extract and Evaluation of Their Anticancer Activity in Human Colorectal Cancer Cells. Ceram. Int. 2023, 49, 32568–32576. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in Food Packaging: Biodegradability and Potential Migration to Food—A Review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.K. Prospects of Using Nanotechnology for Food Preservation, Safety, and Security. J. Food Drug. Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, Q.; Gao, Y.; Wan, S.; Meng, F.; Weng, W.; Zhang, Y. Characterization of Silver Nanoparticles Loaded Chitosan/Polyvinyl Alcohol Antibacterial Films for Food Packaging. Food Hydrocoll. 2023, 136, 108305. [Google Scholar] [CrossRef]
- Pandian, H.; Senthilkumar, K.; Ratnam M, V.; M, N.; S, S. Azadirachta indica Leaf Extract Mediated Silver Nanoparticles Impregnated Nano Composite Film (AgNP/MCC/Starch/Whey Protein) for Food Packaging Applications. Environ. Res. 2023, 216, 114641. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Teoh, Y.L.; Ong, K.M.; Rafflisman Zaidi, N.S.; Mah, S.K. Poly(Vinyl) Alcohol Crosslinked Composite Packaging Film Containing Gold Nanoparticles on Shelf Life Extension of Banana. Food Packag. Shelf Life 2020, 24, 100463. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Hasanin, M.; Hashem, A.H. Eco-Friendly Synthesis of Superhydrophobic Antimicrobial Film Based on Cellulose Acetate/Polycaprolactone Loaded with the Green Biosynthesized Copper Nanoparticles for Food Packaging Application. J. Polym. Environ. 2022, 30, 1820–1832. [Google Scholar] [CrossRef]
- Zhang, W.; Sani, M.A.; Zhang, Z.; McClements, D.J.; Jafari, S.M. High Performance Biopolymeric Packaging Films Containing Zinc Oxide Nanoparticles for Fresh Food Preservation: A Review. Int. J. Biol. Macromol. 2023, 230, 123188. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Zhou, S.; Zhang, R.; Wang, W.; Hou, H. Antimicrobial Starch/Poly(Butylene Adipate-Co-Terephthalate) Nanocomposite Films Loaded with a Combination of Silver and Zinc Oxide Nanoparticles for Food Packaging. Int. J. Biol. Macromol. 2022, 206, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; Abd El-Aziz, M.E.; Morsi, S.M.M. Development and Evaluation of Antimicrobial LDPE/TiO2 Nanocomposites for Food Packaging Applications. Polym. Bull. 2023, 80, 5417–5431. [Google Scholar] [CrossRef]
- Newman, M.D.; Stotland, M.; Ellis, J.I. The Safety of Nanosized Particles in Titanium Dioxide- and Zinc Oxide-Based Sunscreens. J. Am. Acad. Dermatol. 2009, 61, 685–692. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. A Review of Inorganic UV Filters Zinc Oxide and Titanium Dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of Titanium Dioxide Nanoparticles in Cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Simaiti, A.; Xu, M.; Lv, S.; Jiang, H.; He, X.; Fan, Y.; Zhu, S.; Du, B.; Yang, W.; et al. Antagonistic Skin Toxicity of Co-Exposure to Physical Sunscreen Ingredients Zinc Oxide and Titanium Dioxide Nanoparticles. Nanomaterials 2022, 12, 2769. [Google Scholar] [CrossRef] [PubMed]
- Ghamarpoor, R.; Fallah, A.; Jamshidi, M. Investigating the Use of Titanium Dioxide (TiO2) Nanoparticles on the Amount of Protection against UV Irradiation. Sci. Rep. 2023, 13, 9793. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wang, H.; Chen, Y.; Li, Y.; Zhang, J.; Zhang, C.; Lin, B.; Wei, X. Ball-Milling of Titanium Dioxide and Zinc Oxide for Enhanced UV Protection. Front. Mater. 2023, 10, 1273659. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Leret, P.; Álvarez-Docio, C.M.; Del Campo, A.; Fernández, J.F. Enhancement of UV Absorption Behavior in ZnO-TiO2 composites. Boletin de la Sociedad Espanola de Ceramica y Vidrio 2016, 55, 55–62. [Google Scholar] [CrossRef]
- Aldemir Dikici, B.; Claeyssens, F. Basic Principles of Emulsion Templating and Its Use as an Emerging Manufacturing Method of Tissue Engineering Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 875. [Google Scholar] [CrossRef]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef]
- Geng, B.; Li, P.; Fang, F.; Shi, W.; Glowacki, J.; Pan, D.; Shen, L. Antibacterial and Osteogenic Carbon Quantum Dots for Regeneration of Bone Defects Infected with Multidrug-Resistant Bacteria. Carbon 2021, 184, 375–385. [Google Scholar] [CrossRef]
- Rastegar, S.; Mehdikhani, M.; Bigham, A.; Poorazizi, E.; Rafienia, M. Poly Glycerol Sebacate/Polycaprolactone/Carbon Quantum Dots Fibrous Scaffold as a Multifunctional Platform for Cardiac Tissue Engineering. Mater. Chem. Phys. 2021, 266, 124543. [Google Scholar] [CrossRef]
- Shamekhi, M.A.; Mirzadeh, H.; Mahdavi, H.; Rabiee, A.; Mohebbi-Kalhori, D.; Baghaban Eslaminejad, M. Graphene Oxide Containing Chitosan Scaffolds for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2019, 127, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, C.; Zheng, B.; He, J.; Liu, J.; Chen, C.; Lee, I.S.; Wang, X.; Liu, Y. Synergistic Effects on Incorporation of β-Tricalcium Phosphate and Graphene Oxide Nanoparticles to Silk Fibroin/Soy Protein Isolate Scaffolds for Bone Tissue Engineering. Polymers 2020, 12, 69. [Google Scholar] [CrossRef]
- Saleem, J.; Wang, L.; Chen, C. Carbon-Based Nanomaterials for Cancer Therapy via Targeting Tumor Microenvironment. Adv. Healthc. Mater. 2018, 7, e1800525. [Google Scholar] [CrossRef] [PubMed]
- Beyaz, S.; Aslan, A.; Gok, O.; Uslu, H.; Agca, C.A.; Ozercan, I.H. In Vivo, In Vitro and In Silico Anticancer Investigation of Fullerene C60 on DMBA Induced Breast Cancer in Rats. Life Sci. 2022, 291, 120281. [Google Scholar] [CrossRef]
- Ma, Y.; Fu, S.; Tan, Y.; Zhang, A. Design and Synthesis of Highly Fluorescent and Stable Fullerene Nanoparticles as Probes for Folic Acid Detection and Targeted Cancer Cell Imaging. Nanotechnology 2021, 32, 195501. [Google Scholar] [CrossRef]
- Shi, J.; Wang, B.; Wang, L.; Lu, T.; Fu, Y.; Zhang, H.; Zhang, Z. Fullerene (C60)-Based Tumor-Targeting Nanoparticles with “off-on” State for Enhanced Treatment of Cancer. J. Control. Release 2016, 235, 245–258. [Google Scholar] [CrossRef]
- Elshater, A.E.A.; Haridy, M.A.M.; Salman, M.M.A.; Fayyad, A.S.; Hammad, S. Fullerene C60 Nanoparticles Ameliorated Cyclophosphamide-Induced Acute Hepatotoxicity in Rats. Biomed. Pharmacother. 2018, 97, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Naik, K.; Chaudhary, S.; Ye, L.; Parmar, A.S. A Strategic Review on Carbon Quantum Dots for Cancer-Diagnostics and Treatment. Front. Bioeng. Biotechnol. 2022, 10, 882100. [Google Scholar] [CrossRef]
- Lai, C.; Lin, S.; Huang, X.; Jin, Y. Synthesis and Properties of Carbon Quantum Dots and Their Research Progress in Cancer Treatment. Dye. Pigment. 2021, 196, 109766. [Google Scholar] [CrossRef]
- Cutrim, E.S.M.; Vale, A.A.M.; Manzani, D.; Barud, H.S.; Rodríguez-Castellón, E.; Santos, A.P.S.A.; Alcântara, A.C.S. Preparation, Characterization and in Vitro Anticancer Performance of Nanoconjugate Based on Carbon Quantum Dots and 5-Fluorouracil. Mater. Sci. Eng. C 2021, 120, 111781. [Google Scholar] [CrossRef]
- Saljoughi, H.; Khakbaz, F.; Mahani, M. Synthesis of Folic Acid Conjugated Photoluminescent Carbon Quantum Dots with Ultrahigh Quantum Yield for Targeted Cancer Cell Fluorescence Imaging. Photodiagnosis Photodyn. Ther. 2020, 30, 101687. [Google Scholar] [CrossRef] [PubMed]
- Jana, P.; Dev, A. Carbon Quantum Dots: A Promising Nanocarrier for Bioimaging and Drug Delivery in Cancer. Mater. Today Commun. 2022, 32, 104068. [Google Scholar] [CrossRef]
- Moore, M.N.; Sforzini, S.; Viarengo, A.; Barranger, A.; Aminot, Y.; Readman, J.W.; Khlobystov, A.N.; Arlt, V.M.; Banni, M.; Jha, A.N. Antagonistic Cytoprotective Effects of C60 Fullerene Nanoparticles in Simultaneous Exposure to Benzo[a]Pyrene in a Molluscan Animal Model. Sci. Total Environ. 2021, 755, 142355. [Google Scholar] [CrossRef] [PubMed]
- Namadr, F.; Shahyad, S.; Mohammadi, M.T. Fullerene C 60 Nanoparticles Potentiate the Antioxidant Defense System of the Brain and Liver by Increasing Catalase Activity in Normal Rats. Nov. Clin. Med. 2023, 2, 32–38. [Google Scholar] [CrossRef]
- Demir, E. Therapeutic Effect of Curcumin and C60 Fullerene against Hyperglycemia-Mediated Tissue Damage in Diabetic Rat Lungs. J. Bioenerg. Biomembr. 2021, 53, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Beyaz, S.; Aslan, A.; Gok, O.; Agca, C.A.; Ozercan, I.H. Fullerene C60 Attenuates Heart Tissue Inflammation by Modulating COX-2 and TNF-Alpha Signaling Pathways in DMBA Induced Breast Cancer in Rats. Cardiovasc. Toxicol. 2023, 23, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Beyaz, S.; Aslan, A.; Gok, O.; Ozercan, I.H.; Agca, C.A. C60 Nanoparticle Decrease the Inflammatory and Oxidative Responses in 7,12-Dimethylbenz[a]Anthracene (DMBA) Induced Rats Eye Tissue. Biol. Bull. 2023, 50, 790–800. [Google Scholar] [CrossRef]
- Borisenkova, A.A.; Bolshakova, O.I.; Titova, A.V.; Ryabokon, I.S.; Markova, M.A.; Lyutova, Z.B.; Sedov, V.P.; Varfolomeeva, E.Y.; Bakhmetyev, V.V.; Arutyunyan, A.V.; et al. Fullerene C60 Conjugate with Folic Acid and Polyvinylpyrrolidone for Targeted Delivery to Tumor Cells. Int. J. Mol. Sci. 2024, 25, 5350. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, J.; Mulder, R.J.; Ratcliffe, J.; Wang, C.; Wu, B.; Wang, J.; Hao, X. Highly Aqueously Stable C60-polymer Nanoparticles with Excellent Photodynamic Property for Potential Cancer Treatment. Smart Med. 2023, 2, e20230033. [Google Scholar] [CrossRef]
- Kaushal, S.; Pinnaka, A.K.; Soni, S.; Singhal, N.K. Antibody Assisted Graphene Oxide Coated Gold Nanoparticles for Rapid Bacterial Detection and near Infrared Light Enhanced Antibacterial Activity. Sens. Actuators B Chem. 2021, 329, 129141. [Google Scholar] [CrossRef]
- Menazea, A.A.; Ahmed, M.K. Synthesis and Antibacterial Activity of Graphene Oxide Decorated by Silver and Copper Oxide Nanoparticles. J. Mol. Struct. 2020, 1218, 128536. [Google Scholar] [CrossRef]
- Daniluk, K.; Lange, A.; Pruchniewski, M.; Małolepszy, A.; Sawosz, E.; Jaworski, S. Delivery of Melittin as a Lytic Agent via Graphene Nanoparticles as Carriers to Breast Cancer Cells. J. Funct. Biomater. 2022, 13, 278. [Google Scholar] [CrossRef]
- Kesavan, S.; Meena, K.S.; Sharmili, S.A.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Alobaidi, A.S.; Alanzi, K.F.; Vaseeharan, B. Ulvan Loaded Graphene Oxide Nanoparticle Fabricated with Chitosan and D-Mannose for Targeted Anticancer Drug Delivery. J. Drug Deliv. Sci. Technol. 2021, 65, 102760. [Google Scholar] [CrossRef]
- Mihanfar, A.; Targhazeh, N.; Sadighparvar, S.; Darband, S.G.; Majidinia, M.; Yousefi, B. Doxorubicin Loaded Magnetism Nanoparticles Based on Cyclodextrin Dendritic-Graphene Oxide Inhibited MCF-7 Cell Proliferation. Biomol. Concepts 2021, 12, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kim, T.H.; Cho, H.Y.; Luo, J.; Lee, J.M.; Chueng, S.T.D.; Hou, Y.; Yin, P.T.T.; Han, J.; Kim, J.H.; et al. Hybrid Graphene-Gold Nanoparticle-Based Nucleic Acid Conjugates for Cancer-Specific Multimodal Imaging and Combined Therapeutics. Adv. Funct. Mater. 2021, 31, 2006918. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Jothi, V.K.; Natarajan, A.; Rajaram, A.; Ravichandran, S.; Ramalingam, S. Green Synthesis of Copper Oxide Nanoparticles Decorated with Graphene Oxide for Anticancer Activity and Catalytic Applications. Arab. J. Chem. 2020, 13, 6802–6814. [Google Scholar] [CrossRef]
- Turaka, S.; Reddy, K.V.K.; Sahu, R.K.; Katiyar, J.K. Mechanical Properties of MWCNTs and Graphene Nanoparticles Modified Glass Fibre-Reinforced Polymer Nanocomposite. Bull. Mater. Sci. 2021, 44, 194. [Google Scholar] [CrossRef]
- Sagadevan, S.; Lett, J.A.; Weldegebrieal, G.K.; Dowla Biswas, M.R.U.; Oh, W.C.; Alshahateet, S.F.; Fatimah, I.; Mohammad, F.; Al-Lohedan, H.A.; Paiman, S.; et al. Enhanced Gas Sensing and Photocatalytic Activity of Reduced Graphene Oxide Loaded TiO2 Nanoparticles. Chem. Phys. Lett. 2021, 780, 138897. [Google Scholar] [CrossRef]
- Kabeel, A.E.; Sathyamurthy, R.; Manokar, A.M.; Sharshir, S.W.; Essa, F.A.; Elshiekh, A.H. Experimental Study on Tubular Solar Still Using Graphene Oxide Nano Particles in Phase Change Material (NPCM’s) for Fresh Water Production. J. Energy Storage 2020, 28, 101204. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.K.; Rahman, S.; Yadav, A.; Samykano, M.; Tyagi, V.V. Graphene–Silver Hybrid Nanoparticle Based Organic Phase Change Materials for Enhanced Thermal Energy Storage. Sustainability 2022, 14, 3240. [Google Scholar] [CrossRef]
- Kumar Sharma, S.; Kumar Saxena, K. An Outlook on the Influence on Mechanical Properties of AZ31 Reinforced with Graphene Nanoparticles Using Powder Metallurgy Technique for Biomedical Application. Mater. Today Proc. 2022, 56, 2278–2287. [Google Scholar] [CrossRef]
- Maleki-Ghaleh, H.; Siadati, M.H.; Fallah, A.; Koc, B.; Kavanlouei, M.; Khademi-Azandehi, P.; Moradpur-Tari, E.; Omidi, Y.; Barar, J.; Beygi-Khosrowshahi, Y.; et al. Antibacterial and Cellular Behaviors of Novel Zinc-Doped Hydroxyapatite/Graphene Nanocomposite for Bone Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 9564. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Collado, J.L.; García-San-Martín, N.; Molina-Mateo, J.; Torregrosa Cabanilles, C.; Donderis Quiles, V.; Serrano-Aroca, A.; Sabater i Serra, R. Electroactive Calcium-Alginate/Polycaprolactone/Reduced Graphene Oxide Nanohybrid Hydrogels for Skeletal Muscle Tissue Engineering. Colloids Surf. B Biointerfaces 2022, 214, 112455. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, N.; Al-Sarawi, S.; Losic, D.; Mazumdar, J.; Clark, J.; Gronthos, S.; O’Hare Doig, R. Biodegradable and Biocompatible Graphene-Based Scaffolds for Functional Neural Tissue Engineering: A Strategy Approach Using Dental Pulp Stem Cells and Biomaterials. Biotechnol. Bioeng. 2021, 118, 4217–4230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, T.; Zhao, H.; Zhu, G.; Li, F.; Guo, M.; Ran, Q.; Komarneni, S. An Electrochemical Sensor Modified with Novel Nanohybrid of Super-P Carbon Black@zeolitic-Imidazolate-Framework-8 for Sensitive Detection of Carbendazim. Ceram. Int. 2023, 49, 23775–23787. [Google Scholar] [CrossRef]
- Attaallah, R.; Antonacci, A.; Mazzaracchio, V.; Moscone, D.; Palleschi, G.; Arduini, F.; Amine, A.; Scognamiglio, V. Carbon Black Nanoparticles to Sense Algae Oxygen Evolution for Herbicides Detection: Atrazine as a Case Study. Biosens. Bioelectron. 2020, 159, 112203. [Google Scholar] [CrossRef] [PubMed]
- De Lima, G.E.S.; Nalon, G.H.; Santos, R.F.; Ribeiro, J.C.L.; De Carvalho, J.M.F.; Pedroti, L.G.; De Araújo, E.N.D. Microstructural Investigation of the Effects of Carbon Black Nanoparticles on Hydration Mechanisms, Mechanical and Piezoresistive Properties of Cement Mortars. Mater. Res. 2021, 24, e20200539. [Google Scholar] [CrossRef]
- Kuntharin, S.; Harnchana, V.; Klamchuen, A.; Sinthiptharakoon, K.; Thongbai, P.; Amornkitbamrung, V.; Chindaprasirt, P. Boosting the Power Output of a Cement-Based Triboelectric Nanogenerator by Enhancing Dielectric Polarization with Highly Dispersed Carbon Black Nanoparticles toward Large-Scale Energy Harvesting from Human Footsteps. ACS Sustain. Chem. Eng. 2022, 10, 4588–4598. [Google Scholar] [CrossRef]
- Lou, X.; Lu, B.; He, M.; Yu, Y.; Zhu, X.; Peng, F.; Qin, C.; Ding, M.; Jia, C. Functionalized Carbon Black Modified Sulfonated Polyether Ether Ketone Membrane for Highly Stable Vanadium Redox Flow Battery. J. Memb. Sci. 2022, 643, 120015. [Google Scholar] [CrossRef]
- Fan, C.; Dong, Y.; Liu, Y.; Zhang, L.; Wang, D.; Lin, X.; Lv, Y.; Zhang, S.; Song, H.; Jia, D. Mesopore-Dominated Hollow Carbon Nanoparticles Prepared by Simple Air Oxidation of Carbon Black for High Mass Loading Supercapacitors. Carbon 2020, 160, 328–334. [Google Scholar] [CrossRef]
- Janus, Ł.; Radwan-Pragłowska, J.; Piatkowski, M.; Bogdał, D. Facile Synthesis of Surface-Modified Carbon Quantum Dots (CQDs) for Biosensing and Bioimaging. Materials 2020, 13, 3313. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Liu, L.; Sun, T.; Hou, J.; Sun, Y.; Yu, M.; Diao, Y.; Lu, S.; Zhao, W.; Wang, L. Synthesis of Bifunctional Carbon Quantum Dots for Bioimaging and Anti-Inflammation. Nanotechnology 2020, 31, 175102. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.F.; Zheng, Y.Y.; Lee, S.J.; Chen, S.T.; Wu, C.H.; Hsieh, C.T.; Juang, R.S.; Peng, P.Z.; Hsueh, Y.H. N-Doped Carbon Quantum Dots as Fluorescent Bioimaging Agents. Crystals 2021, 11, 789. [Google Scholar] [CrossRef]
- Ratlam, C.; Phanichphant, S.; Sriwichai, S. Development of Dopamine Biosensor Based on Polyaniline/Carbon Quantum Dots Composite. J. Polym. Res. 2020, 27, 183. [Google Scholar] [CrossRef]
- Wu, W.; Huang, J.; Ding, L.; Lin, H.; Yu, S.; Yuan, F.; Liang, B. A Real-Time and Highly Sensitive Fiber Optic Biosensor Based on the Carbon Quantum Dots for Nitric Oxide Detection. J. Photochem. Photobiol. A Chem. 2021, 405, 112963. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, P.; Liu, T.; Pu, H.; Sun, D.W. A Fluorescence Biosensor Based on Single-Stranded DNA and Carbon Quantum Dots for Acrylamide Detection. Food Chem. 2021, 356, 129668. [Google Scholar] [CrossRef] [PubMed]
- Abazar, F.; Noorbakhsh, A. Chitosan-Carbon Quantum Dots as a New Platform for Highly Sensitive Insulin Impedimetric Aptasensor. Sens. Actuators B Chem. 2020, 304, 127281. [Google Scholar] [CrossRef]
- Gao, D.; Zhao, P.; Lyu, B.; Li, Y.; Hou, Y.; Ma, J. Carbon Quantum Dots Decorated on ZnO Nanoparticles: An Efficient Visible-Light Responsive Antibacterial Agents. Appl. Organomet. Chem. 2020, 34, e5665. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Yu, L.; Wu, L.; Hao, X.; Liu, Q.; Lin, L.; Huang, Z.; Ruan, Z.; Weng, S.; et al. Quaternized Carbon Quantum Dots with Broad-Spectrum Antibacterial Activity for the Treatment of Wounds Infected with Mixed Bacteria. Acta Biomater. 2022, 138, 528–544. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Ni, B.J.; Klemeš, J.J.; Bokhari, A.; Hajiaghaei-Keshteli, M. Carbon Quantum Dots-Ag Nanoparticle Membrane for Preventing Emerging Contaminants in Oil Produced Water. J. Water Process Eng. 2022, 50, 103309. [Google Scholar] [CrossRef]
- Sheng, Y.; Dai, W.; Gao, J.; Li, H.; Tan, W.; Wang, J.; Deng, L.; Kong, Y. PH-Sensitive Drug Delivery Based on Chitosan Wrapped Graphene Quantum Dots with Enhanced Fluorescent Stability. Mater. Sci. Eng. C 2020, 112, 110888. [Google Scholar] [CrossRef] [PubMed]
- Zavareh, H.S.; Pourmadadi, M.; Moradi, A.; Yazdian, F.; Omidi, M. Chitosan/Carbon Quantum Dot/Aptamer Complex as a Potential Anticancer Drug Delivery System towards the Release of 5-Fluorouracil. Int. J. Biol. Macromol. 2020, 165, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhou, T.; Yuan, Y.; Natalie Kłodzińska, S.; Zheng, T.; Sternberg, C.; Mørck Nielsen, H.; Sun, Y.; Wan, F. Synthesis of Carbon Quantum Dot-Poly Lactic-Co-Glycolic Acid Hybrid Nanoparticles for Chemo-Photothermal Therapy against Bacterial Biofilms. J. Colloid Interface Sci. 2020, 577, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, A.; Hassan, M.; Mirabet, M.M.; Windhab, N.; Gomes, V.G. 3D-Printed Bioresorbable Poly(Lactic-Co-Glycolic Acid) and Quantum-Dot Nanocomposites: Scaffolds for Enhanced Bone Mineralization and Inbuilt Co-Monitoring. J. Biomed. Mater. Res. A 2022, 110, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Ren, Y.; Sun, X.; Jin, L.; Liu, X.; Chen, H.; Wang, K.; Yu, M.; Zhao, Y. Photoluminescent Functionalized Carbon Quantum Dots Loaded Electroactive Silk Fibroin/PLA Nanofibrous Bioactive Scaffolds for Cardiac Tissue Engineering. J. Photochem. Photobiol. B 2020, 202, 111680. [Google Scholar] [CrossRef] [PubMed]
- Praseetha, P.K.; Vibala, B.V.; Sreedevy, K.; Vijayakumar, S. Aloe-Vera Conjugated Natural Carbon Quantum Dots as Bio-Enhancers to Accelerate the Repair of Chronic Wounds. Ind. Crop. Prod. 2021, 174, 114152. [Google Scholar] [CrossRef]
- Wang, M.; Su, Y.; Liu, Y.; Liang, Y.; Wu, S.; Zhou, N.; Shen, J. Antibacterial Fluorescent Nano-Sized Lanthanum-Doped Carbon Quantum Dot Embedded Polyvinyl Alcohol for Accelerated Wound Healing. J. Colloid Interface Sci. 2022, 608, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, N.; Haghiralsadat, F.; Yazdian, F.; Sadeghian-Nodoushan, F.; Ghasemi, N.; Mazaheri, F.; Pourmadadi, M.; Naghib, S.M. Chitosan/Silk Fibroin/Nitrogen-Doped Carbon Quantum Dot/α-Tricalcium Phosphate Nanocomposite Electrospinned as a Scaffold for Wound Healing Application: In Vitro and in Vivo Studies. Int. J. Biol. Macromol. 2023, 238, 124078. [Google Scholar] [CrossRef] [PubMed]
- Lens, M. Use of Fullerenes in Cosmetics. Recent Pat. Biotechnol. 2009, 3, 118–123. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Fu, S.; Zhang, A. Facile Synthesis of Water-Soluble Fullerene (C60) Nanoparticles via Mussel-Inspired Chemistry as Efficient Antioxidants. Nanomaterials 2019, 9, 1647. [Google Scholar] [CrossRef]
- Emelyantsev, S.; Prazdnova, E.; Chistyakov, V.; Alperovich, I. Biological Effects of C60 Fullerene Revealed with Bacterial Biosensor—Toxic or Rather Antioxidant? Biosensors 2019, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Xu, R.H.J.X.; Yang, N.; Karim, A.A.; Loh, X.J.; Zhang, K. UV Protection and Antioxidant Activity of Nanodiamonds and Fullerenes for Sunscreen Formulations. ACS Appl. Nano Mater. 2019, 2, 7604–7616. [Google Scholar] [CrossRef]
- Asl, A.D.; Bohlooli, S.; Dadkhah, M.; Shirmard, L.R. Topical Delivery of Doxepin Using Liposome Containing Cream: An Emerging Approach in Enhancing Skin Retention. Pak. J. Pharm. Sci. 2023, 36, 1497–1506. [Google Scholar] [PubMed]
- Hui, M.; Jia, X.; Li, X.; Lazcano-Silveira, R.; Shi, M. Anti-Inflammatory and Antioxidant Effects of Liposoluble C60 at the Cellular, Molecular, and Whole-Animal Levels. J. Inflamm. Res. 2023, 16, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Shershakova, N.; Baraboshkina, E.; Andreev, S.; Purgina, D.; Struchkova, I.; Kamyshnikov, O.; Nikonova, A.; Khaitov, M. Anti-Inflammatory Effect of Fullerene C60 in a Mice Model of Atopic Dermatitis. J. Nanobiotechnol. 2016, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhao, Z.; Li, H.; Wu, B.; Huo, J.; Li, L.; Li, X.; Cao, X.; Xia, M.; Wang, C.; et al. Fullerene Nanoparticles for the Treatment of Ulcerative Colitis. Sci. China Life Sci. 2022, 65, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Qiang, R.; Huang, H.; Chen, J.; Shi, X.; Fan, Z.; Xu, G.; Qiu, H. Carbon Quantum Dots Derived from Herbal Medicine as Therapeutic Nanoagents for Rheumatoid Arthritis with Ultrahigh Lubrication and Anti-Inflammation. ACS Appl. Mater. Interfaces 2023, 15, 38653–38664. [Google Scholar] [CrossRef]
- Ghosh, D.; Dutta, G.; Sugumaran, A.; Chakrabarti, G.; Debnath, B. Fullerenes: Bucky Balls in the Therapeutic Application; Springer: Cham, Switzerland, 2023; pp. 1–25. [Google Scholar] [CrossRef]
- Halenova, T.; Raksha, N.; Vovk, T.; Savchuk, O.; Ostapchenko, L.; Prylutskyy, Y.; Kyzyma, O.; Ritter, U.; Scharff, P. Effect of C60 Fullerene Nanoparticles on the Diet-Induced Obesity in Rats. Int. J. Obes. 2018, 42, 1987–1998. [Google Scholar] [CrossRef]
- Beyaz, S.; Aslan, A.; Gok, O.; Ozercan, I.H.; Agca, C.A. Fullerene C60 Protects against 7,12-Dimethylbenz [a] Anthracene (DMBA) Induced-Pancreatic Damage via NF-ΚB and Nrf-2/HO-1 Axis in Rats. Toxicol. Res. 2023, 12, 954–963. [Google Scholar] [CrossRef]
- Hosseini, A.; Abdollahi, M.; Hassanzadeh, G.; Rezayat, M.; Hassani, S.; Pourkhalili, N.; Tabrizian, K.; Khorshidahmad, T.; Beyer, C.; Sharifzadeh, M. Protective Effect of Magnesium-25 Carrying Porphyrin-Fullerene Nanoparticles on Degeneration of Dorsal Root Ganglion Neurons and Motor Function in Experimental Diabetic Neuropathy. Basic Clin. Pharmacol. Toxicol. 2011, 109, 381–386. [Google Scholar] [CrossRef]
- Debnath, S.K.; Srivastava, R. Drug Delivery With Carbon-Based Nanomaterials as Versatile Nanocarriers: Progress and Prospects. Front. Nanotechnol. 2021, 3, 644564. [Google Scholar] [CrossRef]
- Pan, Y.; Sahoo, N.G.; Li, L. The Application of Graphene Oxide in Drug Delivery. Expert Opin. Drug Deliv. 2012, 9, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Jihad, M.A.; Noori, F.T.M.; Jabir, M.S.; Albukhaty, S.; Almalki, F.A.; Alyamani, A.A. Polyethylene Glycol Functionalized Graphene Oxide Nanoparticles Loaded with Nigella Sativa Extract: A Smart Antibacterial Therapeutic Drug Delivery System. Molecules 2021, 26, 3067. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Zhu, Z.; Gan, Z.; Chen, J.; Zhang, X.; Cheng, X.; Wan, Q.; Wang, J. PEGylated Nano-Graphene Oxide as a Nanocarrier for Delivering Mixed Anticancer Drugs to Improve Anticancer Activity. Sci. Rep. 2020, 10, 2717. [Google Scholar] [CrossRef] [PubMed]
- Mahani, M.; Pourrahmani-Sarbanani, M.; Yoosefian, M.; Divsar, F.; Mousavi, S.M.; Nomani, A. Doxorubicin Delivery to Breast Cancer Cells with Transferrin-Targeted Carbon Quantum Dots: An in Vitro and in Silico Study. J. Drug Deliv. Sci. Technol. 2021, 62, 102342. [Google Scholar] [CrossRef]
- Su, W.; Guo, R.; Yuan, F.; Li, Y.; Li, X.; Zhang, Y.; Zhou, S.; Fan, L. Red-Emissive Carbon Quantum Dots for Nuclear Drug Delivery in Cancer Stem Cells. J. Phys. Chem. Lett. 2020, 11, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mater. 2019, 31, e1804838. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; Zhou, L.; Pei, S.; Zhu, Z.; Chen, B. P-Doped Carbon Quantum Dots with Antibacterial Activity. Micromachines 2021, 12, 1116. [Google Scholar] [CrossRef]
- Wu, L.; Gao, Y.; Zhao, C.; Huang, D.; Chen, W.; Lin, X.; Liu, A.; Lin, L. Synthesis of Curcumin-Quaternized Carbon Quantum Dots with Enhanced Broad-Spectrum Antibacterial Activity for Promoting Infected Wound Healing. Biomater. Adv. 2022, 133, 112608. [Google Scholar] [CrossRef]
- Jaworski, S.; Wierzbicki, M.; Sawosz, E.; Jung, A.; Gielerak, G.; Biernat, J.; Jaremek, H.; Łojkowski, W.; Woźniak, B.; Wojnarowicz, J.; et al. Graphene Oxide-Based Nanocomposites Decorated with Silver Nanoparticles as an Antibacterial Agent. Nanoscale Res. Lett. 2018, 13, 116. [Google Scholar] [CrossRef]
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, Characterization, and Antibacterial Activity of Silver Nanoparticle-Decorated Graphene Oxide Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.G.; Hardman, N.J. Nature of Carbon Black Reinforcement of Rubber: Perspective on the Original Polymer Nanocomposite. Polymers 2021, 13, 538. [Google Scholar] [CrossRef] [PubMed]
- Kaliyathan, A.V.; Rane, A.V.; Huskic, M.; Kunaver, M.; Kalarikkal, N.; Rouxel, D.; Thomas, S. Influence of Carbon Black on Cure Properties and Mechanical Strength of Natural Rubber/Butadiene Rubber Blends. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 58, 69–80. [Google Scholar] [CrossRef]
- Talarico, D.; Cinti, S.; Arduini, F.; Amine, A.; Moscone, D.; Palleschi, G. Phosphate Detection through a Cost-Effective Carbon Black Nanoparticle-Modified Screen-Printed Electrode Embedded in a Continuous Flow System. Environ. Sci. Technol. 2015, 49, 7934–7939. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Ye, J.; Zheng, Z.; Li, X.; Chen, C.; Hu, C. Catalytic FeP Decorated Carbon Black as a Multifunctional Conducting Additive for High-Performance Lithium-Sulfur Batteries. Carbon 2021, 172, 96–105. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Mishra, R.K.; Ha, S.K.; Huczko, A. Evolution of Graphene Oxide and Graphene: From Imagination to Industrialization. ChemNanoMat 2018, 4, 598–620. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, X.; Wang, X.; Rodrigues, A.M.; Zhang, R. Reinforcement of the Mechanical Properties in Nitrile Rubber by Adding Graphene Oxide/Silicon Dioxide Hybrid Nanoparticles. J. Appl. Polym. Sci. 2018, 135, 46091. [Google Scholar] [CrossRef]
- Jamnam, S.; Maho, B.; Techaphatthanakon, A.; Ruttanapun, C.; Aemlaor, P.; Zhang, H.; Sukontasukkul, P. Effect of Graphene Oxide Nanoparticles on Blast Load Resistance of Steel Fiber Reinforced Concrete. Constr. Build. Mater. 2022, 343, 128139. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Her, C.H.; Comune, M.; Moia, C.; Lopes, A.; Porporato, P.E.; Vanacker, J.; Lam, M.C.; Steinstraesser, L.; Sonveaux, P.; et al. PLGA Nanoparticles Loaded with Host Defense Peptide LL37 Promote Wound Healing. J. Control. Release 2014, 194, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Shivakumar, H.; Ojha, H. Curcumin-Loaded Liposomes for Wound Healing: Preparation, Optimization, in-Vivo Skin Permeation and Bioevaluation. J. Drug Deliv. Sci. Technol. 2019, 49, 683–691. [Google Scholar] [CrossRef]
- Hou, B.; Qi, M.; Sun, J.; Ai, M.; Ma, X.; Cai, W.; Zhou, Y.; Ni, L.; Hu, J.; Xu, F.; et al. Preparation, Characterization and Wound Healing Effect of Vaccarin-Chitosan Nanoparticles. Int. J. Biol. Macromol. 2020, 165, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C. Mucus and Mucins in Diseases of the Intestinal and Respiratory Tracts. J. Intern. Med. 2019, 285, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Islam, T.; Nurunnabi, M. Mucoadhesive Carriers for Oral Drug Delivery. J. Control. Release 2022, 351, 504–559. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate Coated Chitosan Core-Shell Nanoparticles for Efficient Oral Delivery of Naringenin in Diabetic Animals—An in Vitro and in Vivo Approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef]
- Choudhary, M.; Chhabra, P.; Tyagi, A.; Singh, H. Scar Free Healing of Full Thickness Diabetic Wounds: A Unique Combination of Silver Nanoparticles as Antimicrobial Agent, Calcium Alginate Nanoparticles as Hemostatic Agent, Fresh Blood as Nutrient/Growth Factor Supplier and Chitosan as Base Matrix. Int. J. Biol. Macromol. 2021, 178, 41–52. [Google Scholar] [CrossRef]
- Narisepalli, S.; Salunkhe, S.A.; Chitkara, D.; Mittal, A. Asiaticoside Polymeric Nanoparticles for Effective Diabetic Wound Healing through Increased Collagen Biosynthesis: In-Vitro and in-Vivo Evaluation. Int. J. Pharm. 2023, 631, 122508. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Lopes, A.; Koussoroplis, S.; Payen, V.; Moia, C.; Zhu, H.; Sonveaux, P.; Carmeliet, P.; des Rieux, A.; Vandermeulen, G.; et al. Combined Effects of PLGA and Vascular Endothelial Growth Factor Promote the Healing of Non-Diabetic and Diabetic Wounds. Nanomedicine 2015, 11, 1975–1984. [Google Scholar] [CrossRef]
- Sheir, M.M.; Nasra, M.M.A.; Abdallah, O.Y. Chitosan Alginate Nanoparticles as a Platform for the Treatment of Diabetic and Non-Diabetic Pressure Ulcers: Formulation and in Vitro/in Vivo Evaluation. Int. J. Pharm. 2021, 607, 120963. [Google Scholar] [CrossRef] [PubMed]
- Lopes Rocha Correa, V.; Assis Martins, J.; Ribeiro de Souza, T.; de Castro Nunes Rincon, G.; Pacheco Miguel, M.; Borges de Menezes, L.; Correa Amaral, A. Melatonin Loaded Lecithin-Chitosan Nanoparticles Improved the Wound Healing in Diabetic Rats. Int. J. Biol. Macromol. 2020, 162, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.S.; Eid, H.M.; Elkomy, M.H.; Khames, A.; Hassan, R.M.; Abo El-Ela, F.I.; Yassin, H.A. Berberine Encapsulated Lecithin–Chitosan Nanoparticles as Innovative Wound Healing Agent in Type II Diabetes. Pharmaceutics 2021, 13, 1197. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hameed, A.M. Polydatin-Loaded Chitosan Nanoparticles Ameliorates Early Diabetic Nephropathy by Attenuating Oxidative Stress and Inflammatory Responses in Streptozotocin-Induced Diabetic Rat. J. Diabetes Metab. Disord. 2020, 19, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Wardani, G.; Nugraha, J.; Mustafa, M.R.; Kurnijasanti, R.; Sudjarwo, S.A. Antioxidative Stress and Antiapoptosis Effect of Chitosan Nanoparticles to Protect Cardiac Cell Damage on Streptozotocin-Induced Diabetic Rat. Oxid. Med. Cell Longev. 2022, 2022, 3081397. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hameed, A.M.; Yousef, A.I.; Abd El-Twab, S.M.; El-Shahawy, A.A.G.; Abdel-Moneim, A. Hepatoprotective Effects of Polydatin-Loaded Chitosan Nanoparticles in Diabetic Rats: Modulation of Glucose Metabolism, Oxidative Stress, and Inflammation Biomarkers. Biochemistry 2021, 86, 179–189. [Google Scholar] [CrossRef] [PubMed]
- El-Dakroury, W.A.; Zewail, M.B.; Amin, M.M. Design, Optimization, and in-Vivo Performance of Glipizide-Loaded O-Carboxymethyl Chitosan Nanoparticles in Insulin Resistant/Type 2 Diabetic Rat Model. J. Drug Deliv. Sci. Technol. 2023, 79, 104040. [Google Scholar] [CrossRef]
- Afshar, M.; Dini, G.; Vaezifar, S.; Mehdikhani, M.; Movahedi, B. Preparation and Characterization of Sodium Alginate/Polyvinyl Alcohol Hydrogel Containing Drug-Loaded Chitosan Nanoparticles as a Drug Delivery System. J. Drug Deliv. Sci. Technol. 2020, 56, 101530. [Google Scholar] [CrossRef]
- El-Alfy, E.A.; El-Bisi, M.K.; Taha, G.M.; Ibrahim, H.M. Preparation of Biocompatible Chitosan Nanoparticles Loaded by Tetracycline, Gentamycin and Ciprofloxacin as Novel Drug Delivery System for Improvement the Antibacterial Properties of Cellulose Based Fabrics. Int. J. Biol. Macromol. 2020, 161, 1247–1260. [Google Scholar] [CrossRef]
- Yu, A.; Shi, H.; Liu, H.; Bao, Z.; Dai, M.; Lin, D.; Lin, D.; Xu, X.; Li, X.; Wang, Y. Mucoadhesive Dexamethasone-Glycol Chitosan Nanoparticles for Ophthalmic Drug Delivery. Int. J. Pharm. 2020, 575, 118943. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Liu, T.; Liu, Y.; Wu, X.; Liu, L. Mandarin (Citrus reticulata L.) Essential Oil Incorporated into Chitosan Nanoparticles: Characterization, Anti-Biofilm Properties and Application in Pork Preservation. Int. J. Biol. Macromol. 2021, 185, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Saravanakumar, K.; Sathiyaseelan, A.; Wang, M.H. Chitosan Nanoparticles as Edible Surface Coating Agent to Preserve the Fresh-Cut Bell Pepper (Capsicum annuum L. Var grossum (L.) Sendt). Int. J. Biol. Macromol. 2020, 165, 948–957. [Google Scholar] [CrossRef] [PubMed]
- Tayel, A.A.; Elzahy, A.F.; Moussa, S.H.; Al-Saggaf, M.S.; Diab, A.M. Biopreservation of Shrimps Using Composed Edible Coatings from Chitosan Nanoparticles and Cloves Extract. J. Food Qual. 2020, 2020, 8878452. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Malandrino, G.; Cafiso, V.; Stefani, S.; Geraci, C. Oregano and Thyme Essential Oils Encapsulated in Chitosan Nanoparticles as Effective Antimicrobial Agents against Foodborne Pathogens. Molecules 2021, 26, 4055. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Fabrication of Chitosan-Based Functional Nanocomposite Films: Effect of Quercetin-Loaded Chitosan Nanoparticles. Food Hydrocoll. 2021, 121, 107065. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, Y.; Jin, Y.; Wang, D.; Wang, G.; Zhang, X.; Li, Y.; Lee, S. Ag@MOF-Loaded p-Coumaric Acid Modified Chitosan/Chitosan Nanoparticle and Polyvinyl Alcohol/Starch Bilayer Films for Food Packing Applications. Int. J. Biol. Macromol. 2022, 202, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Dong, H.; Sun, X.; Fan, Y.; Wang, Y.; Huang, F. Development of Glucose Oxidase-Immobilized Alginate Nanoparticles for Enhanced Glucose-Triggered Insulin Delivery in Diabetic Mice. Int. J. Biol. Macromol. 2020, 159, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Karmakar, T.; Ghosh, N.; Basak, S.; Gopal Sahoo, N. Targeting Mangiferin Loaded N-Succinyl Chitosan-Alginate Grafted Nanoparticles against Atherosclerosis—A Case Study against Diabetes Mediated Hyperlipidemia in Rat. Food Chem. 2022, 370, 131376. [Google Scholar] [CrossRef] [PubMed]
- Yeerong, K.; Chantawannakul, P.; Anuchapreeda, S.; Juntrapirom, S.; Kanjanakawinkul, W.; Müllertz, A.; Rades, T.; Chaiyana, W. Chitosan Alginate Nanoparticles of Protein Hydrolysate from Acheta Domesticus with Enhanced Stability for Skin Delivery. Pharmaceutics 2024, 16, 724. [Google Scholar] [CrossRef]
- De Silva, N.D.; Attanayake, A.P.; Karunaratne, D.N.; Arawwawala, L.D.A.M.; Pamunuwa, G.K. Synthesis and Bioactivity Assessment of Coccinia grandis L. Extract Encapsulated Alginate Nanoparticles as an Antidiabetic Drug Lead. J. Microencapsul. 2024, 41, 1–17. [Google Scholar] [CrossRef]
- Yoncheva, K.; Benbassat, N.; Zaharieva, M.M.; Dimitrova, L.; Kroumov, A.; Spassova, I.; Kovacheva, D.; Najdenski, H.M. Improvement of the Antimicrobial Activity of Oregano Oil by Encapsulation in Chitosan–Alginate Nanoparticles. Molecules 2021, 26, 7017. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; de Araújo Gonçalves, M.; de Macedo, L.F.; Torres, A.H.F.; Marena, G.D.; Chorilli, M.; Trovatti, E. Green Nanotechnology for the Development of Nanoparticles Based on Alginate Associated with Essential and Vegetable Oils for Application in Fruits and Seeds Protection. Int. J. Biol. Macromol. 2023, 232, 123351. [Google Scholar] [CrossRef] [PubMed]
- Osanloo, M.; Eskandari, Z.; Zarenezhad, E.; Qasemi, H.; Nematollahi, A. Studying the Microbial, Chemical, and Sensory Characteristics of Shrimp Coated with Alginate Sodium Nanoparticles Containing Zataria Multiflora and Cuminum Cyminum Essential Oils. Food Sci. Nutr. 2023, 11, 2823–2837. [Google Scholar] [CrossRef] [PubMed]
- Cassano, R.; Trombino, S.; Curcio, F.; Sole, R.; Calviello, G.; Serini, S. ROS-Responsive PLGA-NPs for Co-Delivery of DTX and DHA for Colon Cancer Treatment. Int. J. Transl. Med. 2024, 4, 262–277. [Google Scholar] [CrossRef]
- Kim, D.H.; Nguyen, T.N.; Han, Y.M.; Tran, P.; Rho, J.; Lee, J.Y.; Son, H.Y.; Park, J.S. Local Drug Delivery Using Poly(Lactic-Co-Glycolic Acid) Nanoparticles in Thermosensitive Gels for Inner Ear Disease Treatment. Drug Deliv. 2021, 28, 2268–2277. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Fabrication of Poly(Sarcosine), Poly (Ethylene Glycol), and Poly (Lactic-Co-Glycolic Acid) Polymeric Nanoparticles for Cancer Drug Delivery. J. Drug Deliv. Sci. Technol. 2021, 61, 102194. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Small-Howard, A.L.; Fernández-Arévalo, M.; Martín-Banderas, L. Development of Enhanced Drug Delivery Vehicles for Three Cannabis-Based Terpenes Using Poly(Lactic-Co-Glycolic Acid) Based Nanoparticles. Ind. Crop. Prod. 2021, 164, 113345. [Google Scholar] [CrossRef]
- Choe, G.; Lee, M.; Oh, S.; Seok, J.M.; Kim, J.; Im, S.; Park, S.A.; Lee, J.Y. Three-Dimensional Bioprinting of Mesenchymal Stem Cells Using an Osteoinductive Bioink Containing Alginate and BMP-2-Loaded PLGA Nanoparticles for Bone Tissue Engineering. Biomater. Adv. 2022, 136, 212789. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Xu, Y.; Gu, Y.; Yao, Q.; Li, J.; Wang, L. IGF-1-Releasing PLGA Nanoparticles Modified 3D Printed PCL Scaffolds for Cartilage Tissue Engineering. Drug Deliv. 2020, 27, 1106–1114. [Google Scholar] [CrossRef]
- Ghavimi, M.A.; Bani Shahabadi, A.; Jarolmasjed, S.; Memar, M.Y.; Maleki Dizaj, S.; Sharifi, S. Nanofibrous Asymmetric Collagen/Curcumin Membrane Containing Aspirin-Loaded PLGA Nanoparticles for Guided Bone Regeneration. Sci. Rep. 2020, 10, 18200. [Google Scholar] [CrossRef]
- Akolpoğlu Başaran, D.D.; Gündüz, U.; Tezcaner, A.; Keskin, D. Topical Delivery of Heparin from PLGA Nanoparticles Entrapped in Nanofibers of Sericin/Gelatin Scaffolds for Wound Healing. Int. J. Pharm. 2021, 597, 120207. [Google Scholar] [CrossRef] [PubMed]
- Azzazy, H.M.E.S.; Fahmy, S.A.; Mahdy, N.K.; Meselhy, M.R.; Bakowsky, U. Chitosan-Coated PLGA Nanoparticles Loaded with Peganum Harmala Alkaloids with Promising Antibacterial and Wound Healing Activities. Nanomaterials 2021, 11, 2438. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, A.; Macedo, M.H.; Sousa, F.; Arkill, K.; Alexander, C.; Aylott, J.; Sarmento, B. Prediction of the Enhanced Insulin Absorption across a Triple Co-Cultured Intestinal Model Using Mucus Penetrating PLGA Nanoparticles. Int. J. Pharm. 2020, 585, 119516. [Google Scholar] [CrossRef] [PubMed]
- Laddha, U.D.; Kshirsagar, S.J. Formulation of PPAR-Gamma Agonist as Surface Modified PLGA Nanoparticles for Non-Invasive Treatment of Diabetic Retinopathy: In Vitro and in Vivo Evidences. Heliyon 2020, 6, e04589. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, C.; Zhang, F.; Li, Y.; Zhang, B.; Huang, J.; Zhang, Z.; Jin, L. Improved Oral Delivery of Insulin by PLGA Nanoparticles Coated with 5β-Cholanic Acid Conjugated Glycol Chitosan. Biomed. Mater. 2021, 16, 064103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Y.; Fu, H.; Huang, H.; Wu, Z.; Zhao, M.; Yang, X.; Guo, Q.; Duan, Y.; Sun, Y. Multifunctional Tumor-Targeted PLGA Nanoparticles Delivering Pt(IV)/SiBIRC5 for US/MRI Imaging and Overcoming Ovarian Cancer Resistance. Biomaterials 2021, 269, 120478. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.; Sonali; Shekhar, S.; Yadav, B.; Garg, V.; Dutt, R.; Mehata, A.K.; Goswami, P.; Koch, B.; Muthu, M.S.; et al. AS1411 Aptamer/RGD Dual Functionalized Theranostic Chitosan-PLGA Nanoparticles for Brain Cancer Treatment and Imaging. Biomater. Adv. 2024, 160, 213833. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Cao, K.; Jia, R.; Chen, X.; Wu, Y.; Wang, Y.; Cheng, Z.; Xia, H.; Xu, Y.; Xie, Z. Tetramethylpyrazine-Loaded Liposomes Surrounded by Hydrogel Based on Sodium Alginate and Chitosan as a Multifunctional Drug Delivery System for Treatment of Atopic Dermatitis. Eur. J. Pharm. Sci. 2024, 193, 106680. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Chen, P.; Liu, X.; Lian, Y.; Xi, J.; Li, J.; Song, J.; Li, X. Trimethylated Chitosan-Coated Flexible Liposomes with Resveratrol for Topical Drug Delivery to Reduce Blue-Light-Induced Retinal Damage. Int. J. Biol. Macromol. 2023, 252, 126480. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guan, S.; Li, H.; Li, D.; Liu, D.; Wang, J.; Zhu, W.; Xing, G.; Yue, L.; Cai, D.; et al. Polysialic Acid-Functionalized Liposomes for Efficient Honokiol Delivery to Inhibit Breast Cancer Growth and Metastasis. Drug Deliv. 2023, 30, 2181746. [Google Scholar] [CrossRef]
- Taghizadeh, B.; Moradi, R.; Sobhani, B.; Mohammadpanah, H.; Behboodifar, S.; Golmohammadzadeh, S.; Chamani, J.; Maleki, M.; Alizadeh, E.; Zarghami, N.; et al. Development of Nano-Liposomal Human Growth Hormone as a Topical Formulation for Preventing Uvb-Induced Skin Damage. Int. J. Biol. Macromol. 2024, 265, 130641. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, L.; Maia Campos, P.M.B.G.; Oliveira, W.P. Development and Efficacy Evaluation of Innovative Cosmetic Formulations with Caryocar Brasiliense Fruit Pulp Oil Encapsulated in Freeze-Dried Liposomes. Pharmaceutics 2024, 16, 595. [Google Scholar] [CrossRef] [PubMed]
- Shu, G.; Xu, D.; Zhang, W.; Zhao, X.; Li, H.; Xu, F.; Yin, L.; Peng, X.; Fu, H.; Chang, L.J.; et al. Preparation of Shikonin Liposome and Evaluation of Its in Vitro Antibacterial and in Vivo Infected Wound Healing Activity. Phytomedicine 2022, 99, 154035. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Ding, C.; Liu, X.; Zheng, Y.; Zhao, Y.; Zhang, S.; Sun, S.; Peng, Z.; Liu, W. Preparation of Nanocomposite Membranes Loaded with Taxifolin Liposome and Its Mechanism of Wound Healing in Diabetic Mice. Int. J. Biol. Macromol. 2023, 241, 124537. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, Z.; Liu, L.; Hu, Y.; Xiong, Y.; Lu, Y.; Bie, F.; Chen, S.; Zhou, F.; Xu, Y.; et al. 3D-Printed Biomimetic Scaffold with Liposome-Encapsulated SB431542 Promotes Scarless Wound Healing. J. Mater. Sci. Technol. 2024, 208, 38–52. [Google Scholar] [CrossRef]
- Tu, Q.; Li, S.; Zeng, Z.; Liu, Y.; Wang, C.; Chen, S.; Hu, B.; Li, C. Cinnamon Essential Oil Liposomes Modified by Sodium Alginate-Chitosan: Application in Chilled Pork Preservation. Int. J. Food Sci. Technol. 2023, 58, 939–953. [Google Scholar] [CrossRef]
- Cui, H.; Yang, M.; Shi, C.; Li, C.; Lin, L. Application of Xanthan-Gum-Based Edible Coating Incorporated with Litsea Cubeba Essential Oil Nanoliposomes in Salmon Preservation. Foods 2022, 11, 1535. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Tao, J.; Liu, X.; Wen, Z. Baicalin-Liposomes Loaded Polyvinyl Alcohol-Chitosan Electrospinning Nanofibrous Films: Characterization, Antibacterial Properties and Preservation Effects on Mushrooms. Food Chem. 2022, 371, 131372. [Google Scholar] [CrossRef]
- Arora, S.; Layek, B.; Singh, J. Design and Validation of Liposomal ApoE2 Gene Delivery System to Evade Blood-Brain Barrier for Effective Treatment of Alzheimer’s Disease. Mol. Pharm. 2021, 18, 714–725. [Google Scholar] [CrossRef]
- Wang, K.; Shang, F.; Chen, D.; Cao, T.; Wang, X.; Jiao, J.; He, S.; Liang, X. Protein Liposomes-Mediated Targeted Acetylcholinesterase Gene Delivery for Effective Liver Cancer Therapy. J. Nanobiotechnol. 2021, 19, 31. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Quintanar-Guerrero, D.; Del Real, A.; González-Reza, R.M.; Cornejo-Villegas, M.A.; Gutiérrez-Corte, E. Effect of Nano-Edible Coating Based on Beeswax Solid Lipid Nanoparticles on Strawberry’s Preservation. Coatings 2020, 10, 253. [Google Scholar] [CrossRef]
- Alanchari, M.; Mohammadi, M.; Yazdian, F.; Ahangari, H.; Ahmadi, N.; Emam-Djomeh, Z.; Homayouni-Rad, A.; Ehsani, A. Optimization and Antimicrobial Efficacy of Curcumin Loaded Solid Lipid Nanoparticles against Foodborne Bacteria in Hamburger Patty. J. Food Sci. 2021, 86, 2242–2254. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Wu, J.; Qi, G.; Chen, G.; Li, H.; Wang, H. Photodynamic Inactivation Mediated by Curcumin Solid Lipid Nanoparticles on Bacteria and Its Application for Fresh Carrot Juice. Food Bioprocess Technol. 2024, 17, 1294–1308. [Google Scholar] [CrossRef]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.H.; Choi, Y.J. Enhancing the Oral Bioavailability of Curcumin Using Solid Lipid Nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Singh, J.; Kaur, S.; Sandhu, S.; Singh, G.; Kaur, I.P. Enhancing Bioavailability and Stability of Curcumin Using Solid Lipid Nanoparticles (CLEN): A Covenant for Its Effectiveness. Front. Bioeng. Biotechnol. 2020, 8, 879. [Google Scholar] [CrossRef] [PubMed]
- He, X.L.; Yang, L.; Wang, Z.J.; Huang, R.Q.; Zhu, R.R.; Cheng, L.M. Solid Lipid Nanoparticles Loading with Curcumin and Dexanabinol to Treat Major Depressive Disorder. Neural Regen. Res. 2021, 16, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Karamchedu, S.; Tunki, L.; Kulhari, H.; Pooja, D. Morin Hydrate Loaded Solid Lipid Nanoparticles: Characterization, Stability, Anticancer Activity, and Bioavailability. Chem. Phys. Lipids 2020, 233, 104988. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, S.; Narwade, M.; Sheikh, A.; Md, S.; Salve, R.; Gajbhiye, V.; Kesharwani, P.; Gajbhiye, K.R. GLUT1 Transporter-Facilitated Solid Lipid Nanoparticles Loaded with Anti-Cancer Therapeutics for Ovarian Cancer Targeting. Int. J. Pharm. 2023, 637, 122894. [Google Scholar] [CrossRef] [PubMed]
- Affram, K.O.; Smith, T.; Ofori, E.; Krishnan, S.; Underwood, P.; Trevino, J.G.; Agyare, E. Cytotoxic Effects of Gemcitabine-Loaded Solid Lipid Nanoparticles in Pancreatic Cancer Cells. J. Drug Deliv. Sci. Technol. 2020, 55, 101374. [Google Scholar] [CrossRef]
- Smith, T.; Affram, K.; Nottingham, E.L.; Han, B.; Amissah, F.; Krishnan, S.; Trevino, J.; Agyare, E. Application of Smart Solid Lipid Nanoparticles to Enhance the Efficacy of 5-Fluorouracil in the Treatment of Colorectal Cancer. Sci. Rep. 2020, 10, 16989. [Google Scholar] [CrossRef]
- Mostafa, E.S.; Maher, A.; Mostafa, D.A.; Gad, S.S.; Nawwar, M.A.M.; Swilam, N. A Unique Acylated Flavonol Glycoside from Prunus persica (L.) Var. Florida Prince: A New Solid Lipid Nanoparticle Cosmeceutical Formulation for Skincare. Antioxidants 2021, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Ramalho, M.J.; Silva, R.; Silva, V.; Marques-Oliveira, R.; Silva, A.C.; Pereira, M.C.; Loureiro, J.A. Vine Cane Compounds to Prevent Skin Cells Aging through Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Rubiano, S.; Echeverri, J.D.; Salamanca, C.H. Solid Lipid Nanoparticles (SLNs) with Potential as Cosmetic Hair Formulations Made from Otoba Wax and Ultrahigh Pressure Homogenization. Cosmetics 2020, 7, 42. [Google Scholar] [CrossRef]
- Lee, Y.J.; Nam, G.W. Sunscreen Boosting Effect by Solid Lipid Nanoparticles-Loaded Fucoxanthin Formulation. Cosmetics 2020, 7, 14. [Google Scholar] [CrossRef]
- Albuquerque, L.F.F.; Lins, F.V.; Bispo, E.C.I.; Borges, E.N.; Silva, M.T.; Gratieri, T.; Cunha-Filho, M.; Alonso, A.; Carvalho, J.L.; Saldanha-Araujo, F.; et al. Ibrutinib Topical Delivery for Melanoma Treatment: The Effect of Nanostructured Lipid Carriers’ Composition on the Controlled Drug Skin Deposition. Colloids Surf. B Biointerfaces 2024, 237, 113875. [Google Scholar] [CrossRef] [PubMed]
- Ahalwat, S.; Bhatt, D.C.; Rohilla, S.; Jogpal, V.; Sharma, K.; Virmani, T.; Kumar, G.; Alhalmi, A.; Alqahtani, A.S.; Noman, O.M.; et al. Mannose-Functionalized Isoniazid-Loaded Nanostructured Lipid Carriers for Pulmonary Delivery: In Vitro Prospects and In Vivo Therapeutic Efficacy Assessment. Pharmaceuticals 2023, 16, 1108. [Google Scholar] [CrossRef] [PubMed]
- Nicoleti, L.R.; Di Filippo, L.D.; Duarte, J.L.; Luiz, M.T.; Sábio, R.M.; Chorilli, M. Development, Characterization and in Vitro Cytotoxicity of Kaempferol-Loaded Nanostructured Lipid Carriers in Glioblastoma Multiforme Cells. Colloids Surf. B Biointerfaces 2023, 226, 113309. [Google Scholar] [CrossRef] [PubMed]
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Isabel Lema, M.; Javier Otero-Espinar, F. Lactoferrin-Loaded Nanostructured Lipid Carriers (NLCs) as a New Formulation for Optimized Ocular Drug Delivery. Eur. J. Pharm. Biopharm. 2022, 172, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.; Shadambikar, G.; Mehraj, T.; Sulochana, S.P.; Dudhipala, N.; Majumdar, S. Development of α-Tocopherol Succinate-Based Nanostructured Lipid Carriers for Delivery of Paclitaxel. Pharmaceutics 2022, 14, 1034. [Google Scholar] [CrossRef]
- Shaker, S.A.; Alshufta, S.M.; Gowayed, M.A.; El-Salamouni, N.S.; Bassam, S.M.; Megahed, M.A.; El-Tahan, R.A. Propolis-Loaded Nanostructured Lipid Carriers Halt Breast Cancer Progression through MiRNA-223 Related Pathways: An in-Vitro/in-Vivo Experiment. Sci. Rep. 2023, 13, 15752. [Google Scholar] [CrossRef]
- Sherif, A.Y.; Harisa, G.I.; Shahba, A.A.; Alanazi, F.K.; Qamar, W. Optimization of Gefitinib-Loaded Nanostructured Lipid Carrier as a Biomedical Tool in the Treatment of Metastatic Lung Cancer. Molecules 2023, 28, 448. [Google Scholar] [CrossRef]
- Sadeghzadeh, F.; Motavalizadehkakhky, A.; Mehrzad, J.; Zhiani, R.; Homayouni Tabrizi, M. Folic Acid Conjugated-Chitosan Modified Nanostructured Lipid Carriers as Promising Carriers for Delivery of Umbelliprenin to Cancer Cells: In Vivo and in Vitro. Eur. Polym. J. 2023, 186, 111849. [Google Scholar] [CrossRef]
- Imran, M.; Iqubal, M.K.; Imtiyaz, K.; Saleem, S.; Mittal, S.; Rizvi, M.M.A.; Ali, J.; Baboota, S. Topical Nanostructured Lipid Carrier Gel of Quercetin and Resveratrol: Formulation, Optimization, in Vitro and Ex Vivo Study for the Treatment of Skin Cancer. Int. J. Pharm. 2020, 587, 119705. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Cui, Y.; Hao, W.; Fan, Y.; Zhang, J.; Liu, Q.; Jiang, M.; Yang, Y.; Wang, Y.; Gao, C. Ligand-Modified Homologous Targeted Cancer Cell Membrane Biomimetic Nanostructured Lipid Carriers for Glioma Therapy. Drug Deliv. 2021, 28, 2241–2255. [Google Scholar] [CrossRef] [PubMed]
- Dini, A.X.P.; Costa, A.F.; Fávaro, W.J.; Durán, N. Safety Nanocosmetics: Triblock Copolymer Nanostructured Lipid Carriers and Application on Hair Cosmetics. J. Phys. Conf. Ser. 2021, 1953, 012001. [Google Scholar] [CrossRef]
- Ijaz, M.; Akhtar, N. Fatty Acids Based α-Tocopherol Loaded Nanostructured Lipid Carrier Gel: In Vitro and in Vivo Evaluation for Moisturizing and Anti-Aging Effects. J. Cosmet. Dermatol. 2020, 19, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Atapour-Mashhad, H.; Tayarani-Najaran, Z.; Golmohammadzadeh, S. Preparation and Characterization of Novel Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN) Containing Coenzyme Q10 as Potent Antioxidants and Antityrosinase Agents. Heliyon 2024, 10, e31429. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammadi, M.; Ghanbarzadeh, B.; Hanifian, S.; Samadi Kafil, H.; Gharekhani, M.; Falcone, P.M. The Gelatin-Coated Nanostructured Lipid Carrier (NLC) Containing Salvia Officinalis Extract: Optimization by Combined D-Optimal Design and Its Application to Improve the Quality Parameters of Beef Burger. Foods 2023, 12, 3737. [Google Scholar] [CrossRef] [PubMed]
- Talesh, A.A.; Amiri, S.; Radi, M.; Hosseinifarahi, M. Effect of Nanocomposite Alginate-Based Edible Coatings Containing Thymol-Nanoemulsion and/or Thymol-Loaded Nanostructured Lipid Carriers on the Microbial and Physicochemical Properties of Carrot. Int. J. Biol. Macromol. 2024, 129196. [Google Scholar] [CrossRef]
- Radi, M.; Shadikhah, S.; Sayadi, M.; Kaveh, S.; Amiri, S.; Bagheri, F. Effect of Thymus vulgaris Essential Oil-Loaded Nanostructured Lipid Carriers in Alginate-Based Edible Coating on the Postharvest Quality of Tangerine Fruit. Food Bioproc. Technol. 2023, 16, 185–198. [Google Scholar] [CrossRef]
- Borhani, E.A.; Amiri, S.; Radi, M. The Effects of Alginate Coatings Containing Thymol in the Forms of Nanoemulsion and Nanostructured Lipid Carriers on Microbial, Oxidation, and Physicochemical Qualities of Fresh Breast Chicken Meat. Food Bioproc. Technol. 2024. [Google Scholar] [CrossRef]
- Amin, H.; Osman, S.K.; Mohammed, A.M.; Zayed, G. Gefitinib-Loaded Starch Nanoparticles for Battling Lung Cancer: Optimization by Full Factorial Design and in Vitro Cytotoxicity Evaluation. Saudi Pharm. J. 2023, 31, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Alzate, P.; Gerschenson, L.; Flores, S. Ultrasound Application for Production of Nano-Structured Particles from Esterified Starches to Retain Potassium Sorbate. Carbohydr. Polym. 2020, 247, 116759. [Google Scholar] [CrossRef]
- Ou, A.-T.; Zhang, J.-X.; Fang, Y.-F.; Wang, R.; Tang, X.-P.; Zhao, P.-F.; Zhao, Y.-G.; Zhang, M.; Huang, Y.-Z. Disulfiram-Loaded Lactoferrin Nanoparticles for Treating Inflammatory Diseases. Acta Pharmacol. Sin. 2021, 42, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Senapathi, J.; Bommakanti, A.; Mallepalli, S.; Mukhopadhyay, S.; Kondapi, A.K. Sulfonate Modified Lactoferrin Nanoparticles as Drug Carriers with Dual Activity against HIV-1. Colloids Surf. B Biointerfaces 2020, 191, 110979. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.G.R.; Picone, C.S.F. Antimicrobial Activity of Lactoferrin-Chitosan-Gellan Nanoparticles and Their Influence on Strawberry Preservation. Food Res. Int. 2022, 159, 111586. [Google Scholar] [CrossRef]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming Blood–Brain Barrier Transport: Advances in Nanoparticle-Based Drug Delivery Strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, L.; Zhang, Q.; Cheng, Y.; Liu, Y.; Li, R.; Hou, S. Nose-to-Brain Delivery of Self-Assembled Curcumin-Lactoferrin Nanoparticles: Characterization, Neuroprotective Effect and in Vivo Pharmacokinetic Study. Front. Bioeng. Biotechnol. 2023, 11, 1168408. [Google Scholar] [CrossRef]
- Teixeira, M.I.; Lopes, C.M.; Gonçalves, H.; Catita, J.; Silva, A.M.; Rodrigues, F.; Amaral, M.H.; Costa, P.C. Formulation, Characterization, and Cytotoxicity Evaluation of Lactoferrin Functionalized Lipid Nanoparticles for Riluzole Delivery to the Brain. Pharmaceutics 2022, 14, 185. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Gu, G.; Song, Q.; Yao, L.; Hu, Q.; Tu, Y.; Pang, Z.; et al. Lactoferrin-Modified PEG-Co-PCL Nanoparticles for Enhanced Brain Delivery of NAP Peptide Following Intranasal Administration. Biomaterials 2013, 34, 3870–3881. [Google Scholar] [CrossRef]
- Sachdeva, B.; Sachdeva, P.; Negi, A.; Ghosh, S.; Han, S.; Dewanjee, S.; Jha, S.K.; Bhaskar, R.; Sinha, J.K.; Paiva-Santos, A.C.; et al. Chitosan Nanoparticles-Based Cancer Drug Delivery: Application and Challenges. Mar. Drugs 2023, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.; Amin, M.A.; Osman, S.K.; Mohammed, A.M.; Zayed, G. Chitosan Nanoparticles as a Smart Nanocarrier for Gefitinib for Tackling Lung Cancer: Design of Experiment and in Vitro Cytotoxicity Study. Int. J. Biol. Macromol. 2023, 246, 125638. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jia, C.; Xu, Y.; Jiang, Z.; Hu, T.; Li, C.; Cheng, X. Dual-PH Responsive Chitosan Nanoparticles for Improving in Vivo Drugs Delivery and Chemoresistance in Breast Cancer. Carbohydr. Polym. 2022, 290, 119518. [Google Scholar] [CrossRef] [PubMed]
- Schick, J.; Ritchie, R.P.; Restini, C. Breast Cancer Therapeutics and Biomarkers: Past, Present, and Future Approaches. Breast Cancer 2021, 15, 1178223421995854. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R. Lipid-Based Nanoparticles for Drug-Delivery Systems. In Nanocarriers for Drug Delivery: Nanoscience and Nanotechnology in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 249–284. [Google Scholar] [CrossRef]
- Shariare, M.H.; Rahman, M.; Lubna, S.R.; Roy, R.S.; Abedin, J.; Marzan, A.L.; Altamimi, M.A.; Ahamad, S.R.; Ahmad, A.; Alanazi, F.K.; et al. Liposomal Drug Delivery of Aphanamixis Polystachya Leaf Extracts and Its Neurobehavioral Activity in Mice Model. Sci. Rep. 2020, 10, 6938. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, G.S.; Athawale, R.B.; Gude, R.P.; Md, S.; Alhakamy, N.A.; Fahmy, U.A.; Kesharwani, P. Formulation and Development of Transferrin Targeted Solid Lipid Nanoparticles for Breast Cancer Therapy. Front. Pharmacol. 2020, 11, 614290. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.K.; Ismail, A.A.; Kamel, M.A. Nose to Brain Delivery of Astaxanthin–Loaded Nanostructured Lipid Carriers in Rat Model of Alzheimer’s Disease: Preparation, in Vitro and in Vivo Evaluation. Int. J. Nanomed. 2023, 18, 1631–1658. [Google Scholar] [CrossRef]
- Naik, J.B.; Pardeshi, S.R.; Patil, R.P.; Patil, P.B.; Mujumdar, A. Mucoadhesive Micro-/Nano Carriers in Ophthalmic Drug Delivery: An Overview. Bionanoscience 2020, 10, 564–582. [Google Scholar] [CrossRef]
- Garavand, F.; Cacciotti, I.; Vahedikia, N.; Rehman, A.; Tarhan, Ö.; Akbari-Alavijeh, S.; Shaddel, R.; Rashidinejad, A.; Nejatian, M.; Jafarzadeh, S.; et al. A Comprehensive Review on the Nanocomposites Loaded with Chitosan Nanoparticles for Food Packaging. Crit. Rev. Food Sci. Nutr. 2022, 62, 1383–1416. [Google Scholar] [CrossRef]
- Piryaei, M.; Azimi, S. Preparation and Evaluation of Smart Food Packaging Films with Anthocyanin Sardasht Black Grape Based on Astragalus Gummifer and Chitosan Nanoparticles. Int. J. Biol. Macromol. 2024, 254, 127974. [Google Scholar] [CrossRef]
- Amaregouda, Y.; Kamanna, K. Carboxymethyl Cellulose/Starch-Based Films Incorporating Chitosan Nanoparticles for Multifunctional Food Packaging. Cellulose 2024, 31, 2413–2427. [Google Scholar] [CrossRef]
- Wrona, M.; Cran, M.J.; Nerín, C.; Bigger, S.W. Development and Characterisation of HPMC Films Containing PLA Nanoparticles Loaded with Green Tea Extract for Food Packaging Applications. Carbohydr. Polym. 2017, 156, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.Z.; Rahman, M.R.T.; Marcos, B.; Tiwari, B.; Pathania, S. Poly Lactic Acid (PLA) Nanocomposites: Effect of Inorganic Nanoparticles Reinforcement on Its Performance and Food Packaging Applications. Molecules 2021, 26, 1967. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, J.; Villegas, C.; Torres, A.; Vargas, E.; Rodriguez, F.; Baltazar, S.; Prada, A.; Rojas, A.; Romero, J.; Faba, S.; et al. Effect of Functionalized Silica Nanoparticles on the Mass Transfer Process in Active PLA Nanocomposite Films Obtained by Supercritical Impregnation for Sustainable Food Packaging. J. Supercrit. Fluids 2020, 161, 104844. [Google Scholar] [CrossRef]
- Laein, S.S.; Mohajer, F.; Khanzadi, A.; Gheybi, F.; Azizzadeh, M.; Noori, S.M.A.; Mollaei, F.; Hashemi, M. Effect of Alginate Coating Activated by Solid Lipid Nanoparticles Containing Zataria Multiflora Essential Oil on Chicken Fillet’s Preservation. Food Chem. 2024, 446, 138816. [Google Scholar] [CrossRef] [PubMed]
- Katopodi, A.; Detsi, A. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers of Natural Products as Promising Systems for Their Bioactivity Enhancement: The Case of Essential Oils and Flavonoids. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127529. [Google Scholar] [CrossRef]
- Duarte, L.G.R.; Ferreira, N.C.A.; Fiocco, A.C.T.R.; Picone, C.S.F. Lactoferrin-Chitosan-TPP Nanoparticles: Antibacterial Action and Extension of Strawberry Shelf-Life. Food Bioproc. Technol. 2023, 16, 135–148. [Google Scholar] [CrossRef]
- Ghosh, T.; Mondal, K.; Katiyar, V. Lipid Nanoparticles for Edible Food Packaging. In Materials Horizons: From Nature to Nanomaterials; Springer: Singapore, 2021; pp. 191–213. [Google Scholar] [CrossRef]
- Vakili-Ghartavol, M.; Arouiee, H.; Golmohammadzadeh, S.; Naseri, M.; Bandian, L. Edible Coatings Based on Solid Lipid Nanoparticles Containing Essential Oil to Improve Antimicrobial Activity, Shelf-Life, and Quality of Strawberries. J. Stored Prod. Res. 2024, 106, 102262. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Liu, Y.; Zheng, Q.; Tan, W.; Feng, X.; Feng, K.; Hu, W. Application of Cinnamaldehyde Solid Lipid Nanoparticles in Strawberry Preservation. Horticulturae 2023, 9, 607. [Google Scholar] [CrossRef]
- Al-Moghazy, M.; El-sayed, H.S.; Salama, H.H.; Nada, A.A. Edible Packaging Coating of Encapsulated Thyme Essential Oil in Liposomal Chitosan Emulsions to Improve the Shelf Life of Karish Cheese. Food Biosci. 2021, 43, 101230. [Google Scholar] [CrossRef]
- Chen, P.; Ference, C.; Sun, X.; Lin, Y.; Tan, L.; Zhong, T. Antimicrobial Efficacy of Liposome-Encapsulated Citral and Its Effect on the Shelf Life of Shatangju Mandarin. J. Food Prot. 2020, 83, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Assali, M.; Zaid, A.N. Features, Applications, and Sustainability of Lipid Nanoparticles in Cosmeceuticals. Saudi Pharm. J. 2022, 30, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Plyduang, T.; Atipairin, A.; Yoon, A.S.; Sermkaew, N.; Sakdiset, P.; Sawatdee, S. Formula Development of Red Palm (Elaeis guineensis) Fruit Extract Loaded with Solid Lipid Nanoparticles Containing Creams and Its Anti-Aging Efficacy in Healthy Volunteers. Cosmetics 2022, 9, 3. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Song, I.H.; Um, S.H. Role of Physicochemical Properties in Nanoparticle Toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Wu, Y.L.; Gong, Z.; Valiyaveettil, S. Toxicity of Silver Nanoparticles in Zebrafish Models. Nanotechnology 2008, 19, 255102. [Google Scholar] [CrossRef] [PubMed]
- Olugbodi, J.O.; Lawal, B.; Bako, G.; Onikanni, A.S.; Abolenin, S.M.; Mohammud, S.S.; Ataya, F.S.; Batiha, G.E.S. Effect of Sub-Dermal Exposure of Silver Nanoparticles on Hepatic, Renal and Cardiac Functions Accompanying Oxidative Damage in Male Wistar Rats. Sci. Rep. 2023, 13, 10539. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Hung, Y.C.; Liau, I.; Huang, G.S. Assessment of the in Vivo Toxicity of Gold Nanoparticles. Nanoscale Res. Lett. 2009, 4, 858–864. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Jin, T.; Behari, J. Dose-Dependent in-Vivo Toxicity Assessment of Silver Nanoparticle in Wistar Rats. Toxicol. Mech. Methods 2011, 21, 13–24. [Google Scholar] [CrossRef]
- Ali, S.A.; Rizk, M.Z.; Hamed, M.A.; Aboul-Ela, E.I.; El-Rigal, N.S.; Aly, H.F.; Abdel-Hamid, A.H.Z. Assessment of Titanium Dioxide Nanoparticles Toxicity via Oral Exposure in Mice: Effect of Dose and Particle Size. Biomarkers 2019, 24, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, F.; Bwatanglang, I.B.; Al-Lohedan, H.A.; Shaik, J.P.; Al-Tilasi, H.H.; Soleiman, A.A. Influence of Surface Coating towards the Controlled Toxicity of ZnO Nanoparticles In Vitro. Coatings 2023, 13, 172. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflore, O.B.; Ger, T.R.; Hsiao, C. Der Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef] [PubMed]
- Dönmez Güngüneş, Ç.; Şeker, Ş.; Elçin, A.E.; Elçin, Y.M. A Comparative Study on the in Vitro Cytotoxic Responses of Two Mammalian Cell Types to Fullerenes, Carbon Nanotubes and Iron Oxide Nanoparticles. Drug Chem. Toxicol. 2017, 40, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Al Alalaq, M.A.; Al–Hadedee, L.T.; Alrubeii, A.M.S. Effect of Iron Oxide Nanoparticles Prepared by Chemical Method on the Kidneys, Liver and Brain of Male Mice. IOP Conf. Ser. Earth Environ. Sci. 2023, 1252, 012132. [Google Scholar] [CrossRef]
- Abakumov, M.A.; Semkina, A.S.; Skorikov, A.S.; Vishnevskiy, D.A.; Ivanova, A.V.; Mironova, E.; Davydova, G.A.; Majouga, A.G.; Chekhonin, V.P. Toxicity of Iron Oxide Nanoparticles: Size and Coating Effects. J. Biochem. Mol. Toxicol. 2018, 32, e22225. [Google Scholar] [CrossRef] [PubMed]
- Madannejad, R.; Shoaie, N.; Jahanpeyma, F.; Darvishi, M.H.; Azimzadeh, M.; Javadi, H. Toxicity of Carbon-Based Nanomaterials: Reviewing Recent Reports in Medical and Biological Systems. Chem. Biol. Interact. 2019, 307, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Chiticaru, E.A.; Ionita, M. Graphene Toxicity and Future Perspectives in Healthcare and Biomedicine. FlatChem 2022, 35, 100417. [Google Scholar] [CrossRef]
- Jia, P.P.; Sun, T.; Junaid, M.; Yang, L.; Ma, Y.B.; Cui, Z.S.; Wei, D.P.; Shi, H.F.; Pei, D.S. Nanotoxicity of Different Sizes of Graphene (G) and Graphene Oxide (GO) in Vitro and in Vivo. Environ. Pollut. 2019, 247, 595–606. [Google Scholar] [CrossRef]
- Ghosh, S.; Chatterjee, K. Poly(Ethylene Glycol) Functionalized Graphene Oxide in Tissue Engineering: A Review on Recent Advances. Int. J. Nanomed. 2020, 15, 5991–6006. [Google Scholar] [CrossRef]
- Kazempour, M.; Namazi, H.; Akbarzadeh, A.; Kabiri, R. Synthesis and Characterization of PEG-Functionalized Graphene Oxide as an Effective PH-Sensitive Drug Carrier. Artif. Cells Nanomed. Biotechnol. 2019, 47, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Isakovic, A.; Markovic, Z.; Todorovic-Marcovic, B.; Nikolic, N.; Vranjes-Djuric, S.; Mirkovic, M.; Dramicanin, M.; Harhaji, L.; Raicevic, N.; Nikolic, Z.; et al. Distinct Cytotoxic Mechanisms of Pristine versus Hydroxylated Fullerene. Toxicol. Sci. 2006, 91, 173–183. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Bownik, A.; Dudka, J.; Kowal, K.; Ślaska, B. Daphnia Magna Model in the Toxicity Assessment of Pharmaceuticals: A Review. Sci. Total Environ. 2021, 763, 143038. [Google Scholar] [CrossRef] [PubMed]
- Lovern, S.B.; Klaper, R. Daphnia Magna Mortality When Exposed to Titanium Dioxide and Fullerene (C60) Nanoparticles. Environ. Toxicol. Chem. 2006, 25, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Huang, B.; Chen, Z.; Lv, X.; Qian, W.; Zhu, X.; Li, B.; Wang, Z.; Cai, Z. Behavioural and Chronic Toxicity of Fullerene to Daphnia Magna: Mechanisms Revealed by Transcriptomic Analysis. Environ. Pollut. 2019, 255, 113181. [Google Scholar] [CrossRef]
- Lv, X.; Huang, B.; Zhu, X.; Jiang, Y.; Chen, B.; Tao, Y.; Zhou, J.; Cai, Z. Mechanisms Underlying the Acute Toxicity of Fullerene to Daphnia Magna: Energy Acquisition Restriction and Oxidative Stress. Water Res. 2017, 123, 696–703. [Google Scholar] [CrossRef]
- Pesado-Gómez, C.; Serrano-García, J.S.; Amaya-Flórez, A.; Pesado-Gómez, G.; Soto-Contreras, A.; Morales-Morales, D.; Colorado-Peralta, R. Fullerenes: Historical Background, Novel Biological Activities versus Possible Health Risks. Coord. Chem. Rev. 2024, 501, 215550. [Google Scholar] [CrossRef]
- Aschberger, K.; Johnston, H.J.; Stone, V.; Aitken, R.J.; Tran, C.L.; Hankin, S.M.; Peters, S.A.K.; Christensen, F.M. Review of Fullerene Toxicity and Exposure—Appraisal of a Human Health Risk Assessment, Based on Open Literature. Regul. Toxicol. Pharmacol. 2010, 58, 455–473. [Google Scholar] [CrossRef]
- Pikula, K.; Johari, S.A.; Santos-Oliveira, R.; Golokhvast, K. The Comparative Toxic Impact Assessment of Carbon Nanotubes, Fullerene, Graphene, and Graphene Oxide on Marine Microalgae Porphyridium Purpureum. Toxics 2023, 11, 491. [Google Scholar] [CrossRef]
- Pikula, K.; Johari, S.A.; Santos-Oliveira, R.; Golokhvast, K. Toxicity and Biotransformation of Carbon-Based Nanomaterials in Marine Microalgae Heterosigma Akashiwo. Int. J. Mol. Sci. 2023, 24, 10020. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Cheng, W.; Li, Y.; Shi, T.; Jiang, Y.; Wang, T.; Wang, H.; Ren, D.; Zhang, R.; et al. Chronic Carbon Black Nanoparticles Exposure Increases Lung Cancer Risk by Affecting the Cell Cycle via Circulatory Inflammation. Environ. Pollut. 2022, 305, 119293. [Google Scholar] [CrossRef] [PubMed]
- Boland, S.; Hussain, S.; Baeza-Squiban, A. Carbon Black and Titanium Dioxide Nanoparticles Induce Distinct Molecular Mechanisms of Toxicity. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Vesterdal, L.K.; Mikkelsen, L.; Folkmann, J.K.; Sheykhzade, M.; Cao, Y.; Roursgaard, M.; Loft, S.; Møller, P. Carbon Black Nanoparticles and Vascular Dysfunction in Cultured Endothelial Cells and Artery Segments. Toxicol. Lett. 2012, 214, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Guo, A.; Wang, S.; Man, S.; Zhang, Y.; Liu, S.; Liu, Y. From the Lung to the Knee Joint: Toxicity Evaluation of Carbon Black Nanoparticles on Macrophages and Chondrocytes. J. Hazard. Mater. 2018, 353, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, M.; Bhandari, B.; Yang, C. Recent Development of Carbon Quantum Dots: Biological Toxicity, Antibacterial Properties and Application in Foods. Food Rev. Int. 2022, 38, 1513–1532. [Google Scholar] [CrossRef]
- Chen, J.; Sun, D.; Cui, H.; Rao, C.; Li, L.; Guo, S.; Yang, S.; Zhang, Y.; Cao, X. Toxic Effects of Carbon Quantum Dots on the Gut–Liver Axis and Gut Microbiota in the Common Carp Cyprinus Carpio. Environ. Sci. Nano 2022, 9, 173–188. [Google Scholar] [CrossRef]
- Yao, K.; Lv, X.; Zheng, G.; Chen, Z.; Jiang, Y.; Zhu, X.; Wang, Z.; Cai, Z. Effects of Carbon Quantum Dots on Aquatic Environments: Comparison of Toxicity to Organisms at Different Trophic Levels. Environ. Sci. Technol. 2018, 52, 14445–14451. [Google Scholar] [CrossRef] [PubMed]
- Qiang, S.; Zhang, L.; Li, Z.; Liang, J.; Li, P.; Song, J.; Guo, K.; Wang, Z.; Fan, Q. New Insights into the Cellular Toxicity of Carbon Quantum Dots to Escherichia coli. Antioxidants 2022, 11, 2475. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Kashyap, S.; Yadav, U.; Srivastava, A.; Singh, A.V.; Singh, R.K.; Singh, S.K.; Saxena, P.S. Nitrogen Doped Carbon Quantum Dots Demonstrate No Toxicity under in Vitro Conditions in a Cervical Cell Line and in Vivo in Swiss Albino Mice. Toxicol. Res. 2019, 8, 395–406. [Google Scholar] [CrossRef]
- Zhang, S.; Pei, X.; Xue, Y.; Xiong, J.; Wang, J. Bio-Safety Assessment of Carbon Quantum Dots, N-Doped and Folic Acid Modified Carbon Quantum Dots: A Systemic Comparison. Chin. Chem. Lett. 2020, 31, 1654–1659. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is Nano Safe in Foods? Establishing the Factors Impacting the Gastrointestinal Fate and Toxicity of Organic and Inorganic Food-Grade Nanoparticles. NPJ Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef]
- Zoe, L.H.; David, S.R.; Rajabalaya, R. Chitosan Nanoparticle Toxicity: A Comprehensive Literature Review of in Vivo and in Vitro Assessments for Medical Applications. Toxicol. Rep. 2023, 11, 83–106. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.H.; Locatelli, C.; Filippin-Monteiro, F.B.; Zanetti-Ramos, B.G.; Conte, A.; Creczynski-Pasa, T.B. Solid Lipid Nanoparticles Induced Hematological Changes and Inflammatory Response in Mice. Nanotoxicology 2014, 8, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Cambre, M.; Lee, H.J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Li, M.; Zhang, J.; Lee, C.S. Different Strategies for Organic Nanoparticle Preparation in Biomedicine. ACS Mater. Lett. 2020, 2, 531–549. [Google Scholar] [CrossRef]
- Wang, Z.L. Characterization of Nanophase Materials; Wiley-VCH: Weinheim, Germany, 2000; ISBN 3527298371. [Google Scholar]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Mukherjee, B.; Dey, N.S.; Maji, R.; Bhowmik, P.; Das, P.J.; Paul, P. Current Status and Future Scope for Nanomaterials in Drug Delivery. In Application of Nanotechnology in Drug Delivery; IntechOpen: Rijeka, Croatia, 2014. [Google Scholar] [CrossRef]
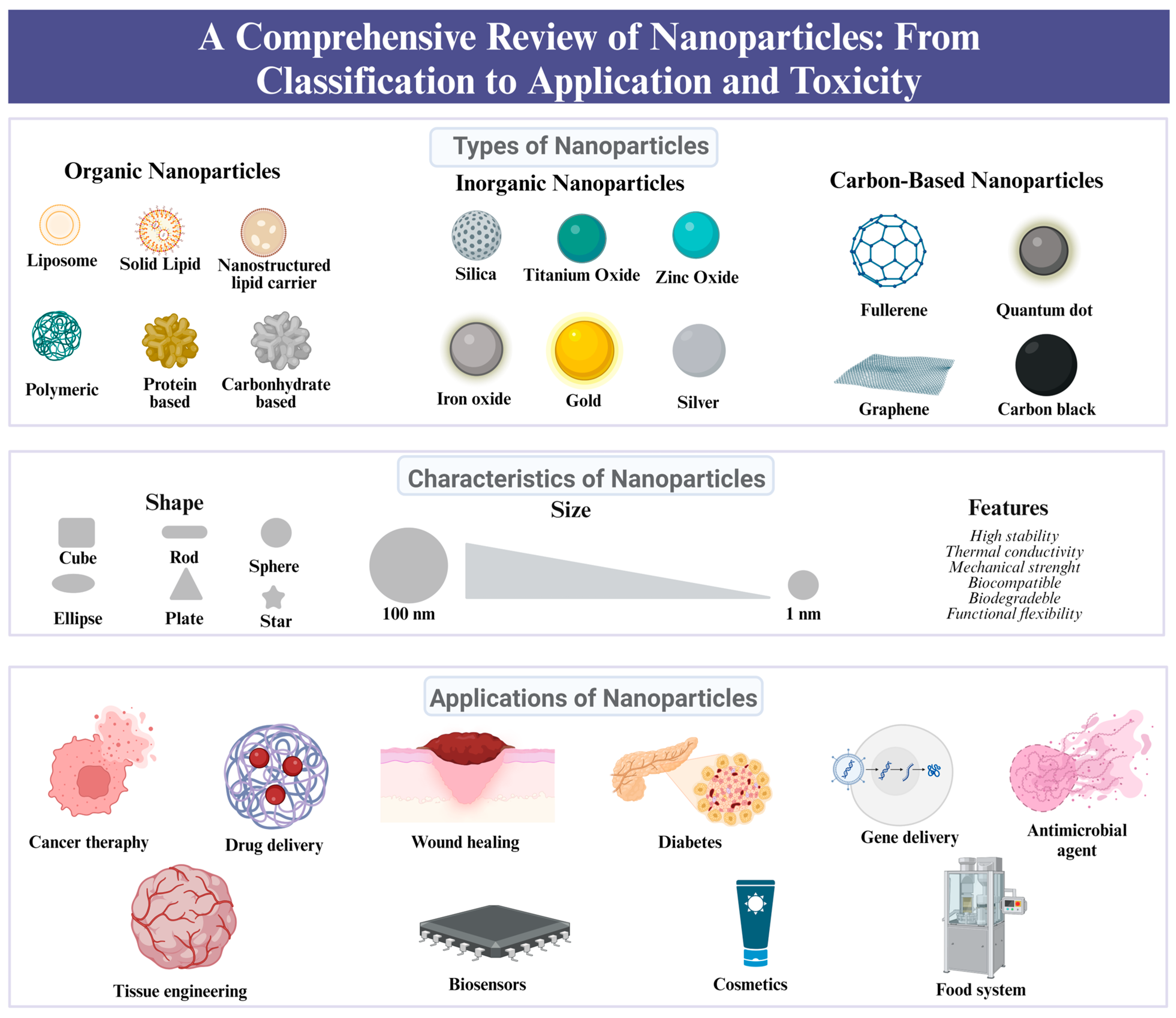

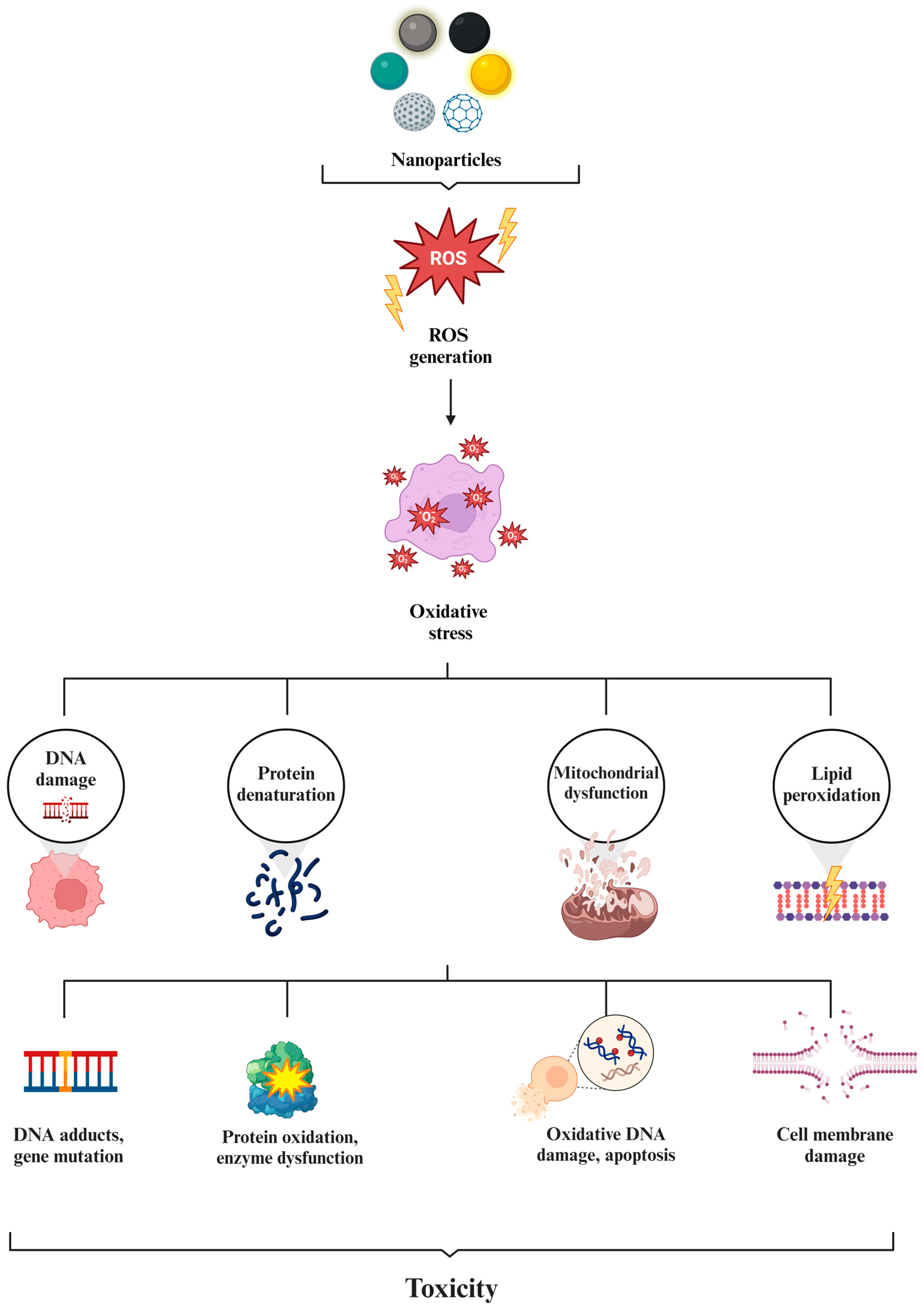
| Type of the NP | Effective Properties | Area of Application | References |
|---|---|---|---|
| Magnetic NP |
|
| [24,97,98,99] |
| Ceramic NP |
|
| [32,34,35] |
| Semiconductor NP |
|
| [36,38,100,101,102] |
| Graphene NP |
|
| [56,103,104,105] |
| Fullerene NP |
|
| [70,106,107,108] |
| Carbon black NP |
|
| [75,109,110] |
| Carbon quantum dots |
|
| [85,94,111] |
| Chitosan NPs |
|
| [112,113,114,115,116] |
| Alginate NPs |
|
| [117,118,119] |
| PLA/PLGA NPs |
|
| [120,121,122,123,124] |
| Liposome NPs |
|
| [92,125,126,127] |
| Solid-lipid NPs |
|
| [128,129,130,131] |
| Nanostructured lipid carriers |
|
| [132,133,134,135] |
| Carbohydrate-based NP |
|
| [117,136,137,138] |
| Protein-based NP |
|
| [139,140,141] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eker, F.; Duman, H.; Akdaşçi, E.; Bolat, E.; Sarıtaş, S.; Karav, S.; Witkowska, A.M. A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity. Molecules 2024, 29, 3482. https://doi.org/10.3390/molecules29153482
Eker F, Duman H, Akdaşçi E, Bolat E, Sarıtaş S, Karav S, Witkowska AM. A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity. Molecules. 2024; 29(15):3482. https://doi.org/10.3390/molecules29153482
Chicago/Turabian StyleEker, Furkan, Hatice Duman, Emir Akdaşçi, Ecem Bolat, Sümeyye Sarıtaş, Sercan Karav, and Anna Maria Witkowska. 2024. "A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity" Molecules 29, no. 15: 3482. https://doi.org/10.3390/molecules29153482
APA StyleEker, F., Duman, H., Akdaşçi, E., Bolat, E., Sarıtaş, S., Karav, S., & Witkowska, A. M. (2024). A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity. Molecules, 29(15), 3482. https://doi.org/10.3390/molecules29153482