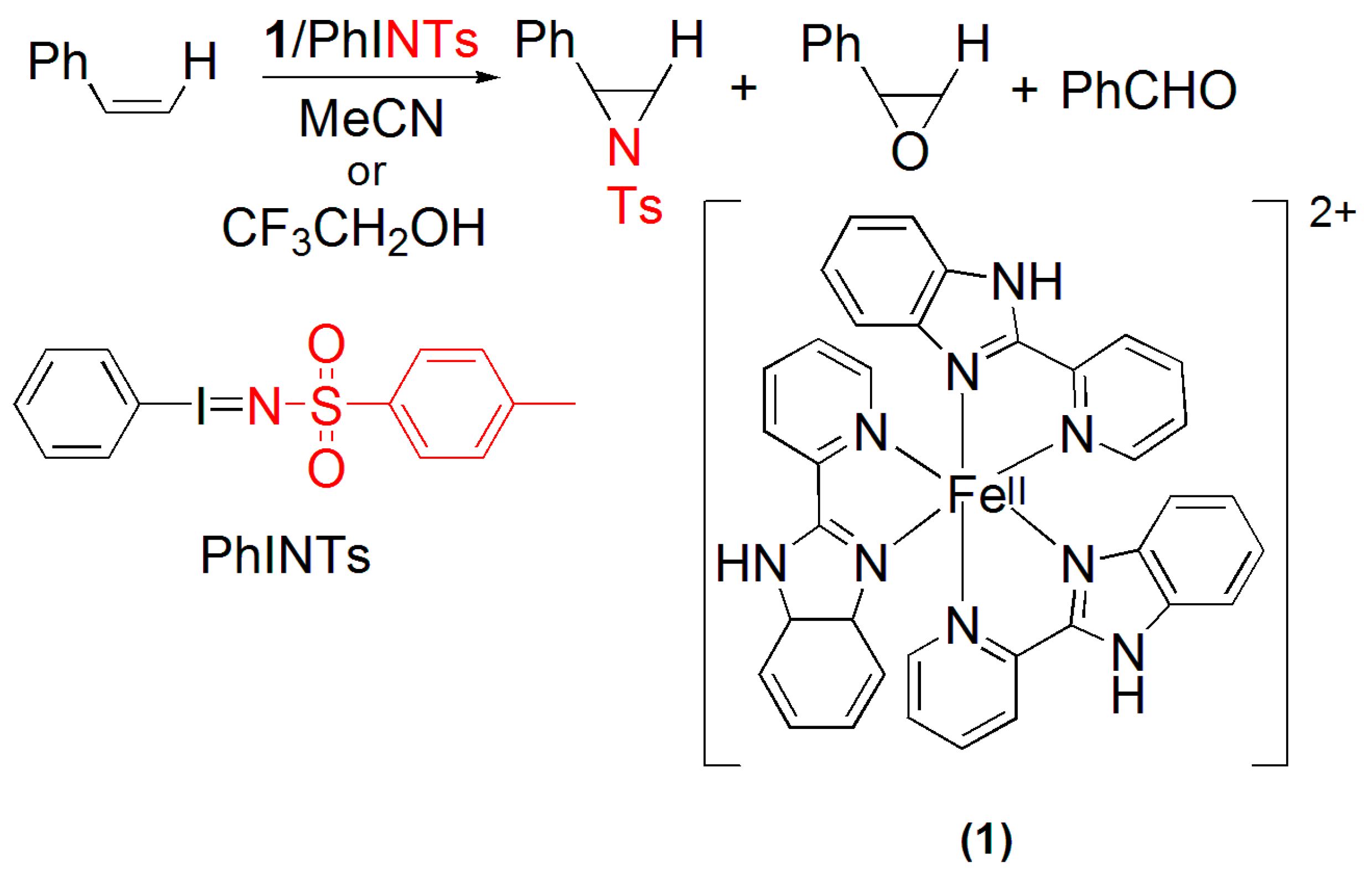
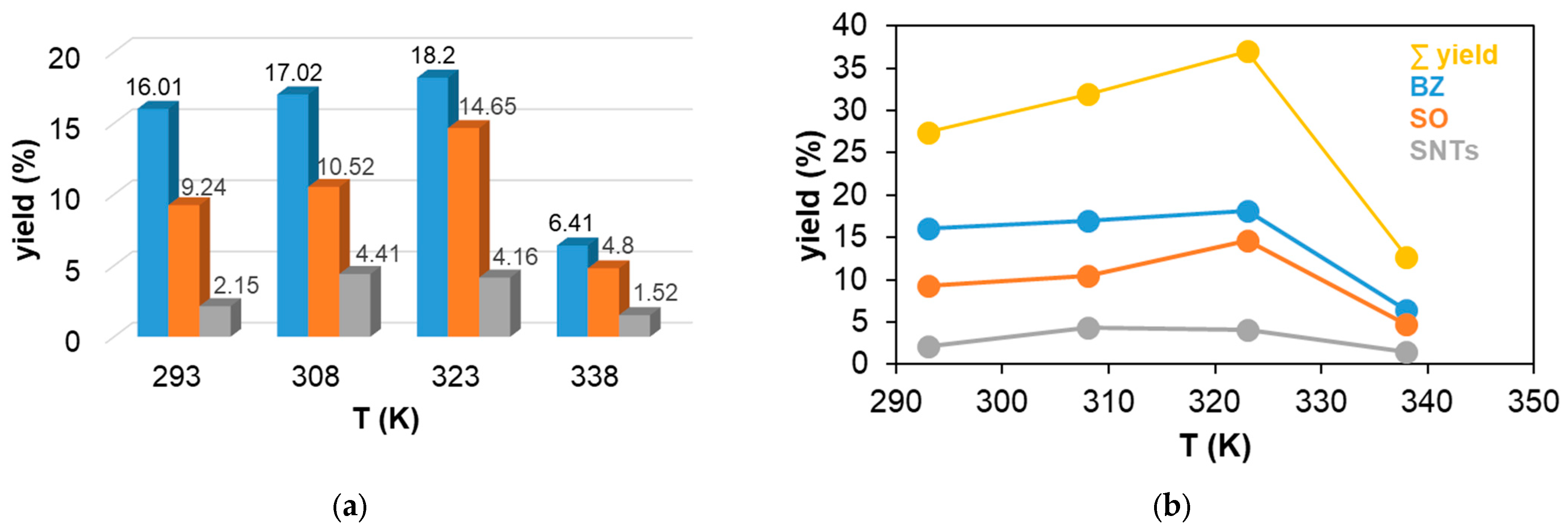Scheme 1.
Products formed during stoichiometric and catalytic aziridination of styrene and structures of PhINTs and [FeII(PBI)3(CF3SO3)2] (1).
Scheme 1.
Products formed during stoichiometric and catalytic aziridination of styrene and structures of PhINTs and [FeII(PBI)3(CF3SO3)2] (1).
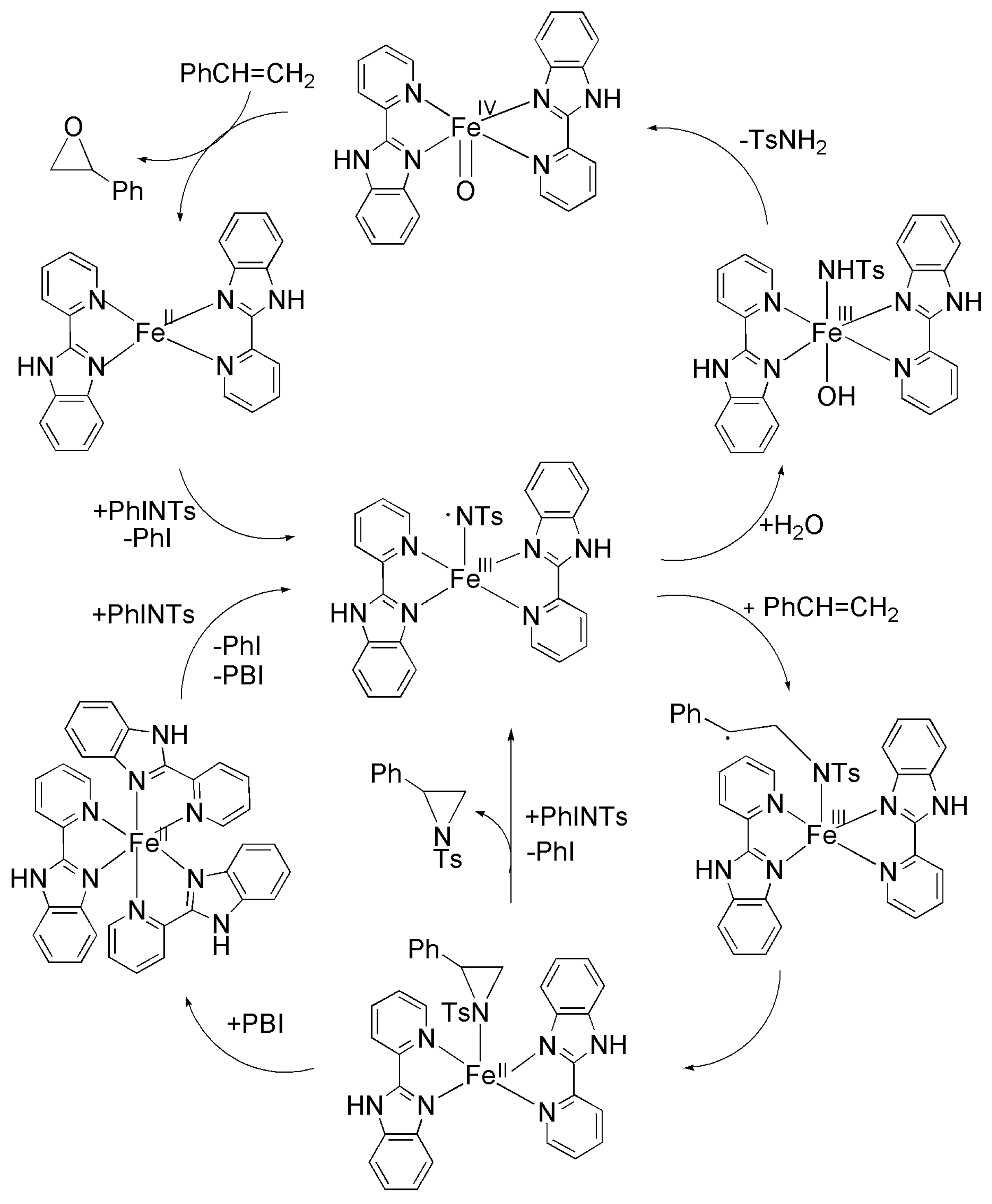
Scheme 2.
The proposed mechanisms for the FeIII(NTs)-mediated aziridination reactions.
Scheme 2.
The proposed mechanisms for the FeIII(NTs)-mediated aziridination reactions.
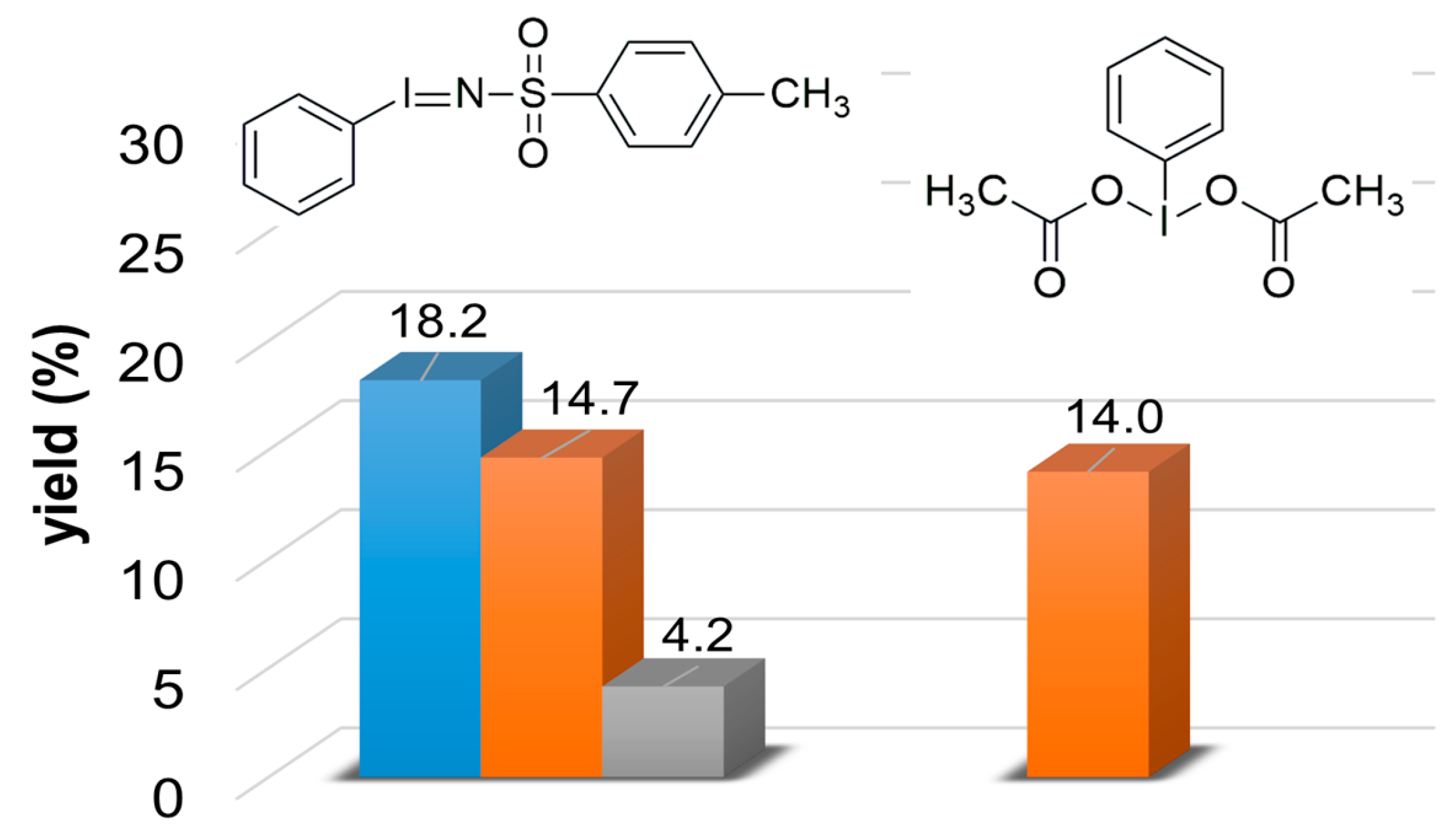
Figure 1.
The yield of products for the catalytic oxidation of styrene at 323 K in MeCN with PhINTs and PhI(OAc)2 (PhIO): benzaldehyde (▪), styrene oxide (▪), and 2-phenyl-1-tosylaziridine (▪). [1]0 = 1 × 10−3 M, [PhIO or PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Figure 1.
The yield of products for the catalytic oxidation of styrene at 323 K in MeCN with PhINTs and PhI(OAc)2 (PhIO): benzaldehyde (▪), styrene oxide (▪), and 2-phenyl-1-tosylaziridine (▪). [1]0 = 1 × 10−3 M, [PhIO or PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
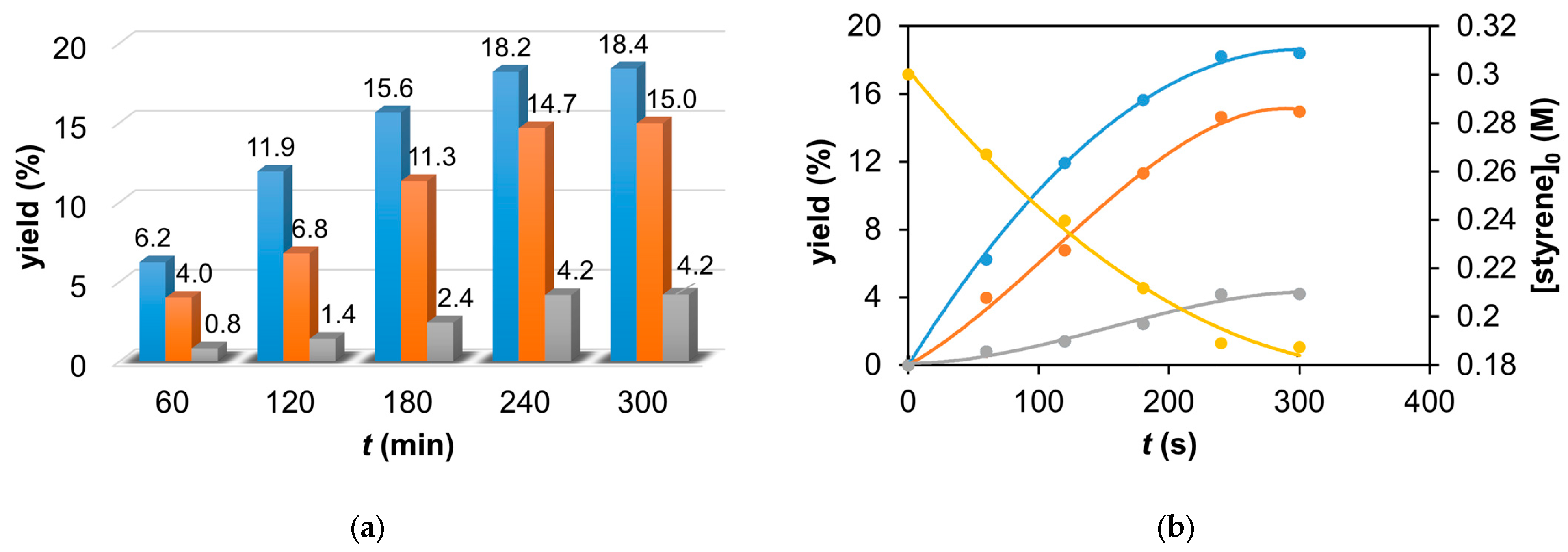
Figure 2.
The catalytic oxidation of styrene at 323 K in MeCN at different times. (a) The yields of benzaldehyde (▪), styrene oxide (▪), and 2-phenyl-1-tosylaziridine (▪) for this reaction. (b) The yields of benzaldehyde (▪), styrene oxide (▪), and 2-phenyl-1-tosylaziridine (▪) and the decrease in the styrene concentration (▪) as a function of time for the catalytic oxidation of styrene at 323 K in MeCN. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Figure 2.
The catalytic oxidation of styrene at 323 K in MeCN at different times. (a) The yields of benzaldehyde (▪), styrene oxide (▪), and 2-phenyl-1-tosylaziridine (▪) for this reaction. (b) The yields of benzaldehyde (▪), styrene oxide (▪), and 2-phenyl-1-tosylaziridine (▪) and the decrease in the styrene concentration (▪) as a function of time for the catalytic oxidation of styrene at 323 K in MeCN. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
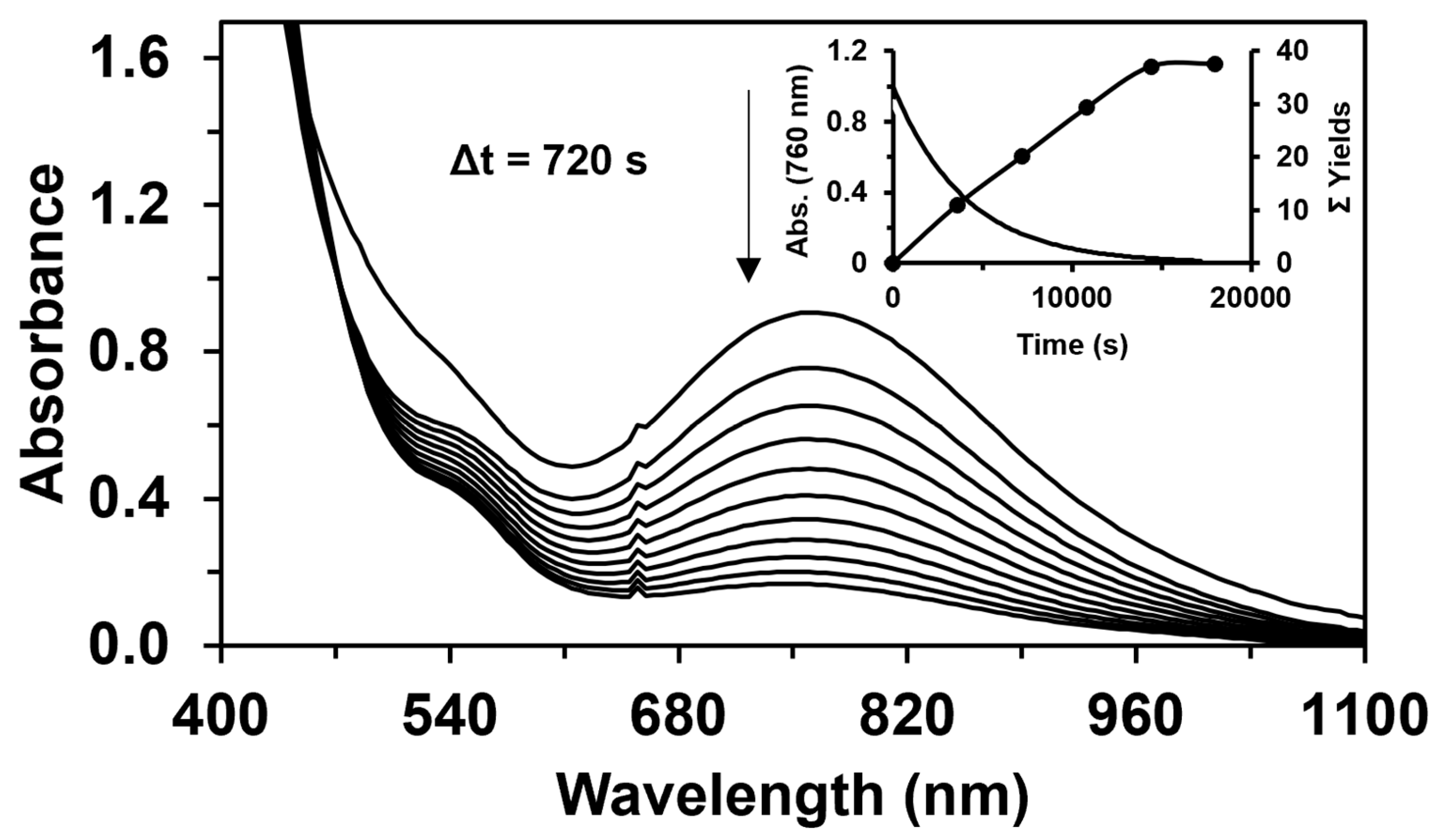
Figure 3.
The self-decay of 1 in the absence of a substrate at 323 K in MeCN. [1]0= 1 × 10−3 M; [PhINTs]0 = 1.2 × 10−3 M. Inset: the change in the absorbance of the 1/PhINTs adduct at 760 nm and the total yield as a function of time for the catalytic oxidation of styrene at 323 K in MeCN. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M.
Figure 3.
The self-decay of 1 in the absence of a substrate at 323 K in MeCN. [1]0= 1 × 10−3 M; [PhINTs]0 = 1.2 × 10−3 M. Inset: the change in the absorbance of the 1/PhINTs adduct at 760 nm and the total yield as a function of time for the catalytic oxidation of styrene at 323 K in MeCN. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M.
Figure 4.
The catalytic oxidation of styrene at different temperatures in MeCN. (a) The yields of Bz, benzaldehyde (▪); SO, styrene oxide (▪); and SNTs, 2-phenyl-1-tosylaziridine (▪) for this reaction. (b) The yields of Bz (▪), SO (▪), and SNTs (▪) and the total yield (▪) as a function of temperature for the catalytic oxidation of styrene in MeCN. [1]0= 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Figure 4.
The catalytic oxidation of styrene at different temperatures in MeCN. (a) The yields of Bz, benzaldehyde (▪); SO, styrene oxide (▪); and SNTs, 2-phenyl-1-tosylaziridine (▪) for this reaction. (b) The yields of Bz (▪), SO (▪), and SNTs (▪) and the total yield (▪) as a function of temperature for the catalytic oxidation of styrene in MeCN. [1]0= 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
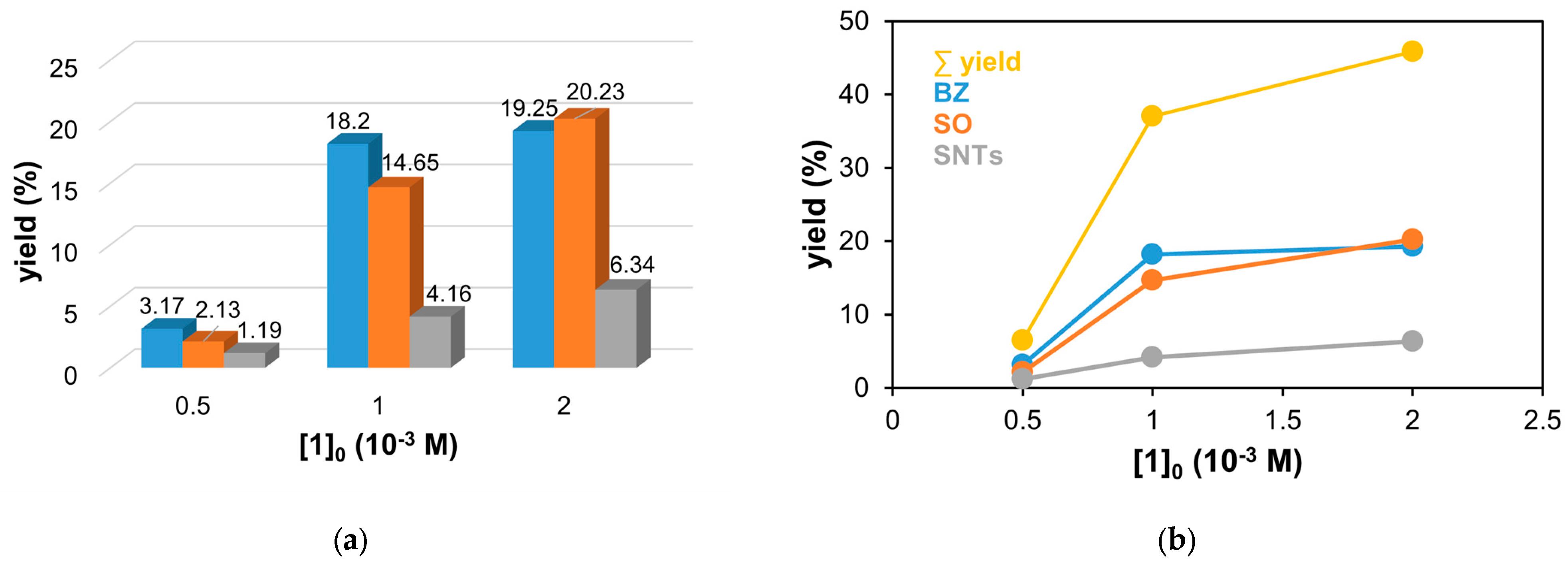
Figure 5.
The catalytic oxidation of styrene at different metal concentrations in MeCN at 323 K. (a) The yields of Bz, benzaldehyde (▪); SO, styrene oxide (▪); and SNTs, 2-phenyl-1-tosylaziridine (▪) for this reaction. (b) The yields of benzaldehyde (▪), styrene oxide (▪), and 2-phenyl-1-tosylaziridine (▪) and the total yield (▪) as a function of the iron concentration for the catalytic oxidation of styrene in MeCN at 323 K. [PhINTs]0 = 1 × 10−1 M; [styrene]0 = 3 × 10−1 M under air.
Figure 5.
The catalytic oxidation of styrene at different metal concentrations in MeCN at 323 K. (a) The yields of Bz, benzaldehyde (▪); SO, styrene oxide (▪); and SNTs, 2-phenyl-1-tosylaziridine (▪) for this reaction. (b) The yields of benzaldehyde (▪), styrene oxide (▪), and 2-phenyl-1-tosylaziridine (▪) and the total yield (▪) as a function of the iron concentration for the catalytic oxidation of styrene in MeCN at 323 K. [PhINTs]0 = 1 × 10−1 M; [styrene]0 = 3 × 10−1 M under air.
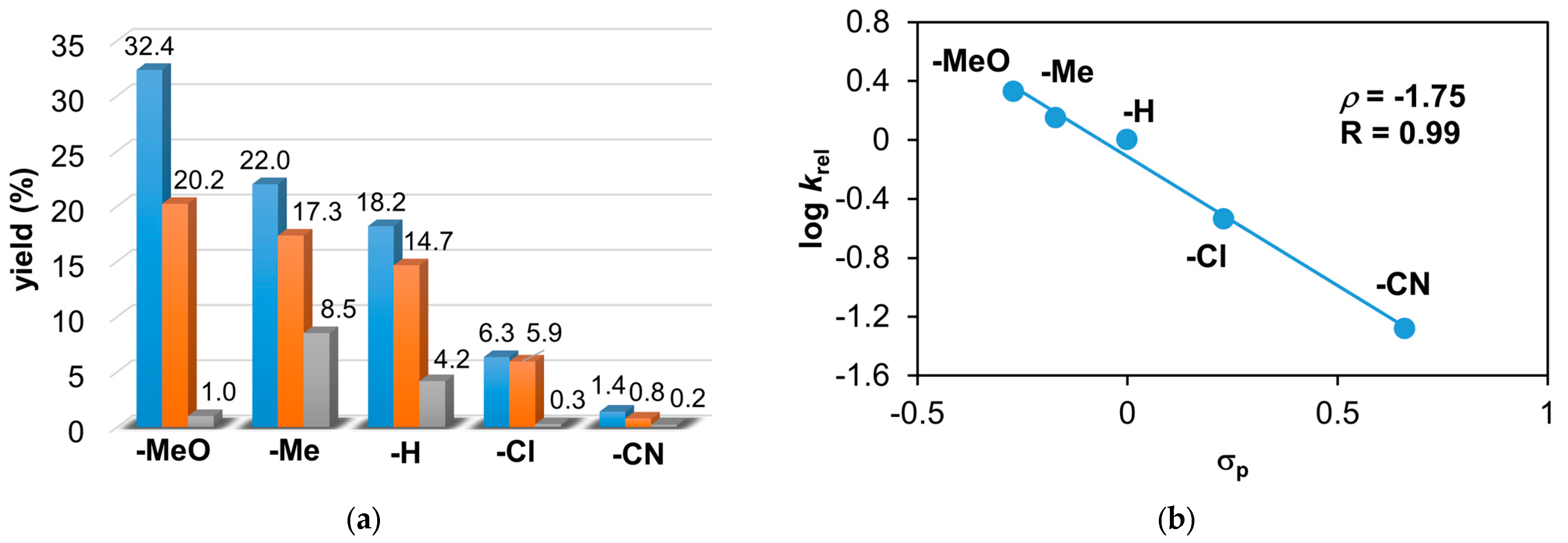
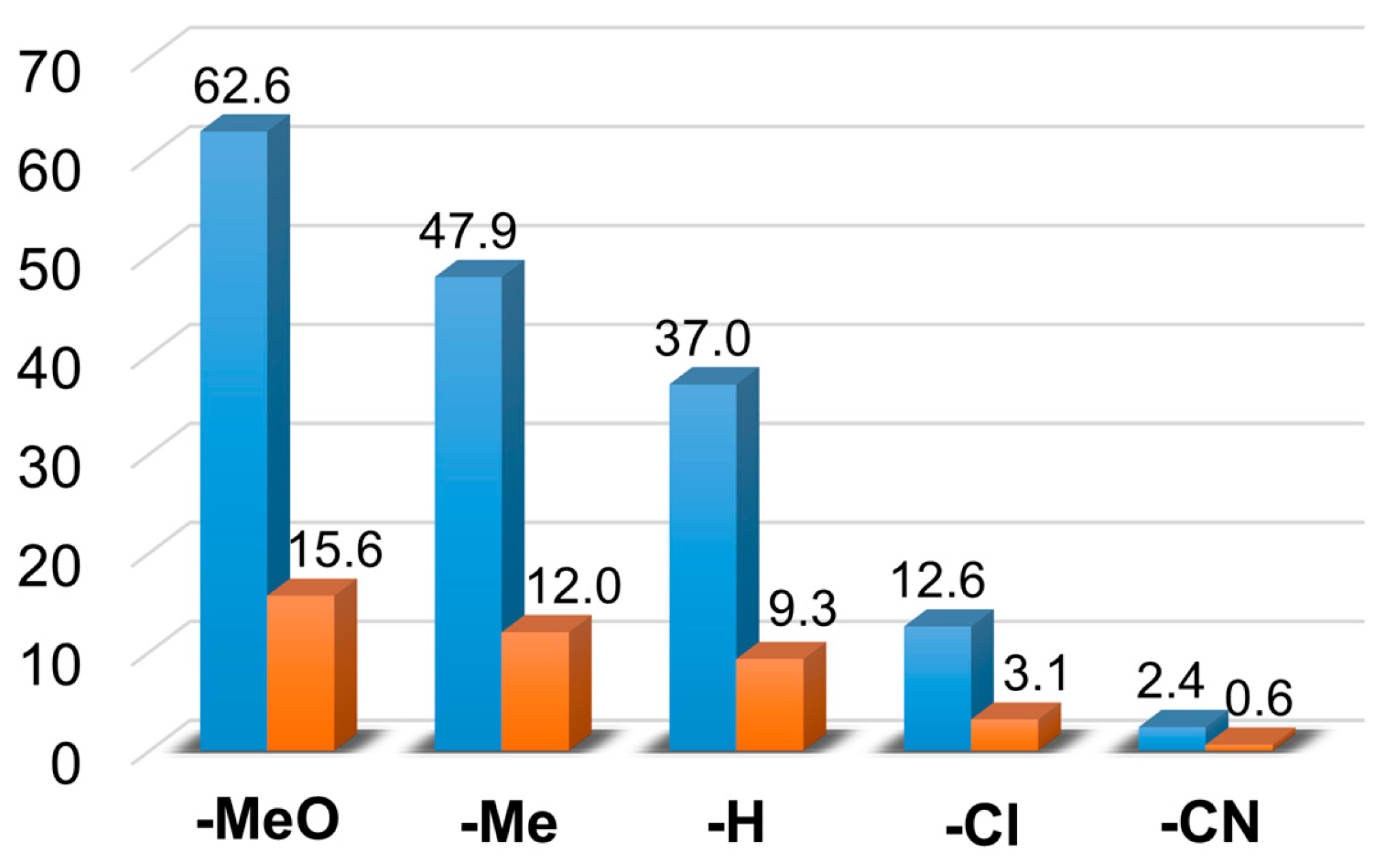
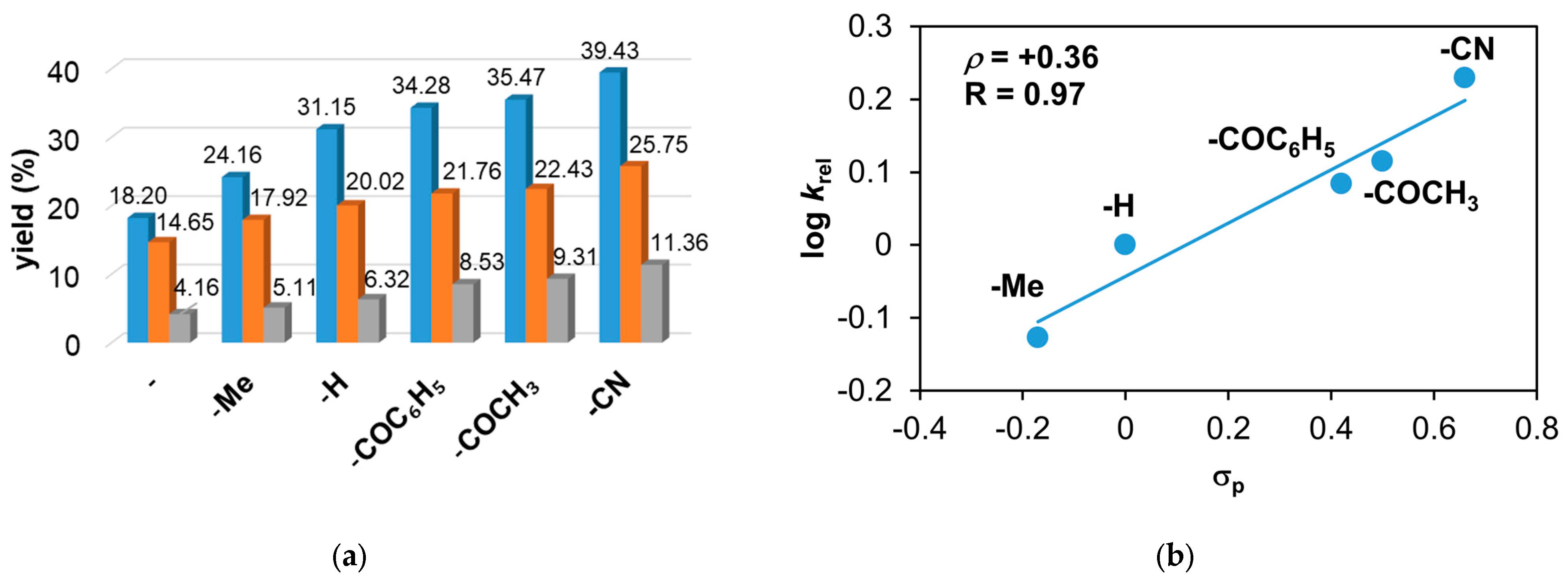
Figure 6.
The catalytic oxidation of different para-substituted styrenes at 323 K in MeCN. (a) The yields of products for this reaction: aldehyde (▪), styrene oxide (▪), and tosylaziridine (▪). (b) The plot of logkrel against σp of para-substituted styrenes. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Figure 6.
The catalytic oxidation of different para-substituted styrenes at 323 K in MeCN. (a) The yields of products for this reaction: aldehyde (▪), styrene oxide (▪), and tosylaziridine (▪). (b) The plot of logkrel against σp of para-substituted styrenes. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
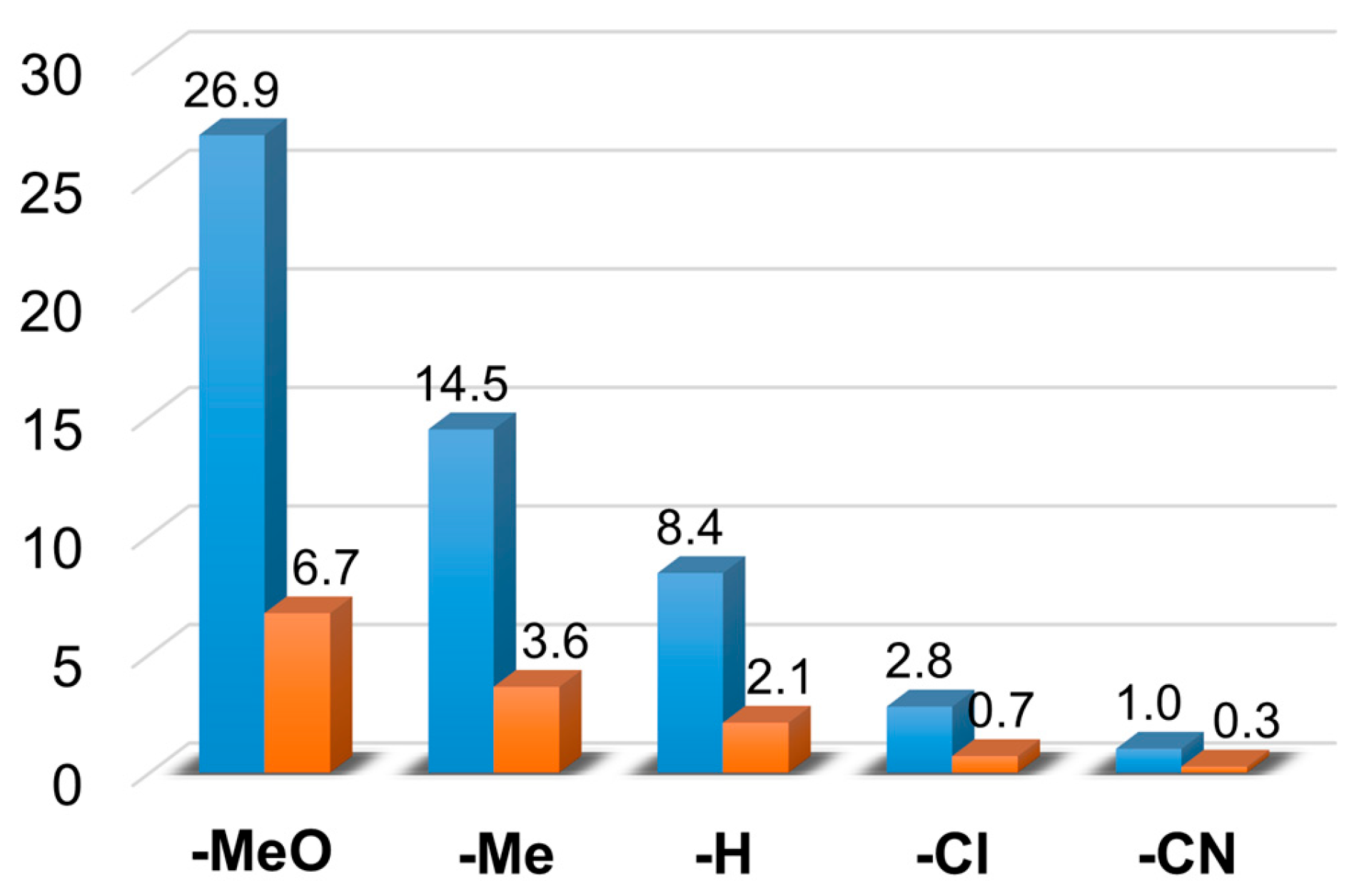
Figure 7.
The TON (▪) and TOF (▪) (1/h) values for the catalytic oxidation of para-substituted styrenes at 323 K in MeCN. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Figure 7.
The TON (▪) and TOF (▪) (1/h) values for the catalytic oxidation of para-substituted styrenes at 323 K in MeCN. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
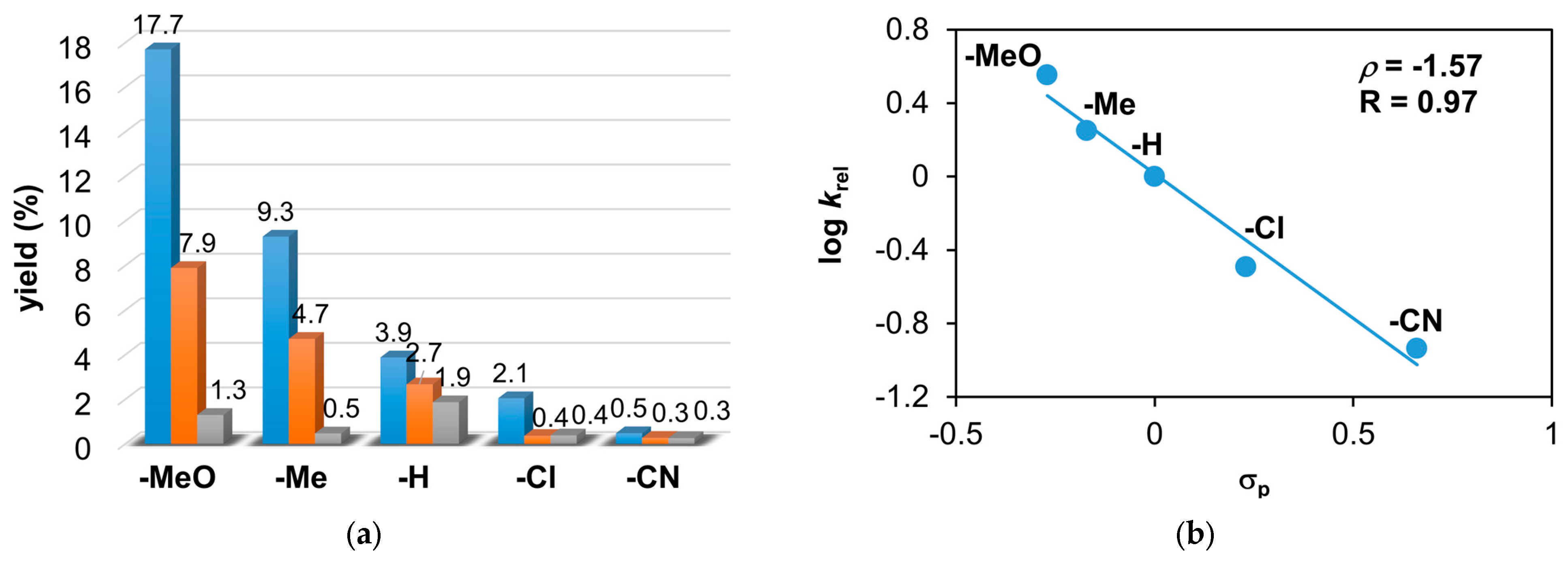
Figure 8.
The catalytic oxidation of different para-substituted styrenes at 323 K in CF3CH2OH. (a) The yields of products for this reaction: aldehyde (▪), styrene oxide (▪), and tosylaziridine (▪), (b) The plot of logkrel against σp of para-substituted styrenes. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Figure 8.
The catalytic oxidation of different para-substituted styrenes at 323 K in CF3CH2OH. (a) The yields of products for this reaction: aldehyde (▪), styrene oxide (▪), and tosylaziridine (▪), (b) The plot of logkrel against σp of para-substituted styrenes. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Figure 9.
The TON (▪) and TOF (▪) (1/h) values for the catalytic oxidation of para-substituted styrenes at 323 K in CF3CH2OH. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Figure 9.
The TON (▪) and TOF (▪) (1/h) values for the catalytic oxidation of para-substituted styrenes at 323 K in CF3CH2OH. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
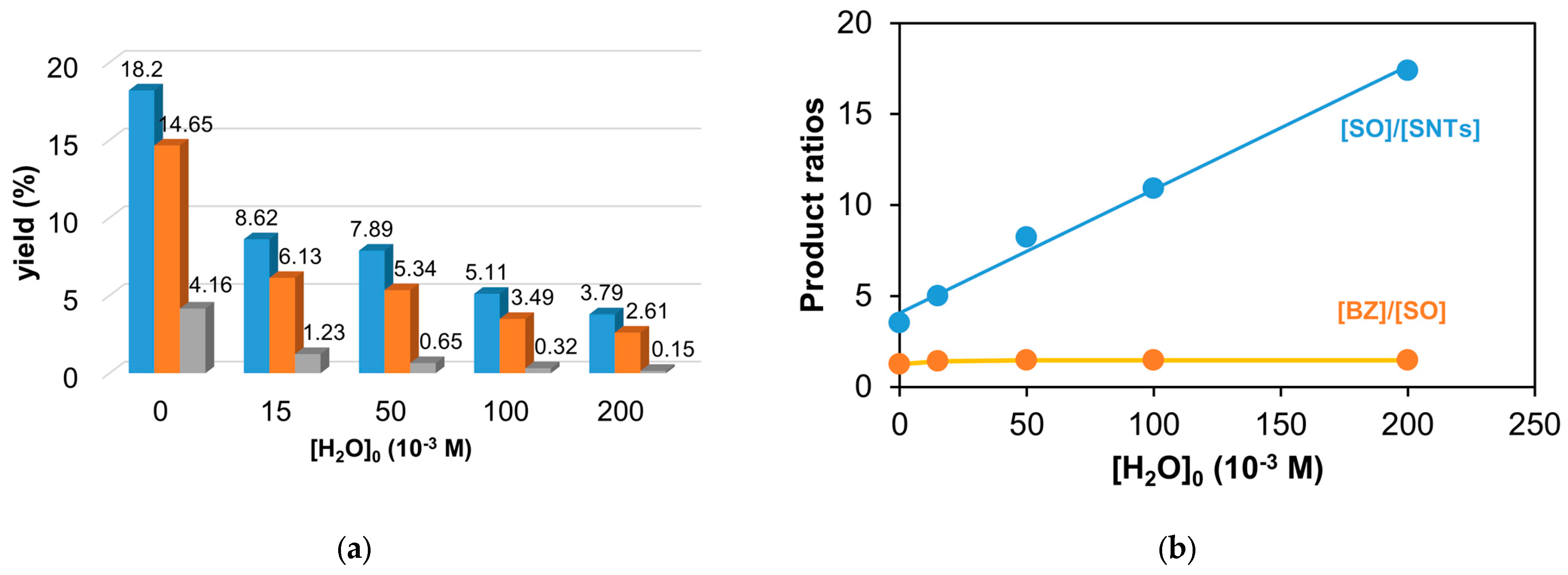
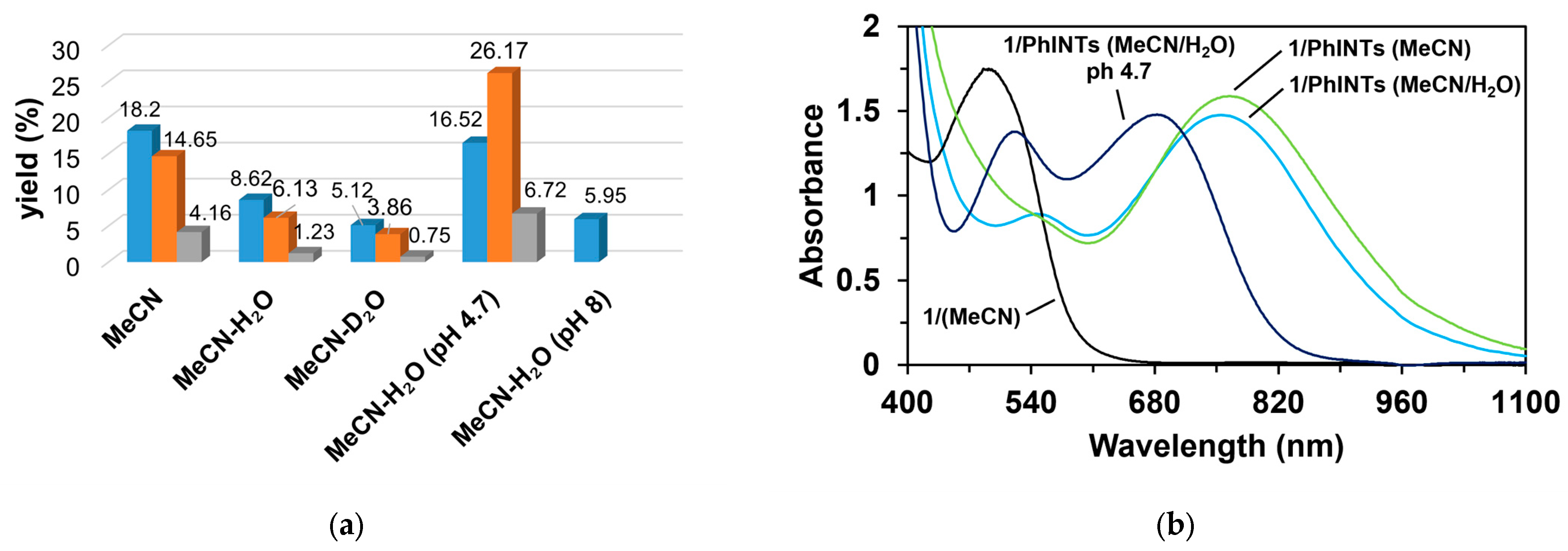
Figure 10.
The effect of water for the catalytic oxidation of styrene. (a) The yields of products for this reaction (Bz, benzaldehyde (▪); SO, styrene oxide (▪); and SNTs, 2-phenyl-1-tosylaziridine (▪)). (b) The epoxide/aziridine ratio and the benzaldehyde/epoxide ratio as functions of the H2O concentration; [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Figure 10.
The effect of water for the catalytic oxidation of styrene. (a) The yields of products for this reaction (Bz, benzaldehyde (▪); SO, styrene oxide (▪); and SNTs, 2-phenyl-1-tosylaziridine (▪)). (b) The epoxide/aziridine ratio and the benzaldehyde/epoxide ratio as functions of the H2O concentration; [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Figure 11.
The effects of water, D2O, and the buffer for the catalytic oxidation of styrene. (a) The yields of products for this reaction: benzaldehyde (▪), styrene oxide (▪), and 2-phenyl-1-tosylaziridine (▪). (b) The change in wavelength due to water and the pH 4.7 buffer. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, [styrene]0 = 3 × 10−1 M, [H2O, D2O]0 = 1.5 × 10−2 M, and [buffer]0 = 2 × 10−1 mL under air.
Figure 11.
The effects of water, D2O, and the buffer for the catalytic oxidation of styrene. (a) The yields of products for this reaction: benzaldehyde (▪), styrene oxide (▪), and 2-phenyl-1-tosylaziridine (▪). (b) The change in wavelength due to water and the pH 4.7 buffer. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, [styrene]0 = 3 × 10−1 M, [H2O, D2O]0 = 1.5 × 10−2 M, and [buffer]0 = 2 × 10−1 mL under air.
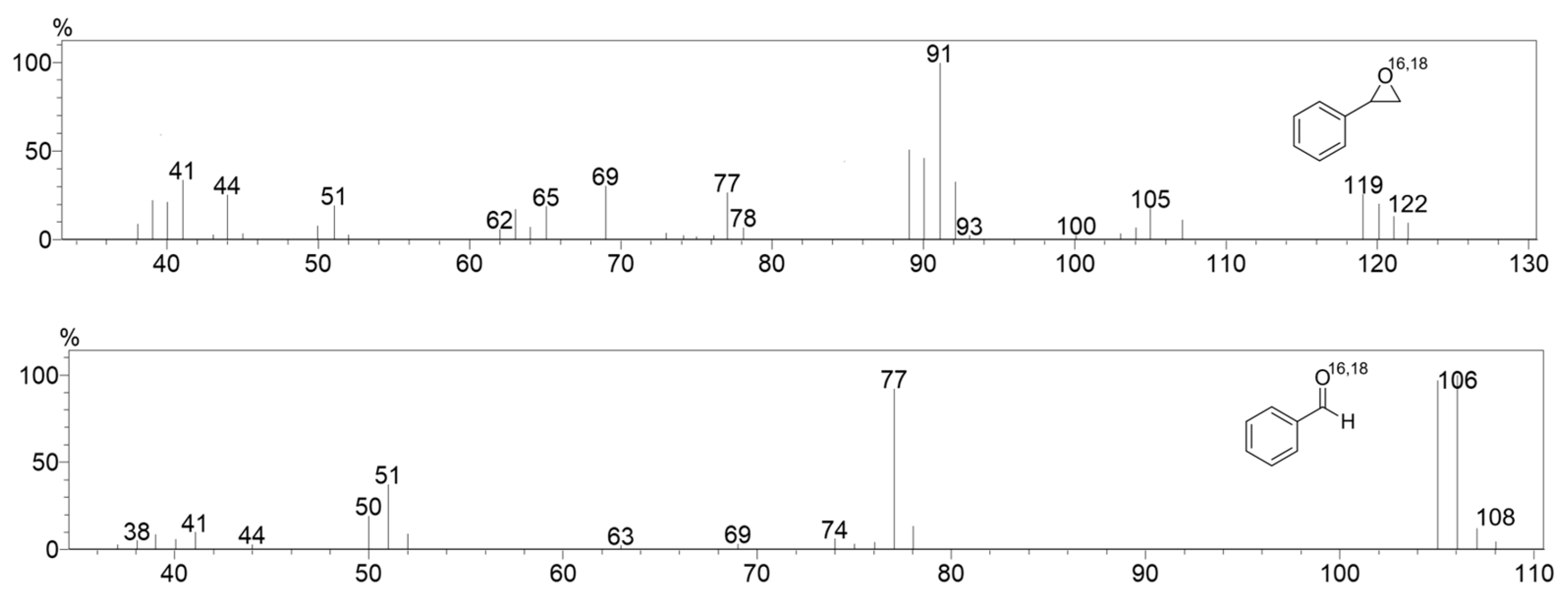
Figure 12.
The GC-MS analysis for the catalytic oxidation of styrene in MeCN at 323 K in the presence of water, H2O18. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, [styrene]0 = 3 × 10−1 M, and [H2O18]0 = 1.5 × 10−2 M under air.
Figure 12.
The GC-MS analysis for the catalytic oxidation of styrene in MeCN at 323 K in the presence of water, H2O18. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, [styrene]0 = 3 × 10−1 M, and [H2O18]0 = 1.5 × 10−2 M under air.
Figure 13.
The catalytic oxidation of different para-substituted pyridines at 323 K in MeCN. (a) The yields of products for this reaction: benzaldehyde (▪), styrene oxide (▪), and 2-phenyl-1-tosylaziridine (▪). (b) The plot of logkrel against σp of para-substituted pyridines. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, [styrene]0 = 3 × 10−1 M, and [para-substituted pyridine]0 = 1 × 10−2 M under air.
Figure 13.
The catalytic oxidation of different para-substituted pyridines at 323 K in MeCN. (a) The yields of products for this reaction: benzaldehyde (▪), styrene oxide (▪), and 2-phenyl-1-tosylaziridine (▪). (b) The plot of logkrel against σp of para-substituted pyridines. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, [styrene]0 = 3 × 10−1 M, and [para-substituted pyridine]0 = 1 × 10−2 M under air.
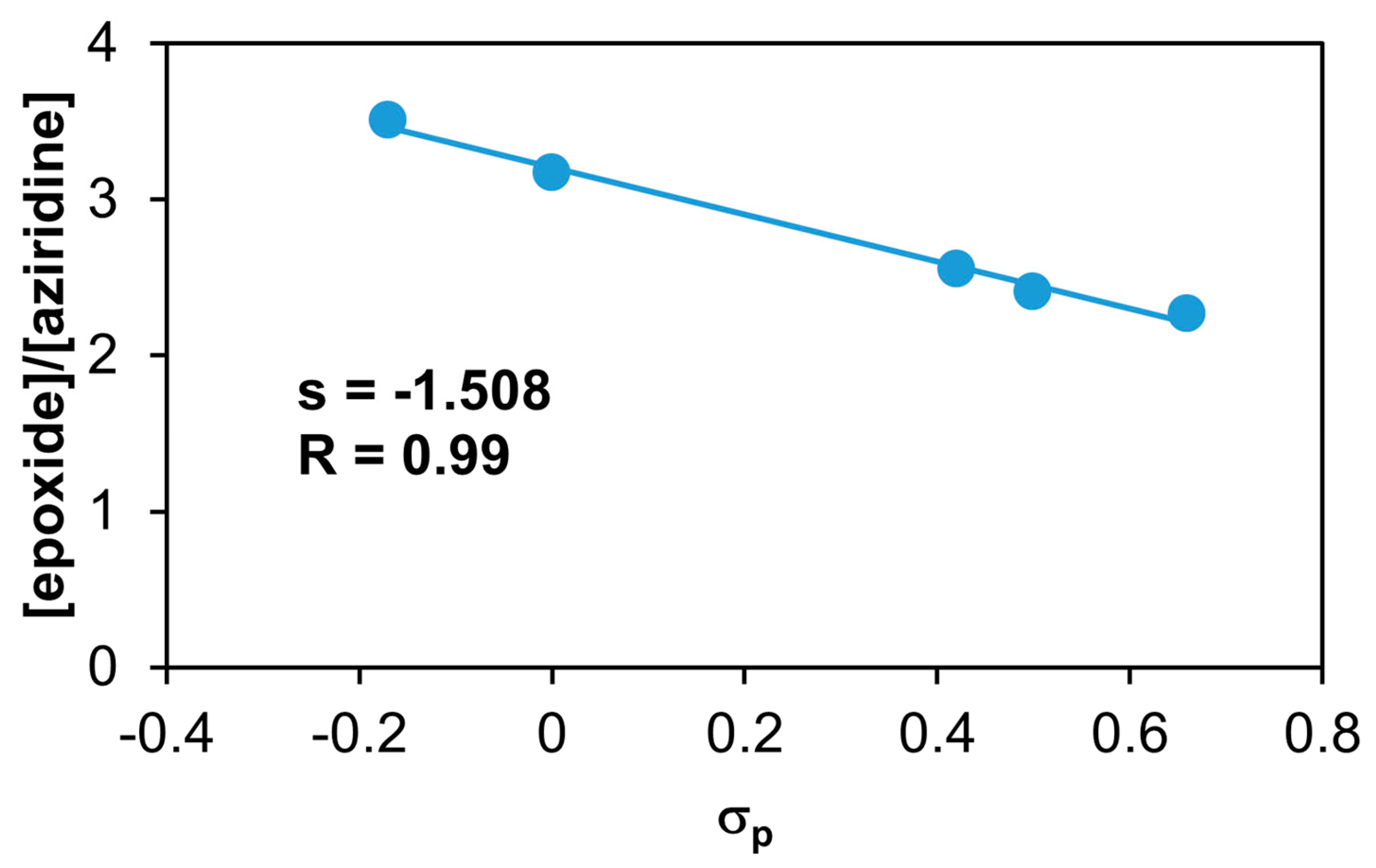
Figure 14.
The catalytic oxidation of different para-substituted pyridines at 323 K in MeCN. The epoxide/aziridine ratio as a function of σp. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, [styrene]0 = 3 × 10−1 M, and [para-substituted pyridine]0 = 1 × 10−2 M under air.
Figure 14.
The catalytic oxidation of different para-substituted pyridines at 323 K in MeCN. The epoxide/aziridine ratio as a function of σp. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, [styrene]0 = 3 × 10−1 M, and [para-substituted pyridine]0 = 1 × 10−2 M under air.
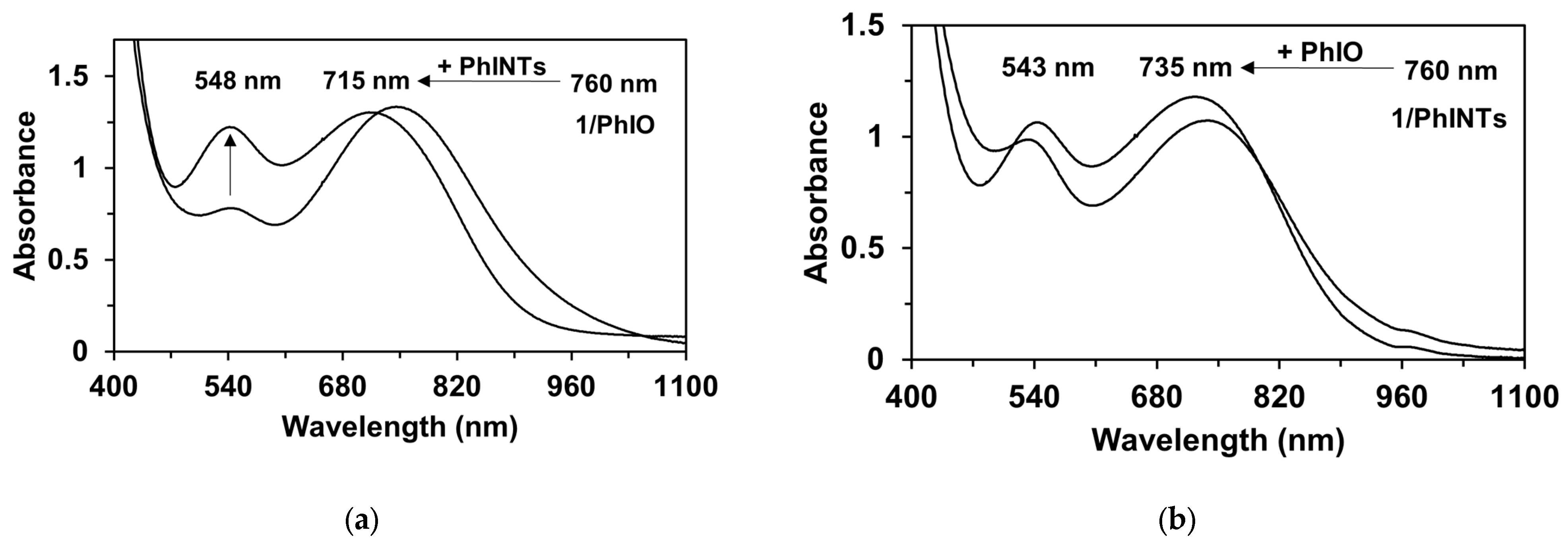
Figure 15.
(a) UV-vis spectral change of FeIII(OIPh) with PhINTs at 293 K in MeCN. FeIII(OIPh) was generated in situ by reaction of 1 with PhI(OAc)2. (b) UV-vis spectral change of FeIII(OINTs) with PhIO at 293 K in MeCN. FeIII(OINTs) was generated in situ by reaction of 1 with PhINTs. [1]0 = 1 × 10−3 M, [PhINTs]0 = 4 × 10−3 M, and [PhIO]0 = 4 × 10−3 M.
Figure 15.
(a) UV-vis spectral change of FeIII(OIPh) with PhINTs at 293 K in MeCN. FeIII(OIPh) was generated in situ by reaction of 1 with PhI(OAc)2. (b) UV-vis spectral change of FeIII(OINTs) with PhIO at 293 K in MeCN. FeIII(OINTs) was generated in situ by reaction of 1 with PhINTs. [1]0 = 1 × 10−3 M, [PhINTs]0 = 4 × 10−3 M, and [PhIO]0 = 4 × 10−3 M.
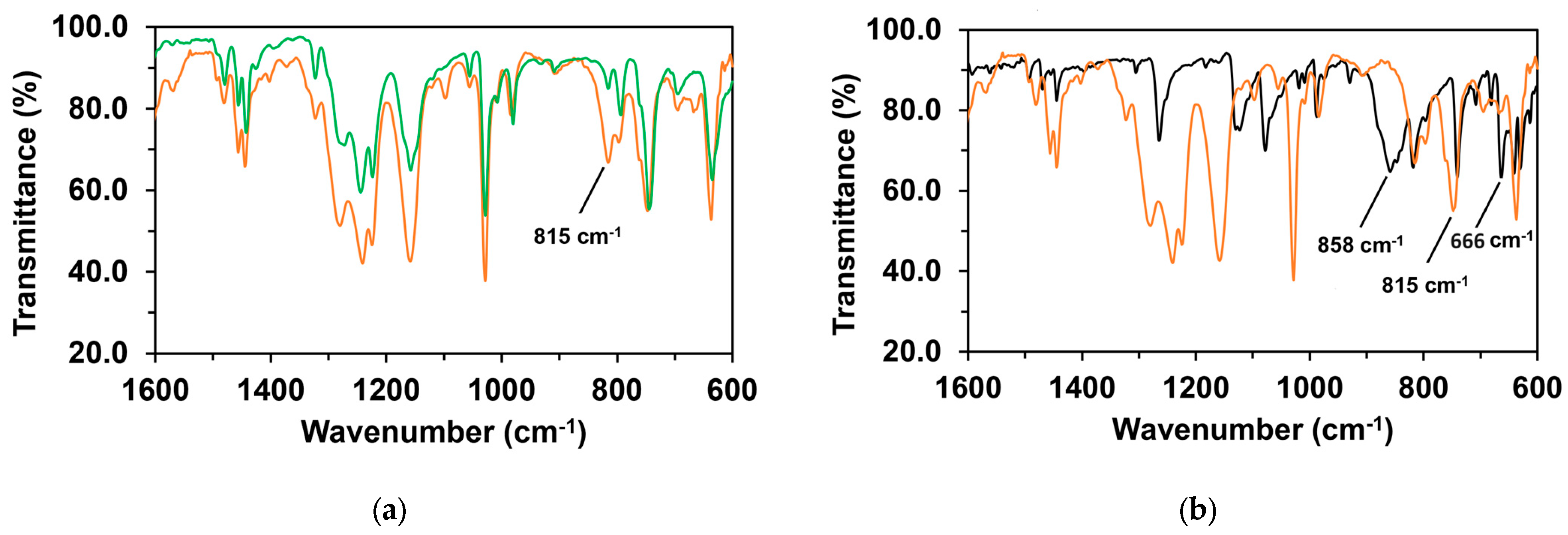
Figure 16.
(a) FT-IR solid spectra of 1 (green) and 1/PhINTs (1:1) adduct (brown). (b) FT-IR solid spectra of PhINTs (black) and 1/PhINTs (1:1) adduct (brown).
Figure 16.
(a) FT-IR solid spectra of 1 (green) and 1/PhINTs (1:1) adduct (brown). (b) FT-IR solid spectra of PhINTs (black) and 1/PhINTs (1:1) adduct (brown).
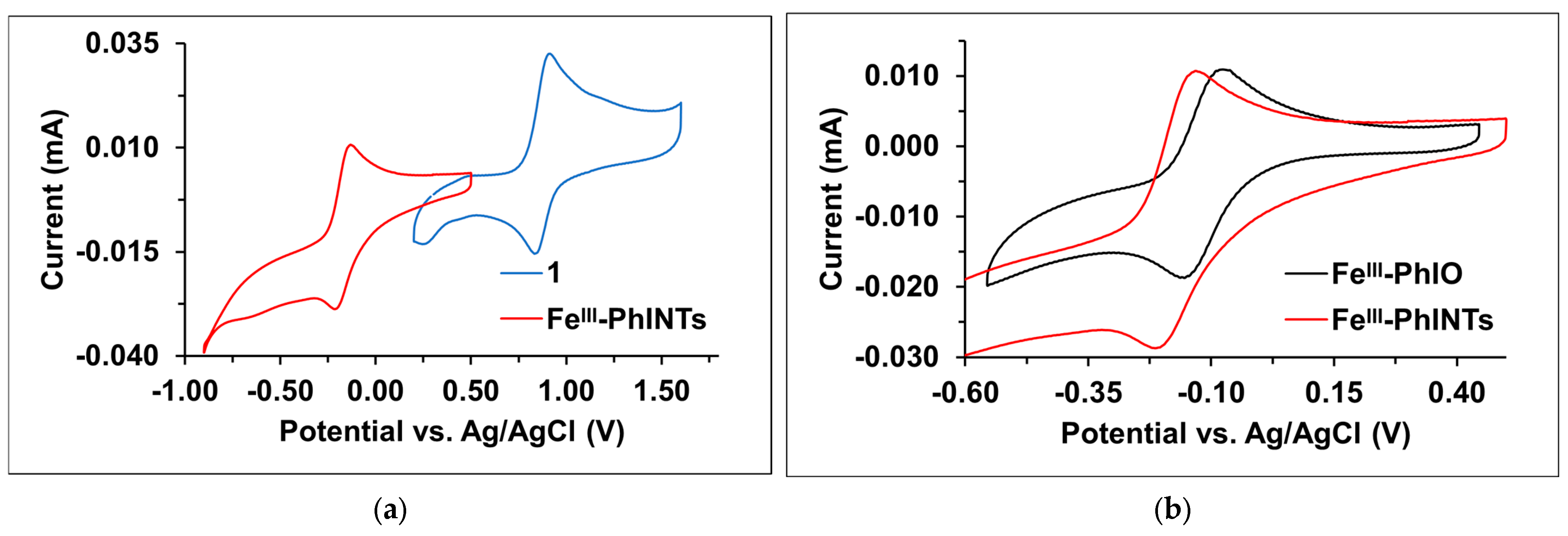
Figure 17.
Cyclic voltammograms at 293 K in MeCN. (a) Cyclic voltammograms of 1 (-) and 1 with PhINTs (-). (b) Cyclic voltammograms of 1 with PhIO (-) and 1 with PhINTs (-). [1]0 = 1.0 × 10−3 M, PhIO = 2.0 × 10−3 M, and PhINTs = 2.0 × 10−3 M in (0.1 M TBAClO4) MeCN (10 cm3); scan rate: 500 mV/s.
Figure 17.
Cyclic voltammograms at 293 K in MeCN. (a) Cyclic voltammograms of 1 (-) and 1 with PhINTs (-). (b) Cyclic voltammograms of 1 with PhIO (-) and 1 with PhINTs (-). [1]0 = 1.0 × 10−3 M, PhIO = 2.0 × 10−3 M, and PhINTs = 2.0 × 10−3 M in (0.1 M TBAClO4) MeCN (10 cm3); scan rate: 500 mV/s.
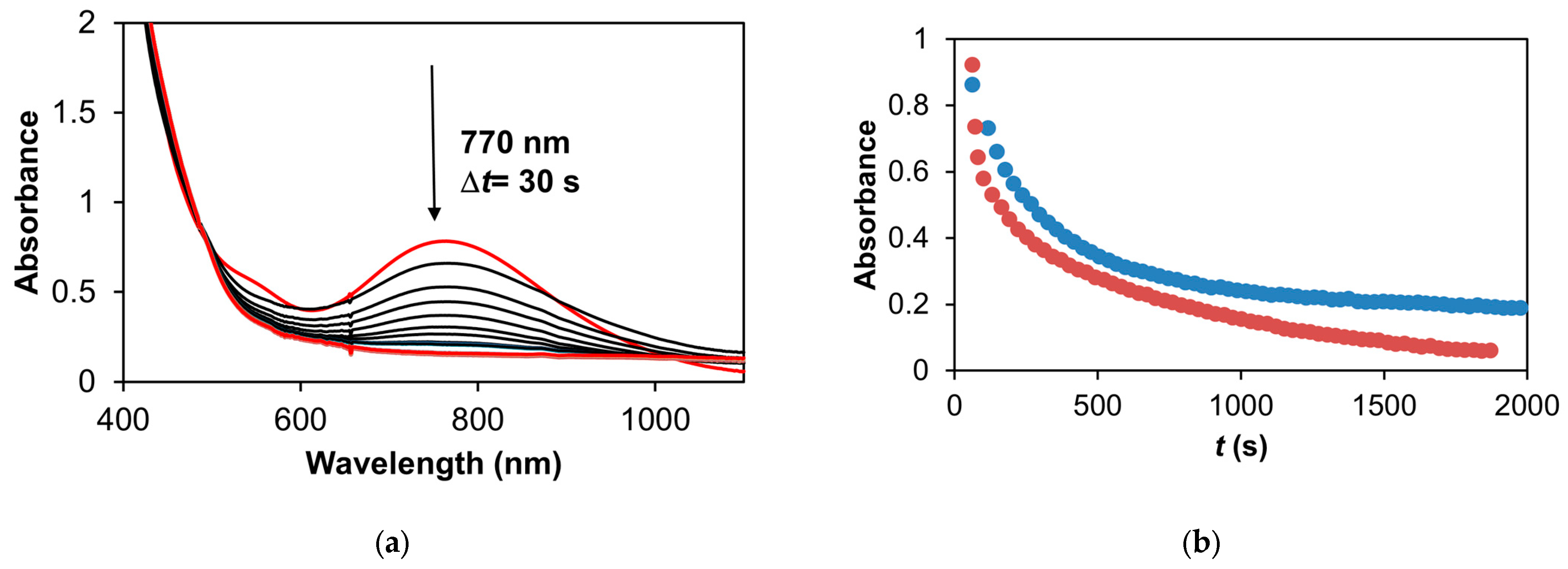
Figure 18.
The stoichiometric oxidation of styrene with the 1/PhINTs adduct at 293 K in MeCN. (a) The UV-vis spectral changes of the 1/PhINTs adduct upon the addition of styrene. (b) The change in absorbance vs. t in the reaction of the 1/PhINTs adduct and styrene at 770 nm in MeCN (•) and in CF3CH2OH (•). [1]0 = 0.5 × 10−3 M, [PhINTs]0 = 1 × 10−3 M, and [styrene]0 = 9 × 10−1 M.
Figure 18.
The stoichiometric oxidation of styrene with the 1/PhINTs adduct at 293 K in MeCN. (a) The UV-vis spectral changes of the 1/PhINTs adduct upon the addition of styrene. (b) The change in absorbance vs. t in the reaction of the 1/PhINTs adduct and styrene at 770 nm in MeCN (•) and in CF3CH2OH (•). [1]0 = 0.5 × 10−3 M, [PhINTs]0 = 1 × 10−3 M, and [styrene]0 = 9 × 10−1 M.
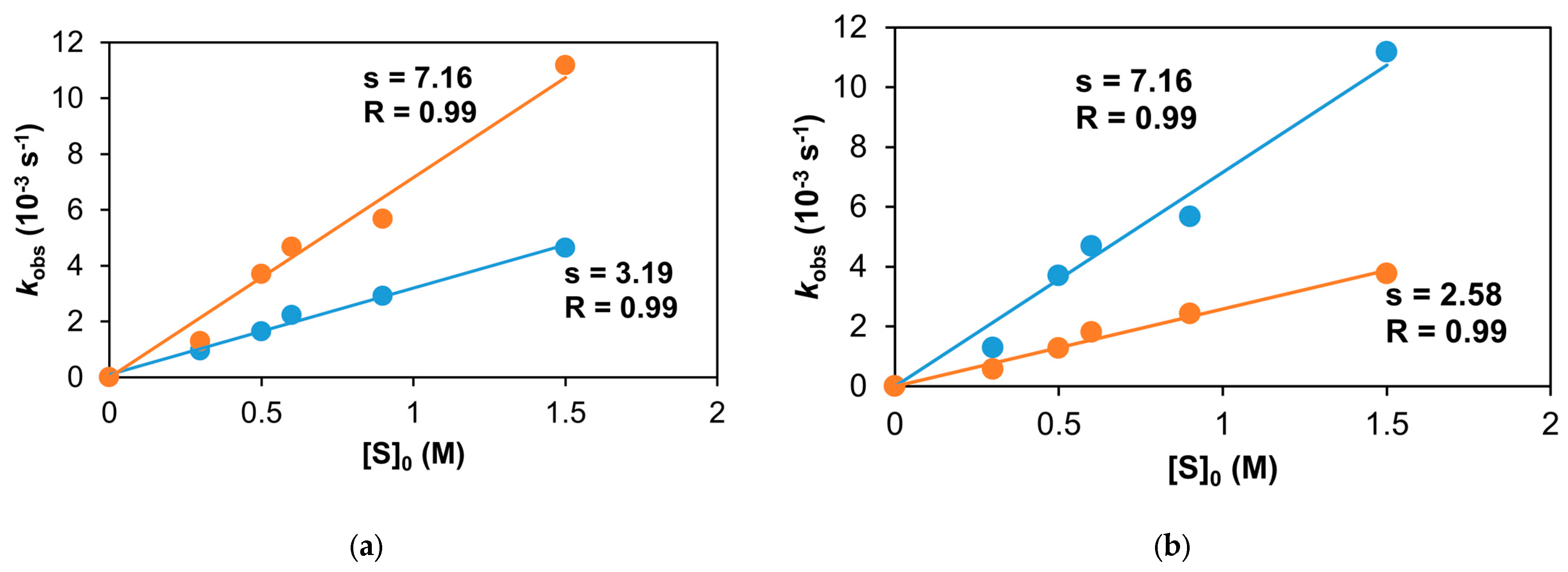
Figure 19.
The stoichiometric oxidation of styrene at 293 K. (a) The reaction rate of 1/PhIO (•) or 1/PhINTs (•) with styrene in MeCN. (b) The reaction rate of the 1/PhINTs adduct with styrene in MeCN (•) or in CF3CH2OH (•) at 293 K. [1]0 = 1 × 10−3 M; [PhINTs]0 = 1 × 10−3 M.
Figure 19.
The stoichiometric oxidation of styrene at 293 K. (a) The reaction rate of 1/PhIO (•) or 1/PhINTs (•) with styrene in MeCN. (b) The reaction rate of the 1/PhINTs adduct with styrene in MeCN (•) or in CF3CH2OH (•) at 293 K. [1]0 = 1 × 10−3 M; [PhINTs]0 = 1 × 10−3 M.
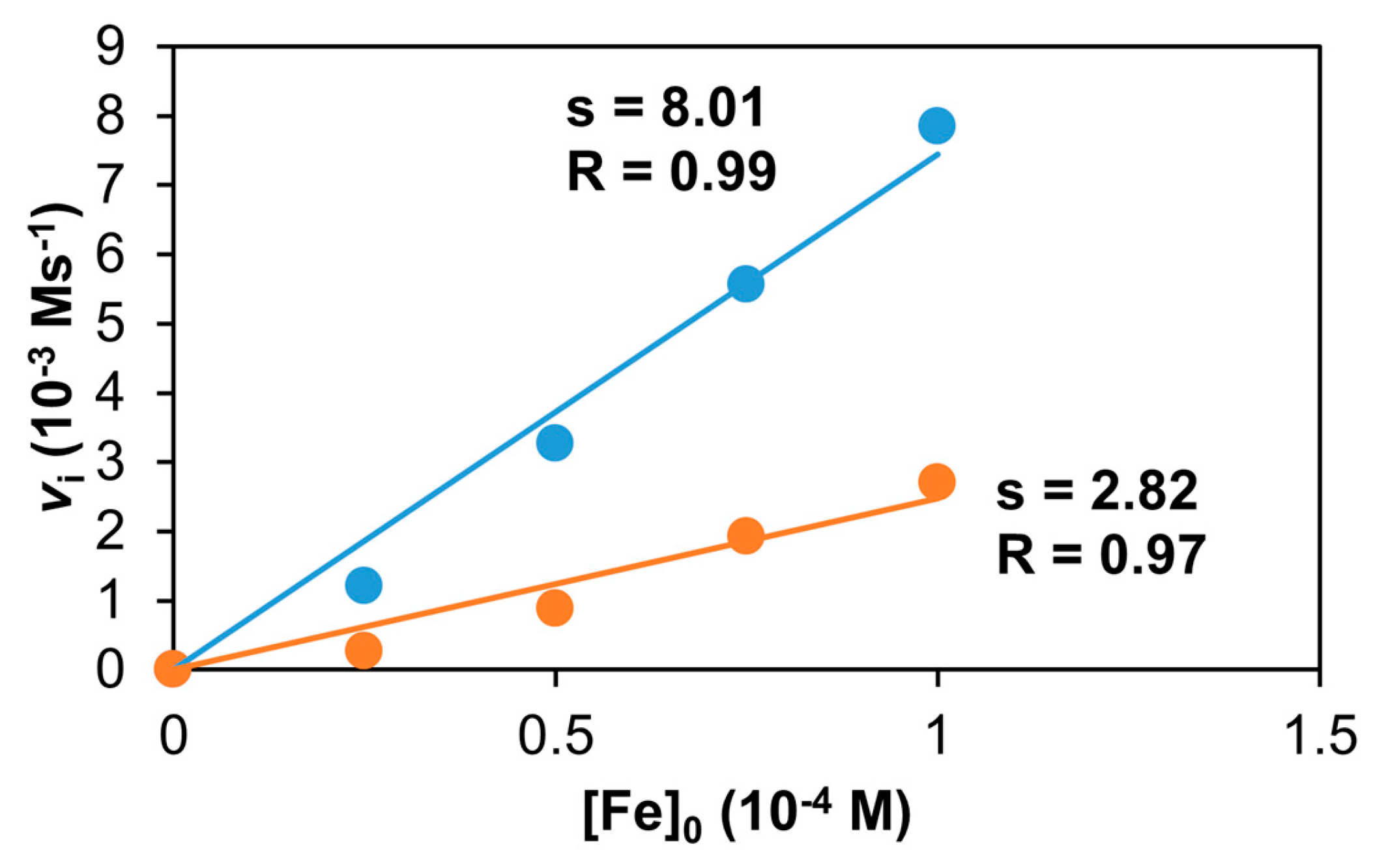
Figure 20.
The dependence of vi on the initial complex concentration in the reaction of the 1/PhINTs adduct and styrene in MeCN (•) or in CF3CH2OH (•) at 293 K. [styrene]0 = 6 × 10−1 M; [PhINTs]0 = 1 × 10−3 M.
Figure 20.
The dependence of vi on the initial complex concentration in the reaction of the 1/PhINTs adduct and styrene in MeCN (•) or in CF3CH2OH (•) at 293 K. [styrene]0 = 6 × 10−1 M; [PhINTs]0 = 1 × 10−3 M.
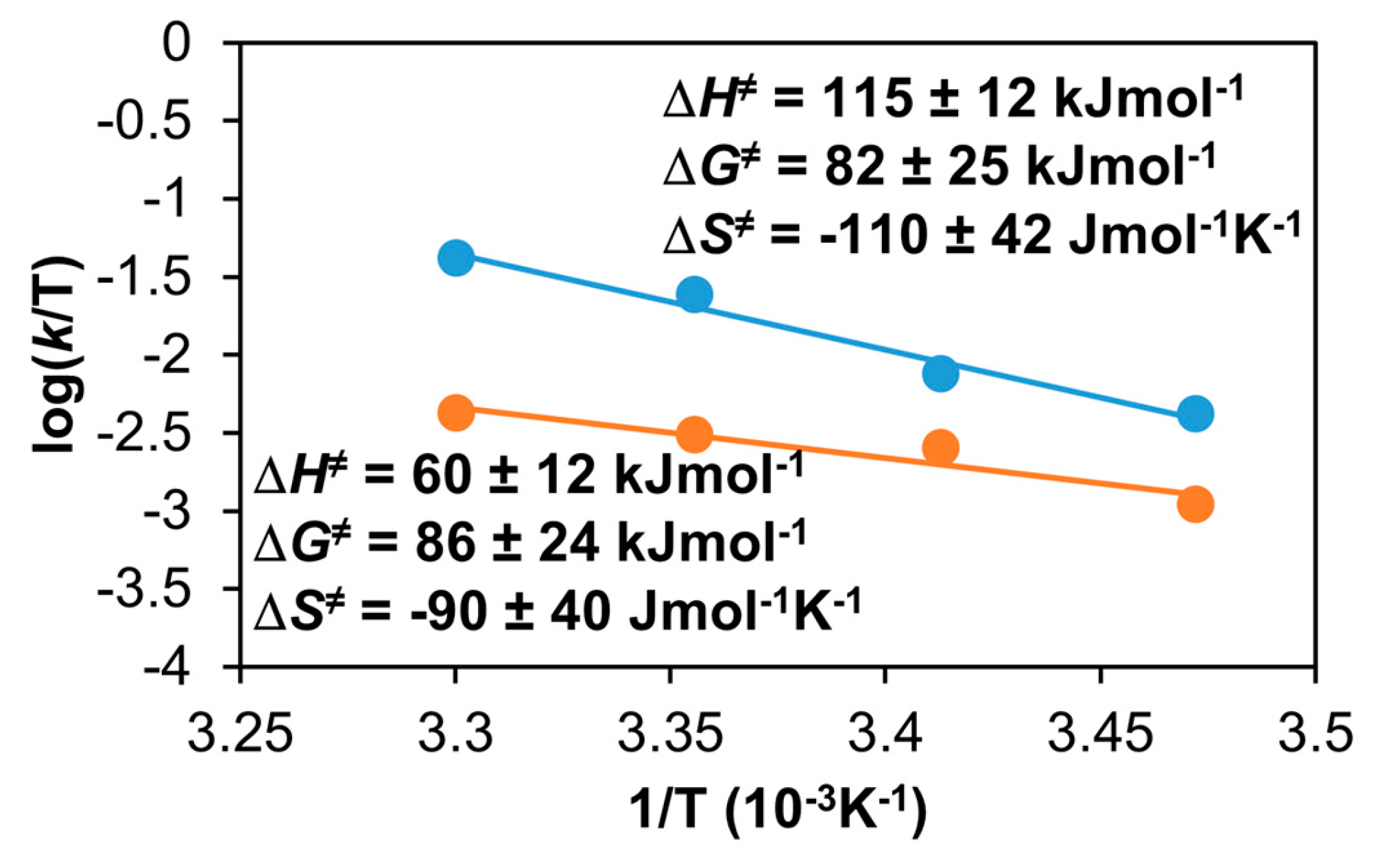
Figure 21.
The Eyring plot of the reaction of the 1/PhINTs adduct with styrene in MeCN (•) or in CF3CH2OH (•). [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−3 M, and [styrene]0 = 1.5 × 100 M.
Figure 21.
The Eyring plot of the reaction of the 1/PhINTs adduct with styrene in MeCN (•) or in CF3CH2OH (•). [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−3 M, and [styrene]0 = 1.5 × 100 M.
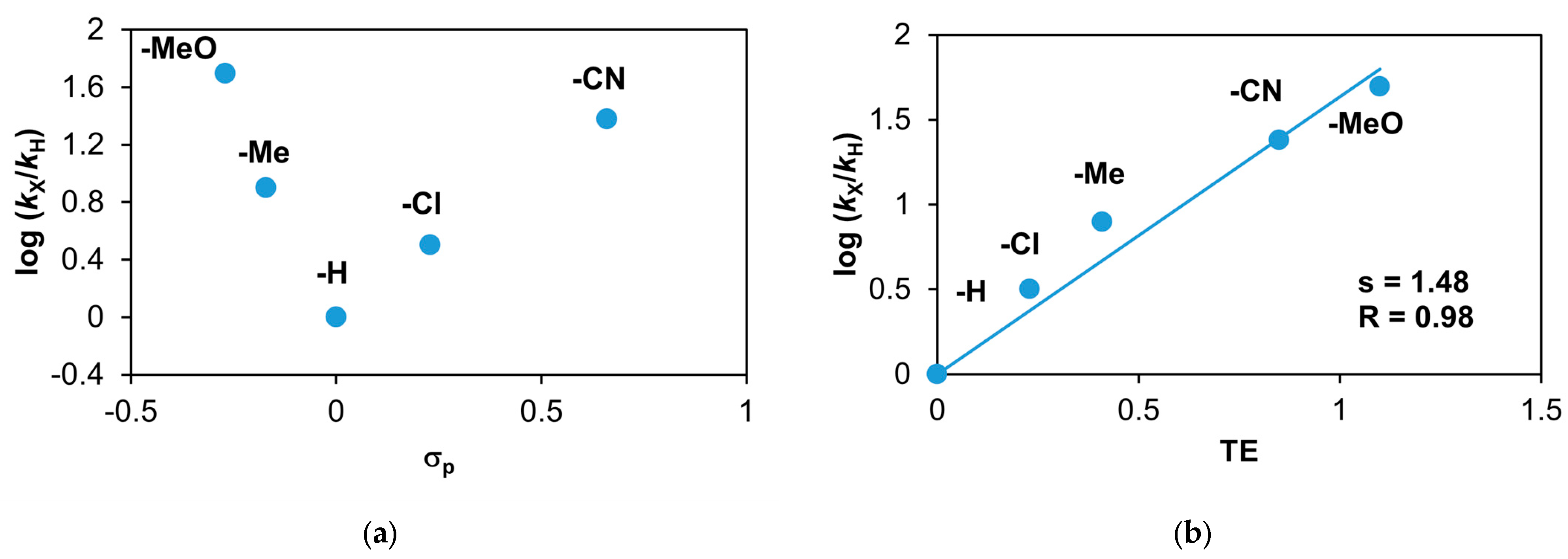
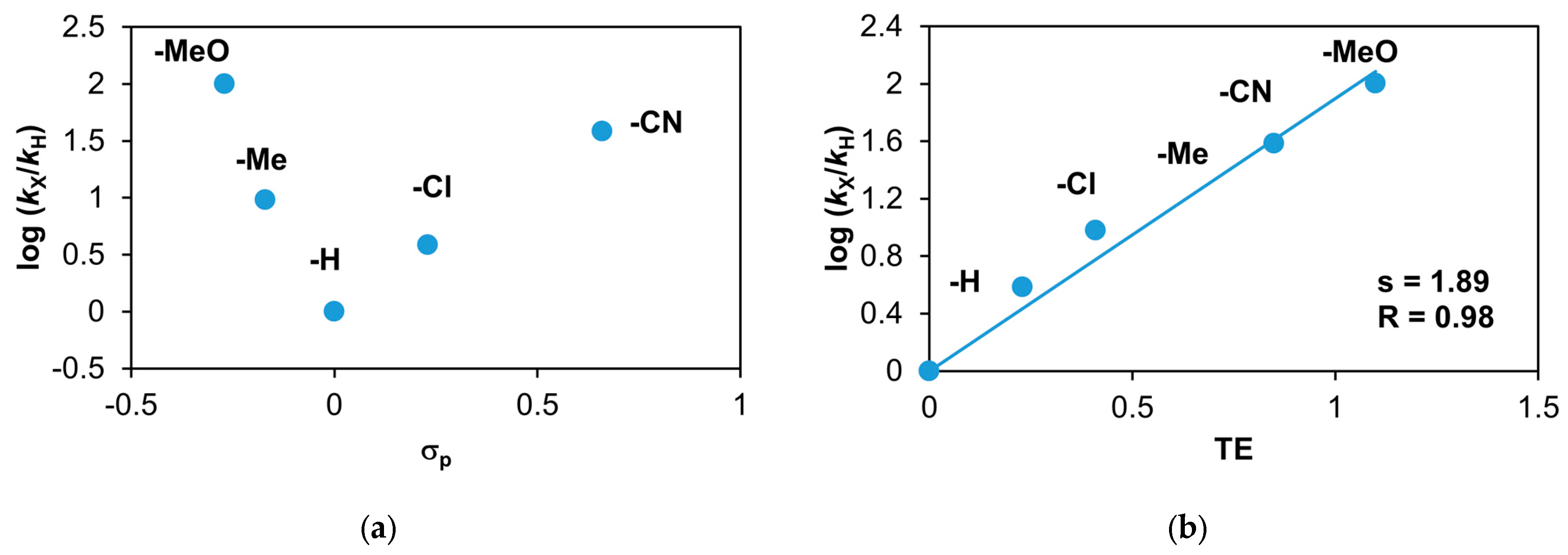
Figure 22.
Stoichiometric oxidation of different para-substituted styrenes at 293 K in MeCN. (a) Plot of log(kX/kH) against σp of para-substituted styrenes. (b) Plot of log(kX/kH) against TE of para-substituted styrenes. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−3 M, and [styrenes]0 = 5 × 10−1 M.
Figure 22.
Stoichiometric oxidation of different para-substituted styrenes at 293 K in MeCN. (a) Plot of log(kX/kH) against σp of para-substituted styrenes. (b) Plot of log(kX/kH) against TE of para-substituted styrenes. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−3 M, and [styrenes]0 = 5 × 10−1 M.
Figure 23.
Stoichiometric oxidation of different para-substituted styrenes at 293 K in CF3CH2OH. (a) Plot of log(kX/kH) against σp of para-substituted styrenes. (b) Plot of log(kX/kH) against TE of para-substituted styrenes. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−3 M, and [styrenes]0 = 5 × 10−1 M.
Figure 23.
Stoichiometric oxidation of different para-substituted styrenes at 293 K in CF3CH2OH. (a) Plot of log(kX/kH) against σp of para-substituted styrenes. (b) Plot of log(kX/kH) against TE of para-substituted styrenes. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−3 M, and [styrenes]0 = 5 × 10−1 M.
Table 1.
The yields of products (benzaldehyde, styrene oxide, and 2-phenyl-1-tosylaziridine) for the catalytic oxidation of styrene at 323 K in MeCN at different times. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Table 1.
The yields of products (benzaldehyde, styrene oxide, and 2-phenyl-1-tosylaziridine) for the catalytic oxidation of styrene at 323 K in MeCN at different times. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
| Entry | t (min) | Yield (%) 1 | Selectivity (%) | TON 2 | TOF (1/h) 3 |
|---|
| BZ | SO | SNTs | BZ | SO | SNTs |
|---|
| 1 | 60 | 6.218 | 3.98 | 0.8 | 57 | 36 | 7 | 10.99 | 2.7495 |
| 2 | 120 | 11.92 | 6.78 | 1.4 | 59 | 34 | 7 | 20.1 | 5.025 |
| 3 | 180 | 15.64 | 11.32 | 2.43 | 53 | 39 | 8 | 29.39 | 7.3475 |
| 4 | 240 | 18.2 | 14.65 | 4.16 | 49 | 40 | 11 | 37.01 | 9.2525 |
| 5 | 300 | 18.4 | 14.95 | 4.19 | 49 | 40 | 11 | 37.54 | 9.385 |
Table 2.
The yields and selectivity of products (Bz, benzaldehyde; SO, styrene oxide; and SNTs, 2-phenyl-1-tosylaziridine) for the catalytic oxidation of styrene at different temperatures in MeCN. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Table 2.
The yields and selectivity of products (Bz, benzaldehyde; SO, styrene oxide; and SNTs, 2-phenyl-1-tosylaziridine) for the catalytic oxidation of styrene at different temperatures in MeCN. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
| Entry | T (K) | Yield (%) 1 | Selectivity (%) | TON 2 | TOF (1/h) 3 |
|---|
| BZ | SO | SNTs | BZ | SO | SNTs |
|---|
| 1 | 293 | 16.01 | 9.24 | 2.15 | 58 | 34 | 8 | 27.4 | 6.85 |
| 2 | 308 | 17.02 | 10.52 | 4.41 | 53 | 33 | 14 | 31.95 | 7.99 |
| 3 | 323 | 18.2 | 14.65 | 4.16 | 49 | 45 | 11 | 37.01 | 9.25 |
| 4 | 338 | 6.41 | 4.8 | 1.52 | 50 | 38 | 12 | 12.73 | 3.18 |
Table 3.
The yields and selectivity of products (benzaldehyde, styrene oxide, and 2-phenyl-1-tosylaziridine) for the catalytic oxidation of styrene in MeCN at 323 K. [PhINTs]0 = 1 × 10−1 M; [styrene]0 = 3 × 10−1 M under air.
Table 3.
The yields and selectivity of products (benzaldehyde, styrene oxide, and 2-phenyl-1-tosylaziridine) for the catalytic oxidation of styrene in MeCN at 323 K. [PhINTs]0 = 1 × 10−1 M; [styrene]0 = 3 × 10−1 M under air.
| Entry | [1]0 (10−3 M) | Yield (%) 1 | Selectivity (%) | TON 2 | TOF (1/h) 3 |
|---|
| BZ | SO | SNTs | BZ | SO | SNTs |
|---|
| 1 | 0.5 | 3.17 | 2.13 | 1.19 | 49 | 33 | 18 | 6.49 | 1.62 |
| 2 | 1 | 18.2 | 14.65 | 4.16 | 49 | 40 | 11 | 37.01 | 9.25 |
| 3 | 2 | 19.25 | 20.23 | 6.34 | 42 | 44 | 14 | 45.82 | 11.45 |
Table 4.
The yields of products (Bz, benzaldehyde; SO, styrene oxide; and SNTs, 2-phenyl-1-tosylaziridine) and calculated TON and TOF values for the catalytic oxidation of para-substituted styrenes at 323 K in MeCN. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Table 4.
The yields of products (Bz, benzaldehyde; SO, styrene oxide; and SNTs, 2-phenyl-1-tosylaziridine) and calculated TON and TOF values for the catalytic oxidation of para-substituted styrenes at 323 K in MeCN. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
| Entry | Substrate | Yield (%) 3 | Selectivity (%) | TON 4 | TOF (1/h) 5 |
|---|
| 4R-S | BZ | SO | SNTs | BZ | SO | SNTs |
|---|
| 1 | 4-methoxystyrene | 32.35 | 20.21 | 10 | 52 | 32 | 16 | 62.56 | 15.64 |
| 2 | 4-methylstyrene | 22 | 17.34 | 8.51 | 46 | 36 | 18 | 47.85 | 11.96 |
| 3 | styrene | 18.2 | 14.65 | 4.16 | 49 | 40 | 11 | 37.01 | 9.25 |
| 4 | styrene 1 | - | - | - | - | - | - | - | - |
| 5 | 4-chlorostyrene | 6.34 | 4.94 | 1.27 | 51 | 39 | 10 | 12.55 | 3.13 |
| 6 | 4-cyanostyrene | 1.4 | 0.77 | 0.21 | 59 | 32 | 9 | 2.38 | 0.59 |
| 7 | α-methylstyrene | 3.19 2 | 5.16 | 0.61 | 35 | 58 | 7 | 8.96 | 2.24 |
| 8 | styrene-d8 | 6.4 | 4.9 | 1.4 | 50 | 39 | 11 | 12.7 | 3.17 |
Table 5.
The yields of products (benzaldehyde, styrene oxide, and 2-phenyl-1-tosylaziridine) and calculated TON and TOF values for the catalytic oxidation of para-substituted styrenes at 323 K in CF3CH2OH. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
Table 5.
The yields of products (benzaldehyde, styrene oxide, and 2-phenyl-1-tosylaziridine) and calculated TON and TOF values for the catalytic oxidation of para-substituted styrenes at 323 K in CF3CH2OH. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, and [styrene]0 = 3 × 10−1 M under air.
| Entry | Substrate | Yield (%) 1 | Selectivity (%) | TON 2 | TOF (1/h) 3 |
|---|
| 4R-S | BZ | SO | SNTs | BZ | SO | SNTs |
|---|
| 1 | 4-methoxystyrene | 17.7 | 7.89 | 1.3 | 66 | 29 | 5 | 26.89 | 6.72 |
| 2 | 4-methylstyrene | 9.3 | 4.71 | 0.47 | 65 | 32 | 3 | 14.48 | 3.62 |
| 3 | styrene | 3.88 | 2.67 | 1.87 | 46 | 32 | 22 | 8.42 | 2.11 |
| 4 | 4-chlorostyrene | 2.05 | 0.36 | 0.38 | 73 | 13 | 14 | 2.79 | 0.69 |
| 5 | 4-cyanostyrene | 0.48 | 0.27 | 0.26 | 48 | 27 | 25 | 1.01 | 0.25 |
Table 6.
The yield and selectivity of products (benzaldehyde, styrene oxide, and 2-phenyl-1-tosylaziridine for the catalytic oxidation of styrene in MeCN at 323 K in the presence of water, D2O, or a buffer. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, [styrene]0 = 3 × 10−1 M, [H2O, D2O]0 = 1.5 × 10−2 M, and [buffer]0 = 2 × 10−1 mL under air.
Table 6.
The yield and selectivity of products (benzaldehyde, styrene oxide, and 2-phenyl-1-tosylaziridine for the catalytic oxidation of styrene in MeCN at 323 K in the presence of water, D2O, or a buffer. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, [styrene]0 = 3 × 10−1 M, [H2O, D2O]0 = 1.5 × 10−2 M, and [buffer]0 = 2 × 10−1 mL under air.
| Entry | MeCN-X | [X]0 (10−3 M) | Yield (%) 1 | Selectivity (%) | TON 2 | TOF (1/h) 3 |
|---|
| X= | | BZ | SO | SNTs | BZ | SO | SNTs |
|---|
| 1 | - | - | 18.2 | 14.65 | 4.16 | 49 | 40 | 11 | 37.01 | 9.25 |
| 2 | H2O (pH 7) | 15 | 8.62 | 6.13 | 1.23 | 54 | 38 | 8 | 15.98 | 3.99 |
| 3 | H2O | 50 | 7.89 | 5.34 | 0.65 | 57 | 38 | 5 | 13.88 | 3.47 |
| 4 | H2O | 100 | 5.11 | 3.49 | 0.32 | 57 | 39 | 4 | 8.92 | 2.23 |
| 5 | H2O | 200 | 3.79 | 2.61 | 0.15 | 58 | 40 | 2 | 6.55 | 1.64 |
| 6 | H2O (pH 4.7) | 15 | 16.52 | 26.17 | 6.72 | 33 | 53 | 14 | 49.41 | 12.35 |
| 7 | H2O (pH 8) | 15 | 5.95 | - | - | 100 | - | - | 5.95 | 1.49 |
| 8 | D2O | 15 | 5.12 | 3.86 | 0.75 | 53 | 40 | 7 | 9.73 | 2.43 |
| 9 | H2O18 | 15 | 9.21 | 7.18 | 0.92 | 53 | 41 | 5 | 17.31 | 4.32 |
Table 7.
The yield and selectivity of products (Bz, benzaldehyde; SO, styrene oxide; and SNTs, 2-phenyl-1-tosylaziridine) for the catalytic oxidation of styrene with different para-substituted pyridines in MeCN at 323 K. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, [styrene]0 = 3 × 10−1 M, and [para-substituted pyridine]0 = 1 × 10−2 M under air.
Table 7.
The yield and selectivity of products (Bz, benzaldehyde; SO, styrene oxide; and SNTs, 2-phenyl-1-tosylaziridine) for the catalytic oxidation of styrene with different para-substituted pyridines in MeCN at 323 K. [1]0 = 1 × 10−3 M, [PhINTs]0 = 1 × 10−1 M, [styrene]0 = 3 × 10−1 M, and [para-substituted pyridine]0 = 1 × 10−2 M under air.
| Entry | Co-Ligand | Yield (%) | Selectivity (%) | TON | TOF (1/h) |
|---|
| 4R-S | BZ | SO | SNTs | BZ | SO | SNTs |
|---|
| 1 | 4-Me-pyridine | 24.16 | 17.915 | 5.11 | 51 | 38 | 11 | 47.18 | 11.79 |
| 2 | pyridine | 31.15 | 20.02 | 6.32 | 54 | 35 | 11 | 57.49 | 14.37 |
| 3 | 4-C(O)C6H5-pyridine | 34.28 | 21.76 | 8.53 | 53 | 34 | 13 | 64.57 | 16.14 |
| 4 | 4-C(O)CH3-pyridine | 35.467 | 22.43 | 9.31 | 53 | 34 | 13 | 67.21 | 16.80 |
| 5 | 4-CN-pyridine | 39.43 | 25.75 | 11.36 | 51 | 34 | 15 | 76.54 | 19.13 |
Table 8.
Redox potential data of 1, 1/PhIO, and 1/PhINTs adducts.
Table 8.
Redox potential data of 1, 1/PhIO, and 1/PhINTs adducts.
| Entry | Complex | Epa (Fe3+/2+) (V) vs. SCE | Epc (Fe3+/2+) (V) vs. SCE | E1/2 (Fe3+/2+) (V) vs. SCE |
|---|
| 1 | [FeII(PBI)3](OTf)2 | +0.934 | +0.870 | +0.902 |
| 2 | [FeII(PBI)3](OTf)2 + PhIO | −0.076 | −0.153 | −0.115 |
| 3 | [FeII(PBI)3](OTf)2 + PhINTs | −0.130 | −0.213 | −0.171 |
Table 9.
Kinetic data for the stoichiometric oxidation of styrene with (PBI)FeIII(PhINTs) in different solvents.
Table 9.
Kinetic data for the stoichiometric oxidation of styrene with (PBI)FeIII(PhINTs) in different solvents.
| Entry | Solvent | [S]0 (M) | T (K) | k′obs
(10−3 s−1) | σ | TE | k2
(10−3 M−1 s−1) |
|---|
| 1 | MeCN | 0.3 | 293 | 1.29 | | | 4.3 |
| 2 | MeCN | 0.5 | 293 | 3.69 | 0 | 1.34 | 7.38 |
| 3 | MeCN | 0.6 | 293 | 4.67 | | | 7.78 |
| 4 | MeCN | 0.9 | 293 | 5.67 | | | 6.3 |
| 5 | MeCN | 1.5 | 293 | 11.18 | | | 7.45 |
| 6 | MeCN | 1.5 | 288 | 6.4 | | | 4.26 |
| 7 | MeCN | 1.5 | 298 | 36.3 | | | 24.2 |
| 8 | MeCN | 1.5 | 303 | 62.7 | | | 41.8 |
| 9 | CF3CH2OH | 0.3 | 293 | 0.57 | | | 1.9 |
| 10 | CF3CH2OH | 0.5 | 293 | 1.26 | 0 | 1.34 | 2.52 |
| 11 | CF3CH2OH | 0.6 | 293 | 1.81 | | | 3.02 |
| 12 | CF3CH2OH | 0.9 | 293 | 2.42 | | | 2.68 |
| 13 | CF3CH2OH | 1.5 | 293 | 3.77 | | | 2.51 |
| 14 | CF3CH2OH | 1.5 | 288 | 1.63 | | | 1.09 |
| 15 | CF3CH2OH | 1.5 | 298 | 4.57 | | | 3.05 |
| 16 | CF3CH2OH | 1.5 | 303 | 6.33 | | | 4.22 |
Table 10.
Kinetic data for stoichiometric oxidation of para-substituted styrenes with 1/PhINTs adduct in different solvents at 293 K.
Table 10.
Kinetic data for stoichiometric oxidation of para-substituted styrenes with 1/PhINTs adduct in different solvents at 293 K.
| Entry | Substrate | Solvent | [S]0 (M) | k′obs (10−3 s−1) | σ | TE | k2 (10−3 M−1 s−1) |
|---|
| 1 | styrenes | MeCN | 0.5 | 3.69 | 0 | 1.34 | 7.38 |
| 2 | 4-methoxystyrene | MeCN | 0.5 | 183 | −0.27 | 1.1 | 366 |
| 3 | 4-methylstyrene | MeCN | 0.5 | 29.3 | −0.17 | 0.41 | 58.6 |
| 4 | 4-chlorostyrene | MeCN | 0.5 | 11.9 | 0.23 | 0.23 | 23.8 |
| 5 | 4-cyanostyrene | MeCN | 0.5 | 88.8 | 0.66 | 0.93 | 177.6 |
| 6 | styrene | CF3CH2OH | 0.5 | 1.26 | 0 | 1.34 | 2.52 |
| 7 | 4-methoxystyrene | CF3CH2OH | 0.5 | 57.53 | −0.27 | 1.1 | 115.06 |
| 8 | 4-methylstyrene | CF3CH2OH | 0.5 | 5.47 | −0.17 | 0.41 | 10.9 |
| 9 | 4-chlorostyrene | CF3CH2OH | 0.5 | 2.2 | 0.23 | 0.23 | 4.4 |
| 10 | 4-cianostyrene | CF3CH2OH | 0.5 | 21.9 | 0.66 | 0.93 | 43.8 |