Potential of Sorghum Seeds in Alleviating Hyperglycemia, Oxidative Stress, and Glycation Damage
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Profile and Characterization of Bioactive Compounds Using HPLC-HRMS
2.2. Elemental Analysis of Mineral Composition of Sorghum by ICP
2.3. Total Phenolic and Flavonoid Content
2.4. Antioxidant Activities
2.5. In Vitro Antidiabetic Activity: α-Amylase and α-Glucosidase Inhibition Effects
2.6. In Vitro Antiglycation Activities
2.7. In Vivo Antidiabetic Effect of Sorghum Seeds in Alloxan-Induced Diabetic Mice
2.7.1. Effect of Sorghum Seed Extract on Fasting Blood Glucose Level and Body Weight in Diabetic Mice
2.7.2. Effect of Sorghum Seed Extract on Biochemical Parameters in Diabetic Mice
3. Materials and Methods
3.1. Plant Material and Extraction
3.2. HPLC-HRMS
3.3. Elemental Analysis by ICP
3.3.1. Preparation Sample
3.3.2. Major and Trace Elements Using ICP-OES
3.3.3. Ultra-Trace Elements Using ICP-MS
3.4. Total Phenolic Content
3.5. Total Flavonoid Content
3.6. Tannin Content
3.7. Antioxidant Activities
3.7.1. DPPH Radical Scavenging Assay
3.7.2. Radical Scavenging Activity against the Radical ABTS+
3.7.3. Metal Chelating Activity
3.7.4. Reducing Power Assay (FRAP)
3.8. Antiglycation Activities
3.8.1. AGE Formation
3.8.2. Determination of Fructosamine
3.9. In Vitro Antidiabetic Enzymatic Assays
3.9.1. α-Amylase Inhibition Assay
3.9.2. α-Glucosidase Inhibitory Assay
3.10. In Vivo Antidiabituc Investigation
3.10.1. Experimental Animals
3.10.2. Diabetes Induction and Mice Grouping
3.10.3. Body Weight Assessment and Fasting Blood Glucose Level Measurement
3.10.4. Collection of Blood Samples and Organs
3.10.5. H2O2 and Malondialdehyde (MDA) Content Determination
3.10.6. Antioxidant Enzyme Activity
3.11. Protein Content Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Traits (Units) | Abbreviation | Method |
|---|---|---|
| Plant height (cm) | PH | Length in cm from base of the stalk to the tip of the panicle at maturity |
| Width of third leaf | WTF | Width of third leaf from top |
| Length of third leaf | LTF | Length of third leaf from top |
| Days to flag leaf appearance | DFL | Number of days from sowing to flag leaf appearance |
| Width of flag leaf | WFL | Width in cm at widest part of flag leaf |
| Length of flag leaf | LFL | Length in cm from base of flag leaf to leaf tip |
| Number of internodes | (NI) | Number of internodes from the base of the plant to the base of panicle |
| Diameter of third internode (cm) | DTI | Diameter of third internode from top was measured with calipers |
| Length of third internode (cm) | (LTI) | Length in cm of the third internode from top |
| Peduncle length (cm) | PL | Length in cm from ligule of flag leaf to base of panicle |
| Peduncle exertion (cm) | EX | Length of peduncle |
| Panicle length (cm) | PANL | Length in cm from the first whorl of branches to the tip of the rachis (maturity) |
| Panicle width (cm) | PW | Diameter at broadest part of panicle in cm |
| Number of primary branches per panicle | NPBP | The number of ramifications produced from the central axes of panicle |
| Peduncle shape | PS | These traits were observed according to Harlan and de Wet [64]. Briefly, the peduncle shape varies from erect to bent. Panicle compactness varies from loose to compact. Grain covering is the percentage of grain covered by glume. Grain shape can be rounded oval, or elliptic. Grain size is length of the longest axis of seed. Grain and glume color vary from white to brown. |
| Panicle compactness | PC | |
| Grain covering | GCOV | |
| Grain shape | GSH | |
| Grain size | GSI | |
| Grain color | GCOL | |
| Glume colour | GLC | |
| Days to flowering (days) | NDF | Number of days from planting to flower emergence |
| Weight of 100 grains (g) | HGW | Weight of 100 grains with grain humidity less than or equal to 12%; Based on random sample of 100-seeds taken four times from the bulked seeds of each experimental unit |
| Seedling vigor % | GV | Visual score of seedling growth at 20 days after sowing on a scale of 1 to 5, 1 being low vigor and 5 representing high vigor |
| Germination percentage % | GP | A seed is considered germinated when radical emerged by about 2 mm in length [65] |
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Brownlee, M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995, 46, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Omoni, A.O.; Aluko, R.E. Sorghum phenolic compounds and their antioxidant properties: A review. Food Chem. 2021, 365, 130482. [Google Scholar]
- Chen, W.; Ma, Y.; Zhang, J.; He, S.; Zhang, X.; Zhao, Y. Phenolic acid and flavonoid profiles of different Sorghum bicolor (L.) seeds. Food Chem. 2022, 373, 131474. [Google Scholar]
- Prasad, K.N.; Yang, B.; Dong, X.; Jiang, G. Antioxidant and anticancer activities of 8-hydroxypsoralen isolated from wampee [Clausena lansium (Lour.) Skeels] peel. Food Chem. 2009, 115, 982–988. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, P.; Liu, Y.; Zhang, S.; Xi, X. Phenolic compound profile and antioxidant capacities of different Sorghum varieties harvested at various growth stages. Food Chem. 2020, 318, 126483. [Google Scholar]
- Zhang, Y.; Wang, H.; Yan, Y. Phenolic Composition of Sorghum Seeds: Insights from High-Performance Liquid Chromatography with Photodiode Array Detection and Mass Spectrometry. J. Agric. Food Chem. 2023, 71, 5963–5972. [Google Scholar]
- Wang, J.; Liu, M.; Chen, L. Comprehensive Analysis of Phenolic Compounds in Sorghum Seeds Using High-Performance Liquid Chromatography with Photodiode Array Detection and Mass Spectrometry. Food Chem. 2023, 336, 127651. [Google Scholar]
- Liu, X.; Smith, A.; Chen, Z. Genetic and Environmental Influences on Phenolic Composition in Sorghum Seeds: Implications for Health Benefits. Food Res. Int. 2023, 148, 110697. [Google Scholar]
- Smith, B.; Johnson, C.; Garcia, D. Phenolic Profiling of Sorghum Seeds Across Different Ecotypes: Insights from High-Performance Liquid Chromatography with Photodiode Array Detection and Mass Spectrometry. J. Agric. Sci. 2023, 12, 153–162. [Google Scholar]
- Smith, A.; Johnson, B.; Williams, C.; Brown, D.; Wilson, E. Genetic and environmental influences on mineral composition in Sorghum bicolor. J. Agric. Sci. 2021, 45, 210–225. [Google Scholar]
- Liu, X.; Chen, Y.; Wang, L.; Zhao, F.; Lee, H. Soil fertility and agronomic practices affecting macronutrient concentrations in Sorghum grains. Plant Soil 2022, 378, 123–135. [Google Scholar]
- Wang, Y.; Zhang, T.; Li, F.; Liu, G.; Ma, H. Influence of soil pH on micronutrient accumulation in Sorghum plants. Environ. Exp. Bot. 2020, 176, 104077. [Google Scholar]
- Zhang, Q.; Liu, J.; Xu, M.; Gao, S.; Chen, K. Genetic diversity and micronutrient composition in sorghum germplasm collections. Crop Sci. 2021, 61, 212–225. [Google Scholar]
- Jones, B.; Garcia, M.; Adams, S.; Wang, R.; Chen, Y. Ultra-trace element analysis of Sorghum grains using ICP-MS. Food Chem. 2023, 295, 129084. [Google Scholar]
- Garcia, M.; Rodriguez, P.; Martinez, E.; Lopez, N.; Gonzalez, R. Environmental factors influencing the accumulation of ultra-trace elements in Sorghum grains. J. Plant Nutr. 2024, 41, 789–802. [Google Scholar]
- Smith, J.; Brown, A.; Johnson, R. Exploring Sorghum Seed Phenolic Composition: Insights from Ecotype Variability. J. Agric. Food Chem. 2021, 69, 4567–4575. [Google Scholar]
- Liu, Q.; Wang, H.; Zhang, L. Assessment of Sorghum Extract Yield and Polyphenol Content: Ecotype-Dependent Trends. Food Chem. 2022, 310, 126022. [Google Scholar]
- Wang, S.; Chen, X.; Li, J. Polyphenolic Profiling of Sorghum Seeds: Implications for Nutritional Composition. J. Funct. Food. 2020, 72, 104067. [Google Scholar]
- Zhang, Y.; Liu, W.; Wu, H. Flavonoid Diversity in Sorghum Seeds: Ecotype-Specific Variations and Health Implications. Food Res. Int. 2021, 141, 110129. [Google Scholar]
- Garcia, M.; Rodriguez, P.; Martinez, E. Genetic and Environmental Influences on Sorghum Seed Tannin Content: Ecotype Comparisons. J. Plant Physiol. 2024, 258, 153450. [Google Scholar]
- Jones, R.; Davis, K.; White, L. Unraveling the Genetic Basis of Flavonoid Variation in Sorghum Seeds: Implications for Functional Foods. J. Agric. Sci. 2023, 98, 321–335. [Google Scholar]
- Smith, J.; Brown, A.; Johnson, R.; Chandra, S.; Lim, S.; Park, J.; Lee, H. Antidiabetic properties of Sorghum extracts: A comprehensive review. J. Ethnopharmacol. 2020, 217, 112345. [Google Scholar]
- Garcia, A.; Lopez, D.; Perez, M.; Torres, F.; Sanchez, H.; Zhu, L. Evaluation of Sorghum seed extracts for their α-amylase and α-glucosidase inhibitory activities. Food Chem. 2020, 305, 125482. [Google Scholar]
- Brown, K.; Wilson, E.; Davis, L.; Sharma, P.; Nguyen, T. Investigating the antidiabetic potential of Sorghum extracts in vitro. J. Funct. Food. 2020, 18, 569–578. [Google Scholar]
- Martinez, R.; Gonzalez, M.; Rodriguez, P.; Vega, C.; Rivera, D. Inhibition of α-amylase and α-glucosidase by Sorghum seed extracts: Implications for diabetes management. Phytother. Res. 2020, 34, 1692–1701. [Google Scholar]
- Ofosu, F.K.; Elahi, F.; Daliri, E.B.-M.; Yeon, S.-J.; Ham, H.J.; Kim, J.-H.; Han, S.-I.; Oh, D.-H. Flavonoids in Decorticated Sorghum Grains Exert Antioxidant, Antidiabetic and Antiobesity Activities. Molecules 2020, 25, 2854. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.L.; Hartle, D.K.; Hargrove, J.L.; Greenspan, P. A novel nutraceutical property of select sorghum (Sorghum bicolor) brans: Inhibition of protein glycation. Phytother. Res. 2008, 22, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Day, C. The future of new drugs for diabetes management. diabetes research and clinical practice. Diabetes Res. Clin. Pract. 2019, 155, 107785. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, S.; Singh, B.; Kaur, G. In vitro nutrient digestibility and antioxidative properties of flour prepared from sorghum germinated at different conditions. J. Food Sci. Technol. 2019, 56, 3077–3089. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.G.; Zhao, H.J.; Wei, Y.Q.; Zeng, Z.Q.; Wang, Z.H. Physiological and biochemical responses of sweet sorghum to cadmium stress and its cadmium accumulation. J. Agric. Sci. Technol. 2019, 23, 30–42. [Google Scholar]
- Sharma, A.K.; Sharma, S. Antidiabetic potential of bioactive compounds from natural sources. J. Tradit. Complement. Med. 2019, 9, 409–420. [Google Scholar]
- Girard, A.L.; Awika, J.M. Sorghum polyphenols and other bioactive components as functional and health promoting food ingredients. J. Cereal Sci. 2018, 84, 112–124. [Google Scholar] [CrossRef]
- Wu, L.; Huang, Z.; Qin, P.; Yao, Y.; Meng, X.; Zou, J.; Zhu, K.; Ren, G. Chemical characterization of a procyanidin-rich extract from sorghum bran and its effect on oxidative stress in tumor inhibition in vivo. J. Agric. Food Chem. 2011, 59, 8609–8615. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Komolafe, Y.O.; Oloyede, O.B.; Ogunbode, S.M.; Adeoye, M.D.; Abdulsalami, I.O.; Nurudeen, Q.O. Polyphenolic extract of Sorghum bicolor grains enhances reactive oxygen species detoxification in N-nitrosodiethylamine-treated rats. Food Sci. Hum. Wellness 2013, 2, 39–45. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, X.; Zhang, X.; Liu, H. Bioactive Compounds and Biological Activities of Sorghum Grains. Foods 2021, 10, 2868. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Ng, K.; Zhang, P.; Warner, R.D.; Shen, S.; Tang, H.-Y.; Liang, Z.; Fangn, Y. In Vitro α-Glucosidase and α-Amylase Inhibitory Activities of Free and Bound Phenolic Extracts from the Bran and Kernel Fractions of Five Sorghum Grain Genotypes. Foods 2020, 9, 1301. [Google Scholar] [CrossRef]
- Li, Y.; Qi, X.; Yang, X.; Zhang, J.; Zhang, Y.; Bai, Y. Antidiabetic activities of Sorghum polysaccharide on type 2 diabetic rats and its mechanism. Int. J. Biol. Macromol. 2020, 161, 659–668. [Google Scholar]
- Johnson, R.J.; Bakris, G.L.; Borghi, C.; Chonchol, M.B.; Feldman, D.; Lanaspa, M.A.; Vassalotti, J.A. Hyper-glycemia, diabetes and COVID-19. Diabetologia 2022, 65, 500–522. [Google Scholar]
- Bouargalne, Y.; Ben Mrid, R.; Bouchmaa, N.; Zouaoui, Z.; Ben Mrid, B.; Kchikich, A.; El Omari, R.; Kabach, I.; Mohamed, N. Genetic diversity for agromorphological traits, phytochemical profile, and antioxidant activity in Moroccan sorghum ecotypes. Sci. Rep. 2022, 12, 589. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Kabach, R.; Mrid, N.; Bouchmaa, Y.; Bouargalne, A.Z.; Nhiri, M. Phytochemical screening, antioxidant and cytotoxic activities of M. vulgare. Int. J. Pharm. Res. 2019, 11, 338–345. [Google Scholar]
- Huang, D.J.; Chun-Der, L.; Hsien-Jung, C.; Yaw-Huei, L. Antioxidant and antiproliferative activities of sweet potato (Ipomoea batatas [L.] LamTainong 57′) constituents. Bot. Stud. 2004, 45, 179–186. [Google Scholar]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Ben Mrid, R.; Bouchmaa, N.; Bouargalne, Y.; Ramdan, B.; Karrouchi, K.; Kabach, I.; El Karbane, M.; Idir, A.; Zyad, A.; Nhiri, M. Phytochemical characterization, antioxidant and in vitro cytotoxic activity evaluation of Juniperus oxycedrus subsp. oxycedrus needles and berries. Molecules 2019, 24, 502. [Google Scholar] [CrossRef] [PubMed]
- Dinis, T.C.; Madeira, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reaction antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Thilavech, T.; Chusak, C. Mesona chinensis Benth extract prevents AGE formation and protein oxidation against fructose-induced protein glycation in vitro. BMC Complement Altern. Med. 2014, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Huang, Z.; Wang, Y.; Wang, Y.; Fu, L.; Su, L. Chinese Bayberry (Myrica rubra) Phenolics Mitigated Protein Gly-coxidation and Formation of Advanced Glycation End-Products: A Mechanistic Investigation. Food Chem. 2021, 361, 130102. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-Q.; Li, M.; Zhu, F.; Liu, F.-L.; Huang, J.-B. Inhibitory potential of trilobatin from Lithocarpus polystachyus Rehd against α-glucosidase and α-amylase linked to type 2 diabetes. Food Chem. 2012, 130, 261–266. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Jeong, Y.-K.; Wang, M.-H.; Lee, W.-Y.; Rhee, H.-I. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition 2005, 21, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Kee, K.T.; Koh, M.; Oong, L.X.; Ng, K. Screening culinary herbs for antioxidant and α- glucosidase inhibitory activities. Int. J. Food Sci. Technol. 2013, 48, 1884–1891. [Google Scholar] [CrossRef]
- Asraoui, F.; Kounnoun, A.; Cacciola, F.; El Mansouri, F.; Kabach, I.; Oulad El Majdoub, Y.; Alibrando, F.; Arena, K.; Trovato, E.; Mondello, L. Phytochemical Profile, Antioxidant Capacity, α-Amylase and α-Glucosidase Inhibitory Potential of Wild Moroccan Inula viscosa (L.) Aiton Leaves. Molecules 2021, 26, 3134. [Google Scholar] [CrossRef] [PubMed]
- Malongane, F.; Phoswa, W.N.; Berejena, T. The effect of indigenous African Diet on inflammatory markers linked to Type 2 Diabetic Mellitus. Hum. Nutr. Metab. 2023, 35, 200236. [Google Scholar] [CrossRef]
- Alema, N.M.; Periasamy, G.; Sibhat, G.G.; Tekulu, G.H.; Hiben, M.G. Antidiabetic activity of extracts of Terminalia brownii Fresen. Stem bark in mice. J. Exp. Pharmacol. 2020, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Menacherry, S.; Rao, A.R. Blood Collection Techniques in Experimental Animals. J. Lab. Anim. Sci. 2020, 50, 112–122. [Google Scholar]
- Bouchmaa, N.; Ben Mrid, R.; Boukharsa, Y.; Nhiri, M.; Ait Mouse, H.; Taoufik, J.; Ansar, M.; Zyad, A. Cytotoxicity of new pyri-dazin-3 (2H)-one derivatives orchestrating oxidative stress in human triple-negative breast cancer (MDA-MB-468). Arch. Pharm. 2018, 351, 1800128. [Google Scholar] [CrossRef]
- Bouchmaa, N.; Ben Mrid, R.; Boukharsa, Y.; Bouargalne, Y.; Nhiri, M.; Idir, A.; Zyad, A. Reactive Oxygen Species-Mediated Apoptosis and Cytotoxicity of Newly Synthesized Pyridazin-3-Ones In P815 (Murin Mastocytoma) Cell Line. Drug Res. 2019, 69, 528–536. [Google Scholar] [CrossRef]
- Ennoury, A.; Roussi, Z.; Nhhala, N.; Zouaoui, Z.; Bouchra, B.; Azzouz, K.; Anass, K.; Kabach, I.; Nhiri, M. Chaste plant extract is a promising biostimulant for tomato plants’ growth under salt stress. Biomass Conv. Bioref. 2022. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Harlan, J.R.; de Wet, J.M. A simplified classification of cultivated sorghum. Crop Sci. 1972, 12, 172–176. [Google Scholar] [CrossRef]
- Mohammadi, G.R. The effect of seed priming on plant traits of late-spring seeded soybean (Glycine max L.). Am. Eurasian J. Agric. Environ. Sci. 2009, 5, 322–326. [Google Scholar]
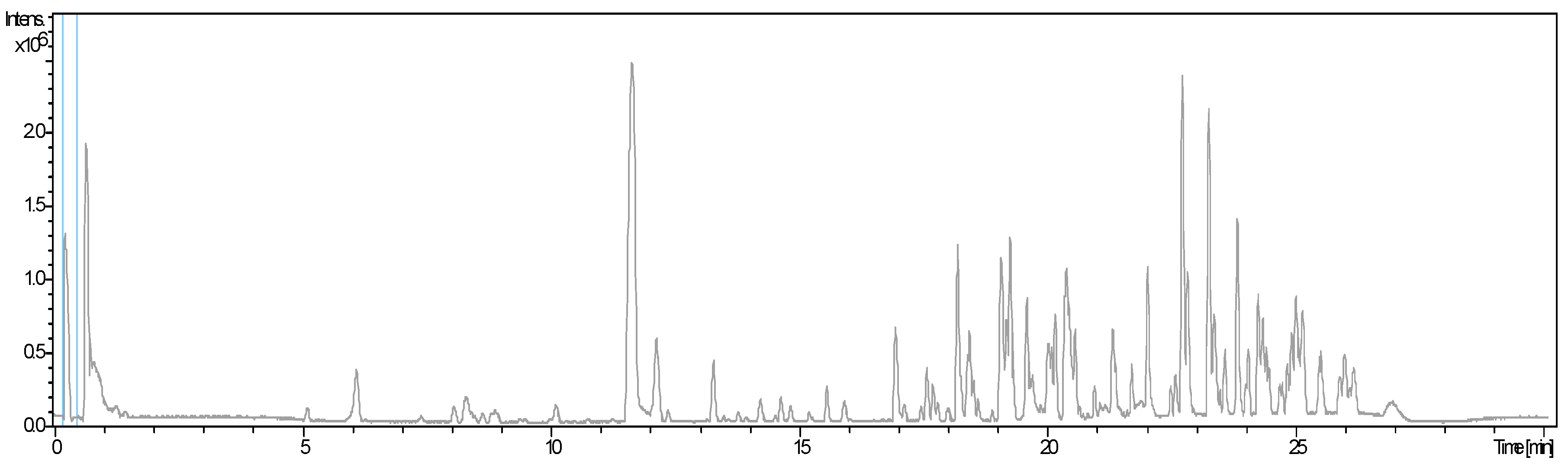
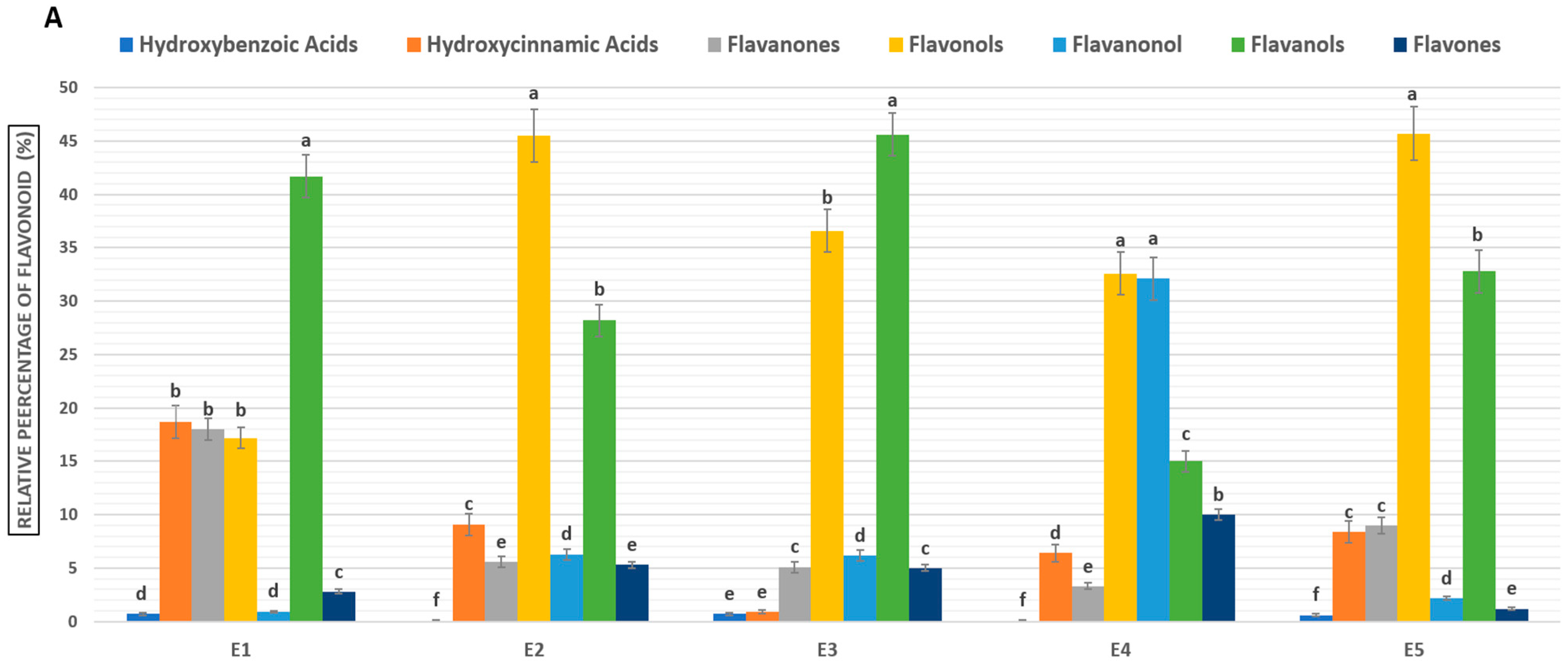

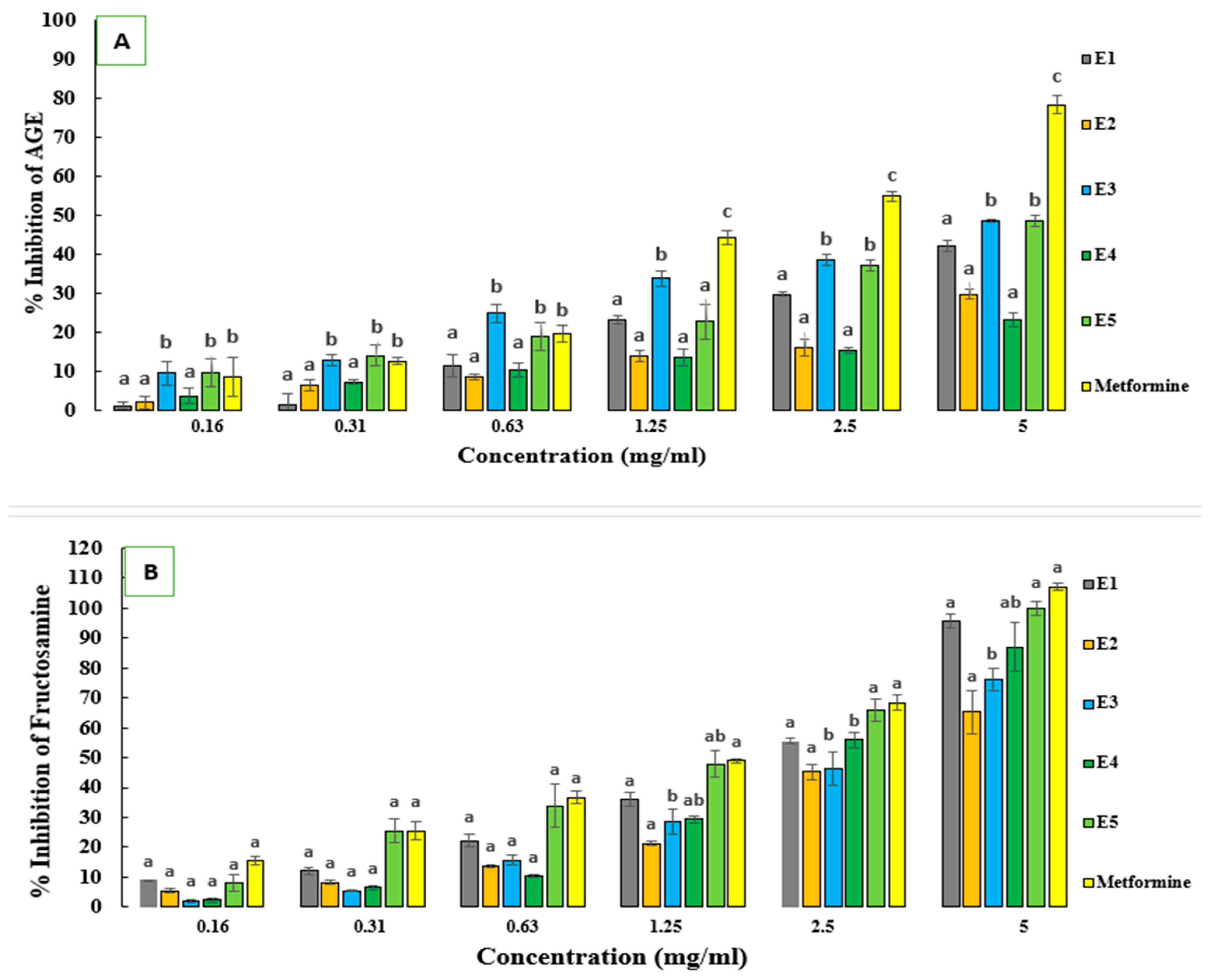

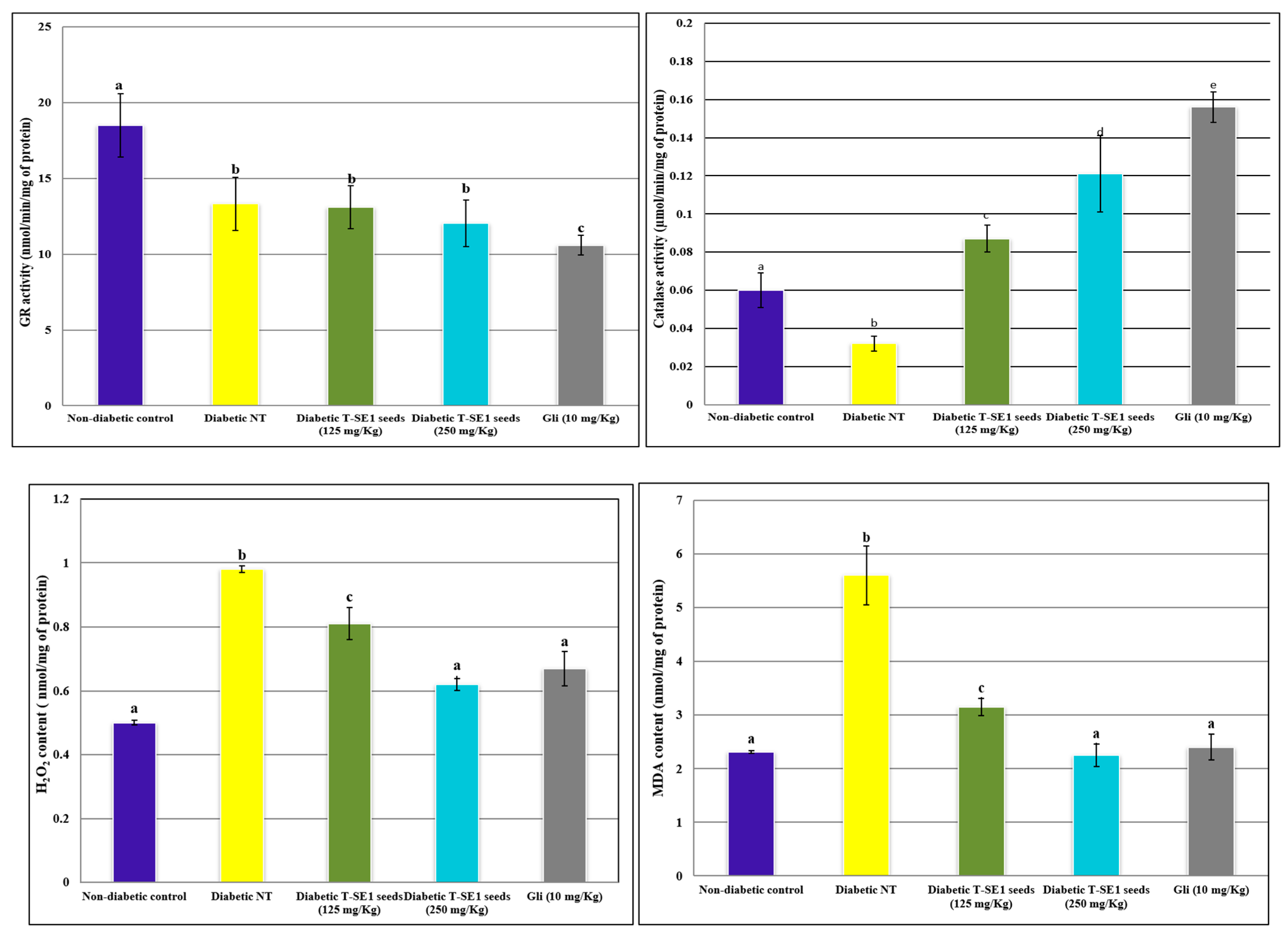
| Ecotypes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak # | tR (min) | [M + H]+ | Name | Family | E1 (%) | E2 (%) | E3 (%) | E4 (%) | E5 (%) |
| 1 | 0.7 | 171.0288 | Gallic acid | I | 0.5 ± 0.1 a | 0.5 ± 0.1 a | 0.4 ± 0.1 a | - | - |
| 2 | 1.1 | 169.0454 | Vanillic acid | I | 0.9 ± 0.2 a | 0.3 ± 0.1 b | - | - | - |
| 3 | 1.5 | 165.0546 | p-coumaric acid | II | 1.1 ± 0.2 a | 1.5 ± 0.2 a | 0.9 ± 0.1 a | 1.0 ± 0.1 a | - |
| 4 | 3.8 | 155.03388 | Protocatechic acid | I | 1.7 ± 0.3 a | 2.9 ± 0.4 b | 1.0 ± 0.2 a | 1.0 ± 0.1 a | 0.8 ± 0.1 a |
| 5 | 5.1 | 579.1497 | Procyanidin B-1 | V | 11.2 ± 1.1 a | 8.4 ± 0.9 b | 10.3 ± 1.0 a | 3.2 ± 0.3 c | 11.5 ± 1.1 a |
| 6 | 6.1 | 139.03897 | Hydroxybenzoic acid | I | - | - | - | - | 1.2 ± 0.2 |
| 7 | 6.1 | 291.0863 | Catechin | V | 53.8 ± 5.4 a | 40.8 ± 4.1 b | 54.6 ± 5.5 a | 19.6 ± 2.0 c | 68.6 ± 6.8 d |
| 8 | 7.0 | 181.0495 | Caffeic acid | II | 0.9 ± 0.1 a | 0.4 ± 0.1 b | 0.9 ± 0.1 a | 0.8 ± 0.1 a | 1.3 ± 0.1 a |
| 9 | 7.1 | 195.06518 | Ferulic acid | II | 1.3 ± 0.2 a | 1.3 ± 0.2 a | 0.6 ± 0.1 b | 0.5 ± 0.1 b | 0.7 ± 0.1 b |
| 10 | 8.2 | 289.07066 | Eriodictyol | IV | 5.6 ± 0.6 a | 6.7 ± 0.9 b | 2.4 ± 0.3 b | 1.0 ± 0.1 c | - |
| 11 | 9.1 | 305.06557 | Taxifolin | V | 6.8 ± 0.7 a | 9.7 ± 1.1 a | 7.9 ± 0.8 a | 38.4 ± 3.9 b | 3.3 ± 0.3 c |
| 12 | 9.4 | 273.07575 | Naringenin | IV | 1.9 ± 0.2 a | 2.4 ± 0.2 a | 0.8 ± 0.1 b | 0.5 ± 0.1 b | - |
| 13 | 9.5 | 271.0601 | Apigenin | VI | 2.3 ± 0.3 a | 4.3 ± 0.4 b | 4.5 ± 0.5 b | 5.7 ± 0.6 b | 1.9 ± 0.2 a |
| 14 | 10.6 | 303.04993 | Quercetin | VI | 0.2 ± 0.1 a | 2.3 ± 0.3 a | 1.9 ± 0.2 a | 9.5 ± 1.0 b | 0.7 ± 0.1 b |
| 15 | 10.6 | 611.16066 | Rutin | IV | 1.6 ± 0.2 a | 2.3 ± 0.3 b | 1.1 ± 0.1 a | - | 1.7 ± 0.2 ab |
| Ecotype | Macroelements (mg/kg) | Microelements (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca | K | Mg | P | S | Fe | Mn | Si | Zn | |
| E1 | 199 ± 0.5 | 510 ± 2.0 | 260 ± 3.0 | 530 ± 2.5 | 100 ± 1.6 | 57 ± 0.3 | 28 ± 0.2 | 396 ± 5.9 | 33 ± 0.6 |
| E2 | 78 ± 0.5 | 300 ± 6.0 | 100 ± 0.9 | 220 ± 0.9 | 100 ± 1.2 | 30 ± 0.4 | 13 ± 0.1 | 408 ± 1.7 | 14 ± 0.1 |
| E3 | 64 ± 0.8 | 240 ± 4.0 | 80 ± 0.7 | 180 ± 1.4 | 100 ± 0.9 | 18 ± 0.2 | 8.7 ± 0.02 | 375 ± 5.2 | 12 ± 0.3 |
| E4 | 104 ± 0.4 | 250 ± 3.0 | 120 ± 0.9 | 250 ± 2.3 | 120 ± 1.3 | 33 ± 0.2 | 16 ± 0.1 | 472 ± 12.1 | 20 ± 0.3 |
| E5 | 125 ± 0.8 | 530 ± 4.2 | 170 ± 5.0 | 420 ± 1.4 | 100 ± 0.5 | 195 ± 2.2 | 22 ± 0.1 | 361 ± 5.7 | 23 ± 0.9 |
| Ecotype | 11B (mg/Kg) | 111Cd (µg/kg) | 59Co (µg/kg) | 52Cr (µg/kg) | 63Cu (mg/Kg) | 95Mo (mg/Kg) | 23Na (mg/Kg) | 60Ni (mg/Kg) | 208Pb (µg/kg) | 78Se (µg/kg) | 51V (µg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| E1 | 1.62 ± 0.03 | <2 | 33 ± 1.38 | 204 ± 3.87 | 4.41 ± 0.04 | 0.52 ± 0.02 | 9.80 ± 0.22 | 0.45 ± 0.01 | 121 ± 1.69 | 12 ± 1.44 | 70 ± 1.58 |
| E2 | 1.09 ± 0.04 | <2 | 16 ± 1.04 | 192 ± 3.07 | 1.91 ± 0.04 | 0.380 ± 0.003 | 3.28 ± 0.22 | 0.340 ± 0.005 | 30 ± 0.72 | 15 ± 1.44 | 41 ± 2.04 |
| E3 | 1.58 ± 0.05 | <2 | 12 ± 0.84 | 68 ± 1.97 | 2.22 ± 0.02 | 0.70 ± 0.01 | 4.83 ± 0.60 | 0.156 ± 0.002 | 29 ± 0.66 | 16 ± 1.71 | 16 ± 1.38 |
| E4 | 2.15 ± 0.03 | <2 | 24 ± 0.98 | 249 ± 2.49 | 2.65 ± 0.05 | 0.96 ± 0.03 | 2.98 ± 0.10 | 0.56 ± 0.02 | 30 ± 0.63 | 23 ± 2.71 | 43 ± 2.92 |
| E5 | 1.48 ± 0.04 | <2 | 98 ± 2.54 | 688 ± 10.32 | 1.36 ± 0.01 | 0.61 ± 0.01 | 2.12 ± 0.02 | 0.67 ± 0.01 | 207 ± 3.72 | 17 ± 1.34 | 397 ± 10.95 |
| Ecotypes | Extract Yield (%) | Polyphenols (TPC) (mg GAE/g dE) | Flavonoids (TFC) (mg QE/g dE) | Tannins EAT (mg QE/mg dE) |
|---|---|---|---|---|
| E1 | 26.73 b | 297 ± 0.008 b | 72 ± 0.002 a | 0.253 ± 0.002 a |
| E2 | 17.05 a | 188 ± 0.006 a | 66 ± 0.005 a | 0.210 ± 0.009 b |
| E3 | 22.49 a | 229 ± 0.006 a | 78 ± 0.002 a | 0.203 ± 0.002 c |
| E4 | 25.23 a | 262 ± 0.005 a | 73 ± 0.001 a | 0.222 ± 0.003 d |
| E5 | 19.12 a | 192 ± 0.005 a | 70 ± 0.001 a | 0.195 ± 0.001 e |
| Ecotypes | Antioxidant Properties (IC50 Values; mg/mL) | |||
|---|---|---|---|---|
| DPPH Scavenging Activity | ABTS | Metal Chelating Activity | Reducing Power (mg AAE/g dE) | |
| E1 | 0.059 ± 0.002 a | 0.090 ± 0.010 a | 2.57 ± 0.10 a | 111 ± 8 a |
| E2 | 0.060 ± 0.001 a | 0.131 ± 0.003 b | 1.816 ± 0.07 b | 121 ± 8 b |
| E3 | 0.059 ± 0.002 a | 0.118 ± 0.002 b | 1.3 ± 0.1 b | 119 ± 3 b |
| E4 | 0.043 ± 0.004 b | 0.118 ± 0.020 b | 2.110 ± 0.007 a | 101 ± 7 c |
| E5 | 0.048 ± 0.001 b | 0.140 ± 0.010 b | 1.297 ± 0.020 b | 90 ± 3 c |
| Trolox | 0.203 ± 0.020 c | 0.086 ± 0.001 c | - | - |
| EDTA | - | - | 0.189 ± 0.030 c | - |
| Ecotypes | IC50 (mg/mL) | |
|---|---|---|
| Alpha-Amylase | Alpha-Glucosidase | |
| Sorghum-E1 | 1.02 ± 0.20 a | 2.013 ± 0.008 a |
| Sorghum-E2 | 1.54 ± 0.03 a | 1.07 ± 0.06 a |
| Sorghum-E3 | 2.87 ± 0.01 a | 1.038 ± 0.040 a |
| Sorghum-E4 | 3.4 ± 0.1 a | 1.889 ± 0.020 a |
| Sorghum-E5 | 2.92 ± 0.06 a | 2.009 ± 0.020 a |
| Acarbose | 0.05 ± 0.02 b | 0.399 ± 0.003 b |
| Treatment | Blood Glucose Level (mg/dL) | Body Weight (g) | ||||||
|---|---|---|---|---|---|---|---|---|
| Time (By Weeks) | Time (By Weeks) | |||||||
| Week 0 | Week 1 | Week 2 | Week 3 | Week 0 | Week 1 | Week 2 | Week 3 | |
| Normal | 78.01 ± 3.2 c | 70.00 ± 3.03 e | 77.65 ± 1.2 c | 73.43 ± 5 d | 21.12 ± 1.87 b | 25.02 ± 4.02 b | 22.91 ± 4.3 b | 23.12 ± 0.23 b |
| Diabetic.NT | 501 ± 9.00 a | 508.02 ± 32.0 a | 467.71 ± 43.9 a | 421.76 ± 26.7 a | 19.23 ± 3.01 a | 17.32 ± 2.3 a | 18.12 ± 3.05 a | 16.88 ± 3.93 a |
| +125 mg/Kg | 532 ± 54 a | 433 ± 12 b | 389.02 ± 65.0 b | 287.89 ± 42 b | 18.87 ± 3.87 a | 18.23 ± 4.02 a | 17.91 ± 3.4 a | 19.54 ± 1.05 a |
| +250 mg/Kg | 356.9 ± 28 b | 350.7 ± 65.1 c | 277.7 ± 23.7 b | 165.54 ± 9.02 c | 17.01 ± 3.65 a | 17.65 ± 3.22 a | 17.13 ± 4.06 a | 19.66 ± 1.24 a |
| Glibenclamide | 444.83 ± 43.02 a | 384.30 ± 54.3 d | 282.02 ± 17.8 b | 199.76 ± 23.1 c | 21.76 ± 4.3 a | 20.22 ± 2.43 a | 19.83 ± 3.90 a | 20.94 ± 3.46 a |
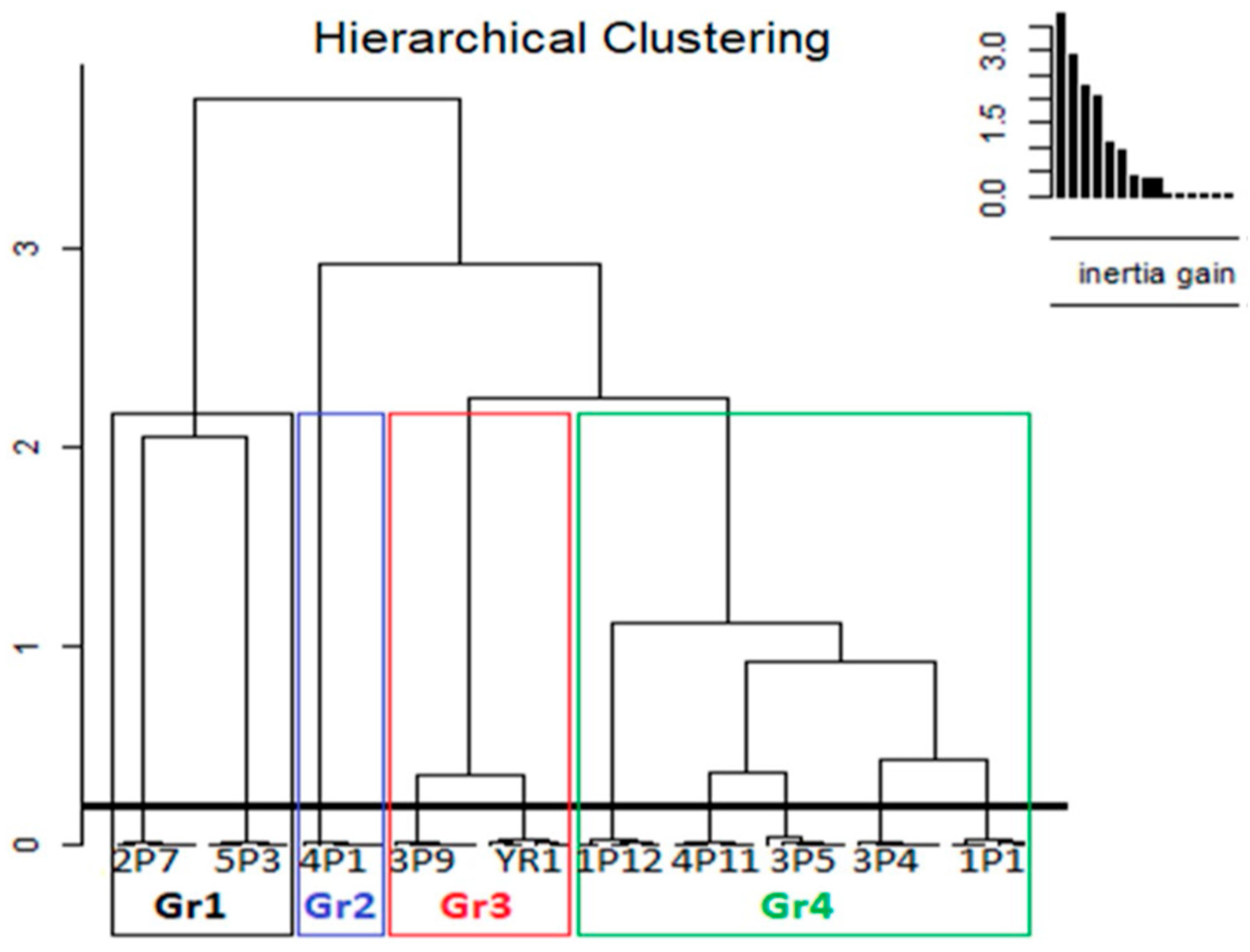
| Abbreviations of the Studied Ecotypes | Extraction Solvent | Designation of Sorghum Ecotype | Groups | Number of Ecotypes Per Group |
|---|---|---|---|---|
| E1 | Ethanol | 1P12 | Gr4 | 5 |
| E2 | Ethanol | 5P3 | Gr1 | 2 |
| E3 | Ethanol | 3P4 | Gr4 | 5 |
| E4 | Ethanol | 3P9 | Gr3 | 2 |
| E5 | Ethanol | 4P1 | Gr2 | 1 |
| ICP-MS Agilent 7800 Operating Conditions | |||
|---|---|---|---|
| Collision/reaction cell gas | - | He | H2 |
| Collision/reaction cell gas flow rate mL.min−1 | - | 4.3 | 4.2 |
| Energy discrimination (V) | 5 | 3 | 5 |
| Nebulizer gas flow rate L.min−1 | 0.99 | ||
| Auxiliary gas flow rate L.min−1 | 0.9 | ||
| Plasma gas flow rate L.min−1 | 15 | ||
| Nebulizer type | Concentric Micromist | ||
| Spray chamber | Quartz Scott double pass | ||
| Injector | Quartz 2.5 mm | ||
| RF Power (W) | 1550 | ||
| Wash (s) | 120 | ||
| Integration time (s) | 1 | ||
| Replicates | 4 | ||
| Sweeps | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben El Mahdi, N.; Lemée, L.; Remaury, Q.B.; Eloy, L.; Nhiri, N.; Lakhssassi, N.; Cacciola, F.; Nhiri, M. Potential of Sorghum Seeds in Alleviating Hyperglycemia, Oxidative Stress, and Glycation Damage. Molecules 2024, 29, 3445. https://doi.org/10.3390/molecules29153445
Ben El Mahdi N, Lemée L, Remaury QB, Eloy L, Nhiri N, Lakhssassi N, Cacciola F, Nhiri M. Potential of Sorghum Seeds in Alleviating Hyperglycemia, Oxidative Stress, and Glycation Damage. Molecules. 2024; 29(15):3445. https://doi.org/10.3390/molecules29153445
Chicago/Turabian StyleBen El Mahdi, Nora, Laurent Lemée, Quentin Blancart Remaury, Lilian Eloy, Naima Nhiri, Naoufal Lakhssassi, Francesco Cacciola, and Mohamed Nhiri. 2024. "Potential of Sorghum Seeds in Alleviating Hyperglycemia, Oxidative Stress, and Glycation Damage" Molecules 29, no. 15: 3445. https://doi.org/10.3390/molecules29153445
APA StyleBen El Mahdi, N., Lemée, L., Remaury, Q. B., Eloy, L., Nhiri, N., Lakhssassi, N., Cacciola, F., & Nhiri, M. (2024). Potential of Sorghum Seeds in Alleviating Hyperglycemia, Oxidative Stress, and Glycation Damage. Molecules, 29(15), 3445. https://doi.org/10.3390/molecules29153445








