Characterization of Bioactive Phenolic Compounds Extracted from Hydro-Distillation By-Products of Spanish Lamiaceae Plants
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Phenolic Compounds in the Solid Residue from Hydro-Distillation of the Aromatic Plants
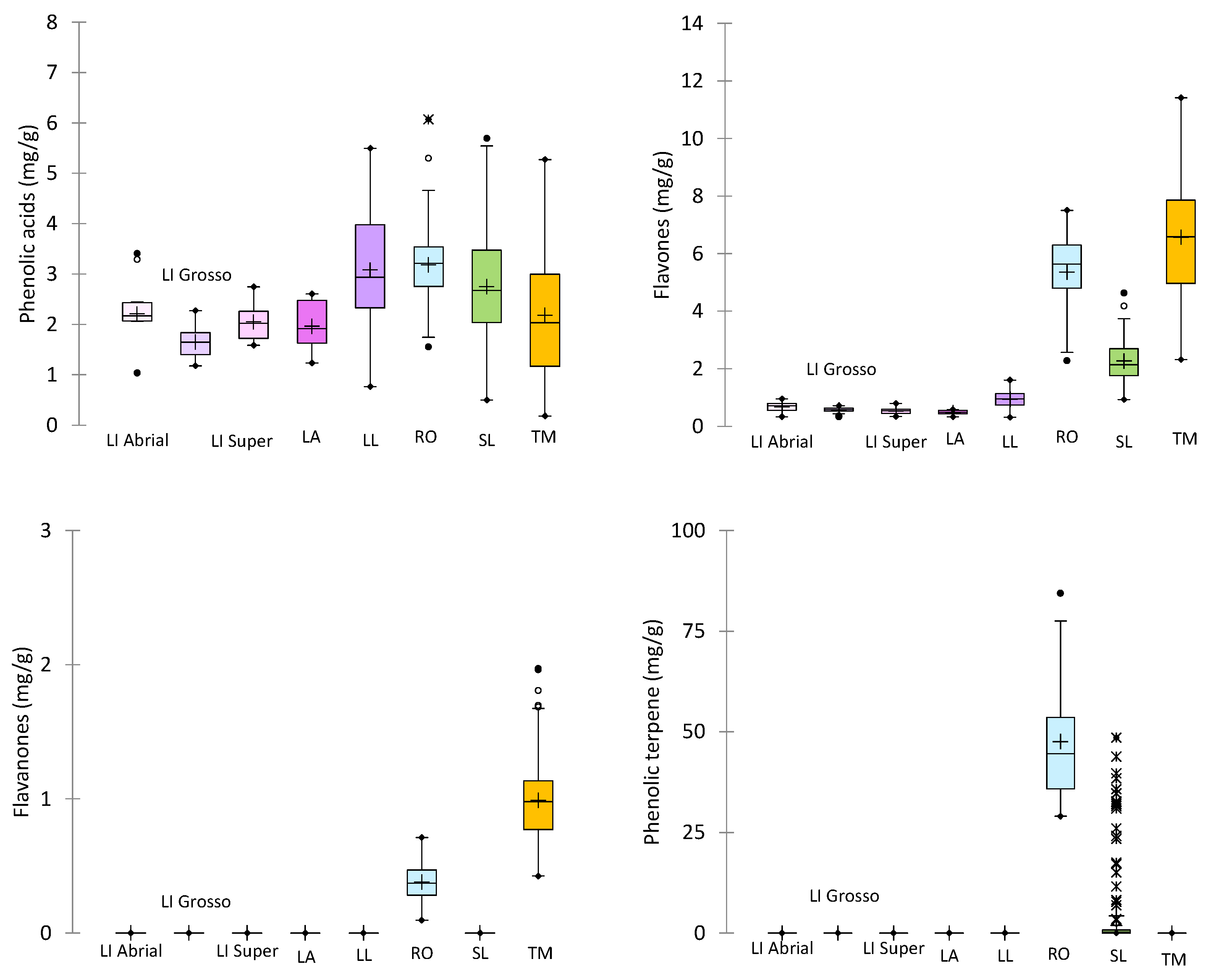
2.1.1. Phenolic Acids
2.1.2. Flavones and Flavonols
2.1.3. Phenolic Terpenes
2.2. Results of the Method Validation
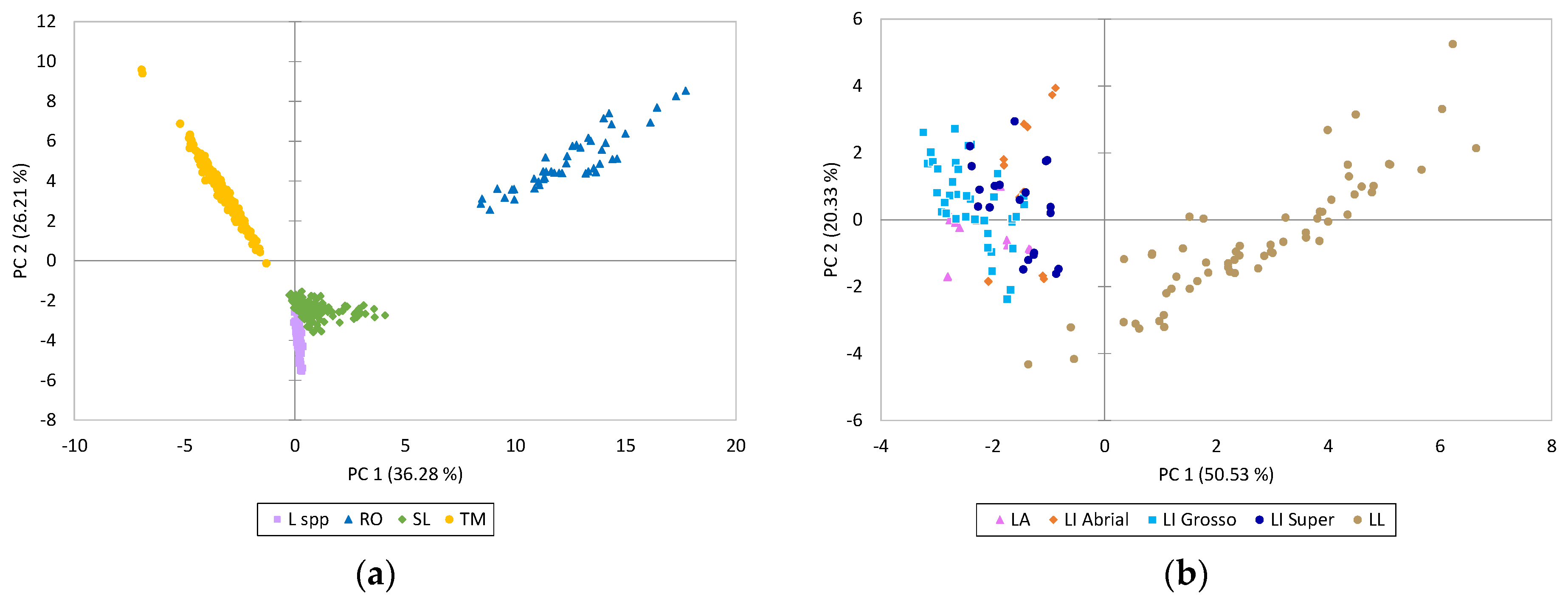
2.3. Quantification of Phenolic Compounds in the Solid Residue from Hydro-Distillation of the Aromatic Plants
3. Materials and Methods
3.1. Plant Samples
3.2. Chemicals
3.3. Solid Residue from the Hydro-Distillation of the Aromatic Plants and Extraction of the Phenolic Compounds
3.4. High-Performance Liquid Chromatography Diode Array Detection (HPLC-DAD) for the Quantification of Phenolic Compounds
3.5. Liquid Chromatography Diode Array Detection and Mass Spectrometer (LC-DAD-MS) for the Identification of Phenolic Compounds
3.6. Method Validation
3.7. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brinckmann, J.A.; Kathe, W.; Berkhoudt, K.; Harter, D.E.V.; Schippmann, U. A new global estimation of medicinal and aromatic plant species in commercial cultivation and their conservation status. Econ. Bot. 2022, 76, 319–333. [Google Scholar] [CrossRef]
- Taghouti, I.; Cristobal, R.; Brenko, A.; Stara, K.; Markos, N.; Chapelet, B.; Hamrouni, L.; Bursic, D.; Bonet, J.A. The market evolution of medicinal and aromatic plants: A global supply chain analysis and an application of the Delphi method in the Mediterranean area. Forests 2022, 13, 808. [Google Scholar] [CrossRef]
- Sharma, A.; Gumber, K.; Gohain, A.; Bhatia, T.; Sohal, H.S.; Mutreja, V.; Bhardwaj, G. Chapter 3—Importance of essential oils and current trends in use of essential oils (aroma therapy, agrofood, and medicinal usage). In Essential Oils. Extraction, Characterization and Applications; Nayik, G.A., Ansari, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 53–83. [Google Scholar] [CrossRef]
- Shabankareh, H.G.; Khorasaninejad, S.; Soltanloo, H.; Shariati, V. Physiological response and secondary metabolites of three lavender genotypes under water deficit. Sci. Rep. 2021, 11, 19164. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.; Martínez, J.R. Essential oils and the circular bioeconomy. In Essential Oils—Recent Advances, New Perspectives and Applications; Viskelis, J., Ed.; IntechOpen: London, UK, 2024; pp. 1–13. [Google Scholar] [CrossRef]
- Saha, A.; Basak, B.B. Scope of value addition and utilization of residual biomass from medicinal and aromatic plants. Ind. Crops Prod. 2020, 145, 111979. [Google Scholar] [CrossRef]
- Galanakis, C.M. Food Waste Recovery: Processing Technologies and Industrial Techniques, 1st ed.; Academic Press-Elsevier Inc.: London, UK, 2015. [Google Scholar]
- Torras-Claveria, L.; Jauregui, O.; Bastida, J. Antioxidant activity and phenolic composition of lavandin (Lavandula x intermedia Emeric ex Loiseleur) waste. J. Agric. Food Chem. 2007, 55, 8436–8443. [Google Scholar] [CrossRef]
- Navarrete, A.; Herrero, M.; Martín, A.; Cocero, M.J.; Ibáñez, E. Valorization of solid wastes from essential oil industry. J. Food Eng. 2011, 104, 196–201. [Google Scholar] [CrossRef]
- Parejo, I.; Viladomat, F.; Bastida, J.; Rosas-Romero, A.; Flerlage, N.; Burillo, J.; Codina, C. Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled mediterranean herbs and aromatic plants. J. Agric. Food Chem. 2002, 50, 6882–6890. [Google Scholar] [CrossRef]
- Asensio-S.-Manzanera, M.C.; Martín, H.; Sanz, M.A.; Herrero, B. Antioxidant activity of Lavandula latifolia, Salvia lavandulifolia and Thymus mastichina collected in Spain. Acta Hortic. 2011, 925, 281–290. [Google Scholar] [CrossRef]
- Santana-Méridas, O.; González-Coloma, A.; Sánchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- Moisa, C.; Copolovici, L.; Bungau, S.; Pop, G.; Imbrea, I.; Lupitu, A.; Nemeth, S.; Copolovici, D. Wastes resulting from aromatic plants distillation. Biosources of antioxidants and phenolic compounds with biological active principles. Farmacia 2018, 66, 289–295. [Google Scholar]
- Ortiz de Elguea-Culebras, G.; Melero Bravo, E.; Sánchez-Vioque, R. Potential sources and methodologies for the recovery of phenolic compounds from distillation residues of Mediterranean aromatic plants. An approach to the valuation of by-products of the essential oil market—A review. Ind. Crops Prod. 2022, 175, 114261. [Google Scholar] [CrossRef]
- Miljanović, A.; Dent, M.; Grbin, D.; Pedisić, S.; Zorić, Z.; Marijanović, Z.; Jerković, I.; Bielen, A. Sage, Rosemary, and Bay Laurel Hydrodistillation By-Products as a Source of Bioactive Compounds. Plants 2023, 12, 2394. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.M.; Algieri, F.; Rodríguez-Nogales, A.; Gálvez, J.; Segura-Carretero, A. Phytochemical profiling of anti-inflammatory Lavandula extracts via RP–HPLC–DAD–QTOF–MS and –MS/MS: Assessment of their qualitative and quantitative differences. Electrophoresis 2018, 39, 1284–1293. [Google Scholar] [CrossRef]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C.G. LC-MS Identification and quantification of phenolic compounds in solid residues from the essential oil industry. Antioxidants 2021, 10, 2016. [Google Scholar] [CrossRef]
- Almela, L.; Sánchez-Muñoz, B.; Fernández-López, J.A.; Roca, M.J.; Rabea, V. Liquid chromatograpic–mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J. Chromatogr. A 2006, 1120, 221–229. [Google Scholar] [CrossRef]
- Clifford, M.N.; Wu, W.; Kirkpatrick, J.; Kuhnert, N. Profiling the chlorogenic acids and other caffeic acid derivatives of herbal chrysanthemum by LC-MSn. J. Agric. Food Chem. 2007, 55, 929–936. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J. Full-Spectrum analysis of bioactive compounds in Rosemary (Rosmarinus officinalis L.) as influenced by different extraction methods. Molecules 2020, 25, 4599. [Google Scholar] [CrossRef]
- Achour, M.; Mateos, R.; Fredj, M.B.; Mtiraoui, A.; Bravo, L.; Saguem, S. A comprehensive characterisation of Rosemary tea obtained from Rosmarinus officinalis L. Collected in a sub-humid area of Tunisia. Phytochem. Anal. 2018, 29, 87–100. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Grayer, R.J.; Eckert, M.R.; Lever, A.; Veitch, N.C.; Kite, G.C.; Paton, A.J. Distribution of exudate flavonoids in the genus Plectranthus. Biochem. Syst. Ecol. 2010, 38, 335–341. [Google Scholar] [CrossRef]
- Psarrou, I.; Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction kinetics of phenolic antioxidants from the hydro distillation residues of rosemary and effect of pretreatment and extraction parameters. Molecules 2020, 25, 4520. [Google Scholar] [CrossRef] [PubMed]
- Cuvelier, M.E.; Richard, H.; Berset, C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chem. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Schwarz, K.; Ternes, W. Antioxidative constituents of Rosmarinus officinalis and Salvia officinalis. II. Isolation of carnosic acid and formation of other phenolic diterpenes. Z. Lebensm. Unters. Forsch. 1992, 195, 99–103. [Google Scholar] [CrossRef]
- Doolaege, E.H.A.; Raes, K.; Smet, K.; Andjelkovic, M.; Van Poucke, C.; De Smet, S.; Verhe, R. Characterization of two unknown compounds in methanol extracts of rosemary oil. J. Agric. Food Chem. 2007, 55, 7283–7287. [Google Scholar] [CrossRef]
- Zhang, Y.; Smuts, J.P.; Dodbiba, E.; Rangarajan, R.; Lang, J.C.; Armstrong, D.W. Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J. Agric. Food Chem. 2012, 60, 9305–9314. [Google Scholar] [CrossRef]
- Bicchi, C.; Binello, A.; Rubiolo, P. Determination of phenolic diterpene antioxidants in rosemary (Rosmarinus officinalis L.) with different methods of extraction and analysis. Phytochem. Anal. 2000, 11, 236–242. [Google Scholar] [CrossRef]
- Troncoso, N.; Sierra, H.; Carvajal, L.; Delpiano, P.; Gunther, G. Fast high-performance liquid chromatography and ultraviolet-visible quantification of principal phenolic antioxidants in fresh rosemary. J. Chromatogr. A 2005, 1100, 20–25. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Arráez-Román, D.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comparison of different extraction procedures for the comprehensive characterization of bioactive phenolic compounds in Rosmarinus officinalis by reversed-phase high-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 7682–7690. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; Ortega-Heras, M.; Cano-Mozo, E. Optimization of a solid-phase extraction method using copolymer sorbents for isolation of phenolic compounds in red wines and quantification by HPLC. J. Agric. Food Chem. 2008, 56, 11560–11570. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Yan-Li, L.; Hong-Cui, T.; Wen-Ting, Z. Preparation and chemical structure identification of methylated metabolites of salvianolic acid A. Chin. J. Anal. Chem. 2014, 42, 65–70. [Google Scholar]
- Li, X.; Yu, C.; Lu, Y.; Gu, Y.; Lu, J.; Xu, W.; Xuan, L.; Wang, Y. Pharmacokinetics, tissue distribution, metabolism, and excretion of depside salts from Salvia miltiorrhiza in rats. Drug Metab. Dispos. 2007, 35, 234–239. [Google Scholar] [CrossRef]
- Ma, L.; Tang, L.; Yi, Q. Salvianolic Acids: Potential Source of Natural Drugs for the Treatment of Fibrosis Disease and Cancer. Front Pharmacol. 2019, 10, 97. [Google Scholar] [CrossRef]
- Koshovyi, O.; Raal, A.; Kovaleva, A.; Myha, M.; Ilina, T.; Borodina, N.; Komissarenko, A. The phytochemical and chemotaxonomic study of Salvia spp. growing in Ukraine. J. Appl. Biol. Biotech. 2020, 8, 29–36. [Google Scholar] [CrossRef]
- Staszek, D.; Orłowska, M.; Waksmundzka-Hajnos, M.; Sajewicz, M.; Kowalska, T. Marker fingerprints originating from TLC and HPLC for selected plants from the Lamiaceae family. J. Liq. Chromatogr. R. T. 2013, 36, 2463–2475. [Google Scholar] [CrossRef]
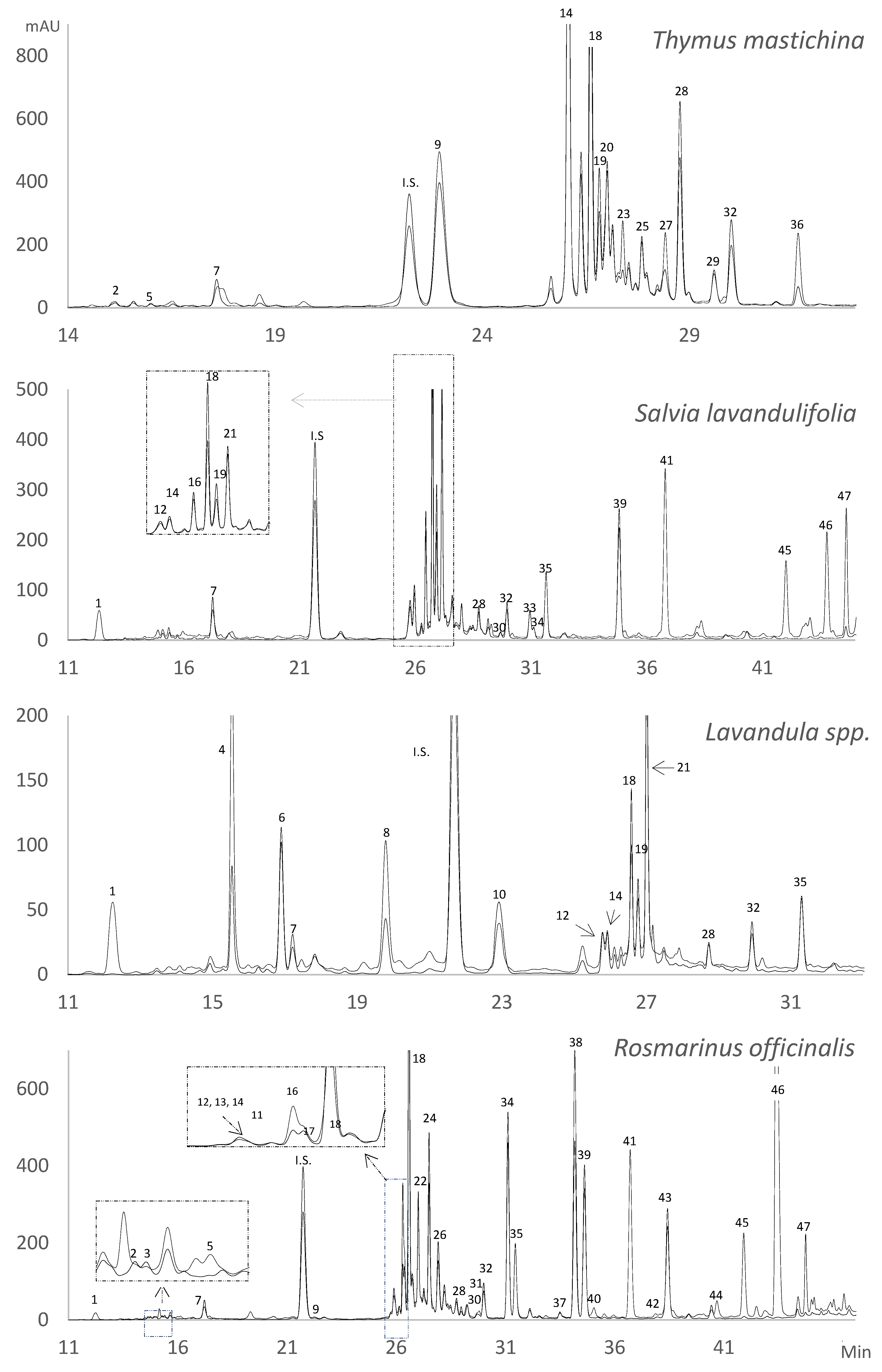
| Peak | Compound | Phenolic Class | λ Max (nm) | [M-H]− | Main Fragments | QW (nm) | IdC b | TM c | SL c | L c | RO c |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Danshensu (3,4-dihydroxyphenyl lactic acid) | Phenolic acid | 282 | 197 | 179, 135 | 280 | S | X e | X | X | |
| 2 | Chlorogenic acid | Phenolic acid | 300sh a, 326 | 353 | 191, 179 | 320 | S | X | X | ||
| 3 | Cryptochlorogenic acid | Phenolic acid | 300sh, 326 | 353 | 191, 179 | 320 | T | X | |||
| 4 | Coumaric acid-O-glucoside | Phenolic acid | 264, 296sh | 325 | 163, 119 | 254 | T | X | |||
| 5 | p-hydroxybenzoic acid | Phenolic acid | 256 | 137 | 254 | S | X | X | |||
| 6 | Ferulic acid-O-glucoside 1 | Phenolic acid | 282sh, 304 | 355 | 193, 149 | 320 | T | X | |||
| 7 | Caffeic acid | Phenolic acid | 298sh, 320 | 179 | 135 | 320 | S | X | X | X | X |
| 8 | Dihydro-p-coumaric acid glucoside | Phenolic acid | 276, 314sh | 327 | 165, 121 | 280 | T | X | |||
| 9 | 6-hydroxyluteolin-7-O-glucoside | Flavone | 282, 344 | 463 | 301 | 350 | T | X | X | ||
| 10 | Ferulic acid-O-glucoside 2 | Phenolic acid | 294, 320 | 355 | 193, 149 | 320 | T | X | |||
| 11 | Rosmarinic acid-3-O-glucoside | Phenolic acid | 288sh, 322 | 521 | 359 | 320 | T | X | |||
| 12 | Luteolin-7-O-rutinoside | Flavone | 254, 266, 350 | 593 | 285 | 350 | S | X | X | X | |
| 13 | Luteolin-7-O-glucuronide | Flavone | 258, 266, 350 | 461 | 285 | 350 | S | X | |||
| 14 | Luteolin-7-O-glucoside | Flavone | 258, 268, 350 | 447 | 285 | 350 | S | X | X | X | X |
| 15 | Naringenin-7-O-glucoside | Flavanone | 284, 332sh | 433 | 271 | 280 | S | X | |||
| 16 | Hesperetin-7-rutinoside (hesperidin) | Flavanone | 284, 334sh | 609 | 301 | 280 | S | X | X | ||
| 17 | Nepetin-7-O-glucoside (nepitrin) | Flavone | 258, 272, 348 | 477 | 315 | 350 | S | X | |||
| 18 | Rosmarinic acid | Phenolic acid | 290sh, 330 | 359 | 197, 179, 161 | 320 | S | X | X | X | X |
| 19 | Apigenin-7-O-glucoside | Flavone | 268, 336 | 431 | 269 | 320 | S | X | X | X | |
| 20 | 6-hydroxyluteolin | Flavone | 252–268, 350 | 301 | 350 | T | X | ||||
| 21 | Salvianolic acid A | Phenolic acid | 288, 310sh, 336sh | 493 | 295 | 280 | S | X | X | ||
| 22 | Hispidulin-7-O-glucoside | Flavone | 276, 334 | 461 | 299 | 350 | T | X | |||
| 23 | Eriodictyol | Flavanone | 288, 334sh | 287 | 151, 135 | 280 | S | X | |||
| 24 | Scutellarein-7-O-glucuronide | Flavone | 270, 338 | 461 | 285 | 350 | T | X | |||
| 25 | Scutellarein | Flavone | 286, 338 | 285 | 350 | S | X | ||||
| 26 | Luteolin-3′-acetyl-O-glucuronide | Flavone | 270, 338 | 503 | 399, 285 | 350 | T | X | |||
| 27 | Naringenin | Flavanone | 290, 332sh | 271 | 280 | S | X | ||||
| 28 | Luteolin | Flavone | 254, 266, 350 | 285 | 350 | S | X | X | X | X | |
| 29 | Kaempferol | Flavonol | 266, 366 | 285 | 350 | S | X | ||||
| 30 | Cirsiliol | Flavone | 256–274, 346 | 329 | 350 | S | X | X | |||
| 31 | Rosmanol | Phenolic terpene | 290 | 345 | 283 | 280 | S | X | |||
| 32 | Apigenin | Flavone | 268, 340 | 269 | 350 | S | X | X | X | X | |
| 33 | Eupatorin | Flavone | 276, 344 | 345 d | 350 | S | X | ||||
| 34 | Cirsimaritin | Flavone | 274, 336 | 313 | 298, 283 | 350 | S | X | X | ||
| 35 | Ladanein | Flavone | 284, 334 | 315 d | 350 | T | X | X | X | ||
| 36 | Sakuranetin | Flavanone | 288, 330sh | 285 | 280 | S | X | ||||
| 37 | Acacetin | Flavone | 268, 334 | 283 | 350 | S | X | ||||
| 38 | Genkwanin | Flavone | 268, 338 | 283 | 350 | S | X | ||||
| 39 | Salvigenin | Flavone | 276, 332 | 329 d | 287 d | 320 | S | X | X | ||
| 40 | (Epi)rosmanol methyl ether | Phenolic terpene | 290 | 359 | 283 | 280 | T | X | |||
| 41 | Carnosol | Phenolic terpene | 284 | 329 | 285 | 280 | S | X | X | ||
| 42 | Rosmadial | Phenolic terpene | 284 | 343 | 299, 285 | 280 | T | X | |||
| 43 | 4′-methoxytectochrysin | Flavone | 268, 332 | 299 d | 350 | T | X | ||||
| 44 | Carnosol isomer | Phenolic terpene | 284 | 329 | 285 | 280 | T | X | |||
| 45 | Carnosic acid derivative | Phenolic terpene | 280 | 301 | 280 | T | X | ||||
| 46 | Carnosic acid | Phenolic terpene | 286 | 331 | 287 | 280 | S | X | X | ||
| 47 | 12-methylcarnosic acid | Phenolic terpene | 284 | 345 | 301, 286 | 280 | T | X |
| Compound | Range (µg/mL) | Calibration a | R2 | LOD b (µg/mL) | LOQ b (µg/mL) | Supplier c | Amounts Added (µg/mL) | Recovery (% RSD, n = 6) | |
|---|---|---|---|---|---|---|---|---|---|
| TM | SL | ||||||||
| Danshensu | 2.0–120 | y = 0.0044x + 0.0012 | 0.9984 | 1.1 | 4.3 | 1 | 24/48 | - | 97/90 |
| Chlorogenic acid | 1.0–10 | y = 0.0130x − 0.0006 | 0.9973 | 0.37 | 1.1 | 1 | 1/2 | 102/100 | |
| p-hydroxybenzoic acid | 0.94–28 | y = 0.0427x + 0.0129 | 0.9996 | 0.07 | 0.92 | 2 | 1/2 | 94/94 | |
| Caffeic acid | 1.0–31 | y = 0.0223x − 0.0010 | 0.9987 | 0.70 | 2.2 | 1 | 4/8 | 105/106 | 120/112 |
| Luteolin-7-O-rutinoside | 1.3–60 | y = 0.0093x − 0.0009 | 0.9997 | 0.78 | 2.3 | 1 | 4/8 | 102/95 | |
| Luteolin-7-O-glucuronide | 2.0–160 | y = 0.0122x + 0.0224 | 0.9991 | 0.33 | 3.4 | 3 | |||
| Naringenin-7-O-glucoside | 2.0–40 | y = 0.0155x + 0.0063 | 0.9998 | 0.11 | 1.3 | 4 | |||
| Rosmarinic acid | 11–123 | y = 0.0104x − 0.0268 | 0.9998 | 3.7 | 6.3 | 1 | 30/60 | 80/81 | 116/98 |
| Apigenin-7-O-glucoside | 2.1–83 | y = 0.0120x + 0.013 | 0.9993 | 0.57 | 0.95 | 4 | |||
| Salvianolic acid A | 2.0–80 | y = 0.0160x + 0.0136 | 0.9977 | 0.96 | 5.2 | 1 | 6/12 | 118/102 | |
| Eriodictyol | 2.1–52 | y = 0.0212x + 0.0088 | 0.9996 | 0.39 | 2.3 | 4 | |||
| Scutellarein | 0.98–39 | y = 0.0212x + 0.0009 | 0.9986 | 0.66 | 2.3 | 4 | |||
| Naringenin | 2.0–40 | y = 0.0230x + 0.0102 | 0.9997 | 0.32 | 1.8 | 4 | |||
| Luteolin | 3.1–78 | y = 0.0200x + 0.0064 | 0.9997 | 0.38 | 2.0 | 1 | |||
| Cirsiliol | 0.42–2.8 | y = 0.0285x − 0.0020 | 0.9974 | 0.14 | 0.30 | 1 | |||
| Kaempferol | 1.0–40 | y = 0.0130x − 0.0010 | 0.9999 | 0.26 | 0.68 | 2 | 7/14 | 60/65 | |
| Rosmanol | 2.2–174 | y = 0.0018x − 0.0009 | 0.9994 | 0.89 | 4.1 | 3 | |||
| Apigenin | 1.1–46 | y = 0.0175x − 0.0060 | 0.9997 | 0.35 | 0.82 | 1 | 3/6 | 94/96 | 108/103 |
| Cirsimaritin | 1.3–60 | y = 0.0072x + 0.0004 | 0.9998 | 0.45 | 1.6 | 1 | |||
| Eupatorin | 0.96–19 | y = 0.0226x − 0.0011 | 0.9996 | 0.23 | 0.65 | 4 | 4/8 | 110/116 | |
| Sakuranetin | 2–40 | y = 0.0201x − 0.0067 | 0.9999 | 0.18 | 1.4 | 4 | |||
| Salvigenin | 1.0–60 | y = 0.0147x − 0.0247 | 0.9952 | 3.7 | 7.3 | 5 | |||
| Carnosol | 2.0–200 | y = 0.0023x + 0.0038 | 0.9985 | 0.69 | 3.5 | 4 | 30/60 | 140/135 | |
| Carnosic acid | 10–200 | y = 0.0010x − 0.0062 | 0.9974 | 10 | 19 | 4 | 80/160 | 64/67 | |
| Ferulic acid | 3.9–90 | y = 0.0254x − 0.0046 | 0.9998 | 0.54 | 2.2 | 2 | |||
| trans-cinnamic acid | 5.9–59 | y = 0.0564x − 0.0094 | 0.9997 | 0.10 | 0.71 | 2 | |||
| Repetibility (%RSD, n = 3) | Reproducibility (%RSD, n = 9) | |||||||
|---|---|---|---|---|---|---|---|---|
| Phenolic Compounds | TM | SL | LL | RO | TM | SL | LL | RO |
| Phenolic acids | ||||||||
| Danshensu | - | 5.0 | 2.9 | 5.3 | - | 6.4 | 7.4 | 5.4 |
| Chlorogenic acid | 2.2 | - | - | 2.9 | 6.5 | - | - | 3.5 |
| Cryptochlorogenic acid | - | - | - | 3.4 | - | - | - | 6.5 |
| Coumaric acid-O-glucoside | - | - | 2.6 | - | - | - | 8.1 | - |
| p-hydroxybenzoic acid | 1.2 | - | - | 6.1 | 5.6 | - | - | 7.6 |
| Ferulic acid-O-glucoside 1 | - | - | 2.6 | - | - | - | 7.6 | - |
| Caffeic acid | 1.6 | 2.6 | 1.0 | 1.6 | 3.7 | 4.7 | 6.4 | 3.0 |
| Dihydro-p-coumaric acid glucoside | - | - | 6.4 | - | - | - | 8.1 | - |
| Ferulic acid-O-glucoside 2 | - | - | 2.2 | - | - | - | 7.2 | - |
| Rosmarinic acid-3-O-glucoside | - | - | - | 4.6 | - | - | - | 5.4 |
| Rosmarinic acid | 2.4 | 2.1 | 3.5 | 2.1 | 6.4 | 5.1 | 7.4 | 2.3 |
| Salvianolic acid A | - | 2.7 | 2.0 | - | - | 6.7 | 6.6 | - |
| Flavones | ||||||||
| 6-hydroxyluteolin-7-O-glucoside | 2.2 | - | - | 4.0 | 5.8 | - | - | 6.2 |
| Luteolin-7-O-rutinoside | - | 2.9 | 3.8 | 5.5 | - | 5.5 | 5.8 | 6.2 |
| Luteolin-7-O-glucuronide | - | - | - | 4.1 | - | - | - | 7.6 |
| Luteolin-7-O-glucoside | 1.7 | 3.5 | 3.3 | 1.8 | 6.2 | 7.5 | 7.3 | 4.8 |
| Nepetin 7-O-glucoside | - | - | - | 2.0 | - | - | - | 5.0 |
| Apigenin-7-O-glucoside | 1.6 | 3.8 | 3.5 | - | 6.8 | 6.7 | 9.3 | - |
| 6-hydroxyluteolin | 1.1 | - | - | - | 6.8 | - | - | - |
| Hispidulin-7-O-glucoside | - | - | - | 5.3 | - | - | - | 6.9 |
| 6-hydroxyapigenin-7-O-β-glucoside | 1.4 | - | - | - | 7.0 | - | - | - |
| Scutellarein-7-O-glucuronide | - | - | - | 2.9 | - | - | - | 5.2 |
| Scutellarein | 3.6 | - | - | - | 5.9 | - | - | - |
| Luteolin-3′-acetyl-O-glucuronide | - | - | - | 2.2 | - | - | - | 5.2 |
| Luteolin | 0.8 | 3.8 | 3.5 | 2.6 | 4.6 | 5.1 | 5.6 | 3.6 |
| Cirsiliol | - | 5.9 | - | 1.9 | - | 6.1 | - | 3.4 |
| Apigenin | 0.9 | 5.4 | 4.3 | 1.5 | 3.7 | 7.3 | 8.7 | 3.1 |
| Eupatorin | - | 2.4 | - | - | - | 4.3 | - | - |
| Cirsimaritin | - | 2.1 | - | 1.5 | - | 3.9 | - | 2.6 |
| Ladanein | - | 5.1 | 5.8 | 1.7 | - | 5.2 | 8.7 | 3.1 |
| Acacetin | - | - | - | 2.6 | - | - | - | 5.5 |
| Genkwanin | - | - | - | 3.0 | - | - | - | 3.2 |
| Salvigenin | - | 3.5 | - | 2.7 | - | 4.7 | - | 3.2 |
| 4′-methoxytectochrysin | - | - | - | 4.5 | - | - | - | 4.6 |
| Flavanones | ||||||||
| Naringenin-7-O-glucoside | <LQ | - | - | - | <LQ | - | - | - |
| Hesperidin | - | 6.2 | - | 6.0 | - | 9.3 | - | 8.2 |
| Eriodictyol | 3.2 | - | - | - | 5.8 | - | - | - |
| Naringenin | 2.6 | - | - | - | 3.8 | - | - | - |
| Sakuranetin | 4.5 | - | - | - | 5.6 | - | - | - |
| Flavonols | ||||||||
| Kaempferol | 1.3 | - | - | - | 4.0 | - | - | - |
| Phenolic terpene | ||||||||
| Rosmanol | - | - | - | 7.3 | - | - | - | 7.6 |
| (Epi)rosmanol methyl ether | - | - | - | 5.5 | - | - | - | 9.4 |
| Rosmadial | - | - | - | 4.7 | - | - | - | 7.1 |
| Carnosol | - | 3.1 | - | 2.7 | - | 4.1 | - | 2.8 |
| Carnosol isomer | - | - | - | 3.4 | - | - | - | 4.0 |
| Carnosic acid derivative | - | - | - | 5.3 | - | - | - | 6.7 |
| Carnosic acid | - | <LQ | - | 3.3 | - | <LQ | - | 5.3 |
| 12-methylcarnosic acid | - | - | - | 4.0 | - | - | - | 5.1 |
| Phenolic Compounds | RO | TM | SL | LL | LA | LI_Abrial | LI_Grosso | LI_Super |
|---|---|---|---|---|---|---|---|---|
| Phenolic acids | ||||||||
| Danshensu | 307 ± 55 | - | 679 ± 285 | 1051 ± 451 | 593 ± 123 | 879 ± 311 | 457 ± 146 | 678 ± 254 |
| Chlorogenic acid | 44 ± 34 | 37 ± 17 | - | - | - | - | - | - |
| Cryptochlorogenic acid | 19 ± 7 | - | - | - | - | - | - | - |
| Coumaric acid-O-glucoside | - | - | - | 412 ± 163 | 100 ± 32 | 161 ± 50 | 156 ± 33 | 195 ± 28 |
| p-hydroxybenzoic acid | 16 ± 10 | 35 ± 18 | - | - | - | - | - | - |
| Ferulic acid-O-glucoside 1 | - | - | - | 66 ± 30 | 131 ± 44 | 167 ± 58 | 132 ± 29 | 147 ± 28 |
| Caffeic acid | 88 ± 14 | 108 ± 32 | 123 ± 31 | 54 ± 19 | 79 ± 14 | 63 ± 18 | 114 ± 46 | 93 ± 54 |
| Dihydro-p-coumaric acid glucoside | - | - | - | 244 ± 97 | 69 ± 12 | 116 ± 37 | 107 ± 20 | 104 ± 22 |
| Ferulic acid-O-glucoside 2 | - | - | - | 57 ± 29 | 158 ± 54 | 193 ± 75 | 164 ± 32 | 154 ± 37 |
| Rosmarinic acid-3-O-glucoside | 421 ± 284 | - | - | - | - | - | - | - |
| Rosmarinic acid | 2283 ± 720 | 1997 ± 1158 | 1486 ± 670 | 599 ± 272 | 579 ± 173 | 343 ± 109 | 235 ± 70 | 290 ± 67 |
| Salvianolic acid A | - | - | 457 ± 273 | 596 ± 304 | 252 ± 98 | 285 ± 135 | 282 ± 98 | 385 ± 113 |
| Flavones | ||||||||
| 6-hydroxyluteolin-7-O-glucoside | 40 ± 38 | 1600 ± 751 | - | - | - | - | - | - |
| Luteolin-7-O-rutinoside | 61 ± 63 | - | 298 ± 161 | 158 ± 75 | 91 ± 20 | 150 ± 61 | 122 ± 23 | 146 ± 32 |
| Luteolin-7-O-glucuronide | 99 ± 91 | - | - | - | - | - | - | - |
| Luteolin-7-O-glucoside | 79 ± 67 | 2235 ± 903 | 195 ± 84 | 385 ± 118 | 14 ± 13 | 52 ± 19 | 45 ± 13 | 62 ± 20 |
| Nepetin 7-O-glucoside | 352 ± 190 | - | - | - | - | - | - | - |
| Apigenin-7-O-glucoside | - | 793 ± 285 | 426 ± 179 | 273 ± 70 | 153 ± 21 | 255 ± 98 | 162 ± 32 | 133 ± 72 |
| 6-hydroxyluteolin | - | 440 ± 242 | - | - | - | - | - | - |
| Hispidulin-7-O-glucoside | 302 ± 62 | - | - | - | - | - | - | - |
| 6-hydroxyapigenin-7-O-β-glucoside | - | 215 ± 75 | - | - | - | - | - | - |
| Scutellarein-7-O-glucuronide | 584 ± 151 | - | - | - | - | - | - | - |
| Scutellarein | - | 201 ± 155 | - | - | - | - | - | - |
| Luteolin-3′-acetyl-O-glucuronide | 364 ± 122 | - | - | - | - | - | - | - |
| Luteolin | 131 ± 43 | 636 ± 323 | 157 ± 52 | 42 ± 24 | 30 ± 10 | 49 ± 22 | 64 ± 17 | 60 ± 22 |
| Cirsiliol | 56 ± 37 | - | 20 ± 14 | - | - | - | - | - |
| Apigenin | 195 ± 82 | 665 ± 233 | 145 ± 61 | 29 ± 10 | 95 ± 35 | 100 ± 44 | 81 ± 21 | 64 ± 18 |
| Eupatorin | - | - | 95 ± 48 | - | - | - | - | - |
| Cirsimaritin | 1032 ± 509 | - | 382 ± 328 | - | - | - | - | - |
| Ladanein | 234 ± 141 | - | 74 ± 71 | 46 ± 14 | 93 ± 33 | 72 ± 20 | 93 ± 25 | 70 ± 18 |
| Acacetin | 127 ± 100 | - | - | - | - | - | - | - |
| Genkwanin | 606 ± 339 | - | - | - | - | - | - | - |
| Salvigenin | 398 ± 239 | - | 531 ± 343 | - | - | - | - | - |
| 4′-methoxytectochrysin | 692 ± 308 | - | - | - | - | - | - | - |
| Flavanones | ||||||||
| Naringenin-7-O-glucoside | - | 87 ± 85 | - | - | - | - | - | - |
| Hesperidin | 380 ± 148 | - | 41 ± 86 | - | - | - | - | - |
| Eriodictyol | - | 311 ± 132 | - | - | - | - | - | - |
| Naringenin | - | 310 ± 94 | - | - | - | - | - | - |
| Sakuranetin | - | 279 ± 103 | - | - | - | - | - | - |
| Flavonols | ||||||||
| Kaempferol | - | 268 ± 147 | - | - | - | - | - | - |
| Phenolic terpene | ||||||||
| Rosmanol | 218 ± 190 | - | - | - | - | - | - | - |
| (Epi)rosmanol methyl ether | 657 ± 434 | - | - | - | - | - | - | - |
| Rosmadial | 189 ± 205 | - | - | - | - | - | - | - |
| Carnosol | 6862 ± 1511 | - | 1055 ± 2203 | - | - | - | - | - |
| Carnosol isomer | 1809 ± 1605 | - | - | - | - | - | - | - |
| Carnosic acid derivative | 6937 ± 2416 | - | 559 ± 1703 | - | - | - | - | - |
| Carnosic acid | 26,703 ± 9259 | - | 867 ± 2687 | - | - | - | - | - |
| 12-Methylcarnosic acid | 4174 ± 1789 | - | 1769 ± 4541 | - | - | - | - | - |
| Total phenolic compounds | 56,459 ± 1375 | 10,002 ± 3193 | 9301 ± 11,089 | 4015 ± 1343 | 2436 ± 463 | 2884 ± 859 | 2213 ± 341 | 2582 ± 431 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Magariño, S.; Bueno-Herrera, M.; Asensio-S.-Manzanera, M.C. Characterization of Bioactive Phenolic Compounds Extracted from Hydro-Distillation By-Products of Spanish Lamiaceae Plants. Molecules 2024, 29, 5285. https://doi.org/10.3390/molecules29225285
Pérez-Magariño S, Bueno-Herrera M, Asensio-S.-Manzanera MC. Characterization of Bioactive Phenolic Compounds Extracted from Hydro-Distillation By-Products of Spanish Lamiaceae Plants. Molecules. 2024; 29(22):5285. https://doi.org/10.3390/molecules29225285
Chicago/Turabian StylePérez-Magariño, Silvia, Marta Bueno-Herrera, and M. Carmen Asensio-S.-Manzanera. 2024. "Characterization of Bioactive Phenolic Compounds Extracted from Hydro-Distillation By-Products of Spanish Lamiaceae Plants" Molecules 29, no. 22: 5285. https://doi.org/10.3390/molecules29225285
APA StylePérez-Magariño, S., Bueno-Herrera, M., & Asensio-S.-Manzanera, M. C. (2024). Characterization of Bioactive Phenolic Compounds Extracted from Hydro-Distillation By-Products of Spanish Lamiaceae Plants. Molecules, 29(22), 5285. https://doi.org/10.3390/molecules29225285






