Optimization of Enzymolysis Modification Conditions of Dietary Fiber from Bayberry Pomace and Its Structural Characteristics and Physicochemical and Functional Properties
Abstract
1. Introduction
2. Results and Discussion
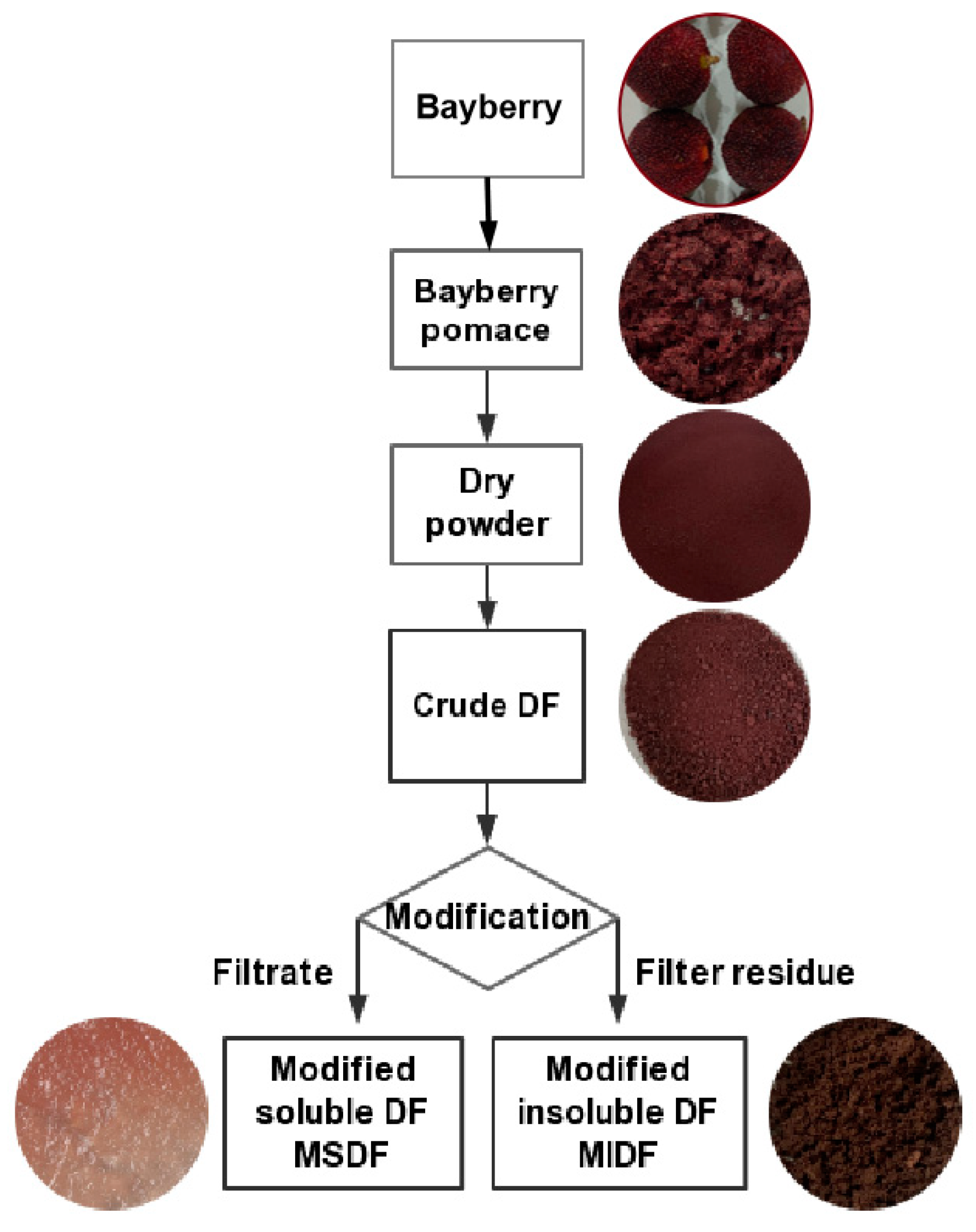
2.1. The Modification of DF from Bayberry Pomace
2.1.1. Effect of Fluid–Material Ratio on the MSDF Yield and WHC
2.1.2. Effect of Cellulase Dosage on the MSDF Yield and WHC
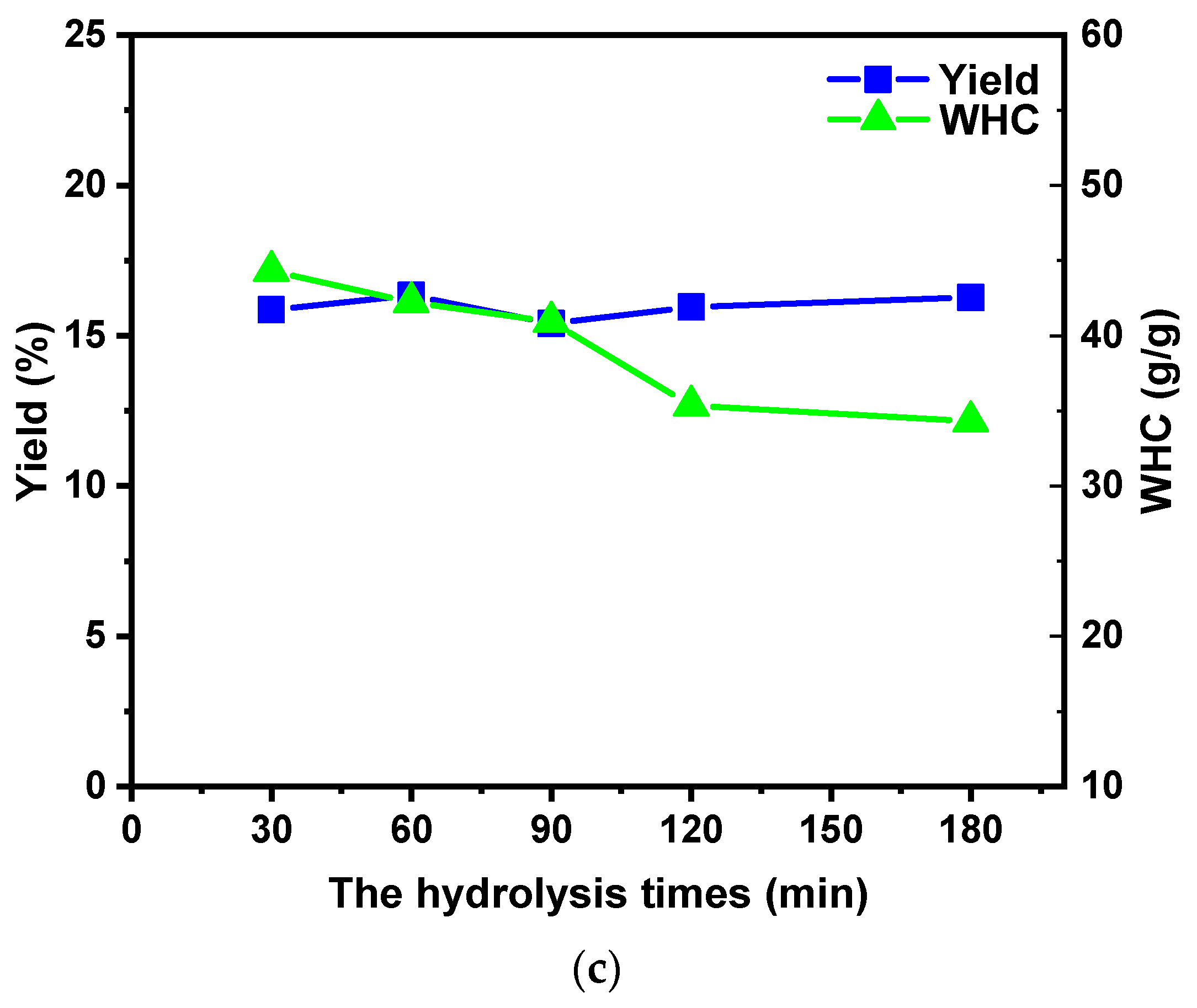
2.1.3. Effect of Hydrolysis Time on the MSDF Yield and WHC
2.2. Orthogonal Test to Optimize the Modification Process
2.3. Physicochemical Properties
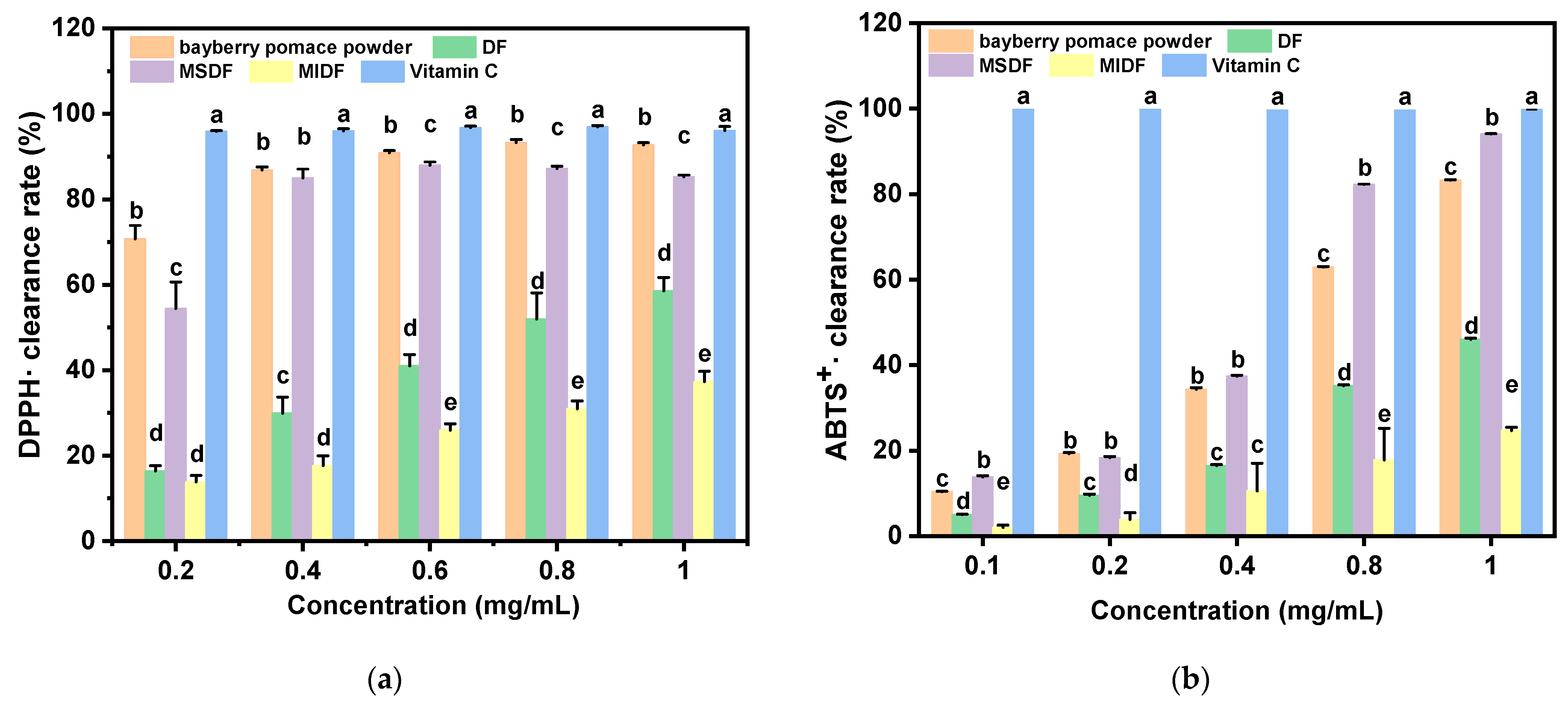
2.4. The Radical Scavenging Capacity
2.5. Hypoglycemic Capacity In Vitro
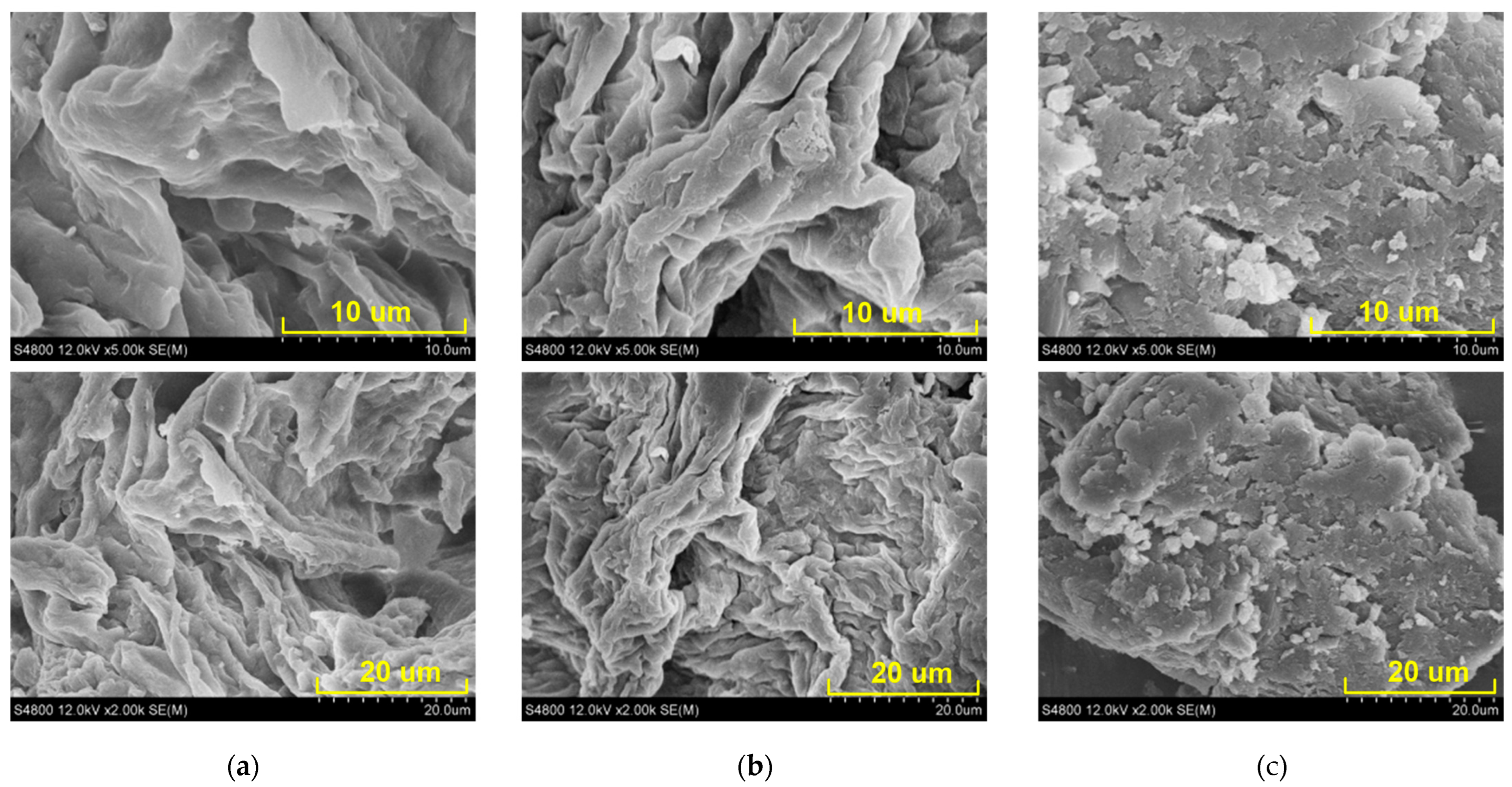
2.6. SEM Analysis
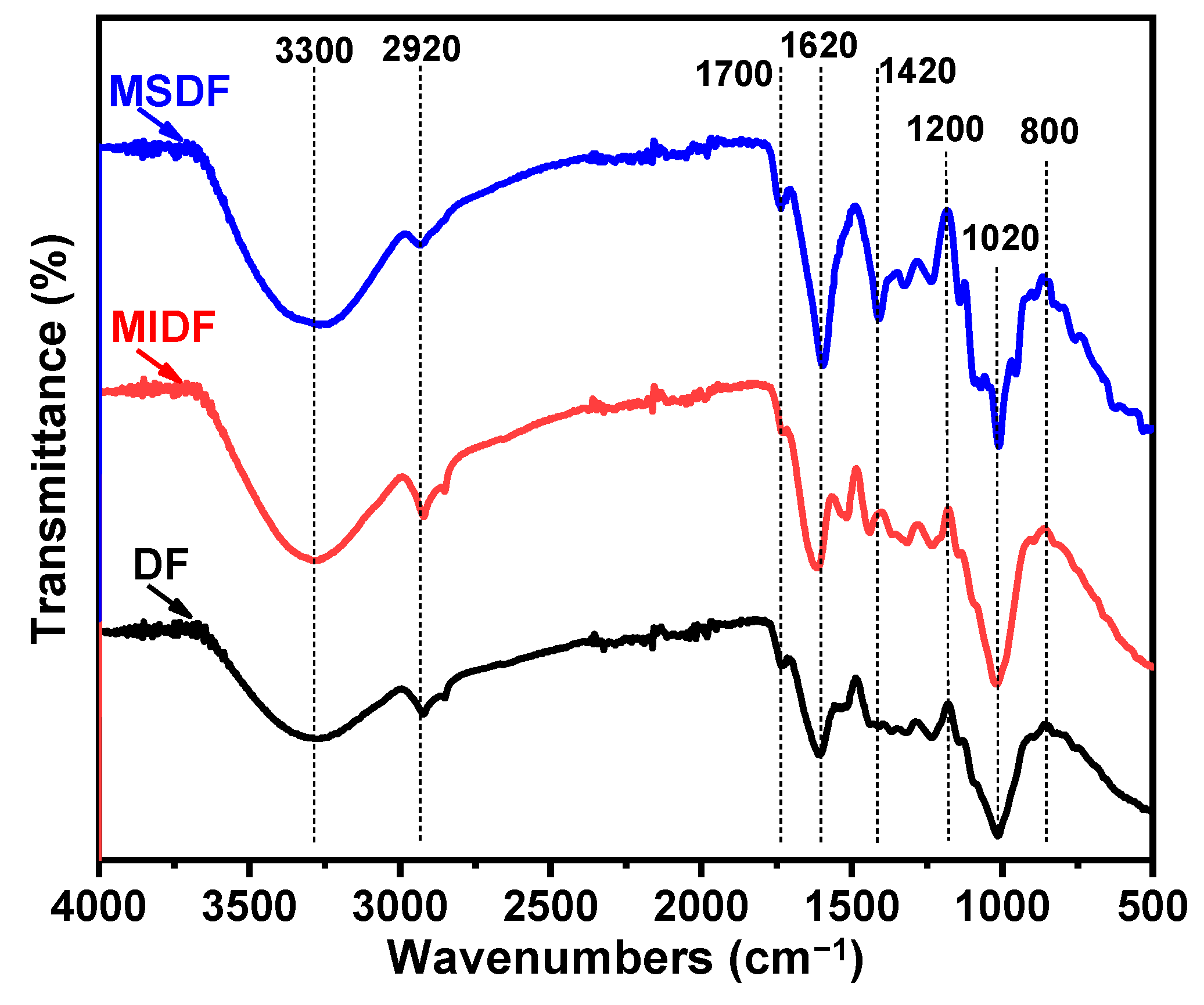
2.7. FTIR Analysis
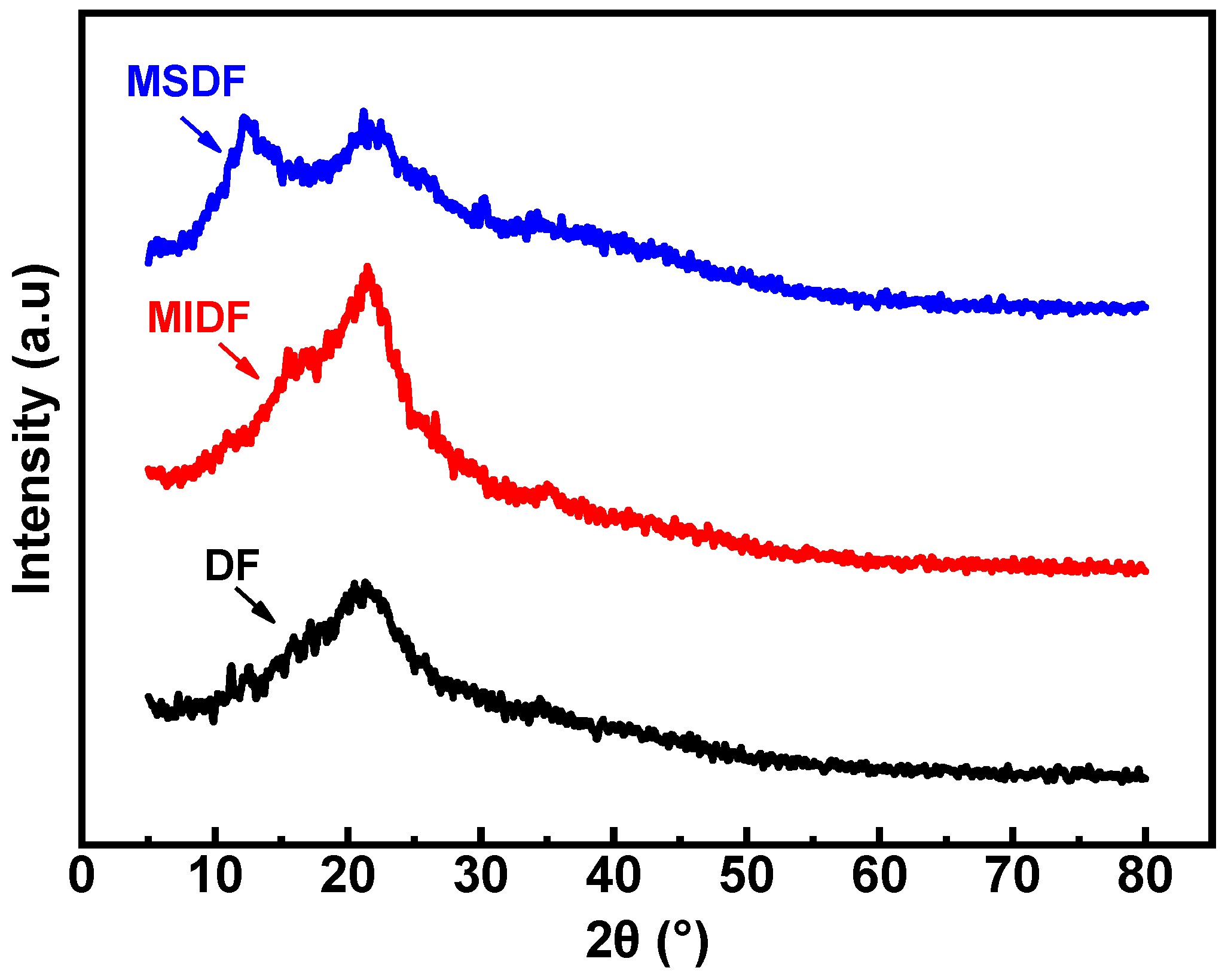
2.8. XRD Analysis
3. Materials and Methods
3.1. Materials
3.2. Extraction of Bayberry Pomace DF
3.3. Modification and Optimization of Bayberry Pomace DF
3.4. Physicochemical Properties
3.4.1. WHC Analysis
3.4.2. OHC Analysis
3.4.3. SC Analysis
3.4.4. GAC Analysis
3.4.5. CEC Analysis
3.5. Radical Scavenging Activities In Vitro
3.6. Hypoglycemic Capacities In Vitro
3.6.1. α-Amylase Inhibitory Activity Analysis (AIA)
3.6.2. α-Glucosidase Inhibitory Activity Analysis
3.7. SEM Analysis
3.8. Fourier-Transform Infrared (FT-IR) Spectroscopy Analysis
3.9. X-ray Diffraction (XRD) Analysis
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, S.L.; Fang, X.J.; Wu, W.J.; Gao, Y.; Chen, H.J.; Yang, H.L.; Gao, H.Y. Effects of different drying methods on antioxidant ability and flavor of bayberry pomace powder. Acta Agric. Zhejiangensis 2023, 35, 1440–1451. [Google Scholar]
- Sun, C.D.; Huang, H.Z.; Xu, C.J.; Li, X.; Chen, K.S. Biological activities of extracts from Chinese bayberry (Myrica rubra Sieb. et Zucc.): A Review. Plant Foods Hum. Nutr. 2013, 68, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.H.; Cao, Y.P.; Chen, S.G.; Fang, Z.X.; Chen, J.C.; Liu, D.H.; Ye, X.Q. Juices processing characteristics of Chinese bayberry from different cultivars. Food Sci. Nutr. 2019, 7, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Jin, Z.N.; Xu, Y.S.; Sha, R.Y.; Mao, J.W.; Jiang, Z.L. Chinese bayberry Jiaosu fermentation—Changes of mycobiota composition and antioxidant properties. Int. J. Food Eng. 2020, 17, 455–463. [Google Scholar] [CrossRef]
- Cai, H.L.; You, S.Y.; Xu, Z.Y.; Li, Z.M.; Guo, J.J.; Ren, Z.Y.; Fu, C.L. Novel extraction methods and potential applications of polyphenols in fruit waste: A review. J. Food Meas. Charact. 2021, 15, 3250–3261. [Google Scholar] [CrossRef]
- Li, Y.N.; Yu, Y.S.; Wu, J.J.; Xu, Y.J.; Xiao, G.S.; Li, L.; Liu, H.R. Comparison the structural, physicochemical, and prebiotic properties of Litchi pomace dietary fibers before and after modification. Foods 2022, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Kong, L.C.; Yin, C.P.; Jiang, D.H.; Jiang, J.Q.; He, J.; Xiao, W.X. Extraction optimization by response surface methodology, purification and principal antioxidant metabolites of red pigments extracted from bayberry (Myrica rubra) pomace. LWT-Food Sci. Technol. 2013, 51, 343–347. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Jiang, J.; Yue, Y.; Feng, Z.P.; Chen, J.C.; Ye, X.Q. Influence of mixed probiotics on the the bioactive composition, antioxidant activity and appearance of fermented red bayberry pomace. LWT 2020, 133, 110076. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Lv, J.M.; Gu, Y.; He, Y.K.; Chen, J.C.; Zhou, Z.Q.; Ye, X.Q. Polysaccharides of Chinese bayberry pomace wine: Structural characteristics, antioxidant activity and influence on the bayberry wine. Food Biosci. 2022, 50, 102025. [Google Scholar] [CrossRef]
- Sezer, D.B.; Ahmed, J.; Sumnu, G.; Sahin, S. Green processing of sour cherry (Prunus cerasus L.) pomace: Process optimization for the modification of dietary fibers and property measurements. J. Food Meas. Charact. 2021, 15, 3015–3025. [Google Scholar] [CrossRef]
- Siddiqui, H.; Sultan, Z.; Yousuf, O.; Malik, M.; Younis, K. A review of the health benefits, functional properties, and ultrasound-assisted dietary fiber extraction. Bioact. Carbohydr. Diet. Fibre. 2023, 30, 100356. [Google Scholar] [CrossRef]
- Daou, C.; Zhang, H. Functional and physiological properties of total, soluble, and insoluble dietary fibres derived from defatted rice bran. J. Food Sci. Technol. 2014, 51, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.Z.; Hong, T.; Zheng, M.J.; Yang, Y.F.; Zhu, Y.B.; Jiang, Z.D.; Ni, H.; Li, Q.B. High-pressure homogenization treatment of red seaweed Bangia fusco-purpurea affects the physicochemical, functional properties and enhances in vitro anti-glycation activity of its dietary fibers. Innov. Food Sci. Emerg. Technol. 2023, 86, 103369. [Google Scholar] [CrossRef]
- Meng, X.M.; Liu, F.; Xiao, Y.; Cao, J.W.; Wang, M.; Duan, X.C. Alterations in physicochemical and functional properties of buckwheat straw insoluble dietary fiber by alkaline hydrogen peroxide treatment. Food Chem. X 2019, 3, 100029. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Liang, Y.T.; Lin, H.S.; Chen, Y.; Xie, J.H.; Ai, F.L.; Yan, Z.W.; Hu, X.B.; Yu, Q. Fermentation of grapefruit peel by an efficient cellulose-degrading strain, (Penicillium YZ-1): Modification, structure and functional properties of soluble dietary fiber. Food Chem. 2023, 420, 136123. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt, V.H.; Ötles, S. Effect of food processing on the physicochemical properties of dietary fibre. Acta Sci. Pol. Technol. Aliment. 2016, 15, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Ain, H.B.U.; Saeed, F.; Ahmed, A.; Khan, M.A.; Niaz, B.; Tufail, T. Improving the physicochemical properties of partially enhanced soluble dietary fiber through innovative techniques: A coherent review. J. Food Process. Preserv. 2019, 43, e13917. [Google Scholar]
- Kaur, S.; Panesar, P.S.; Chopra, H.K. Extraction of dietary fiber from kinnow (Citrus reticulata) peels using sequential ultrasonic and enzymatic treatments and its application in development of cookies. Food Biosci. 2023, 54, 102891. [Google Scholar] [CrossRef]
- Gan, J.; Xie, L.; Peng, G.; Xie, J.; Chen, Y.; Yu, Q. Systematic review on modification methods of dietary fiber. Food Hydrocoll. 2021, 119, 106872. [Google Scholar] [CrossRef]
- He, Y.Y.; Li, W.; Zhang, X.Y.; Li, T.T.; Ren, D.F.; Lu, J. Physicochemical, functional, and microstructural properties of modified insoluble dietary fiber extracted from rose pomace. J. Food Sci. Technol. 2020, 57, 1421–1429. [Google Scholar] [CrossRef]
- Ullah, I.; Yin, T.; Xiong, S.B.; Huang, Q.L.; Din, Z.; Zhang, J.; Javaid, A.B. Effects of thermal pre-treatment on physicochemical properties of nano-sized okara (soybean residue) insoluble dietary fiber prepared by wet media milling. J. Food Eng. 2018, 237, 18–26. [Google Scholar] [CrossRef]
- Wang, S.Q.; Fang, Y.Q.; Xu, Y.B.; Zhu, B.; Piao, J.G.; Zhu, L.L.; Yao, L.M.; Liu, K.H.; Wang, S.C.; Zhang, Q.Y.; et al. The effects of different extraction methods on physicochemical, functional and physiological properties of soluble and insoluble dietary fiber from Rubus chingii Hu. Fruits. J Funct. Foods 2022, 93, 105081. [Google Scholar] [CrossRef]
- Chen, H.; Pu, J.S.; Liu, D.; Yu, W.S.; Shao, Y.Y.; Yang, G.W.; Xiang, Z.H.; He, N.J. Anti-Inflammatory and antinociceptive properties of flavonoids from the fruits of black mulberry (Morus nigra L.). PLoS ONE 2016, 11, e0153080. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-S.; Chi, C.-S.; Huang, S.-S.; Shie, P.-H.; Lin, T.-H.; Huang, G.-J. Antioxidant, analgesic, and anti-inflammatory activities of the ethanolic extracts of Taxillus liquidambaricola. J. Ethnopharmacol. 2011, 137, 1161–1171. [Google Scholar] [CrossRef]
- Afrazeh, M.; Tadayoni, M.; Abbasi, H.; Sheikhi, A. Extraction of dietary fibers from bagasse and date seed, and evaluation of their technological properties and antioxidant and prebiotic activity. J. Food Meas. Charact. 2021, 15, 1949–1959. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, M.; Bai, T.M.; Chen, D.W.; Zhang, Q.; Lin, D.R.; Liu, Y.T.; Liu, A.P.; Huang, Z.Q.; Qin, W. Comparative study on the structure, physicochemical, and functional properties of dietary fiber extracts from quinoa and wheat. LWT 2021, 149, 111816. [Google Scholar] [CrossRef]
- Hu, S.Z.; Yin, J.Y.; Nie, S.P.; Wang, J.Q.; Phillips, G.O.; Xie, M.Y.; Cui, S.W. In vitro evaluation of the antioxidant activities of carbohydrates. Bioact. Carbohydr. Diet. Fibre. 2016, 7, 19–27. [Google Scholar] [CrossRef]
- Zhou, S.H.; Cheng, J.Y.; Ye, X.Q. Antioxidant activity and dietary fiber function of bayberry pomace. J. Chinese Ins. Food Sci. Technol. 2009, 9, 52–58. [Google Scholar]
- Guo, W.W.; Beta, T. Phenolic acid composition and antioxidant potential of insoluble and soluble dietary fibre extracts derived from select whole-grain cereals. Food Res. Int. 2013, 51, 518–525. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Tian, J.H.; Yang, W.H.; Chen, S.G.; Liu, D.H.; Fang, H.T.; Zhang, H.L.; Ye, X.Q. Inhibition mechanism of ferulic acid against α-amylase and α-glucosidase. Food Chem. 2020, 317, 126346. [Google Scholar] [CrossRef]
- Han, L.; Wang, H.Q.; Cao, J.W.; Li, Y.L.; Jin, X.Y.; He, C.A. Inhibition mechanism of α-glucosidase inhibitors screened from Tartary buckwheat and synergistic effect with acarbose. Food Chem. 2023, 420, 136102. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Feng, Z.Q.; Niu, Y.G.; Yu, L.L. Structural, rheological and functional properties of modified soluble dietary fiber from tomato peels. Food Hydrocoll. 2018, 77, 557–565. [Google Scholar] [CrossRef]
- Chen, M.S.; Guo, L.P.; Nsor-Atindana, J.; Goff, H.D.; Zhang, W.X.; Mao, J.; Zhong, F. The effect of viscous soluble dietary fiber on nutrient digestion and metabolic responses I: In vitro digestion process. Food Hydrocoll. 2020, 107, 105971. [Google Scholar] [CrossRef]
- Repin, N.; Cui, S.W.; Goff, H.D. Impact of dietary fibre on in vitro digestibility of modified tapioca starch: Viscosity effect. Bioact. Carbohydr. Diet. Fibre. 2018, 15, 2–11. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.F.; Yu, W.Y.; Gao, H.; Szeto, I.M.Y.; Feng, H.T.; Liu, X.B.; Wang, Y.T.; Sun, L.J. Soluble dietary fibres decrease α-glucosidase inhibition of epigallocatechin gallate through affecting polyphenol-enzyme binding interactions. Food Chem. 2023, 409, 135327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bai, B.; Yan, Y.; Liang, J.; Guan, X. Bound polyphenols from red Quinoa prevailed over free polyphenols in reducing postprandial blood glucose rises by inhibiting α-glucosidase activity and starch digestion. Nutrients 2022, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Carlos Andrés, A.V. An Aggregated Understanding of Alkaline Hydrogen Peroxide (AHP) Pretreatment of Biomass and Hemicellulose Conversion. Doctoral Dissertation, Washington State University, Pullman, WC, USA, 2016. [Google Scholar]
- Chen, Y.; Ye, R.; Yin, L.; Zhang, N. Novel blasting extrusion processing improved the physicochemical properties of soluble dietary fiber from soybean residue and in vivo evaluation. J. Food Eng. 2014, 120, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Suo, K.K.; Wang, P.; Li, X.; Hao, L.M.; Zhu, J.Q.; Yi, J.J.; Kang, Q.Z.; Huang, J.Y.; Lu, J.K. Modification of wheat bran insoluble and soluble dietary fibers with snail enzyme. Food Sci. Hum. Well. 2021, 10, 356–361. [Google Scholar] [CrossRef]
- Khawas, P.; Deka, S.C. Isolation and characterization of cellulose nanofibers from culinary banana peel using high-intensity ultrasonication combined with chemical treatment. Carbohydr. Polym. 2016, 237, 608–616. [Google Scholar] [CrossRef]
- Canga, E.M.; Dudak, F.C. Characterization of cellulose acetate/gum Arabic fibers loaded with extract of Viburnum opulus L. fruit. LWT 2019, 110, 247–254. [Google Scholar] [CrossRef]
- Geng, N.N.; Wang, H.J.; Zhang, Y.; Song, J.F.; Li, Y.; Wu, C.E. Physicochemical, structural, and functional properties of microfluidic modified dietary fiber from fresh corn bracts. J. Cereal. Sci. 2023, 112, 103731. [Google Scholar] [CrossRef]
- Dong, W.J.; Wang, D.D.; Hu, R.S.; Rong, Y.Z.; Lv, L.S. Chemical composition, structural and functional properties of soluble dietary fiber obtained from coffee peel using different extraction methods. Food Res. Int. 2020, 136, 109497. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.M.; Zhao, Y.Y.; Zhang, Z.; Wang, L.; Du, J.M.; Zhang, S.X. Optimization of chemical extraction conditions of dietary fiber from cistanche deserticola residues and Its structural characteristics and physicochemical and functional properties. Molecules 2023, 28, 7604. [Google Scholar] [CrossRef]
- Yan, K.J.; Liu, J.L.; Yan, W.S.; Wang, Q.; Huo, Y.X.; Feng, S.S.; Zhang, L.L.; Hu, Q.P.; Xu, J.G. Effects of alkaline hydrogen peroxide and cellulase modifications on the physicochemical and functional properties of Forsythia suspensa dietary fiber. Molecules 2023, 28, 7164. [Google Scholar] [CrossRef]
- Kanwar, P.; Yadav, R.B.; Yadav, B.S. Cross-linking, carboxymethylation and hydroxypropylation treatment to sorghum dietary fiber: Effect on physicochemical, micro structural and thermal properties. Int. J. Biol. Macromol. 2023, 233, 123638. [Google Scholar] [CrossRef]
- Zhu, Y.; Chu, J.X.; Lu, Z.X.; Lv, F.X.; Bie, X.M.; Zhang, C.; Zhao, H.Z. Physicochemical and functional properties of dietary fiber from foxtail millet (Setaria italic) bran. J. Cereal Sci. 2018, 79, 456–461. [Google Scholar] [CrossRef]
- Tan, X.; Cheng, X.; Ma, B.; Cui, F.; Wang, D.; Shen, R.; Li, X.; Li, J. Characterization and Function Analysis of Soluble Dietary Fiber Obtained from Radish Pomace by Different Extraction Methods. Molecules 2024, 29, 500. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Liu, H.; Zhang, Y.Q.; Xu, Z.G.; Sui, X. Optimization of extraction technology of soluble dietary fiber from Flammulina valutipes by response surface methodology. China Brew. 2017, 36, 137–141. [Google Scholar]
- Gu, Q.; Gao, X.; Zhou, Q.Q.; Li, Y.Q.; Li, G.Q.; Li, P. Characterization of soluble dietary fiber from citrus peels (Citrus unshiu), and its antioxidant capacity and beneficial regulating effect on gut microbiota. Int. J. Biol. Macromol. 2023, 246, 125715. [Google Scholar] [CrossRef]
- Li, S.X.; Wang, J.F.; Zhang, L.L.; Zheng, Y.; Ma, G.R.; Sun, X.M.; Yuan, J.F. Preparation of Dendrobium officinale flower anthocyanin and extended lifespan in Caenorhabditis elegans. Molecules 2022, 27, 8608. [Google Scholar] [CrossRef]


| Level | Factors | ||
|---|---|---|---|
| A (Fluid–Material Ratio) | B (Cellulase Dosage) | C (Hydrolysis Time) | |
| −1 | 20 mL/g | 50 U/g | 30 min |
| 0 | 100 mL/g | 100 U/g | 60 min |
| 1 | 200 mL/g | 200 U/g | 120 min |
| Test Number | A | B | C | WHC (g/g) | MSDF Yield (%) |
|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 45.53 | 15.36 |
| 2 | −1 | 0 | 0 | 50.44 | 14.10 |
| 3 | −1 | 1 | 1 | 30.78 | 18.62 |
| 4 | 0 | −1 | 0 | 50.23 | 15.18 |
| 5 | 0 | 0 | 1 | 41.77 | 14.44 |
| 6 | 0 | 1 | −1 | 40.41 | 12.78 |
| 7 | 1 | −1 | 1 | 25.09 | 17.52 |
| 8 | 1 | 0 | −1 | 41.54 | 16.90 |
| 9 | 1 | 1 | 0 | 32.73 | 13.84 |
| k1 | 42.25 | 40.28 | 42.49 | ||
| k2 | 44.14 | 44.58 | 44.47 | ||
| k3 | 33.12 | 34.64 | 32.54 | ||
| R | 11.02 | 9.94 | 11.92 | ||
| Factor priority | C > A > B | ||||
| Optimal condition | A2B2C2 | ||||
| WHC (g/g) | OHC (g/g) | SC (mL/g) | GAC (mmol/g) | CEC (mmol/g) × 10−1 | |
|---|---|---|---|---|---|
| DF | 3.68 ± 0.20 c | 2.21 ± 0.03 b | 6.13 ± 0.19 b | 0.89 ± 0.02 a | 2.35 ± 0.03 b |
| MIDF | 5.77 ± 0.02 b | 2.47 ± 0.07 a | 6.67 ± 0.12 b | 0.59 ± 0.25 ab | 2.99 ± 0.13 a |
| MSDF | 54.13 ± 0.77 a | 1.94 ± 0.04 c | 7.57 ± 0.4 a | 0.31 ± 0.08 b | 2.81 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Ruan, Q.; Sun, X.; Yuan, J. Optimization of Enzymolysis Modification Conditions of Dietary Fiber from Bayberry Pomace and Its Structural Characteristics and Physicochemical and Functional Properties. Molecules 2024, 29, 3415. https://doi.org/10.3390/molecules29143415
Zhang Z, Ruan Q, Sun X, Yuan J. Optimization of Enzymolysis Modification Conditions of Dietary Fiber from Bayberry Pomace and Its Structural Characteristics and Physicochemical and Functional Properties. Molecules. 2024; 29(14):3415. https://doi.org/10.3390/molecules29143415
Chicago/Turabian StyleZhang, Zhaolin, Qin Ruan, Xiaoming Sun, and Jianfeng Yuan. 2024. "Optimization of Enzymolysis Modification Conditions of Dietary Fiber from Bayberry Pomace and Its Structural Characteristics and Physicochemical and Functional Properties" Molecules 29, no. 14: 3415. https://doi.org/10.3390/molecules29143415
APA StyleZhang, Z., Ruan, Q., Sun, X., & Yuan, J. (2024). Optimization of Enzymolysis Modification Conditions of Dietary Fiber from Bayberry Pomace and Its Structural Characteristics and Physicochemical and Functional Properties. Molecules, 29(14), 3415. https://doi.org/10.3390/molecules29143415






