Production of Resistant Starch by Roasting Retrograded Starch with Glucose
Abstract
1. Introduction
2. Discussion of Results
2.1. Determination of Particle Size Distribution via Gel Chromatography
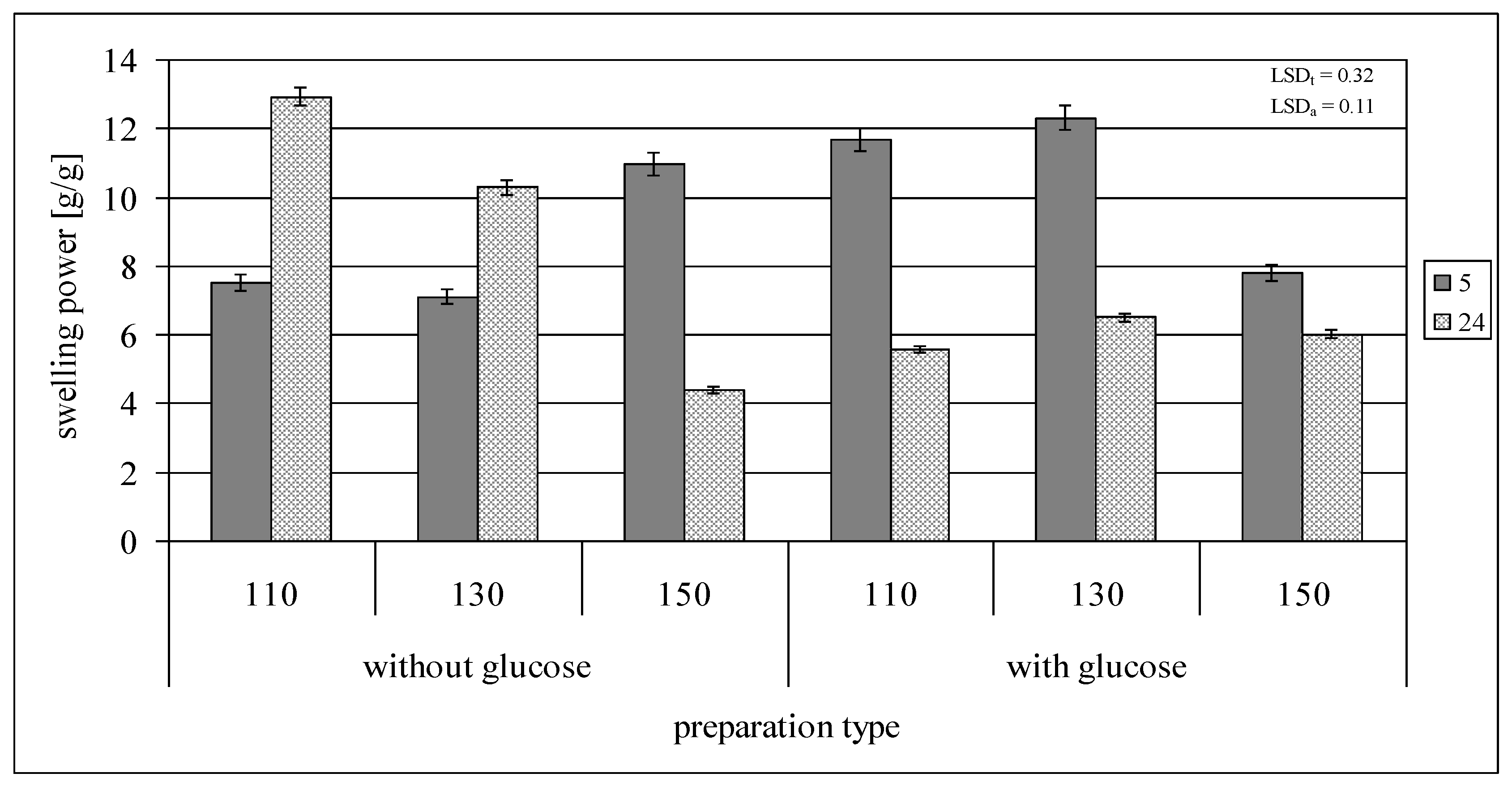
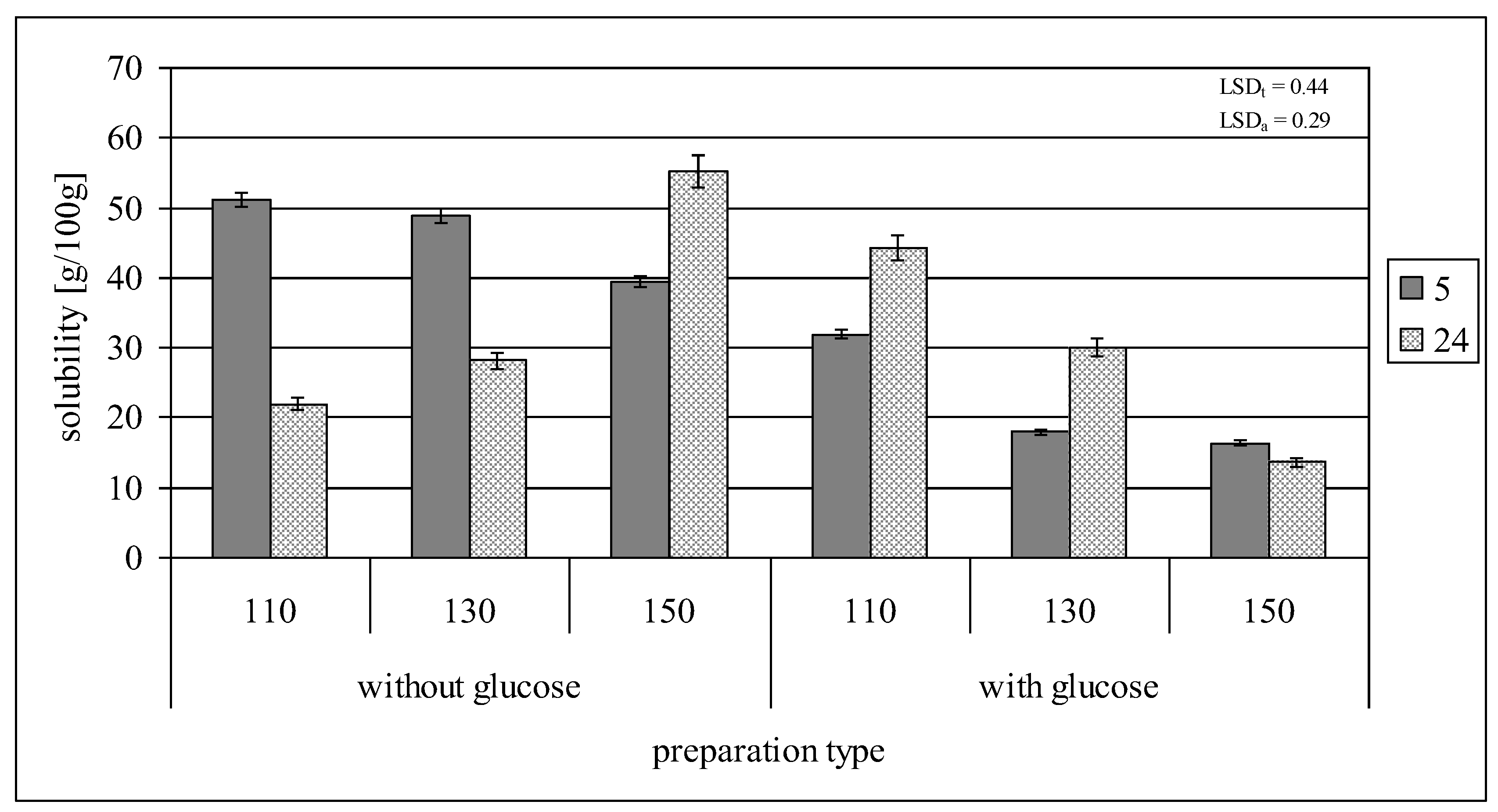
2.2. Swelling Power and Solubility in Water
2.3. Differential Scanning Colorimetry
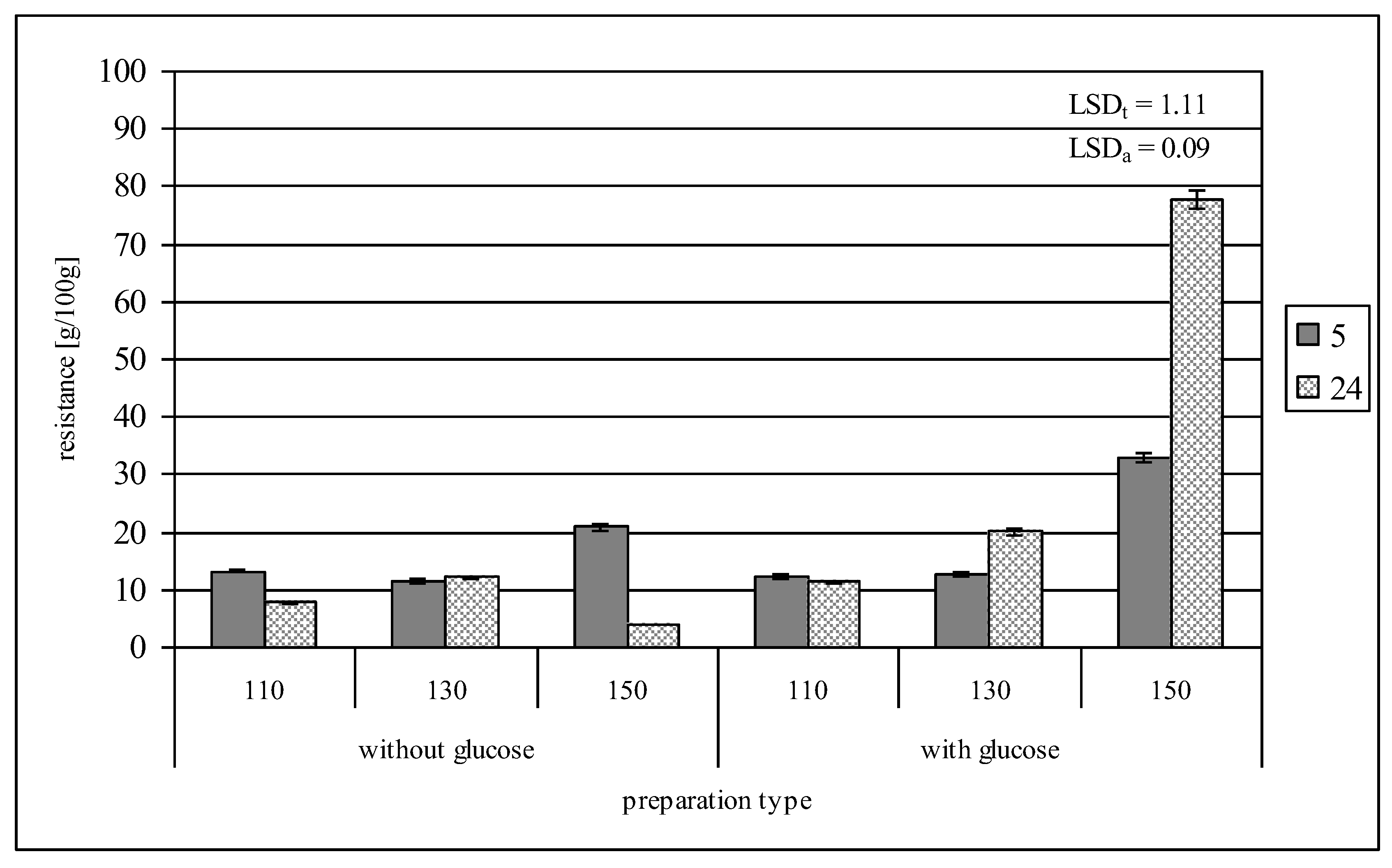
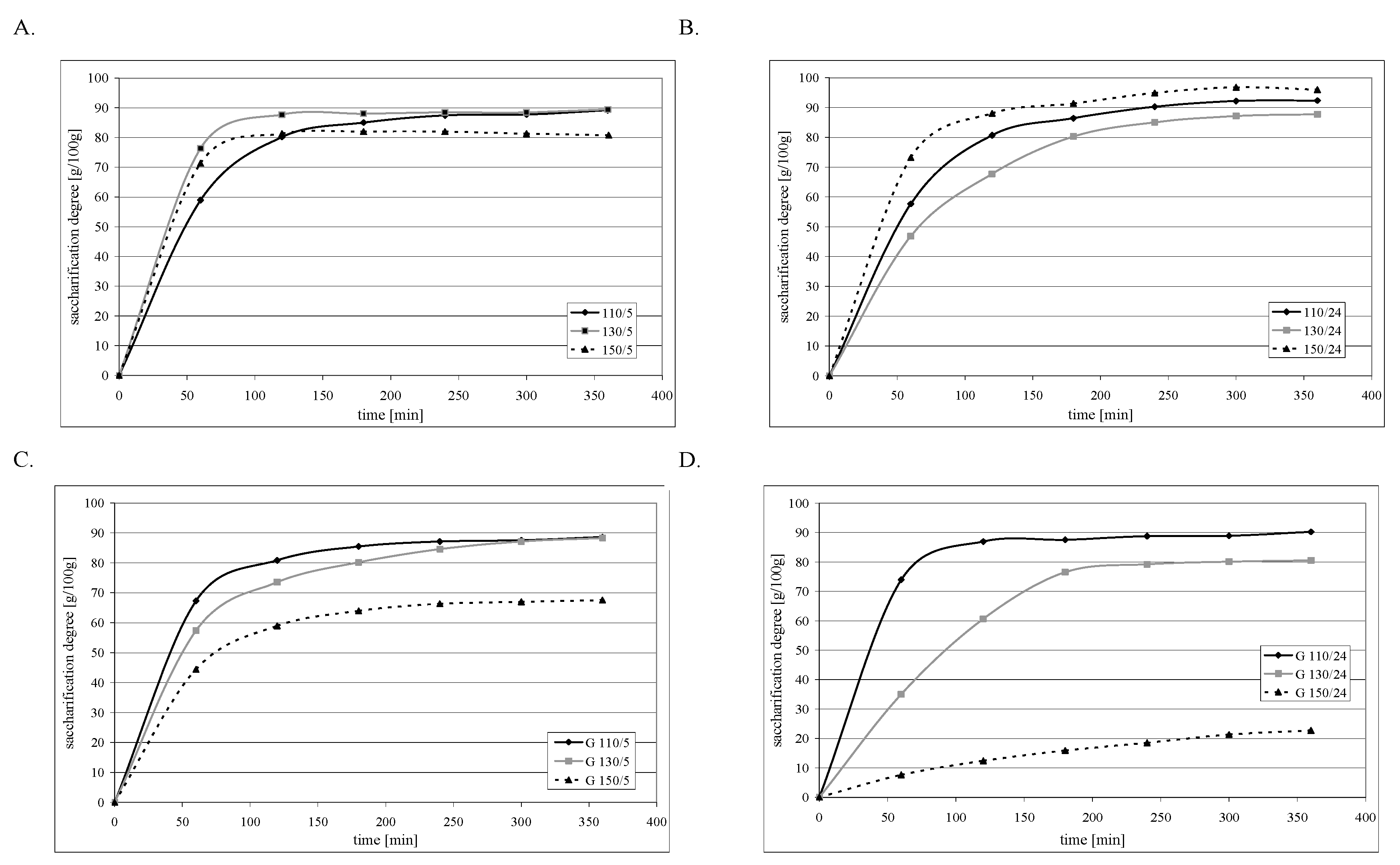
2.4. Resistance to Amyloglucosidase
3. Materials and Methods
3.1. Materials
3.2. Production of Modified Starch Preparations
3.3. Determination of Particle Size Distributions via Gel Chromatography
3.4. Swelling Power and Solubility of Starch Preparations in Water at a Temperature of 80 °C
3.5. Determination of the Characteristics of Phase Transitions of Starch Preparations with Differential Scanning Calorimetry (DSC)
3.6. Determination of the Resistance of Starch Preparations to Amyloglucosidase
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sui, Z.; Kong, X. Physical Modifications of Starch; Springer Nature Singapore Pte Ltd.: Singapore, 2018. [Google Scholar]
- Rudrapatnam, N.T. Starch—Value Addition by Modification. Crit. Rev. Food Sci. Nutr. 2005, 45, 371–384. [Google Scholar]
- Apriyanto, A.; Compart, J.; Fettke, J. A review of starch, a unique biopolymer—Structure, metabolism and in planta modifications. Plant Sci. 2022, 318, 111–123. [Google Scholar] [CrossRef]
- Thompson, D.B. Strategies for the manufacture of resistant starch. Trends Food Sci. Technol. 2000, 11, 245–253. [Google Scholar] [CrossRef]
- Jiang, F.; Du, C.; Jiang, W.; Wang, L.; Du, S. The preparation, formation, fermentability and applications of resistant starch. Int. J. Biol. Macromol. 2020, 150, 1155–1161. [Google Scholar] [CrossRef]
- Ciudad-Muler, M.; Fernández-Ruiz, V.; Matallana-González, C.; Morales, P. Dietary fiber sources and human benefits: The case study of cereal and pseudocereals. Adv. Food Nutr. Res. 2019, 90, 83–123. [Google Scholar]
- Haralampu, S.G. Resistant starch—A review of the physical properties and biological impact of RS3. Carbohydr. Polym. 2000, 41, 285–292. [Google Scholar] [CrossRef]
- Chang, Q.; Zheng, B.; Zhang, Y.; Zeng, H. A comprehensive review of the factors influencing the formation of retrograded starch. Int. J. Biol. Macromol. 2021, 186, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Falsafi, S.R.; Maghsoudlou, Y.; Aalami, M.; Jafari, S.M.; Raeisi, M. Physicochemical and morphological properties of resistant starch type 4 prepared under ultrasound and conventional conditions and their in-vitro and in-vivo digestibilities. Ultrason. Sonochem. 2019, 53, 110–119. [Google Scholar] [CrossRef]
- Patterson, M.A.; Maiya, M.; Stewart, M.L. Resistant starch content in foods commonly consumed in the United States: A narrative review. J. Acad. Nutr. Diet. 2020, 120, 230–244. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Mello El Halal, S.L.; Dias, A.R.G.; Zavareze, E.D. Physical modification of starch by heat-moisture treatment and annealing and their applications: A review. Carbohydr. Polym. 2021, 274, 118665. [Google Scholar] [CrossRef]
- Han, H.; Hou, J.; Yang, N.; Zhang, Y.; Chen, H.; Zhang, Z.; Shen, Y.; Huang, S.; Guo, S. Insight on the changes of cassava and potato starch granules during gelatinization. Int. J. Biol. Macromol. 2019, 126, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.; Storsley, J.; Ames, N. Effect of heat treatments on starch pasting, particle size and color of whole-grain barley. Cereal Chem. 2017, 94, 325–332. [Google Scholar] [CrossRef]
- Din, Z.; Xiong, H.; Fei, P. Physical and chemical modification of starches: A review. Crit. Rev. Food Sci. Nutr. 2015, 57, 2691–2705. [Google Scholar]
- Dereje, B. Composition, morphology and physicochemical properties of starches derived from indigenous Ethiopian tuber crops: A review. Int. J. Biol. Macromol. 2021, 187, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Jayakody, L.; Hoover, R. Effect of annealing on the molecular structure and physicochemical properties of starches from different botanical origins—A review. Carbohydr. Polym. 2008, 74, 691–703. [Google Scholar] [CrossRef]
- Neeraj; Siddiqui, S.; Dalal, N.; Srivastva, A.; Pathera, A.K. Physicochemical, morphological, functional, and pasting properties of potato starch as a function of extraction methods. J. Food Meas. Charact. 2021, 15, 2805–2820. [Google Scholar] [CrossRef]
- Hoover, R. Starch retrogradation. Food Rev. Int. 1995, 11, 331–346. [Google Scholar] [CrossRef]
- Zhu, F. Relationships between amylopectin internal molecular structure and physicochemical properties of starch. Trends Food Sci. Technol. 2018, 78, 234–242. [Google Scholar] [CrossRef]
- Ninni, L.; Meirelles, A.J.A.; Maurer, G. Thermodynamic properties of aqueous solutions of maltodextrins from laser-light scattering, calorimetry and isopiestic investigations. Carbohydr. Polym. 2005, 59, 289–303. [Google Scholar] [CrossRef]
- Lu, X.; Xu, R.; Zhan, J.; Chen, L.; Jin, Z.; Tian, Y. Pasting, rheology, and fine structure of starch for waxy rice powder with high-temperature baking. Int. J. Biol. Macromol. 2020, 146, 620–626. [Google Scholar] [CrossRef]
- Putseys, J.A.; Derde, L.J.; Lamberts, L.; Östman, E.; Björck, I.M.; Delcour, J.A. Functionality of short chain amylose-lipid complexes in starch-water systems and their impact on in vitro starch degradation. J. Agric. Food Chem. 2010, 58, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Yokoyama, W.; Wang, Q.; Shoemaker, C.F. Rice starch, amylopectin, and amylose: molecular weight and solubility in dimethyl sulfoxide-based solvents. J. Agric. Food Chem. 2006, 54, 2320–2326. [Google Scholar] [CrossRef]
- Jackson, D.S. Solubility behavior of granular corn starches in methyl sulfoxide (DMSO) as measured by High Performance Size Exclusion Chromatography. Starch 1991, 43, 422–427. [Google Scholar] [CrossRef]
- Frankel, J.; Hutter, R. A Practical Treatise on the Manufacture of Starch, Glucose, Starch-Sugar, and Dextrine; Reprint of the Original, First Published in 1881; Verlag Publisher: Berlin, Germany, 2024; pp. 1–335. [Google Scholar]
- Trithavisup, K.; Krusong, K.; Tananuwong, K. In-depth study of the changes in properties and molecular structure of cassava starch during resistant dextrin preparation. Food Chem. 2019, 297, 124–131. [Google Scholar] [CrossRef]
- Okhuma, K.; Matsuda, I.; Katta, Y.; Hanno, Y. Pyrolysis of starch and its digestibility by enzymes—Characterization of indigestible dextrin. J. Jpn. Soc. Starch Sci. (Starch Sci.) 1990, 37, 107–114. [Google Scholar]
- Bertoft, E. Understanding starch structure: Recent progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Shah, M.A.; Schweiggert-Weisz, U.; Gul, K.; Wani, I.A. Rice Starch Diversity: Effects on structural, morphological, thermal, and physicochemical properties—A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 417–436. [Google Scholar] [CrossRef]
- Yashini, M.; Khushbu, S.; Madhurima, N.; Sunil, C.K. Thermal properties of different types of starch: A review. Crit. Rev. Food Sci. Nutr. 2022, 64, 4373–4396. [Google Scholar] [CrossRef]
- Gryszkin, A.; Zięba, T.; Kapelko, M.; Buczek, A. Effect of thermal modifications of potato starch on its selected properties. Food Hydrocoll. 2014, 40, 122–127. [Google Scholar] [CrossRef]
- Zięba, T.; Szumny, A.; Kapelko, M. Properties of retrograded and acetylated starch preparations. Part 1. Structure, susceptibility to amylase, and quality of gelatinization. LWT Food Sci. Technol. 2011, 44, 1314–1320. [Google Scholar] [CrossRef]
- Villas-Boas, F.; Facchinatto, W.M.; Colnago, L.A.; Volanti, D.P.; Franco, C.M.L. Effect of amylolysis on the formation, the molecular, crystalline and thermal characteristics and the digestibility of retrograded starches. Int. J. Biol. Macromol. 2020, 163, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Liu, P. Starch gelatinization, retrogradation, and enzyme susceptibility of retrograded starch: Effect of amylopectin internal molecular structure. Food Chem. 2020, 316, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Zięba, T.; Kapelko, M.; Szumny, A. Effect of preparation method on the properties of potato starch acetates with an equal degree of substitution. Carbohydr. Polym. 2013, 94, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Behall, K.M.; Scholfield, D.J.; Canary, J. Effect of starch structure on glucose and insulin responses in adults. Am. J. Clin. Nutr. 1988, 47, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Tovar, J.; Granfeldt, Y.; Bjoerck, I.M. Effect of processing on blood glucose and insulin responses to starch in legumes. J. Agric. Food Chem. 1992, 40, 1846–1851. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Zaragoza, E.; Riquelme-Navarrete, M.J.; Sánchez-Zapata, E.; Pérez-Álvarez, J.A. Resistant starch as functional ingredient: A review. Food Res. Int. 2010, 43, 931–942. [Google Scholar] [CrossRef]
- Raigond, P.; Ezekiel, R.; Raigond, B. Resistant starch in food: A review. J. Sci. Food Agric. 2015, 95, 1968–1978. [Google Scholar] [CrossRef]
- Buksa, K.; Nowotna, A.; Ziobro, R.; Gambuś, H. Rye flour enriched with arabinoxylans in rye bread making. Food Sci. Technol. Int. 2015, 21, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Praznik, W.; Buksa, K.; Ziobro, R.; Gambuś, H.; Nowotna, A. The effect of long-term alkali treatment on the molecular characteristics of native and extruded starches at 35 °C. Starch 2012, 64, 890–897. [Google Scholar] [CrossRef]
- Richter, M.; Augustat, S.; Schierbaum, F. Ausgewählte Methoden der Stärke Chemie; VEB Fachbuch Verlag Leipzig: Leipzig, Germany, 1968; pp. 110–112. [Google Scholar]
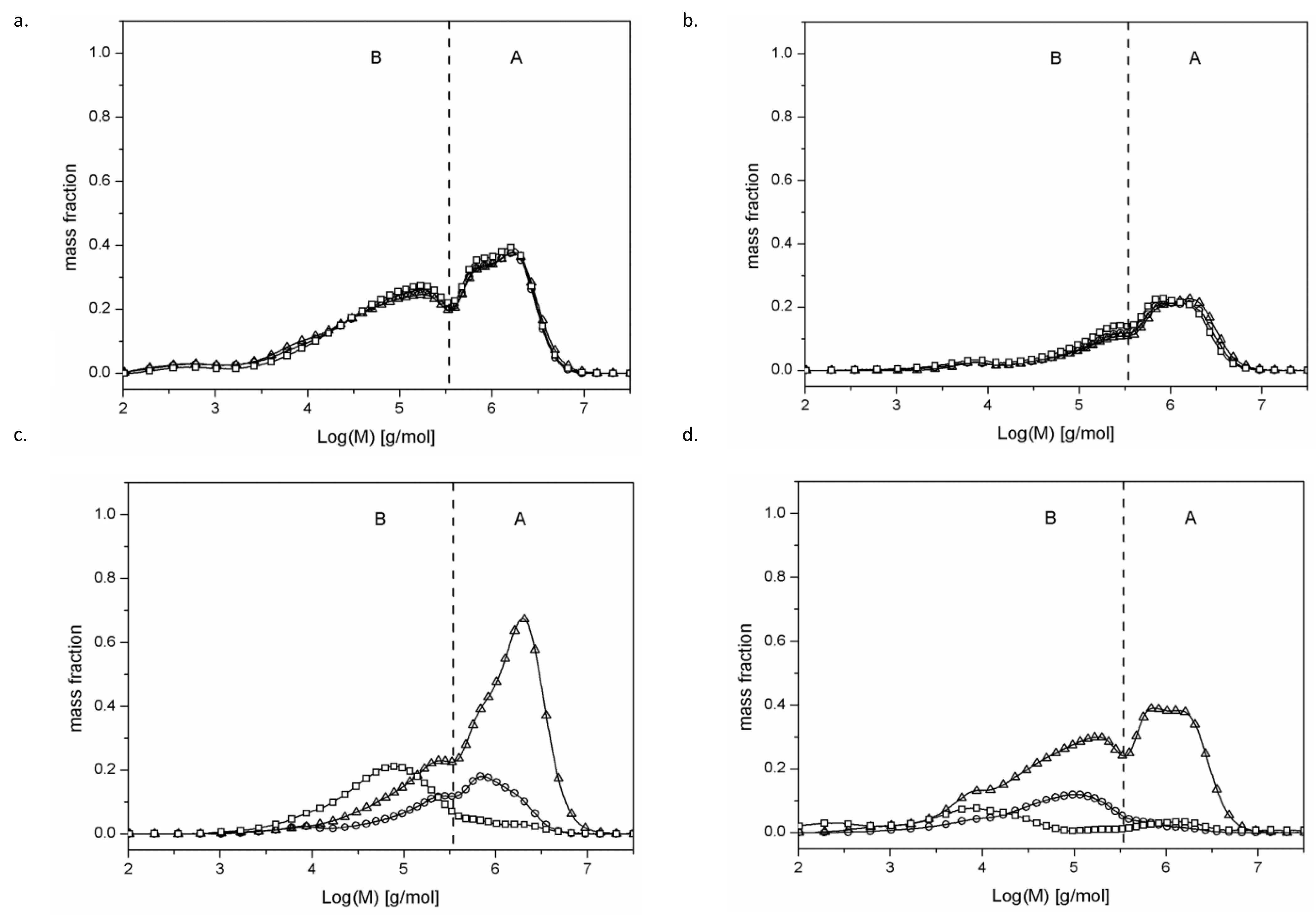
| 110/5 | 130/5 | 150/5 | 110/24 | 130/24 | 150/24 | |||||||
| Mw [g × 103/mol] | Ð | Mw [g × 103/mol] | Ð | Mw [g × 103/mol] | Ð | Mw [g × 103/mol] | Ð | Mw [g × 103/mol] | Ð | Mw [g × 103/mol] | Ð | |
| whole | 777 | 12.9 | 721 | 11.3 | 741 | 9.9 | 1022 | 8.5 | 926 | 8.8 | 830 | 9.3 |
| A | 1454 | 1.6 | 1363 | 1.5 | 1373 | 1.5 | 1463 | 1.6 | 1358 | 1.5 | 1287 | 1.5 |
| B | 114 | 9.1 | 117 | 8.7 | 122 | 6.5 | 155 | 3.6 | 155 | 5.3 | 152 | 6.4 |
| G110/5 | (*) G130/5 | (*) G150/5 | G110/24 | (-) G130/24 | (-) G150/24 | |||||||
| Mw [g × 103/mol] | Ð | Mw [g × 103/mol] | Ð | Mw [g × 103/mol] | Ð | Mw [g × 103/mol] | Ð | Mw [g × 103/mol] | Ð | Mw [g × 103/mol] | Ð | |
| whole | 1253 | 7.2 | * 726 | 7.5 | * 258 | 6.6 | 684 | 12.1 | - | - | - | |
| A | 1724 | 1.6 | * 1155 | 1.5 | * 1236 | 1.7 | 1330 | 1.5 | - | - | - | |
| B | 156 | 3.0 | * 159 | 4.0 | * 99 | 3.4 | 117 | 6.3 | - | - | - | |
| Trait | Retrograded Starch |
|---|---|
| Molar mass MW [g × 103/mol] | 1643 |
| Mw—fraction A [g × 103/mol] | 2415 |
| Mw—fraction B [g × 103/mol] | 192 |
| Swelling power [g/g] | 22.87 ± 0.57 |
| Solubility [g/100 g] | 14.84 ± 0.37 |
| Initial pasting temperature [°C] | 45.12 ± 1.12 |
| Final pasting temperature [°C] | 53.34 ± 1.33 |
| Phase transition heat [J/g] | 6.04 ± 0.15 |
| Resistance to amyloglucosidase [g/100 g] | 10.04 ± 0.24 |
| Type of Starch Preparation | Roasting Temperature [°C] | Initial Temperature of Gelatinization [°C] | LSDT | Final Temperature of Gelatinization [°C] | LSDT | Enthalpy of Gelatinization [J/g] | LSDT | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Roasting Time [h] | Roasting Time [h] | Roasting Time [h] | ||||||||
| 5 | 24 | 5 | 24 | 5 | 24 | |||||
| without glucose | 110 | 48.77 c | 45.85 d | 0.75 | 47.09 a | 64.48 b | 0.96 | 6.43 c | 6.01 c | 1.27 |
| 130 | 50.45 a | 46.74 c | 64.64 b | 64.53 ab | 4.24 b | 6.68 a | ||||
| 150 | 50.64 c | 50.18 a | 67.28 a | 66.70 ab | 7.80 a | 7.91 a | ||||
| with glucose | 110 | 50.21 b | 51.46 a | 65.89 b | 67.66 a | 8.04 a | 7.29 b | |||
| 130 | 49.15 b | 49.05 b | 63.86 a | 67.07 a | 5.90 a | 6.54 a | ||||
| 150 | 46.94 ab | 47.06 a | 65.70 b | 66.50 b | 3.70 b | 0.48 c | ||||
| LSDA | 0.68 | 0.83 | 1.57 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapelko-Żeberska, M.; Zięba, T.; Meisel, M.; Buksa, K.; Gryszkin, A. Production of Resistant Starch by Roasting Retrograded Starch with Glucose. Molecules 2024, 29, 2883. https://doi.org/10.3390/molecules29122883
Kapelko-Żeberska M, Zięba T, Meisel M, Buksa K, Gryszkin A. Production of Resistant Starch by Roasting Retrograded Starch with Glucose. Molecules. 2024; 29(12):2883. https://doi.org/10.3390/molecules29122883
Chicago/Turabian StyleKapelko-Żeberska, Małgorzata, Tomasz Zięba, Marta Meisel, Krzysztof Buksa, and Artur Gryszkin. 2024. "Production of Resistant Starch by Roasting Retrograded Starch with Glucose" Molecules 29, no. 12: 2883. https://doi.org/10.3390/molecules29122883
APA StyleKapelko-Żeberska, M., Zięba, T., Meisel, M., Buksa, K., & Gryszkin, A. (2024). Production of Resistant Starch by Roasting Retrograded Starch with Glucose. Molecules, 29(12), 2883. https://doi.org/10.3390/molecules29122883






