Immunomodulatory Effect of Cordyceps militaris Polysaccharide on RAW 264.7 Macrophages by Regulating MAPK Signaling Pathways
Abstract
1. Introduction
2. Results and Discussion
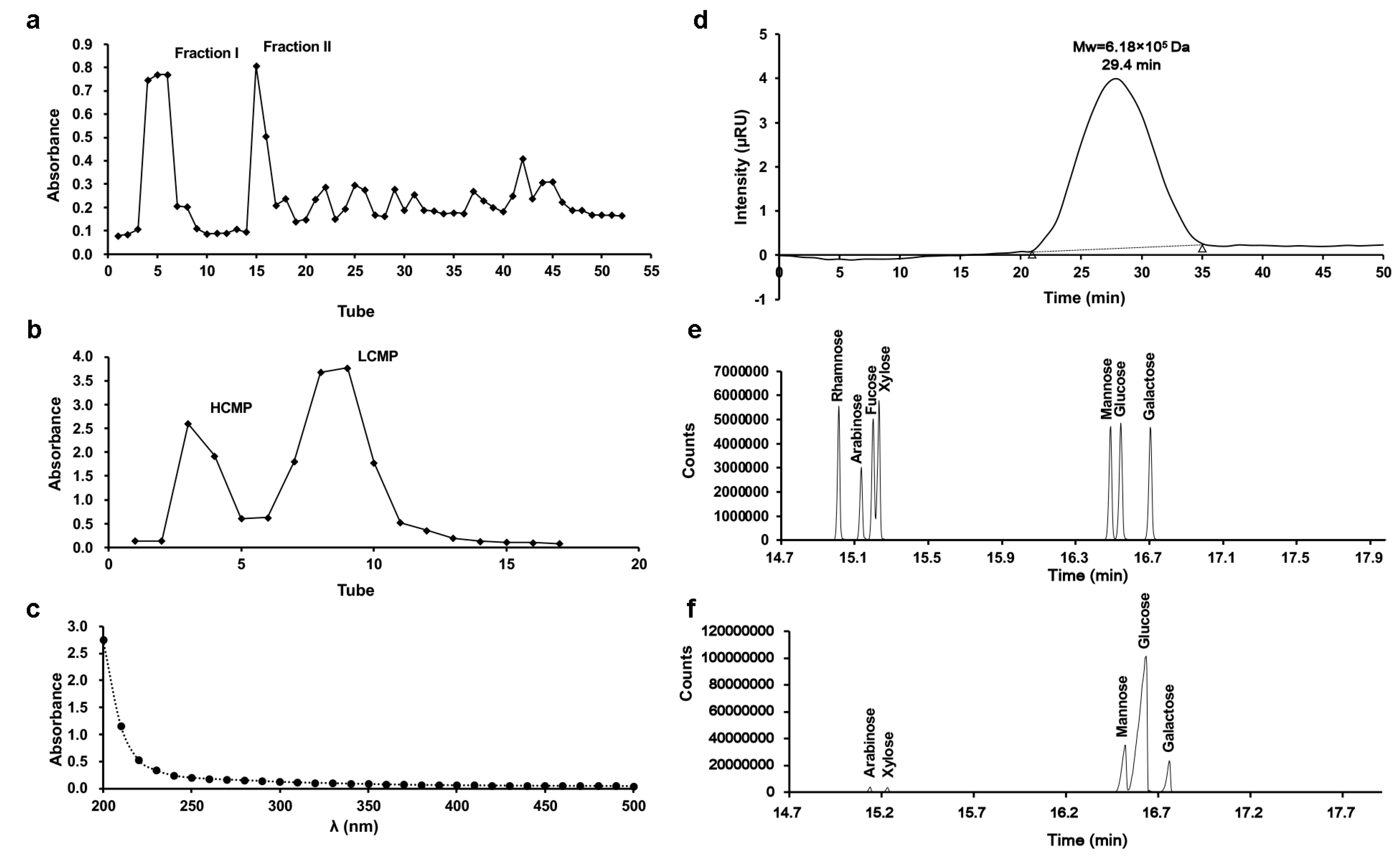
2.1. Isolation of HCMP
2.2. Mw Distribution and Monosaccharide Composition of HCMP
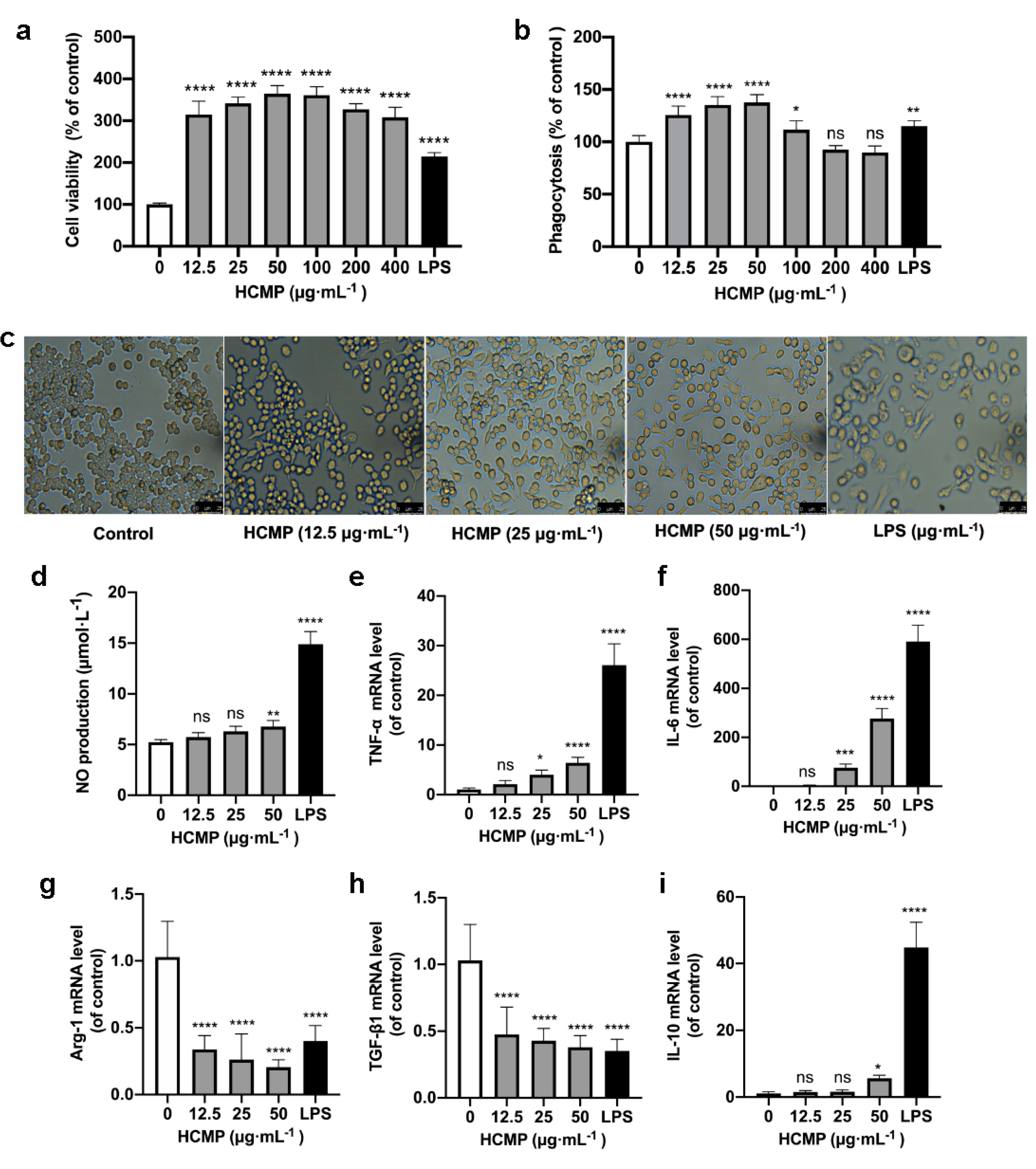
2.3. Effects of HCMP on the Growth of RAW 264.7 Macrophages
2.4. HCMP Inducing the Activation of RAW 264.7 Macrophages
2.5. HCMP Inducing Production and mRNA Expression of Inflammation-Related Molecules in RAW 264.7 Macrophages
2.6. The Effects of HCMP on Inflammation-Related Molecules in an Inflammatory Environment
2.7. HCMP Activating MAPK Signaling Pathway
3. Materials and Methods
3.1. Materials
3.2. Isolation and Purification of C. militaris Polysaccharides
3.3. Mw Determination of HCMP
3.4. Monosaccharide Composition Determination of HCMP
3.5. Culture of RAW 264.7 Macrophages
3.6. Cell Viability Assay
3.7. Cell Phagocytosis Assay
3.8. Cell Morphology Assessment
3.9. NO Assay
3.10. RT-qPCR Assay
3.11. Western Blotting
3.12. Validation of HCMP Inducing MAPK Signaling Pathways
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, W.; Han, R.; Li, H.; Song, J.; Yan, H.; Li, G.; Liu, A.; Cao, X.; Guo, J.; Zhai, S. Agronomic traits and molecular marker identification of wheat-Aegilops caudata addition lines. Front. Plant Sci. 2017, 8, 1743. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, X.; Yang, Y.; Ayivi-Tosuh, S.M.; Wang, F.; Li, H.; Wang, G. A polysaccharide isolated from the fruits of Physalis alkekengi L. induces RAW264. 7 macrophages activation via TLR2 and TLR4-mediated MAPK and NF-κB signaling pathways. Int. J. Biol. Macromol. 2019, 140, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Huang, Y.; Bian, Y.; Wong, J.H.; Ng, T.; Wang, H. Hypoglycemic activity of the fungi Cordyceps militaris, Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens in streptozotocin-induced diabetic rats. Appl. Microbiol. Biotechnol. 2006, 72, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, S.; Du, M. Cordycepin from Cordyceps militaris prevents hyperglycemia in alloxan-induced diabetic mice. Nutr. Res. 2015, 35, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.C.; Koyyalarnudi, S.R.; Hughes, J.M.; Khoo, C.; Bailey, T.; Marripudi, K.; Park, J.P.; Kim, J.H.; Song, C.H. Antioxidant and immunomodulating activities of exo- and endopolysaccharide fractions from submerged mycelia cultures of culinary-medicinal mushrooms. Int. J. Med. Mushrooms 2013, 15, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.Y.; Lin, Y.H.; Yeh, E.L.; Lo, H.C.; Hsu, T.H.; Su, C.C. Cordycepin and a preparation from Cordyceps militaris inhibit malignant transformation and proliferation by decreasing EGFR and IL-17RA signaling in a murine oral cancer model. Oncotarget 2017, 8, 93712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q.; Chen, Y.; Liang, C.L.; Liu, H.; Qiu, F.; Dai, Z. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed. Pharmacother. 2020, 121, 109570. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Qin, T.; Qiu, F.; Song, Y.; Lin, D.; Ma, Y.; Li, J.; Huang, Y. Immunomodulatory effects of hydroxyethylated Hericium erinaceus polysaccharide on macrophages RAW264.7. Int. J. Biol. Macromol. 2017, 105, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Zhen, D.; Su, L.; Miao, Y.; Zhao, F.; Ren, G.; Mahfuz, S.; Song, H. Purification, partial characterization and inducing tumor cell apoptosis activity of a polysaccharide from Ganoderma applanatum. Int. J. Biol. Macromol. 2018, 115, 10–17. [Google Scholar] [CrossRef]
- Yan, J.K.; Yu, Y.B.; Wang, C.; Cai, W.D.; Wu, L.X.; Yang, Y.; Zhang, H.N. Production, physicochemical characteristics, and in vitro biological activities of polysaccharides obtained from fresh bitter gourd (Momordica charantia L.) via room temperature extraction techniques. Food Chem. 2021, 337, 127798. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y.; Cui, Y.; Liu, H.; Dong, C.; Sun, Y. Structural characterization, antioxidant and immunomodulatory activities of a neutral polysaccharide from Cordyceps militaris cultivated on hull-less barley. Carbohydr. Polym. 2020, 235, 115969. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gong, Z.; Su, Y.; Lin, J.; Tang, K. Cordyceps fungi: Natural products, pharmacological functions and developmental products. J. Pharm. Pharmacol. 2009, 61, 279–291. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zheng, Q.; Guo, L.; Huang, J.; Yun, F.; Huang, S.; Lin, J. Structural characterization and immune-enhancing activity of a novel high-molecular-weight polysaccharide from Cordyceps militaris. Int. J. Biol. Macromol. 2020, 145, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Xie, L.; Xie, J.; Shen, M. Sulfated Chinese yam polysaccharide enhances the immunomodulatory activity of RAW 264.7 cells via the TLR4-MAPK/NF-κB signaling pathway. Food Funct. 2022, 13, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, B.; Wang, Z.; Li, M.; Zhao, W. Natural polysaccharides with immunomodulatory activities. Mini Rev. Med. Chem. 2020, 20, 96–106. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, C.; Guo, Y.; Zhou, W.; Zhang, Y. Toll-like receptor 4-related immunostimulatory polysaccharides: Primary structure, activity relationships, and possible interaction models. Carbohydr. Polym. 2016, 149, 186–206. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yajima, T.; Saito, K.; Nishimura, H.; Fushimi, T.; Ohshima, Y.; Tsukamoto, Y.; Yoshikai, Y. Immunostimulating properties of intragastrically administered Acetobacter-derived soluble branched (1, 4)-β-D-glucans decrease murine susceptibility to Listeria monocytogenes. Infect. Immun. 2004, 72, 7005–7011. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Hou, Y.; Song, L.; Zhu, S.; Lin, F.; Bai, Y. D-Mannose enhanced immunomodulation of Periodontal Ligament stem cells via inhibiting IL-6 secretion. Stem Cells Int. 2018, 2018, 7168231. [Google Scholar] [CrossRef]
- Zhu, L.; Li, W.; Fan, Z.; Ye, X.; Lin, R.; Ban, M.; Ren, L.; Chen, X.; Zhang, D. Immunomodulatory activity of polysaccharide from Arca granosa Linnaeus via TLR4/MyD88/NFκB and TLR4/TRIF signaling pathways. J. Funct. Foods 2021, 84, 104579. [Google Scholar] [CrossRef]
- Lee, J.S.; Synytsya, A.; Kim, H.B.; Choi, D.J.; Lee, S.; Lee, J.; Kim, W.J.; Jang, S.; Park, Y.I. Purification, characterization and immunomodulating activity of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Int. Immunopharmacol. 2013, 17, 858–866. [Google Scholar] [CrossRef]
- Michel, T.; Vanhoutte, P.M. Cellular signaling and NO production. Pflug. Arch. Eur. J. Phy. 2010, 459, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zou, Y.; Li, Q.; Mao, R.; Shao, X.; Jin, D.; Zheng, D.; Zhao, T.; Zhu, H.; Zhang, L. Immunomodulatory effects of a polysaccharide purified from Lepidium meyenii Walp. on macrophages. Process Biochem. 2016, 51, 542–553. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Jia, S.; Huang, L.; Wang, Z.; Li, C.; Xie, M. Immunomodulatory effects of an acetylated Cyclocarya paliurus polysaccharide on murine macrophages RAW264. 7. Int. J. Biol. Macromol. 2017, 98, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Wu, J.; Zhao, M.; Sun, W.; Sun, J.; Li, H.; Huang, M. Immunomodulatory activity of a novel polysaccharide extracted from Huangshui on THP-1 cells through NO production and increased IL-6 and TNF-α expression. Food Chem. 2020, 330, 127257. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, J.; Chen, F.; Chen, X.; Zhou, Z.; Wang, H. Activation of RAW264. 7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohyd. Polym. 2015, 121, 388–402. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Yang, J.Y.; Guo, Z.J.; Wu, Q.; Zhang, L.D.; Zhou, X.W. RNA-seq analysis reveals an immunomodulatory peptide from highland barley activating RAW264. 7 macrophages via TNF/NF-κB signaling pathway. Funct. Integr. Genomic. 2023, 23, 253. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Peasura, N.; Laohakunjit, N.; Kerdchoechuen, O.; Vongsawasdi, P.; Chao, L.K. Assessment of biochemical and immunomodulatory activity of sulphated polysaccharides from Ulva intestinalis. Int. J. Biol. Macromol. 2016, 91, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.m.; Xu, S.s.; Li, L.; Pan, T.m.; Shi, C.l.; Liu, H.; Cao, M.j.; Su, W.j.; Liu, G.m. In vitro and in vivo immunomodulatory activity of sulfated polysaccharide from Porphyra haitanensis. Carbohydr. Polym. 2017, 165, 189–196. [Google Scholar] [CrossRef]
- Bai, J.B.; Ge, J.C.; Zhang, W.J.; Liu, W.; Luo, J.P.; Xu, F.Q.; Wu, D.L.; Xie, S.Z. Physicochemical, morpho-structural, and biological characterization of polysaccharides from three Polygonatum spp. RSC Adv. 2021, 11, 37952–37965. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the Interleukin-10 Receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Kotenko, S.V. The family of IL-10-related cytokines and their receptors: Related, but to what extent? Cytokine Growth Factor Rev. 2002, 13, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Z.; Chang, Y.Z.; He, Z.M.; Chen, L.; Zhou, X.W. Immunomodulatory activity of Ganoderma lucidum immunomodulatory protein via PI3K/Akt and MAPK signaling pathways in RAW264.7 cells. J. Cell Physiol. 2019, 234, 23337–23348. [Google Scholar] [CrossRef] [PubMed]
- Diling, C.; Xin, Y.; Chaoqun, Z.; Jian, Y.; Xiaocui, T.; Jun, C.; Ou, S.; Yizhen, X. Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota. Oncotarget 2017, 8, 85838. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.H.; Li, H.M.; Yu, W.Y.; Yu, C.H. Iridin revented against lipopolysaccharide-induced inflammatory responses of macrophages via inactivation of PKM2-mediated glycolytic pathways. J. Inflamm. Res. 2021, 14, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Mitsi, E.; Kamng’ona, R.; Rylance, J.; Solórzano, C.; Jesus Reiné, J.; Mwandumba, H.C.; Ferreira, D.M.; Jambo, K.C. Human alveolar macrophages predominately express combined classical M1 and M2 surface markers in steady state. Respir. Res. 2018, 19, 66. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Wei, J.; Besner, G.E. M1 to M2 macrophage polarization in heparin-binding epidermal growth factor-like growth factor therapy for necrotizing enterocolitis. J. Surg. Res. 2015, 197, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yuan, F.; Wang, K.; Song, D.; Zhang, W. Modulatory effects of the acid polysaccharide fraction from one of anamorph of Cordyceps sinensis on Ana-1 cells. J. Ethnopharmacol. 2012, 142, 739–745. [Google Scholar] [CrossRef]
- Bi, S.; Huang, W.; Chen, S.; Huang, C.; Li, C.; Guo, Z.; Yang, J.; Zhu, J.; Song, L.; Yu, R. Cordyceps militaris polysaccharide converts immunosuppressive macrophages into M1-like phenotype and activates T lymphocytes by inhibiting the PD-L1/PD-1 axis between TAMs and T lymphocytes. Int. J. Biol. Macromol. 2020, 150, 261–280. [Google Scholar] [CrossRef]
- Bi, S.; Jing, Y.; Zhou, Q.; Hu, X.; Zhu, J.; Guo, Z.; Song, L.; Yu, R. Structural elucidation and immunostimulatory activity of a new polysaccharide from Cordyceps militaris. Food Funct. 2018, 9, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Tar. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shepherd, E.G.; Nelin, L.D. MAPK phosphatases-regulating the immune response. Nat. Rev. Immunol. 2007, 7, 202–212. [Google Scholar] [CrossRef]
- Chen, N.; Xia, P.; Li, S.; Zhang, T.; Wang, T.T.; Zhu, J. RNA sensors of the innate immune system and their detection of pathogens. IUBMB Life 2017, 69, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Hua, K.F.; Lin, C.C.; Lin, C.H.; Hsu, J.; Wong, C.H. Extract of reishi polysaccharides induces cytokine expression via TLR4-modulated protein kinase signaling pathways. J. Immunol. 2004, 173, 5989–5999. [Google Scholar] [CrossRef] [PubMed]
- McGruder, E.D.; Moore, G.M. Use of lipopolysaccharide (LPS) as a positive control for the evaluation of immunopotentiating drug candidates in experimental avian colibacillosis models. Res. Vet. Sci. 1999, 66, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Shen, T.; Wang, G.; You, L.; Zhang, L.; Ren, H.; Hu, W.; Qiang, Q.; Wang, X.; Ji, L.; Gu, Z. Polysaccharide from wheat bran induces cytokine expression via the toll-like receptor 4-mediated p38 MAPK signaling pathway and prevents cyclophosphamide-induced immunosuppression in mice. Food Nutr. Res. 2017, 61, 1344523. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, Y.; Yang, Y.; Tao, L. Aloperine suppresses LPS-induced macrophage activation through inhibiting the TLR4/NF-κB pathway. Inflamm. Res. 2020, 69, 375–383. [Google Scholar] [CrossRef]
- Lu, J.; Chen, X.; Xu, X.; Liu, J.; Zhang, Z.; Wang, M.; Li, X.; Chen, H.; Zhao, D.; Wang, J.; et al. Active polypeptides from Hirudo inhibit endothelial cell inflammation and macrophage foam cell formation by regulating the LOX-1/LXR-α/ABCA1 pathway. Biomed. Pharmacother. 2019, 115, 108840. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, J.; Wei, Y.; Yu, G.; Li, Q. Characterization of a neutral polysaccharide from pumpkin (Cucurbita moschata Duch) with potential immunomodulatory activity. Int. J. Biol. Macromol. 2021, 188, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Hou, C.; Shi, C.; Lin, Z.; Liao, W.; Yuan, E. A polysaccharide isolated and purified from Platycladus orientalis (L.) Franco leaves, characterization, bioactivity and its regulation on macrophage polarization. Carbohydr. Polym. 2019, 213, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Gao, Z.; Ji, K.; Li, X.; Wu, J.; Liu, Y.; Wang, X.; Liang, H.; Liu, Y.; Li, X. The in vitro and in vivo anti-inflammatory effect of osthole, the major natural coumarin from Cnidium monnieri (L.) Cuss, via the blocking of the activation of the NF-κB and MAPK/p38 pathways. Phytomedicine 2019, 58, 152864. [Google Scholar] [CrossRef] [PubMed]



| Gene | Direction | Primer Sequence (5′→3′) |
|---|---|---|
| β-actin | Forward | ATCGTGCGGGACATCAAGG |
| Reverse | TCGTTGCCGATGGTGATGAC | |
| TNF-α | Forward | GGGGATTATGGCTCAGGGTC |
| Reverse | CGAGGCTCCAGTGAATTCGG | |
| IL-6 | Forward | CATGTTCTCTGGGAAATCGTGG |
| Reverse | AACGCACTAGGTTTGCCGAGTA | |
| Arg-1 | Forward | CAGAAGAATGGAAGAGTCAG |
| Reverse | CAGATATGCAGGGAGTCACC | |
| TGF-β1 | Forward | ACAGCACCAATTGTCCAAGTTTC |
| Reverse | CGGTGCATGCATAGCCTTGT | |
| IL-10 | Forward | CCAAGCCTTATCGGAAATGA |
| Reverse | TCCTGAGGGTCTTCAGCTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yang, J.; Guo, Z.; Li, Q.; Zhang, L.; Zhao, L.; Zhou, X. Immunomodulatory Effect of Cordyceps militaris Polysaccharide on RAW 264.7 Macrophages by Regulating MAPK Signaling Pathways. Molecules 2024, 29, 3408. https://doi.org/10.3390/molecules29143408
Liu Y, Yang J, Guo Z, Li Q, Zhang L, Zhao L, Zhou X. Immunomodulatory Effect of Cordyceps militaris Polysaccharide on RAW 264.7 Macrophages by Regulating MAPK Signaling Pathways. Molecules. 2024; 29(14):3408. https://doi.org/10.3390/molecules29143408
Chicago/Turabian StyleLiu, Yan, Jiayi Yang, Zhijian Guo, Qizhang Li, Lida Zhang, Lingxia Zhao, and Xuanwei Zhou. 2024. "Immunomodulatory Effect of Cordyceps militaris Polysaccharide on RAW 264.7 Macrophages by Regulating MAPK Signaling Pathways" Molecules 29, no. 14: 3408. https://doi.org/10.3390/molecules29143408
APA StyleLiu, Y., Yang, J., Guo, Z., Li, Q., Zhang, L., Zhao, L., & Zhou, X. (2024). Immunomodulatory Effect of Cordyceps militaris Polysaccharide on RAW 264.7 Macrophages by Regulating MAPK Signaling Pathways. Molecules, 29(14), 3408. https://doi.org/10.3390/molecules29143408








