Comparison among Different Green Extraction Methods of Polyphenolic Compounds from Exhausted Olive Oil Pomace and the Bioactivity of the Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Yield of Extraction
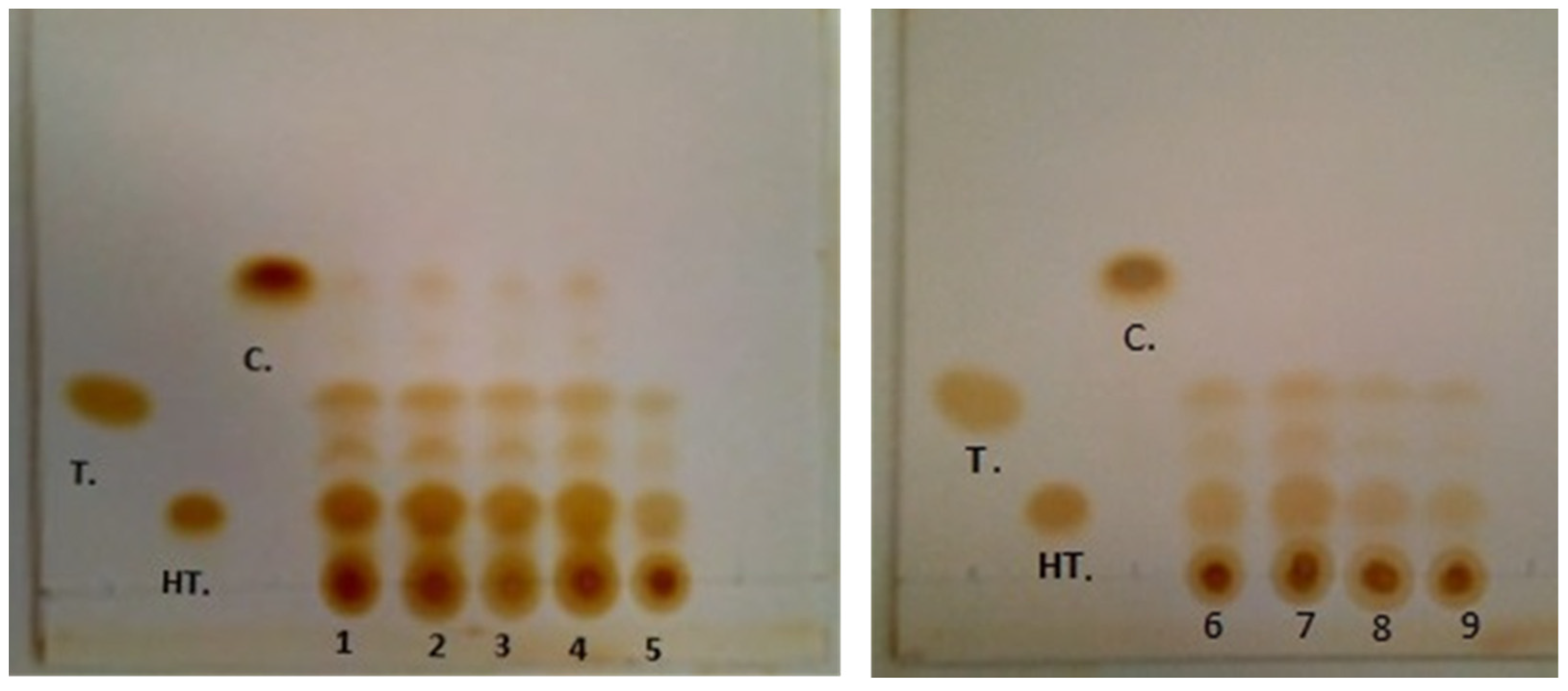
2.2. Thin Layer Chromatography (TLC)
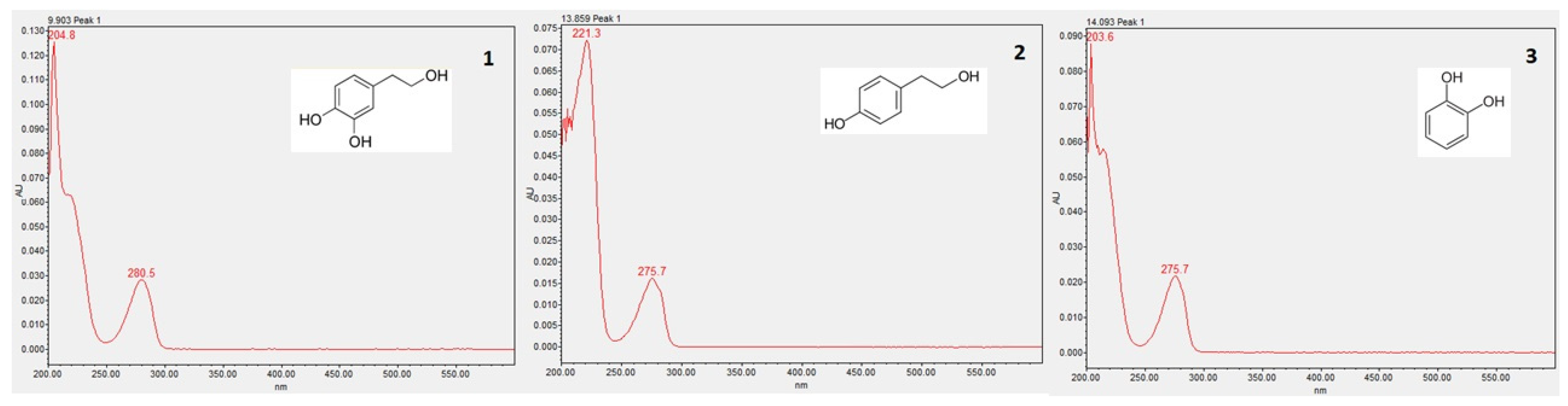
2.3. High-Performance Liquid Chromatography and Diode-Array Detector (HPLC-DAD)
2.4. Total Phenolic Content (TPC) and Antioxidant Activity
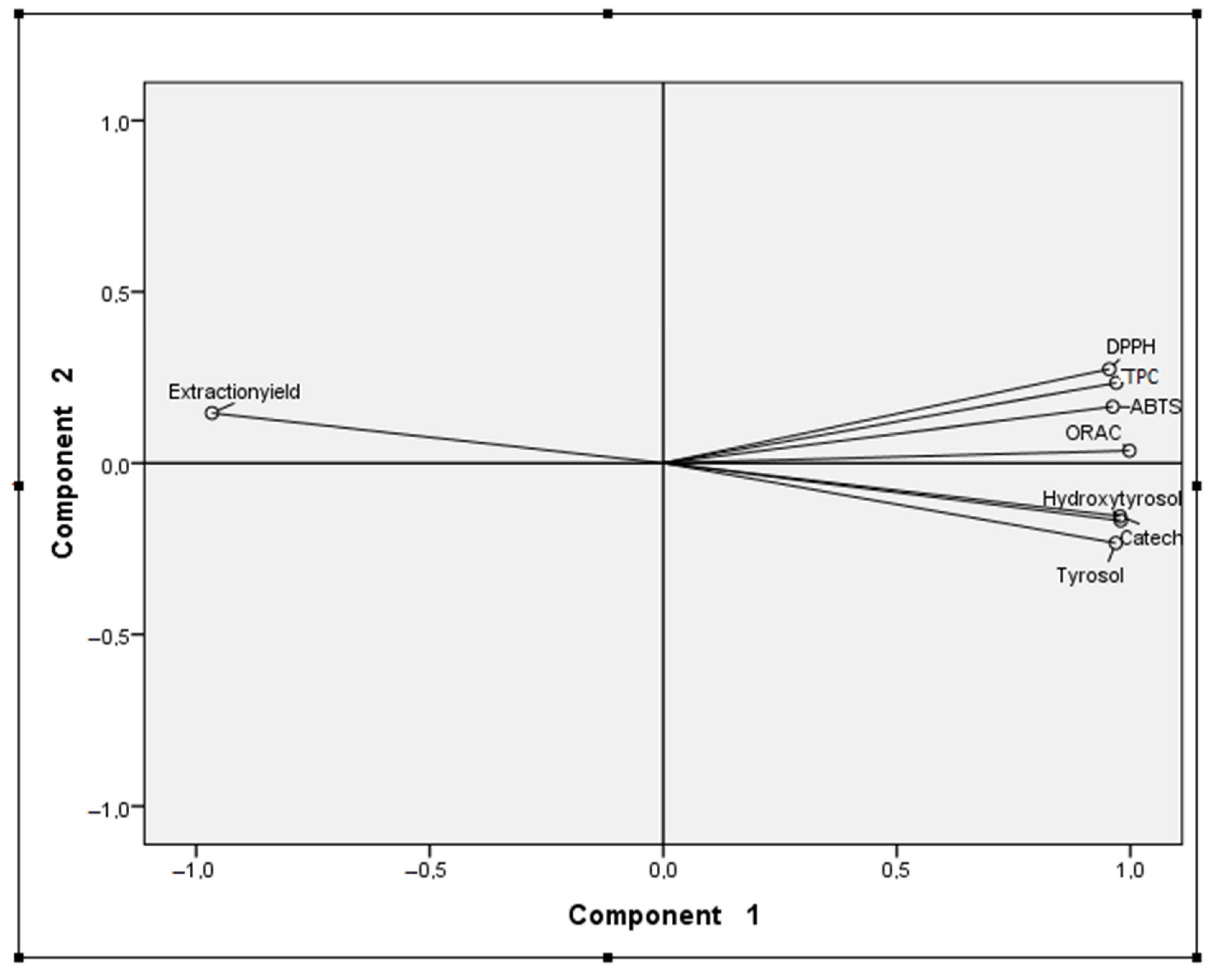
2.5. Principal Components Analysis (PCA)
2.6. Antimicrobial Analysis
3. Materials and Methods
3.1. Plant Material
3.2. Dry weight (DW) Determination
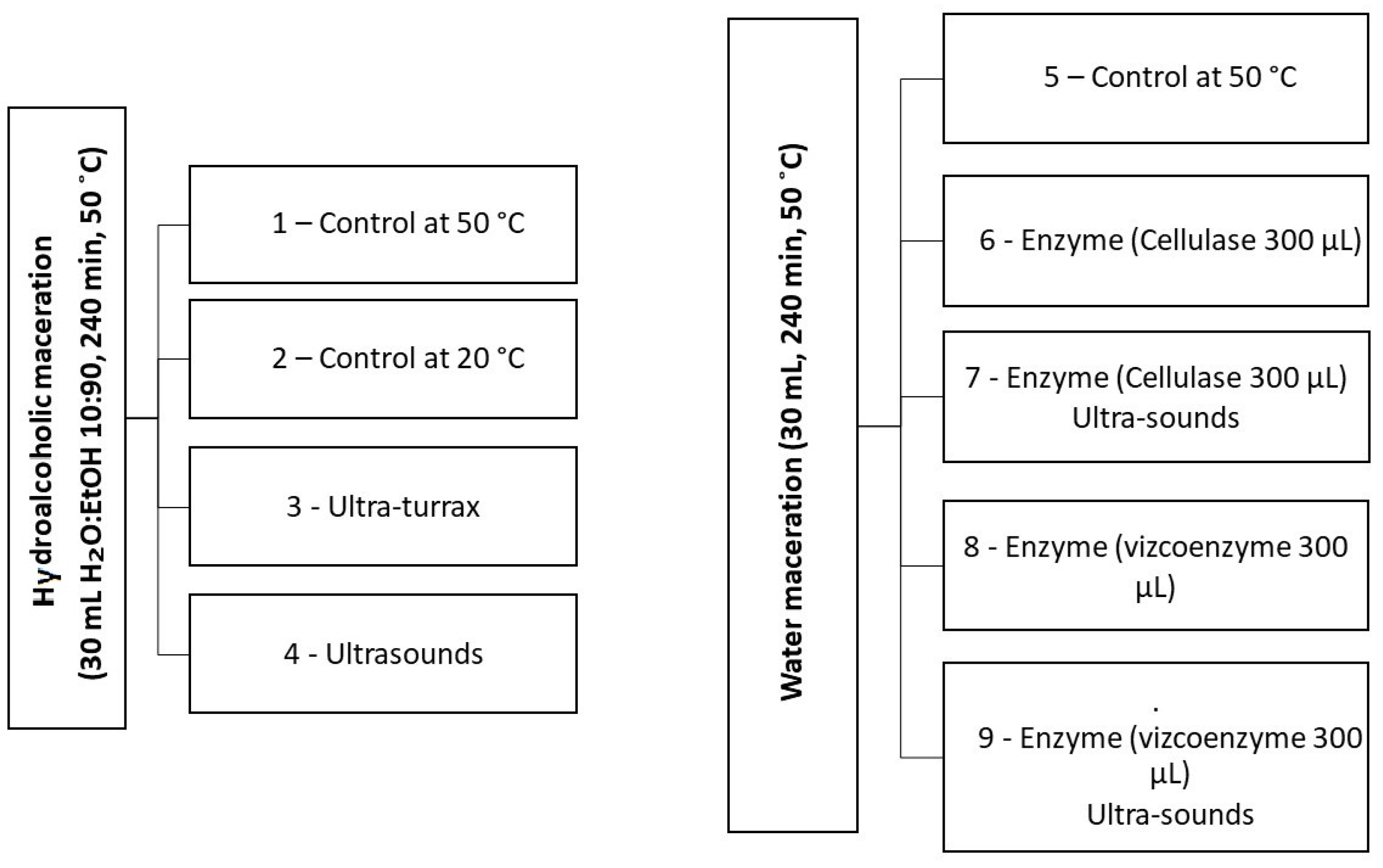
3.3. Extraction of Bioactive Compounds
3.3.1. Conventional Solvent Extraction
3.3.2. Ultra-Turrax-Assisted Extraction
3.3.3. Ultrasound-Assisted Extraction (USAE)
3.3.4. Enzyme-Assisted Extraction (EAE)
3.3.5. USAE and Enzymes Extraction
3.3.6. Yield of Extraction
3.4. Phenolic Compounds Identification and Quantification
3.4.1. TLC Analysis
3.4.2. HPLC-DAD Analysis
3.5. Total Phenolic Content Determination
3.6. Antioxidant Activity Determination
3.6.1. The ABTS Method
3.6.2. The DPPH Method
3.6.3. The Oxygen Radical Absorbance Capacity Method (ORAC)
3.7. Antimicrobial Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otero, P.; Garcia-Oliveira, P.; Carpena, M.; Barral-Martinez, M.; Chamorro, F.; Echave, J.; Garcia-Perez, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; et al. Applications of By-Products from the Olive Oil Processing: Revalorization Strategies Based on Target Molecules and Green Extraction Technologies. Trends Food Sci. Technol. 2021, 116, 1084–1104. [Google Scholar] [CrossRef]
- Klisović, D.; Novoselić, A.; Režek Jambrak, A.; Brkić Bubola, K. The Utilisation Solutions of Olive Mill By-Products in the Terms of Sustainable Olive Oil Production: A Review. Int. J. Food Sci. Technol. 2021, 56, 4851–4860. [Google Scholar] [CrossRef]
- Dantas Palmeira, J.; Araújo, D.; Mota, C.C.; Alves, R.C.; Oliveira, M.B.P.P.; Ferreira, H.M.N. Fermentation as a Strategy to Valorize Olive Pomace, a By-Product of the Olive Oil Industry. Fermentation 2023, 9, 442. [Google Scholar] [CrossRef]
- Doula, M.K.; Moreno-Ortego, J.L.; Tinivella, F.; Inglezakis, V.J.; Sarris, A.; Komnitsas, K. Chapter 2—Olive Mill Waste: Recent Advances for the Sustainable Development of Olive Oil Industry. In Olive Mill Waste; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 29–56. [Google Scholar]
- Garcia-Garcia, G.; Stone, J.; Rahimifard, S. Opportunities for Waste Valorisation in the Food Industry—A Case Study with Four UK Food Manufacturers. J. Clean. Prod. 2019, 211, 1339–1356. [Google Scholar] [CrossRef]
- Scicutella, F.; Cucu, M.A.; Mannelli, F.; Pastorelli, R.; Daghio, M.; Paoli, P.; Pazzagli, L.; Turini, L.; Mantino, A.; Luti, S.; et al. Rumen microbial community and milk quality in Holstein lactating cows fed olive oil pomace as part in a sustainable feeding strategy. Animal 2023, 17, 100815. [Google Scholar] [CrossRef] [PubMed]
- Albendea, P.; Tres, A.; Rafecas, M.; Vichi, S.; Solà-Oriol, D.; Verdú, M.; Guardiola, F. Effect of feeding olive pomace acid oil on pork lipid composition, oxidative stability, colour, and sensory acceptance. Animal 2023, 17, 100879. [Google Scholar] [CrossRef] [PubMed]
- Verge-Mèrida, G.; Solà-Oriol, D.; Tres, A.; Verdú, M.; Farré, G.; Garcés-Narro, C.; Barroeta, A.C. Olive pomace oil and acid oil as alternative fat sources in growing-finishing broiler chicken diets. Poult. Sci. 2022, 101, 102079. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Deng, Y.; Wang, X.; Cheng, J. Enhanced Extraction of Hydroxytyrosol, Maslinic Acid and Oleanolic Acid from Olive Pomace: Process Parameters, Kinetics and Thermodynamics, and Greenness Assessment. Food Chem. 2019, 276, 662–674. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A Natural Compound with Promising Pharmacological Activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Pastor, R.; Bouzas, C.; Tur, J.A. Beneficial Effects of Dietary Supplementation with Olive Oil, Oleic Acid, or Hydroxytyrosol in Metabolic Syndrome: Systematic Review and Meta-Analysis. Free Radic. Biol. Med. 2021, 172, 372–385. [Google Scholar] [CrossRef]
- Salucci, S.; Burattini, S.; Battistelli, M.; Buontempo, F.; Canonico, B.; Martelli, A.M.; Papa, S.; Falcieri, E. Tyrosol Prevents Apoptosis in Irradiated Keratinocytes. J. Dermatol. Sci. 2015, 80, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Paulo, F.; Santos, L. Inclusion of Hydroxytyrosol in Ethyl Cellulose Microparticles: In Vitro Release Studies under Digestion Conditions. Food Hydrocoll. 2018, 84, 104–116. [Google Scholar] [CrossRef]
- Martins, V.F.R.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Valorisation of Micro/Nanoencapsulated Bioactive Compounds from Plant Sources for Food Applications Towards Sustainability. Foods 2023, 12, 32. [Google Scholar] [CrossRef]
- Miralles, P.; Chisvert, A.; Salvador, A. Determination of Hydroxytyrosol and Tyrosol by Liquid Chromatography for the Quality Control of Cosmetic Products Based on Olive Extracts. J. Pharm. Biomed. Anal. 2014, 102, 157–161. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pawlowski, S.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P.; Velizarov, S. Valorization of Olive Pomace by a Green Integrated Approach Applying Sustainable Extraction and Membrane-Assisted Concentration. Sci. Total Environ. 2019, 652, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cruz, I.; Cara, C.; Romero, I.; Castro, E.; Gullón, B. Valorisation of Exhausted Olive Pomace by an Ecofriendly Solvent Extraction Process of Natural Antioxidants. Antioxidants 2020, 9, 1010. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cruz, I.; Del Mar Contreras, M.; Carvalheiro, F.; Duarte, L.C.; Roseiro, L.B.; Romero, I.; Castro, E. Recovery of Bioactive Compounds from Industrial Exhausted Olive Pomace through Ultrasound-Assisted Extraction. Biology 2021, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.; Karnaouri, A.; Templis, C.C.; Papayannakos, N.; Topakas, E. Valorization of exhausted olive pomace for the production of omega-3 fatty acids by Crypthecodinium cohnii. Waste Manag. 2020, 118, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.B.; Oliveira, A.L.; Costa, C.; Nunes, J.; Vicente, A.A.; Pintado, M. Total and Sustainable Valorisation of Olive Pomace Using a Fractionation Approach. Appl. Sci. 2020, 10, 6785. [Google Scholar] [CrossRef]
- Xu, W.J.; Zhai, J.W.; Cui, Q.; Liu, J.Z.; Luo, M.; Fu, Y.J.; Zu, Y.G. Ultra-Turrax Based Ultrasound-Assisted Extraction of Five Organic Acids from Honeysuckle (Lonicera japonica Thunb.) and Optimization of Extraction Process. Sep. Purif. Technol. 2016, 166, 73–82. [Google Scholar] [CrossRef]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Bibi, A. Fungal Cellulase; Production and Applications: Minireview. LIFE Int. J. Health Life-Sci. 2018, 4, 19–36. [Google Scholar] [CrossRef]
- Guan, X.; Yao, H. Optimization of Viscozyme L-Assisted Extraction of Oat Bran Protein Using Response Surface Methodology. Food Chem. 2008, 106, 345–351. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Zhang, C.; Li, W. Ultrasound-Assisted Enzyme Catalyzed Hydrolysis of Olive Waste and Recovery of Antioxidant Phenolic Compounds. Innov. Food Sci. Emerg. Technol. 2017, 44, 224–234. [Google Scholar] [CrossRef]
- Morais, R.M.S.C.; Morais, A.M.M.B.; Dammak, I.; Bonilla, J.; Sobral, P.J.A.; Laguerre, J.C.; Afonso, M.J.; Ramalhosa, E.C.D. Functional Dehydrated Foods for Health Preservation. J. Food Qual. 2018, 29, 1739636. [Google Scholar] [CrossRef]
- Cravotto, C.; Fabiano-Tixier, A.S.; Claux, O.; Abert-Vian, M.; Tabasso, S.; Cravotto, G.; Chemat, F. Towards Substitution of Hexane as Extraction Solvent of Food Products and Ingredients with No Regrets. Foods 2022, 11, 3412. [Google Scholar] [CrossRef] [PubMed]
- Stramarkou, M.; Missirli, T.V.; Kyriakopoulou, K.; Papadaki, S.; Angelis-Dimakis, A.; Krokida, M. The Recovery of Bioactive Compounds from Olive Pomace Using Green Extraction Processes. Resources 2023, 12, 77. [Google Scholar] [CrossRef]
- Capasso, R.; Evidente, A.; Scognamiglio, F. A Simple Thin Layer Chromatographic Method to Detect the Main Polyphenols Occurring in Olive Oil Vegetation Waters. Phytochem. Anal. 1992, 3, 270–275. [Google Scholar] [CrossRef]
- Miklavčič Višnjevec, A.; Baker, P.; Charlton, A.; Preskett, D.; Peeters, K.; Tavzes, Č.; Kramberger, K.; Schwarzkopf, M. Developing an Olive Biorefinery in Slovenia: Analysis of Phenolic Compounds Found in Olive Mill Pomace and Wastewater. Molecules 2021, 26, 7. [Google Scholar] [CrossRef]
- Niknam, S.M.; Kashaninejad, M.; Escudero, I.; Sanz, M.T.; Beltrán, S.; Benito, J.M. Valorization of Olive Mill Solid Residue through Ultrasound-Assisted Extraction and Phenolics Recovery by Adsorption Process. J. Clean. Prod. 2021, 316, 128340. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; del Mar Contreras, M.; Romero, I.; Castro, E. A biorefinery approach to obtain antioxidants, lignin and sugars from exhausted olive pomace. J. Ind. Eng. Chem. 2021, 96, 356–363. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Coelho, M.; Veiga, M.; Costa, E.M.; Silva, S.; Nunes, J.; Vicente, A.A.; Pintado, M. Are Olive Pomace Powders a Safe Source of Bioactives and Nutrients? J. Sci. Food Agric. 2021, 101, 1963–1978. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005. [Google Scholar]
- Macedo, G.A.; Santana, Á.L.; Crawford, L.M.; Wang, S.C.; Dias, F.F.G.; de Mour Bell, J.M.L.N. Integrated Microwave- and Enzyme-Assisted Extraction of Phenolic Compounds from Olive Pomace. LWT 2021, 138, 110621. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Campos, D.A.; Nunes, C.; Ribeiro, S.; Nunes, J.; Oliveira, A.; Pintado, M. Polyphenol Extraction by Different Techniques for Valorisation of Non-Compliant Portuguese Sweet Cherries towards a Novel Antioxidant Extract. Sustainability 2020, 12, 5556. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Oxidants and Antioxidants Part A. Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marin-Arroyo, M.R. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese Medicinal Plants: Dependence of Final Antioxidant Capacity and Phenol Content on Extraction Features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, A.A.; Oliveira, A.; Ribeiro, T.B.; Ribeiro, S.; Nunes, C.; Gómez-García, R.; Nunes, J.; Pintado, M. Impact of Extraction Process in Non-Compliant ‘Bravo de Esmolfe’ Apples towards the Development of Natural Antioxidant Extracts. Appl. Sci. 2021, 11, 5916. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial Activity of Pomegranate Peel Extracts Performed by High Pressure and Enzymatic Assisted Extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.I.; Melo, A.; Caleja, C.; Pereira, E.; Finimundy, T.C.; Afonso, T.B.; Silva, S.; Ivanov, M.; Soković, M.; Tavaria, F.K.; et al. Evaluation of Antimicrobial and Antioxidant Activities of Alginate Edible Coatings Incorporated with Plant Extracts. Coatings 2023, 13, 1487. [Google Scholar] [CrossRef]



| Extraction Experiment | Yield of Extraction |
|---|---|
| 1—50 °C H2O:EtOH | 0.110 ± 0.02 b |
| 2—20 °C H2O:EtOH | 0.083 ± 0.003 b |
| 3—Ultra Turrax + 50 °C H2O:EtOH | 0.106 ± 0.02 b |
| 4—Ultrasounds + 50 °C H2O:EtOH | 0.107 ± 0.004 b |
| 5—50 °C H2O | 0.153 ± 0.002 b |
| 6—Enzyme (cellulase) + 50 °C H2O | 0.512 ± 0.0006 a |
| 7—Ultrasounds + Enzyme (cellulase) + 50 °C H2O | 0.455 ± 0.09 a |
| 8—Enzyme (viscoenzyme) + 50 °C H2O | 0.493 ± 0.007 a |
| 9—Ultrasounds + Enzyme (viscoenzyme) + 50 °C H2O | 0.492 ± 0.003 a |
| Extraction Method | Advantages | Disadvantages |
|---|---|---|
| Water extraction |
|
|
| Hydroethanolic extraction |
|
|
| Ultrasound extraction |
|
|
| Ultra-Turrax extraction |
|
|
| Enzyme extraction |
|
|
| Standards | Extracts Obtained in the Different Experiments | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Hydroxytyrosol (0.13 a) | 0.12 a | 0.12 a | 0.12 a | 0.12 a | 0.12 a | 0.12 a | 0.12 a | 0.12 a | 0.12 a |
| Tyrosol (0.30 b) | 0.31 b | 0.31 b | 0.31 b | 0.31 b | 0.29 b | 0.29 b | 0.29 b | 0.29 b | 0.29 b |
| Catechol (0.51 c) | 0.51 c | 0.51 c | 0.51 c | 0.51 c | 0 | 0 | 0 | 0 | 0 |
| Compound | LOD | LOQ |
|---|---|---|
| (mg/100 mg extract) | ||
| Hydroxytyrosol | 0.002 | 0.005 |
| Tyrosol | 0.008 | 0.025 |
| Catechol | 0.007 | 0.021 |
| Extraction Experiment | Hydroxytyrosol (mg/100 mg Extract) | Hydroxytyrosol (mg/g DW Olive Oil Pomace) | Tyrosol (mg/100 mg Extract) | Tyrosol (mg/g DW Olive Oil Pomace) | Catechol (mg/100 Mgextract) | Catechol (mg/g DW Olive Oilpomace) | Total Phenolic Content (mg/100 mg Extract) | Total Phenolic Content (mg/g DW Olive Oil Pomace) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.725 ± 0.115 b | 1.904 ± 0.127 e | 0.69 ± 0.052 b | 0.761 ± 0.057 d,e | 0.084 ± 0.006 c | 0.093 ± 0.006 e,f | 2.499 ± 0.162 c | 2.758 ± 0.179 e |
| 2 | 1.609 ± 0.103 b | 1.329 ± 0.085 f | 0.737 ± 0.095 b | 0.609 ± 0.078 e | 0.083 ± 0.009 c | 0.069 ± 0.007 f | 2.876 ± 0.092 b | 3.050 ± 0.097 d,e |
| 3 | 2.003 ± 0.087 a | 2.124 ± 0.091 d,e | 0.778 ± 0.046 b | 0.825 ± 0.048 c,d | 0.095 ± 0.012 b,c | 0.101 ± 0.013 d,e | 2.429 ± 0.176 c | 2.007 ± 0.146 f |
| 4 | 2.021 ± 0.287 a | 2.168 ± 0.307 c,d,e | 0.987 ± 0.094 a | 1.058 ± 0.101 b,c | 0.121 ± 0.005 a | 0.13 ± 0.005 a,b,c | 3.129 ± 0.341 a | 3.356 ± 0.366 b,c,d |
| 5 | 1.596 ± 0.135 b | 2.448 ± 0.207 a,b,c | 0.731 ± 0.066 b | 1.121 ± 0.101 a,b | 0.098 ± 0.015 b | 0.15 ± 0.023 a,b,c | 2.425 ± 0.209 c | 3.719 ± 0.320 a,b,c |
| 6 | 0.49 ± 0.080 c | 2.23 ± 0.368 b,c,d | 0.211 ± 0.047 c | 0.96 ± 0.211 b,c | 0.028 ± 0.004 d | 0.126 ± 0.017 b,c,d | 0.729 ± 0.129 d | 3.316 ± 0.588 a,b |
| 7 | 0.496 ± 0.028 c | 2.539 ± 0.143 a,b | 0.223 ± 0.026 c | 1.14 ± 0.131 a,b | 0.029 ± 0.003 d | 0.147 ± 0.015 a,b,c | 0.748 ± 0.054 d | 3.826 ± 0.276 a,b |
| 8 | 0.541 ± 0.031 c | 2.669 ± 0.151 a | 0.257 ± 0.031 c | 1.27 ± 0.156 a | 0.032 ± 0.005 d | 0.159 ± 0.026 a | 0.830 ± 0.063 d | 4.098 ± 0.304 a |
| 9 | 0.506 ± 0.043 c | 2.491 ± 0.212 a,b | 0.218 ± 0.028 c | 1.075 ± 0.138 b | 0.024 ± 0.007 d | 0.118 ± 0.036 c,d,e | 0.748 ± 0.064 d | 3.684 ± 0.313 a,b,c |
| Extraction Experiment | TPC (mg Gallic Acid Equivalent/100 mg Dried Extract) | ABTS | DPPH | ORAC |
|---|---|---|---|---|
| (µmol Trolox Equivalent/100 mg Dried Extract) | ||||
| 1 | 8.116 ± 0.465 c | 58.421 ± 3.095 b | 30.458 ± 2.489 b | 187.625 ± 17.707 b,c |
| 2 | 6.813 ± 0.347 d | 37.415 ± 6.986 c | 25.408 ± 1.776 c | 170.896 ± 14.017 c |
| 3 | 8.743 ± 0.533 b | 57.152 ± 4.079 b | 30.873 ± 1.322 b | 205.297 ± 4.122 a,b |
| 4 | 7.666 ± 0.245 c | 63.528 ± 0.34 a,b | 25.099 ± 2.161 c | 201.182 ± 8.79 a,b |
| 5 | 10.159 ± 0.741 a | 69.155 ± 7.703 a | 38.121 ± 1.614 a | 215.522 ± 18.908 a |
| 6 | 3.057 ± 0.141 f | 18.760 ± 3.381 d | 10.601 ± 0.968 d | 69.336 ± 4.963 d |
| 7 | 3.658 ± 0.245 e,f | 18.665 ± 3.194 d | 9.873 ± 0.847 d | 66.555 ± 4.407 d |
| 8 | 3.73 ± 0.342 e | 24.103 ± 1.497 d | 10.439 ± 0.36 d | 63.979 ± 4835 d |
| 9 | 3.543 ± 0.293 e,f | 24.102 ± 1.497 d | 11.201 ± 0.755 d | 71.459 ± 6.156 d |
| Bacteria | Inhibition (%) by Extracts from the Ultrasounds-Assisted Hydroalcoholic Extraction (Experiment 4) | ||
|---|---|---|---|
| 1% | 2% | 3% | |
| Escherichia coli | 0.00 d | 7.65 ± 2.30 b | 5.20 ± 1.92 c |
| Yersinia enterocolitica | 0.72 ± 1.04 d | 7.93 ± 0.96 b | 10.98 ± 1.14 c |
| Salmonela enterica serovar enteriditis | 8.20 ± 0.54 c | 8.35 ± 0.71 b | 10.45 ± 0.59 c |
| Staphylococcus aureus | 2.78 ± 3.96 d | 18.68 ± 5.62 b | 42.67 ± 16.03 b |
| Bacillus cereus | 14.78 ± 1.19 b | 37.52 ± 16.5 a | 67.21 ± 1.97 a |
| Listeria monocytogenes | 20.79 ± 2.92 a | 36.45 ± 2.7 a | 59.83 ± 5.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, V.F.R.; Ribeiro, T.B.; Lopes, A.I.; Pintado, M.E.; Morais, R.M.S.C.; Morais, A.M.M.B. Comparison among Different Green Extraction Methods of Polyphenolic Compounds from Exhausted Olive Oil Pomace and the Bioactivity of the Extracts. Molecules 2024, 29, 1935. https://doi.org/10.3390/molecules29091935
Martins VFR, Ribeiro TB, Lopes AI, Pintado ME, Morais RMSC, Morais AMMB. Comparison among Different Green Extraction Methods of Polyphenolic Compounds from Exhausted Olive Oil Pomace and the Bioactivity of the Extracts. Molecules. 2024; 29(9):1935. https://doi.org/10.3390/molecules29091935
Chicago/Turabian StyleMartins, Valter F. R., Tânia B. Ribeiro, Ana I. Lopes, Manuela E. Pintado, Rui M. S. C. Morais, and Alcina M. M. B. Morais. 2024. "Comparison among Different Green Extraction Methods of Polyphenolic Compounds from Exhausted Olive Oil Pomace and the Bioactivity of the Extracts" Molecules 29, no. 9: 1935. https://doi.org/10.3390/molecules29091935
APA StyleMartins, V. F. R., Ribeiro, T. B., Lopes, A. I., Pintado, M. E., Morais, R. M. S. C., & Morais, A. M. M. B. (2024). Comparison among Different Green Extraction Methods of Polyphenolic Compounds from Exhausted Olive Oil Pomace and the Bioactivity of the Extracts. Molecules, 29(9), 1935. https://doi.org/10.3390/molecules29091935