The Antisolvent Precipitation of CuZnOx Mixed Oxide Materials Using a Choline Chloride-Urea Deep Eutectic Solvent
Abstract
1. Introduction
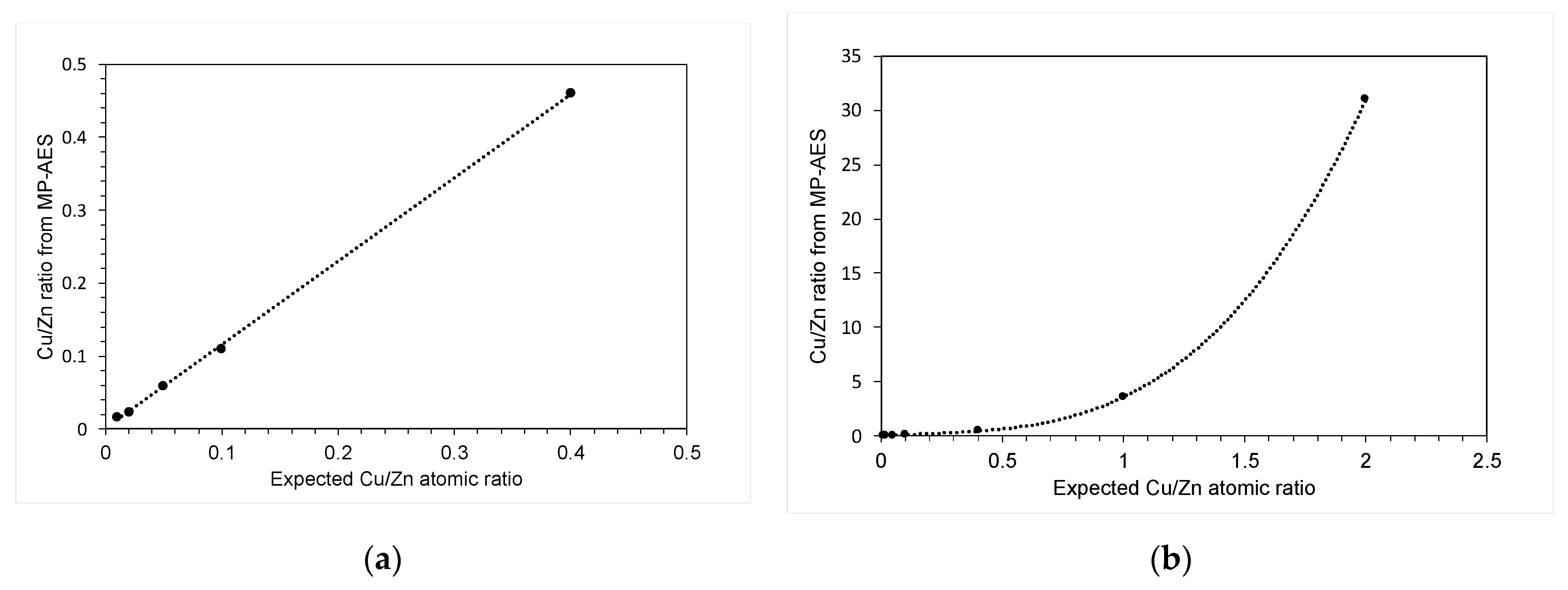
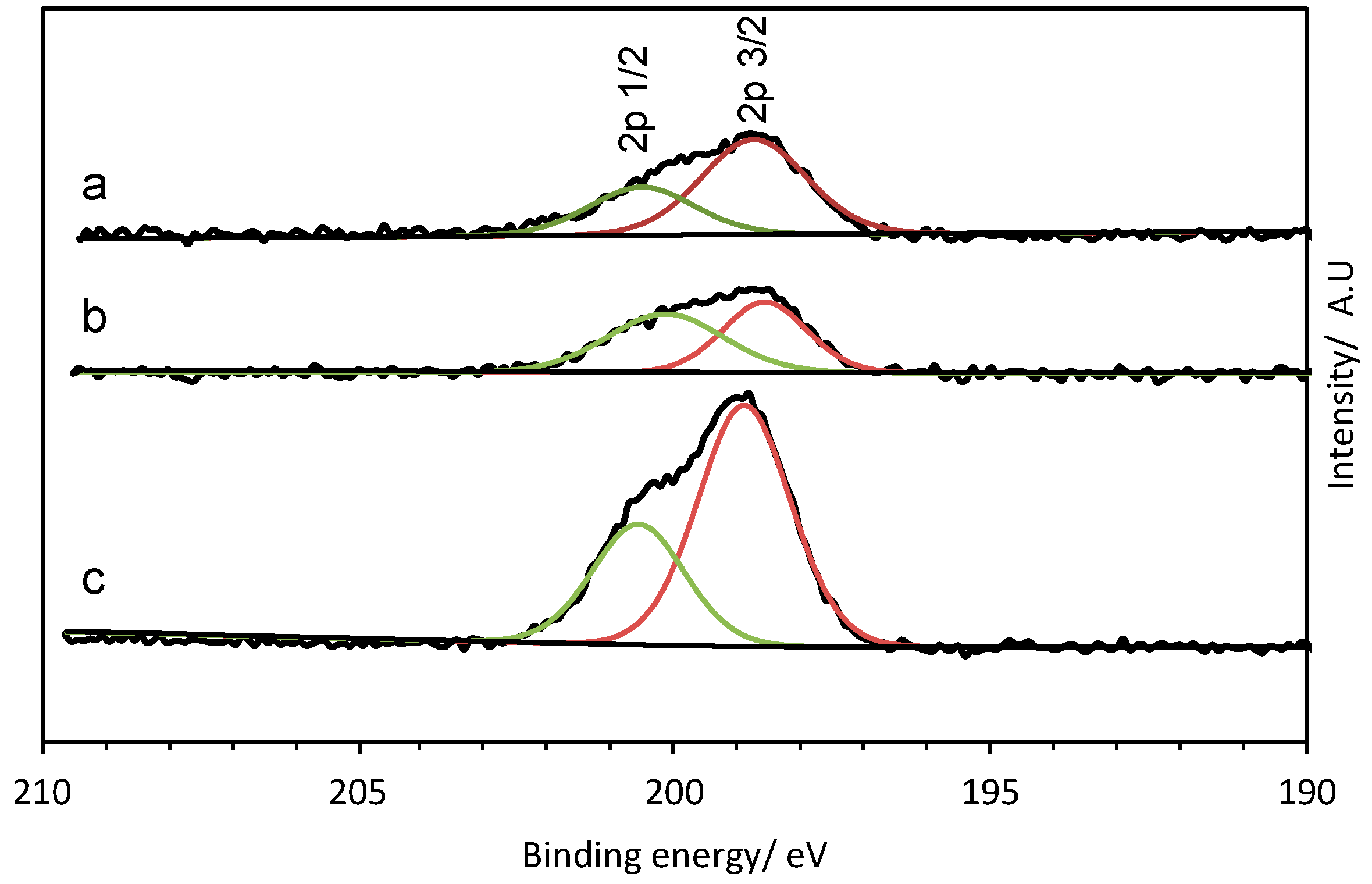
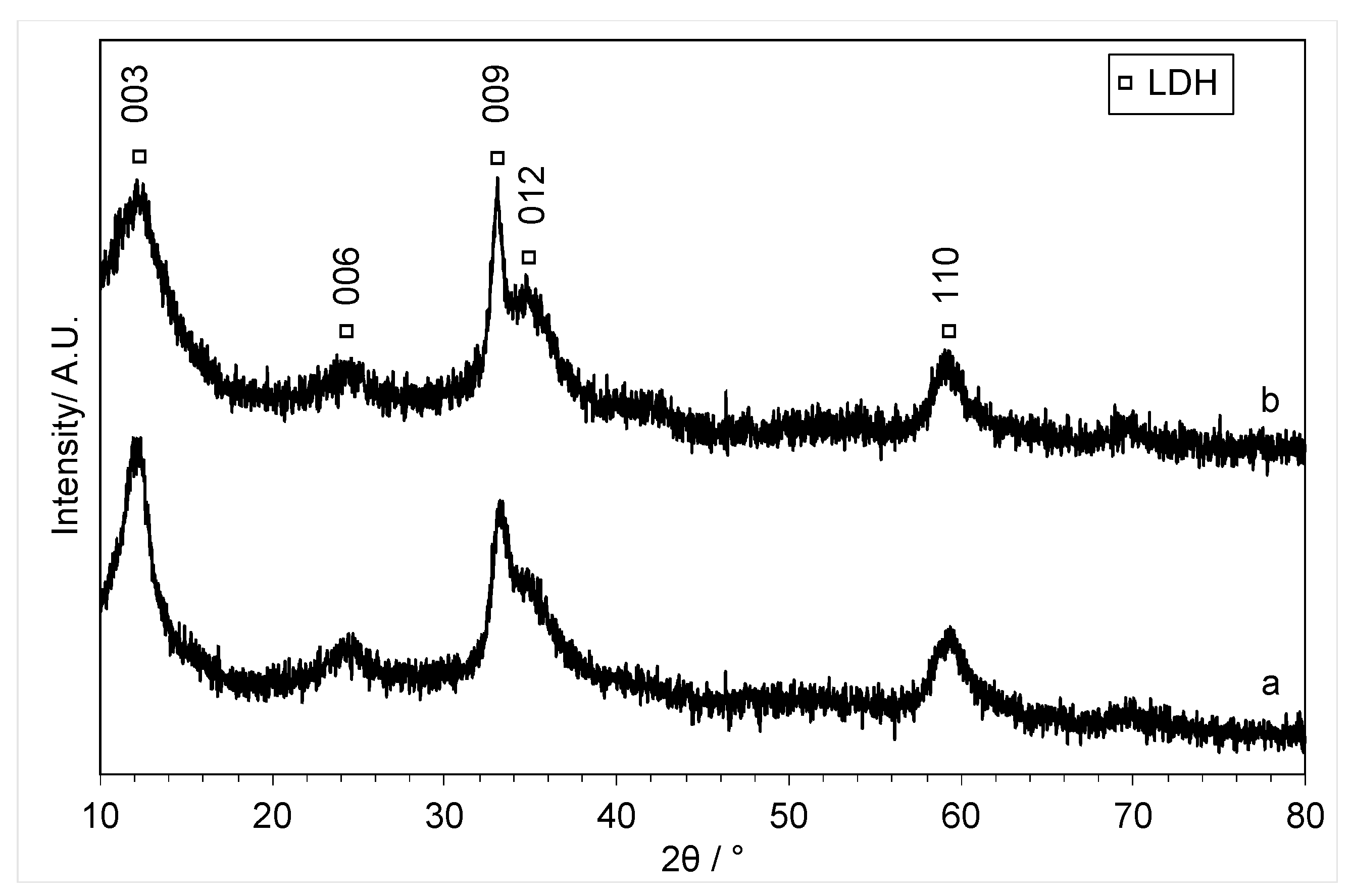
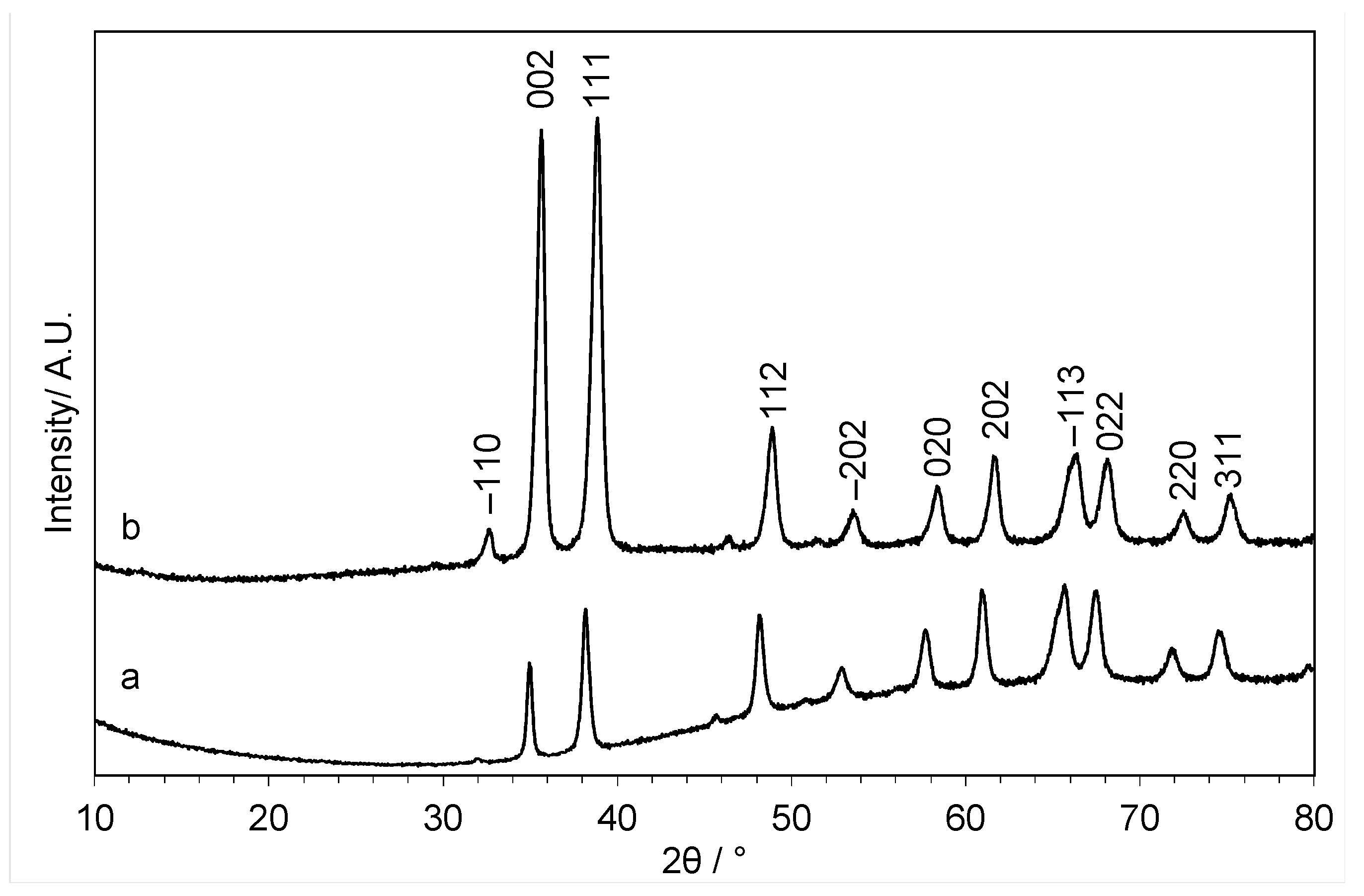
2. Results and Discussion
3. Materials and Methods
3.1. Choline Chloride-Urea Deep Eutectic Solvent Synthesis
3.2. Copper Zinc Oxide Synthesis Using Choline Chloride-Urea Deep Eutectic Solvent
3.2.1. Method One
Fast Mixing
Slow Mixing
3.2.2. Method Two
3.3. Material Characterisation
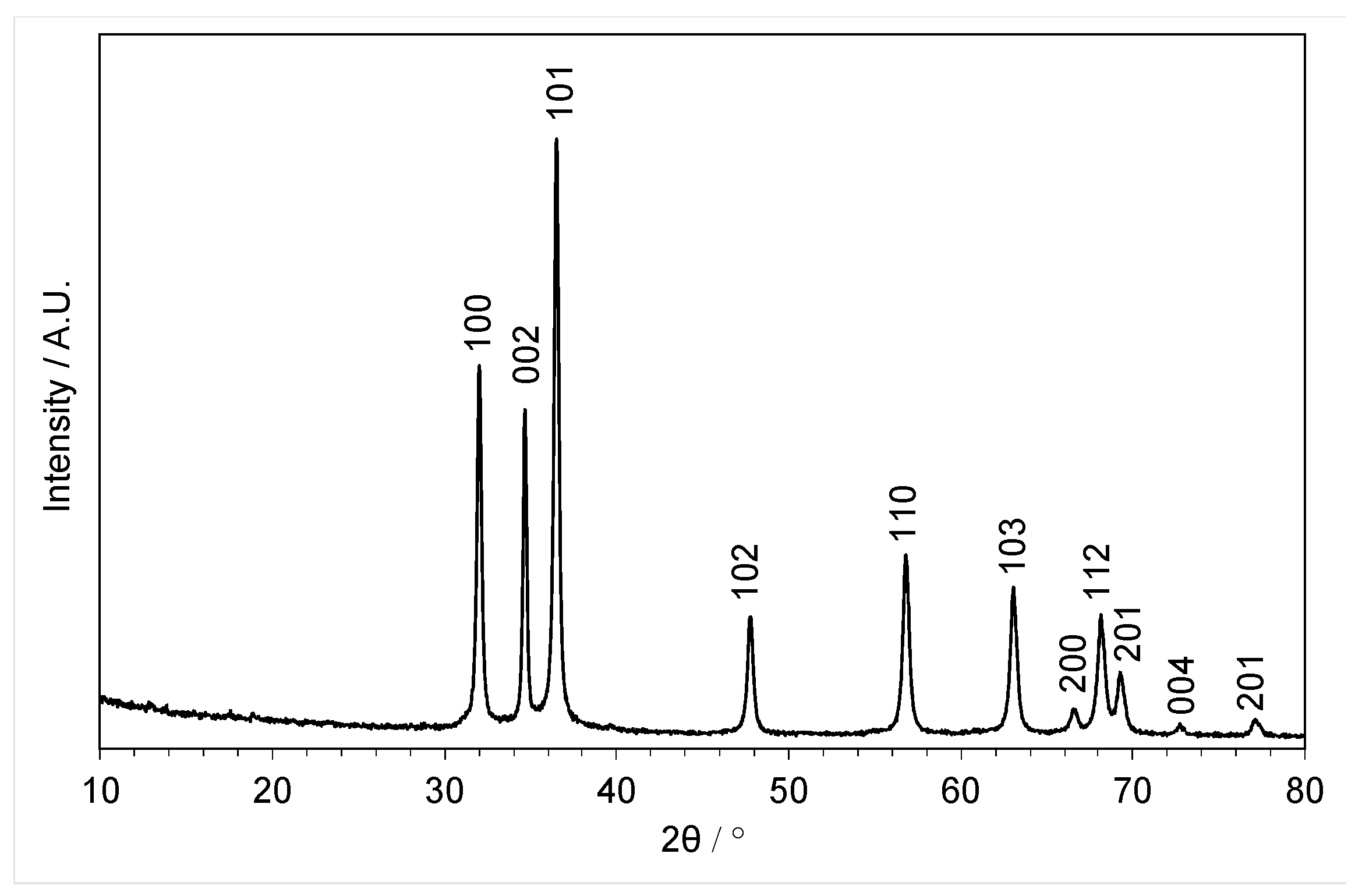
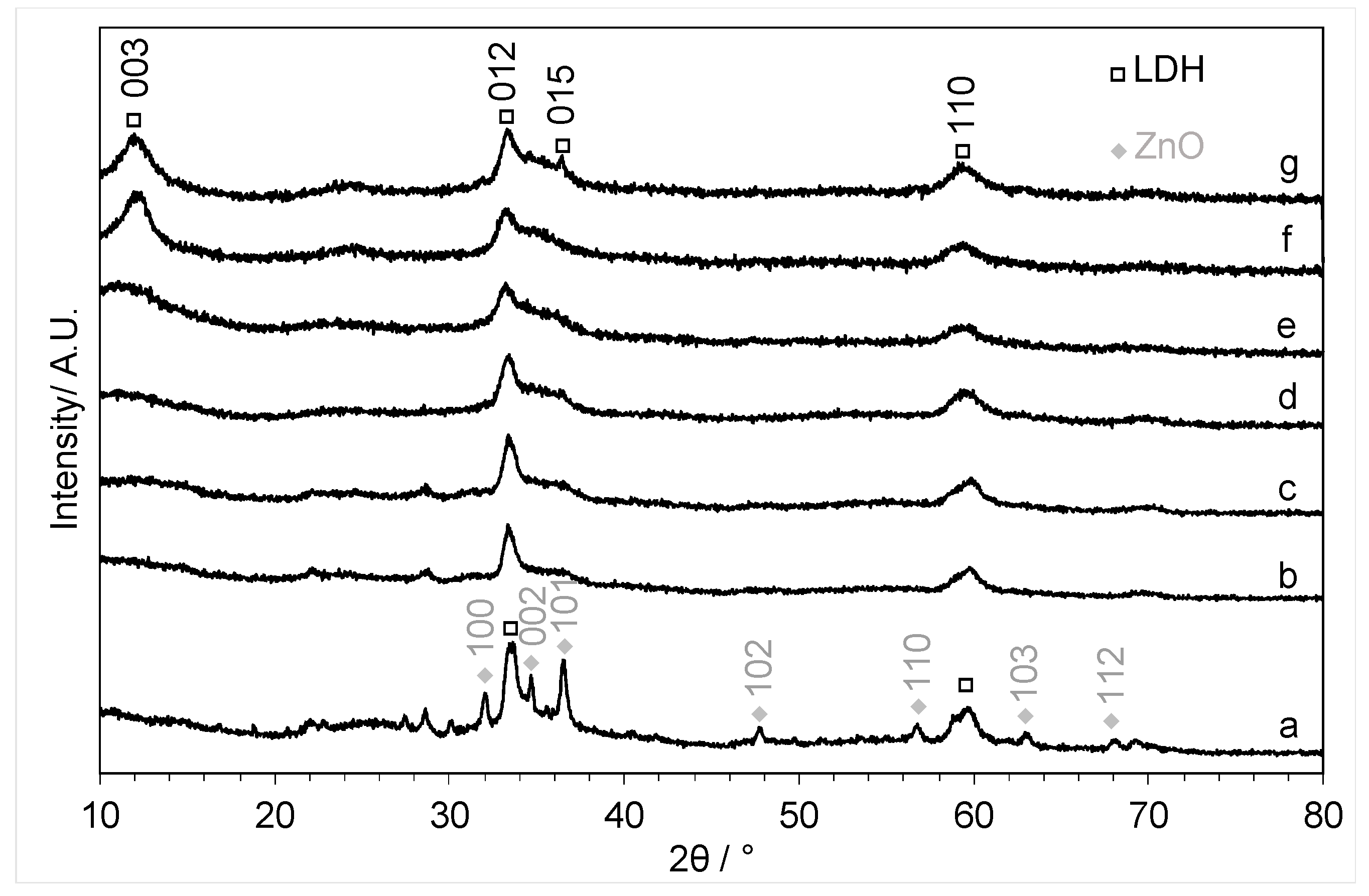
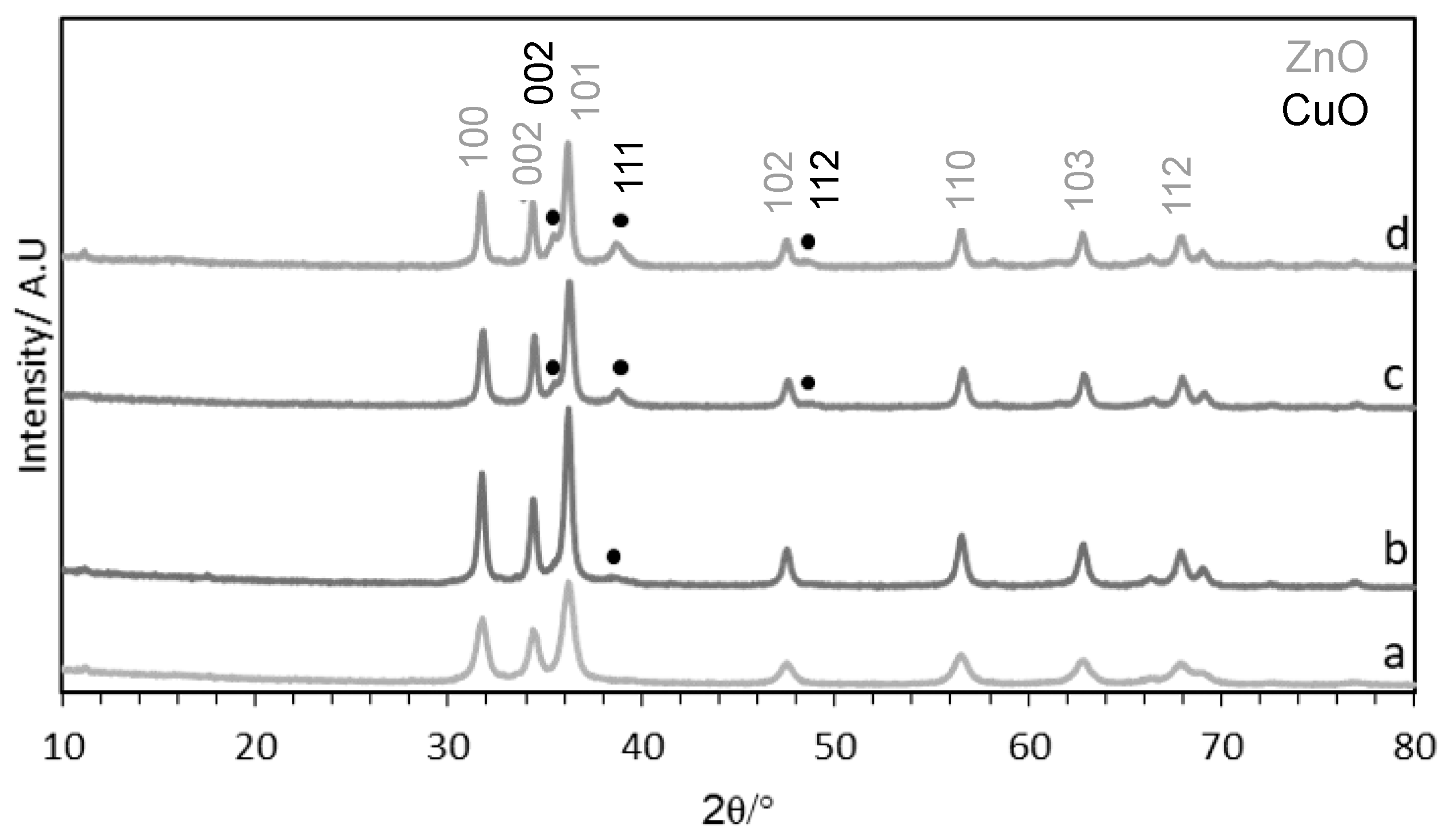
3.3.1. Powder X-ray Diffraction
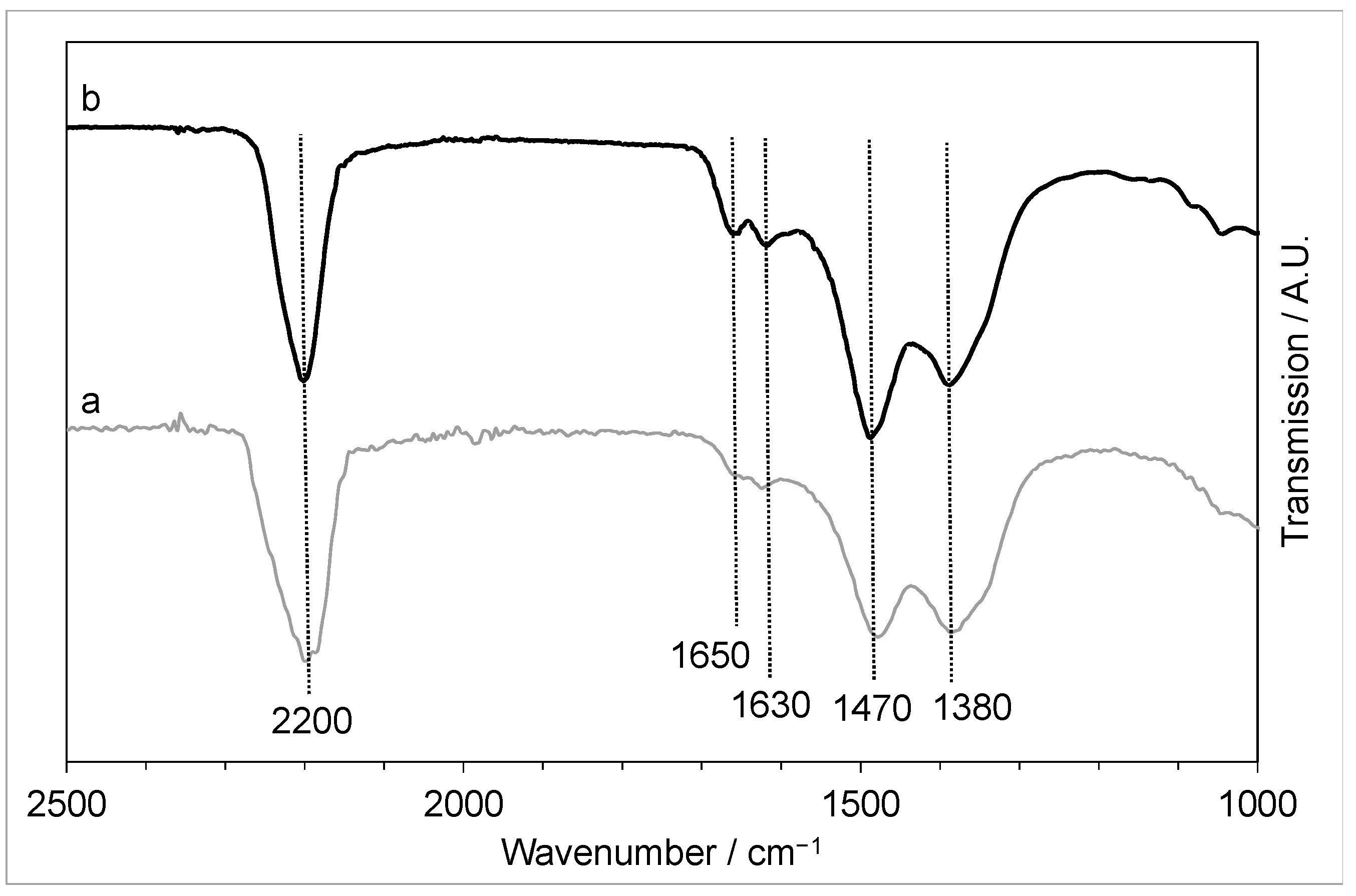
3.3.2. FTIR
3.3.3. Microwave Plasma Atomic Emission Spectroscopy
3.3.4. X-ray Photoelectron Spectroscopy
3.3.5. Surface Area Determination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joshi, K.; Rawat, M.; Gautam, S.K.; Singh, R.G.; Ramola, R.C.; Singh, F. Band gap widening and narrowing in Cu-doped ZnO thin films. J. Alloys Compd. 2016, 680, 252–258. [Google Scholar] [CrossRef]
- Tarwal, N.L.; Gurav, K.V.; Mujawar, S.H.; Sadale, S.B.; Nam, K.W.; Bae, W.R.; Moholkar, A.V.; Kim, J.H.; Patil, P.S.; Jang, J.H. Photoluminescence and photoelectrochemical properties of the spray deposited copper doped zinc oxide thin films. Ceram. Int. 2014, 40, 7669–7677. [Google Scholar] [CrossRef]
- Wang, F.; Lin, W.; Wang, L.-Z.; Ge, Y.-M.; Zhang, X.-T.; Lin, H.-R.; Huang, W.W.; Huang, J.-Q.; Cao, W. Magnetic properties of the Cu-doped ZnO: Experiments and theory. Acta. Phys. Sin. Chin. Ed. 2014, 63, 157502. [Google Scholar] [CrossRef]
- Li, X.-L.; Xu, X.-H.; Quan, Z.-Y.; Guo, J.-F.; Wu, H.-S.; Gehring, G.A. Role of donor defects in enhancing ferromagnetism of Cu-doped ZnO films. J. Appl. Phys. 2009, 105, 103914. [Google Scholar] [CrossRef]
- Jiang, G.; Li, X.; Che, Y.; Lv, Y.; Liu, F.; Wang, Y.; Wang, X. Antibacterial and anticorrosive properties of CuZnO@RGO waterborne polyurethane coating in circulating cooling water. Environ. Sci. Pollut. Res. 2019, 26, 9027–9040. [Google Scholar] [CrossRef] [PubMed]
- Abinaya, C.; Mayandi, J.; Osborne, J.; Frost, M.; Ekstrum, C.; Pearce, J.M. Inhibition of growth of S. epidermidis by hydrothermally synthesized ZnO nanoplates. Mater. Res. Express 2017, 4, 075401. [Google Scholar] [CrossRef]
- Mirmohseni, A.; Rastgar, M.; Olad, A. Preparation of PANI–CuZnO ternary nanocomposite and investigation of its effects on polyurethane coatings antibacterial, antistatic, and mechanical properties. J. Nanostruct. Chem. 2018, 8, 473–481. [Google Scholar] [CrossRef]
- Gigi, S.; Naor, T.; Waiskopf, N.; D Stone, D.; Natan, M.; Jacobi, G.; Levi, A.; Remennik, S. Photoactive Antimicrobial CuZnO Nanocrystals. J. Phys. Chem. C 2022, 126, 18683–18691. [Google Scholar] [CrossRef]
- Ali, H.T.; Mongi Amami, M.; Rehman, U.; Mahmood, K.; Yusuf, M.; Ikram, S.; Ali, A.; Amin, N.; Javaid, K.; Arshad, M.I. Investigating the thermoelectric power generation performance of ZnCuO: A p-type mixed-metal oxide system. J. Phys. Chem. Solid 2022, 163, 110535. [Google Scholar] [CrossRef]
- Zeraatkish, Y.; Jafarian, M.; Gobal, F.; Mahjani, M.G. Synthesis and elucidation of electrochemical characteristics of nanorods, microsized and nanosized CuO as cathode materials for Zn/CuO alkaline battery. J. Solid State Electrochem. 2015, 19, 2155–2165. [Google Scholar] [CrossRef]
- Smith, P.J.; Kondrat, S.A.; Carter, J.H.; Chater, P.A.; Bartley, J.K.; Taylor, S.H.; Spencer, M.S.; Hutchings, G.J. Supercritical antisolvent precipitation of amorphous copper-zinc georgeite and acetate precursors for the preparation of ambient-pressure water-gas-shift copper/zinc oxide catalysts. ChemCatChem 2017, 9, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Kondrat, S.A.; Smith, P.J.; Wells, P.P.; Chater, P.A.; Davies, T.E.; Lu, L.; Bartley, J.K.; Taylor, S.H.; Spencer, M.S.; Kiely, C.J.; et al. Stable amorphous georgeite as a precursor to a high-activity catalyst. Nature 2016, 531, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, P.; Antoniak-Jurak, K.; Prochniak, W.; Wiercioch, P.; Konkol, M.; Bicki, R.; Michalska, K.; Walczak, M. The Evaluation of Synthesis Route Impact on Structure, Morphology and LT-WGS Activity of Cu/ZnO/Al2O3 catalysts. Catal. Lett. 2017, 147, 1422–1433. [Google Scholar] [CrossRef]
- Dang, S.S.; Yang, H.Y.; Gao, P.; Wang, H.; Li, X.P.; Wei, W.; Sun, Y.H. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation. Catal. Today 2019, 330, 61–75. [Google Scholar] [CrossRef]
- Brzezińska, M.; Keller, N.; Ruppert, A.M. Self-tuned properties of CuZnO catalysts for hydroxymethylfurfural hydrodeoxygenation towards dimethylfuran production. Catal. Sci. Technol. 2020, 10, 658–670. [Google Scholar] [CrossRef]
- Küksal, A.; Klemm, E.; Emig, G. Reaction kinetics of the liquid-phase hydrogenation of succinic anhydride on CuZnO-catalysts with varying copper-to-zinc ratios in a three-phase slurry reactor. Appl. Catal. A 2002, 228, 237–251. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Memon, R.; Gado, L.F.; Khandaker, M.U.; Almukhlifi, H.A.; Modafer, Y.; Bashir, N.; Abida, O.; Alshammari, F.A.; et al. Structural, Optical, and Renewable Energy-Assisted Photocatalytic Dye Degradation Studies of ZnO, CuZnO, and CoZnO Nanostructures for Wastewater Treatment. Separations 2023, 10, 184. [Google Scholar] [CrossRef]
- He, S.; Hou, P.; Petropoulos, E.; Feng, Y. High Efficient Visible-Light Photocatalytic Performance of Cu/ZnO/rGO Nanocomposite for Decomposing of Aqueous Ammonia and Treatment of Domestic Wastewater. Front. Chem. 2018, 6, 219. [Google Scholar] [CrossRef] [PubMed]
- Prieto, G.; de Jong, K.P.; de Jongh, P.E. Towards ‘greener’ catalyst manufacture: Reduction of wastewater from the preparation of Cu/ZnO/Al2O3 methanol synthesis catalysts. Catal. Today 2013, 215, 142–151. [Google Scholar] [CrossRef]
- Cho, C.R.; Hwang, J.Y.; Kim, J.P.; Jeong, S.Y.; Jang, M.S.; Lee, W.J.; Kim, D.H. Ferromagnetism of heteroepitaxial Zn1-xCuxO films grown on n-GaN substrates. Jpn. J. Appl. Phys. 2004, 43, L1383. [Google Scholar] [CrossRef]
- Chen, L.-C.; Hsieh, C.-A.; Zhang, X. Electrical Properties of CZO Films Prepared by Ultrasonic Spray Pyrolysis. Materials 2014, 7, 7304–7313. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Wang, H.; Liang, F.; Shao, L. Reversible switching of ferromagnetism in ZnCuO nanorods by electric field. Appl. Phys. Lett. 2015, 106, 142402. [Google Scholar] [CrossRef]
- Wallace, W.T.; Hayward, J.S.; Ho, C.-Y.; Marsh, A.R.; Tariq, A.; Bartley, J.K. Triethylamine–Water as a Switchable Solvent for the Synthesis of Cu/ZnO Catalysts for Carbon Dioxide Hydrogenation to Methanol. Top. Catal. 2021, 64, 984–991. [Google Scholar] [CrossRef]
- Hartley, J.M.; Ip, C.M.; Forrest, G.C.H.; Singh, K.; Gurman, S.J.; Ryder, K.S.; Abbott, A.P.; Frisch, G. EXAFS Study into the Speciation of Metal Salts Dissolved in Ionic Liquids and Deep Eutectic Solvents. Inorg. Chem. 2014, 53, 6280–6288. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Harris, R.C.; Holyoak, F.; Frisch, G.; Hartley, J.; Jenkin, G.R.T. Electrocatalytic recovery of elements from complex mixtures using deep eutectic solvents. Green Chem. 2015, 17, 2172–2179. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Comm. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Abo-Hamad, A.; Hayyan, M.; AlSaadi, M.A.; Hashim, M.A. Potential applications of deep eutectic solvents in nanotechnology. Chem. Eng. J. 2015, 273, 551–567. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; McKenzie, K.J.; Obi, S.U. Solubility of Metal Oxides in Deep Eutectic Solvents Based on Choline Chloride. J. Chem. Eng. Data 2006, 51, 1280–1282. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Shikotra, P. Selective Extraction of Metals from Mixed Oxide Matrixes Using Choline-Based Ionic Liquids. Inorg. Chem. 2005, 44, 6497–6499. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Hsu, Y.J.; Wong, D.S.H.; Lu, S.Y. Growth of ZnO Nanostructures with Controllable Morphology Using a Facile Green Antisolvent Method. J. Phys. Chem. C 2010, 114, 8867–8887. [Google Scholar] [CrossRef]
- Dong, J.Y.; Lin, W.H.; Hsu, Y.J.; Wong, D.S.H.; Lu, S.Y. Ultrafast formation of ZnO mesocrystals with excellent photocatalytic activities by a facile Tris-assisted antisolvent process. Crystengcomm 2011, 13, 6218–6222. [Google Scholar] [CrossRef]
- Velazquez-Herrera, F.D.; Fetter, G.; Rosato, V.; Pereyra, A.M.; Basaldella, E.I. Effect of structure, morphology and chemical composition of Zn-Al, Mg/Zn-Al and Cu/Zn-Al hydrotalcites on their antifungal activity against A. niger. J. Environ. Chem. Eng. 2018, 6, 3376–3383. [Google Scholar] [CrossRef]
- Li, D.L.; Cai, Y.B.; Ding, Y.Y.; Li, R.L.; Lu, M.M.; Jiang, L.L. Layered double hydroxides as precursors of Cu catalysts for hydrogen production by water-gas shift reaction. Int. J. Hydrogen Energy 2015, 40, 10016–10025. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.D.; Wang, X.L.; Tu, J.P. Spinel type CoFe oxide porous nanosheets as magnetic adsorbents with fast removal ability and facile separation. J. Colloid Interface Sci. 2015, 454, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yao, X.Q.; Geng, Y.R.; Zhou, Q.; Lu, X.M.; Zhang, S.J. Deep eutectic solvents as highly active catalysts for the fast and mild glycolysis of poly(ethylene terephthalate) (PET). Green Chem. 2015, 17, 2473–2479. [Google Scholar] [CrossRef]
- Vasquez, R.P. CuCl by XPS. Surf. Sci. Spectra 1993, 2, 138–143. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Bartley, J.K.; Webster, J.M.; Lopez-Sanchez, J.A.; Gilbert, D.J.; Kiely, C.J.; Carley, A.F.; Howdle, S.M.; Sajip, S.; Calderelli, S.; et al. Amorphous vanadium phosphate catalyst from supercritical antisolvent precipitation. J. Catal. 2001, 197, 232–235. [Google Scholar] [CrossRef]
- Miedziak, P.J.; Tang, Z.; Davies, T.E.; Enache, D.I.; Bartley, J.K.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Taylor, S.H.; Hutchings, G.J. Ceria prepared using supercritical antisolvent precipitation: A green support for gold-palladium nanoparticles for the selective catalytic oxidation of alcohols. J. Mat. Chem. 2009, 19, 8619–8627. [Google Scholar] [CrossRef]
- Marin, R.P.; Kondrat, S.A.; Davies, T.E.; Morgan, D.J.; Enache, D.I.; Coombes, G.B.; Taylor, S.H.; Bartley, J.K.; Hutchings, G.J. Novel cobalt zinc oxide Fischer-Tropsch catalysts synthesised using supercritical anti-solvent precipitation. Catal. Sci. Technol. 2014, 4, 1970–1978. [Google Scholar] [CrossRef][Green Version]
- Marin, R.P.; Kondrat, S.A.; Pinnell, R.K.; Davies, T.E.; Golunski, S.; Bartley, J.K.; Hutchings, G.J.; Taylor, S.H. Green preparation of transition metal oxide catalysts using supercritical CO2 anti-solvent precipitation for the total oxidation of propane. Appl. Catal. B 2013, 140–141, 671–679. [Google Scholar] [CrossRef]
- Phoohinkong, W.; Foophow, T.; Pecharapa, W. Synthesis and characterization of copper zinc oxide nanoparticles obtained via metathesis process. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 035003. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallace, W.T.; Hayward, J.S.; Marsh, A.R.; Bartley, J.K. The Antisolvent Precipitation of CuZnOx Mixed Oxide Materials Using a Choline Chloride-Urea Deep Eutectic Solvent. Molecules 2024, 29, 3357. https://doi.org/10.3390/molecules29143357
Wallace WT, Hayward JS, Marsh AR, Bartley JK. The Antisolvent Precipitation of CuZnOx Mixed Oxide Materials Using a Choline Chloride-Urea Deep Eutectic Solvent. Molecules. 2024; 29(14):3357. https://doi.org/10.3390/molecules29143357
Chicago/Turabian StyleWallace, William T., James S. Hayward, Amy R. Marsh, and Jonathan K. Bartley. 2024. "The Antisolvent Precipitation of CuZnOx Mixed Oxide Materials Using a Choline Chloride-Urea Deep Eutectic Solvent" Molecules 29, no. 14: 3357. https://doi.org/10.3390/molecules29143357
APA StyleWallace, W. T., Hayward, J. S., Marsh, A. R., & Bartley, J. K. (2024). The Antisolvent Precipitation of CuZnOx Mixed Oxide Materials Using a Choline Chloride-Urea Deep Eutectic Solvent. Molecules, 29(14), 3357. https://doi.org/10.3390/molecules29143357





