Abstract
Among the challenges related to rechargeable magnesium batteries (RMBs) still not resolved are positive electrode materials with sufficient charge storage and rate capability as well as stability and raw material resources. Out of the materials proposed and studied so far, vanadium oxides stand out for these requirements, but significant further improvements are expected and required. They will be based on new materials and an improved understanding of their mode of operation. This report provides a critical review focused on this material, which is embedded in a brief overview on the general subject. It starts with the main strategic ways to design layered vanadium oxides cathodes for RMBs. Taking these examples in more detail, the typical issues and challenges often missed in broader overviews and reviews are discussed. In particular, issues related to the electrochemistry of intercalation processes in layered vanadium oxides; advantageous strategies for the development of vanadium oxide composite cathodes; their mechanism in aqueous, “wet”, and dry non-aqueous aprotic systems; and the possibility of co-intercalation processes involving protons and magnesium ions are considered. The perspectives for future development of vanadium oxide-based cathode materials are finally discussed and summarized.
1. Introduction
Magnesium ion batteries are among the promising power sources that can provide high energy density combined with increased environmental and operational safety at lower device costs mostly because of the high magnesium content in the Earth’s crust [1,2,3,4,5,6]. The Mg/Mg2+ system provides metallic anodes offering a high theoretical specific capacity (3834 mAh·cm−3), which is higher than that of metallic lithium (2061 mAh·cm−3). It also provides an attractively low electrode potential (−2.37 V) and forms metallic deposits without dendrites [1,4,5,6].
Due to these advantages, rechargeable magnesium ion batteries have attracted considerable and growing research interest in the field of post-lithium secondary batteries [7]. For comparison, Table 1 collects pertinent data on relevant negative electrode materials.
Systems with both non-aqueous and aqueous electrolytes are considered, in addition to the extended window of electrochemical stability available with water-in-salt electrolytes [8] has been discussed and extended into a quasi-solid state concept [9]. A first pouch cell demonstration has been reported [10].
Challenges for developing a practical magnesium full cell are related to all components, including in particular Mg2+-containing electrolytes that would remain electrochemically stable with both a Mg anode and cathodes. Nonaqueous rechargeable magnesium batteries also suffer from the complicated and moisture-sensitive electrolyte chemistry at magnesium electrode. Practical realization of a RMB is, in particular, handicapped by the absence of high-performance electrode materials due to the intrinsically slow Mg2+-ion diffusion in solids.

Table 1.
Selected data of relevant negative electrode materials for metal ion batteries [11].
Table 1.
Selected data of relevant negative electrode materials for metal ion batteries [11].
| Element | Atomic Mass | E0, SHE/V | Gravimetric Capacity/mAh·g−1 | Volumetric Capacity/mAh·cm−3 |
|---|---|---|---|---|
| Li | 6.94 | −3.040 | 3860 | 2061 |
| Na | 23.0 | −2.713 | 1165 | 1129 |
| K | 39.1 | −2.924 | 685 | 610 |
| Mg | 24.31 | −2.356 | 2206 | 3834 |
| Ca | 40.08 | −2.840 | 1337 | 2072 |
| Zn | 65.41 | −0.763 | 820 | 5855 |
| Al | 26.98 | −1.676 | 2980 | 8046 |
Critical characteristics, such as specific capacity, rate capability, and cycling stability of RMBs, are strongly affected by the intrinsic electrochemical properties of the cathode, i.e., the positive electrode (following an earlier recommendation by Huggins [12,13]), and, in particular, the materials they are made of. There is continuous progress in the improvements of their properties.
Several earlier reviews [14,15,16,17] as well as selective overviews [10,18,19,20,21,22,23] on RMBs are available. They address cathode-related issues like electrode reaction kinetics particularly relevant for rate capability and mass utilization [24] and detrimental effects of particular properties attributed to Mg2+-ions [25], highlight potential advantages of layered compounds [26], and provide mostly brief considerations and discussions of aspects concerning different intercalation-type cathode materials (like transition metal oxides, phosphates, chalcogenides, and Prussian blue analogues), but they are usually very short on considerations of their properties and mostly without an in-depth analysis of the reasons for the differences in functional properties (like specific capacity and power) and mechanism of intercalation processes regarding the latter class of materials. A focal issue is the high charge density of the magnesium ion with associated problems of strong interactions with a host material used in the positive electrode and associated slow diffusion. This also affects interfacial processes at the electrode/solution interface, and possibly even the electrolyte solution.
The number of investigations on different cathode materials for RMBs is rapidly increasing every year. Several reviews are published each year summarizing experimental progress on various cathode materials suitable for RMBs (transition metal oxides, sulfides, selenides, and other layered materials) [10,19,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. However, given the large number of material classes and research areas, they are discussed too superficially, without more detailed consideration of the existing challenges.
Some more specialized reviews discuss and analyze reaction mechanisms in cathodes structure–kinetics correlations, strategies for improving the electrode kinetics [24], the role of the electrolyte composition in the intercalation processes of Mg2+-ions [41], cathode materials and electrolytes for aqueous magnesium batteries [42], nanostructured cathode materials [43], and employing graphene and graphene-based materials to improve cathode materials [44].
Some reviews on RMBs have been dedicated to vanadium oxides solely. The structural characteristics and electrochemical performance of vanadium-based materials as RMB cathodes are discussed in [6,45,46]. The role of structural water molecules in vanadium oxide was analyzed in [47], the developments in application of vanadium oxide bronzes in metal ion batteries were discussed in [48], and the intercalation mechanisms in vanadium oxide were discussed in [49,50].
In this review, we will focus on the recent investigations of one of the promising and widely studied groups of cathode materials—layered vanadium oxides, including a systematic discussion of the achievements in their development and their possible improvement, the existing opinions on the mechanisms of charge–discharge processes in aqueous, “wet”, and dry non-aqueous electrolytes, and strategies to optimize functional properties of these cathode materials. We believe that this specialized and detailed review will help researchers to optimize the functional properties of these cathode materials.
2. Vanadium Oxide-Based Cathodes
Why did we select vanadium oxides as cathode materials for RMBs for this review?
Although the radius of Mg2+-ions (0.72 Å) is similar to that of Li+-ions (0.76 Å), the intercalation of Mg2+-ions into the same host materials is more difficult due to the difference in charge. The large charge/radius ratio of Mg2+-ions results in their strong electrostatic interactions with cathode materials, making intercalation and diffusion processes sluggish. As a result, most cathode materials suitable for RMBs exhibit a low degree of magnesiation, large voltage hysteresis, and low rate capabilities. Therefore, it is important to develop high-performance cathode materials for RMBs.
Vanadium-based materials have high theoretical capacity and energy density resulting from the multiple valence states of vanadium. Many vanadium-based materials have a layered structure, and the interlayer can be enlarged and adjusted to favor the intercalation and diffusion of Mg2+-ions into the cathode material.
Among vanadium-based materials, vanadium oxides are the most common and have numerous advantages for multivalent metal ion batteries. Vanadium oxides are cheap and abundant. Vanadium oxides with different structures have a variety of valence states (V+3/V+4/V+5) and can be easily synthesized by a variety of methods. Vanadium oxide polymorphs with different lattice symmetries have different electronic properties and thus different metal ion insertion thermodynamics and kinetics. Different synthetic strategies offer possibilities to synthesize different polymorphic structures and to fit the obtained layered structures to different interlayer distances and to weaken (i.e., screen) the strong electrostatic interaction of Mg2+-ions with the host lattice.
Among other cathode materials, the vanadium pentoxide family has been considered promising for RMBs due to the high theoretical capacity of V2O5 (294 mAh·g−1, considering 1 mol of Mg2+ per mol of V2O5) and high working voltage of ≈2.4 V, resulting in a high specific energy (>600 Wh·kg−1) [6,51,52,53]. Orthorhombic V2O5 was the first of the vanadium oxides to be investigated for Mg2+ intercalation.
In recent years, researchers have focused on other types of vanadium oxides like V2O5·nH2O, MxV2O5·nH2O (with M: intercalated foreign metal ion) [54], VO2 [55,56], and others, and also specially designed mixed-valence composites, such as V3O7/VO2 [57].
In the thermodynamically most stable α-phase, V2O5 has a layered structure consisting of alternating edge- and corner-sharing distorted VO5 trigonal-bipyramidal coordination polyhedrons stacked along the c-axis with an interlayer spacing of 4.37 Å (Figure 1a). Vanadium oxide gels (V2O5·nH2O) have bilayer crystal structures comprised of layered square-pyramidal VO5 polyhedra in which V5+ is coordinated with five oxygen atoms. Water molecules are bound between the layers of V2O5 (Figure 1b). The reported water content n in V2O5·nH2O is usually about 1.6–2.0 moles per mole of V2O5 [58]. The layered structure provides channels for ion insertion and de-insertion (Figure 1b).
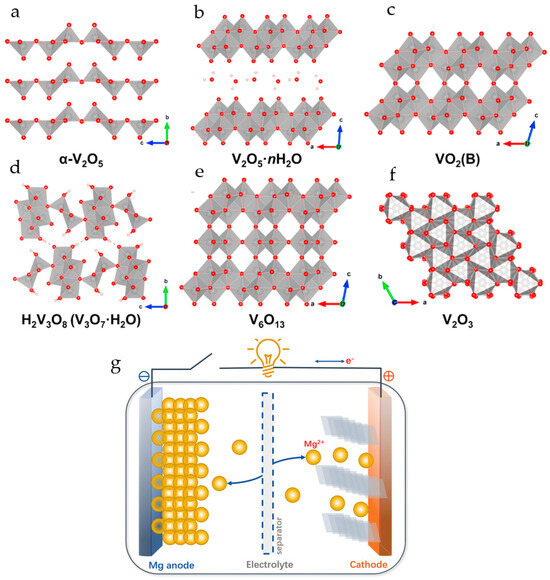
Figure 1.
Crystal structures of vanadium oxides: (a) α-V2O5, (b) V2O5·nH2O, (c) VO2(B), (d) H2V3O8 (V3O7·H2O), (e) V6O13, (f) V2O3. (Reprinted with permission from [45]. Copyright 2023, Elsevier). (g) The working principle of the RMB. (Reprinted with permission from [21]. Copyright 2020, John Wiley and Sons).
Brookite-phase vanadium dioxide VO2(B) (Figure 1c) has a stable open framework. The crystal structure of VO2(B) consists of corner- and edge-sharing VO6 octahedra that provide three plausible migration channels along the c- and b-axes. It has a monoclinic symmetry with a space group of C2/m. Because the b-axis is the shortest unit cell dimension, crystals grow predominantly along the b-axis, forming one-dimensional morphologies such as nanowires, nanorods, and nanobelts. VO2(B) is a promising candidate for RMBs due to its high theoretical capacity of 323 mAh·g−1 per one electron transfer for the V4+/V3+ redox coupe [56].
Hydrated vanadium oxide (H2V3O8, V3O7·H2O, HVO) (Figure 1d) has an orthorhombic crystal structure and comprises V3O8 layers, consisting of edge- and corner-shared VO5 square pyramids and VO6 octahedra. In contrast to van der Waals forces in V2O5, vanadium oxide layers in HVO are linked by hydrogen bonds, which decrease the volume expansion/contraction during cycling.
The crystal structure of V6O13 (Figure 1e) is composed of corner- and edge-sharing distorted VO6 octahedra, which form alternating single and double vanadium oxide layers connected by shared corners. V6O13 has high electrical conductivity at room temperature and has a high theoretical capacity of 417 mAh·g−1 when high-valent vanadium (V5+/V4+) is all reduced to V3+.
The vanadium trioxide V2O3 (Figure 1f) possesses a tunnel-like 3D structure, which determines its good ion intercalation performance. The theoretical capacity of V2O3 is 357.5 mAh g−1 and 715.1 mAh g−1 for one- and two-electron redox processes.
Consideration of this class of insertion-type compounds allows to highlight the main strategic ways to design layered metal oxide cathodes and to consider, on these examples, the typical issues and mechanistic details often missing within broader overviews and reviews.
In particular, these issues should be focused on (i) the electrochemistry of intercalation processes in layered oxides (vanadium oxide V2O5 and its modifications), their mechanism in aqueous, wet, and dry non-aqueous aprotic systems (PC, AN), the possibility of co-intercalation processes involving protons and magnesium ions; and (ii) the dependence of electrochemical properties on the modification of the interlayer space due to the inclusion of foreign metal ions, organic molecules (polymeric and others), and water molecules.
The basis for the selection of a suitable inorganic host material in metal ion battery technology is the reversible electrochemical intercalation processes associated with electrochemical redox processes with the recharging of metal ions and the accompanying reversible processes of insertion/removal of electrolyte cations into/from the host electrode material (Figure 1g). The driving force of these processes is the gradient of electrochemical potentials realized in the host materials. The three main factors affecting the intercalation and diffusion of metal ions are (i) steric factors, depending on the geometric size of intercalated metal ions and crystal structures providing the interstitial voids along ion hopping trajectories, (ii) desolvation of ions at injection, and (iii) electrostatic and other interactions of intercalated ions with the host materials.
Several innovative approaches have been proposed for layered oxide cathodes to successfully overcome all these challenges in the case of Mg2+-intercalation. Among them is the strategy based on a significant increase in the interlayer spacing in layered materials by the synthesis of layered materials pre-intercalated/doped with foreign metal ions or organic molecules, which could facilitate Mg2+-intercalation and diffusion in the host material. Another important feature of the increased interlayer spacing is the possible intercalation of solvated magnesium ions, avoiding the sluggish desolvation process at the interface.
Additional attention is given to the retention of water molecules in the host structures and the co-insertion of water molecules in the case of aqueous or wet non-aqueous electrolytes. The degree of reversible Mg2+-insertion strongly depends on the “lubrication” of the layered channels. The selected strategic approaches are usually combined with more common measures, such as nanostructuring of materials, which results in shortening the solid state diffusion path for highly polarized Mg2+ in the host and increasing the surface area of the active grains. In the following, we discuss these issues in more detail, based on experimental examples from published original works.
2.1. Orthorhombic Vanadium Pentoxide V2O5
Orthorhombic vanadium pentoxide (α-V2O5) was the first compound in the family of vanadium oxides to be proposed for use as cathode in RMBs. Early works showed that its typical layered crystal structure with weak van der Waals forces is suitable for tuning the interlayer space, making it accessible for intercalation of Mg2+-ions [29,59,60,61].
The electrochemical intercalation of Mg2+-ions into orthorhombic V2O5 in a solid state RMB composed of a metallic Mg anode, a solid Mg-montmorillonite electrolyte, and a V2O5 cathode has been confirmed [59]. The quantitative estimation of magnesium intercalation was reported [60], where the authors reported that 0.66 mol of Mg can be intercalated into 1 mol of V2O5 by chemical intercalation in the dibutylmagnesium/heptane solution, which corresponds to a high specific capacity of 194 mAh·g−1. This value is consistent with the capacity determined electrochemically in a 1 M Mg(ClO4)2/THF electrolyte.
A stronger indication that V2O5 is a potential cathode material for RMBs was the first publication on the ability of α-V2O5 to reversibly electrochemically intercalate Mg2+ in 1 M Mg(ClO4)2/AN electrolytes with water added to AN [61]. It was shown that the insertion of Mg2+ into V2O5 depends on the ratio between the amounts of H2O and Mg2+ and on the absolute amount of H2O in the electrolyte solution. The preferential solvation of Mg2+-ions by water molecules facilitated the insertion process. The highest capacities of up to 170 Ah·kg−1 were obtained in acetonitrile solutions containing 1 M Mg(ClO4)2 + 1 M H2O; however, the electrode had low cycling stability—only about 50 Ah·kg−1 was retained after 20 cycles.
To optimize the diffusion pathways of Mg2+ in V2O5 during the charge–discharge, orthorhombic V2O5 with particle size in the range of 20 to 50 nm obtained via a combustion flame-chemical vapor condensation process was suggested [62]. The reversible capacity of the V2O5 in 0.5 M Mg(ClO4)2/PC was 180 mAh·g−1 at a current density 7.6 mA·g−1.
During the discharge process, Mg2+-ions are gradually inserted into the interlayer of V2O5 and become coordinated with the VO5 pyramids, and the oxidation state of vanadium decreases from +5 to +4. The V4+/V5+ redox pair endows V2O5 with a high potential of 2.66 V vs. Mg/Mg2+. The overall reaction of the electrochemical insertion of Mg2+ ions into V2O5 as a RMB cathode material can be written as
V2O5 has a high theoretical specific capacity of 294 mAh·g−1 when 1 mol of Mg2+ ion is inserted (x = 1) [28].
V2O5 forms metastable polymorphs (β-, γ-, δ-, ε-, and ζ-V2O5) in addition to the thermodynamically stable phase (α-V2O5). Some of them have been the subject of theoretical and experimental studies concerning RMBs.
First-principles calculations were performed to investigate Mg intercalation in the α- and δ-polymorphs of V2O5 [63]. It was supposed that the conversion of fully demagnesiated stable α-V2O5 into a two-phase structure consisting of fully magnesiated δ-V2O5 and fully demagnesiated α-V2O5 phases during the discharge process results in slow diffusion kinetics in V2O5 electrodes. It was shown that since the calculated α-phase migration barriers indicate poor Mg mobility, reversible Mg intercalation is only possible at very low rates and in small particles, and the δ-V2O5 polymorph exhibits superior Mg mobility, provided that the δ-V2O5 host structure can remain stable or metastable over a wide Mg concentration range. The first-principles calculations have also shown that the Mg2+-ion diffusion barrier in δ-V2O5 (~0.6–0.8 eV) is lower than that in α-V2O5 (~0.975–1.1 eV) [64].
The DFT calculations have also shown that V2O5 undergoes a structural transformation from the α-phase to the ε-phase (ε-Mg0.5V2O5) and δ-phase (δ-MgV2O5) as the concentration of intercalated Mg2+-ions is increased [65].
The DTF calculations have shown a significant reduction in barriers for Mg diffusion in the β-V2O5 phase (0.65 eV) compared to α-V2O5 (>1 eV), leading to possibly fast charge–discharge rates of β-V2O5 as a cathode material for Mg2+-ion batteries [66]. β-V2O5 synthesized at high pressure demonstrated large voltage hysteresis in a dry (<20 ppm of H2O) 0.1 M Mg(ClO4)2/AN electrolyte and a maximum discharge capacity of 361 mAh·g−1 in GCD tests at C/25 [67].
The migration barriers for Mg2+ in ζ-V2O5 calculated by DFT were 0.62–0.86 eV [68], also suggesting its applicability in RMB cathodes. The metastable ζ-V2O5 obtained by topochemical extraction of the Ag+ ion from ζ-Ag0.33V2O5 also demonstrated large voltage hysteresis in Mg(TFSI)2/AN electrolyte, and displayed a specific capacity of 90 mAh g−1 with insertion of up to 0.33 Mg2+ per formula unit [69]. Nanosized ζ-V2O5 had a discharge capacity of 130 mAh·g−1 and lower voltage hysteresis [70].
The experimental investigations also indicated possible multi-stage transformation during the Mg2+-intercalation. Mg ion intercalation into nanoscale films of V2O5 deposited on Pt was studied [71]. It was shown that highly reversible Mg insertion/de-insertion is possible within V2O5 thin films. In the 0.5 M Mg(ClO4)2/AN electrolyte, the V2O5 thin film was cycled over a potential range of 2.2–3.0 V vs. Mg/Mg2+, and had an initial capacity of 180 mAh·g−1 at a 0.15 mV s−1 scan rate, the capacity retention after 36 cycles was ~83%. The electrodes had 100% coulombic efficiency. The differential capacity plots (dQ/dV − V) obtained for low current density galvanostatic cycling revealed four different Mg2+-insertion stages or processes with different thermodynamic and kinetic characteristics.
In order to check the inherent ability of α-V2O5 to intercalate Mg2+, electrochemical tests were conducted in an ionic liquid electrolyte in [72]. The α-V2O5 cathode prepared from commercial vanadium oxide powder had a low reversible capacity of only 16 mAh·g−1 at 25 °C, which increased to 295 mAh·g−1 (at C/5) at 110 °C. This capacity corresponds to reversible intercalation of 1 mol Mg2+ per unit formula. After 1 cycle at 110 °C, the electrochemical activity of α-V2O5 at room temperature was significantly enhanced (specific capacity of about 95 mAh·g−1 at C/5). The composition of the cathode after the thermal activation was Mg0.2V2O5; accordingly, the activation resulted in an expanded interlayer spacing with Mg-pillaring, facilitating fast Mg2+-migration. The reversible intercalation of Mg2+ into α-V2O5 was also observed during the characterization of the material composition, crystal structure, and redox state.
Thin films of orthorhombic V2O5 were grown on fluorine-doped tin oxide (FTO) glass electrodes using AACVD, and studied in aqueous solutions of MgCl2 [73]. The material showed a specific discharge capacity as high as 427 mAh·g−1 at a high current density of 5.9 A·g−1 with 82% capacity retention after 2000 cycles. At a current density of 2.4 A·g−1, the specific capacity was 170 mAh·g−1, and when the current was returned to 5.9 A·g−1, the cathode delivered 425 mAh·g−1, corresponding to a capacity retention of 99%. The increase in the specific discharge capacity with increasing specific current was explained by the fast diffusion kinetics of Mg2+ in the metal oxide framework.
Several recent studies of V2O5 nanoclusters have shown that the use of vanadium-based nanoscale materials can improve the diffusion of Mg2+-ions and provide high reversibility of intercalation processes. Highly dispersed vanadium oxide nanoclusters supported on porous carbon frameworks were synthesized using the ambient hydrolysis deposition method in order to improve the electrical conductivity of V2O5 [74]. The composite with 45 wt.% V2O5 had a surface area and pore volume of 593 m2·g−1 and 2.8 cm3·g−1, respectively. Reversible intercalation of Mg2+ with an initial capacity of 350 mAh·g−1 (per V2O5) or 180 mAh·g−1 (per composite weight) at 40 mA·g−1 within the voltage range of 0.5–2.8 V was observed in 0.2 M [Mg2(µ-Cl)2(DME)4][AlCl4]2 in DME. During the subsequent five cycles, the capacity of the composite electrode decreased to 160 mAh·g−1 (per V2O5) or 90 mAh·g−1 (per composite).
2.2. Nanostructured Vanadium Oxides
The findings confirming slow diffusion kinetics in V2O5 and low capacities pointed at the necessity of modifying the structure of V2O5 for Mg battery electrode applications.
In most papers it was noted that strong electrostatic interactions between divalent Mg2+-ions and lattice oxygen cause slow diffusion of Mg2+ in α-V2O5, and this is one of the main reasons for the unsatisfactory magnesium storage properties. Nanostructuring can increase the active surface area and reduce the diffusion distance to improve the electrochemical performance of α-V2O5. Various nanostructured vanadium oxide cathode materials with superior electrochemical performance were synthesized.
Hydrothermally synthesized V2O5 nanowires (Figure 2a) were used as a cathode in a magnesium ion system with 1 M Mg(ClO4)2/AN as an electrolyte and MgxMo6S8 (x ≈ 2) as an anode [75]. V2O5 nanowires provided an initial discharge/charge capacity of 103/110 mAh·g−1 and a maximum discharge capacity of 130 mAh·g−1 in the sixth cycle at a C/20 rate in a full cell.
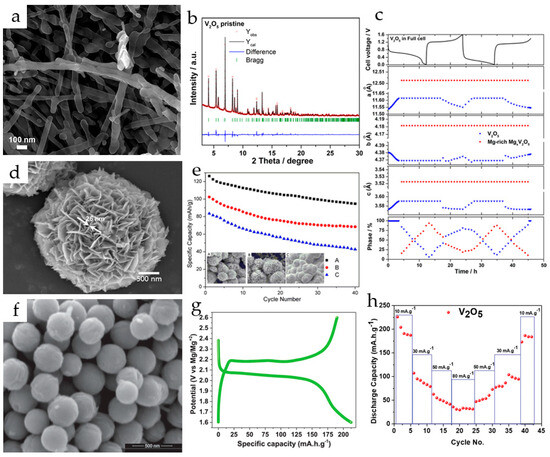
Figure 2.
(a) SEM image of V2O5 nanowires, (b) synchrotron diffraction pattern of V2O5 nanowires, (c) structural parameters and phase ratios from diffraction patterns with Rietveld refinement during the first two charge–discharge cycles for V2O5. (Reprinted with permission from [75]. Copyright 2019, American Chemical Society.) (d) SEM image of V2O5 microspheres, (e) cyclic performance of V2O5 microspheres with the diameters of 3 (A), 7 (B), and 15 (C) μm. (Reprinted with permission from [76]. Copyright 2019, Springer). (f) SEM (scale 500 nm) image of V2O5 spheres, (g) first galvanostatic profile of V2O5 spheres at 10 mA·g−1, (h) rate performance of V2O5 spheres. (Reprinted with permission from [77]. Copyright 2020, John Wiley and Sons).
The synchrotron diffraction pattern of the pristine V2O5 nanowires (Figure 2b) showed a high degree of crystallinity in the material. All reflections were indexed in the orthorhombic α-V2O5 structure model with space group Pmn21 and lattice parameters a = 11.511 Å, b = 4.373 Å and c = 3.565 Å. The mechanism of Mg2+ insertion and extraction in V2O5 during the first two cycles was studied by in operando synchrotron diffraction (Figure 2c). It was shown that during the first discharge, the V2O5 phase accommodates Mg2+-ions via a solid solution mechanism up to the stoichiometry of Mg0.14V2O5, while the lattice parameters a and c increased and b decreased. During further magnesium uptake, a decrease in the amount of Mg0.14V2O5 phase and an increase in the Mg0.6V2O5 phase with constant cell parameters for both phases were observed; at the end of the first discharge, the phase ratio of Mg0.14V2O5:Mg0.6V2O5 was 13:87. In the second cycle, the material showed almost the same behavior during discharge, and at the end of the second charge V2O5 returned to its original structure.
Flower-like V2O5 microspheres composed of 25 nm thick nanosheets (Figure 2d) were synthesized via a surfactant-assisted hydrothermal procedure in [76]. The cathode produced from V2O5 microspheres with an average diameter of 3 μm delivered the initial discharge capacity of 126.2 mAh·g−1 at 50 mA·g−1 in 0.25 M Mg(AlCl2EtBu)2/THF, demonstrated good cycling stability (90.7 mAh g−1 after 80 cycles) and enhanced rate capability (60 mAh·g−1 at 200 mA·g−1). The improved electrochemical performance of the V2O5 microflowers was explained by the increased specific surface area (13.7 m2·g−1) and flexibility. The microspheres with larger size (7 and 15 μm) or structural irregularities demonstrated lower capacities and cycling stability (Figure 2e). The charge–discharge mechanism was investigated by X-ray diffraction and X-ray photoelectron spectroscopy.
Monodisperse spherical V2O5 particles with a diameter of 230–250 nm were obtained in [77] (Figure 2f). The V2O5 spheres exhibited an initial discharge capacity of 225 mAh·g−1 (Figure 2g) and a stabile discharge capacity of 190 mAh·g−1 at 10 mA·g−1 in a dry Mg(ClO4)2/AN electrolyte. The rate performance of V2O5 spheres (Figure 2h) and long-term cycling stability at different current rates were good, and the specific discharge capacity of 55 mAh·g−1 was achieved at 50 mA·g−1 with 5% and 13% capacity fading after 50 and 100 cycles, respectively. The retention of 95% coulombic efficiency after 100 cycles and the stability of the phase and morphology confirmed the stability of the material during Mg2+-ion intercalation/deintercalation.
V5O12·6H2O nanoflowers formed by self-assembly of nanosheets were obtained by a one-step solvothermal method (Figure 3a) in [78]. The synthesized V5O12·6H2O had uniform morphology with an average size of a nanoflower of 650 nm while the individual nanosheets were about 25 nm thick (Figure 3b). For comparison, V5O12·6H2O nanoparticles were also synthesized. The galvanostatic tests revealed that both V5O12·6H2O nanoflowers and nanoparticles showed a similar activation process, the nanoparticles having a much lower capacity (Figure 3c). It was shown that the structural water in the interlayer of V5O12·6H2O improved the stability of the crystal structure and created more active sites for Mg2+ storage. The nanoflower morphology increased the active surface area, and enhanced the contact between the electrode material and the electrolyte, improving the diffusion kinetics of Mg2+. The V5O12·6H2O nanoflowers had a high initial specific capacity of 234.3 mAh·g−1 and reversible capacity after activation of 153.2 mAh·g−1 at 10 mA·g−1 (in a 1:1 mixture of 0.8M PhMgCl and 0.4M AlCl3 in anhydrous THF electrolyte) (Figure 3d), a good rate capability in the current range 50–500 mA·g−1 (Figure 3e), and long cycling stability (73.8% capacity retention after 1500 cycles at 100 mA·g−1, i.e., 0.017% capacity loss per cycle). It was proposed that the layered structure of the V5O12·6H2O nanoflowers can accommodate the volume changes during the intercalation/deintercalation of Mg2+, and the interlayer water molecules can serve as pillars enhancing the structural stability.
Ultrathin 2D V6O13 nanosheets were synthesized via a microwave-assisted method [79]. Two pairs of redox peaks observed in CVs at 0.9/0.68 V and 1.15/1.33 V (vs. Mg/Mg2+) correspond to the magnesiation/demagnesiation process, suggesting that it is a two-step process. The V6O13 nanosheet cathode delivered a maximum discharge specific capacity of 324 mAh·g−1 at 20 mA·g−1 in 1 M Mg(ClO4)2/AN, corresponding to 3.22 mol of Mg2+ ions’ insertion per unit formula of V6O13. At current densities of 40, 60, and 80 mA·g−1, the reversible specific capacities were 278, 244, and 214 mAh·g−1, respectively, indicating the good rate capability of the material. A high reversible capacity of 200 mAh·g−1 was retained after 30 cycles at 40 mA·g−1 with a ~100% coulombic efficiency.
Vanadium oxide nanotubes are advantageous for Mg2+-intercalation due to their large interlayer distance, open ends, and large inner and outer diameters. The shape of the tubes is favorable for an ion insertion process because the diffusion path in the solid is shorter and the heterogeneous kinetics are faster at higher surface-to-bulk ratios. In addition, the tubes can provide electrolyte-filled channels or voids for fast Mg2+-ion transportation to the insertion sites.
VOx nanotubes (with the nominal composition of VO2.37[C18H40N]0.26) were obtained from V2O5 and octadecylamine through a hydrothermal reaction [80]. The nanotubes had open ends and were 1–3 μm long, and their outer/inner diameter was 60–100 nm/15–40 nm, respectively. In an 0.25 M Mg(AlBu2Cl2)2/THF electrolyte, the material exhibited good cycle performance, and the initial specific discharge capacity was 81 mAh·g−1 at 5 mA·g−1. The Mg2+-insertion into the VOx nanotubes was confirmed by the XPS and XPD results.
VOx nanotubes were obtained using a microwave-assisted hydrothermal method and low and high concentrations of the amine template, denoted as HT and LT, respectively [81]. The amount of the amine template did not affect the morphology of the VOx nanotubes; both types of nanotube had an average outer diameter of 80–100 nm and were 1–5 μm long. The performance of VOx nanotubes with various oxidation states (V3+/V4+/V5+) as a cathode for RMB was studied in 0.5 M Mg(ClO4)2/AN solution. The kinetics of magnesium insertion and extraction depended highly on the oxidation state and bonding structure of the nanotubes’ surface. The formation of V3+-ions in highly reduced VOx nanotubes resulted in high initial discharge capacity (218 mAh·g−1 for HT-VOx and 230 mAh·g−1 for LT-VOx at 0.2 C (60 mA·g−1)), excellent cycling performance, and lower charge transfer resistance at the electrode/electrolyte interface compared to VOx nanotubes containing vanadium ions of higher oxidation states. Although the initial capacities of HT-VOx were lower than those of LT-VOx, HT-VOx demonstrated better cycling performance, with capacity retention of 70.8% after 20 cycles. The coulombic efficiency of both materials increased while cycling.
The Mg2+-ion insertion/extraction performance of VOx-NTs was investigated in Mg(ClO4)2 tetramethylsilane-ethyl acetate (TMS-EA) electrolyte in comparison with an AN electrolyte [82]. The VOx-NTs were prepared using a microwave-assisted hydrothermal method starting with V2O5 and octadecylamine. As seen in the SEM and TEM images (Figure 3f,g), the VOx-NTs were open-ended, multilayered tubular structures, with alternating arrangements of VOx and amine layers. VOx-NTs showed an initial capacity of more than 200 mAh·g−1 in de-aerated 0.5 M Mg(ClO4)2/AN electrolyte solution, but had poor capacity retention. Superior cycling performance and coulombic efficiency were observed for the VOx-NTs in TMS and TMS-EA, although the initial discharge capacities in TMS-based electrolytes were lower (Figure 3h). It was shown that the TMS-EA solvent improved the cell performance due to the higher stability of TMS against oxidation and the strong Mg2+ coordination ability of EA. The initial discharge capacity of VOx-NT in TMS-EA solution was 124 mAh·g−1 at 0.2 C (60 mA·g−1), and the capacity retention was 70% after 80 cycles.
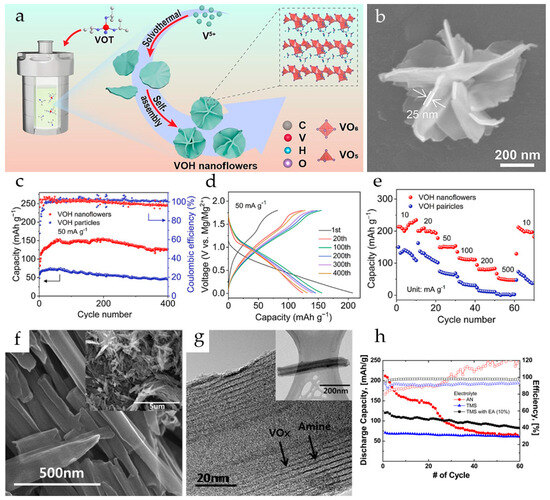
Figure 3.
(a) Scheme of the preparation of V5O12·6H2O nanoflowers, (b) SEM of a V5O12·6H2O nanoflower, (c) cycling performance of V5O12·6H2O nanoflowers and nanoparticles at 50 mA·g−1, (d) discharge/charge profiles of V5O12·6H2O nanoflowers, (e) rate capability of V5O12·6H2O nanoflowers and nanoparticles. (Reprinted with permission from [78]. Copyright 2023, Elsevier). (f,g) High-resolution and (inset) low-resolution SEM and TEM images of VOx-NTs, (h) discharge capacity and coulombic efficiency of VOx-NT electrodes in AN, TMS, and TMS-EA electrolytes. (Reprinted with permission from [82]. Copyright 2016, American Chemical Society).
The electrochemical Mg2+-ion intercalation and extraction in multi-walled vanadium oxide nanotubes VOx-NTs was investigated in [83]. The VOx-NTs had an open-ended structure of multilayer scrolls with an outer diameter of 50 nm and a length of a few µm, and the VOx interlayer spacing was 2.7 nm. The initial specific discharge capacity for VOx-NTs in 1 M Mg(ClO4)2/AN and in 1 M Mg(TFSI)2/G2 was 146 ± 36 and 33 ± 12 mAh·g−1 at 5 mA·g−1, respectively, since, as a rule, the cells with the Mg(TFSI)2/G2 electrolyte provide lower initial capacities compared to Mg(ClO4)2/AN. In both electrolytes, the VOx-NTs demonstrated poor capacity retention. The intercalation of Mg2+-ions was confirmed by ICP-AES. In operando scattering studies have shown that Mg2+-ion intercalation into VOx-NTs is accompanied by an increase in the interlayer spacing and a loss of the long-range stacking order.
2.3. Bilayer V2O5
V2O5 gels can be synthesized by a sol–gel process from a variety of precursors. Xerogels and aerogels differ in the drying process that removes the solvent from the nanoscale pores and capillaries after the material is synthesized. Xerogels are dried at ambient conditions, resulting in a more compact and dense material than aerogels. Aerogels are dried by filling the pores and capillaries with a supercritical fluid and then cooling so that the pores are filled with a gas. This process allows the pores to remain intact and increases the surface area of the material. V2O5 aerogel has a surface area ~30 times larger than that of V2O5 xerogel [27].
V2O5 gels have an enhanced capacity for insertion/extraction of magnesium ions due to their water content. Furthermore, the increased surface area supports more efficient diffusion and provides more sites for insertion/extraction of magnesium ions, thus increasing the ability of the material to enable high-rate charge and discharge. In addition, the capacity increases due to the large surface area because there is less isolated bulk material that the intercalating ions cannot reach.
Amorphous V2O5 aerogels with high surface area, consisting of interpenetrating networks of water and V2O5 ribbons, are capable of accommodating 0.6–2 moles of Mg2+ per mole of V2O5 as a result of chemical insertion [84], or two moles formally corresponding to the reduction of V5+ to the V3+ state [85].
Bilayer V2O5·nH2O is generally considered to be amorphous due to the lack of long-range structural order, but at the nanoscale, a well-organized repetition of bilayers is observed. The synthesis route affects the morphology of V2O5·nH2O. The reduced structural order, wide interlayer space, and short diffusion length in V2O5·nH2O allow reversible cation accommodation [86]. The interlayer structural water can increase the interlayer spacing, which is favorable for the intercalation of Mg2+. The structural water molecules can partially shield the charge of Mg2+, which leads to the improvement in the diffusion kinetics of Mg2+ in bilayer V2O5 [86].
The insertion of Mg2+ into V2O5 xerogel and V2O5 xerogel/C composite in aqueous Mg(NO3)2 electrolytes was studied [87]. The intercalation/deintercalation reaction of Mg2+- ions was much faster in the case of the V2O5 xerogel/C composite as compared to the pure V2O5 xerogel. The addition of 10% carbon black during the synthesis of V2O5 xerogel/C composite allowed a significant improvement in the electrochemical behavior of this electrode material; the specific capacity of V2O5 xerogel/C composite in Mg(NO3)2 was 107 mAh·g−1, while pure V2O5 xerogel yielded 50 mAh·g−1.
V2O5 xerogel was prepared under microwave (MW) irradiation [88]. The structure and electrochemical properties of the V2O5 xerogel were compared with those of V2O5 prepared by conventional heat treatment. XRD revealed that the V2O5 xerogel prepared by MW irradiation was low-crystalline. The first discharge capacity of V2O5 prepared by MW irradiation was 175 mAh·g−1 at a 0.1 C rate in 0.3 M Mg(ClO4)2/PC solution, and the second discharge capacity increased to 463 mAh·g−1, indicating that Mg2+-insertion increased after the first cycle. The capacity of V2O5 prepared by heat treatment at 200 °C was 138 and 190 mAh·g−1 for the first and the second cycles, respectively, and the capacity of V2O5 prepared by heat treatment at 300 °C was 77 mAh·g−1.
An amorphous V2O5 xerogel/graphite composite containing 10 wt.% synthetic graphite showed an initial deintercalation capacity of 77 mAh·g−1 at 10 mV·s−1 (~18 C rate) in Mg(NO3)2 aqueous electrolyte [89]. After ten cycles, the value decreased to 63.5 mAh·g−1. In GCD tests, a relatively high magnesium storage capacity of 62, 53, 47, and 44 mAh·g−1 was obtained at 2, 3, 4, and 5 A·g−1, respectively.
The scenario of Mg2+ and H2O co-intercalation in nanocrystalline V2O5 xerogel was analyzed by first-principles calculations [90]. The models of fully relaxed structures of the fully magnesiated and demagnesiated V2O5 xerogel, where two individual V2O5 layers are bound by long interlayer V–O bonds and the intercalated Mg atoms and H2O molecules are located in the space between two bilayers, are shown in Figure 4a,b.
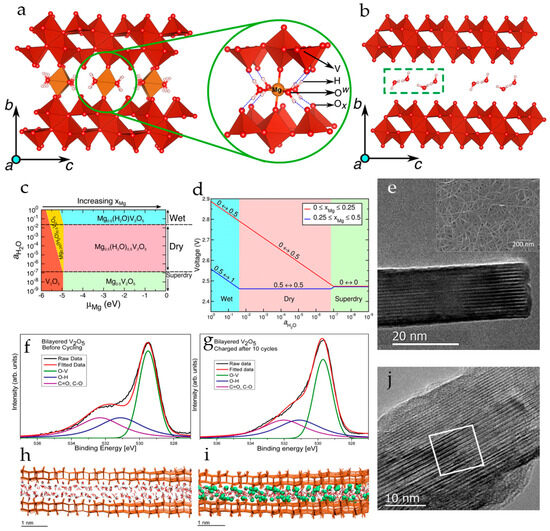
Figure 4.
Structures of (a) fully magnesiated (xMg = 0.5) and (b) fully demagnesiated xerogel with one H2O molecule per formula unit of V2O5, (c) potential phase diagram at 0 K of Mg-intercalated V2O5 xerogel as a function of water content in the electrolyte and Mg chemical potential (μMg = 0 corresponds to full magnesiation), (d) average Mg insertion voltage for low and high Mg concentrations as a function of water content in the electrolyte (aH2O). (Reprinted with permission from [90]. Copyright 2016, American Chemical Society). (e) HRTEM of a nanoribbon of bilayered V2O5 (inset—SEM of the nanoribbon architecture on a carbon nanofoam substrate), (f,g) XPS spectra of bilayered V2O5 before and after 10 cycles of charging with Mg2+-ions, and (h) MD simulation of the V2O5 bilayer immersed in water and (i) V2O5 bilayer (V in the +4 state) in the presence of Mg2+ ions in the presence of water, (j) HRTEM image of Mg-enriched V2O5. (Reprinted with permission from [91]. Copyright 2015, American Chemical Society).
Analysis of the stable phases of Mg-intercalated V2O5 xerogel at different voltages and in electrolytes with different water contents revealed a range of concentrations of intercalated Mg-ions in the V2O5 xerogel and H2O in the electrolyte where there is no thermodynamic driving force for the water molecules to shuttle with Mg2+-ions during electrochemical cycling (Figure 4c,d). It was also demonstrated that shuttling of water molecules with the Mg2+-ions in wet electrolytes yields higher voltages than in dry electrolytes.
Highly hydrated nanoribbons of bilayer V2O5 with a large interlayer distance (13.5 Å) were electrodeposited on porous highly conductive carbon nanofoam [91]. This method also allowed effective incorporation of defects, water, and hydroxyl groups, which in turn promoted electron transfer and (de)intercalation of highly charged Mg2+ ions. The “as-prepared” individual V2O5 nanoribbon is shown in an HRTEM image in Figure 4e. Mg2+ ions were incorporated into the as-prepared bilayer V2O5 cathodes by galvanostatic discharge at 20 μA to the potential 0.2 V (vs. Mg/Mg2+). The specific capacity of 240 mAh·g−1 obtained after this preconditioning procedure suggests reduction of vanadium V5+ to V4+. The electrode demonstrated reversible Mg2+ (de)intercalation in 1 M Mg(ClO4)2/AN electrolyte with a capacity of 150 mAh·g−1 at C/15 rate. The O 1s XPS spectra before cycling and after 10 cycles of charging showed that strongly bound structural hydroxyl groups remain in the structure of bilayer V2O5 during cycling (Figure 4f,g), and act as a lubricant for reversible (de)intercalation of solvated Mg2+ ions between the layers of V2O5. Molecular dynamics simulations of a three-bilayer V2O5 model, shown in Figure 4h,i, suggested that upon insertion of Mg2+-ions into the V2O5 structures, the spacing between bilayers decreases from ~12 to ~11 Å due to the interaction of Mg2+-ions with bilayer apical oxygen and structural hydroxyl groups, but a significant amount of H2O molecules remains in the structure, solvating the Mg2+ ions. The HRTEM image (Figure 4j) showed that after Mg2+-ions were intercalated, the layered V2O5 structure featured a spacing of 6.0 to 6.4 Å, and the bilayer distance was not uniform across the whole imaged area.
The reversible insertion/extraction of Mg2+ into the V2O5·nH2O electrode accompanied by the co-intercalation of solvent molecules was demonstrated [92]. The V2O5·nH2O achieved a discharge capacity of 50 mAh·g−1 (0.25 Mg per V2O5·nH2O) in a Mg(TFSI)2/G2 (diglyme) electrolyte at a current density of 20 mA·cm−2. It was shown that the process of Mg2+ intercalation into V2O5·nH2O involves the formation of new phases, while the bilayer spacing expands/contracts. The large interlayer spacing of bilayer V2O5 allowed the reversible co-intercalation of Mg2+ and solvent molecules.
Bilayered nanostructured V2O5·nH2O 2D nanopapers with oxygen defects (BL-HVOd NPS) with improved Mg2+ insertion/extraction kinetics were obtained [93]. The interlayer water molecules effectively stabilized the expanded interlayer spacing (10.6 Å) in BL-HVOd NPS and shielded the electrostatic interaction between Mg2+-ions and BL-HVOd NPS lattice, improving diffusion kinetics during repeated cycling (the log(DMg2+) value was in the range from −8.3 to −9.5 during charge and discharge). The nanopaper structure of BL-HVOd NPS enhanced electrolyte/electrode contact and reduced the diffusion path of Mg2+-ions, improving the rate performance. Cells with a BL-HVOd NPS cathode, AC anode, and 2 M Mg(CF3SO3)2 aqueous electrolyte demonstrated a reversible capacity of 162.8 mAh·g−1 at 0.2 A·g−1 and high cyclic stability with 88% capacity retention after 2000 cycles at 10 A·g−1. This was attributed to the contribution of oxygen defects.
Bilayer V2O5 is a promising cathode material for RMBs, but the low electronic conductivity of bilayer V2O5 is still a challenge that hinders its further application in RMBs. The main strategy to solve this problem is the modification of V2O5 with highly conductive materials or the pre-intercalation of metal ions to improve the conductivity of bilayer V2O5 and promote the ionic and electronic transport.
2.4. Nanocomposites of V2O5 with Conductive Carbons
The use of conductive carbons as additives for the improvement in electrical conductivity of V2O5 has also been studied in a large number of works.
Mg2+ ion intercalation into a V2O5/carbon composite, consisting of aggregated carbon particles (30–100 nm) covered with a thin layer (<100 nm) of V2O5 xerogel, in 1 M Mg(ClO4)2/AN electrolyte, was reported in [94,95]. Mg2+ insertion into V2O5 xerogel/C composites and accompanying structural changes were studied [94]. In the cyclic voltammogram for the V2O5 xerogel/C composite in 1 M Mg(ClO4)2/AN, two broad cathodic peaks associated with the Mg2+-insertion process were observed, pointing at the existence of two different Mg intercalation sites in the V2O5 xerogel. Ex situ FT-IR spectra suggested that Mg2+ is inserted into inner layer sites of V2O5 at −0.15 V and into the interlayer sites at −0.65 V vs. Ag/Ag+. The reversibility of Mg2+ intercalation/deintercalation was confirmed by FT-IR and XRD. The V2O5/carbon composite showed higher capacity than a conventional V2O5 electrode. It was assumed that the Mg2+ diffusion into the interlayer is slow, but since the V2O5/C composite has a large interlayer distance and short diffusion length compared to the normal xerogel of V2O5, the interlayer site could be utilized more effectively for Mg2+-insertion.
With a V2O5 gel/carbon composite, insertion of 1.84 mol Mg per 1 mol V2O5 in the first cycle at a scan rate of 0.1 mV·s−1 was found, resulting in a specific capacity of 540 mAh·g−1 (per V2O5 mass) [95]. In GCD tests, at a current density of 1.0 A·g−1 the composite electrode showed a capacity of about 600 mAh·g−1, and at a high current density of 20 A·g−1 the capacity of about 300 mAh·g−1 was maintained. The capacity of the carbon-free V2O5 xerogel electrode at 1.0 A·g−1 was only 150 mAh·g−1, and at 20 A·g−1 it was less than 10% of the capacity of the composite electrode. Thus, homogeneous mixing of a V2O5 sol with carbon particles, resulting in the formation of a thin layer of V2O5 gel around the carbon particles, was effective in improving the Mg2+ intercalation behavior. The reduction in the Mg2+ diffusion length in V2O5 and the improvement in the electronic conductivity by the carbon particles resulted in high capacities, especially at high current densities. The composites of vanadium oxide with graphene are expected to advance the RMB performance by providing rapid electron transport and easy Mg2+-diffusion due to the large surface area and excellent structural stability.
The synthesis of V2O5/graphene (GNP) nanoparticles by a ball milling technique was attempted in [96] to enhance the electrochemical performance of V2O5. The full cell with a Mg anode, (V2O5)1-x/GNPx cathode, and MgNO3∙6H2O/tetraethylene glycol dimethyl ether electrolyte yielded an initial capacity of ~90 mAh·g−1 at a low current density, while Mg/V2O5 cells exhibited the initial discharge capacity of ~100 mAh·g−1 under the same testing conditions. The capacities dropped to half the initial value on the second cycle and then the cells short-circuited, probably due to the incompatible electrolyte.
A composite of V2O5 with graphene oxide (GO/V2O5) was prepared by a solvothermal method [97]. SEM revealed that the V2O5 microparticles were wrapped around GO sheets, and TEM micrographs demonstrated the tight contact between GO and V2O5 microparticles. The good contact and dispersion of the composite components provided improved electrochemical performance of the material. The GO/V2O5 cathode was tested in coin cells with a Mg foil anode and 0.25 M Mg(AlCl2EtBu)2/THF as an electrolyte. The GCD measurements showed an initial discharge capacity of GO/V2O5 of 178 mAh·g−1 at 0.2 C, which faded to 160 and 150 mAh·g−1 in the second and third cycle, respectively, due to formation of a Mg passivation layer, but still remained at 140 mAh·g−1 after 20 cycles.
rGO-decorated hydrated V2O5 nanowire composites with different contents of crystal water have been synthesized by a sol–gel method with subsequent freeze-drying [98]. The V2O5·nH2O/rGO aerogel with a water content of 12.3% and large lattice spacing of 1.13 nm had a highly porous, interconnected 3D structure with V2O5 nanowires anchored on rGO (Figure 5a). The V2O5·nH2O/rGO electrode showed a capacity of 280 mAh·g−1 at 100 mA·g−1 in a 0.5 M Mg(TFSI)2/AN electrolyte, a capacity retention of 81% after 200 cycles, and a coulombic efficiency of 99%. At 50 mA·g−1 the capacity was 320 mAh·g−1. The nanocomposite displayed a wide working temperature range (~ 30–55 °C). As shown in Figure 5b, at 55 °C a high discharge capacity of ~200 mAh·g−1 was achieved, much higher than the capacity of 120 mAh·g−1 at room temperature. A reversible capacity of 40 mAh·g−1 was delivered at −30 °C. The improved electrochemical performance of the material was attributed to the shielding effect of crystal water as well as the synergistic effects of rGO, which provided Mg diffusion pathways, high surface area, and structural stability.
A three-dimensional structure of V2O3 nanoparticles with reduced graphene oxide (rGO) with improved conductivity and structural stability was prepared by spray-drying of a dispersed solution [99]. In the 0.3 M Mg(TFSI)2/AN electrolyte solution, V2O3@rGO microspheres demonstrated a high specific capacity (291.3 mAh·g−1 at 50 mA·g−1) and improved rate performance (185.3 mAh·g−1 at a high current density of 2 A·g−1) compared to pure V2O3 (~80 mAh·g−1). Excellent cycling stability (88.5% capacity retention after 1000 cycles at 0.5 A·g−1) with coulombic efficiency close to 100% was achieved. V2O3@rGO delivered a capacity of 472.1 mAh·g−1 in the GITT study, and the calculated diffusion coefficient of Mg2+ in the V2O3@rGO structure was 3.4 × 10−11 cm2·s−1.
A hierarchical V2O3@C structure with an accordion-like vanadium oxide/carbon heterointerface was synthesized from vanadium-based metal organic frameworks (Figure 5c) [100]. The mesoporous V2O3@C nanorods had a length of 250–300 nm, elongated pores of 30–50 nm (Figure 5d), and a BET surface area of 114.6 m2·g−1. The V2O3@C demonstrated a capacity of 354.8 mAh·g−1 at a current density of 500 mA·g−1 and an ultra-high capacity of 130.4 mAh·g−1 at 50 A·g−1 in 0.3 M Mg(TFSI)2/AN(H2O) with a coulombic efficiency of 99.6% and a capacity retention of 60.0% (1000 cycles, 500 mA·g−1). To verify the advantages of hierarchical accordion-like heterointerfaces, the rate performance of the V2O3@C electrodes was compared with that of bulk-V2O3/AC and bulk-V2O5/AC electrodes prepared with activated carbon in the same ratio as in V2O3@C. As shown in Figure 5e, the specific capacity of bulk-V2O3/AC at 200 mA·g−1 (203.2 mAh·g−1) is four times higher than of bulk-V2O5/AC (54.9 mAh·g−1) due to the anodic hydration of the V2O3 phase, occurring in bulk-V2O3/AC upon the first charging. The crystalline structure of V2O3 was reconstructed into a V3O7∙H2O@C through an anodic hydration reaction upon the first cycle. The cell was disassembled under an inert atmosphere and the V3O7∙H2O@C electrode was retested in a 0.3 M Mg(TFSI)2/AN electrolyte, where the specific capacity of the V3O7∙H2O@C electrode was 259.3 mAh·g−1 at 500 mA·g−1. The capacity difference of 33.9 mAh·g−1 between the two electrolytes indicated that the capacity contributed by protons in the water-containing electrolyte is about 13% of the total capacity. The higher overpotential of the V3O7∙H2O@C electrode in water-free electrolyte solution resulted from the slower diffusion of Mg2+-ions. In a full cell with a Mg anode and a 0.3 M Mg(TFSI)2 + 0.25 M MgCl2/DME electrolyte within the voltage range of 2.25 V vs. Mg/Mg2+, the V2O3@C exhibited a capacity of 245.1 mAh·g−1.
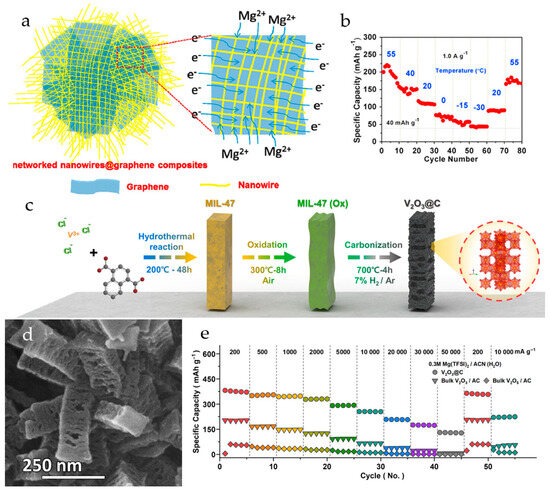
Figure 5.
(a) Scheme of V2O5·nH2O@rGO nanocomposite with electron/ion transport pathways, (b) cycling performance of V2O5·nH2O@rGO at 1.0 A·g−1, (c) discharge capacity V2O5·nH2O@rGO at various temperatures at 1.0 A·g−1. (Reprinted with permission from [98]. Copyright 2015, Elsevier). (c) Scheme of the synthesis of V2O3@C from MOFs, (d) SEM image of V2O3@C, (e) rate capabilities of V2O3@C, bulk-V2O3/AC and bulk-V2O5/AC. (Reprinted with permission from [100]. Copyright 2024, John Wiley and Sons).
2.5. Foreign Cation Pre-Intercalated Vanadium Oxides
The rich valence state variation of vanadium and the easy distortion of V–O polyhedra lead to the ability of V–O structure adaptation to incorporate different cations. The resulting derivatives with an A–V–O structures (A—metal ion or ) are called vanadates or foreign cation pre-intercalated vanadium oxides.
The pre-intercalation strategy has been widely recognized as a way to improve the properties of vanadium oxide cathodes for RMBs by increasing the interlayer space due to the pillaring effect combined often with improved stability of the obtained structures, allowing reversible insertion/extraction of Mg2+-ions. The analysis of the results of different works shows that, in most cases of pre-intercalated ions, a dual effect of structure expansion and void hydration takes place.
It should be noted that the pre-intercalation of cations, with its positive effects on increasing the interlayer spacing of V2O5 bilayers, also has some drawbacks. The most important one is that the introduction of electrochemically inactive cations leads to a decrease in specific capacitance. Therefore, the necessary optimization of cathode materials requires a balance between structural and stability factors on the one hand, and the improvement of specific capacities on the other hand.
The differences in the active material composition (especially water content), electrode processing and morphology, and electrolyte system make it difficult to compare results from different groups.
Among the cations used for pre-intercalation of vanadium oxides for RMBs are mono-, bi-, and tri-valent ions: Na+ [101,102,103,104], [105,106], Mg2+ [107,108,109,110], Mn2+ [111], Ca2+ [112], and Al3+ [113].
NaV3O8·1.69H2O nanobelts were synthesized from commercial V2O5 powder by a solvothermal procedure under ambient conditions (Figure 6a) [102]. The cells with a Mg anode and all-phenyl complex electrolyte demonstrated an initial discharge capacity of 150 mAh·g−1 and 110 mAh·g−1 after five cycles at a current density of 10 mA·g−1. The GCD curves of NaV3O8·1.69H2O at different current densities (Figure 6b) showed several plateaus during the charging and discharge process, due to the multistep redox reactions responsible for the insertion/extraction of Mg2+ ions. The limited specific capacity of the cells was attributed to the trapping of Mg2+ ions in the lattice of NaV3O8. The cathodes demonstrated high cyclic stability with 80% capacity retention after 100 cycles at 50 mA·g−1.
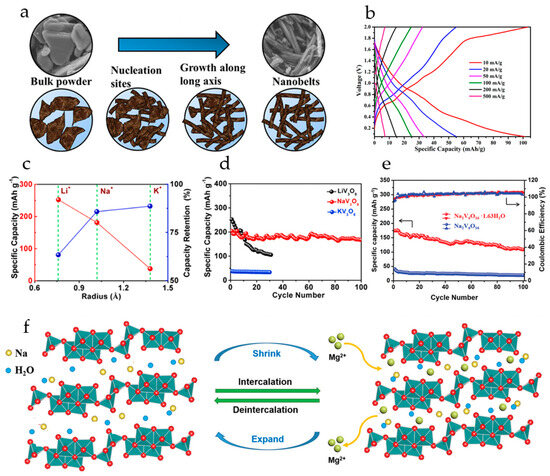
Figure 6.
(a) Scheme of the growth mechanism of the NaV3O8·1.69H2O nanobelts from V2O5, (b) charge–discharge curves of NaV3O8·1.69H2O at current densities 10–500 mA·g−1. (Reprinted with permission from [102]. Copyright 2018 American Chemical Society). (c) Specific capacity of alkali ion (Li+, Na+, K+) pre-intercalated V3O8 at 100 mA·g−1 and the capacity retention after 30 cycles, (d) cycling performance of alkali ion pre-intercalated V3O8 at 100 mA·g−1. (Reprinted with permission from [101]. Copyright 2019, Elsevier). (e) Cyclic performance of Na2V6O16 and Na2V6O16·1.63H2O at 50 mA·g−1, (f) scheme of Mg2+ intercalation/deintercalation into Na2V6O16·1.63H2O during the charge–discharge processes. (Reprinted with permission from [104]. Copyright 2020, John Wiley and Sons).
Pre-intercalating of alkali ions (Li+, Na+, K+) into layered vanadium oxide was studied in [101]. It was shown that the interlayer spacing of Li-, Na-, K-intercalated V3O8 increased regularly with the size of the pre-intercalated cations. The cycling performance of cathodes for Mg2+ storage was enhanced with the bigger radius of pre-intercalated ion (Figure 6c); after 30 cycles the capacity retention of KV3O8 was 88.6%, compared to 85.78% of NaV3O8 and 42.2% of LiV3O8, although the specific capacity of KV3O8 was the lowest (37.56 mAh·g−1). The initial specific capacity of LiV3O8 and NaV3O8 at 100 mA·g−1 was 252.2 and 204.16 mAh·g−1, respectively (Figure 6d). Based on structural analysis and electrochemical tests, it was concluded that pre-intercalation with Na+ resulted in a more stable interlayer structure, allowing free Mg2+ diffusion and preventing destructive collapse of the layers during the charge–discharge.
The interlayer spacing of V2O5 was expanded in [103] by introducing Na+-ions in the crystal lattice. NaV6O15 (NVO) free of crystal water was synthesized and studied as a cathode material for RMBs with anhydrous 0.5 M Mg(ClO4)2/AN electrolyte solution. It was shown that the introduction of Na+-ions enhanced the diffusion kinetics of Mg2+ ions (DMg2+ during the discharge process was in the range of 7.55 × 10−13 to 2.41 × 10−11 cm2·s−1 according to GITT), and improved the stability of the layered structure. NVO exhibited high initial discharge capacity of 213.4 mAh·g−1 at the current density of 10 mA·g−1, good capacity retention (87% after 100 cycles at 20 mA·g−1), and good rate capability. The ex situ XRD showed that the mechanism of Mg2+ storage in NVO is reversible intercalation/de-intercalation. The DFT calculation results indicated that during the intercalation process, Mg2+-ions tend to occupy the semi-occupied sites of Na+ in the NVO.
Water-pillared Na2V6O16·1.63H2O nanowires obtained in [104] displayed high performance in magnesium storage. In a cell with a Mg(TFSI)2/DME electrolyte and an AC anode, Na2V6O16·1.63H2O exhibited a high specific capacity of 175 mAh·g−1 at 0.05 A·g−1, long-term cycling performance, and ≈100% coulombic efficiency. Under the same testing conditions, annealed Na2V6O16 delivered a low specific capacity of only 40 mAh·g−1 (Figure 6e). Na2V6O16·1.63H2O cathodes demonstrated a discharge voltage plateau at around 2.0 V (vs. Mg/Mg2+), ascribed to the Mg2+ ion intercalation into the bilayer structure. According to ex situ XRD analysis, Na2V6O16·1.63H2O possesses a very stable structure for reversible Mg2+ ion intercalation/deintercalation. During the discharge, Mg2+-intercalation is accompanied by shrinkage of the interlayer space (Figure 6f). During subsequent charge–discharge processes, the Mg2+-ions can reversibly intercalate/deintercalate into the Na2V6O16·1.63H2O layers. The water molecules acting as “pillars” stabilize the layered structure and effectively shield the high charge density of Mg2+-ions.
Magnesiated V2O5 xerogel with the formula Mg0.1V2O5·1.8H2O was synthesized in [109] by a low-temperature procedure. The material had an initial discharge capacity of 300 mAh·g−1 in 0.5 M Mg(TFSI)2/AN. A capacity of ~250 mAh·g−1 was maintained over eight cycles at a C/10 rate, consistent with an insertion of 1 equivalent of Mg2+ per formula unit. No change in the interlayer distance (12.3 Å) was observed upon cycling of Mg0.1V2O5·1.8H2O electrodes.
Mg-inserted V2O5·xerogel was prepared via an ion removal sol–gel method in [114]. The Mg0.1V2O5·2.35H2O cathode displayed a high electrode potential of 3.0 V (vs. Mg/Mg2+), high energy density of 420 mWh·g−1, and a discharge capacity of 140 mAh·g−1 at a 0.1 C rate.
Mg2+ pre-intercalated hydrated vanadium oxide nanowires with bilayer structure, Mg0.3V2O5·1.1H2O, were synthesized in [110] from α-V2O5 using a hydrothermal approach. The reaction of α-V2O5 with H2O2 lowers the valence state of V, resulting in structural transformation of VO5 square pyramids to VO6 octahedra, and a large number of H2O molecules are embedded into the interlayer. This results in a bilayer structure with a larger interlayer space, providing ample channels for the subsequent insertion of Mg2+ ions. As a result of the coordination between Mg2+ and lattice oxygen, the insertion of Mg2+ ions into the layers leads to layer slippage and shrinkage (Figure 7a). In order to investigate the role of Mg2+ ions and lattice water molecules, V2O5·nH2O and Mg0.3V2O5 nanowires were also prepared. In Mg0.3V2O5·1.1H2O, the intercalated Mg2+ ions work together with crystal water to provide wide channels for electrolyte ion transport during the charge–discharge process. Mg0.3V2O5·1.1H2O possessed high electronic conductivity, fast Mg2+ reaction kinetics, and good structural stability. The charge and discharge curves of Mg0.3V2O5·1.1H2O, V2O5·nH2O and Mg0.3V2O5 at 0.1 A·g−1 in a cell with 0.3 M Mg(TFSI)2/AN electrolyte and an activated carbon anode are shown in Figure 7b. Mg0.3V2O5·1.1H2O exhibited three discharge voltage plateaus at 3.02, 2.25, and 1.40 V (vs. Mg/Mg2+), which can be attributed to the multi-step Mg2+ intercalation in the bilayer structure. Mg0.3V2O5·1.1H2O delivered high capacity (164 mAh·g−1 at 0.1 A·g−1), corresponding to the insertion of 0.5 Mg2+ ions per formula unit. V2O5·nH2O had a similar charge–discharge curve, with a lower discharge capacity of 114 mAh·g−1. For Mg0.3V2O5, no obvious plateaus were observed on the charge–discharge curve and it had the lowest discharge capacity (91 mAh·g−1). As can be seen in Figure 7c, Mg0.3V2O5·1.1H2O demonstrated good rate performance (50 mAh·g−1 at 4.0 A·g−1) and high cycling stability (capacity retention of 80.0% after 10,000 cycles at 2.0 A·g−1). In a full RMB cell with a Na2Ti3O7 anode and the Mg0.3V2O5·1.1H2O cathode, the specific capacity of about 62 mAh·g−1 at the current density of 100 mA·g−1 was achieved, and the battery demonstrated a stable voltage plateau of 1.5 V and a cycle life of 100 cycles.
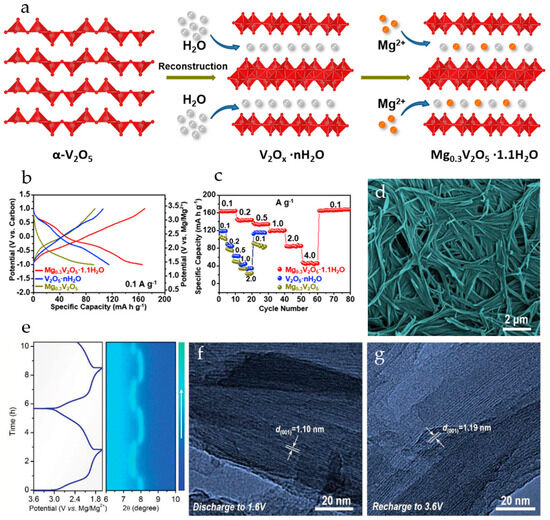
Figure 7.
(a) Scheme of consecutive incorporation of water and magnesium to form the bilayer Mg0.3V2O5·1.1H2O, (b) charge–discharge curves, and (c) rate performance of Mg0.3V2O5·1.1H2O, V2O5·nH2O, and Mg0.3V2O5 (Reprinted with permission from [110]. Copyright 2019, Elsevier). (d) SEM image of MgVOH electrode, (e) in situ XRD contour map of the MgxV5O12·nH2O electrode and corresponding high-resolution TEM images of (f) discharged and (g) recharged MgxV5O12·nH2O electrode (Reprinted with permission from [108]. Copyright 2020, John Wiley and Sons).
Mg2+-pillared hydrated vanadium oxide MgxV5O12·nH2O nanofibers (Figure 7d), with expanded interlayer spacing were obtained in [108]. Mg2+ pillars and structural H2O molecules stabilized the material framework and promoted Mg2+ diffusion during cycling. At a current density of 0.05 A·g−1 the MgxV5O12·nH2O electrode provided a high capacity of 160 mAh·g−1, corresponding to 1.3 Mg2+ inserted per V5O12 structural unit. The MgxV5O12·nH2O electrode showed excellent long-term stability (81% capacity retention after 10,000 cycles at 2 A·g−1), the coulombic efficiency was >99%. In situ XRD measurements (Figure 7e) had shown that the MgxV5O12·nH2O electrode undergoes dynamic structural changes upon Mg2+-intercalation/deintercalation. The (001) diffraction peak shifted to a higher angle region during the discharge process (reduction), indicating the gradual contraction of the interlayer spacing. During charging (oxidation), the (001) peak returned to the initial position by the end of a deep charge. The Mg2+-intercalation caused a decrease in interlayer spacing to 1.10 nm, and it recovered to 1.19 nm upon Mg2+ deintercalation (Figure 7f,g).
Expanding the interlayer spacing of layered materials is an effective way to improve Mg2+-ion storage performance. On the one hand, as the interlayer spacing increases, the interaction between intercalated guest ions and host lattices weakens, which greatly improves the mobility of cations in cathode materials. On the other hand, the number of available sites in cathode materials also increases due to the enlarged lattice spaces, leading to improved reversibility of Mg2+-ion storage capacity.
2.6. Vanadium Oxides Pre-Intercalated with Organic Molecules and Conducting Polymers
The intercalation of conducting polymers into vanadium oxides has been shown to be advantageous for improving the functional properties of electrode materials. Conducting polymers intercalated into layered vanadium oxide can increase the interlayer spacing and stabilize the layered structures by reducing the coulombic interactions between the guest cations and the host framework, as has been shown on numerous examples for aqueous Zn-ion batteries [115].
Rechargeable magnesium batteries with a V2O5 xerogel cathode with polyethylene oxide (PEO) incorporated between the oxide layers showed a significant improvement in the reversible capacity of magnesium ions [116]. PEO was introduced in the V2O5 interlayer during the V2O5 sol–gel synthesis (Figure 8a,b); this approach allowed the expansion of the interlayer spacing in V2O5 and the reduction in the interaction of intercalated divalent Mg2+ with the host lattice. X-ray diffraction showed that the interlayer spacing in V2O5 xerogel was increased to 12.6–13.6 Å by the inclusion of PEO. Galvanostatic charge–discharge profiles of V2O5 xerogel and V2O5-PEO nanocomposites with different amounts of PEO are shown in Figure 8c. In a cell with a Mg anode and a dry Mg(ClO4)2/AN electrolyte, the V2O5-PEO-1 nanocomposite exhibited a discharge capacity of 100.3 mAh·g−1 at 10 mA·g−1, ~5 times higher than of V2O5 xerogel (and ~2 times higher than of V2O5-PEO-2), improved stability, and enhanced rate capability. The Mg2+-ion capacity for the V2O5 xerogel, V2O5-PEO-1, and V2O5-PEO-2 cathode materials was determined as 0.06, 0.34, and 0.18 per V2O5 formula unit (i.e., x in MgxV2O5), respectively. In cyclic voltammograms, the V2O5-PEO-1 nanocomposite showed higher currents compared to V2O5 xerogel and V2O5-PEO-2, indicating that the introduction of an optimal PEO ratio within V2O5 improved the Mg ion charge storage performance. The Mg2+ diffusion coefficient in V2O5-PEO-1 nanocomposite was 4.7 × 10−11 cm2·s−1, that is, 2 times higher than the Mg2+ diffusion coefficient in the V2O5 xerogel. The Mg2+ diffusion coefficient in V2O5-PEO-2 was 2.5 × 10−12 cm2·s−1, lower than that for the V2O5 xerogel. Therefore, Mg2+-ion diffusion was not only influenced by the interlayer spacing (which is the largest for V2O5-PEO-2), but also by the interlayer composition. It was suggested that a higher PEO ratio in the composite could result in lower charge storage for reasons such as a lower diffusion rate of Mg2+-ions in PEO-rich regions, lower electronic conductivity due to the insulating nature of PEO, and coordination of PEO molecules with the V2O5 lattice, blocking sites for Mg2+ intercalation.
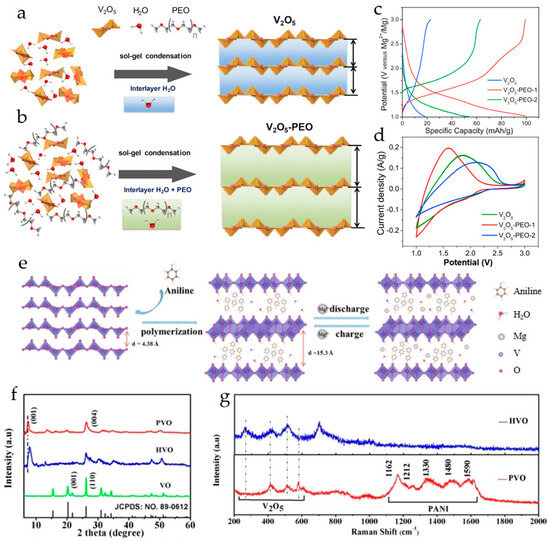
Figure 8.
(a) Scheme of the growth of hydrated V2O5 nanosheets by condensation of [VO4]3− polyanions in aqueous solution without PEO and (b) with PEO, (c) GCD profiles of V2O5, V2O5-PEO-1, and V2O5-PEO-2 at a current density of 10 mA·g−1, (d) cyclic voltammograms of V2O5, V2O5-PEO-1, and V2O5-PEO-2 at a scan rate of 2.5 mV·s−1. (Reprinted with permission [116]. Copyright 2017, Elsevier). (e) Scheme of the synthesis of the PVO superlattice, (f) XRD patterns of VO, HVO, and PVO, (g) Raman spectra of HVO and PVO. (Reprinted with permission [117]. Copyright 2021, John Wiley and Sons).
PANI-V2O5 2D organic–inorganic superlattices were synthesized and tested as cathode materials for RMBs in [117]. PANI-V2O5 (PVO) was synthesized via the intercalation of aniline monomers and subsequent interlayer polymerization (Figure 8e). In the acidic environment, the anilinium cations diffused into the interlayer space of V2O5 and underwent oxidative polymerization, V2O5 being a mild oxidizing agent. Compared to the XRD pattern of V2O5 (VO), a new diffraction peak of hydrated V2O5 (HVO) was located at 7.08°, confirming that the interlayer spacing of HVO is greater than that of VO, and for PVO, the new diffraction peak was shifted to a lower angle 6.47°, indicating larger interlayer spacing in PVO than in HVO and the insertion of PANI (Figure 8f). The formation of protonated PANI in PVO was confirmed by the Raman spectra (Figure 8g). PANI not only expanded the interlayer spacing of V2O5, but also provided additional charge storage sites. Benefitting from the above features, the PVO demonstrated high capacity (280 mAh·g−1 at a current density of 0.1 A·g−1) and excellent rate performance (135 mAh·g−1 at a current density of 4 A·g−1) in 0.3 M Mg(CF3SO3)2/AN electrolyte. The PVO cathode delivered capacities of 275, 250, 220, 175, 155, and 130 mAh·g−1 at current densities of 0.1, 0.2, 0.5, 1.0, 2.0, and 4.0 A·g−1, respectively. The PVO had an excellent cycling stability, and the retained capacity after 500 cycles at 4 A·g−1 was 80 mAh·g−1.
Polyaniline was in situ intercalated into V6O13 [118]. With the increase in the amount of aniline monomer, PANI-V6O13 composites with different enlarged d-spacings (11.8, 13.4, and 14.7 Å) were obtained. The contents of V6O13 and PA in the PA100-V6O13 with 13.4 Å d-spacing were 88.18% and 9.60%, respectively. In the cell with an activated carbon anode and a Mg(TFSI)2/DME electrolyte, the PA100-V6O13 sample demonstrated the highest reversible capacity (195 mAh·g−1 at 0.1 A·g−1), with a retention of 173 mAh·g−1 after 100 cycles. PANI-V6O13 exhibited high rate capability (46 mAh·g−1 at 10 A·g−1) and outstanding cycling stability (2500 cycles at 5 A·g−1). The higher reversible capacity of Mg2+ storage of PA100-V6O13 was mainly ascribed to the expanded interlayer spacing. The sample PA150-V6O13 with a higher polyaniline content and a larger interlayer spacing had higher initial discharge capacity but less satisfactory cycling performance, probably due to poor structural stability which reduced the capacity retention. The Mg2+ diffusion coefficients in PANI-V6O13 calculated from GITT data varied over the entire insertion/extraction processes and were in the range from 2.1 × 10−9 to 7.5 × 10−12 and from 2.7 × 10−10 to 3.2 × 10−11 for the insertion and extraction processes, respectively. Thus, in polyaniline-intercalated V6O13, the Mg2+ intercalation kinetics were enhanced due to the π-conjugated polyaniline molecules weakening strong coulombic interactions between Mg2+-ions and anions in the host material.
The layered V2O5-PEDOT composite with an enlarged interlayer spacing of 9.86 Å was synthesized in [119] via in situ polymerization of 3,4-ethylenedioxythiophene and sequential intercalation of PEDOT and cetyltrimethylammonium bromide (CTAB) into V2O5. In the XRD pattern of the as-prepared V2O5-PEDOT (VOP), a new peak appears at 8.96°, corresponding to an increased interlayer spacing of 9.86 Å, much larger than the d-spacing of V2O5 (4.38 Å). The intercalation of PEDOT pillars results in dark coloring of the VOP powder; these pillars change the structure of bulk V2O5 by drastic volume expansion, resulting in delamination of the VOP into a nest-like structure of interlaced nanowires about 50 nm in diameter, as seen in the SEM image (Figure 9a–c). The magnesium storage performance of V2O5-PEDOT cathode was evaluated in a cell with a Mg anode and all-phenyl complex APC-CTAB/THF electrolyte. The CTAB was added for further improvement in the Mg2+ diffusion kinetics. Two pairs of broad redox peaks located at around 1.25/0.80 and 1.78/1.57 V (vs. Mg/Mg2+) were observed in the cyclic voltammograms of V2O5-PEDOT. The reversible capacity of V2O5-PEDOT in the full cell was 288.7 mAh·g−1 at 0.1 A·g−1, and the electrode demonstrated high cyclability (over 500 cycles at 0.5 A·g−1 with capacity retention 68%).
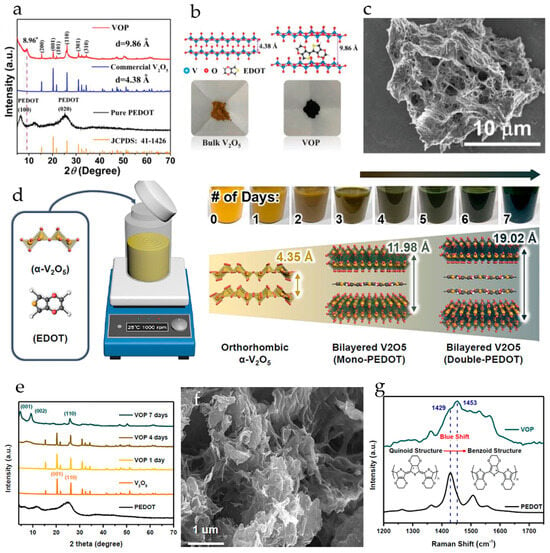
Figure 9.
(a) XRD patterns of PEDOT, commercial V2O5 and as-prepared VOP composite, (b) schematic structure and optical images of commercial V2O5 and VOP composite, (c) SEM image of VOP composite. (Reprinted with permission [119]. Copyright 2021, John Wiley and Sons). (d) Schematic representation of the VOP synthesis, (e) XRD patterns of pristine V2O5, PEDOT, and as-prepared VOP, (f) SEM of VOP, (g) Raman spectra of PEDOT and blue-shifted VOP. (Reprinted with permission [120]. Copyright 2023, Elsevier).
A V2O5/PEDOT (VOP) composite was synthesized by intercalating poly-3,4-ethylenedioxythiophene into V2O5 under stirring at room temperature for 1 week (Figure 9d) [120]. After 4 days of stirring, a new diffraction peak appeared in the XRD pattern of VOP at 7.6°, corresponding to an interlayer spacing of 11.98 Å. After 7 days of stirring, two new peaks appeared at 4.65° and 9.32°, corresponding to an interlayer spacing of 19.02 Å of the (001) plane and 9.49 Å of the (002) plane (Figure 9e). It was observed that the bilayer structure of orthorhombic V2O5 was retained even after the insertion of PEDOT. The porous structure of the as-prepared VOP resulting from the aggregation of the bilayer structures is shown in the SEM image (Figure 9f). The electronic coupling of PEDOT with V2O5 interlayers led to a change in PEDOT structure from the quinoid to the benzoid, and the corresponding blue shift of the principal peaks of PEDOT was observed in UV-vis spectra (Figure 9g). A reversible and fast Mg2+ ion insertion/extraction in/out of an enlarged interlayer spacing of 19.02 Å (in the charged state at +1.0 V) and 20.16 Å (in the discharge state at −1.0 V) was achieved. The VOP electrodes in 0.3 M Mg(TFSI)2/AN delivered a high specific capacity of 339.7 mAh·g−1 at 0.1 A·g−1, high rate capacity of 256.3 mAh·g−1 at 0.5 A·g−1, and long-term cyclic stability with a 0.065% decay rate and high capacity of 172.5 mAh·g−1 after 500 cycles. VOP delivered much higher specific capacities (320, 271, 220, 172, and 117 mAh·g−1) than pristine V2O5 (72.0, 67, 58, 50, and 43 mAh·g−1) at the current densities of 0.1, 0.2, 0.5, 1.0, and 2.0 A·g−1, respectively. The enhancing effect of water activation on the kinetics was confirmed. When 3 M H2O was added to 0.3 M Mg(TFSI)2/AN, the discharge capacities of VOP at 0.1 A·g−1 increased from 165 to 348 mAh·g−1, and the capacity of pristine V2O5 increased from 82 to 113 mAh·g−1. The improvement in the electrochemical performance of VOP and pristine V2O5 was attributed to the lowered desolvation energy of the solvated Mg2+-ions.
V2O5·H2O was synthesized and intercalated with polyacrylonitrile (PAN) by hydrothermal synthesis procedure [121]. The exact chemical composition of the PAN-intercalated oxide was V2O5·0.34H2O-PAN, and that of vanadium oxide used for comparison was V2O5·0.33H2O. According to XRD data, all peaks of the V2O5-PAN and V2O5·H2O were consistent with double-layer V2O5 (JCPDS No. 40-1296). The layer spacing increased from 9.91 Å in the pure V2O5·H2O to 10.63 Å in V2O5-PAN. It was shown that intercalation of PAN also induced cation reduction and generated abundant anion vacancies, thus improving ion diffusion and electron transfer kinetics. V2O5-PAN electrodes had perfect structural stability and electrochemical reversibility during charge–discharge. After stabilization, V2O5-PAN achieved a high specific discharge capacity of ~180 mAh·g−1 at 50 mA·g−1 in 0.5 M Mg(TFSI)2/AN electrolyte, and its life-span at 2 A·g−1 was 18,000 cycles.
The general tendency of positive effects observed with the introduction of conducting polymer molecules is explained by the formation of “pillars” expanding the interlayers, and also by the improvement in electrical conductivity and the effect of electrostatic shielding of the host from the high charge density of Mg2+. However, to date, the role of the composition/nature and the optimal fraction of conducting polymers introduced into the layered structure need further investigation.
3. Mechanisms of Intercalation and Role of Structural Water
The general operating principle of RMBs in dry aprotic electrolytes is thought to be similar to that of other metal ion batteries. It is based on the reversible intercalation/deintercalation of Mg2+-ions into the active material of the cathode. However, there is some controversy in the studies of the mechanism of Mg2+ intercalation in dry electrolytes; the reaction mechanism of α-V2O5 in RMBs with a dry electrolyte is not well understood.
A systematic study of Mg insertion into orthorhombic V2O5 was performed by combining electrochemical and structural methods [122]. The results for an electrochemically cycled V2O5 cathode in a full cell with Mg metal anode obtained by atomic-resolution transmission electron microscopy showed the local formation of the theoretically predicted ε-Mg0.5V2O5 phase; however, the intercalation level of Mg was low.
The investigations of the electrochemical behavior of orthorhombic α-V2O5 in dry and “wet” alkyl carbonate-based electrolytes and the insertion-driven structural changes of the active phase studied by ex situ X-ray diffraction showed that proton intercalation dominates the reaction of α-V2O5, even in a dry electrolyte, and the intercalation of Mg2+-ions is negligible [123].
In operando studies of the reaction mechanism of α-V2O5 cathodes in RMBs by synchrotron diffraction and in situ X-ray absorption near-edge spectroscopy (XANES) together with ex situ Raman and X-ray photoelectron spectroscopy [75] have shown the reversibility of magnesium ion intercalation and provided information on the evolution of the crystal structure and the change in oxidation degrees during charge–discharge cycling. It was shown that α-V2O5 transforms to a Mg-poor phase (Mg0.14V2O5) during discharging, and then undergoes a two-phase transition.
There are three related issues, the role of which in the case of vanadium oxide cathodes for RMBs should be considered in more detail:
- (i)
- Crystalline V2O5 is capable of reversible intercalation of Mg2+ in “wet” organic electrolytes, containing enough water molecules to coordinate to Mg2+-ions and shield their high charge during intercalation, thus allowing faster diffusion of Mg2+-ions within the host material.
- (ii)
- The incompatibility of metallic Mg anodes with wet electrolytes leads to the focus of the research on the bilayer V2O5, hydrated, and water-pillared vanadium oxides, where water molecules are naturally present in the lattice [104,108,111]. The interlayer water molecules effectively shield the interaction between Mg2+ and the host lattice, improving the reversibility of Mg2+-ion intercalation/deintercalation and structural stability of the cathode material during cycling.
Following the pioneering work [61], many researchers noted that V2O5 exhibits improved capacity in aprotic electrolytes containing small amounts of water. In particular, the study of the electrochemical performance of V2O5 cathodes in Mg(ClO4)2/PC and Mg(TFSI)2)/G2 electrolytes [124,125] showed that controllable amounts of water lead to increased specific capacities.
Water molecule intercalation into V2O5 structures improves Mg2+-ion diffusion kinetics due to enlarged interlayer spacing. In addition, the water molecules in the cathode increase electrostatic shielding, which is a very important factor for improving the migration rate of Mg2+-ions within the host material [47,124].
The experimental observations of the positive influence of water molecules in non-aqueous electrolytes on the charge storage properties of V2O5 (higher currents in CVs, higher capacities, lower polarization) were later extended by the experimental results pointing to the mechanism in which both protons and magnesium ions participate as charge carriers in the redox process.
Some works demonstrate co-intercalation of protons together with magnesium ions, providing higher capacity of vanadium oxide cathodes. A number of works demonstrate the dominant role of reversible proton insertion in the capacity of RMB in aqueous electrolytes and non-aqueous electrolytes containing small amounts of water.
The study of the influence of water additive in the organic electrolyte had shown that the presence of water enables higher capacities for an α-V2O5 cathode [125]. The capacity achieved for the wet electrolyte was ~260 mAh·g−1, which is close to the theoretical value. In dry electrolyte, a discharge capacity of only ~60 mAh·g−1 was achieved. Solid state NMR revealed that the major contribution towards the higher capacity in high water-containing electrolyte solution (1 M Mg(TFSI)2/G2 with 2600 ppm H2O) originates from the reversible proton insertion. On lowering the water level of the electrolyte (1 M Mg(TFSI)2/G2 with 15 ppm H2O), the evidence of reversible Mg intercalation was obtained by EDX of the discharged V2O5, which demonstrated the presence of a higher concentration of Mg in the discharged state compared to the charged and pristine V2O5 electrodes.
The intercalation of Mg2+ in α-V2O5 was again investigated in dry and water-containing organic electrolytes [126]. An improved electrochemical performance was observed after adding water to a dry organic electrolyte; this effect was explained by the co-intercalation of protons and Mg2+. The discharged product of α-V2O5 in a 0.5 M Mg(ClO4)2 + 2.0 M H2O/AN electrolyte was Mg0.17HxV2O5 (x = 0.66–1.16), indicating that the capacity is mainly due to proton intercalation.
The magnesium ion storage capability of a VO2(B) cathode in dry and wet 0.5 M Mg(ClO4)2/AN electrolytes was studied in [56]. The water content in dry and wet electrolytes was 40 and 650 ppm, respectively. In the dry electrolyte, VO2(B) deliveredan initial discharge capacity of 50.6 mAh·g−1 at a current density of 20 mA·g−1, with a coulombic efficiency of 94.4%. Under the same electrochemical test conditions, the initial discharge capacity of VO2(B) in the wet electrolyte was 251.0 mA·g−1, with a coulombic efficiency of 99.4 %. The high discharge capacity observed in the wet electrolyte was attributed to predominant proton intercalation instead of magnesium intercalation. It was also shown that VO2(B) may suit as a high-performance cathode material only if its particles are relatively small, to exclude long distances for Mg2+-ion diffusion along the diffusion channels within the material.
The strongly polarizing nature of Mg2+-ions leads to slow intercalation kinetics. The structural water in host electrode materials facilitates migration of Mg2+-ions due to the “lubricating” effect; in addition, water molecules act as charge screening media to reduce the coulombic repulsion between the ions and the host lattice.
The enhanced insertion reaction of Mg2+-ions into the V2O5(H2O)y (2 < y < 3) xerogel, containing single and/or double layers of H2O molecules in the interlayer space of the oxide, was reported as early as 1992 in [127]. Bonded H2O molecules allowed smooth insertion and de-insertion of Mg2+-ions during the electrochemical cycling of V2O5 xerogel electrodes in a dry 1 M Mg(ClO4)2/AN electrolyte. The specific charge density 170 Ah·kg−1 was measured in the first cycle for V2O5 xerogel, while less than 50 Ah·kg−1 was observed in the first cycle for pure V2O5.
Layered H2V3O8 nanowires demonstrated high Mg2+-ion storage with a capacity of 304.2 mAh·g−1 at 50 mA·g−1 in a three-electrode cell with 0.5 M Mg(ClO4)2/AN electrolyte and activated carbon cloth as counter and reference electrodes [128]. In the cyclic voltammograms at a scan rate of 0.2 mV·s−1, one cathodic peak at ~2.0 V and two anodic peaks at 2.3 and 2.5 V (vs. Mg/Mg2+) were observed, which were ascribed to the insertion and extraction of magnesium ions. GCD curves showed that H2V3O8 nanowires possess a high working voltage platform of about 2.0 V. H2V3O8 nanowires demonstrated 85.9% capacity retention after 20 cycles at 50 mA·g−1.
Hydrated vanadium oxide H2V3O8 nanowires were synthesized via a one-step hydrothermal method [129]. The H2V3O8 nanowires had a diameter of 100–200 nm (Figure 10a,b) and an interlayer spacing of 0.34 nm (Figure 10c), corresponding to the d(011) spacing of H2V3O8. The material exhibited reversible magnesiation/demagnesiation with an initial discharge capacity of 80 mAh·g−1 and 231 mAh·g−1 at 10 mA·g−1 at 25 °C and 60 °C, respectively (Figure 10d), and an average discharge voltage of ~1.9 V (vs. Mg/Mg2+) in a dry (48 ppm H2O) 0.5 M Mg(ClO4)2/AN electrolyte. The discharge capacities of H2V3O8 at 60 °C were 231, 201, 170, and 97 mAh·g−1 at the current densities of 10, 20, 40, and 80 mA·g−1, respectively (Figure 10e). The capacity observed at 25 °C in dry electrolyte was much lower than the value of 304.2 mAh·g−1 reported for H2V3O8 in [128] due to the difference in the water content in the organic electrolyte. In the wet electrolyte (5790 ppm H2O), it increased to ~260 mAh·g−1. The structural water of H2V3O8 (V3O7·H2O) remained stable during cycling. The chemical formula for the discharged electrode at 60 °C was calculated as Mg1.22H2V3O8, considering that one electron charge transfer corresponds to 94.75 mAh·g−1 per formula unit of H2V3O8. The analysis of the discharged electrode suggested the chemical formula Mg0.97H2V3O8 (Figure 10f). The difference of ~20% was explained by the thorough washing of the electrodes during the sample preparation for elemental analysis, when the bulk-intercalated Mg2+-ions remained and the surface ions were washed out. A homogeneous distribution of Mg2+-ions was observed in the vanadium oxide particles of the discharged electrode, while they were absent in the initial or charged samples (Figure 10 g–i).
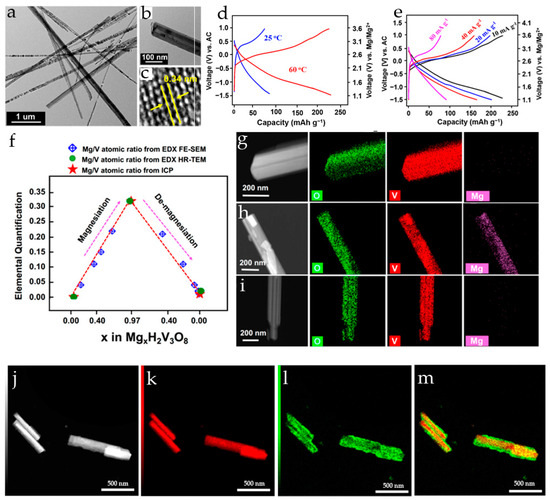
Figure 10.
(a,b) TEM images of H2V3O8 nanowires, (c) HR-TEM image of H2V3O8 nanowires, (d) initial GCD profiles of H2V3O8 in 0.5 M Mg(ClO4)2/AN at 25 and 60 °C at a current density of 10 mA·g−1, (e) initial GCD profiles H2V3O8 at 60 °C at various current densities, (f) Mg/V atomic ratios for MgxH2V3O8 electrodes during the discharge–charge cycle, (g) FE-TEM EDX elemental mapping of initial (x = 0) electrode, (h,i) FE-TEM EDX elemental mapping of discharged (x = 0.97), and charged (x = 0) electrodes. (Reprinted with permission from [129]. Copyright 2018, American Chemical Society). (j) HAADF STEM image of HVO nanofibers cycled in wet electrolyte, (k,l) corresponding V-K edge and Mg-K edge EDX intensity maps, (m) overlay of V-K and Mg-K edge EDX intensity maps. (Reprinted with permission from [130]. Copyright 2022, Elsevier).
It was shown that the kinetic and the electrochemical properties of H2V3O8 (HVO) are improved by adding water to a dry organic electrolyte [130]. HVO nanofibers were synthesized by exfoliation of V2O5 followed by a hydrothermal process. H2V3O8 delivered an initial specific capacity of 303 mAh·g−1 at a current density of 50 mA·g−1 in a wet Mg(ClO4)2·3.1H2O/AN electrolyte, four times higher than in a dry electrolyte (67 mAh·g−1). Although the presence of water in the aprotic electrolyte was beneficial for the specific capacity, the capacity retention in the wet electrolyte was 80% after 30 cycles at 50 mA·g−1 and 61% after 200 cycles at 100 mA·g−1, while in the dry electrolyte the capacity retention was 99% after 30 cycles at 50 mA·g−1 and 109% after 200 cycles at 100 mA·g−1, demonstrating the long-term stability and the reversibility of the charge–discharge process in the dry electrolyte. In an “ambient” Mg(ClO4)2·1.4H2O/AN electrolyte, the HVO cathode delivered an initial specific capacity of 167 mAh·g−1 at a current density of 50 mA·g−1 and showed a capacity retention of 73% after 30 cycles, suggesting that the water content in the electrolyte affects the reversibility of the charge–discharge processes. The structural changes in HVO as a function of the water content in the electrolyte were studied by in operando XRD during galvanostatic cycling. It was observed that the HVO structure almost fully recovered after two discharge/charge cycles in dry electrolyte, while in the presence of water, irreversible changes in the lattice parameters occurred in the initial cycle followed by a reversible discharge/charge cycle. In the presence of water, the changes in lattice parameters and unit cell volume during discharge/charge were 8 times larger, supporting the hypothesis that H2O molecules from a hydration shell co-intercalate with Mg2+ ions, thus leading to a decrease in the cycling stability of HVO in electrolytes with water content. In the wet and “ambient” electrolytes, the insertion of ~0.60 equivalent Mg2+ into HVO took place. In the wet electrolyte, STEM/EDX (Figure 10 j–m) revealed that the inserted Mg2+-ions were mainly accumulated close to the surface and at the edges of the HVO fibers, but did not diffuse into the bulk. This explained the high reversibility of the insertion process and the small lattice parameter changes.
Introducing water molecules into the lattice material together with cation pre-intercalation is also an effective strategy for improving the electrochemical performance of V2O5 in RMBs. Mn2+-ions were introduced into the hydrated vanadium oxide by a hydrothermal method in [111]. The experimental results revealed the synergetic effect of both Mn2+ ions and crystal water in the Mn0.04V2O5·1.17H2O nanobelts, improving the structural and cycle stability of the cathode in the Mg(TFSI)2/AN electrolyte. The Mn0.04V2O5·1.17H2O material exhibited a high specific capacity (145 mAh·g−1 at 50 mA·g−1), good rate capability (50 mAh·g−1 at 4 A·g−1), and long cycle stability (82% capacity retention after 10,000 cycles at 2 A·g−1), better than that of water-free Mn0.04V2O5. The in situ and ex situ material characterizations revealed that the structure of Mn0.04V2O5·1.17H2O was stable during the charge–discharge process.
Water-lubricated and aluminum ion-pillared vanadate H11Al2V6O23.2 (HAlVO) was reported as a high-performance cathode material for Mg2+ ion storage [113]. The capacity fade mechanism of water-free aluminum vanadate AlV3O9 (AlVO) was also investigated. The charge transfer process in water-lubricated and water-free aluminum vanadates was analyzed by DFT calculations, and the different charge transfer processes in the two materials and the charge shielding effect of the water molecule in HAlVO were revealed. The comparison of the cyclic voltammograms of HAlVO and AlVO showed that the shape of the CVs and the position of the redox peaks of the two materials are different: HAlVO had more intense reduction and oxidation peaks than AlVO (Figure 11a). HAlVO displayed higher initial specific capacity and a more stable cycle performance (165 mAh·g−1 in the 1st and 50th cycles), while AlVO exhibited a capacity of only 125 mAh·g−1 in the 1st cycle, which decreased to 105 mAh·g−1 in the 50th cycle (Figure 11b,c). Water-lubricated HAlVO had better cycle performance at all current densities. The diffusion coefficients were obtained for HAlVO and AlVO from GITT curves at different stages of Mg2+ insertion. At the initial stage of Mg2+ insertion, DHAlVO was 4.45 × 10−11 cm2·s−1 and DAlVO was 2.77 × 10−11 cm2·s−1. When the amount of inserted Mg2+ reached 100%, DHAlVO decreased to 1.01 × 10−11 cm2·s−1 and DAlVO to 2.12 × 10−12 cm2·s−1. The faster decrease in the diffusion rate of Mg2+ in AlVO than in HAlVO was explained by larger interlayer spacing of HAlVO and the shielding effect of interlayer water molecules, providing more active storage sites for Mg2+, enough diffusion space, and less charge repulsion when a large amount of Mg2+-ions is intercalated into HAlVO. The scheme of reversible insertion/extraction of Mg2+ in HAlVO is shown in Figure 11d. Pre-intercalated Al3+ ions act as “pillars” which stabilize the V-O layered structure both in discharged and charge states, which improves the cyclic stability of the electrode. When Mg2+ ions are inserted, the water molecules in the interlayer space facilitate achieving local electroneutrality and a lower Mg diffusion barrier; this improves the rate performance of HAlVO. The DFT calculation results suggested that the water molecules also reduce the insertion energy barrier of Mg2+, thus improving the specific capacity of HAlVO. The charge transfer occurs mainly from the Mg2+ to O of the water molecule, and less from Mg2+ to O in the V-O layer, thus probably preventing the structural decay of the cathode during cycling.
Figure 11.
(a) The first two CVs of HAlVO and AlVO at the scan rate of 1 mV·s−−1, (b) the first and fiftieth charge and discharge curves of HAlVO and AlVO at the current density of 100 mA·g−1, (c) rate performance of HAlVO and AlVO at the current densities of 0.1–4.0 A·g−1, (d) schematic of the reversible insertion/extraction of Mg2+ ions in water-lubricated HAlVO. (Reprinted with permission from [113]. Copyright 2022, John Wiley and Sons). (e) Migration of Mg2+ ions along the a direction in the interlayer of MVOH during the H2O-Mg2+ waltz-like shuttle, (f) in situ Raman spectra of the MVOH cathode at different discharge/charge states, (g) depth profile of H+, (h,i) TOF-SIMS imaging of H+, O+, Mg+, MgOH+, MgVO−, and CF3SO3− in fully discharged and charged electrode [107].
A Mg2+ pre-intercalated hydrated V10O24·nH2O layered material was obtained [107]. Mg0.75V10O24·4H2O (MVOH) had an interlayer spacing of 13.9 Å, much larger than that of the hydrated MgxV5O12 (11.9 Å) reported in [108]. The nanoflower morphology greatly increased the specific surface area of the MVOH (33.07 m2·g−1) and provided short diffusion paths for Mg2+ from bulk solution to active sites, accelerating the insertion/extraction of Mg2+. The diffusion coefficient of Mg2+ in the MVOH calculated from the EIS data was as high as 1.08 × 10−9 cm2·s−1, indicating fast migration kinetics inside the MVOH crystal lattice. In a three-electrode cell with a platinum counter electrode, a saturated calomel reference electrode (SCE), and 2 M Mg(CF3SO3)2 aqueous electrolyte, the MVOH cathode exhibited a high discharge capacity of 350 mAh·g−1 (at 0.05 A·g−1) and 70 mAh·g−1 at a higher current density of 4 A·g−1. The full cell with a MVOH cathode, perylene-3,4,9,10-tetracarboxylic dianhydride (PTCDA) as an anode, and 2 M Mg(CF3SO3)2 in PEG/H2O (1:1) electrolyte, delivered a discharge capacity of 133 mAh·g−1 at a current density of 0.02 A·g−1 and 42 mAh·g−1 at a high current density of 4 A·g−1, demonstrating high rate capability and satisfactory cycling stability with a capacity retention of 62% after 5000 cycles at 4 A·g−1. DFT calculations performed on the basis of structural characterization results demonstrated that pre-intercalated Mg2+-cations and structural H2O molecules stabilize the layered structure of MVOH. Based on the determined pre-intercalated structure of MVOH, DFT calculations were performed to identify the energetically favorable sites for Mg2+ to occupy and the nearest neighboring site for Mg2+ to hop to. Based on the relaxed structures, the waltz-like like shuttle mechanism of H2O molecules coordinated with Mg2+ transport was demonstrated during the charge–discharge of the MVOH cathode. It was shown that the Mg2+-ion migrates together with the coordinated water molecules, which adjust their orientation by rotating or flipping to facilitate the zig-zag migration of Mg2+-ions (Figure 11e). After the extraction of Mg2+ from MVOH, the Mg2+-H2O bonds break, and the lattice H2O molecules rearrange and form H-bonds in the interlayer of MVOH to stabilize the lamellar structure. To verify the Mg2+ shuttling of lattice H2O molecules, the MVOH cathode was investigated by in situ Raman spectroscopy during discharging/charging, and a variation in the peak at 871 cm−1, corresponding to the V-OH2 bonds, at different discharged/charged states, was observed (Figure 11f). To further verify the Mg2+ shuttle function of lattice H2O molecules, the fully discharged and charged MVOH cathode was analyzed by time of flight–secondary ion mass spectrometry (TOF-SIMS). The much higher intensity of the MgVO− signal in the discharged MVOH than in the charged MVOH indicates successful insertion of Mg2+ during discharge, and the higher MgOH+ signal in the discharged MVOH than in the charged MVOH indicates strong coordination of lattice H2O molecules with Mg2+-ions inserted during discharge, confirming the H2O–Mg2+ shuttle mechanism. The same intensity and distribution of the H+ signal in discharged and charged MVOH indicate that the H2O-Mg2+ waltz-like shuttle mechanism eliminates the co-insertion or co-extraction of H+ together with Mg2+ (Figure 11g–i).
To sum up, the investigations of vanadium oxide-based cathodes in RMBs show that the water content, even in trace amounts, has an important influence on the mechanism of intercalation processes in organic electrolytes. Although the exact mechanism of the effect of the water molecules present in organic electrolytes on the increase in the specific capacity of vanadium oxide-based cathodes is not fully understood, it has been emphasized by many researchers. The following explanations have been proposed:
- (i)
- Water molecules can solvate the magnesium ions and shield their divalent charge to facilitate insertion into the interlayer spaces of VO-based cathodes.
- (ii)
- Water molecules inserted together with magnesium ions solvate the interstitial space and inner -V=O groups, and can lubricate Mg2+ diffusion.
- (iii)
- Insertion or co-insertion of protons (from water molecules) takes place.
- (iv)
- Water molecules can react with the surface -V=O groups to form stable structural hydroxyl groups to reduce the migration barrier of magnesium insertion.
A better understanding of these effects is also necessary for the proper design of compatible cathodes and electrolytes.
For more illustrative comparison, the main electrochemical characteristics of selected vanadium oxide-based cathode materials are listed in Table 2.

Table 2.
Summary of electrochemical performance of selected vanadium oxide-based cathodes in RMBs.
4. Conclusions
RMBs are probably one of the most promising next-generation energy storage technologies due to their high theoretical energy density, high safety, abundant resources, and low cost. The design of novel cathode materials for RMBs is the focus of investigations for the future development of RMBs.
Vanadium oxide-based cathode materials present many advantages, including low cost, high safety, and great opportunity to tune the ion transport channels, which resulted in their improved electrochemical performance in RMBs.
Although great progress has been made in the development of vanadium oxide-based cathodes for RMBs, a number of challenges remain to be overcome in the future. The main challenge in the development of cathodes for RMBs is the sluggish kinetics of Mg2+-ion intercalation and diffusion in electrode materials due to the strong interaction between doubly charged Mg2+-ions and vanadium oxide host materials.
For the development of new cathodes with improved electrochemical performance, different synthetic procedures that allow new morphological modifications of vanadium oxide-based materials, pre-intercalation of metal ions and organic (mono- and polymeric) molecules into the interlayer space can be proposed. Pre-intercalated species act as pillars between the layers, stabilize the crystal structure of the material, and prevent amorphization and dissolution of oxides during repeated insertion/extraction of Mg2+-ions. A pre-intercalation strategy allows the acquisition of materials with increased reversibility of Mg2+ (de)intercalation processes and increased interlayer/channel distances, which significantly improve the Mg2+-ion diffusion kinetics and possibly reduce the desolvation barriers. Deeper understanding of the effects of different pre-intercalation strategies, their optimization, and their combination with other approaches will facilitate the achievement of high-performance RMB cathodes. Further searching for possible modifiers that act as pillars represents a perspective means of cathode improvement.
Modification of vanadium oxides with highly conductive materials, such as nanocarbons, and especially conducting polymers, is also a promising means of enhancing the intrinsic conductivity of vanadium-based electrode materials and facilitating Mg2+ migration.
Excessive removal of interlayer water results in the loss of capacity of highly crystalline vanadium oxide structures. Mild annealing of vanadium oxides during synthesis should be considered to preserve an adequate amount of crystal water necessary to lubricate the interlayer space and weaken an interaction between oxygen sub-lattices and Mg2+ ions, providing electrostatic shielding. In combination with pre-intercalated metal ions or organic molecules, interlayer water also plays an important role in maintaining sufficient interlayer space. The role that traces of water in the organic electrolytes play in facilitating Mg2+ ion insertion also needs to be elucidated for further design of high-performance RMBs.
In addition to designing cathode materials with improved electrochemical performance, electrode/electrolyte interphase control is an important issue for stable electrochemical performance in RMBs. More attention should also be paid to the development of electrolytes compatible with cathodes and anodes.
Author Contributions
All authors contributed equally to this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (grant № 24-23-00224).
Data Availability Statement
There are no research data to share.
Acknowledgments
On of us (R.H.) appreciates the generous hospitality of Y. Wu at Southeast University in Nanjing, China, during the preparation of this manuscript and inspiring discussions on the topic with Weijia Fan.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AN | acetonitrile |
| APC | all-phenyl complex electrolyte/phenylmagnesium chloride (PhMgCl + AlCl3) |
| CTAB | cetyltrimethylammonium bromide |
| DME | dimethoxyethane |
| EA | ethyl acetate |
| G2 | diglyme |
| PC | propylenecarbonate |
| PEDOT | poly(3,4-ethylenedioxythiophene) |
| PY14TFSI | 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide |
| TFSI | trifluoromethylsulfonylimide |
| THF | tetrahydrofurane |
| TMS | tetramethylsilane |
References
- Yoshimoto, N.; Yakushiji, S.; Ishikawa, M.; Morita, M. Rechargeable magnesium batteries with polymeric gel electrolytes containing magnesium salts. Electrochim. Acta 2003, 48, 2317. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652. [Google Scholar] [CrossRef]
- Levi, E.; Gofer, Y.; Aurbach, D. On the way to rechargeable Mg batteries: The challenge of new cathode materials. Chem. Mater. 2010, 22, 860. [Google Scholar] [CrossRef]
- Yoo, H.D.; Shterenberg, I.; Gofer, Y.; Gershinsky, G.; Pour, N.; Aurbach, D. Mg rechargeable batteries: An on-going challenge. Energ. Environ. Sci. 2013, 6, 2265. [Google Scholar] [CrossRef]
- Medina, A.; Pérez-Vicente, C.; Alcántara, R. Advancing towards a practical magnesium ion battery. Materials 2021, 14, 7488. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.F.; Li, H.Y.; Ren, W.; Gao, D.; Chen, F.; Diao, J.; Xie, B.; Huang, G.; Wang, J.; Pan, F. A critical review of vanadium-based electrode materials for rechargeable magnesium batteries. J. Mater. Sci. Technol. 2023, 153, 56–74. [Google Scholar] [CrossRef]
- Kulova, T.L.; Fateev, V.N.; Seregina, E.A.; Grigoriev, A.S. A brief review of post-lithium-ion batteries. Int. J. Electrochem. Sci. 2020, 15, 7242–7259. [Google Scholar] [CrossRef]
- Holze, R. Between the Electrodes of a Supercapacitor: An Update on Electrolytes. Adv. Mater. Sci. Technol. 2024, 6, 0627771. [Google Scholar] [CrossRef]
- Leong, K.W.; Pan, W.; Yi, X.; Luo, S.; Zhao, X.; Zhang, Y.; Wang, Y.; Mao, J.; Chen, Y. Next-generation magnesium-ion batteries: The quasi-solid-state approach to multivalent metal ion storage. Sci. Adv. 2023, 9, eadh1181. [Google Scholar] [CrossRef]
- Blázquez, J.A.; Maca, R.R.; Leonet, O.; Azaceta, E.; Mukherjee, A.; Zhao-Karger, Z.; Li, Z.Y.; Kovalevsky, A.; Fernandez-Barquin, A.; Mainar, A.R.; et al. A practical perspective on the potential of rechargeable Mg batteries. Energy Environ. Sci. 2023, 16, 1964–1981. [Google Scholar] [CrossRef]
- Wu, Y.; Holze, R. Electrochemical Energy Conversion and Storage; WILEY-VCH: Weinheim, Germany, 2022. [Google Scholar]
- Huggins, R.A. Advanced Batteries—Materials Science Aspects; Springer: New York, NY, USA, 2009. [Google Scholar]
- Huggins, R.A. Energy Storage; Springer: New York, NY, USA, 2010. [Google Scholar]
- Shterenberg, I.; Salama, M.; Gofer, Y.; Levi, E.; Aurbach, D. The challenge of developing rechargeable magnesium batteries. MRS Bull. 2014, 39, 453–460. [Google Scholar] [CrossRef]
- Saha, P.; Datta, M.K.; Velikokhatnyi, O.I.; Manivannan, A.; Alman, D.; Kumta, P.N. Rechargeable magnesium battery: Current status and key challenges for the future. Prog. Mater. Sci. 2014, 66, 1–86. [Google Scholar] [CrossRef]
- Mohtadi, R.; Mizuno, F. Magnesium batteries: Current state of the art, issues and future perspectives. Beilstein J. Nanotechnol. 2014, 5, 1291–1311. [Google Scholar] [CrossRef]
- Massé, R.C.; Uchaker, E.; Cao, G. Beyond Li-ion: Electrode materials for sodium- and magnesium-ion batteries. Sci. China Mater. 2015, 58, 715–766. [Google Scholar] [CrossRef]
- You, C.; Wu, X.; Yuan, X.; Chen, Y.; Liu, L.; Zhu, Y.; Fu, L.; Wu, Y.; Guo, Y.G.; van Ree, T. Advances in rechargeable Mg batteries. J. Mater. Chem. A 2020, 8, 25601–25625. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Z.; Shi, H.; Du, A.; Sun, M.; Cui, G. Progress and perspective on rechargeable magnesium-ion batteries. Sci. China Chem. 2024, 67, 214–246. [Google Scholar] [CrossRef]
- Shah, R.; Mittal, V.; Matsil, E.; Rosenkranz, A. Magnesium-ion batteries for electric vehicles: Current trends and future perspectives. Adv. Mechan. Eng. 2021, 13, 16878140211003398. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, S.; Li, T.; Su, D.; Guo, S.; Wang, G. Recent advances in rechargeable magnesium-based batteries for high-efficiency energy storage. Adv. Energy Mater. 2020, 10, 1903591. [Google Scholar] [CrossRef]
- Liu, F.; Wang, T.; Liu, X.; Fan, L.Z. Challenges and recent progress on key materials for rechargeable magnesium batteries. Adv. Energy Mater. 2021, 11, 2000787. [Google Scholar] [CrossRef]
- Das, A.; Balakrishnan, N.T.M.; Sreeram, P.; Fatima, M.J.J.; Joyner, J.D.; Thakur, V.K.; Pullanchiyodan, A.; Ahn, J.H.; Raghavan, P. Prospects for magnesium ion batteries: A comprehensive materials review. Coord. Chem. Rev. 2024, 502, 215593. [Google Scholar] [CrossRef]
- Chen, S.; Fan, S.; Li, H.; Shi, Y.; Yang, H.Y. Recent advances in kinetic optimizations of cathode materials for rechargeable magnesium batteries. Coord. Chem. Rev. 2022, 466, 214597. [Google Scholar] [CrossRef]
- Kwon, B.J.; Lapidus, S.H.; Vaughey, J.T.; Ceder, G.; Cabana, J.; Key, B. Design strategies of spinel oxide frameworks enabling reversible mg-ion intercalation. Acc. Chem. Res. 2024, 57, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Li, H.; Zhang, H.; Zhang, Y.; Wang, K. Layered materials in the magnesium ion batteries: Development history, materials structure, and energy storage mechanism. Adv. Funct. Mater. 2023, 33, 2301974. [Google Scholar] [CrossRef]
- Huie, M.M.; Bock, D.; Takeuchi, E.S.; Marschilok, A.C.; Takeuchi, K.J. Cathode materials for magnesium and magnesium-ion based batteries. Coord. Chem. Rev. 2015, 287, 15–27. [Google Scholar] [CrossRef]
- Canepa, P.; Sai Gautam, G.; Hannah, D.C.; Malik, R.; Liu, M.; Gallagher, K.G.; Persson, K.A.; Ceder, G. Odyssey of multivalent cathode materials: Open questions and future challenges. Chem. Rev. 2017, 117, 4287–4341. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Gao, T.; Hou, S.; Wang, C. A critical review of cathodes for rechargeable Mg batteries. Chem. Soc. Rev. 2018, 47, 8804–8841. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; MacFarlane, D.R.; Kar, M. Mg cathode materials and electrolytes for rechargeable Mg batteries: A review. Batter. Supercaps 2019, 2, 115–127. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; Jiang, H.; Parkin, I.P.; Shearing, P.R.; Brett, D.J.L. Cathode design for aqueous rechargeable multivalent ion batteries: Challenges and opportunities. Adv. Funct. Mater. 2021, 31, 2010445. [Google Scholar] [CrossRef]
- Pryke, J.J.; Kennard, R.M.; Cussen, S.A. Cathodes for Mg batteries: A condensed review. Energy Rep. 2022, 8, 83–88. [Google Scholar] [CrossRef]
- Li, Z.; Häcker, J.; Fichtner, M.; Zhao-Karger, Z. Cathode materials and chemistries for magnesium batteries: Challenges and opportunities. Adv. Energy Mater. 2023, 13, 2300682. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, S.; Wu, Y.; Li, Y. Cathode Materials for Rechargeable Magnesium-Ion Batteries: A Review. Acta Phys. Chim. Sin. 2023, 39, 2205050. [Google Scholar] [CrossRef]
- Kotobuki, M.; Yan, B.; Lu, L. Recent progress on cathode materials for rechargeable magnesium batteries. Energy Storage Mater. 2023, 54, 227–253. [Google Scholar] [CrossRef]
- Shi, M.; Li, T.; Shang, H.; Zhang, D.; Qi, H.; Huang, T.; Xie, Z.; Qi, J.; Wie, F. A critical review of inorganic cathode materials for rechargeable magnesium ion batteries. J. Energy Storage 2023, 68, 107765. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, Z.; Zhang, Z.; Du, A.; Dong, S.; Li, Z.; Li, G.; Cui, G. Current design strategies for rechargeable magnesium-based batteries. ACS Nano 2021, 15, 15594. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, Y.; Zhang, Q.; Zhao, S.; Hu, Z.; Chou, S.L. Recent progress on layered cathode materials for nonaqueous rechargeable magnesium batteries. Small 2021, 17, 201902767. [Google Scholar] [CrossRef]
- Xu, R.; Gao, X.; Chen, Y.; Chen, X.; Cui, L. Research status and prospect of rechargeable magnesium ion batteries cathode materials. Chin. Chem. Lett. 2024, 35, 109852. [Google Scholar] [CrossRef]
- Maroni, F.; Dongmo, S.; Gauckler, C.; Marinaro, M.; Wohlfahrt-Mehrens, M. Through the maze of multivalent-ion batteries: A critical review on the status of the research on cathode materials for Mg2+ and Ca2+ ions insertion. Batter. Supercaps 2021, 4, 1221–1251. [Google Scholar] [CrossRef]
- Attias, R.; Sharon, D.; Goffer, Y.; Aurbach, D. Critical review on the unique interactions and electroanalytical challenges related to cathodes—Solutions interfaces in non-aqueous Mg battery prototypes. ChemElectroChem 2021, 8, 3229. [Google Scholar] [CrossRef]
- Li, M.; Ding, Y.; Sun, Y.; Ren, Y.; Yang, J.; Yin, B.; Li, H.; Zhang, S.; Ma, T. Emerging rechargeable aqueous magnesium ion battery. Mater. Rep. Energy 2022, 2, 100161. [Google Scholar] [CrossRef]
- Javed, M.; Shah, A.; Nisar, J.; Shahzad, S.; Haleem, A.; Shah, I. Nanostructured design cathode materials for magnesium-ion batteries. ACS Omega 2024, 9, 4229–4245. [Google Scholar] [CrossRef]
- Mensing, J.P.; Lomas, T.; Tuantranont, A. Advances in rechargeable magnesium batteries employing graphene-based materials. Carbon 2022, 197, 264–281. [Google Scholar] [CrossRef]
- Zhang, X.; Li, D.; Ruan, Q.; Liu, L.; Wang, B.; Xiong, F.; Huang, C.; Chu, P.K. Vanadium-based cathode materials for rechargeable magnesium batteries. Mater. Today Energy 2023, 32, 101232. [Google Scholar] [CrossRef]
- Guo, W.; Fu, D.; Song, H.; Wang, C. Advanced V-based materials for multivalent-ion storage applications. Energy Mater 2024, 4, 400026. [Google Scholar] [CrossRef]
- Johnston, B.; Henry, H.; Kim, N.; Lee, S.B. Mechanisms of water-stimulated Mg2+ intercalation in vanadium oxide: Toward the development of hydrated vanadium oxide cathodes for Mg batteries. Front. Energy Res. 2021, 8, 611391. [Google Scholar] [CrossRef]
- Esparcia, E., Jr.; Joo, J.; Lee, J. Vanadium oxide bronzes as cathode active materials for non-lithium-based batteries. CrystEngComm 2021, 23, 5267–5283. [Google Scholar] [CrossRef]
- Alcantara, R.; Lavela, P.; Edstrom, K.; Fichtner, M.; Le, T.K.; Floraki, C.; Aivaliotis, D.; Vernardou, D. Metal-ion intercalation mechanisms in vanadium pentoxide and its new perspectives. Nanomaterials 2023, 13, 3149. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Dose, W.M.; Boruah, B.D.; De Volder, M. In situ and operando analyses of reaction mechanisms in vanadium oxides for Li-, Na-, Zn-, and Mg-ions batteries. Adv. Mater. Technol. 2022, 7, 2100799. [Google Scholar] [CrossRef]
- Hu, P.; Hu, P.; Vu, T.D.; Li, M.; Wang, S.; Ke, Y.; Zeng, X.; Mai, L.; Long, Y. Vanadium oxide: Phase diagrams, structures, synthesis, and applications. Chem. Rev. 2023, 123, 4353–4415. [Google Scholar] [CrossRef] [PubMed]
- Cestarolli, D.T.; Guerra, E.M. Vanadium pentoxide (V2O5): Their obtaining methods and wide applications. In Transition Metal Compounds—Synthesis, Properties and Applications; Haider, S., Haider, A., Eds.; IntechOpen: London, UK, 2021; pp. 1–15. [Google Scholar]
- Kuang, C.; Zeng, W.; Li, Y. A review of electrode for rechargeable magnesium-ion batteries. J. Nanosci. Nanotechnol. 2019, 19, 12–25. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Cheng, Q.; Liao, F.; Yang, G.; Lu, X. Interlayer engineering of preintercalated layered oxides as cathode for emerging multivalent metal-ion batteries: Zinc and beyond. Energy Storage Mater. 2021, 38, 397–437. [Google Scholar] [CrossRef]
- Luo, T.; Liu, Y.; Su, H.; Xiao, R.; Huang, L.; Xiang, Q.; Zhou, Y.; Chen, C. Nanostructured-VO2(B): A high-capacity magnesium-ion cathode and its electrochemical reaction mechanism. Electrochim. Acta 2018, 260, 805–813. [Google Scholar] [CrossRef]
- Setiawan, D.; Chae, M.S.; Hong, S.T. Re-evaluating the magnesium-ion storage capability of vanadium dioxide, VO2(B): Uncovering the influence of water content on the previously overestimated high capacity. ChemSusChem 2023, 16, e202300758. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xu, H.; Bian, H.; Ge, Q.; Li, D.; Wang, A.; Sun, D. Synergistic effect of V3O7/VO2 film electrodes as high-performance cathode materials for magnesium-ion batteries. Solid State Sci. 2024, 149, 107445. [Google Scholar] [CrossRef]
- Livage, J. Vanadium pentoxide gels. Chem. Mater. 1991, 3, 578–593. [Google Scholar] [CrossRef]
- Yu, W.H.; Wang, D.Z.; Zhu, B.; Wang, S.J.; Xue, L.X. Insertion of bi-valence cations Mg2+ and Zn2+ into V2O5. Solid State Commun. 1987, 61, 271–273. [Google Scholar] [CrossRef]
- Gregory, T.D.; Hoffman, R.J.; Winterton, R.C. Nonaqueous electrochemistry of magnesium: Applications to energy storage. J. Electrochem. Soc. 1990, 137, 775–780. [Google Scholar] [CrossRef]
- Novák, P.; Desilvestro, J. Electrochemical insertion of magnesium in metal oxides and sulfides from aprotic electrolytes. J. Electrochem. Soc. 1993, 140, 140–144. [Google Scholar] [CrossRef]
- Amatucci, G.G.; Badway, F.; Singhal, A.; Beaudoin, B.; Skandan, G.; Bowmer, T.; Plitz, I.; Pereira, N.; Chapman, T.; Jaworski, R. Investigation of yttrium and polyvalent ion intercalation into nanocrystalline vanadium oxide. J. Electrochem. Soc. 2001, 148, A940–A950. [Google Scholar] [CrossRef]
- Gautam, G.S.; Canepa, P.; Abdellahi, A.; Urban, A.; Malik, R.; Ceder, G. The intercalation phase diagram of Mg in V2O5 from first-principles. Chem. Mater. 2015, 27, 3733–3742. [Google Scholar]
- Gautam, G.S.; Canepa, P.; Malik, R.; Liu, M.; Persson, K.; Ceder, G. First-principles evaluation of multi-valent cation insertion into orthorhombic V2O5. Chem. Commun. 2015, 51, 13619–13622. [Google Scholar] [CrossRef]
- Xiao, R.; Xie, J.; Luo, T.; Huang, L.; Zhou, Y.; Yu, D.; Chen, C.; Liu, Y. Phase transformation and diffusion kinetics of V2O5 electrode in rechargeable Li and Mg batteries: A first-principle study. J. Phys. Chem. C 2018, 122, 1513–1521. [Google Scholar] [CrossRef]
- Kulish, V.V.; Manzhos, S. Comparison of Li, Na, Mg and Al-ion insertion in vanadium pentoxides and vanadium dioxides. RSC Adv. 2017, 7, 18643–18649. [Google Scholar] [CrossRef]
- Trócoli, R.; Parajuli, P.; Frontera, C.; Black, A.P.; Alexander, G.C.B.; Roy, I.; Arroyo-de Dompablo, M.E.; Klie, R.F.; Cabana, J.; Palacín, M.R. β-V2O5 as magnesium intercalation cathode. ACS Appl. Energy Mater. 2022, 5, 11964–11969. [Google Scholar] [CrossRef]
- Parija, A.; Liang, Y.; Andrews, J.L.; De Jesus, L.R.; Prendergast, D.; Banerjee, S. Topochemically de-intercalated phases of V2O5 as cathode materials for multivalent intercalation batteries: A first-principles evaluation. Chem. Mater. 2016, 28, 5611–5620. [Google Scholar] [CrossRef]
- Andrews, J.L.; Mukherjee, A.; Yoo, H.D.; Parija, A.; Marley, P.M.; Fakra, S.; Prendergast, D.; Cabana, J.; Klie, R.F.; Banerjee, S. Reversible Mg-ion insertion in a metastable one-dimensional polymorph of V2O5. Chem 2018, 4, 564–585. [Google Scholar] [CrossRef]
- Johnson, I.D.; Nolis, G.; Yin, L.; Yoo, H.D.; Parajuli, P.; Mukherjee, A.; Andrews, J.L.; Lopez, M.; Klie, R.F.; Banerjee, S.; et al. Enhanced charge storage of nanometric ζ-V2O5 in Mg electrolytes. Nanoscale 2020, 12, 22150–22160. [Google Scholar] [CrossRef]
- Gershinsky, G.; Yoo, H.D.; Gofer, Y.; Aurbach, D. Electrochemical and spectroscopic analysis of Mg2+ intercalation into thin film electrodes of layered oxides: V2O5 and MoO3. Langmuir 2013, 29, 10964–10972. [Google Scholar] [CrossRef]
- Yoo, H.D.; Jokisaari, J.R.; Yu, Y.S.; Kwon, B.J.; Hu, L.; Kim, S.; Han, S.D.; Lopez, M.; Lapidus, S.H.; Nolis, G.M.; et al. Intercalation of magnesium into a layered vanadium oxide with high capacity. ACS Energy Lett. 2019, 4, 1528–1534. [Google Scholar] [CrossRef]
- Drosos, C.; Jia, C.; Mathew, S.; Palgrave, R.G.; Moss, B.; Kafizas, A.; Vernardou, D. Aerosol-assisted chemical vapor deposition of V2O5 cathodes with high rate capabilities for magnesium-ion batteries. J. Power Sources 2018, 384, 355–359. [Google Scholar] [CrossRef]
- Cheng, Y.; Shao, Y.; Raju, V.; Ji, X.; Mehdi, B.L.; Han, K.S.; Engelhard, M.H.; Li, G.; Browning, N.D.; Mueller, K.T.; et al. Molecular storage of Mg ions with vanadium oxide nanoclusters. Adv. Funct. Mater. 2016, 26, 3446–3453. [Google Scholar] [CrossRef]
- Fu, Q.; Sarapulova, A.; Trouillet, V.; Zhu, L.; Fauth, F.; Mangold, S.; Welter, E.; Indris, S.; Knapp, M.; Dsoke, S.; et al. In operando synchrotron diffraction and in operando X-ray absorption spectroscopy investigations of orthorhombic V2O5 nanowires as cathode materials for Mg-ion batteries. J. Am. Chem. Soc. 2019, 141, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Pan, M.; Zou, J.; Guo, R.; Zeng, X.; Ding, W. Surfactant induced formation of flower-like V2O5 microspheres as cathode materials for rechargeable magnesium batteries. Ionics 2019, 25, 5889–5897. [Google Scholar] [CrossRef]
- Mukherjee, A.; Taragin, S.; Aviv, H.; Perelshtein, I.; Noked, M. Rationally designed vanadium pentoxide as high capacity insertion material for Mg-ion. Adv. Funct. Mater. 2020, 30, 2003518. [Google Scholar] [CrossRef]
- Man, Y.; Fei, Y.; Duan, L.; Tian, R.; Li, A.; Yuan, Z.; Zhou, X. Self-assembled vanadium oxide nanoflowers as a high-efficiency cathode material for magnesium-ion batteries. Chem. Eng. J. 2023, 472, 145118. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Xiao, S.; Zhai, H.; Zhu, Y.; Cao, C. Microwave-assisted synthesis of metallic V6O13 nanosheet as high-capacity cathode for magnesium storage. Mater. Lett. 2022, 308, 131279. [Google Scholar] [CrossRef]
- Jiao, L.; Yuan, H.; Wang, Y.; Cao, J.; Wang, Y. Mg intercalation properties into open-ended vanadium oxide nanotubes. Electrochem. Commun. 2005, 7, 431–436. [Google Scholar] [CrossRef]
- Kim, R.H.; Kim, J.S.; Kim, H.J.; Chang, W.S.; Han, D.W.; Lee, S.S.; Doo, S.G. Highly reduced VOx nanotube cathode materials with ultra-high capacity for magnesium ion batteries. J. Mater. Chem. A 2014, 2, 20636. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, R.H.; Yun, D.J.; Lee, S.S.; Doo, S.G.; Kim, D.Y.; Kim, H. Cycling stability of a VOx nanotube cathode in mixture of ethyl acetate and tetramethylsilane-based electrolytes for rechargeable Mg-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 26657–26663. [Google Scholar] [CrossRef] [PubMed]
- Christensen, C.K.; Bojesen, E.D.; Sørensen, D.R.; Kristensen, J.H.; Mathiesen, J.K.; Iversen, B.B.; Ravnsbæk, D.B. Structural evolution during Li- and Mg-intercalation in vanadium oxide nanotube electrodes for battery applications. ACS Appl. Nano Mater. 2018, 1, 5071–5082. [Google Scholar] [CrossRef]
- Tang, P.E.; Sakamoto, J.S.; Baudrin, E.; Dunn, B. V2O5 aerogel as a versatile host for metal ions. J. Non-Cryst. Solids 2004, 350, 67–72. [Google Scholar] [CrossRef]
- Le, D.B.; Passerini, S.; Coustier, F.; Guo, J.; Soderstrom, T.; Owens, B.B.; Smyrl, W.H. Intercalation of polyvalent cations into V2O5 aerogels. Chem. Mater. 1998, 10, 682–684. [Google Scholar] [CrossRef]
- Moretti, A.; Passerini, S. Bilayered nanostructured V2O5·nH2O for metal batteries. Adv. Energy Mater. 2016, 6, 1600868. [Google Scholar] [CrossRef]
- Stojković, I.; Cvjetićanin, N.; Markovic, S.; Mitrić, M.; Mentus, S. Electrochemical behavior of V2O5 xerogel and V2O5 xerogel/C composite in an aqueous LiNO3 and Mg(NO3)2 solutions. Acta Phys. Pol. A 2010, 117, 837–840. [Google Scholar] [CrossRef]
- Inamoto, M.; Kurihara, H.; Yajima, T. Electrode performance of vanadium pentoxide xerogel prepared by microwave irradiation as an active cathode material for rechargeable magnesium batteries. Electrochemistry 2012, 80, 421–422. [Google Scholar] [CrossRef]
- Vujković, M.; Pašti, I.; Simatović, I.; Šljukić, B.; Milenković, M.; Mentus, S. The influence of intercalated ions on cyclic stability of V2O5/graphite composite in aqueous electrolytic solutions: Experimental and theoretical approach. Electrochim. Acta 2015, 176, 130–140. [Google Scholar] [CrossRef]
- Sai Gautam, G.; Canepa, P.; Richards, W.D.; Malik, R.; Ceder, G. Role of structural H2O in intercalation electrodes: The case of Mg in nanocrystalline xerogel-V2O5. Nano Lett. 2016, 16, 2426–2431. [Google Scholar] [CrossRef] [PubMed]
- Tepavcevic, S.; Liu, Y.; Zhou, D.; Uti, B.; Maser, J.; Zuo, X.; Chan, H.; Král, P.; Johnson, C.S.; Stamenkovic, V.; et al. Nanostructured layered cathode for rechargeable Mg-ion batteries. ACS Nano 2015, 9, 8194–8205. [Google Scholar] [CrossRef] [PubMed]
- Sa, N.Y.; Kinnibrugh, T.L.; Wang, H.; Gautam, G.S.; Chapman, G.W.; Vaughey, J.T.; Key, B.; Fister, T.T.; Freeland, J.W.; Proffit, D.L.; et al. Structural evolution of reversible Mg insertion into a bilayer structure of V2O5·nH2O xerogel material. Chem. Mater. 2016, 28, 2962–2969. [Google Scholar] [CrossRef]
- He, Q.; Wang, H.; Bai, J.; Liao, Y.; Wang, S.; Chen, L. Bilayered nanostructured V2O5 nH2O xerogel constructed 2D nano-papers for efficient aqueous zinc/magnesium ion storage. J. Colloid Interf. Sci. 2024, 662, 490–504. [Google Scholar] [CrossRef]
- Imamura, D.; Miyayama, M. Characterization of magnesium-intercalated V2O5/carbon composites. Solid State Ion. 2003, 161, 173–180. [Google Scholar] [CrossRef]
- Imamura, D.; Miyayama, M.; Hibino, M.; Kudo, T. Mg intercalation properties into V2O5 gel/carbon composites under high-rate condition. J. Electrochem. Soc. 2003, 150, A753–A758. [Google Scholar] [CrossRef]
- Sheha, E.; Makled, M.; Nouman, W.M.; Bassyouni, A.; Yaghmour, S.; Abo-Elhassan, S. Vanadium oxide/graphene nanoplatelet as a cathode material for Mg-ion battery. Graphene 2016, 5, 178–188. [Google Scholar] [CrossRef]
- Du, X.; Huang, G.; Qin, Y.; Wang, L. Solvothermal synthesis of GO/V2O5 composites as a cathode material for rechargeable magnesium batteries. RSC Adv. 2015, 5, 76352–76355. [Google Scholar] [CrossRef]
- An, Q.; Li, Y.; Yoo, H.D.; Chen, S.; Ru, Q.; Mai, L.; Yao, Y. Graphene decorated vanadium oxide nanowire aerogel for long cycle-life magnesium battery cathodes. Nano Energy 2015, 18, 265–272. [Google Scholar] [CrossRef]
- Pei, C.Y.; Jin, M.D.; Yin, Y.M.; Xiong, F.Y.; Jiang, Y.L.; Yuan, X.F.; Wang, F.; An, Q.Y. Intercalation-type V2O3 with fast Mg2+ diffusion kinetics for high-capacity and long-life Mg-ion storage. ACS Sustain. Chem. Eng. 2020, 8, 16164–16171. [Google Scholar] [CrossRef]
- Jang, G.; Joe, Y.S.; Lee, S.J.; Cho, H.G.; Baek, S.H.; Xiong, P.; Shin, K.H.; Yeon, J.S.; Kang, M.S.; Oh, S.H.; et al. Crystal reconstruction of V2O3/carbon heterointerfaces via anodic hydration for ultrafast and reversible Mg-ion battery cathodes. InfoMat 2024, 6, e12517. [Google Scholar] [CrossRef]
- Tang, H.; Xiong, F.; Jiang, Y.; Pei, C.; Tan, S.; Yang, W.; Li, M.; An, Q.; Mai, L. Alkali ions pre-intercalated layered vanadium oxide nanowires for stable magnesium ions storage. Nano Energy 2019, 58, 347–354. [Google Scholar] [CrossRef]
- Rashad, M.; Zhang, H.; Asif, M.; Feng, K.; Li, X.; Zhang, H. Low-Cost Room-Temperature Synthesis of NaV3O8·1.69H2O Nanobelts for Mg Batteries. ACS Appl. Mater. Interfaces 2018, 10, 4757–4766. [Google Scholar] [CrossRef]
- Wu, D.; Zeng, J.; Hua, H.; Wu, J.; Yang, Y.; Zhao, J. NaV6O15: A promising cathode material for insertion/extraction of Mg2+ with excellent cycling performance. Nano Res. 2020, 13, 335–343. [Google Scholar] [CrossRef]
- Sun, R.; Ji, X.; Luo, C.; Hou, S.; Hu, P.; Pu, X.; Cao, L.; Mai, L.; Wang, C. Water-pillared sodium vanadium bronze nanowires for enhanced rechargeable magnesium ion storage. Small 2020, 16, 2000741. [Google Scholar] [CrossRef]
- Muthuraj, D.; Sarkar, A.; Panda, M.R.; Adil, M.; Sagdeo, A.; Mitra, S. Zirconium-doped vanadium oxide and ammonium linked layered cathode to construct a full-cell magnesium-ion battery: A Realization and structural, electrochemical Study. Batter. Supercaps 2021, 4, 1757–1770. [Google Scholar] [CrossRef]
- Esparcia, E.A.; Chae, M.S.; Ocon, J.D.; Hong, S.T. Ammonium vanadium bronze (NH4V4O10) as a high-capacity cathode material for nonaqueous magnesium-ion batteries. Chem. Mater. 2018, 30, 3690–3696. [Google Scholar] [CrossRef]
- Ma, X.F.; Zhao, B.Q.; Liu, H.; Tan, J.; Li, H.Y.; Zhang, X.; Diao, J.; Yue, J.; Huang, G.; Wang, J. H2O-Mg2+ waltz-like shuttle enables high-capacity and ultralong-life magnesium-ion batteries. Adv. Sci. 2024, 11, 2401005. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, G.; Yin, J.; Lei, Y.; Emwas, A.H.; Yu, X.; Mohammed, O.F.; Alshareef, H.N. Hydrated MgxV5O12 cathode with improved Mg2+ storage performance. Adv. Energy Mater. 2020, 10, 2002128. [Google Scholar] [CrossRef]
- Lee, S.H.; DiLeo, R.A.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Sol gel based synthesis and electrochemistry of magnesium vanadium oxide: A promising cathode material for secondary magnesium ion batteries. ECS Electrochem. Lett. 2014, 3, A87–A90. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, X.; Li, Q.; Zhang, G.; Xiong, F.; Tan, S.; Wei, Q.; Lu, J.; Li, J.; An, Q. Vanadium oxide pillared by interlayer Mg2+ ions and water as ultralong-life cathodes for magnesium-ion batteries. Chem 2019, 5, 1194–1209. [Google Scholar] [CrossRef]
- Deng, X.; Xu, Y.; An, Q.; Xiong, F.; Tan, S.; Wu, L.; Mai, L. Manganese ion pre-intercalated hydrated vanadium oxide as a high-performance cathode for magnesium ion batteries. J. Mater. Chem. A 2019, 7, 10644–10650. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, X.; Luo, X.; Ding, Z.; Indris, S.; Sarapulova, A.; Meng, Z.; Desmau, M.; Wang, Z.; Hua, W. Ca2+ pre-intercalated bilayered vanadium oxide for high-performance aqueous Mg-ion batteries. Energy Storage Mater. 2024, 66, 103212. [Google Scholar] [CrossRef]
- Tang, H.; Chao, F.; Chen, H.; Jia, R.; Luo, H.; Xiong, F.; Yao, X.; Zhang, W.; Zuo, C.; Wang, J. Water-lubricated aluminum vanadate for enhanced rechargeable magnesium ion storage. Small 2022, 18, 2203525. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Pelliccione, C.J.; Lee, S.H.; Takeuchi, E.S.; Takeuchi, K.J.; Marschilok, A.C. Sol-gel synthesized magnesium vanadium oxide, MgxV2O5·nH2O: The role of structural Mg2+ on battery performance. J. Electrochem. Soc. 2016, 163, A1941–A1943. [Google Scholar] [CrossRef]
- Tolstopyatova, E.G.; Kamenskii, M.A.; Kondratiev, V.V. Vanadium oxide–conducting polymers composite cathodes for aqueous zinc-ion batteries: Interfacial design and enhancement of electrochemical performance. Energies 2022, 15, 8966. [Google Scholar] [CrossRef]
- Perera, S.D.; Archer, R.B.; Damin, C.A.; Mendoza-Cruz, R.; Rhodes, C.P. Controlling interlayer interactions in vanadium pentoxide-poly(ethylene oxide) nanocomposites for enhanced magnesium-ion charge transport and storage. J. Power Sources 2017, 343, 580–591. [Google Scholar] [CrossRef]
- Zuo, C.; Xiao, Y.; Pan, X.; Xiong, F.; Zhang, W.; Long, J.; Dong, S.; An, Q.; Luo, P. Organic-inorganic superlattices of vanadium oxide@polyaniline for high-performance magnesium-ion batteries. ChemSusChem 2021, 14, 2093–2099. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Ou, J.; Chen, L.; Sun, W.; Fu, F.; Zhang, H.; Chen, H. Interlayer engineering in V6O13 nanobelts toward superior Mg-ion storage. Inorg. Chem. Front. 2022, 10, 544–551. [Google Scholar] [CrossRef]
- Yao, Z.; Yu, Y.; Wu, Q.; Cui, M.; Zhou, X.; Liu, J.; Li, C. Maximizing magnesiation capacity of nanowire cluster oxides by conductive macromolecule pillaring and multication intercalation. Small 2021, 17, 2102168. [Google Scholar] [CrossRef] [PubMed]
- Joe, Y.S.; Kang, M.S.; Jang, G.; Lee, S.J.; Nakhanivei, P.; Baek, S.H.; Kim, Y.K.; Jeong, G.; Kim, H.S.; Park, H.S. Intercalation of bilayered V2O5 by electronically coupled PEDOT for greatly improved kinetic performance of magnesium ion battery cathodes. Chem. Eng. J. 2023, 460, 141706. [Google Scholar] [CrossRef]
- Deng, R.; Wang, Z.; Tan, S.; Huang, X.; Gao, Z.; Fang, Y.; Chen, C.; Wang, R.; Xu, C.; Huang, G.; et al. Optimization of the electrode reaction kinetics of V2O5 via polyacrylonitrile intercalation for high-performance magnesium batteries. Chem. Eng. J. 2024, 489, 151095. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sa, N.; Phillips, P.J.; Burrell, A.; Vaughey, J.; Klie, R.F. Direct investigation of Mg intercalation into the orthorhombic V2O5 cathode using atomic-resolution transmission electron microscopy. Chem. Mater. 2017, 29, 2218–2226. [Google Scholar] [CrossRef]
- Verrelli, R.; Black, A.P.; Pattanathummasid, C.; Tchitchekova, D.S.; Ponrouch, A.; Oro-Sol, J.; Frontera, C.; Bard, F.; Rozier, P.; Palacín, M.R. On the strange case of divalent ions intercalation in V2O5. J. Power Sources 2018, 407, 162–172. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, X. Electrochemical insertion of magnesium ions into V2O5 from aprotic electrolytes with varied water content. J. Colloid Interface Sci. 2004, 278, 160–165. [Google Scholar] [CrossRef]
- Sa, N.; Wang, H.; Proffit, D.L.; Lipson, A.L.; Key, B.; Liu, M.; Feng, Z.; Fister, T.T.; Ren, Y.; Sun, C.J.; et al. Is α-V2O5 a cathode material for Mg insertion batteries? J. Power Sources 2016, 323, 44–50. [Google Scholar] [CrossRef]
- Lim, S.C.; Lee, J.; Kwak, H.H.; Heo, J.W.; Chae, M.S.; Ahn, D.; Jang, Y.H.; Lee, H.; Hong, S.T. Unraveling the magnesium-ion intercalation mechanism in vanadium pentoxide in a wet organic electrolyte by structural determination. Inorg. Chem. 2017, 56, 7668–7678. [Google Scholar] [CrossRef] [PubMed]
- Novák, P.; Scheifele, W.; Joho, F.; Haas, O. Electrochemical insertion of magnesium into hydrated vanadium bronzes. J. Electrochem. Soc. 1995, 142, 2544–2550. [Google Scholar] [CrossRef]
- Tang, H.; Xu, N.; Pei, C.; Xiong, F.; Tan, S.; Luo, W.; An, Q.; Mai, L. H2V3O8 nanowires as high-capacity cathode materials for magnesium-based battery. ACS Appl. Mater. Interfaces 2017, 9, 28667–28673. [Google Scholar] [CrossRef] [PubMed]
- Rastgoo-Deylami, M.; Chae, M.S.; Hong, S.T. H2V3O8 as a high energy cathode material for nonaqueous magnesium-ion batteries. Chem. Mater. 2018, 30, 7464–7472. [Google Scholar] [CrossRef]
- Söllinger, D.; Redhammer, G.J.; Schoiber, J.; Zickler, G.A.; Pokrant, S. Exploring the insertion properties of Mg2+ in H2V3O8 as a function of the water content in the organic electrolyte. Electrochim. Acta 2022, 434, 141294. [Google Scholar] [CrossRef]
- Jiao, L.; Yuan, H.; Si, Y.; Wang, Y.; Cao, J.; Gao, X.; Zhao, M.; Zhou, X.; Wang, Y. Electrochemical insertion of magnesium in open-ended vanadium oxide nanotubes. J. Power Sources 2006, 156, 673–676. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).