1. Introduction
The photocatalytic reduction of CO
2 into carbonaceous fuels has attracted much attention because it offers a feasible strategy for reducing the consumption of fossil fuels and preventing the emission of greenhouse gases [
1,
2,
3,
4]. Various types of semiconductor-based photocatalysts, such as TiO
2, BiVO
4, In
2O
3, and WO
3, have been employed to transform CO
2 into high-value fuels, such as CO, CH
3OH, HCOOH, and CH
4 [
5,
6,
7]. However, the performance of these photocatalysts is limited by their insufficient active sites, low band gap energies, and high charge-carrier recombination rate. Hence, the elaborate design of advanced photocatalysts with outstanding performance and excellent stability is of great importance.
Metal sulfides are fascinating photocatalytic materials because they possess excellent photosensitivity, low redox potential, appropriate band edges, moderate band potential, high stability, good charge-carrier mobility, etc. In
2S
3 and CuInS
2, in particular, have emerged as promising catalysts owing to their visible-light response and appropriate band gaps (In
2S
3: 2–2.3 eV; CuInS
2: 1.57 eV) for CO
2 conversion [
8,
9,
10,
11]. For example, In
2S
3-based photocatalysts, such as In
2S
3/In
2O
3/rGO [
7], Bi
2S
3@In
2S
3 [
12], and In
2S
3/C
3N
4 [
13], can transform CO
2 into CO, CH
4, or C
2H
4. CuInS
2-based photocatalysts, such as Ti
3C
2 MXene@TiO
2/CuInS
2 [
14], ZnIn
2S
4/CuInS
2 [
15], and CuInS
2/ZnS [
16], also exhibit photocatalytic activity for the reduction of CO
2 to CO and H
2. Heterojunctions have recently been demonstrated to maximize the performance of In
2S
3/CuInS
2 photocatalysts and increase their ability for CO
2 reduction [
17,
18]. However, the synthesis of the accurate composition and morphological/structural design of multielement CuInS
2 semiconductor materials on the surface of In
2S
3 is challenging because of the difficulty of balancing the various reactivities of multiple reactants. Several studies have pointed out that the accurate composition and morphological/structural design of semiconductors are effective strategies for obtaining photocatalysts with outstanding performance and excellent stability [
19,
20]. Thus, further explorations of suitable strategies for designing special morphological In
2S
3/CuInS
2 photocatalysts are necessary.
Photocatalysts with three-dimensional flower-like structures are intriguing materials that have recently shown great potential for photocatalytic CO
2 reduction because of their intrinsic properties, which include a large surface area enriched with exposed active sites, which promotes CO
2 capture and conversion catalysis, a lessened perpendicular charge-transfer distance, which prevents electron (e
−)–hole (h
+) recombination, and internal reflection/dispersion effects, which reinforce light utilization [
21,
22]. However, the synthesis of flower-like micro/nanocrystals is challenging because it requires the refined tuning of nucleation, growth of particles, and surface binding dynamics for selective crystal face exposure. Hence, common strategies to prepare semiconducting flower-like micro/nanocrystals with controllable compositions and structures are highly desirable for achieving outstanding photocatalysts for carbonaceous fuel production.
Cation-exchange reactions, in which the bonding cations of the host sublattice are replaced by external mobile cations, present a promising method for fabricating hollow photocatalysts based on the Kirkendall effect [
23,
24]. For instance, using the Kirkendall effect driven by cation exchange between Cu
+ and In
3+, Li et al. synthesized controllable compositions and morphology/structures of hollow CuInS
2 nanododecahedrons [
25]. This type of cation exchange not only allows for multiple cations to be wholly or partly replaced with each other, but also achieves powerful synthetic control of the composition of the desired materials. Furthermore, semiconductor structures preserve the anisotropic shapes of the as-prepared templates. Wang et al. prepared various hollow multinary metal sulfides, including binary compounds (CdS, ZnS, Ag
2S, PbS, SnS), ternary compounds (CuInS
2, Zn
xCd
1−xS), and quaternary compounds (single-atom Pt-anchored Zn
xCd
1−xS, Zn
xCd
1−xS-Pt
1), using Cu
2−xS nanocubes as the initial template. In this case, Cu
+ was replaced by cations, the Cu
2−xS nanocube framework was conserved, and precise control of distinct elementary compositions was achieved by adjusting the ratio and type of the metal cation source, all of which significantly improved the photocatalytic performance of the resulting catalysts [
26]. It has been argued that this is a simple but efficient approach to attain precise compositions and morphological/structural of multicomponent semiconductor materials.
In this work, sulfur vacancy (Vs)-bearing In2S3/CuInS2 with microflower heterojunctions (denoted Vs-In2S3/CuInS2) were formed in situ using an In2S3 microsphere template-directed synthesis and metal ion exchange reaction. Hybrid photocatalysts with flower-like microspheres were obtained using hydrothermally synthesized In2S3 microspheres as a template, followed by Ostwald ripening growth during the metal cation exchange of Cu+ and In3+. The optimal heterostructured Vs-In2S3/CuInS2 microflowers exhibited excellent activity, with CO and CH4 evolution rates of 80.3 and 11.8 μmol g−1 h−1, respectively, under visible-light irradiation; these values are roughly 4 and 6.8 times higher than those reported for pristine In2S3, respectively. The enhanced photocatalytic performance of the Vs-In2S3/CuInS2 catalysts can be attributed to the synergistic effects of the following factors: (i) the constructed heterojunctions accelerate charge-carrier separation; (ii) the flower-like microspheres exhibit highly uniform morphologies and compositions, which enhance electron transport and light harvesting; and (iii) the vs. may trap excited electrons and, thus, inhibit charge-carrier recombination.
2. Results and Discussion
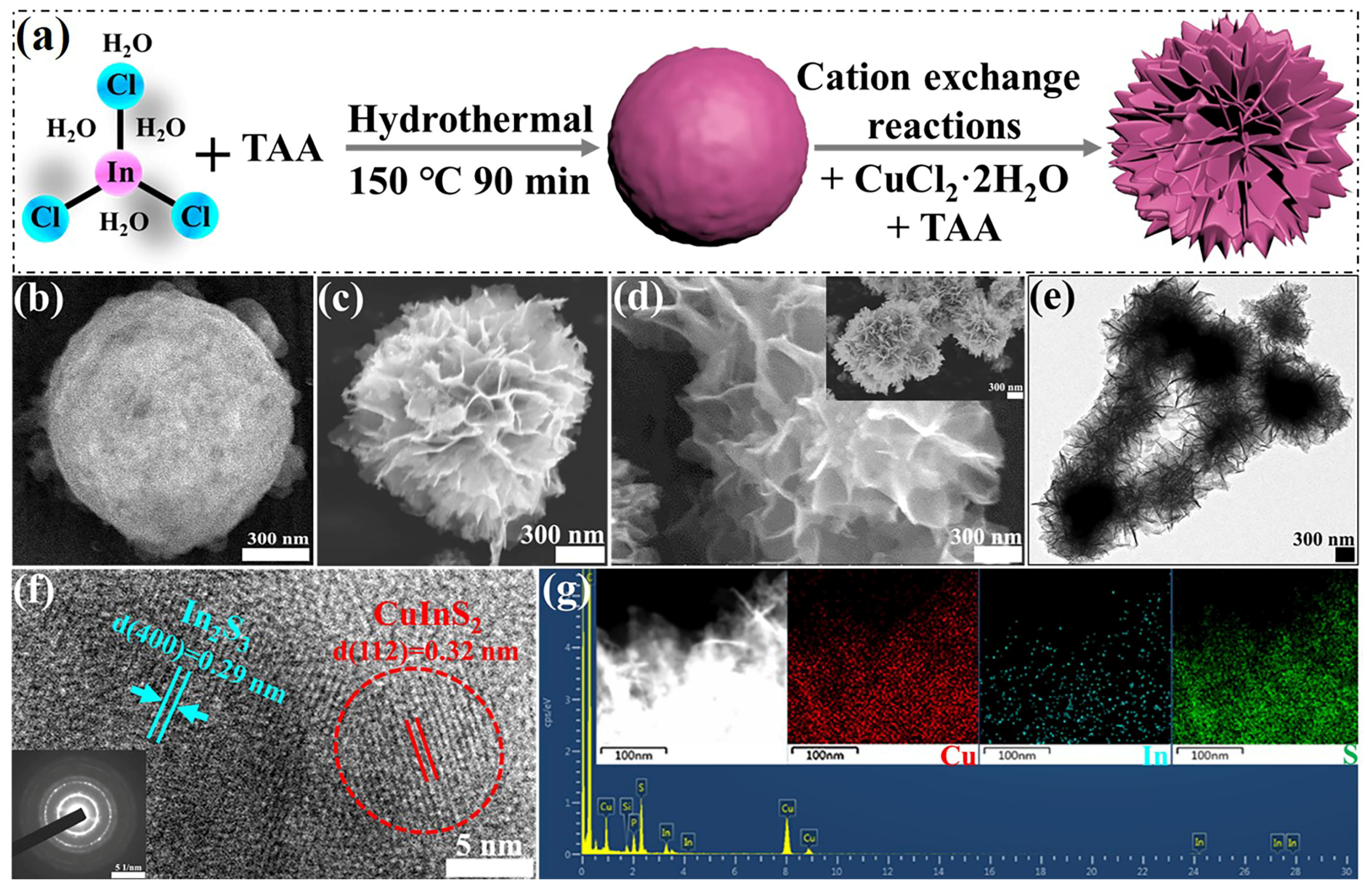
Figure 1a shows a schematic of the synthetic process for the Vs-In
2S
3/CuInS
2 composites by using an In
2S
3 microsphere template-assisted and cation exchange-mediated growth strategy. Typically, In
2S
3 template materials with controllable morphology are first synthesized by a facile low-temperature solvothermal method. The mixed solution, including an appropriate amount of the preprepared In
2S
3, CuCl
2·2H
2O, and thioacetamide (TAA), is then heated. The SEM image in
Figure 1b shows that the as-prepared In
2S
3 has a microspherical structure with a rough and uniform surface. The spherical architectures are composed of small nanoparticles, and the average diameter of all the spheres is in the range of 1–2 μm.
Figure 1c,d show SEM images of Vs-In
2S
3/CuInS
2, while
Figure 1e shows its TEM image. The images reveal that the as-derived Vs-In
2S
3/CuInS
2 maintains the spherical shape of the In
2S
3 microsphere template and that all of the spheres have a uniform morphology with an average diameter of 1–2 μm. However, the surfaces of the microspheres exhibit numerous flakes (thickness, <15 nm) aligned with each other. The unique morphology of Vs-In
2S
3/CuInS
2 may be derived from Ostwald ripening growth during the metal cation exchange of Cu
+ and In
3+. The HRTEM image shown in
Figure 1f reveals lattice spacings of 0.32 and 0.29 nm, which correspond to the (112) crystal plane of CuInS
2 and the (400) crystal plane of In
2S
3, respectively; these findings imply that CuInS
2 is orthotopically grown on the surface of the In
2S
3 microspheres, leading to the successful preparation of Vs-In
2S
3/CuInS
2. The selected area electron diffraction (SAED;
Figure 1f, inset) pattern of the Vs-In
2S
3/CuInS
2 composite shows that the flower-like microspheres are polycrystalline, indicating that CuInS
2 is generated along with Ostwald ripening growth spatial orientation during the cation-exchange process; this finding is consistent with the TEM results. The elemental composition of the composites, which includes Cu, In, and S, is confirmed by the elemental mappings shown in
Figure 1g.
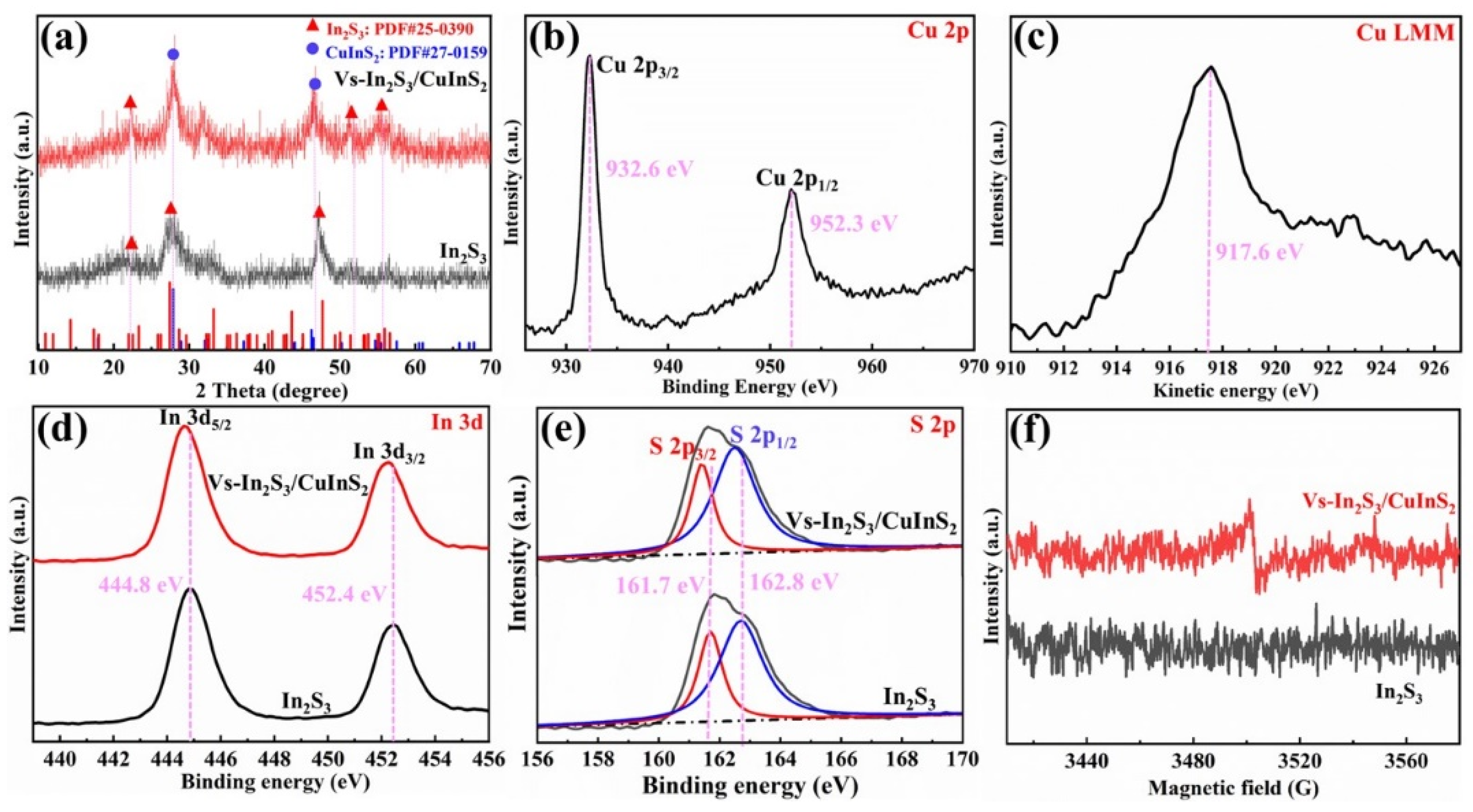
The XRD patterns shown in
Figure 2a reveal that all the diffraction peaks of In
2S
3 can be well indexed to the standard card (JCPDS No. 25-0390), and no other phase is observed (
Figure 2a). Furthermore, most of the diffraction peaks in the XRD pattern of Vs-In
2S
3/CuInS
2 can be indexed to the composite phase of In
2S
3 and CuInS
2 (In
2S
3: JCPDS No. 25-0390; CuInS
2: JCPDS No. 27-0159).
XPS was used to investigate the elemental composition and valence environment of the samples. The survey XPS profile of Vs-In
2S
3/CuInS
2 reveals the existence of Cu, In, and S (
Figure S1). The two peaks in the Cu 2p spectrum (
Figure 2b) with binding energies of 932.6 and 952.3 eV are assigned to Cu 2p
3/2 and Cu 2p
1/2 of Cu
+, respectively. These peaks coincide with the literature description of the valence state of Cu in CuInS
2, and verify the successful growth of CuInS
2 on In
2S
3. The peak in the Cu LMM (Auger electron) spectrum (
Figure 2c) at 917.6 eV reveals that the oxidation number of Cu is +1. The peaks in the XPS profile of In 3d (
Figure 2d) at 444.8 and 452.4 eV are ascribed to the core lines of the In–S bond. The binding energy of In 3d in In
2S
3 is 0.10 eV lower than that in Vs-In
2S
3/CuInS
2 owing to the existence of CuInS
2. The S 2p spectra of In
2S
3 and Vs-In
2S
3/CuInS
2 can be deconvoluted into two peaks with binding energies of 162.8 and 161.7 eV, which are consistent with S
2− in the crystal lattice. The binding energy of S 2p in In
2S
3 is 0.10 eV lower than that in Vs-In
2S
3/CuInS
2, probably because of the existence of vs. or CuInS
2 (
Figure 2e). The presence of vs. was further confirmed by EPR spectroscopy. Several vs. are formed on the In
2S
3/CuInS
2 surface because it is S-deficient owing to the sharing of S atoms (
Figure 2f). Numerous studies have shown that vs. defects can not only regulate the electronic structure/state of a photocatalyst but also serve as active sites to adsorb and activate CO
2 molecules, thus leading to improved photoactivity [
27,
28].
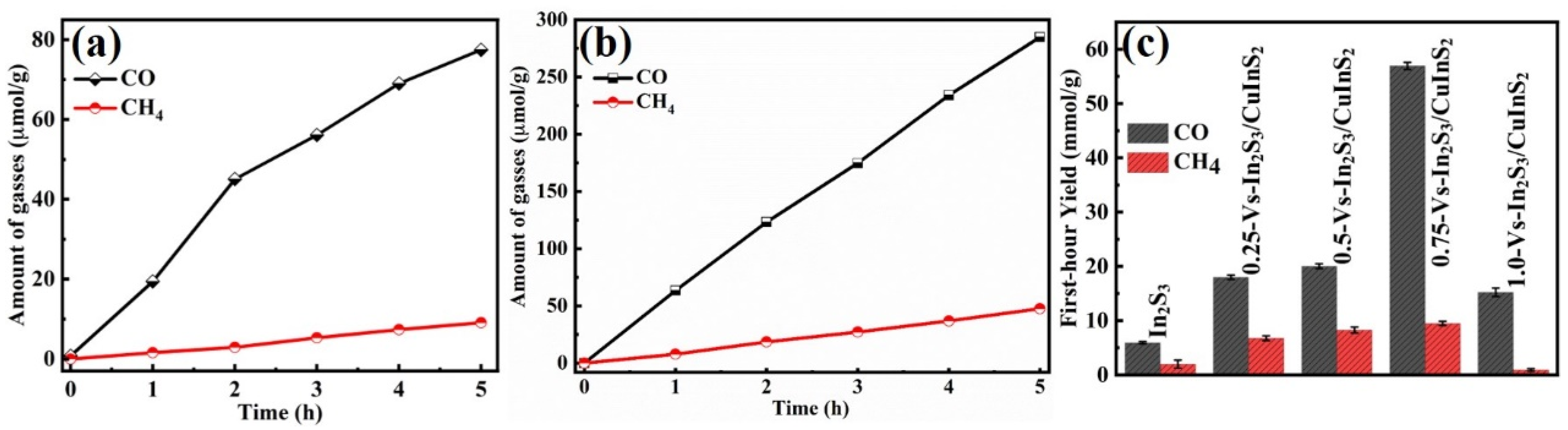
Photocatalytic CO
2 conversion was performed in the presence of water vapor under simulated solar irradiation (
Figure 3). CO as the major product and a small amount of CH
4 as the minor product were detected over In
2S
3 and Vs-In
2S
3/CuInS
2. In
2S
3 could produce 20 μmol g
−1 CO and 1.73 μmol g
−1 CH
4 after 1 h (
Figure 3a), and these yields gradually increase with increasing irradiation time. Compared with that of In
2S
3, the photocatalytic activity of Vs-In
2S
3/CuInS
2 is significantly improved, and the yields of CO and CH
4 over the catalyst gradually increase as the amount of CuCl
2·2H
2O increases from 0.25 to 0.75 g (
Figure 3b). Maximum yields of 80.3 μmol g
−1 CO and 11.8 μmol g
−1 CH
4 are achieved over 0.75-Vs-In
2S
3/CuInS
2 within 1 h; these yields are 4 and 6.8 times greater than those produced over In
2S
3, respectively. However, the use of 1.0 g of CuCl
2·2H
2O leads to a reduction in activity, possibly because excess vs. defects may accelerate the recombination of photogenerated carriers (
Figure 3c). The enhanced photocatalytic performance of the Vs-In
2S
3/CuInS
2 composite could be attributed to its flower-like microsphere structure, which features a large surface area that could promote the exposure of active sites and increase charge separation owing to the short charge-transport distance from the bulk to the surface. Moreover, the existence of vs. may trap excited electrons, thereby inhibiting charge-carrier recombination.
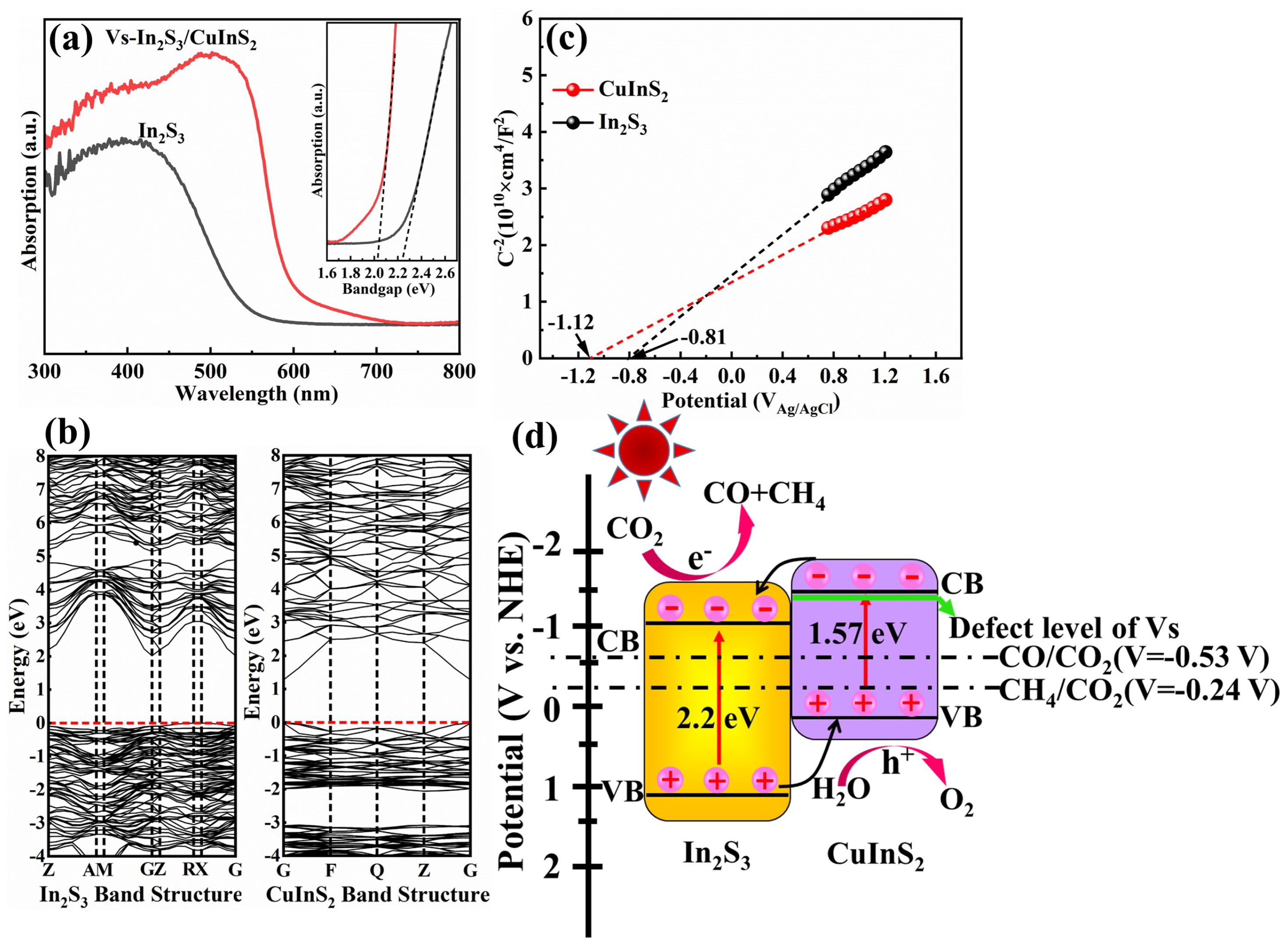
Considering that the photocatalytic behavior of semiconductors is closely related to their optical properties, UV-visible absorption spectroscopy was conducted on the samples (
Figure 4a). Vs-In
2S
3/CuInS
2 shows significant absorption over nearly the entire visible region up to 700 nm. Its corresponding band gap energy is estimated to be 2.02 eV (
Figure 4a, inset), which is lower than that of pure In
2S
3 (2.2 eV) owing to the low band gap energy of CuInS
2. The calculated band gap energy of CuInS
2 is 1.2 eV (
Figure 4b, right), which approximately matches the literature value of 1.57 eV [
11]. Theoretical calculations indicate that the band gap energy of In
2S
3 is approximately 2.13 eV, which closely matches the experimental result (
Figure 4b, left). These results illustrate the reliability of the experimental data. Additionally, the absorption intensity of Vs-In
2S
3/CuInS
2 is improved compared with that of In
2S
3 because of its flower-like microsphere structure. The flat band potentials (E
FB) of the samples were acquired from the tangent of their Mott–Schottky curves at a frequency of 1000 Hz (
Figure 4c). The E
FB values of In
2S
3 and CuInS
2 are −0.81 and −1.12 eV, respectively. In general, the E
FB is approximately 0.2 eV more positive than the conduction band edge (E
CB) of n-type semiconductors. Thus, the E
CB values of In
2S
3 and CuInS
2 can be estimated to be −1.01 and −1.32 eV, respectively. According to the equation E
VB = E
CB + E
g, the minimum positions of the corresponding conduction bands (CBs) of In
2S
3 and CuInS
2 may occur at 1.19 and 0.25 eV, respectively. The energy band structures of In
2S
3 and CuInS
2 are illustrated in
Figure 4d; the well-aligned bands shown in the figure meet the photocatalytic CO
2 reduction potential. Under light irradiation, the electrons are excited from the VB to the CBs of In
2S
3 and CuInS
2. Owing to the formation of heterojunctions, the excited electrons of CuInS
2 transfer to the CBs of In
2S
3. The photoinduced electrons then react with CO
2 to form CO and CH
4. Simultaneously, the photoinduced holes could transfer from the valence band of the In
2S
3 to that of CuInS
2. NaSO
3, as the sacrificial agent, reacts with holes to further inhibit the recombination of photoinduced electrons and holes [
29]. Moreover, previous work has reported that when vacancies are generated, a defect energy level will form in the forbidden band and the higher the sulfur vacancy concentration, the closer the defect level is to the CB position [
27]. The EPR test (
Figure 2d) verified that Vs exist in the In
2S
3/CuInS
2 sample; therefore, a defect energy level forms below the CBs of CuInS
2 (
Figure 4d), which could easily trap the photogenerated charge carriers.
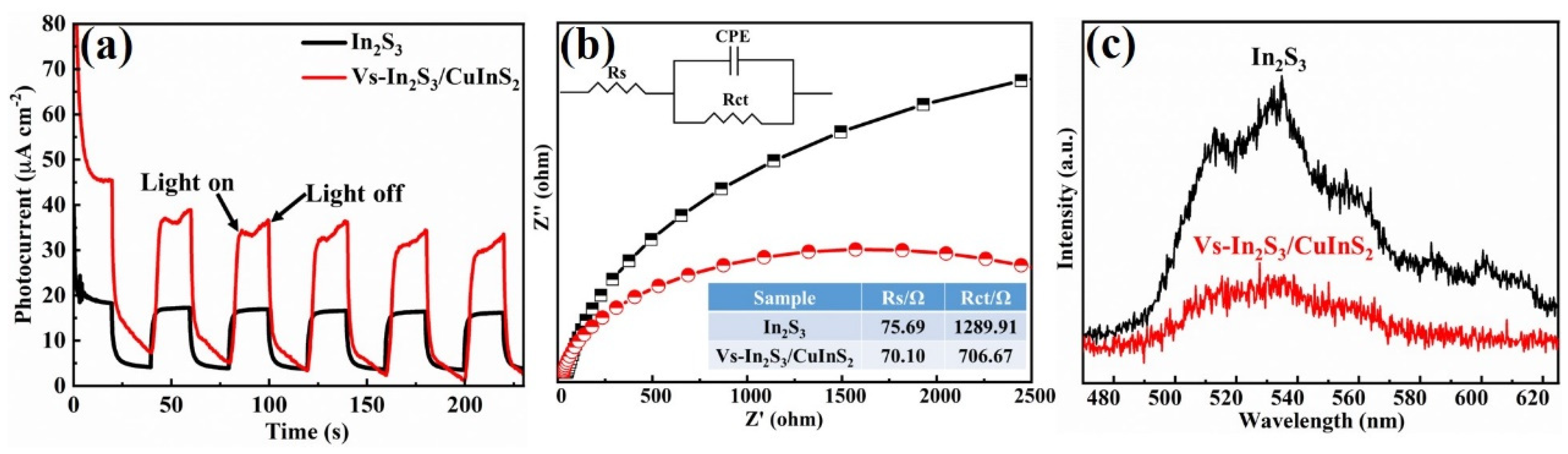
Figure 5a shows the transient photocurrent measurements of the as-prepared In
2S
3 and Vs-In
2S
3/CuInS
2 photoelectrodes under intermittent light irradiation; here, the light was switched on and off at 250 s intervals. The transient photocurrent for Vs-In
2S
3/CuInS
2 is higher than that for In
2S
3, indicating a higher charge-separation efficiency of the photogenerated carriers when using the Vs-In
2S
3/CuInS
2 composite. This result implies that the formation of a flower-like microsphere structure and heterojunctions enhance the separation efficiency of photoinduced charges, leading to improved photocatalytic performance. The Nyquist plots shown in
Figure 5b reveal that the extent of photocurrent enhancement exhibited by Vs-In
2S
3/CuInS
2 could be correlated with the reduction in the charge-transfer resistance of the material. Fitting the experimental data to an equivalent resistance/capacitance circuit model (
Figure 4a, inset) reveals that compared with In
2S
3, Vs-In
2S
3/CuInS
2 presents lower charge-transfer resistance, indicating easier charge transfer on the catalyst surface owing to the existence of a flower-like microsphere structure, Vs, and heterojunctions in the composite. The room-temperature steady-state PL of the developed catalyst was further investigated to understand its photogenerated charge-transfer mechanism. The intensity of the room-temperature steady-state PL peak of Vs-In
2S
3/CuInS
2 is weaker than that of In
2S
3 (
Figure 5c), indicating that the recombination efficiency of the photogenerated electrons and holes is low. Based on the above analyses, the enhanced photocatalytic performance of the Vs-In
2S
3/CuInS
2 catalysts could be attributed to the synergistic effect of the following factors: (i) the formation of a flower-like microsphere structure with high morphological and compositional uniformity, which enhances light harvesting and electron transport; (ii) the acceleration of charge-carrier separation by the constructed heterojunctions; and (iii) the introduction of Vs, which may trap excited electrons and inhibit charge-carrier recombination.
To reveal the underlying reasons for the improved CO
2 photoreduction performances over the Vs-In
2S
3/CuInS
2 heterostructure, a possible mechanism of selective photocatalytic CO
2 reduction is proposed. Prior reports [
30,
31] indicate that the selectivity of methane during CO
2 reduction is strongly related to the reaction path of CO* to CHO*, which determines whether the CO* intermediates desorb to CO (g) or continue protonation to CHO* to yield CH
4. Xie et al. [
31] report that the reaction energy from CO* to CHO* of the In
2S
3 slab is much higher than that from CO* to CO (g), consistent with the low activity towards CH
4 during CO
2 photoreduction in
Figure 3a. Compared with that of In
2S
3, the photocatalytic activity of Vs-In
2S
3/CuInS
2 is significantly improved, its products are still CO and CH
4, and the yields of CO always exceed those of CH
4 (
Figure 3b). These results suggest that the reaction energy from CO* to CO (g) and CO* to CHO* of the Vs-In
2S
3/CuInS
2 heterostructure slab was distinctly lower than In
2S
3, which could be attributed to the optimized carrier dynamics on the hybrid photocatalyst slab, including the generation and separation of the carrier. Furthermore, vs. may act as adsorption sites for CO
2 [
28], conducive to the further hydrogenation of the CO intermediate into CH
4. To understand the deep behavior of the photocatalytic reduction of CO
2, this aspect needs to be further studied in the future.
In short, this study offers a significant contribution to the literature because we not only confirmed the feasibility of the design of heterostructures on demand, but also demonstrated the benefits of such heterostructures for improving the photochemical properties of In2S3-based photocatalysts. However, satisfactory photocatalytic efficiency in applications such as solar energy conversion, environmental remediation, chemosensors, etc., has not been achieved until now. Thus, intensive investigations should be carried out in the future, in order to provide scientific references for maximizing the photocatalytic efficiencies of sulfide semiconductors.
3. Experimental Section
3.1. Materials
Indium chloride tetrahydrate (InCl3·4H2O, AR) and copper chloride dihydrate (CuCl2·2H2O, AR) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China. Thioacetamide (TAA, AR) was provided by Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. All reagents used during the experiment were as provided without further purification.
3.2. Synthesis of In2S3 and Vs-In2S3/CuInS2
The In2S3 crystals were prepared using a facile one-pot solvothermal method. Briefly, 37 mg of InCl3⋅4H2O and 15 mg of TAA were dissolved in 40 mL of ethanediol under ultrasonication. The mixture was then transferred into a hydrothermal autoclave (50 mL in capacity) and maintained at 150 °C for 90 min. The In2S3 microspheres were obtained by multiple centrifugations and thoroughly washed with water and ethanol, using a centrifugal speed of 4000 r/min and maintaining time of 1 min. Vs-In2S3/CuInS2 flower-like microspheres were subsequently synthesized using an In2S3 template-assisted and cation-exchange strategy. Typically, an appropriate amount of In2S3 was added to a 40 mL ethanediol solution containing CuCl2·2H2O and TAA under continuous stirring. The solution was then transferred to a sealed Teflon-lined autoclave and heated to 150 °C for 90 min. After the reaction was completed, the products were collected by centrifugation, washed several times with deionized water, and dried at 60 °C in a vacuum. The mass of CuCl2·2H2O was changed to 0.25, 0.5, 0.75, and 1.0 g, and the same procedures were performed to obtain Vs-In2S3/CuInS2 with different Cu masses; these products were denoted as 0.25-Vs-In2S3/CuInS2, 0.50-Vs-In2S3/CuInS2, 0.75-Vs-In2S3/CuInS2, and 0.10-Vs-In2S3/CuInS2, respectively.
3.3. Characterizations
X-ray diffraction (XRD, Rigaku D/MAX-Ultima III, Tokyo, Japan) was used to investigate the purity information and crystallographic phase of the as-prepared powder samples. The XRD pattern was recorded by using Cu-ka radiation (λ = 0.154178 nm) at 40 kV and 40 mA with a scan rate of 10° min−1. The morphology of nanomaterials was observed by field-emission scanning electron microscopy (FESEM, FEI NOVA NANOSEM 230). Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were captured using JEM 200CX TEM apparatus. Chemical states were investigated by X-ray photoelectron spectroscopy (XPS; K-Alpha, Thermo Fisher Scientific, Waltham, MA, USA); here, the spectra obtained were standardized according to the binding energy of the adventitious C 1s peak at 284.8 eV. A UV-vis spectrophotometer (UV-3600iPlus, Shimadzu, San Jose, CA, USA) was used to record the UV-visible diffuse reflectance of the samples. The sulfur vacancy signals were examined using spin-trapping electron paramagnetic resonance (EPR, EXM-10/12, Bruker, Bremen, Germany) measurements. The photoluminescence (PL) decay profiles of the samples were analyzed using single-particle confocal fluorescence spectroscopy measurements (PicoHarp300, Berlin, Germany).
3.4. Photoelectrochemical Measurements
Photoelectrochemical measurements were performed using electrochemical analysis (CHI-630D, Shanghai Chenhua, Shanghai, China) in a standard three-electrode system. The electrolyte was 0.5 M NaSO4 aqueous solution. The as-prepared In2S3 or Vs-In2S3/CuInS2 sample was used as the working electrode. A saturated Ag/AgCl electrode and a Pt foil were used as the reference and counter electrodes, respectively. The preparation process of In2S3 and Vs-In2S3/CuInS2 bulk samples was as follows. In2S3 or Vs-In2S3/CuInS2 catalyst ink was first prepared by dispersing 10 mg of the as-prepared catalyst in 1 mL of ethanol under sonication. Then, 50 μL of the ink was evenly spread onto a piece of pretreated fluorine-doped tin oxide (FTO) glass within a 1 cm2 area and dried at room temperature (25 °C). Thus, the catalysts were attached to the FTO surface. The transient photocurrent test curves exhibited ohmic characteristics, confirming the formation of an ohmic back contact between the sample and FTO. The working area of the electrode was 1 × 1 cm2, and the scan rate was 5 mV s−1. Electrochemical impedance spectroscopy (EIS) was performed using a PAR2273 workstation (Princeton Applied Research, USA) under AM 1.5G illumination. The Mott–Schottky curves of the samples were measured in 0.5 M Na2SO4 aqueous solution using an electrochemical workstation (CHI-630D, Shanghai Chenhua, Shanghai, China).
3.5. Photocatalytic Activity Measurements
Photocatalytic reduction of CO2 was performed on a photoreaction system. A 4–5 mg powder catalyst was dissolved under ultrasound for 10 min in the prepared solution, including 2 mL of deionized H2O and 1 mL of NaSO3 in a 460 mL reactor with a quartz glass cover. After the air in the reaction system was cleared away, high-purity CO2 was introduced into the system until the pressure reached 70 kPa and circulated for 60 min to achieve uniform distribution of CO2 gas. The temperature of photocatalytic CO2 reduction was set at 10 °C by cooling the water circulation system, which can promote the adsorption of CO2. A 300 W xenon arc lamp was used as the light source for the photocatalytic reaction. During irradiation, approximately 1 mL of gas was collected from the reaction cell at specific time intervals for CO and CH4 concentration analysis using a gas chromatograph (GC-2014C, Shimadzu Corp., Kyoto, Japan).
3.6. Density Functional Theory Calculation Details
The calculations are performed within the framework of density functional theory (DFT) using a basis set consisting of plane waves. The electron–ion interactions are described by ultrasoft pseudopotentials and electron exchange, and correlation energies are calculated with the Perdew–Burke–Ernzerhof (PBE) formulation of the generalized gradient approximation (GGA). The geometric structure is optimized with the Broyden–Fletcher–Goldfarb–Shanno (BFGS) method, the forces on each ion converge to less than 0.01 eV/Å, and the stress is less than 0.02 GPa. All the atoms of the structures are fully relaxed to their equilibrium positions with an energy convergence of 5 × 10−6 eV, the atomic displacement is less than 5 × 10−4 Å, and the self-consistent field (SCF) tolerance is 5 × 10−7 eV. The kinetic energy cutoff (In2S3: 350 eV; CuInS2: 440 eV) of the plane wave basis is used throughout, and the Brillouin zone is sampled with special k-points of a 3 × 3 × 1 grid for In2S3 and a 5 × 5 × 2 grid for CuInS2 based on Monkhorst–Pack.