Self-Assembly of Rhein and Matrine Nanoparticles for Enhanced Wound Healing
Abstract
1. Introduction
2. Results
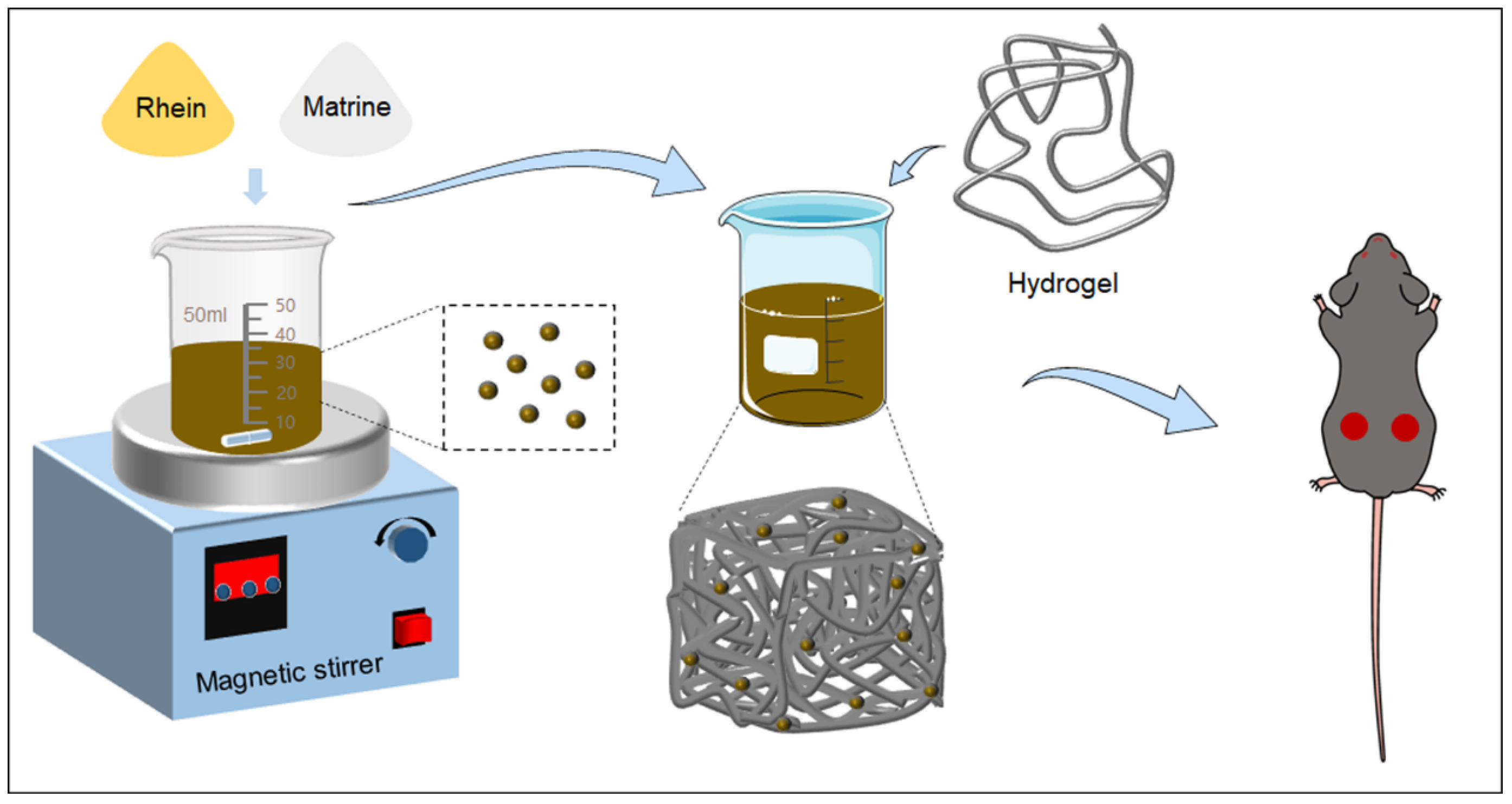
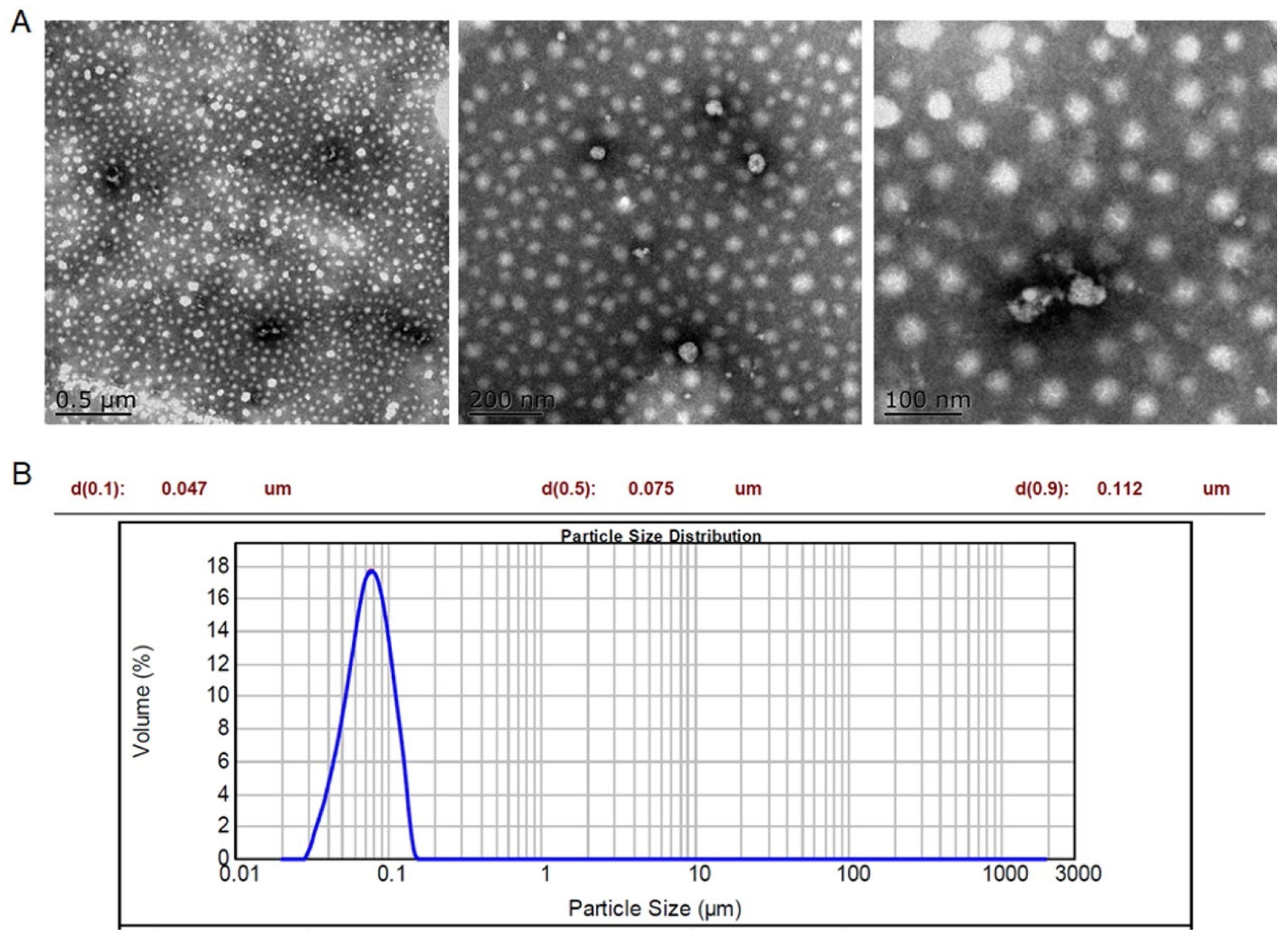
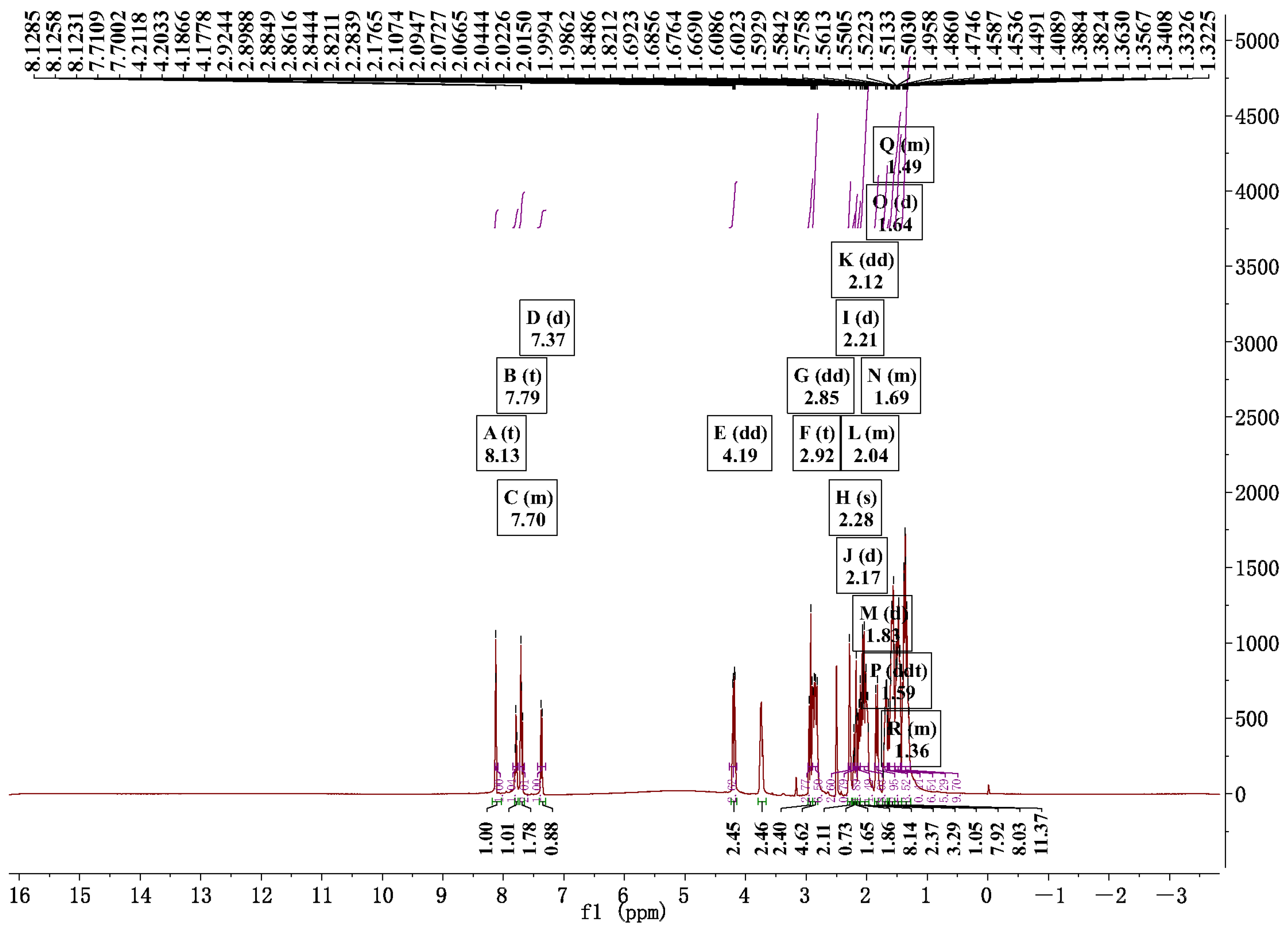
2.1. Preparation and Characterization of RM NPs
2.2. Formation of RM NPs
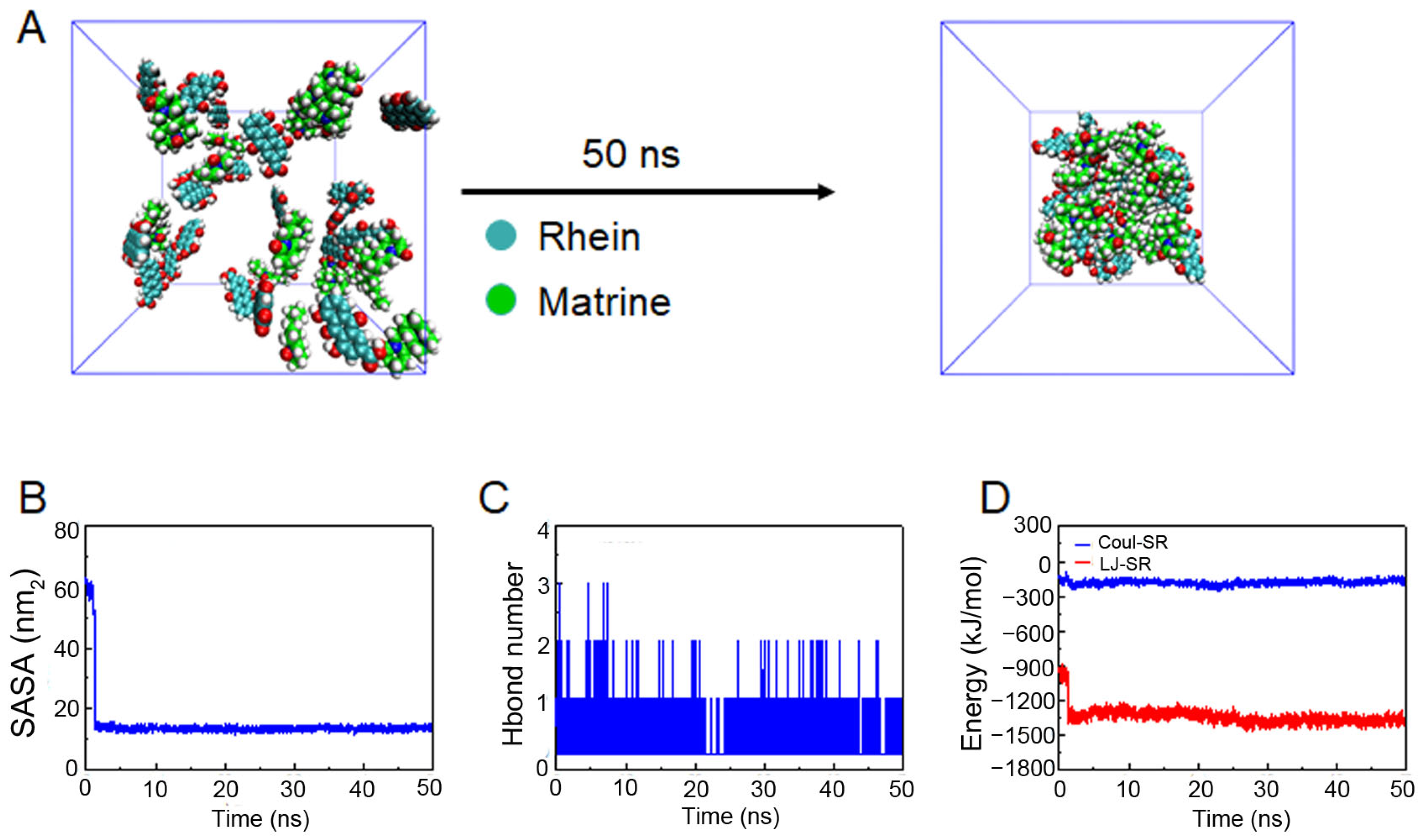
2.3. Molecular Dynamics Simulation Analysis of RM NPs
2.4. Intermolecular Interaction Modes of RM NPs
2.5. Characterization of GelMA Hydrogel Loaded with RM NPs
2.6. Effect of RM NPs on Wound Healing in Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of RM NPs
4.3. Characterization of the RM NPs
4.4. Molecular Dynamics Simulation Analysis of RM NPs
4.5. Preparation and Characterization of GelMA Hydrogels Loaded with RM NPs
4.6. Drug-Release Test
4.7. Animal
4.8. Effect of RM NP Wound Healing in Mice
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mei, H.; Cai, S.; Huang, D.; Gao, H.; Cao, J.; He, B. Carrier-Free Nanodrugs with Efficient Drug Delivery and Release for Cancer Therapy: From Intrinsic Physicochemical Properties to External Modification. Bioact. Mater. 2021, 8, 220–240. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, Y.; Wang, C.; Liu, Z.; Zhang, X.; An, F.; Diao, X.; Hao, X.; Zhang, X. Carrier-Free, Functionalized Drug Nanoparticles for Targeted Drug Delivery. Chem. Commun. 2012, 48, 8120–8122. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef] [PubMed]
- Karaosmanoglu, S.; Zhou, M.; Shi, B.; Zhang, X.; Williams, G.R.; Chen, X. Carrier-Free Nanodrugs for Safe and Effective Cancer Treatment. J. Control. Release 2021, 329, 805–832. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Huang, X.; Tian, X.; Yuan, Z.; Lu, J.; Nie, X.; Wang, P.; Lei, H.; Wang, P. Natural Small-Molecule-Based Carrier-Free Self-Assembly Library Originated from Traditional Chinese Herbal Medicine. ACS Omega 2022, 7, 43510–43521. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, X.; Yang, C.; Diao, Y. Target Characterization of Kaempferol Against Myocardial Infarction Using Novel In Silico Docking and DARTS Prediction Strategy. Int. J. Mol. Sci. 2021, 22, 12908. [Google Scholar] [CrossRef] [PubMed]
- Zhi, K.; Wang, J.; Zhao, H.; Yang, X. Self-Assembled Small Molecule Natural Product Gel for Drug Delivery: A Breakthrough in New Application of Small Molecule Natural Products. Acta Pharm. Sin. B 2020, 10, 913–927. [Google Scholar] [CrossRef]
- Tian, X.; Wang, P.; Li, T.; Huang, X.; Guo, W.; Yang, Y.; Yan, M.; Zhang, H.; Cai, D.; Jia, X.; et al. Self-Assembled Natural Phytochemicals for Synergistically Antibacterial Application from the Enlightenment of Traditional Chinese Medicine Combination. Acta Pharm. Sin. B 2020, 10, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, Z.; Wang, R.; Zhong, K.; Zhang, K.; Zhang, N.; Liu, W.; Feng, F.; Qu, W. Review of Natural Phytochemical-Based Self-Assembled Nanostructures for Applications in Medicine. ACS Appl. Nano Mater. 2022, 5, 3146–3169. [Google Scholar] [CrossRef]
- Wei, D.; Yang, H.; Zhang, Y.; Zhang, X.; Wang, J.; Wu, X.; Chang, J. Nano-Traditional Chinese Medicine: A Promising Strategy and Its Recent Advances. J. Mater. Chem. B 2022, 10, 2973–2994. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yuan, L.; Gao, S.; Wang, Z.; Tang, Y.; Chen, C.; Zhao, C.-Q.; Fu, X. Self-Assembled Nanodrug Delivery Systems for Anti-Cancer Drugs from Traditional Chinese Medicine. Biomater. Sci. 2024, 12, 1662–1692. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Liu, Y.; Lan, J.; Li, T.; He, Y.; Li, Z.; Zhang, T.; Ding, Y. Self-Assembled Nanoparticles from Xie-Bai-San Decoction: Isolation, Characterization and Enhancing Oral Bioavailability. Int. J. Nanomed. 2024, 19, 3405–3421. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Wang, Y.; Kong, L.; Han, C. Carrier-Free Nanoplatforms from Natural Plants for Enhanced Bioactivity. J. Adv. Res. 2023, 50, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, F.P.; Huang, Y.R.; Li, H.D.; Cao, X.Y.; You, Y.; Meng, Z.F.; Sun, K.Y.; Shen, X.Y. Matrine suppresses NLRP3 inflammasome activation via regulating PTPN2/JNK/SREBP2 pathway in sepsis. Phytomedicine 2023, 109, 154574. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Fan, R.; Wu, H.; Yao, H.; Yan, Y.; Liu, J.; Ran, L.; Sun, Z.; Yi, L.; Dang, L.; et al. Directed Self-Assembly of Herbal Small Molecules into Sustained Release Hydrogels for Treating Neural Inflammation. Nat. Commun. 2019, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shi, Y.; Ni, Z.; Yu, T.; Chen, Z. Preparation of a Self-Assembled Rhein-Doxorubicin Nanogel Targeting Mitochondria and Investigation on Its Antihepatoma Activity. Mol. Pharm. 2022, 19, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Pyun, J.; Fréchet, J.M.; Hawker, C.J.; Frank, C.W. The Dramatic Effect of Architecture on the Self-Assembly of Block Copolymers at Interfaces. Langmuir 2005, 21, 10444–10458. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Wang, B.; Sun, K.; Li, F.; Liu, Q.; Yu, X.A.; Jiang, L.; Wang, L. Self-Assembled Matrine-PROTAC Encapsulating Zinc(II) Phthalocyanine with GSH-Depletion-Enhanced ROS Generation for Cancer Therapy. Molecules 2024, 29, 1845. [Google Scholar] [CrossRef]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell. 2022, 29, 1161–1180. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Criollo-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Heredia, J.B. Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules 2023, 28, 598. [Google Scholar] [CrossRef] [PubMed]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Niu, J.; Chong, Y.S.; Huang, Y.F.; Chu, Y.; Xie, S.Y.; Jiang, Z.H.; Peng, L.H. Porous microspheres as promising vehicles for the topical delivery of poorly soluble asiaticoside accelerate wound healing and inhibit scar formation in vitro & in vivo. Eur. J. Pharm. Biopharm. 2016, 109, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhang, L.; Lin, J.; Wang, L. Self-Assembly Behavior of pH- and Thermosensitive Amphiphilic Triblock Copolymers in Solution: Experimental Studies and Self-Consistent Field Theory Simulations. J. Phys. Chem. B 2008, 112, 12666–12673. [Google Scholar] [CrossRef]
- Shinoda, W.; DeVane, R.; Klein, M.L. Computer Simulation Studies of Self-Assembling Macromolecules. Curr. Opin. Struct. Biol. 2012, 22, 175–186. [Google Scholar] [CrossRef]
- Hao, X.; Yan, W.; Yang, J.; Bai, Y.; Qian, H.; Lou, Y.; Ju, P.; Zhang, D. Matrine@chitosan-D-proline nanocapsules as antifouling agents with antibacterial properties and biofilm dispersibility in the marine environment. Front. Microbiol. 2022, 13, 950039. [Google Scholar] [CrossRef]
- Folliero, V.; Dell’Annunziata, F.; Roscetto, E.; Amato, A.; Gasparro, R.; Zannella, C.; Casolaro, V.; De Filippis, A.; Catania, M.R.; Franci, G.; et al. Rhein: A novel antibacterial compound against Streptococcus mutans infection. Microbiol Res. 2022, 261, 127062. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound Repair and Regeneration: Mechanisms, Signaling, and Translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. New ways to boost molecular dynamics simulations. J. Comput. Chem. 2015, 36, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Darden, T.A.; York, D.M.; Pedersen, L.G. Particle Mesh Ewald: An Nlog(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1992, 98, 10089–10092. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular Dynamics with Coupling to an External Bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Martonák, R.; Laio, A.; Parrinello, M. Predicting Crystal Structures: The Parrinello-Rahman Method Revisited. Phys. Rev. Lett. 2003, 90, 075503. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Zang, R.; Qiu, Y.; Yang, N.; Liu, M.; Wei, S.; Xu, X.; Diao, Y. Self-Assembly of Rhein and Matrine Nanoparticles for Enhanced Wound Healing. Molecules 2024, 29, 3326. https://doi.org/10.3390/molecules29143326
Wu X, Zang R, Qiu Y, Yang N, Liu M, Wei S, Xu X, Diao Y. Self-Assembly of Rhein and Matrine Nanoparticles for Enhanced Wound Healing. Molecules. 2024; 29(14):3326. https://doi.org/10.3390/molecules29143326
Chicago/Turabian StyleWu, Xunxun, Ranqing Zang, Yiting Qiu, Ni Yang, Meiyan Liu, Site Wei, Xianxiang Xu, and Yong Diao. 2024. "Self-Assembly of Rhein and Matrine Nanoparticles for Enhanced Wound Healing" Molecules 29, no. 14: 3326. https://doi.org/10.3390/molecules29143326
APA StyleWu, X., Zang, R., Qiu, Y., Yang, N., Liu, M., Wei, S., Xu, X., & Diao, Y. (2024). Self-Assembly of Rhein and Matrine Nanoparticles for Enhanced Wound Healing. Molecules, 29(14), 3326. https://doi.org/10.3390/molecules29143326






