Experimental and Theoretical Investigation of the Inhibitor Efficiency of Eucalyptus globulus Leaf Essential Oil (EuEO) on Mild Steel Corrosion in a Molar Hydrochloric Acid Medium
Abstract
1. Introduction
2. Result and Discussion
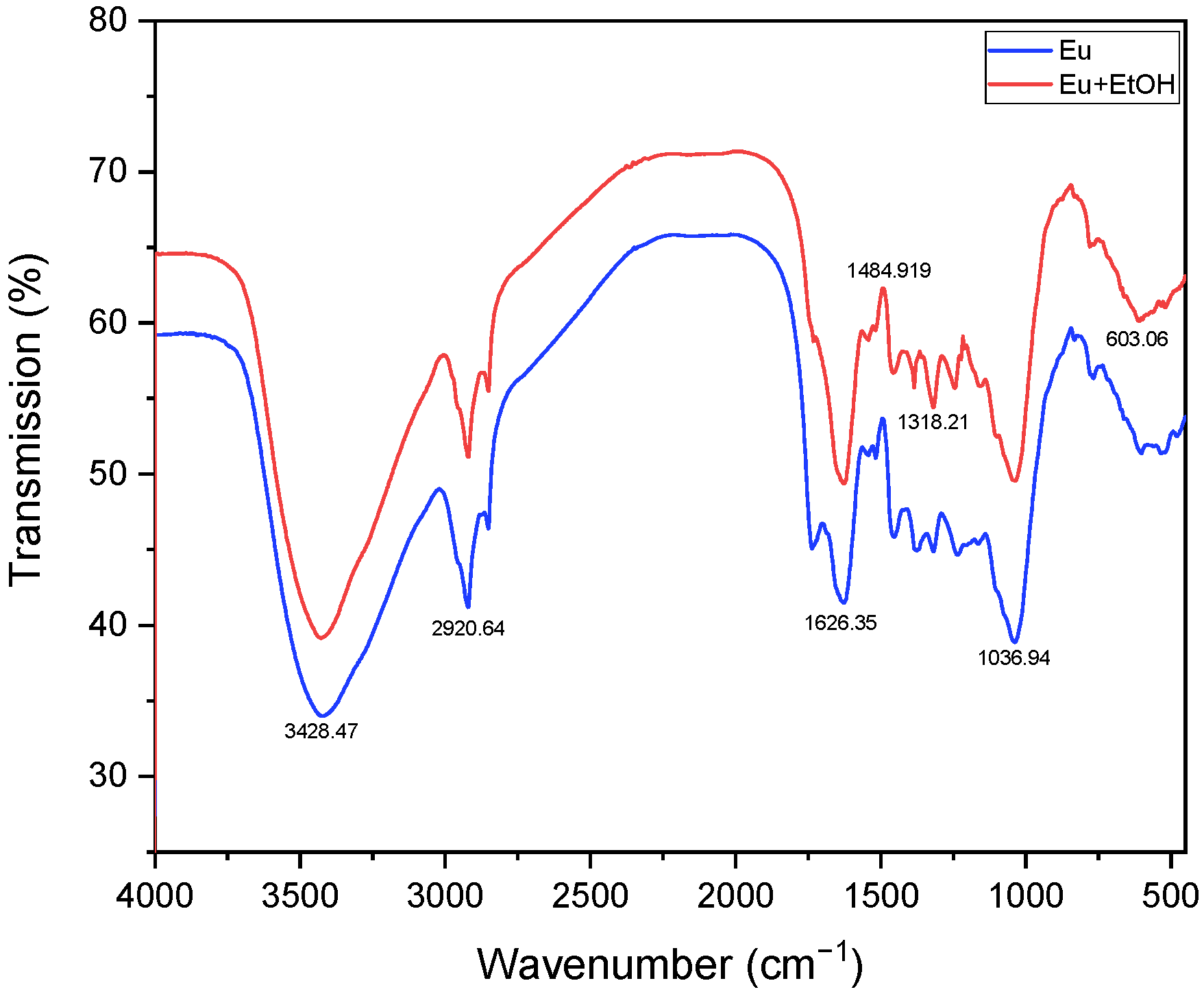
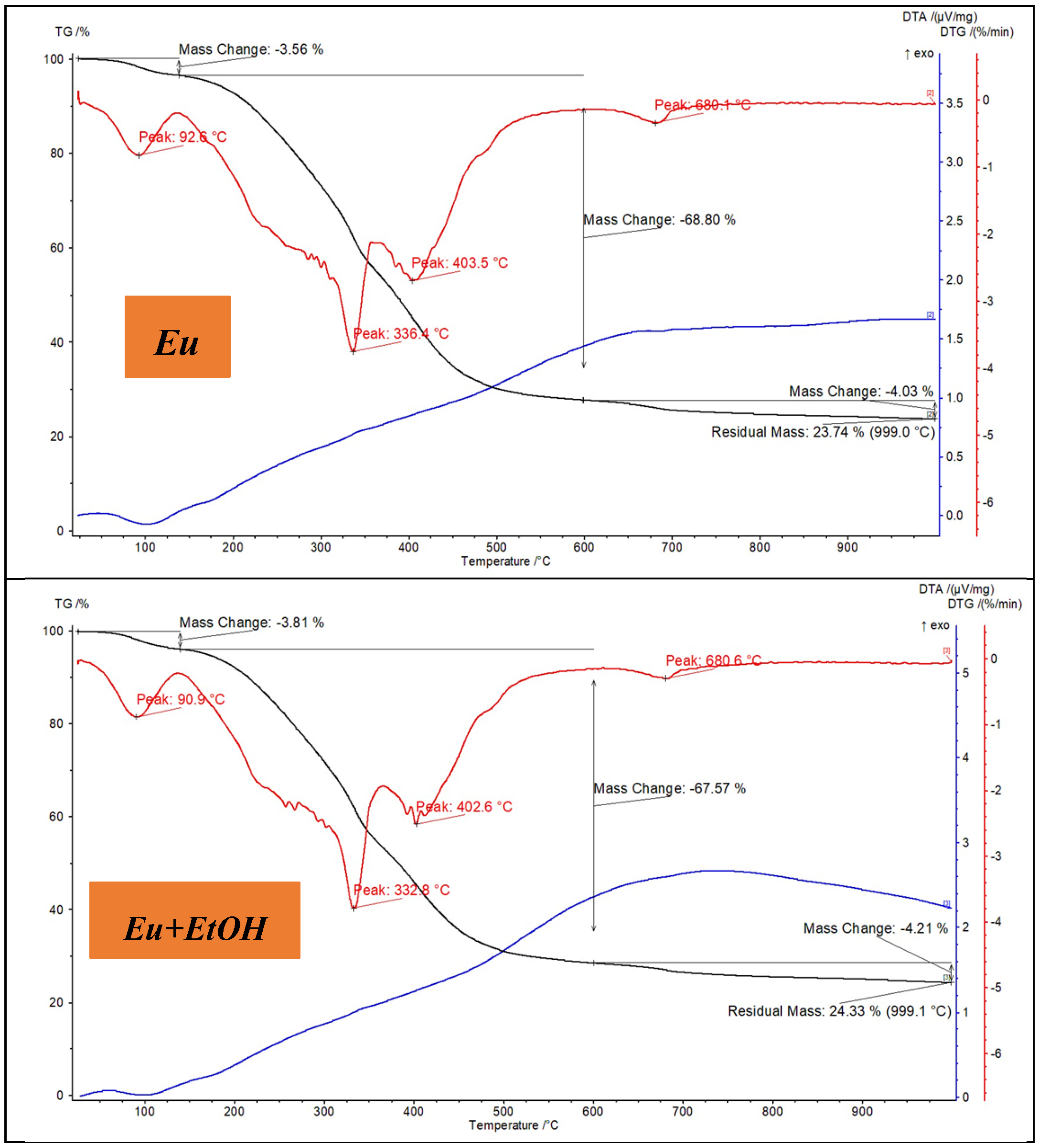
2.1. Characterization of Eu and EuEO
2.2. Essential Oil Composition
2.3. Electrochemical Study
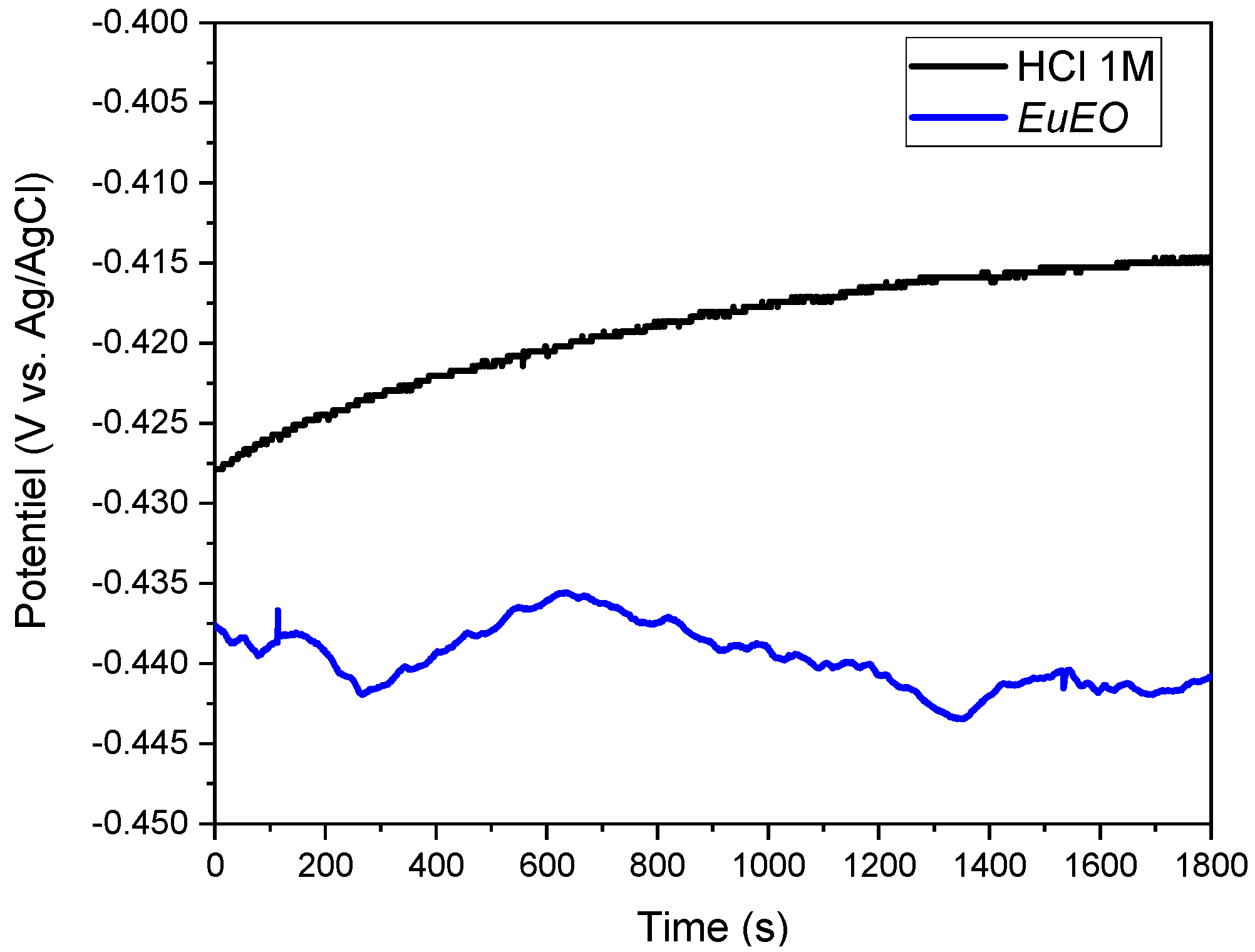
2.3.1. Open Circuit Potential (OCP) Monitoring
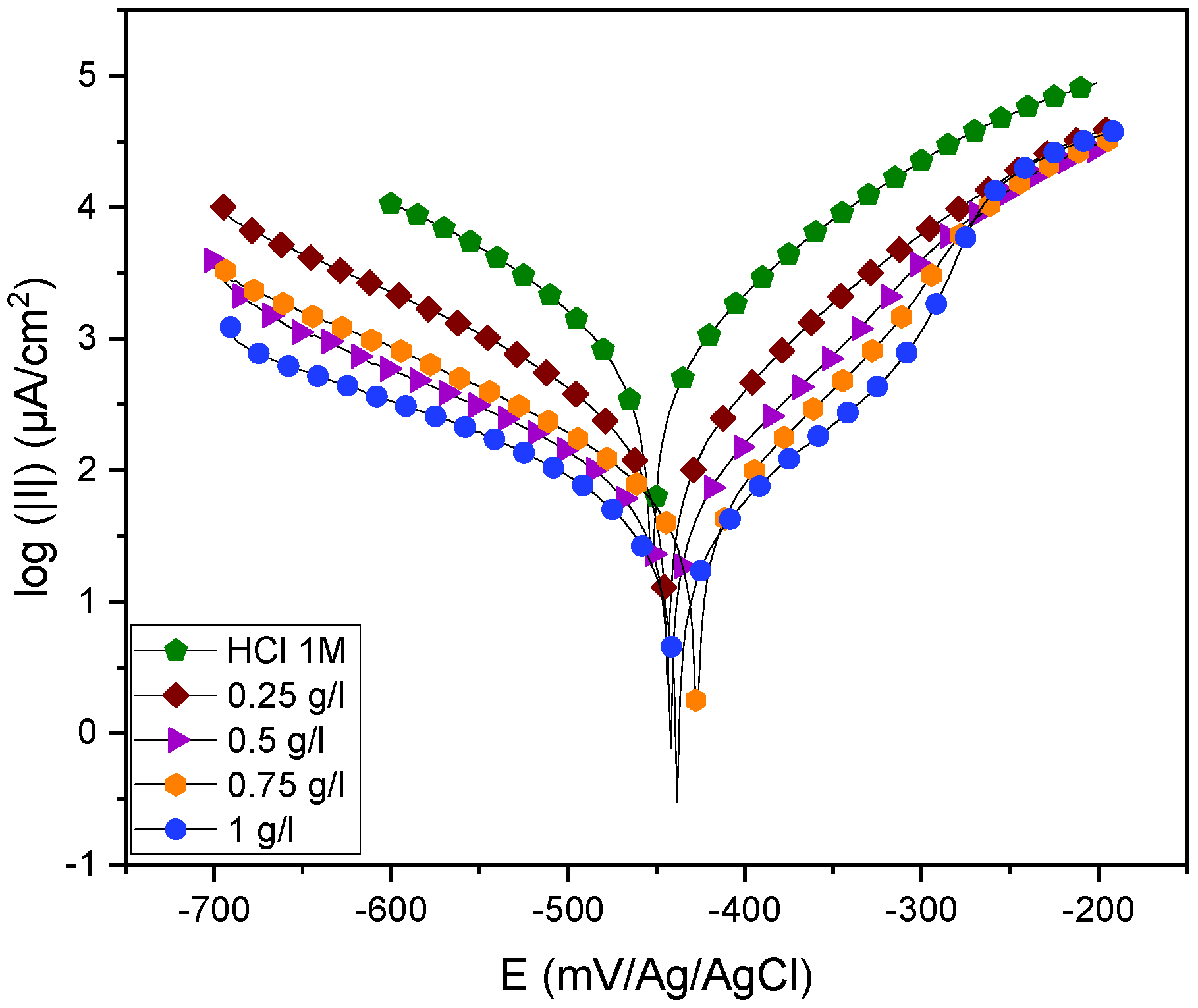
2.3.2. Polarization Curves
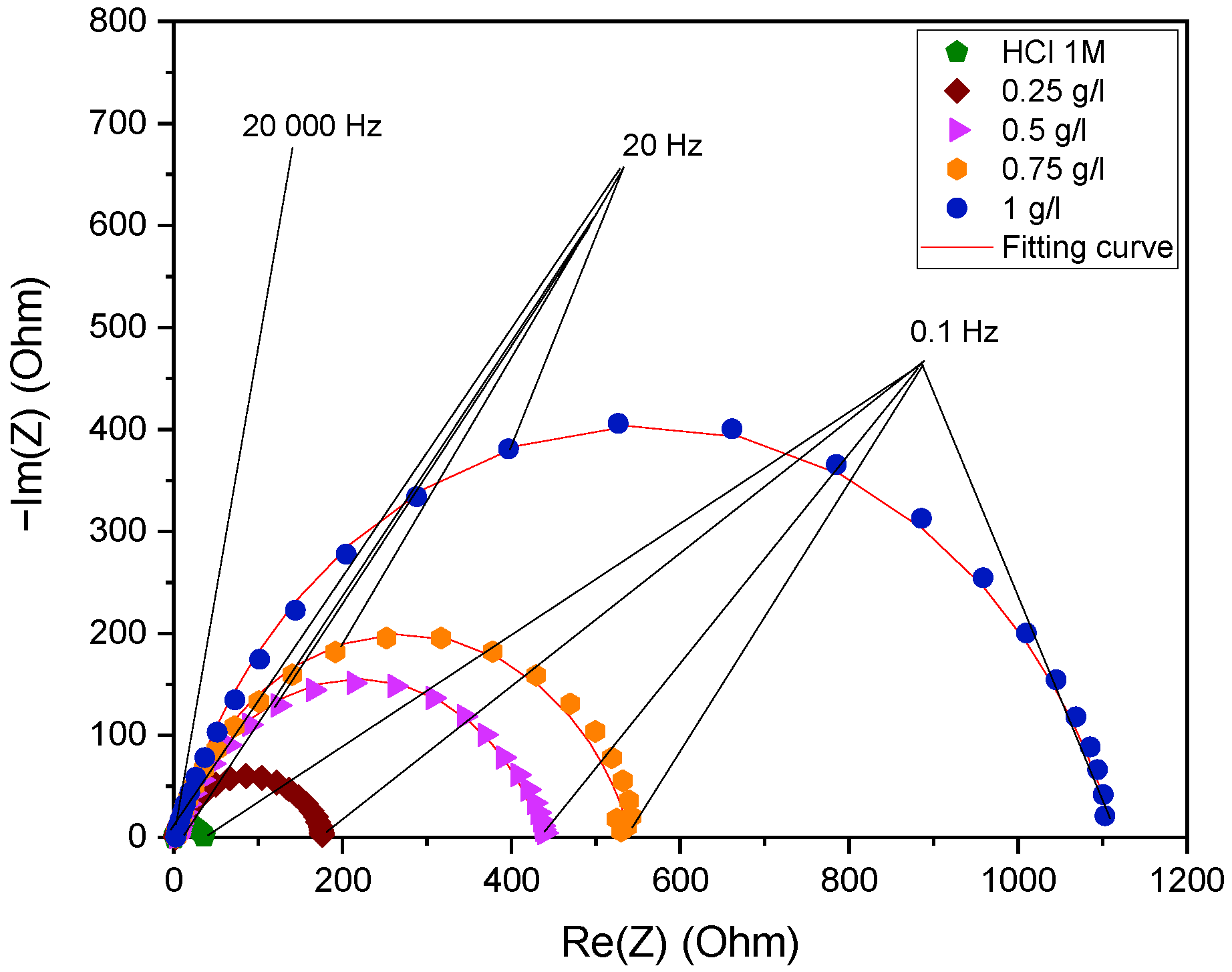
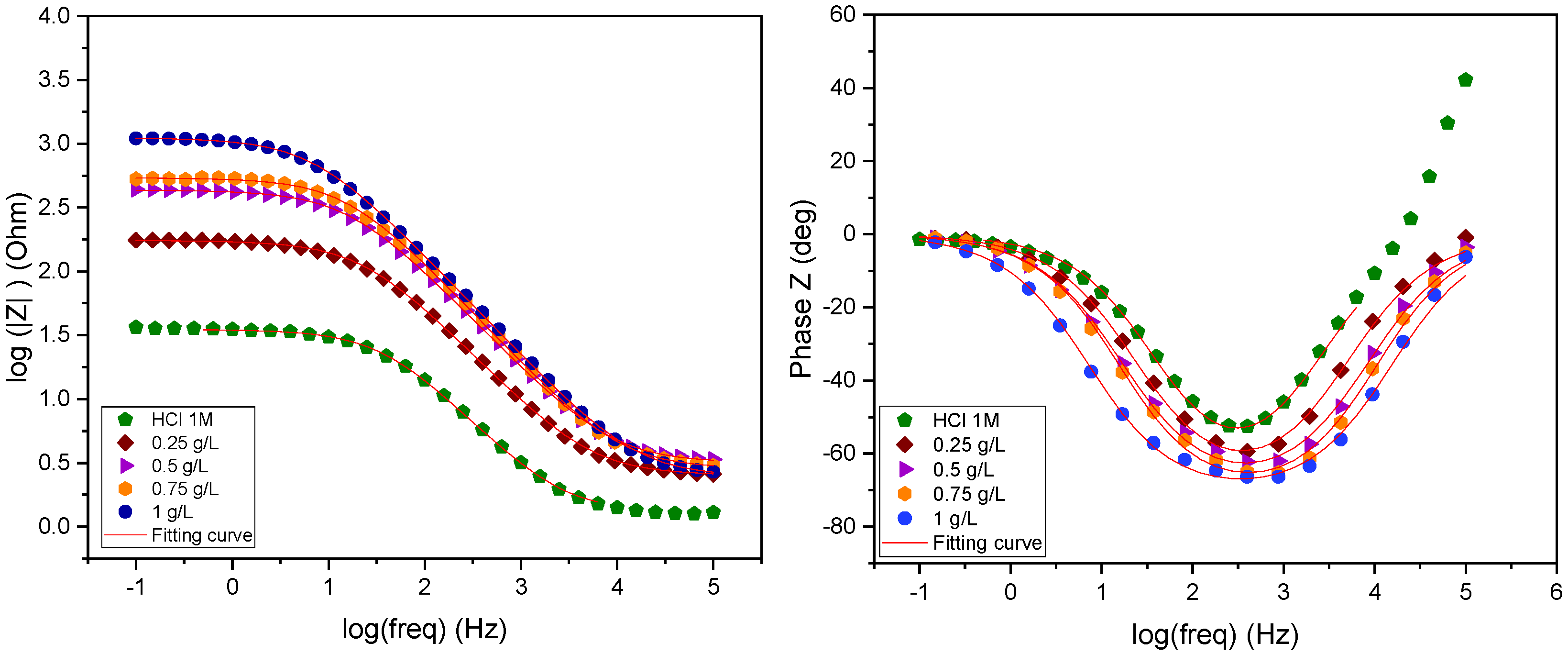
2.3.3. Electrochemical Impedance Spectroscopy Measurements
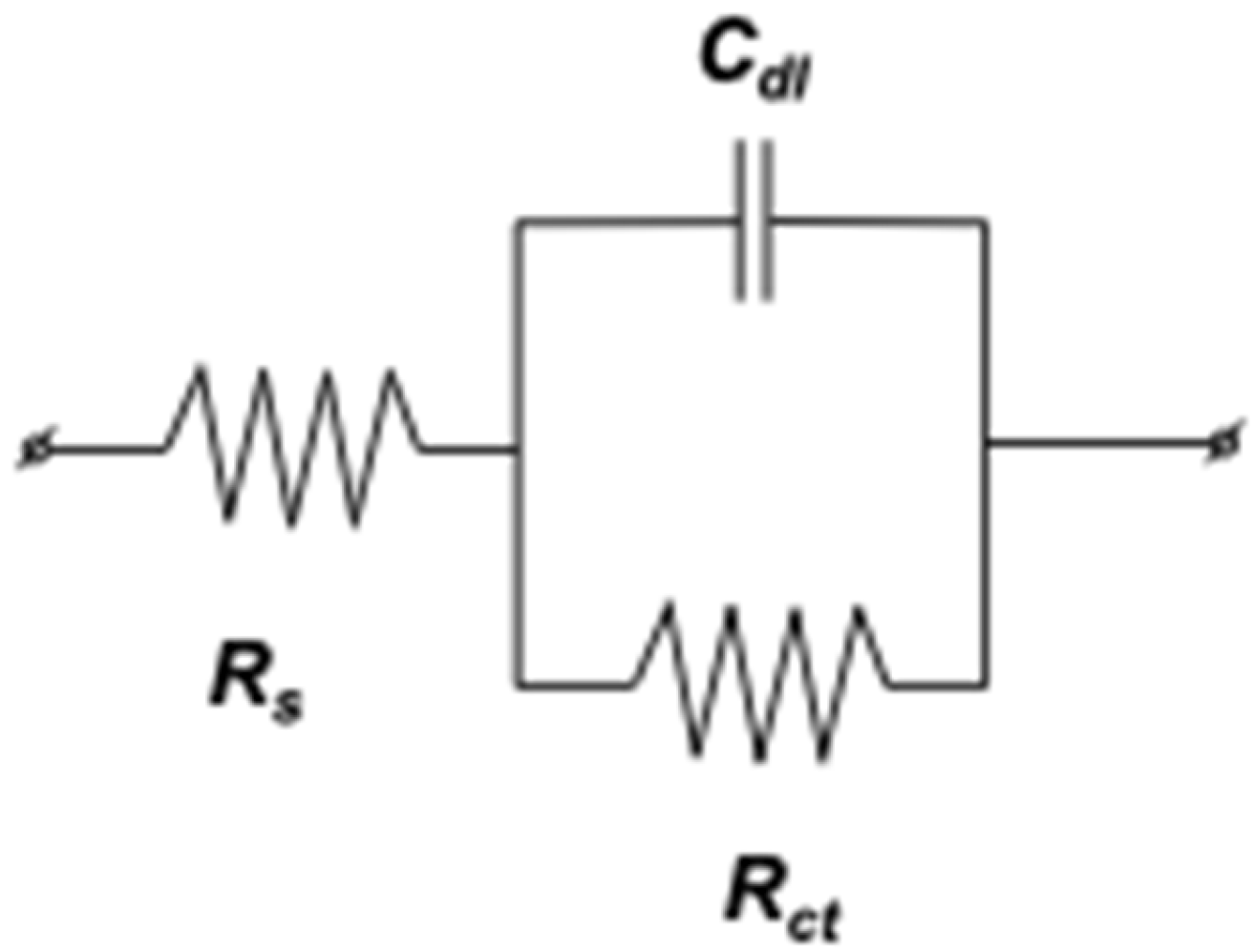
2.3.4. Mild Steel/1 M HCl Interface Equivalent Electrical Circuits
2.3.5. Representation of Impedance Diagrams on the Nyquist and Bode Planes
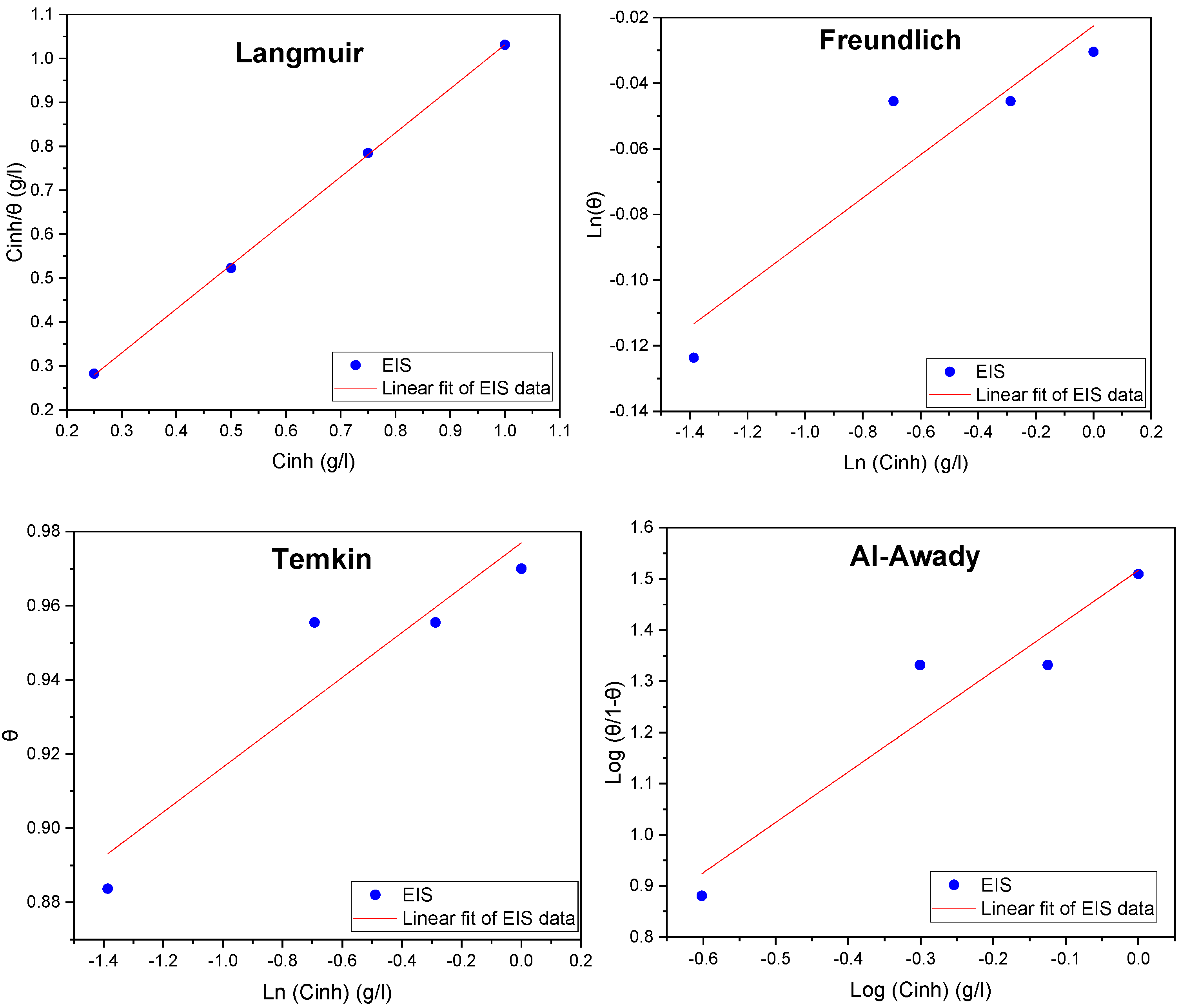
2.4. Adsorption Isotherms
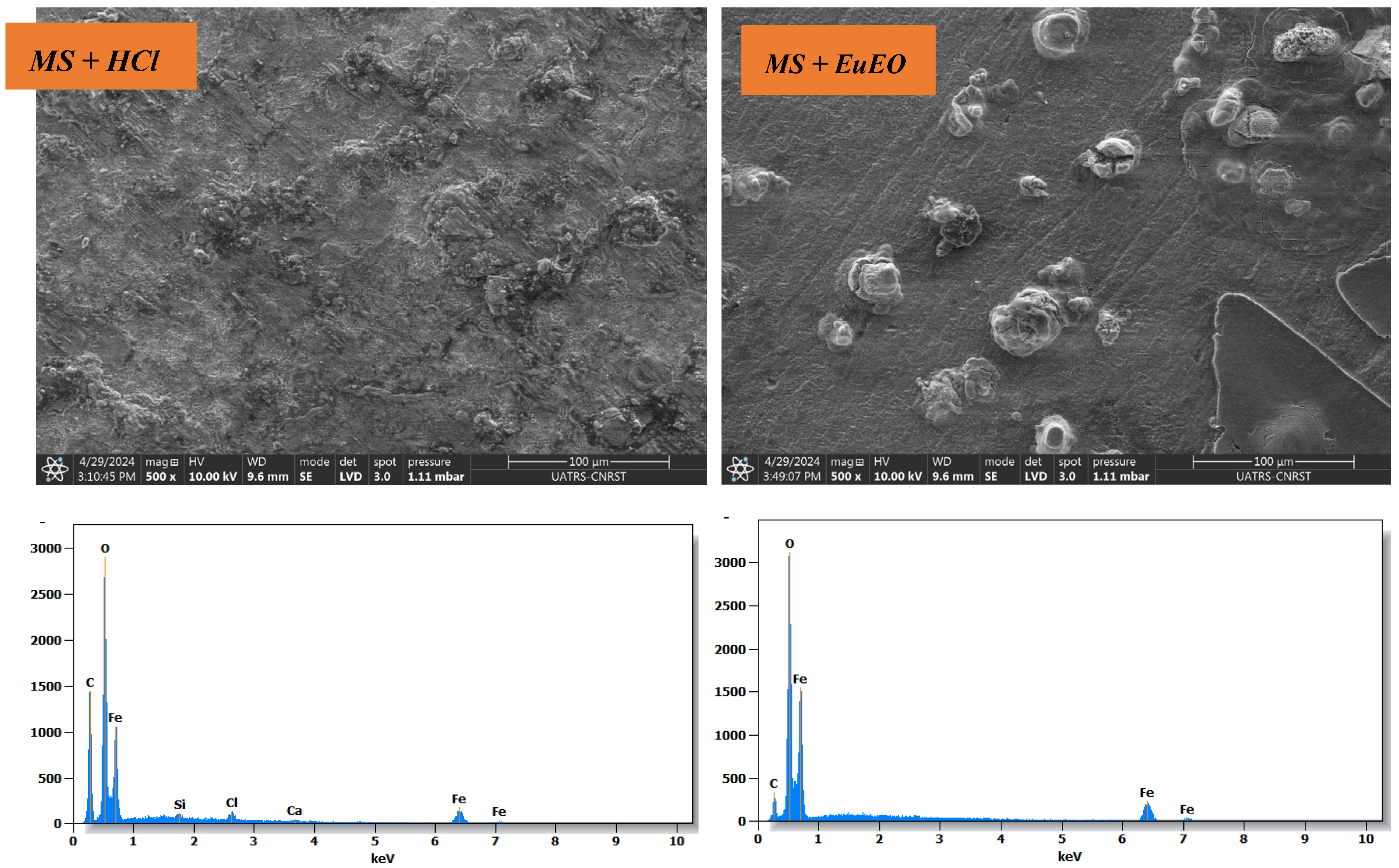
2.5. Surface Analysis
2.6. Evaluation of the Quantum Chemical Calculations
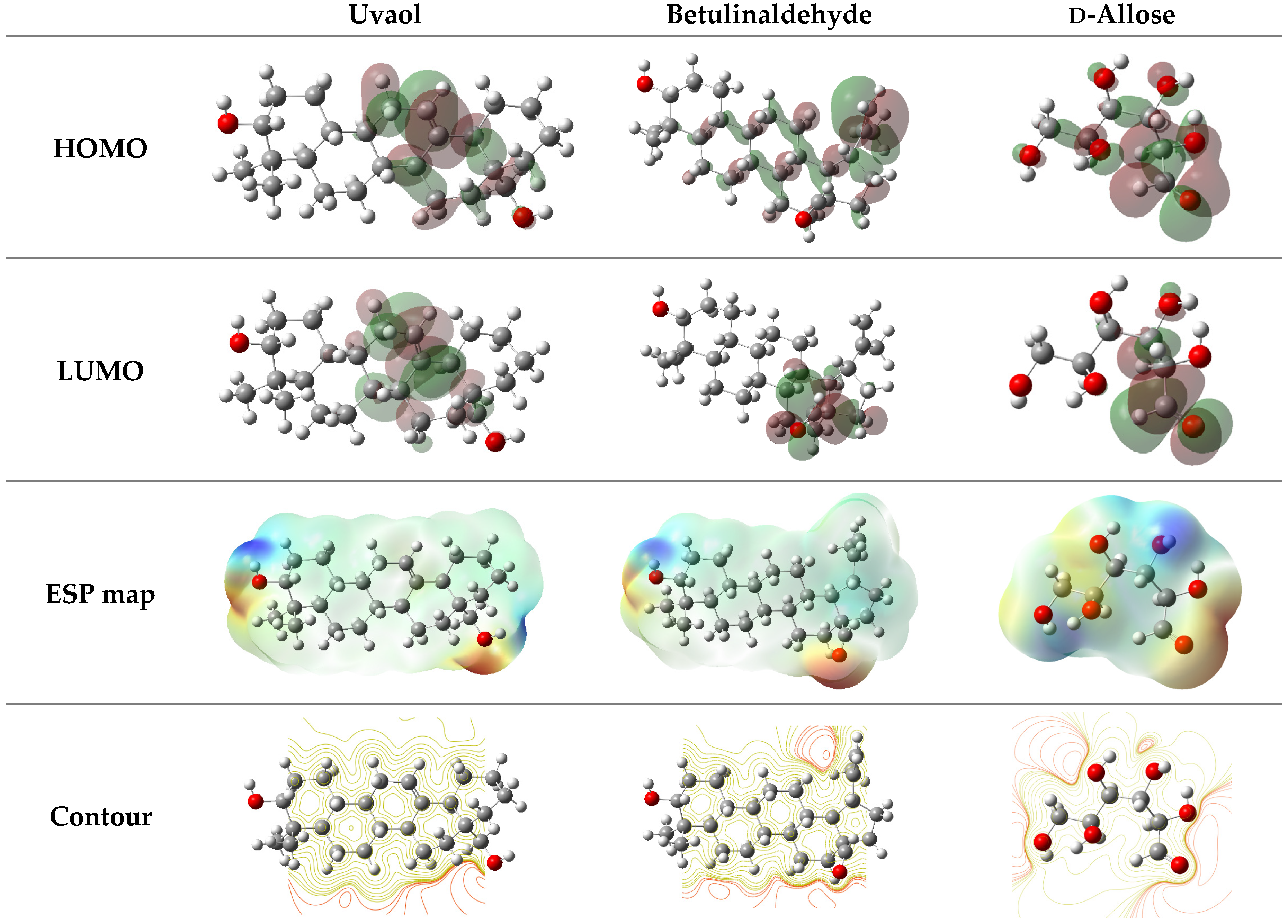
2.6.1. DFT Calculations
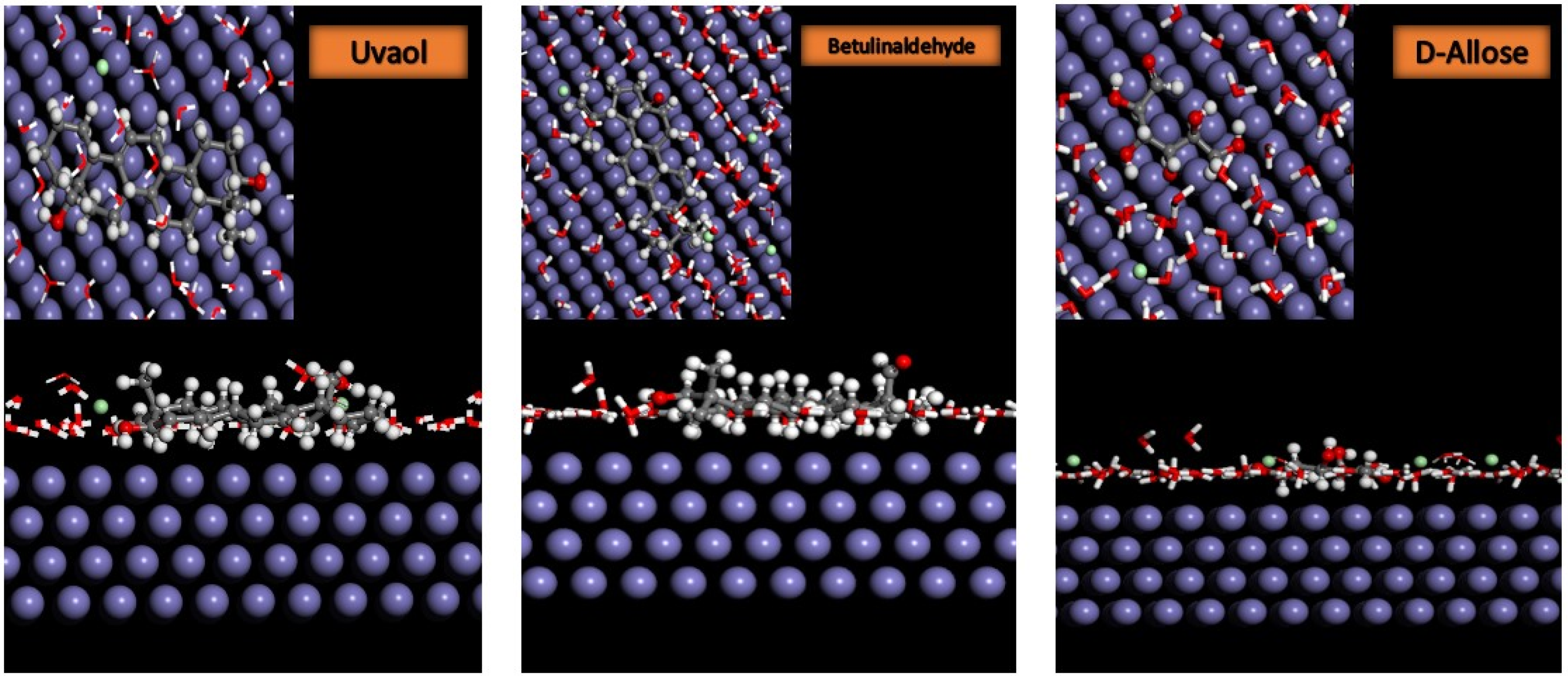
2.6.2. Monte Carlo Simulation
3. Materials and Methods
3.1. Botanical Description of Eucalyptus
3.2. Eucalyptus Leaf Powder Preparation
3.3. Characterization of Eu and EuEO
- Using a Quanta 200 FEl-XRF device, environmental scanning electron microscopy (ESEM) in conjunction with an energy-dispersive X-ray (EDX) was utilized for microanalysis and morphology assessment. One of its main advantages is that it can evaluate the surface of wet or soft samples and scan materials that are delicate or hydrated. Moreover, a high-resolution picture of the powder’s surface features and microstructure is produced [64].
- Using KBr pellets, the FTIR spectra of the powdered eucalyptus leaves were displayed on a Nicolet Avatar 320 spectrophotometer. A total of 32 scans were recorded in transmittance mode between 4000 and 400 cm−1, with a resolution of 4 cm−1 [65].
- Thermogravimetric analysis (TGA) and differential thermal analysis (DTA) were performed using an SDT Q600 TA instrument in air, with heating from 30 °C to 1000 °C at a rate of 10 °C/min; alternatively, heating was carried out on Al2O3 crucibles using a Netzsch STA 449 F1 Jupiter thermal analyzer under a N2 atmosphere (100 mL/min) from 25 °C to 1000 °C at a rate of 10 °C/min. Data were processed via Netzsch Proteus 6.1 Thermal Analysis software [65].
3.4. Eucalyptus Leaf Essential Oil Extraction (EuEO)
3.5. GC/MS Analysis
3.6. Mild Steel Preparation
3.7. Electrolytic Medium
3.8. Electrochemical Method
3.9. Computational Study
3.10. Monte Carlo Simulation
3.11. Surface Analysis
4. Conclusions
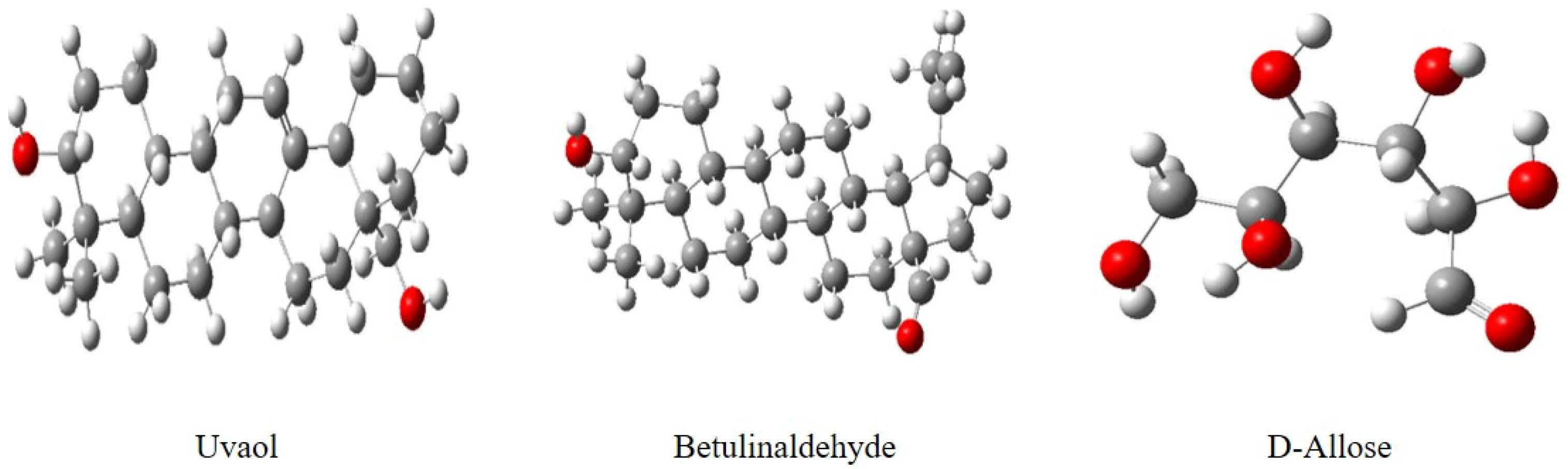
- The GC-MS analysis identified the major active chemicals in Eucalyptus globulus essential oil (EuEO): d-Allose (12.94%), Betulinaldehyde (31.75%), and Uvaol (32.17%).
- EuEO exhibited potent corrosion inhibition for mild steel in molar hydrochloric acid, achieving an inhibitory efficiency of up to 97% at a 1 g/L concentration.
- Significant adsorption of the EuEO molecules onto the mild steel surface was observed in the acidic medium.
- Polarization studies suggested that EuEO acts as a mixed-type inhibitor, blocking both cathodic and anodic reactions.
- Electrochemical impedance spectroscopy (EIS) indicated that EuEO significantly decreases the double-layer capacitance and increases charge transfer resistance.
- Surface analysis confirmed that EuEO forms a protective coating on the mild steel surface, adhering effectively.
- Monte Carlo (MC) dynamic simulations and density functional theory (DFT) simulations showed that EuEO functions as both an electron donor and acceptor to and from the iron surface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salama, M.; Vaz, M.F.; Colaço, R.; Santos, C.; Carmezim, M. Biodegradable iron and porous iron: Mechanical properties, degradation behaviour, manufacturing routes and biomedical applications. J. Funct. Biomater. 2022, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Procópio, L. The role of biofilms in the corrosion of steel in marine environments. World J. Microbiol. Biotechnol. 2019, 35, 73. [Google Scholar] [CrossRef] [PubMed]
- Alamri, A.H. Localized corrosion and mitigation approach of steel materials used in oil and gas pipelines—An overview. Eng. Fail. Anal. 2020, 116, 104735. [Google Scholar] [CrossRef]
- Maleki, E.; Bagherifard, S.; Bandini, M.; Guagliano, M. Surface post-treatments for metal additive manufacturing: Progress, challenges, and opportunities. Addit. Manuf. 2021, 37, 101619. [Google Scholar] [CrossRef]
- Ren, H.; Liu, Y.; Gong, Z.; Tan, B.; Deng, H.; Xiong, J.; Shao, P.; Dai, Q.; Cao, J.; Marzouki, R. Pumpkin leaf extract crop waste as a new degradable and environmentally friendly corrosion inhibitor. Langmuir 2024, 40, 5738–5752. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Sustainable inhibitors for corrosion mitigation in aggressive corrosive media: A comprehensive study. J. Bio-Tribo-Corros. 2021, 7, 67. [Google Scholar] [CrossRef]
- Tan, B.; Liu, Y.; Gong, Z.; Zhang, X.; Chen, J.; Guo, L.; Xiong, J.; Liu, J.; Marzouki, R.; Li, W. Pyracantha fortuneana alcohol extracts as biodegradable corrosion inhibitors for copper in H2SO4 media. J. Mol. Liq. 2024, 397, 124117. [Google Scholar] [CrossRef]
- Daoudi, W.; El Aatiaoui, A.; Falil, N.; Azzouzi, M.; Berisha, A.; Olasunkanmi, L.O.; Dagdag, O.; Ebenso, E.E.; Koudad, M.; Aouinti, A.; et al. Essential oil of Dysphania ambrosioides as a green corrosion inhibitor for mild steel in HCl solution. J. Mol. Liq. 2022, 363, 119839. [Google Scholar] [CrossRef]
- Bathily, M.; Ngom, B.; Gassama, D.; Tamba, S. Review on essential oils and their corrosion-inhibiting properties. Am. J. Appl. Chem. 2021, 9, 65–73. [Google Scholar] [CrossRef]
- Thakur, A.; Assad, H.; Kaya, S.; Kumar, A. Plant extracts as environmentally sustainable corrosion inhibitors II. In Eco-Friendly Corrosion Inhibitors; Elsevier: Amsterdam, The Netherlands, 2022; pp. 283–310. [Google Scholar]
- Suvin, P.S.; Gupta, P.; Horng, J.H.; Kailas, S.V. Evaluation of a comprehensive non-toxic, biodegradable and sustainable cutting fluid developed from coconut oil. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2021, 235, 1842–1850. [Google Scholar] [CrossRef]
- Gupta, D.K.; Awasthi, L.; Das, A.K.; Yadav, B.; Ghimire, A.; Yadav, A.P. Corrosion inhibition effect of acidic extract of bark of eucalyptus globulus on mild steel. Tribhuvan Univ. J. 2020, 35, 1–10. [Google Scholar] [CrossRef]
- Lazrak, J.; El Assiri, E.H.; Arrousse, N.; El-Hajjaji, F.; Taleb, M.; Rais, Z.; Farah, A.; Ramzi, A.; Hammouti, B. Origanum compactum essential oil as a green inhibitor for mild steel in 1 M hydrochloric acid solution: Experimental and Monte Carlo simulation studies. Mater. Today Proc. 2021, 45, 7486–7493. [Google Scholar] [CrossRef]
- Mehdipour, M.; Ramezanzadeh, B.; Arman, S.Y. Electrochemical noise investigation of Aloe plant extract as green inhibitor on the corrosion of stainless steel in 1 M H2SO4. J. Ind. Eng. Chem. 2015, 21, 318–327. [Google Scholar] [CrossRef]
- El Messaoudi, N.; Ciğeroğlu, Z.; Şenol, Z.M.; Kazan-Kaya, E.S.; Fernine, Y.; Gubernat, S.; Lopicic, Z. Green synthesis of CuFe2O4 nanoparticles from bioresource extracts and their applications in different areas: A review. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–22. [Google Scholar]
- Cichosz, S.; Masek, A. Cellulose fibers hydrophobization via a hybrid chemical modification. Polymers 2019, 11, 1174. [Google Scholar] [CrossRef] [PubMed]
- Şenol, Z.M.; Messaoudi, N.E.; Fernine, Y.; Keskin, Z.S. Bioremoval of rhodamine B dye from aqueous solution by using agricultural solid waste (almond shell): Experimental and DFT modeling studies. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–14. [Google Scholar]
- Dai, F.; Zhuang, Q.; Huang, G.; Deng, H.; Zhang, X. Infrared spectrum characteristics and quantification of OH groups in coal. ACS Omega 2023, 8, 17064–17076. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Mai, T.T.T.; Nguyen, N.B.; Dang, T.D.; Le, M.L.P.; Dang, T.T. A novel method for preparing microfibrillated cellulose from bamboo fibers. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 015016. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Chao, Y.; Nawawi, D.S.; Akiyama, T.; Yokoyama, T.; Matsumoto, Y. Analysis of lignin aromatic structure in wood based on the IR spectrum. J. Wood Chem. Technol. 2012, 32, 294–303. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Şenol, Z.M.; Elma, E.; El Messaoudi, N.; Mehmeti, V. Performance of cross-linked chitosan-zeolite composite adsorbent for removal of Pb2+ ions from aqueous solutions: Experimental and Monte Carlo simulations studies. J. Mol. Liq. 2023, 391, 123310. [Google Scholar] [CrossRef]
- Van Riessen, A.; Rickard, W.; Sanjayan, J. Thermal properties of geopolymers. In Geopolymers; Woodhead Publishing: Cambridge, UK, 2009; pp. 315–342. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Kumar, L.; Alam, M.S.; Meena, C.L.; Jain, R.; Bansal, A.K. Fexofenadine hydrochloride. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press: Cambridge, MA, USA, 2009; Volume 34, pp. 153–192. [Google Scholar]
- Chen, D.; Cen, K.; Zhuang, X.; Gan, Z.; Zhou, J.; Zhang, Y.; Zhang, H. Insight into biomass pyrolysis mechanism based on cellulose, hemicellulose, and lignin: Evolution of volatiles and kinetics, elucidation of reaction pathways, and characterization of gas, biochar and bio-oil. Combust. Flame 2022, 242, 112142. [Google Scholar] [CrossRef]
- Ghavami, N.; Özdenkçi, K.; Salierno, G.; Björklund-Sänkiaho, M.; De Blasio, C. Analysis of operational issues in hydrothermal liquefaction and supercritical water gasification processes: A review. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–28. [Google Scholar]
- Elbhnsawi, N.A.; Elwakil, B.H.; Hassanin, A.H.; Shehata, N.; Elshewemi, S.S.; Hagar, M.; Olama, Z.A. Nano-chitosan/eucalyptus oil/cellulose acetate nanofibers: Manufacturing, antibacterial and wound healing activities. Membranes 2023, 13, 604. [Google Scholar] [CrossRef]
- Belhachemi, A.; Maatoug, M.H.; Canela-Garayoa, R. GC-MS and GC-FID analyses of the essential oil of Eucalyptus camaldulensis grown under greenhouses differentiated by the LDPE cover-films. Ind. Crop. Prod. 2022, 178, 114606. [Google Scholar] [CrossRef]
- Joshi, A.; Sharma, A.; Bachheti, R.K.; Pandey, D.P. A comparative study of the chemical composition of the essential oil from Eucalyptus globulus growing in Dehradun (India) and around the world. Orient. J. Chem 2016, 32, 331–340. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, Z.; Guo, L.; AlObaid, A.A. Batatas lam leaf extract as a biodegradable corrosion inhibitor for copper in 0.5 M H2SO4 solution. Sustain. Chem. Pharm. 2024, 39, 101601. [Google Scholar] [CrossRef]
- Azzouni, D.; Haldhar, R.; Salim, R.; Ech-chihbi, E.; Mrani, S.A.; Rais, Z.; Azam, M.; Kim, S.-C.; Taleb, M. Adsorption treatment residues as novel ecological corrosion inhibitors applied to mild steel in a molar hydrochloric acid medium: Experimental studies and surface characterization. Mater. Today Commun. 2023, 35, 106181. [Google Scholar] [CrossRef]
- Hamdouch, A.; Anejjar, A.; Bijla, L.; Gharby, S.; Asdadi, A.; Chebli, B.; Salghi, R.; Hassani, L.I. Corrosion inhibition of carbon steel by Vitex agnus castus leaves essential oils from the oasis of Tata. Moroc. J. Chem. 2023, 11, 105–118. [Google Scholar]
- Tan, B.; Gong, Z.; He, W.; Xiong, J.; Guo, L.; Marzouki, R. Insight into the anti-corrosion mechanism of crop waste Arachis hypogaea L. leaf extract for copper in sulfuric acid medium. Sustain. Chem. Pharm. 2024, 38, 101449. [Google Scholar] [CrossRef]
- Shahraki, M.; Dehdab, M.; Elmi, S. Theoretical studies on the corrosion inhibition performance of three amine derivatives on carbon steel: Molecular dynamics simulation and density functional theory approaches. J. Taiwan Inst. Chem. Eng. 2016, 62, 313–321. [Google Scholar] [CrossRef]
- Tan, J.; Guo, L.; Lv, T.; Zhang, S. Experimental and computational evaluation of 3-indolebutyric acid as a corrosion inhibitor for mild steel in sulfuric acid solution. Int. J. Electrochem. Sci. 2015, 10, 823–837. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Guo, L.; Zheng, X.; Xiang, B.; Chen, S. Experimental and theoretical studies of four allyl imidazolium-based ionic liquids as green inhibitors for copper corrosion in sulfuric acid. Corros. Sci. 2017, 119, 68–78. [Google Scholar] [CrossRef]
- El Faydy, M.; Benhiba, F.; Lakhrissi, B.; Touhami, M.E.; Warad, I.; Bentiss, F.; Zarrouk, A. The inhibitive impact of both kinds of 5-isothiocyanatomethyl-8-hydroxyquinoline derivatives on the corrosion of carbon steel in acidic electrolyte. J. Mol. Liq. 2019, 295, 111629. [Google Scholar] [CrossRef]
- Jiang, C.; Silva, S.M.; Fan, S.; Wu, Y.; Alam, M.T.; Liu, G.; Gooding, J.J. Aryldiazonium salt derived mixed organic layers: From surface chemistry to their applications. J. Electroanal. Chem. 2017, 785, 265–278. [Google Scholar] [CrossRef]
- Xie, X.; Hosni, B.; Chen, C.; Wu, H.; Li, Y.; Chen, Z.; Verdy, C.; Kedim, O.E.; Zhong, Q.; Addad, A.; et al. Corrosion behavior of cold sprayed 7075Al composite coating reinforced with TiB2 nanoparticles. Surf. Coat. Technol. 2020, 404, 126460. [Google Scholar] [CrossRef]
- Abd El-Raouf, M.; Khamis, E.A.; Abou Kana, M.T.; Negm, N.A. Electrochemical and quantum chemical evaluation of new bis (coumarins) derivatives as corrosion inhibitors for carbon steel corrosion in 0.5 M H2SO4. J. Mol. Liq. 2018, 255, 341–353. [Google Scholar] [CrossRef]
- Ituen, E.; James, A.; Akaranta, O.; Sun, S. Eco-friendly corrosion inhibitor from Pennisetum purpureum biomass and synergistic intensifiers for mild steel. Chin. J. Chem. Eng. 2016, 24, 1442–1447. [Google Scholar] [CrossRef]
- Mrani, S.A.; Salim, R.; Arrousse, N.; El Abiad, C.; Radi, S.; Saffaj, T.; Taleb, M. Computational, SEM/EDX and experimental insights on the adsorption process of novel Schiff base molecules on mild steel/1 M HCl interface. J. Mol. Liq. 2022, 368, 120648. [Google Scholar] [CrossRef]
- Jokar, M.; Farahani, T.S.; Ramezanzadeh, B. Electrochemical and surface characterizations of morus alba pendula leaves extract (MAPLE) as a green corrosion inhibitor for steel in 1 M HCl. J. Taiwan Inst. Chem. Eng. 2016, 63, 436–452. [Google Scholar] [CrossRef]
- Frazão, C.; Barros, J.; Camões, A.; Alves, A.C.; Rocha, L. Corrosion effects on pullout behavior of hooked steel fibers in self-compacting concrete. Cem. Concr. Res. 2016, 79, 112–122. [Google Scholar] [CrossRef]
- Benali, O.; Benmehdi, H.; Hasnaoui, O.; Selles, C.; Salghi, R. Green corrosion inhibitor: Inhibitive action of tannin extract of Chamaerops humilis plant for the corrosion of mild steel in 0.5 M H2SO4. J. Mater. Environ. Sci. 2013, 4, 127–138. [Google Scholar]
- Hmamou, D.B.; Salghi, R.; Zarrouk, A.; Hammouti, B.; Al-Deyab, S.S.; Bazzi, L.; Zarrok, H.; Chakir, A.; Bammou, L. Corrosion inhibition of steel in 1 M hydrochloric acid medium by chamomile essential oils. Int. J. Electrochem. Sci. 2012, 7, 2361–2373. [Google Scholar] [CrossRef]
- Guo, L.; Xin, H.; Zhang, Z.; Zhang, X.; Ye, F. Microstructure modification of Y2O3 stabilized ZrO2 thermal barrier coatings by laser glazing and the effects on the hot corrosion resistance. J. Adv. Ceram. 2020, 9, 232–242. [Google Scholar] [CrossRef]
- Olasunkanmi, L.O.; Ebenso, E.E. Experimental and computational studies on propanone derivatives of quinoxalin-6-yl-4, 5-dihydropyrazole as inhibitors of mild steel corrosion in hydrochloric acid. J. Colloid Interface Sci. 2020, 561, 104–116. [Google Scholar] [CrossRef]
- Benbouguerra, K.; Chafai, N.; Chafaa, S.; Touahria, Y.I.; Tlidjane, H. New α-Hydrazinophosphonic acid: Synthesis, characterization, DFT study and in silico prediction of its potential inhibition of SARS-CoV-2 main protease. J. Mol. Struct. 2021, 1239, 130480. [Google Scholar] [CrossRef] [PubMed]
- Haque, J.; Ansari, K.R.; Srivastava, V.; Quraishi, M.A.; Obot, I.B. Pyrimidine derivatives as novel acidizing corrosion inhibitors for N80 steel useful for petroleum industry: A combined experimental and theoretical approach. J. Ind. Eng. Chem. 2017, 49, 176–188. [Google Scholar] [CrossRef]
- Ijuo, G.A.; Chahul, H.F.; Eneji, I.S. Kinetic and thermodynamic studies of corrosion inhibition of mild steel using Bridelia ferruginea extract in acidic environment. J. Adv. Electrochem. 2016, 2, 107–112. [Google Scholar]
- Cao, Z.; Tang, Y.; Cang, H.; Xu, J.; Lu, G.; Jing, W. Novel benzimidazole derivatives as corrosion inhibitors of mild steel in the acidic media. Part II: Theoretical studies. Corros. Sci. 2014, 83, 292–298. [Google Scholar] [CrossRef]
- Karaoui, M.; Zarrouk, A.M.; Hsissou, R.; Alami, M.; Assouag, M. Performance of organic molecules as corrosion inhibitors for CS: A comprehensive review. Anal. Bioanal. Electrochem. 2022, 14, 535–556. [Google Scholar]
- Salvestrini, S.; Ambrosone, L.; Kopinke, F.D. Some mistakes and misinterpretations in the analysis of thermodynamic adsorption data. J. Mol. Liq. 2022, 352, 118762. [Google Scholar] [CrossRef]
- Zhang, H.H.; Chen, Y. Experimental and theoretical studies of benzaldehyde thiosemicarbazone derivatives as corrosion inhibitors for mild steel in acid media. J. Mol. Struct. 2019, 1177, 90–100. [Google Scholar] [CrossRef]
- El Hajjaji, F.; Abrigach, F.; Hamed, O.; Hasan, A.R.; Taleb, M.; Jodeh, S.; Rodríguez-Castellón, E.; Yuso, M.D.V.M.d.; Algarra, M. Corrosion resistance of mild steel coated with orgainc material containing pyrazol moiety. Coatings 2018, 8, 330. [Google Scholar] [CrossRef]
- Dennington, R.D.I.I.; Keith, T.A.; Millam, J.M. GaussView, version 6.0.16; Semichem Inc.: Shawnee Mission, KS, USA, 2016.
- Erdoğan, Ş.; Safi, Z.S.; Kaya, S.; Işın, D.Ö.; Guo, L.; Kaya, C. A computational study on corrosion inhibition performances of novel quinoline derivatives against the corrosion of iron. J. Mol. Struct. 2017, 1134, 751–761. [Google Scholar] [CrossRef]
- Kumar, U.P.; Albrakaty, R.H.; Wazzan, N.; Obot, I.B.; Safi, Z.S.; Shanmugan, S.; Liang, T. Insight into the nature of the ionic interactions between some aldehydes and Ni-W alloy: A theoretical study. Mater. Today Commun. 2020, 22, 100693. [Google Scholar] [CrossRef]
- Wazzan, N.; Obot, I.B.; Fagieh, T.M. The role of some triazoles on the corrosion inhibition of C1020 steel and copper in a desalination descaling solution. Desalination 2022, 527, 115551. [Google Scholar] [CrossRef]
- Berrani, A.; Lrhorfi, L.A.; Bengueddour, R. The effect of the methanol extracts of various parts of vitex agnus castus on the corrosion of e24 steel in a neutral medium (nacl 3.5%). J. Chem. Technol. Metall. 2020, 55, 459. [Google Scholar]
- Ehsani, A.; Mahjani, M.G.; Hosseini, M.; Safari, R.; Moshrefi, R.; Shiri, H.M. Evaluation of Thymus vulgaris plant extract as an eco-friendly corrosion inhibitor for stainless steel 304 in acidic solution by means of electrochemical impedance spectroscopy, electrochemical noise analysis and density functional theory. J. Colloid Interface Sci. 2017, 490, 444–451. [Google Scholar] [CrossRef]
- Rosson, E.; Sgarbossa, P.; Mozzon, M.; Venturino, F.; Bogialli, S.; Glisenti, A.; Talon, A.; Moretti, E.; Carturan, S.M.; Bertani, R.; et al. Novel correlations between spectroscopic and morphological properties of activated carbons from waste coffee grounds. Processes 2021, 9, 1637. [Google Scholar] [CrossRef]
- El-Hajjaji, F.; Messali, M.; De Yuso, M.M.; Rodríguez-Castellón, E.; Almutairi, S.; Bandosz, T.J.; Algarra, M. Effect of 1-(3-phenoxypropyl) pyridazin-1-ium bromide on steel corrosion inhibition in acidic medium. J. Colloid Interface Sci. 2019, 541, 418–424. [Google Scholar] [CrossRef]
- Mrani, S.A.; Arrousse, N.; Haldhar, R.; Lahcen, A.A.; Amine, A.; Saffaj, T.; Kim, S.-C.; Taleb, M. In Silico approaches for some sulfa drugs as eco-friendly corrosion inhibitors of iron in aqueous medium. Lubricants 2022, 10, 43. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Arrousse, N.; Salim, R.; Kaddouri, Y.; Zahri, D.; El Hajjaji, F.; Touzani, R.; Taleb, M.; Jodeh, S. The inhibition behavior of two pyrimidine-pyrazole derivatives against corrosion in hydrochloric solution: Experimental, surface analysis and in silico approach studies. Arab. J. Chem. 2020, 13, 5949–5965. [Google Scholar] [CrossRef]
- Rekkab, S.; Zarrok, H.; Salghi, R.; Zarrouk, A.; Bazzi, L.; Hammouti, B.; Zougagh, M. Green corrosion inhibitor from essential oil of Eucalyptus globulus (Myrtaceae) for C38 steel in sulfuric acid solution. J. Mater. Environ. Sci. 2012, 3, 613–627. [Google Scholar]
- Ansari, A.; Ou-Ani, O.; Oucheikh, L.; Youssefi, Y.; Chebabe, D.; Oubair, A.; Znini, M. Experimental, Theoretical Modeling and Optimization of Inhibitive Action of Ocimum Basilicum Essential Oil as Green Corrosion Inhibitor for C38 Steel in 0.5 M H2SO4 Medium. Chem. Afr. 2022, 5, 37–55. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Abu-Dief, A.M.; Mohamed, M.A. Corrosion inhibition of carbon steel pipelines by some novel Schiff base compounds during acidizing treatment of oil wells studied by electrochemical and quantum chemical methods. J. Mol. Struct. 2017, 1130, 522–542. [Google Scholar] [CrossRef]
- Dahmani, K.; Galai, M.; Ech-Chebab, A.; Al-Zaqri, N.; Ouakki, M.; Elgendy, A.; Ez-Zriouli, R.; Kim, S.-C.; Touhami, M.E.; Cherkaoui, M. Investigating the Inhibitory Properties of Cupressus sempervirens Extract against Copper Corrosion in 0.5 M H2SO4: Combining Quantum (Density Functional Theory Calculation–Monte Carlo Simulation) and Electrochemical-Surface Studies. ACS Omega 2023, 8, 24218–24232. [Google Scholar] [CrossRef]
- Mrani, S.A.; Ech-Chihbi, E.; Salim, R.; Daoui, S.; Benchat, N.; Saffaj, T.; Zarrouk, A.; Taleb, M. Experimental, theoretical and MC simulation investigations of the inhibitory efficiency of novel non-toxic pyridazine derivatives inhibition on carbon steel in 1 M HCl solution. J. Mol. Liq. 2023, 382, 122043. [Google Scholar] [CrossRef]
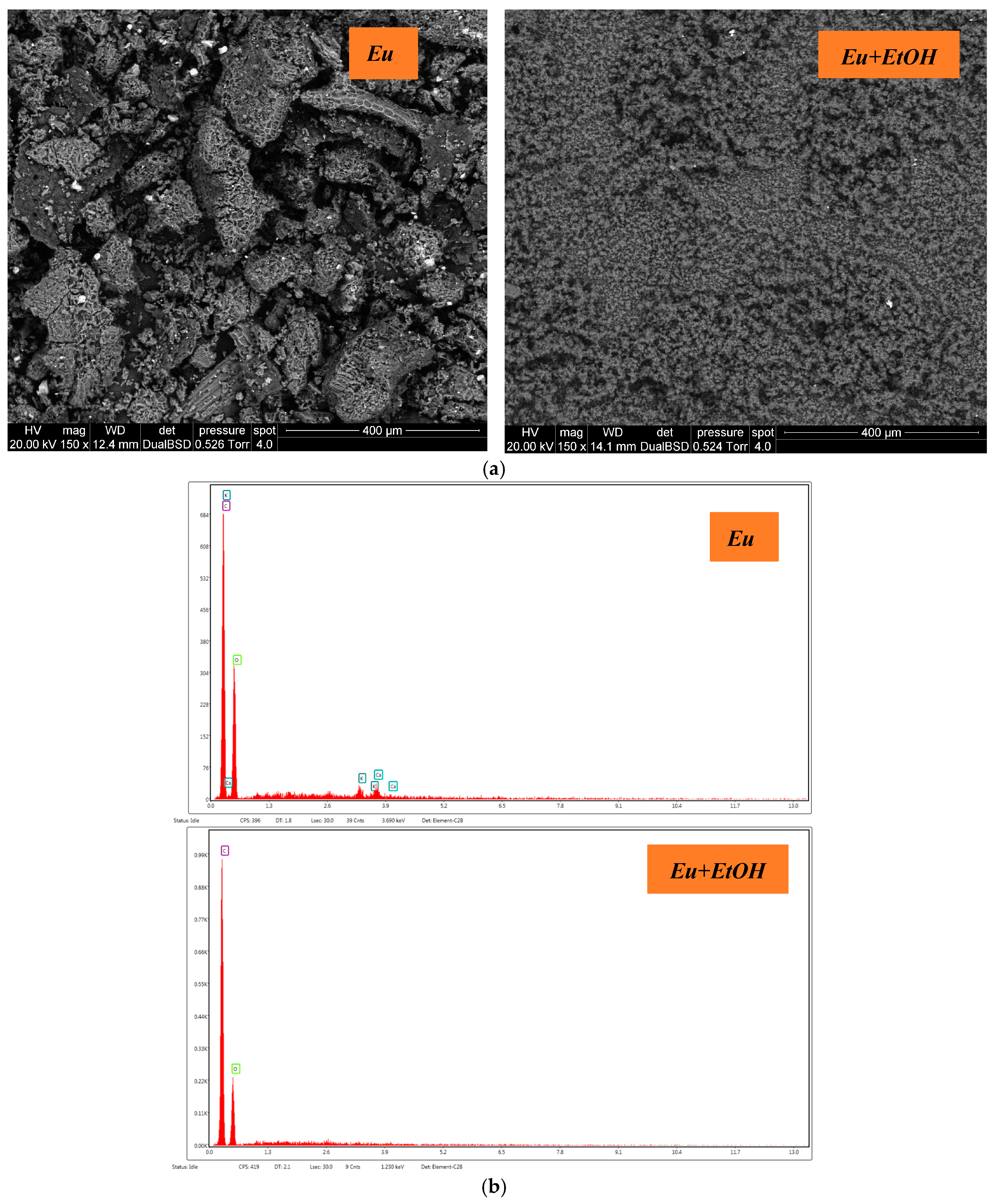
| Element | O | C | K | Ca |
|---|---|---|---|---|
| Eu | 46.5 | 49.66 | 1.63 | 2.21 |
| Eu+EtOH | 64.53 | 35.47 | - | - |
| Name | Molecular Formula | Structure | Molar Mass (g/mol) | |
|---|---|---|---|---|
| a | Uvaol | C30H50O2 | 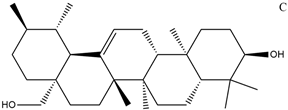 | 442.71 |
| b | Betulinaldehyde | C30H48O2 | 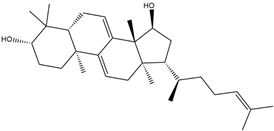 | 440.36 |
| c | d-Allose | C6H12O6 | 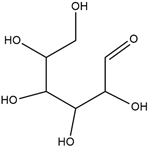 | 180.06 |
| Concentration (g/L) | −Ecorr (mV/Ag/AgCl) | icorr (µA.m−2) | −βc (mV.dec−1) | −βa (mV.dec−1) | IEpdp (%) | |
|---|---|---|---|---|---|---|
| HCl 1 M | - | 451.506 | 793.261 | 123.8 | 84.5 | - |
| EuEO | 1.00 | 437.847 | 23.775 | 107.7 | 97.9 | 97 |
| 0.75 | 422.626 | 35.334 | 103.4 | 76.8 | 95 | |
| 0.50 | 445.678 | 35.235 | 87.4 | 61.4 | 95 | |
| 0.25 | 441.017 | 92.280 | 88.9 | 64.2 | 88 |
| Medium | Concentration g/L | Rs (Ω.cm2) | Rp (Ω.cm2) | Q (µF.sn−1) | ndl | Cdl (µF.cm−2) | IEIES % |
|---|---|---|---|---|---|---|---|
| HCl 1 M | - | 1.22 | 33.97 | 315 | 0.82 | 117 | - |
| EuEO | 1.00 | 2.451 | 1113 | 42.46 | 0.799 | 19.69 | 97 |
| 0.75 | 2.849 | 538.6 | 44.24 | 0.811 | 18.47 | 94 | |
| 0.50 | 3.154 | 431.5 | 56.37 | 0.795 | 21.63 | 92 | |
| 0.25 | 2.52 | 172.4 | 10.55 | 0.797 | 38.04 | 80 |
| Isotherms | R2 | Parameters | Kads (L/g) | ΔG0ads (kJ/mol) | |
|---|---|---|---|---|---|
| Langmuir | 0.999 | Slope | 1.002 | 34.36 | −25.87 |
| Freundlich | 0.870 | Z | 0.065 | 0.95 | −16.98 |
| Temkin | 0.874 | a | −8.26 | 1.02 × 107 | −57.09 |
| El-Awady | 0.961 | 1/y | 1.02 | 30.68 | −25.59 |
| Element | O | C | Fe | Ca | Cl | Si |
|---|---|---|---|---|---|---|
| MS | 28.9 | 10 | 58 | 0.8 | 1.8 | 0.6 |
| MS + EuEO | 25.3 | 2.3 | 72.5 | - | - | - |
| Parameters | Uvaol | Betulinaldehyde | d-Allose |
|---|---|---|---|
| EHOMO (eV) | −6.0949 | −6.6873 | −7.1510 |
| ELUMO (eV) | −0.8305 | −0.9293 | −0.9911 |
| ΔEgap (eV) | 5.2644 | 7.6166 | 8.1421 |
| η (eV) | 2.6322 | 3.8083 | 4.0711 |
| σ (eV−1) | 0.3799 | 0.2626 | 0.2456 |
| χ (eV) | 3.4627 | 2.8790 | 3.0800 |
| ΔN110 | 0.2578 | 0.2548 | 0.2137 |
| μ (D) | 3.6619 | 4.5603 | 4.4922 |
| Systems | Total Energy (kcal/mol) | Eads (Inhibitor) (kcal/mol) | EH2O (Inhibitor) (kcal/mol) |
|---|---|---|---|
| Fe(110)/a/100H2O | −2803.122 | −4980.527 | −35.61 |
| Fe(110)/b/100H2O | −2833.019 | −5015.946 | −33.80 |
| Fe(110)/c/100H2O | −2658.588 | −4944.691 | −33.13 |
| Elements | Fe | Si | C | Mn | S | P | Al |
|---|---|---|---|---|---|---|---|
| (%) Mass | 99.21 | 0.38 | 0.21 | 0.05 | 0.05 | 0.09 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azzouni, D.; Alaoui Mrani, S.; Bertani, R.; Alanazi, M.M.; En-nabety, G.; Taleb, M. Experimental and Theoretical Investigation of the Inhibitor Efficiency of Eucalyptus globulus Leaf Essential Oil (EuEO) on Mild Steel Corrosion in a Molar Hydrochloric Acid Medium. Molecules 2024, 29, 3323. https://doi.org/10.3390/molecules29143323
Azzouni D, Alaoui Mrani S, Bertani R, Alanazi MM, En-nabety G, Taleb M. Experimental and Theoretical Investigation of the Inhibitor Efficiency of Eucalyptus globulus Leaf Essential Oil (EuEO) on Mild Steel Corrosion in a Molar Hydrochloric Acid Medium. Molecules. 2024; 29(14):3323. https://doi.org/10.3390/molecules29143323
Chicago/Turabian StyleAzzouni, Dounia, Soukaina Alaoui Mrani, Roberta Bertani, Mohammed M. Alanazi, Ghizlan En-nabety, and Mustapha Taleb. 2024. "Experimental and Theoretical Investigation of the Inhibitor Efficiency of Eucalyptus globulus Leaf Essential Oil (EuEO) on Mild Steel Corrosion in a Molar Hydrochloric Acid Medium" Molecules 29, no. 14: 3323. https://doi.org/10.3390/molecules29143323
APA StyleAzzouni, D., Alaoui Mrani, S., Bertani, R., Alanazi, M. M., En-nabety, G., & Taleb, M. (2024). Experimental and Theoretical Investigation of the Inhibitor Efficiency of Eucalyptus globulus Leaf Essential Oil (EuEO) on Mild Steel Corrosion in a Molar Hydrochloric Acid Medium. Molecules, 29(14), 3323. https://doi.org/10.3390/molecules29143323






