Spin Polarization Enhances the Catalytic Activity of Monolayer MoSe2 for Oxygen Reduction Reaction
Abstract
1. Introduction
2. Results and Discussion
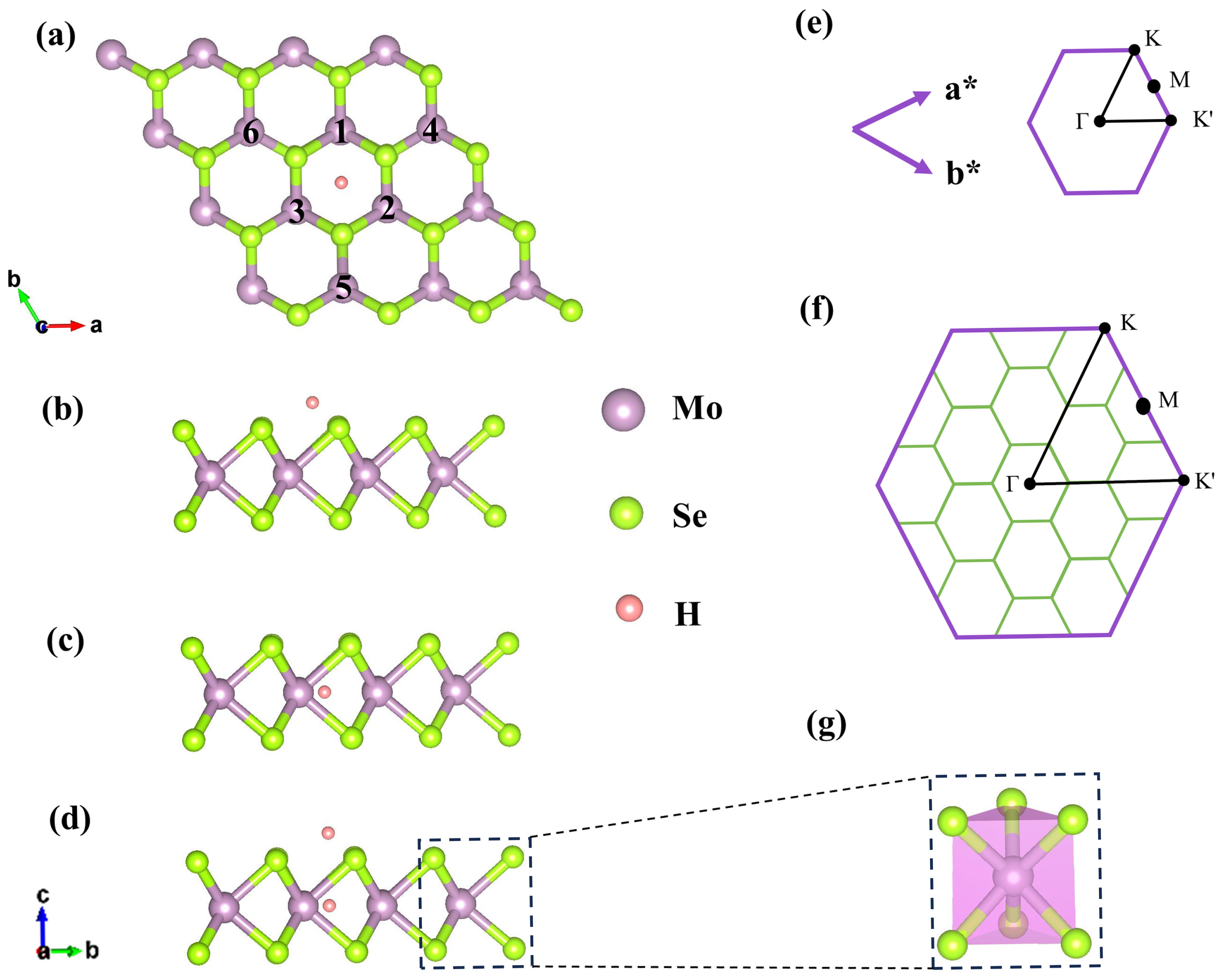
2.1. Geometric Structure
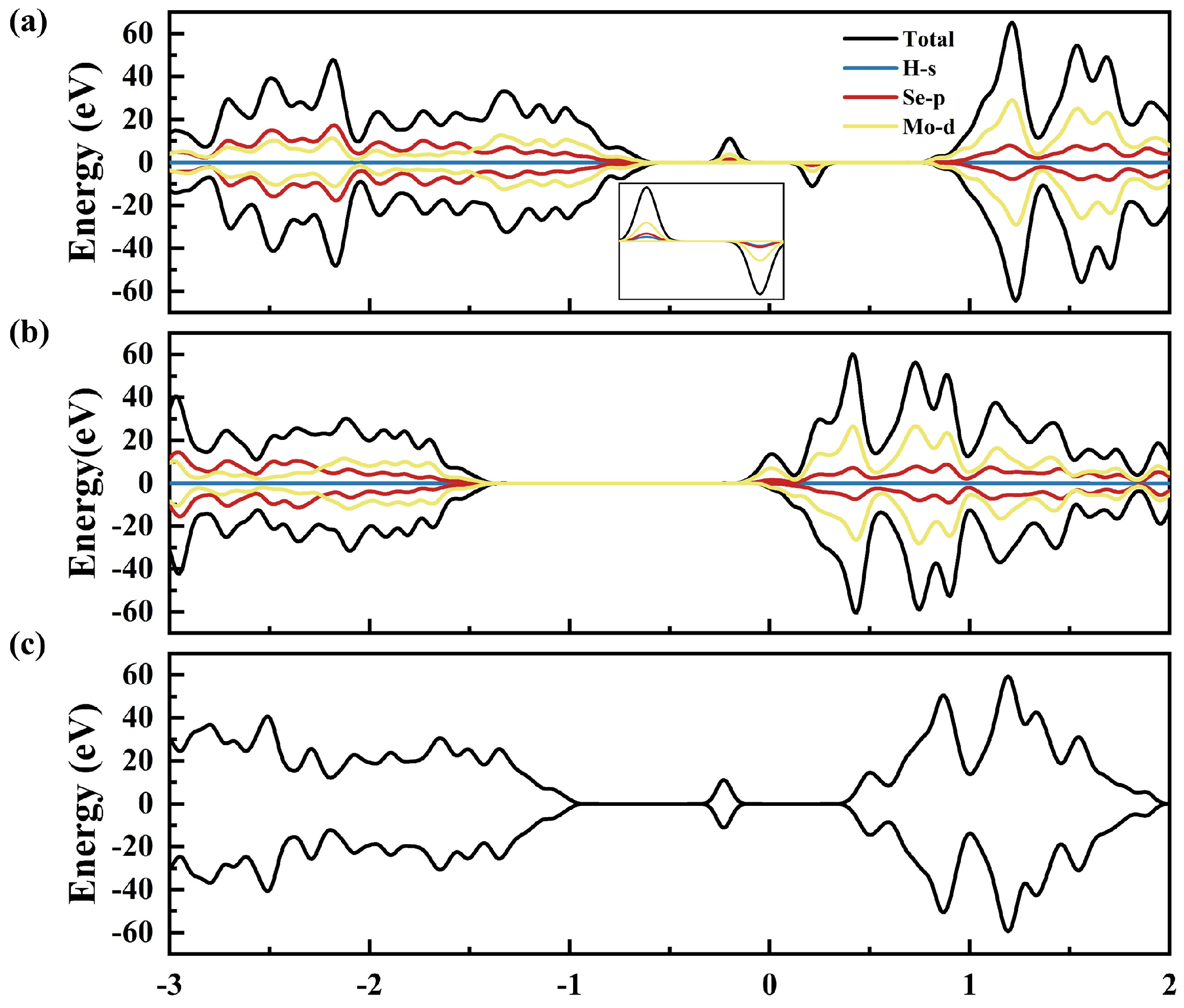
2.2. Electronic Structure
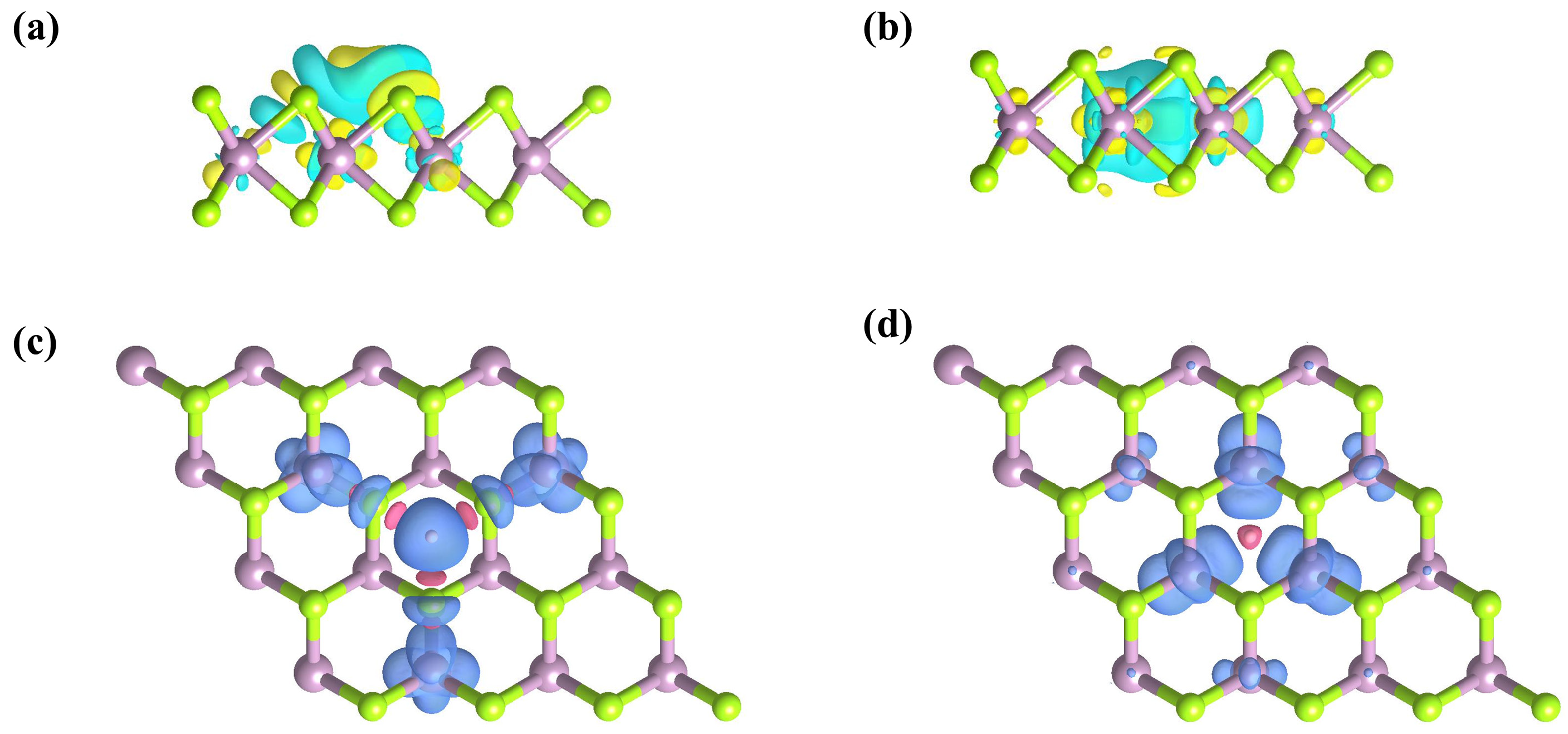
2.3. Oxygen Reduction Reaction Analysis
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, F.; Wang, Y.C.; Wu, Z.P.; Chen, G.; Yang, F.; Zhu, S.; Siddharth, K.; Kong, Z.; Lu, A.; Li, J.C.; et al. Recent Advances in Electrocatalysts for Proton Exchange Membrane Fuel Cells and Alkaline Membrane Fuel Cells. Adv. Mater. 2021, 33, 2006292. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, S.; Priest, C.; Shi, Q.; Wu, G. Atomically dispersed metal–nitrogen–carbon catalysts for fuel cells: Advances in catalyst design, electrode performance, and durability improvement. Chem. Soc. Rev. 2020, 49, 3484–3524. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 17832–17852. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.K.; Zeng, Y.J.; Liu, W.; Tang, L.M.; Chen, K.Q. Topological Phonons and Thermoelectric Conversion in Crystalline Materials. Adv. Funct. Mater. 2024, 2401684. [Google Scholar] [CrossRef]
- Eftekhari, A. Molybdenum diselenide (MoSe2) for energy storage, catalysis, and optoelectronics. Appl. Mater. Today 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Lihter, M.; Graf, M.; Iveković, D.; Zhang, M.; Shen, T.H.; Zhao, Y.; Macha, M.; Tileli, V.; Radenovic, A. Electrochemical Functionalization of Selectively Addressed MoS2 Nanoribbons for Sensor Device Fabrication. ACS Appl. Nano Mater. 2021, 4, 1076–1084. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Zhang, J.; Lou, X.W.D. Recent Advances on Transition Metal Dichalcogenides for Electrochemical Energy Conversion. Adv. Mater. 2021, 33, 2008376. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Matadamas-Ortiz, P.; Ortiz-Herrera, J.; López-Chávez, E.; Solorza-Feria, O.; Medina, D. Current progress of Pt-based ORR electrocatalysts for PEMFCs: An integrated view combining theory and experiment. Mater. Today Phys. 2021, 19, 100406. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Liang, Z.; Zou, R.; Guo, S. Earth-Abundant Nanomaterials for Oxygen Reduction. Angew. Chem. Int. Ed. 2016, 55, 2650–2676. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Li, Z.; Yang, B.; Ling, M.; Gao, X.; Lu, J.; Shi, Q.; Lei, L.; Wu, G.; et al. Designing 3d dual transition metal electrocatalysts for oxygen evolution reaction in alkaline electrolyte: Beyond oxides. Nano Energy 2020, 77, 105162. [Google Scholar] [CrossRef]
- Peng, Y.; Zhu, L.; Li, C.; Hu, J.; Lu, Y.; Fu, J.; Cui, F.; Wang, X.; Cao, A.; Ji, Q.; et al. Highly Stable Vertically Oriented 2H-NbS2 Nanosheets on Carbon Nanotube Films toward Superior Electrocatalytic Activity. Adv. Energy Mater. 2024, 14, 2302510. [Google Scholar] [CrossRef]
- Luxa, J.; Mazánek, V.; Pumera, M.; Lazar, P.; Sedmidubskỳ, D.; Callisti, M.; Polcar, T.; Sofer, Z. 2H→ 1T phase engineering of layered tantalum disulfides in electrocatalysis: Oxygen reduction reaction. Chem. Eur. J. 2017, 23, 8082–8091. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.H.; Kong, X.Y.; Ng, B.J.; Soo, H.S.; Mohamed, A.R.; Chai, S.P. Recent Advances in Defect-Engineered Transition Metal Dichalcogenides for Enhanced Electrocatalytic Hydrogen Evolution: Perfecting Imperfections. ACS Omega 2023, 8, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, Y.; Yu, J.; Ye, Q.; Yang, L.; Li, Y.; Fan, H.J. Biaxially strained MoS2 nanoshells with controllable layers boost alkaline hydrogen evolution. Adv. Mater. 2022, 34, 2202195. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, J.; Zhang, Y.; Sun, Y.; Ma, K.; Xie, Y.; Zheng, W.; Tian, Z.; Kang, Z.; Zhang, Y. Vacancy defects in 2D transition metal dichalcogenide electrocatalysts: From aggregated to atomic configuration. Adv. Mater. 2023, 35, 2206576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, W.; Li, C.; He, L. First-principles study of nonmetal doped monolayer MoSe2 for tunable electronic and photocatalytic properties. Sci. Rep. 2017, 7, 17088. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ding, Z.K.; Zeng, B.W.; Luo, N.N.; Zeng, J.; Tang, L.M.; Chen, K.Q. Ab initio Boltzmann approach to coupled magnon-phonon thermal transport in ferromagnetic crystals. Phys. Rev. B 2023, 107, 104303. [Google Scholar] [CrossRef]
- Xiao, W.H.; Yang, K.; D’Agosta, R.; Xu, H.R.; Ouyang, G.; Zhou, G.; Chen, K.Q.; Tang, L.M. High-mobility two-dimensional MA2N4 (M = Mo, W; A = Si, Ge) family for transistors. Phys. Rev. B 2024, 109, 115427. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Sun, M.; Qi, Q.; Luo, S.; Song, H.; Xiao, J.; Huang, B.; Leung, M.K.; Zhang, Y. Ultrastable bimetallic Fe2Mo for efficient oxygen reduction reaction in pH-universal applications. Nano Res. 2022, 15, 4950–4957. [Google Scholar] [CrossRef]
- He, R.; Wang, D.; Luo, N.; Zeng, J.; Chen, K.Q.; Tang, L.M. Nonrelativistic Spin-Momentum Coupling in Antiferromagnetic Twisted Bilayers. Phys. Rev. Lett. 2023, 130, 046401. [Google Scholar] [CrossRef]
- Pan, H.; Tang, L.M.; Chen, K.Q. Quantum mechanical modeling of magnon-phonon scattering heat transport across three-dimensional ferromagnetic/nonmagnetic interfaces. Phys. Rev. B 2022, 105, 064401. [Google Scholar] [CrossRef]
- Huang, Q.; Sheng, H. Magnetic-Field-Induced Spin Regulation in Electrocatalytic Reactions. Chem. Eur. J. 2024, 30, e202400352. [Google Scholar] [CrossRef]
- Zhong, W.; Qiu, Y.; Shen, H.; Wang, X.; Yuan, J.; Jia, C.; Bi, S.; Jiang, J. Electronic Spin Moment as a Catalytic Descriptor for Fe Single-Atom Catalysts Supported on C2N. J. Am. Chem. Soc. 2021, 143, 4405–4413. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Qi, R.; Hu, Y.; Zhang, J.; Zhao, H.; Zhang, J.; Zhao, Y. Enhanced Fe 3d delocalization and moderate spin polarization in FeNi atomic pairs for bifunctional ORR and OER electrocatalysis. Appl. Catal. B 2021, 285, 119778. [Google Scholar] [CrossRef]
- Marbaniang, P.; Patil, I.; Lokanathan, M.; Parse, H.; Catherin Sesu, D.; Ingavale, S.; Kakade, B. Nanorice-like Structure of Carbon-Doped Hexagonal Boron Nitride as an Efficient Metal-Free Catalyst for Oxygen Electroreduction. ACS Sustain. Chem. Eng. 2018, 6, 11115–11122. [Google Scholar] [CrossRef]
- Cao, S.; Cao, C.; Tian, S.; Chen, J.H. Enhancement of spin-orbit coupling and magnetic scattering in hydrogenated graphene. Phys. Rev. B 2021, 104, 125422. [Google Scholar] [CrossRef]
- Ding, Z.K.; Zeng, Y.J.; Pan, H.; Luo, N.; Tang, L.M.; Zeng, J.; Chen, K.Q. Robustness and scattering behavior of topological phonons in crystalline materials. Phys. Rev. B 2024, 109, 245104. [Google Scholar] [CrossRef]
- Cao, S.; Cao, C.; Tian, S.; Chen, J.H. Evidence of tunable magnetic coupling in hydrogenated graphene. Phys. Rev. B 2020, 102, 045402. [Google Scholar] [CrossRef]
- Zhu, Z.; Peelaers, H.; Van de Walle, C.G. Hydrogen intercalation in MoS2. Phys. Rev. B 2016, 94, 085426. [Google Scholar] [CrossRef]
- Liu, W.; Ding, Z.K.; Luo, N.; Zeng, J.; Tang, L.M.; Chen, K.Q. Phononic hybrid-order topology in semihydrogenated graphene. Phys. Rev. B 2024, 109, 115422. [Google Scholar] [CrossRef]
- Lin, Z.; Fu, B.; An, Y. Effects of defects and anions on the geometry, electronic structures and exchange interaction of Fe-doped 2H-MoSe2 monolayer. Appl. Surf. Sci. 2020, 528, 146960. [Google Scholar] [CrossRef]
- Miller, T.; Mueller, M.; Chiang, T.C. Band folding and energy-gap formation in Ag-Au superlattices. Phys. Rev. B 1989, 40, 1301. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Chen, M.; Weinert, M. Layer k-projection and unfolding electronic bands at interfaces. Phys. Rev. B 2018, 98, 245421. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W.T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.Y.; Calle-Vallejo, F.; Hansen, H.A.; Martínez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]


| Site | (Å) | (Å) | (meV) | (meV) | (meV) |
|---|---|---|---|---|---|
| A | 3.26 | 2.09 | 35 | −20 | 55 |
| C | 1.95 | 2.57 | 25 | 10 | 15 |
| AC | – | – | – | 0 | 0 |
| Site | Point Group | s | p | d |
|---|---|---|---|---|
| A | C3v | a1 | a1⊕e | a1⊕e |
| C | D3h | a′1 | a″2⊕e′ | a′1⊕e′⊕e″ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, D.; Wang, D.; Wang, Y.; Tang, L.; Chen, K. Spin Polarization Enhances the Catalytic Activity of Monolayer MoSe2 for Oxygen Reduction Reaction. Molecules 2024, 29, 3311. https://doi.org/10.3390/molecules29143311
Shu D, Wang D, Wang Y, Tang L, Chen K. Spin Polarization Enhances the Catalytic Activity of Monolayer MoSe2 for Oxygen Reduction Reaction. Molecules. 2024; 29(14):3311. https://doi.org/10.3390/molecules29143311
Chicago/Turabian StyleShu, Dan, Dan Wang, Yan Wang, Liming Tang, and Keqiu Chen. 2024. "Spin Polarization Enhances the Catalytic Activity of Monolayer MoSe2 for Oxygen Reduction Reaction" Molecules 29, no. 14: 3311. https://doi.org/10.3390/molecules29143311
APA StyleShu, D., Wang, D., Wang, Y., Tang, L., & Chen, K. (2024). Spin Polarization Enhances the Catalytic Activity of Monolayer MoSe2 for Oxygen Reduction Reaction. Molecules, 29(14), 3311. https://doi.org/10.3390/molecules29143311








