Exploring Selenide Synthesis Pathways for Optimizing Energy Conversion
Abstract
1. Introduction
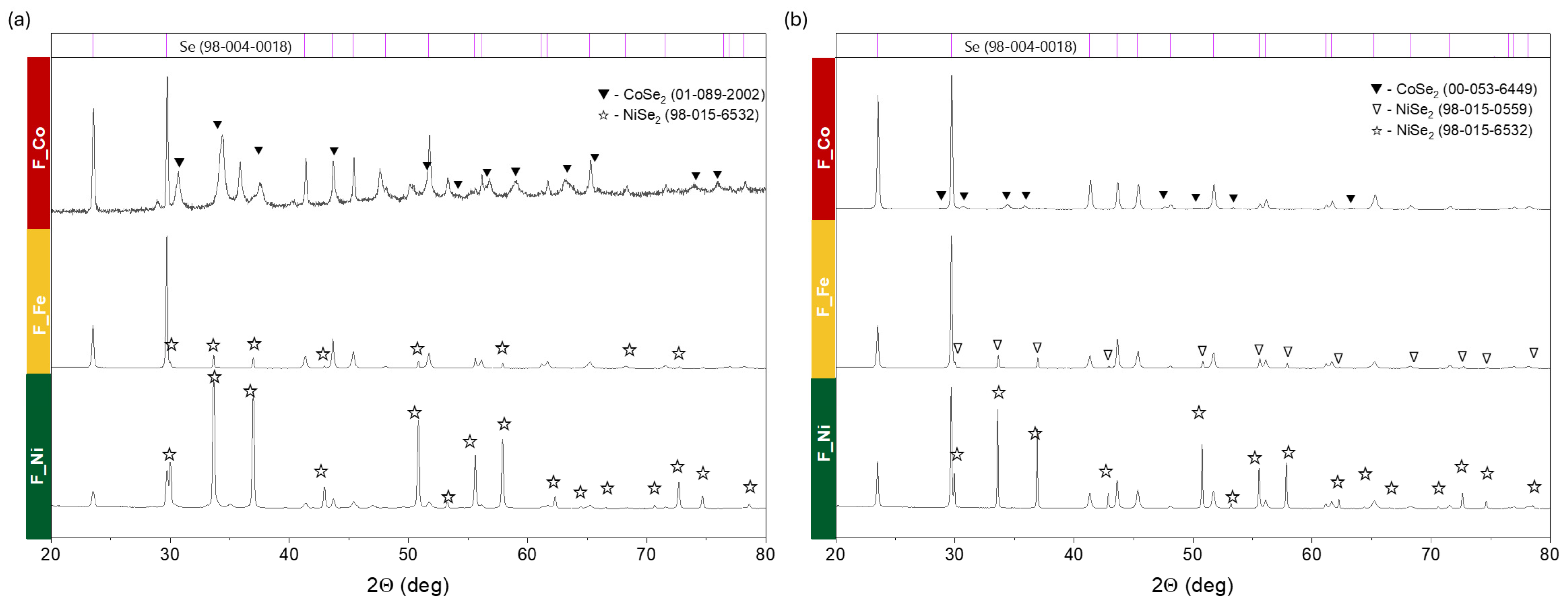
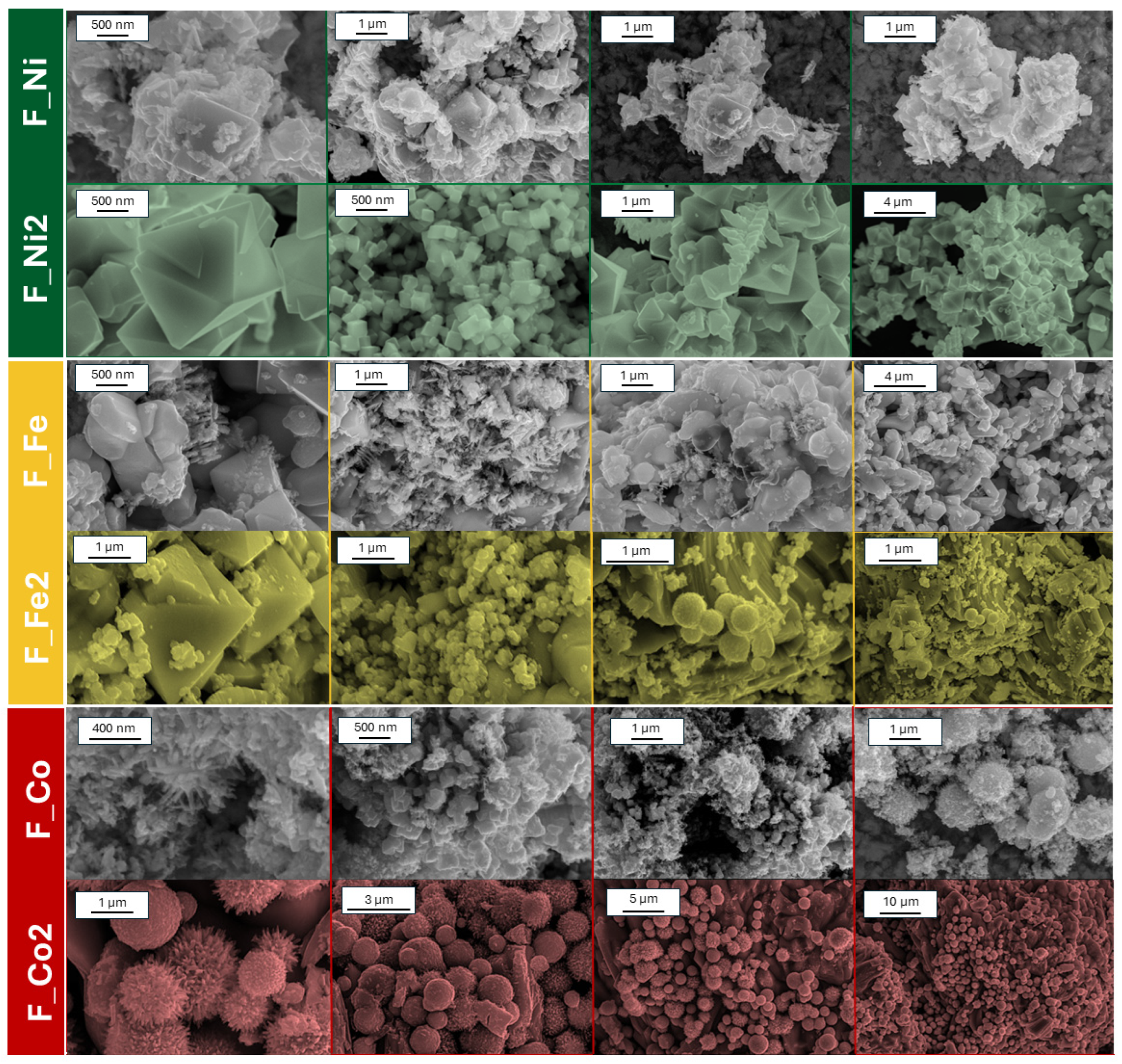
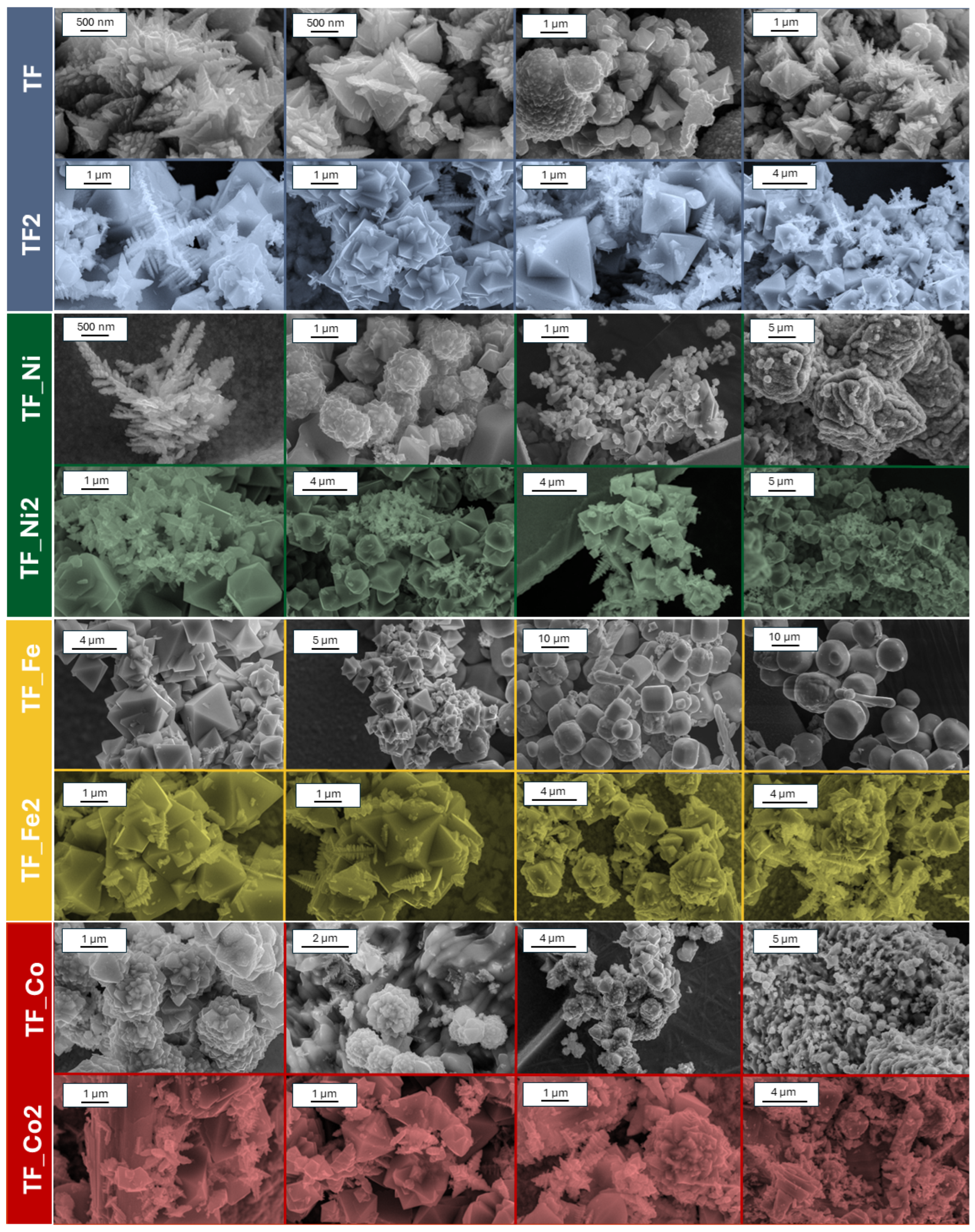
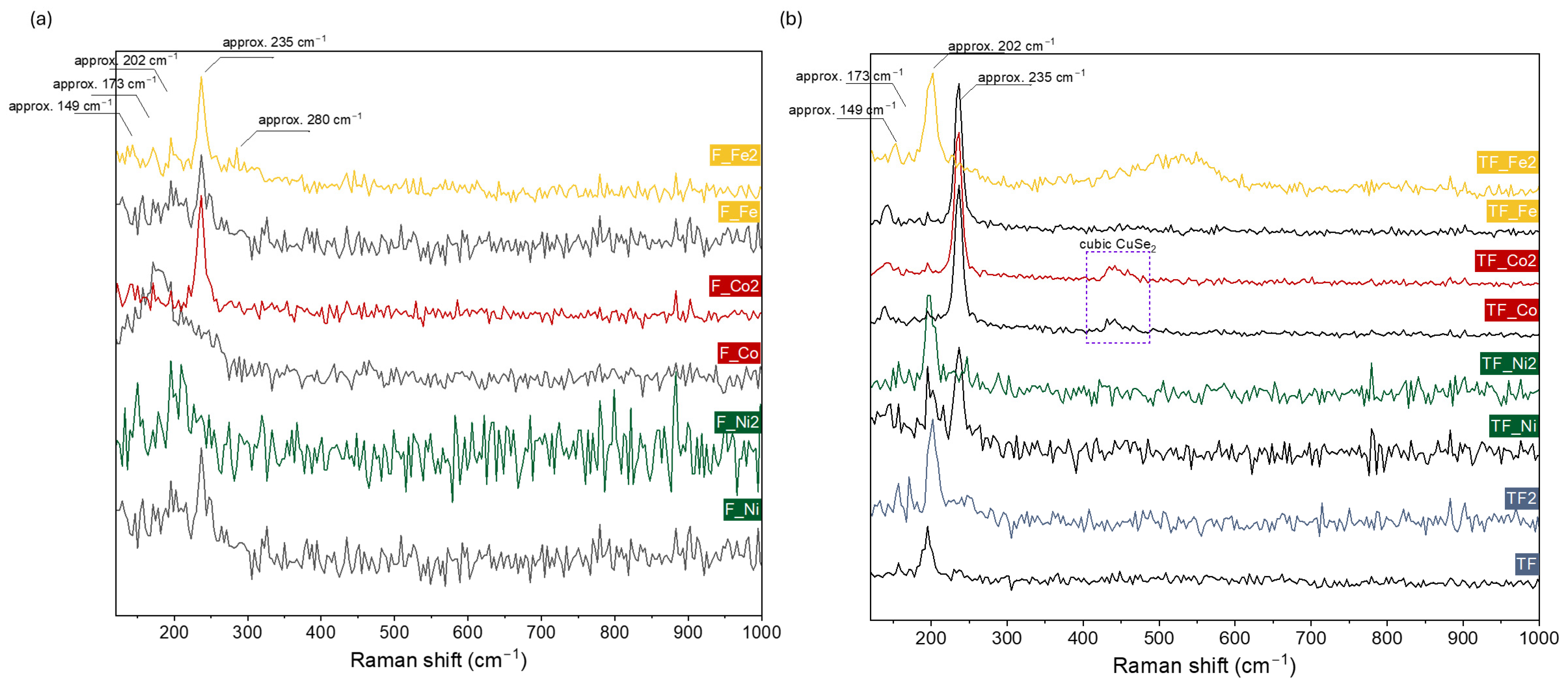
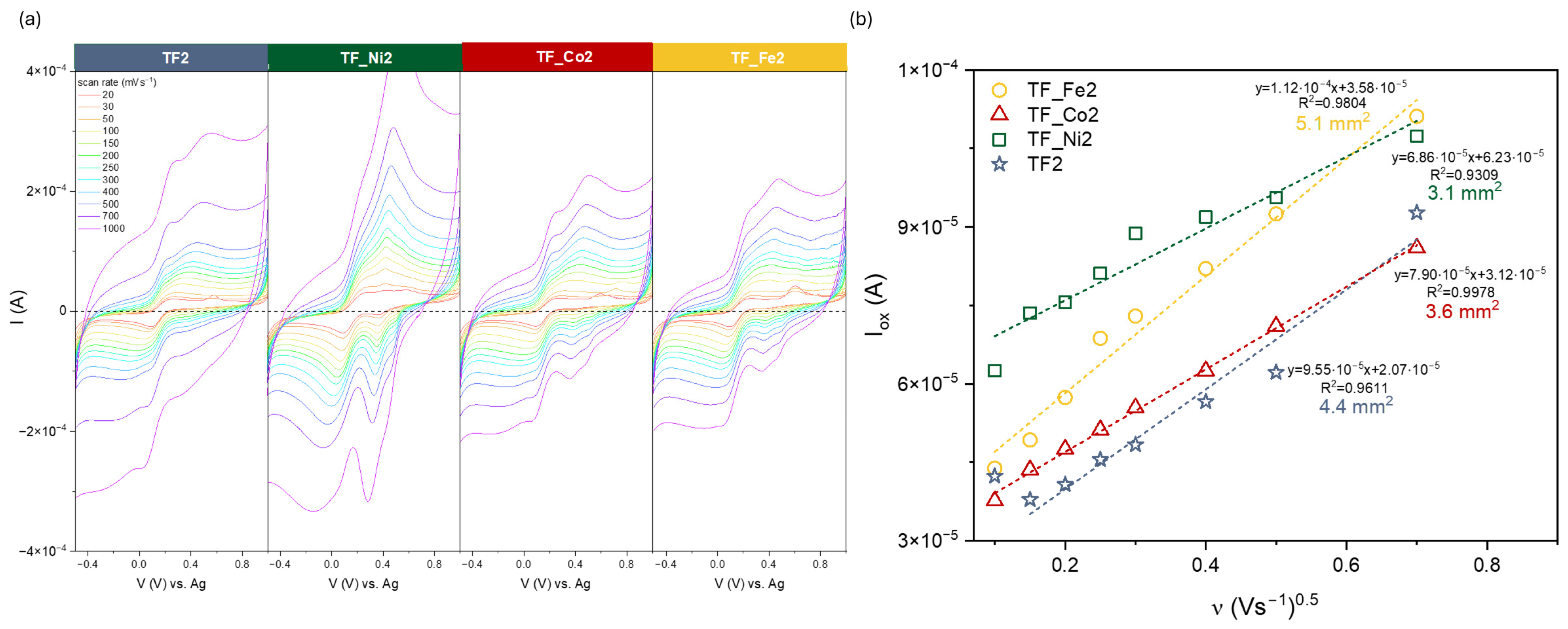
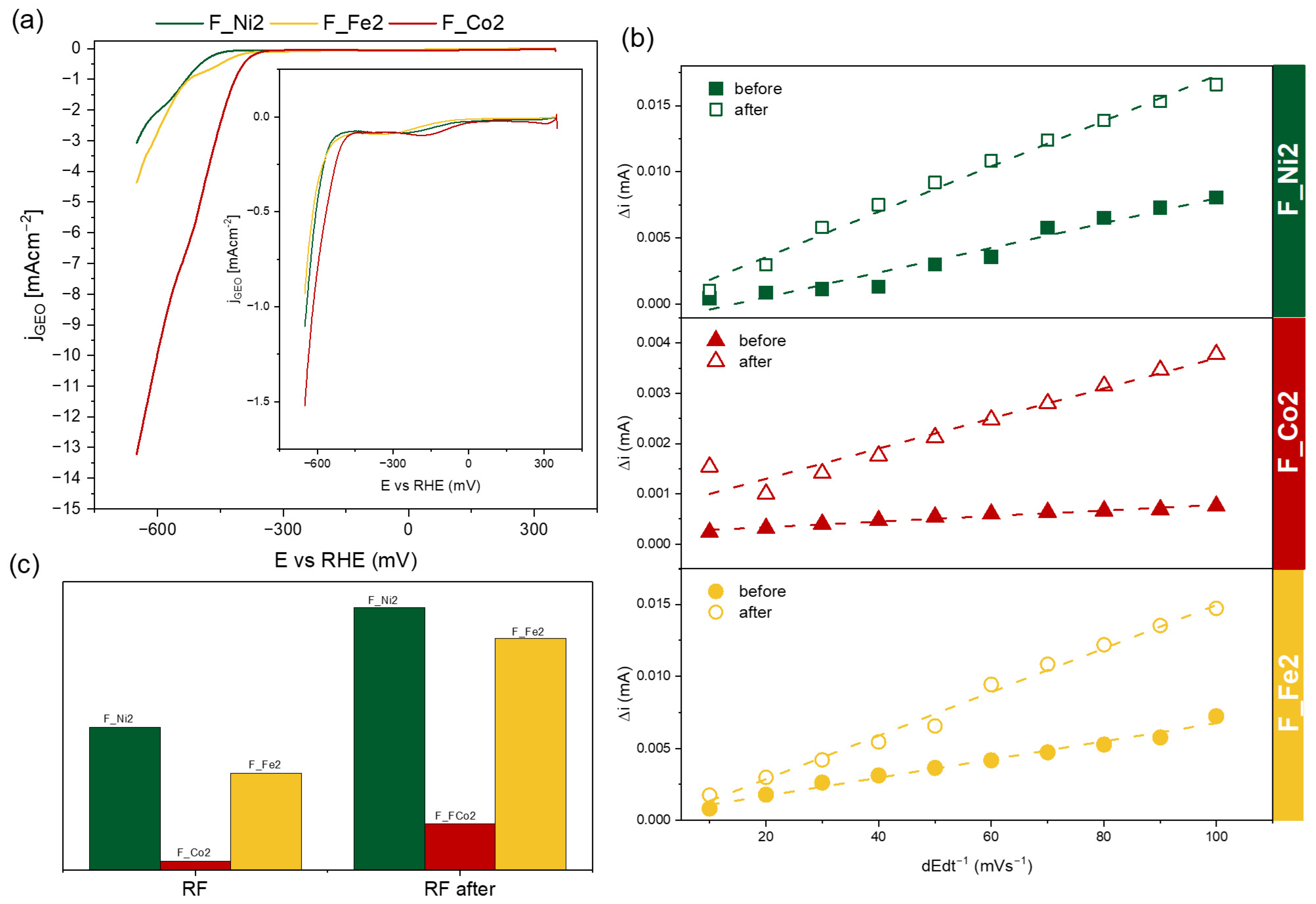
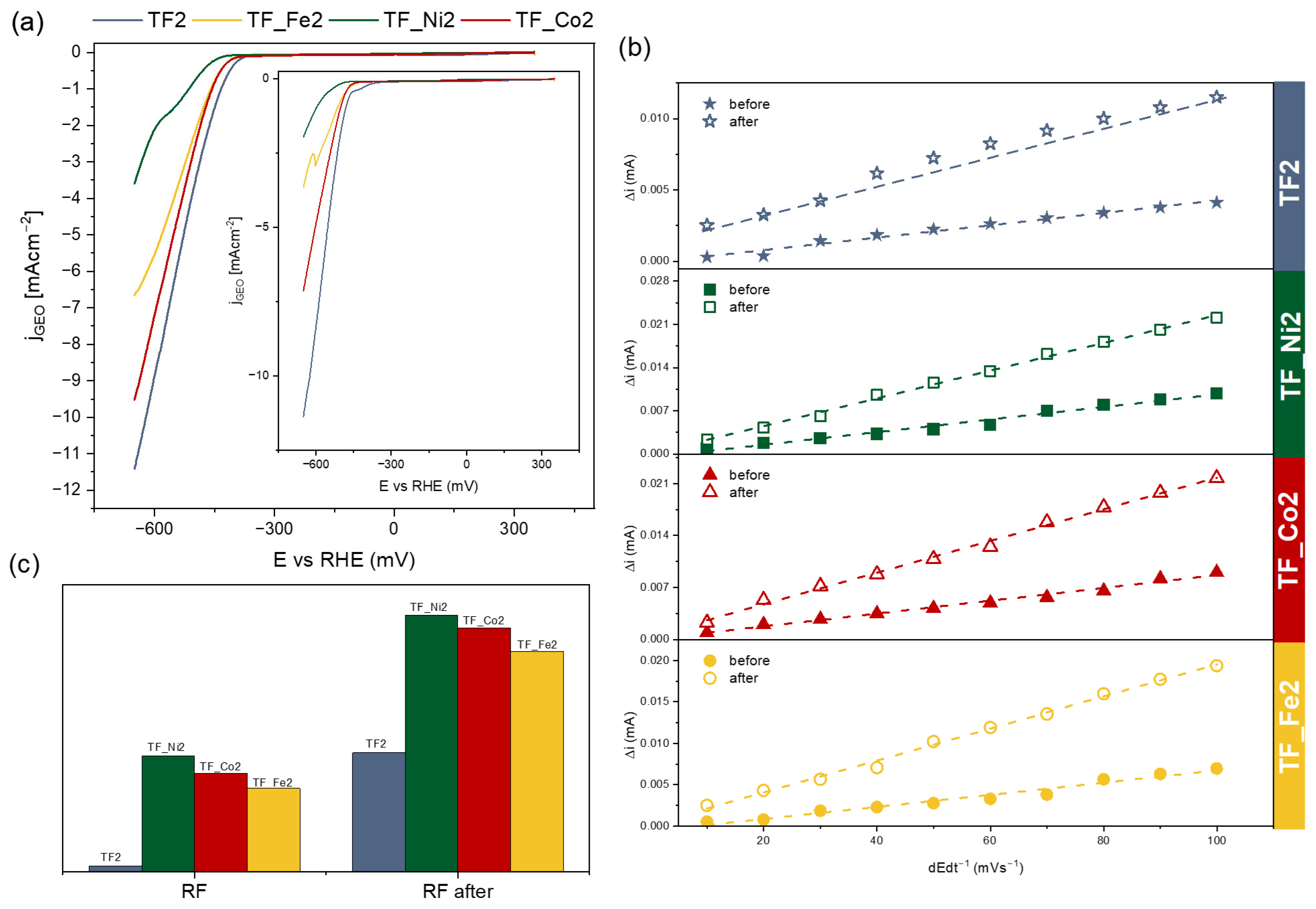
2. Results and Discussion
3. Experimental Section
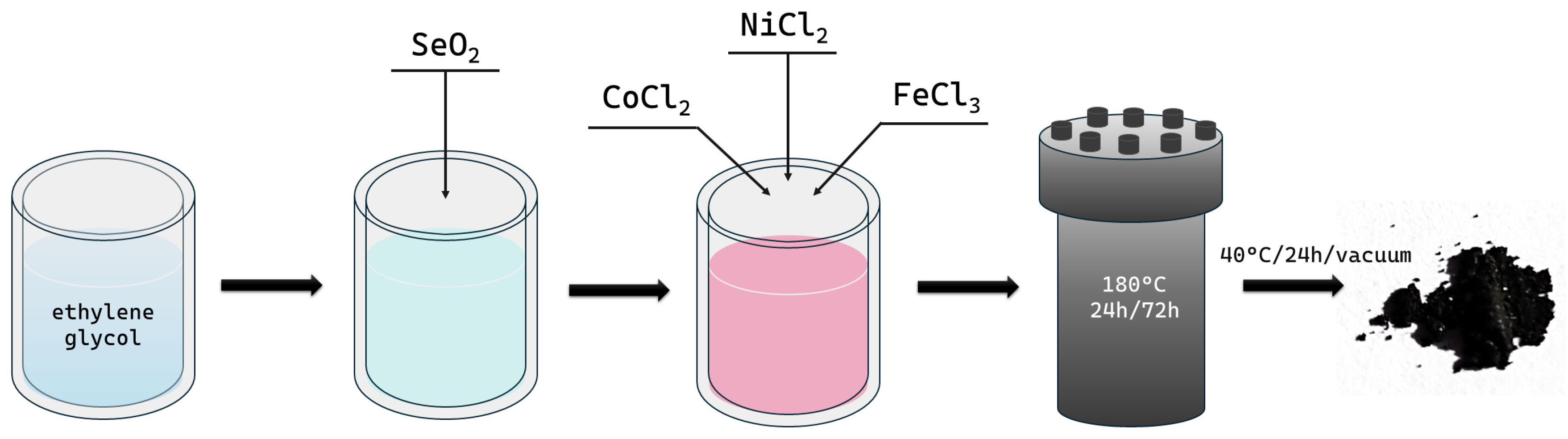
3.1. Synthesis
3.2. Materials Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.-X. Kinetics of Hydrogen Evolution Reaction on Hydrogen Storage Alloy Electrode in Alkaline Solution and Effects of Surface Modification on the Electrocatalytic Activity for Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2001, 26, 603–608. [Google Scholar] [CrossRef]
- Sarac, B.; Zadorozhnyy, V.; Berdonosova, E.; Ivanov, Y.P.; Klyamkin, S.; Gumrukcu, S.; Sarac, A.S.; Korol, A.; Semenov, D.; Zadorozhnyy, M.; et al. Hydrogen Storage Performance of the Multi-Principal-Component CoFeMnTiVZr Alloy in Electrochemical and Gas-Solid Reactions. RSC Adv. 2020, 10, 24613–24623. [Google Scholar] [CrossRef]
- Cheng, N.; Stambula, S.; Wang, D.; Banis, M.N.; Liu, J.; Riese, A.; Xiao, B.; Li, R.; Sham, T.K.; Liu, L.M.; et al. Platinum Single-Atom and Cluster Catalysis of the Hydrogen Evolution Reaction. Nat. Commun. 2016, 7, 13638. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, Z.; Liu, H.; Kang, X.; Ge, S.; Li, S.; Gan, L.; Liu, B. Why Do Platinum Catalysts Show Diverse Electrocatalytic Performance? Fundam. Res. 2023, 3, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.N.; Prats, H.; Toudahl, K.K.; Mørch Secher, N.; Chan, K.; Kibsgaard, J.; Chorkendorff, I. Is There Anything Better than Pt for HER? ACS Energy Lett. 2021, 6, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, J.; Xu, K.; Geng, R. Constructing NiSe2@MoS2nano-Heterostructures on a Carbon Fiber Paper for Electrocatalytic Oxygen Evolution. RSC Adv. 2021, 11, 26928–26936. [Google Scholar] [CrossRef] [PubMed]
- Di, F.; Wang, X.; Farid, S.; Ren, S. CoS2 with Carbon Shell for Efficient Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2023, 48, 17758–17768. [Google Scholar] [CrossRef]
- Yuan, B.; Luan, W.; Tu, S.T. One-Step Synthesis of Cubic FeS2 and Flower-like FeSe2 Particles by a Solvothermal Reduction Process. Dalton Trans. 2012, 41, 772–776. [Google Scholar] [CrossRef]
- Song, D.; Wang, H.; Wang, X.; Yu, B.; Chen, Y. NiSe2 Nanoparticles Embedded in Carbon Nanowires as Highly Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. Electrochim. Acta 2017, 254, 230–237. [Google Scholar] [CrossRef]
- Yu, B.; Jin, J.; Wu, H.; Wang, S.; Xia, Q.; Liu, H. Iron and Nickel Doped CoSe2 as Efficient Non Precious Metal Catalysts for Oxygen Reduction. Int. J. Hydrog. Energy 2017, 42, 236–242. [Google Scholar] [CrossRef]
- Li, X.; Babar, P.; Patil, K.; Kale, S.; Jo, E.; Chen, X.; Hussain, Z.; Kim, J.H.; Yoo, Y.T. Bifunctional Ni–Fe–CoSe2 Nanosheets Electrodeposited on Ni Foam for Efficient Catalysis of the Oxidation of Water and Urea. Mater. Chem. Phys. 2022, 287, 126310. [Google Scholar] [CrossRef]
- Zlotea, C.; Sow, M.A.; Ek, G.; Couzinié, J.P.; Perrière, L.; Guillot, I.; Bourgon, J.; Møller, K.T.; Jensen, T.R.; Akiba, E.; et al. Hydrogen Sorption in TiZrNbHfTa High Entropy Alloy. J. Alloys Compd. 2019, 775, 667–674. [Google Scholar] [CrossRef]
- Zhang, G.; Ming, K.; Kang, J.; Huang, Q.; Zhang, Z.; Zheng, X.; Bi, X. High Entropy Alloy as a Highly Active and Stable Electrocatalyst for Hydrogen Evolution Reaction. Electrochim. Acta 2018, 279, 19–23. [Google Scholar] [CrossRef]
- Mikuła, A.; Kubowicz, M.; Mazurków, J.; Mars, K.; Smialkowski, M.; Apfel, U.P.; Radecka, M. Tailoring the Electrocatalytic Activity of Multicomponent (Co,Fe,Ni)9S8−xSex Pentlandite Solid Electrodes. J. Mater. Chem. A Mater. 2023, 11, 7526–7538. [Google Scholar] [CrossRef]
- Mikuła, A.; Kubowicz, M.; Smialkowski, M.; Sanden, S.; Apfel, U.P. Tuning the Electrocatalytic Properties of Trimetallic Pentlandites: Stability and Catalytic Activity as a Function of Material Form and Selenium Concentration. ACS Mater. Lett. 2024, 6, 1581–1592. [Google Scholar] [CrossRef]
- Mikuła, A.; Kurek, T.; Kożusznik, M.; Nieroda, P. Cu2−xS and Cu2−xSe Alloys: Investigating the Influence of Ag, Zn, and Ni Doping on Structure and Transport Behavior. Metals 2024, 14, 360. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; He, R.; Yu, F.; Sun, J.; Wang, F.; Lan, Y.; Ren, Z.; Chen, S. One-Step Synthesis of Self-Supported Porous NiSe2/Ni Hybrid Foam: An Efficient 3D Electrode for Hydrogen Evolution Reaction. Nano Energy 2016, 20, 29–36. [Google Scholar] [CrossRef]
- Campos, C.E.M.; De Lima, J.C.; Grandi, T.A.; Machado, K.D.; Pizani, P.S. Structural Studies of Cobalt Selenides Prepared by Mechanical Alloying. Physica B 2002, 324, 409–418. [Google Scholar] [CrossRef]
- Bither, T.; Prewitt, C.; Gilison, J.; Bierstedt, P.; Flippen, R.; Young, H. New Transition Metal Dichalcogenides Formed at High Pressure. Solid. State Commun. 1966, 4, 533–535. [Google Scholar] [CrossRef]
- Bastola, E.; Bhandari, K.P.; Matthews, A.J.; Shrestha, N.; Ellingson, R.J. Elemental Anion Thermal Injection Synthesis of Nanocrystalline Marcasite Iron Dichalcogenide FeSe2 and FeTe2. RSC Adv. 2016, 6, 69708–69714. [Google Scholar] [CrossRef]
- Khan, M.D.; Malik, M.A.; Revaprasadu, N. Progress in Selenium Based Metal-Organic Precursors for Main Group and Transition Metal Selenide Thin Films and Nanomaterials. Coord. Chem. Rev. 2019, 388, 24–47. [Google Scholar] [CrossRef]
- Ndlwana, L.; Raleie, N.; Dimpe, K.M.; Ogutu, H.F.; Oseghe, E.O.; Motsa, M.M.; Msagati, T.A.M.; Mamba, B.B. Sustainable Hydrothermal and Solvothermal Synthesis of Advanced Carbon Materials in Multidimensional Applications: A Review. Materials 2021, 14, 5094. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Singh, M.; Moumen, A.; Duina, G.; Comini, E. 1D Titanium Dioxide: Achievements in Chemical Sensing. Materials 2020, 13, 2974. [Google Scholar] [CrossRef] [PubMed]
- Hühn, J.; Carrillo-Carrion, C.; Soliman, M.G.; Pfeiffer, C.; Valdeperez, D.; Masood, A.; Chakraborty, I.; Zhu, L.; Gallego, M.; Yue, Z.; et al. Selected Standard Protocols for the Synthesis, Phase Transfer, and Characterization of Inorganic Colloidal Nanoparticles. Chem. Mater. 2017, 29, 399–461. [Google Scholar] [CrossRef]
- Aslan, E.; Sarilmaz, A.; Yanalak, G.; Ozel, S.S.; Ozel, F.; Patir, I.H. Transition Metal–Incorporated Tungsten-Based Ternary Refractory Metal Selenides (MWSex; M = Fe, Co, Ni, and Mn) as Hydrogen Evolution Catalysts at Soft Interfaces. Mater. Today Energy 2020, 18, 100510. [Google Scholar] [CrossRef]
- Cao, X.; Hong, Y.; Zhang, N.; Chen, Q.; Masud, J.; Zaeem, M.A.; Nath, M. Phase Exploration and Identification of Multinary Transition-Metal Selenides as High-Efficiency Oxygen Evolution Electrocatalysts through Combinatorial Electrodeposition. ACS Catal. 2018, 8, 8273–8289. [Google Scholar] [CrossRef]
- Grigoriev, M.V.; Solovyov, L.A.; Ruseikina, A.V.; Aleksandrovsky, A.S.; Chernyshev, V.A.; Velikanov, D.A.; Garmonov, A.A.; Molokeev, M.S.; Oreshonkov, A.S.; Shestakov, N.P.; et al. Quaternary Selenides EuLnCuSe3: Synthesis, Structures, Properties and In Silico Studies. Int. J. Mol. Sci. 2022, 23, 1503. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Shen, J.-N.; Shi, Y.-F.; Li, L.-H.; Chen, L. Quaternary Rare-Earth Selenides with Closed Cavities: Cs[RE9Mn4Se18] (RE = Ho–Lu). Inorg. Chem. Front. 2015, 2, 298–305. [Google Scholar] [CrossRef]
- Oledzka, M.; Ramanujachary, K.V.; Greenblatt, M. Synthesis and Characterization of New Quaternary Selenides with ThCr2Si2-Type Structure: ACuMnSe2 (A = K, Rb, Cs). Mater. Res. Bull. 1998, 33, 855–866. [Google Scholar] [CrossRef]
- Yue, H.; Yang, D.; Yu, B.; Lu, Y.; Zhang, W.; Chen, Y. Porous Interwoven CoSe2/C Microsphere: A Highly Efficient and Stable Nonprecious Electrocatalyst for Hydrogen Evolution Reaction. J. Mater. Sci. 2019, 54, 14123–14133. [Google Scholar] [CrossRef]
- Mikuła, A.; Kożusznik, M.; Mars, K.; Cieślak, J.; Sanden, S.; Apfel, U.-P. Cobalt-Rich Multimetallic Selenides-Exploring Relationships between Chemical Composition, Temperature Treatment, and Electrocatalytic Performance of Solid Electrodes. Chem. Mater. 2024, 36, 4571–4582. [Google Scholar] [CrossRef]
- Zegkinoglou, I.; Zendegani, A.; Sinev, I.; Kunze, S.; Mistry, H.; Jeon, H.S.; Zhao, J.; Hu, M.Y.; Alp, E.E.; Piontek, S.; et al. Operando Phonon Studies of the Protonation Mechanism in Highly Active Hydrogen Evolution Reaction Pentlandite Catalysts. J. Am. Chem. Soc. 2017, 139, 14360–14363. [Google Scholar] [CrossRef]
- Smialkowski, M.; Siegmund, D.; Stier, K.; Hensgen, L.; Checinski, M.P.; Apfel, U.-P. Trimetallic Pentlandites (Fe,Co,Ni)9S8 for the Electrocatalytical HER in Acidic Media. ACS Mater. Au 2022, 2, 474–481. [Google Scholar] [CrossRef]


| System | Material | Synthesis | Application | References |
|---|---|---|---|---|
| binary | WSex | hot-injection | hydrogen evolution catalysts | [25] |
| Ni3Se2 FeSe | electrodeposition | oxygen evolution reaction | [26] | |
| Cu2-xSe | solid-state reaction | thermoelectric materials | [16] | |
| FeSe2 | Schlenk line techniques | optoelectronic devices | [20] | |
| NiSe2 | thermal selenization | hydrogen evolution reaction | [17] | |
| ternary | WSex, CoWSex FeWSex MnWSex | hot-injection | hydrogen evolution catalysts | [25] |
| (Ni0.85Fe0.15)3Se4 (Ni0.24Co0.76)Se (Co0.1Fe0.9)3Se4 | electrodeposition | oxygen evolution reaction | [26] | |
| quaternary | Co3Fe3Ni3Se8 | solid-state reaction | hydrogen evolution reaction | [14] |
| EuLnCuSe2 | thermolysis | - | [27] | |
| Cs[RE9Mn4Se18] (RE = Ho–Lu) | solid-state reaction | - | [28] | |
| ACuMnSe2 (A = K, Rb, Cs) | thermal salt melting | - | [29] |
| Sample | EASA (mm2) | From HER Analysis | |||
|---|---|---|---|---|---|
| η2 | η10 | RF | RF after | ||
| F_Ni2 | 6.6 | −593 | - | 7.43·10−4 | 1.37·10−3 |
| F_Co2 | 2.5 | −440 | −601 | 4.42·10−5 | 2.93·10−4 |
| F_Fe2 | 4.9 | −572 | - | 5.03·10−4 | 1.20·10−3 |
| TF2 | 4.4 | −461 | −620 | 4.42·10−5 | 8.32·10−4 |
| TF_Ni2 | 3.1 | −593 | - | 8.12·10−4 | 1.79·10−3 |
| TF_Co2 | 3.6 | −484 | - | 6.87·10−4 | 1.70·10−3 |
| TF_Fe2 | 5.1 | −493 | - | 5.83·10−4 | 1.54·10−3 |
| Sample | Initial Chemical Composition | Reaction Time | ||||
|---|---|---|---|---|---|---|
| Se | Ni | Fe | Co | EG | ||
| mmol | mL | h | ||||
| F_Ni | 1 | 2 | - | - | 160 | 24 |
| F_Fe | - | 2 | - | |||
| F_Co | - | - | 2 | |||
| F_Ni2 | 1 | 2 | - | - | 160 | 72 |
| F_Fe2 | - | 2 | - | |||
| F_Co2 | - | - | 2 | |||
| TF | 3 | 1 | 1 | 1 | 160 | 24 |
| TF_Ni | 2 | 1 | 1 | |||
| TF_Fe | 1 | 2 | 1 | |||
| TF_Co | 1 | 1 | 2 | |||
| TF2 | 3 | 1 | 1 | 1 | 160 | 72 |
| TF_Ni2 | 2 | 1 | 1 | |||
| TF_Fe2 | 1 | 2 | 1 | |||
| TF_Co2 | 1 | 1 | 2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusior, A.; Wieczorek, F.; Dechnik, J.; Mikuła, A. Exploring Selenide Synthesis Pathways for Optimizing Energy Conversion. Molecules 2024, 29, 3310. https://doi.org/10.3390/molecules29143310
Kusior A, Wieczorek F, Dechnik J, Mikuła A. Exploring Selenide Synthesis Pathways for Optimizing Energy Conversion. Molecules. 2024; 29(14):3310. https://doi.org/10.3390/molecules29143310
Chicago/Turabian StyleKusior, Anna, Fabian Wieczorek, Jakub Dechnik, and Andrzej Mikuła. 2024. "Exploring Selenide Synthesis Pathways for Optimizing Energy Conversion" Molecules 29, no. 14: 3310. https://doi.org/10.3390/molecules29143310
APA StyleKusior, A., Wieczorek, F., Dechnik, J., & Mikuła, A. (2024). Exploring Selenide Synthesis Pathways for Optimizing Energy Conversion. Molecules, 29(14), 3310. https://doi.org/10.3390/molecules29143310






