First-Principles Investigation on the Tunable Electronic Structures and Photocatalytic Properties of AlN/Sc2CF2 and GaN/Sc2CF2 Heterostructures
Abstract
1. Introduction
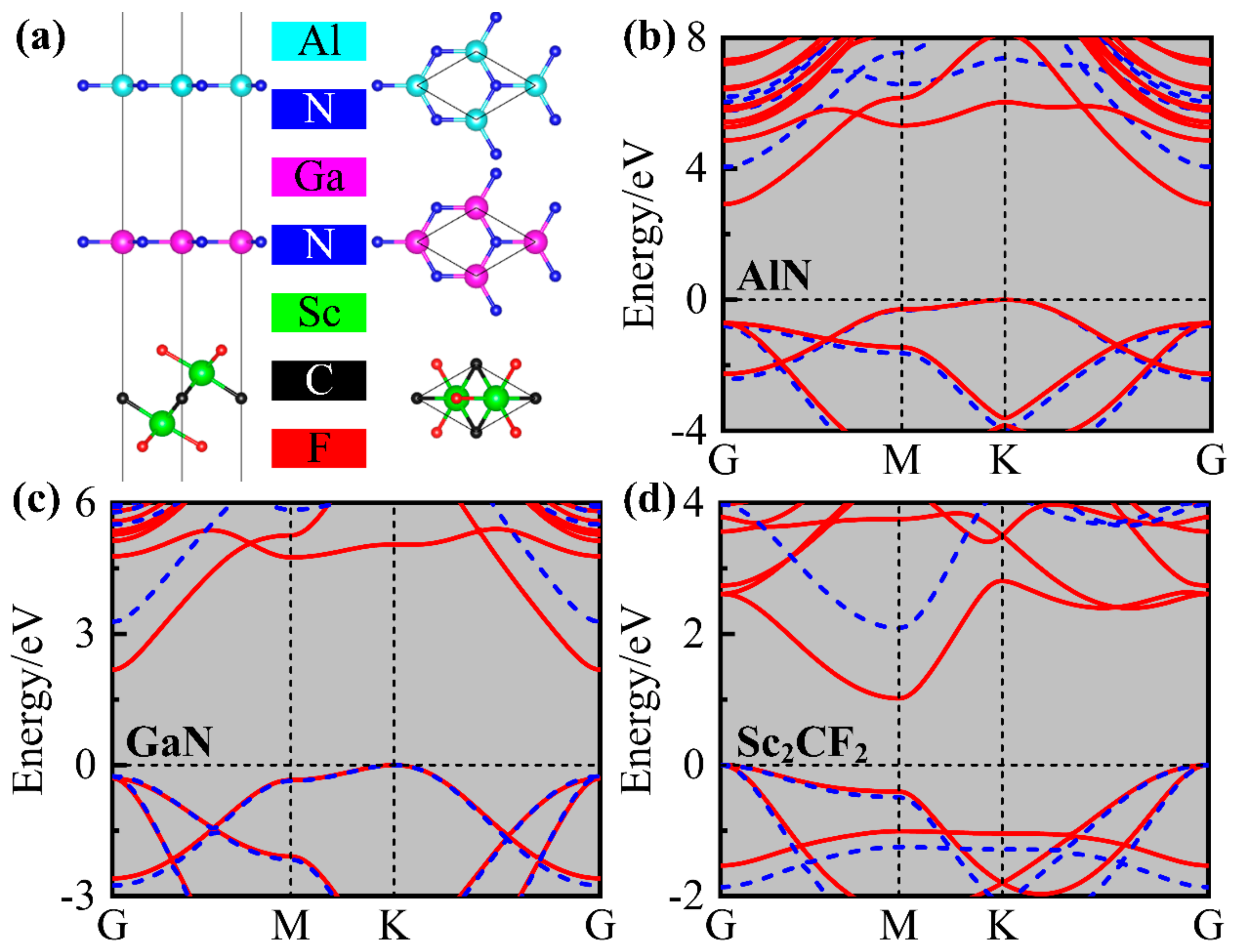
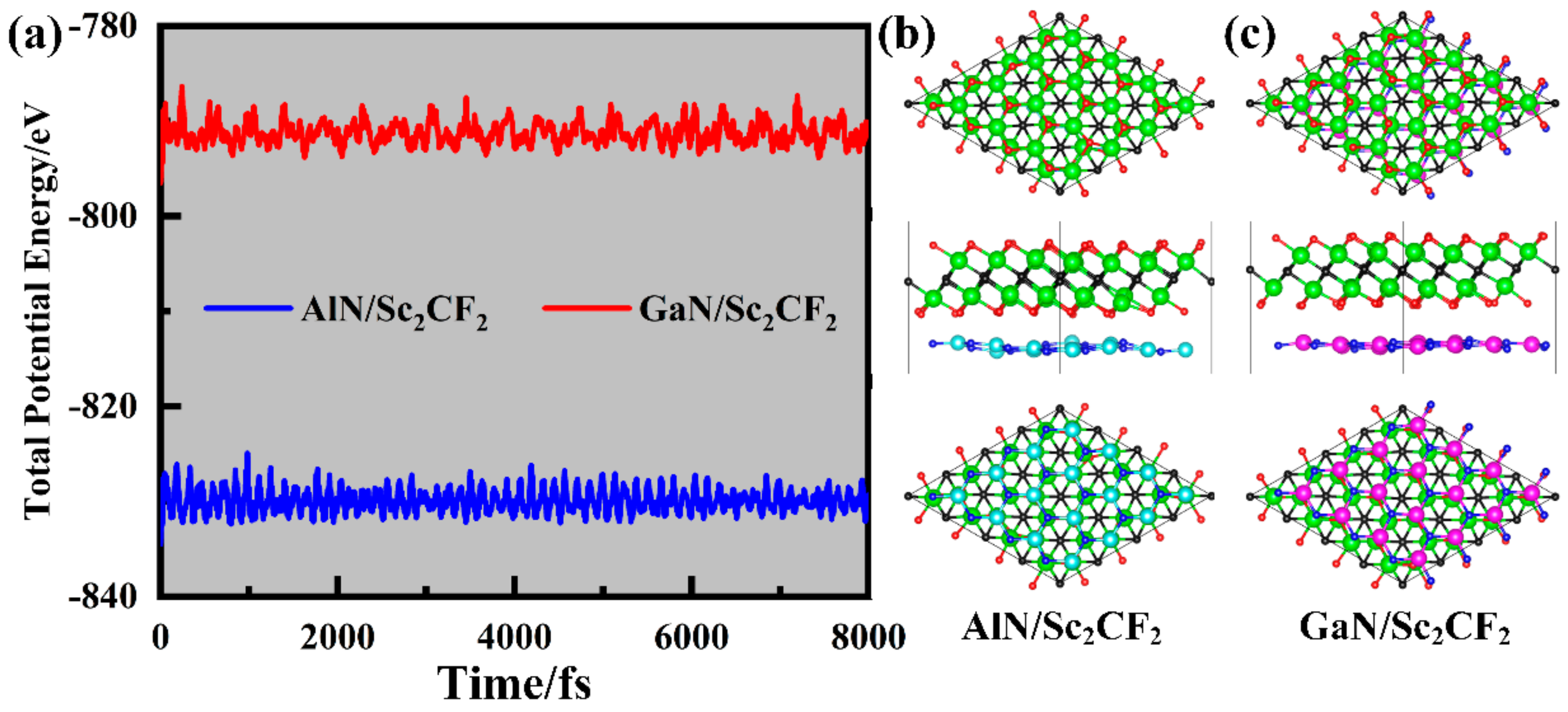
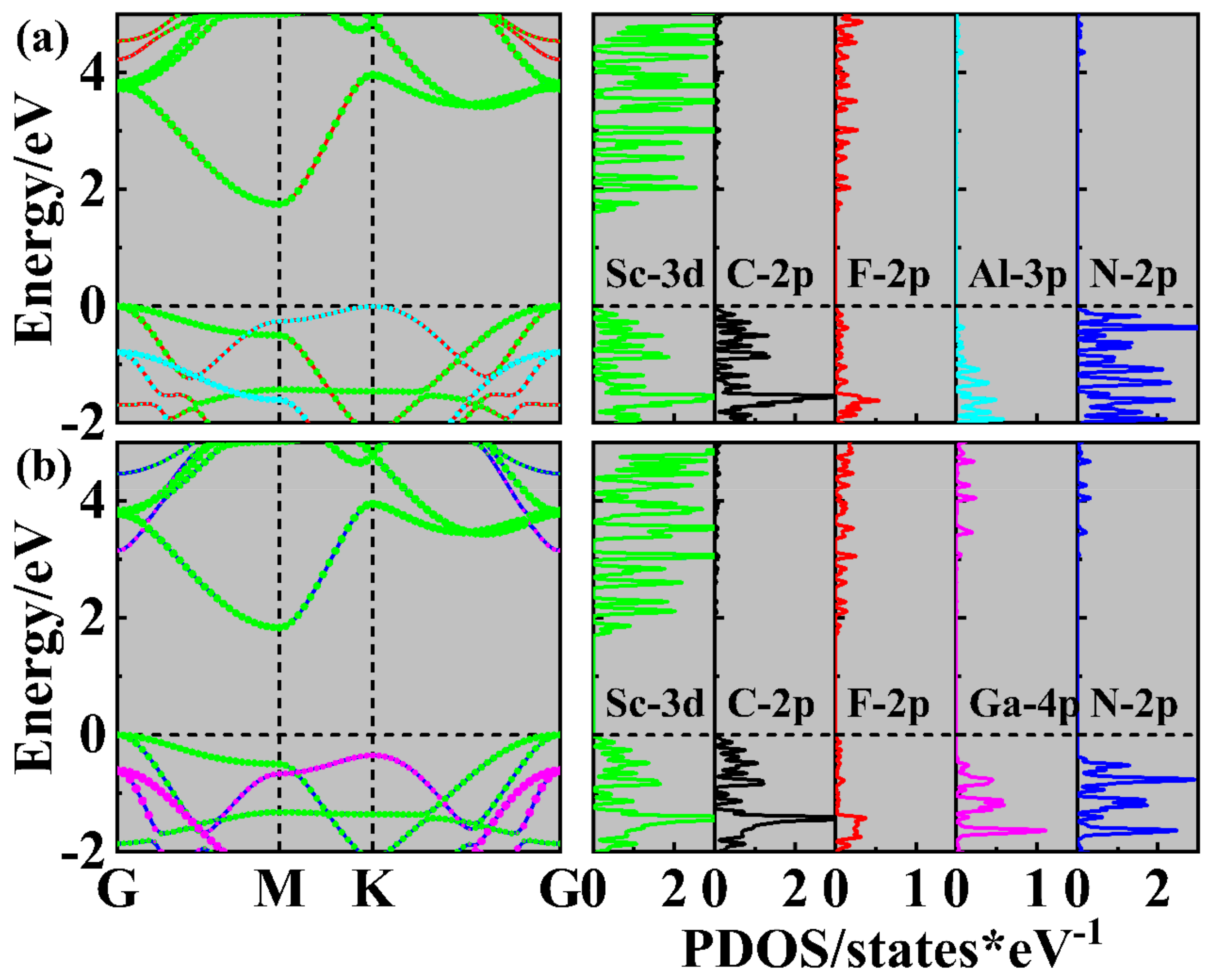
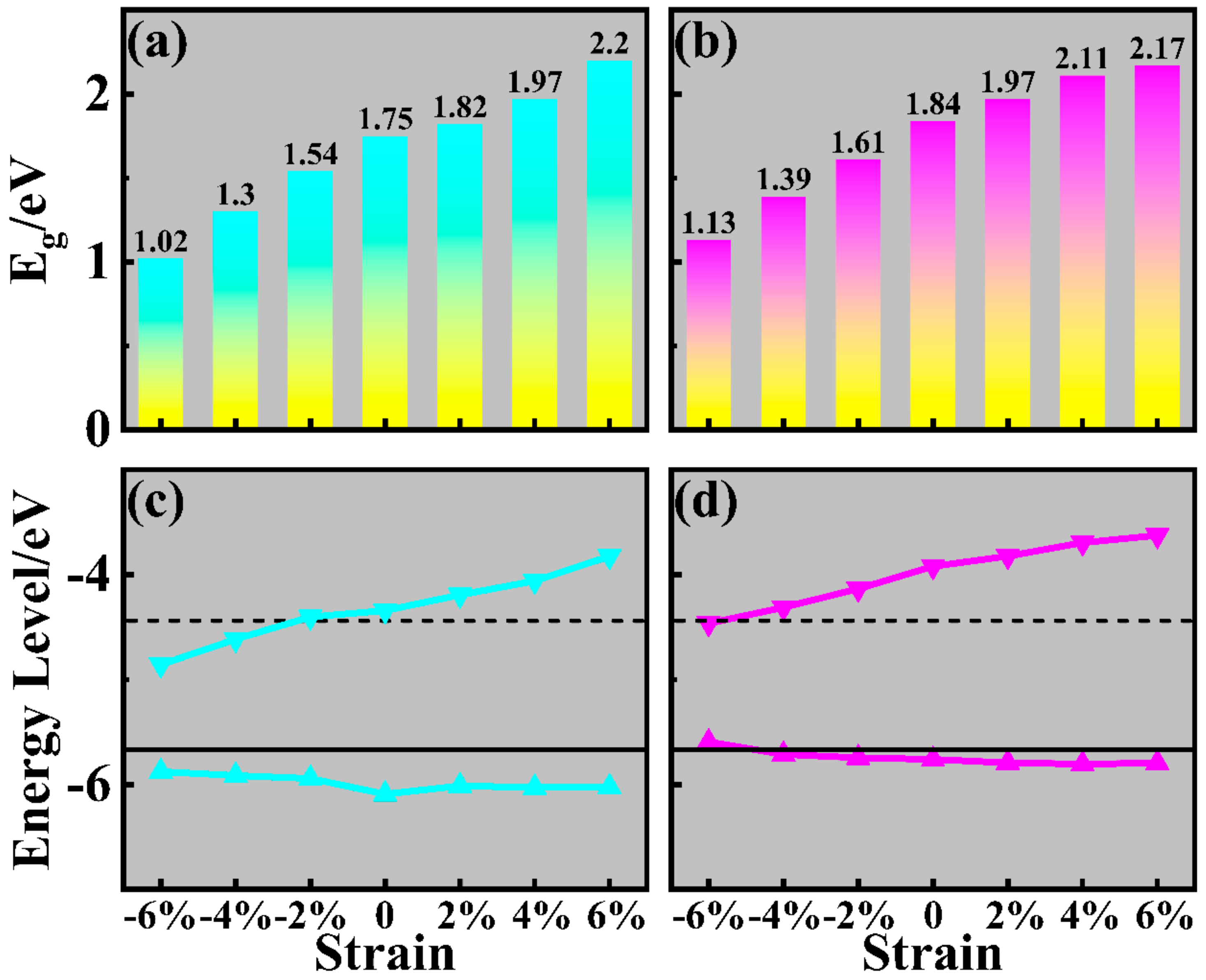
2. Results and Discussions
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Osterloh, F.; Wang, X.; Mallouk, T.; Maeda, K. Photocatalytic Water Splitting. Nat. Rev. Methods Primers 2023, 3, 42. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.; Miao, R.; Song, W.; Suib, S. Photocatalytic Water Splitting-The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Sa, B.; Ahuja, R. Review of Two-Dimensional Materials for Photocatalytic Water Splitting from a Theoretical Perspective. Catal. Sci. Technol. 2017, 7, 545–559. [Google Scholar] [CrossRef]
- Tao, X.; Zhao, Y.; Wang, S.; Li, C.; Li, R. Recent Advances and Perspectives for Solar-Driven Water Splitting Using Particulate Photocatalysts. Chem. Soc. Rev. 2022, 51, 3561–3608. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, X.; Rong, C.; Zhong, A.; Liu, S.; Zhao, D. On the Origin and Nature of Internal Methyl Rotation Barriers: An Information-Theoretic Approach Study. Theor. Chem. Acc. 2022, 141, 68. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, S.; Rong, C.; Zhong, A.; Liu, S. Toward Understanding the Isomeric Stability of Fullerenes with Density Functional Theory and the Information-Theoretic Approach. ACS Omega 2018, 3, 17986–17990. [Google Scholar] [CrossRef]
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a Photocatalyst for Water Splitting—An Experimental and Theoretical Review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef] [PubMed]
- Acar, C.; Dincer, I.; Naterer, G. Review of Photocatalytic Water-Splitting Methods for Sustainable Hydrogen Production: Review: Photocatalysis for Sustainable Hydrogen. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Tang, J.; Durrant, J.; Klug, D. Mechanism of Photocatalytic Water Splitting in TiO2. Reaction of Water with Photoholes, Importance of Charge Carrier Dynamics, and Evidence for Four-Hole Chemistry. J. Am. Chem. Soc. 2008, 130, 13885–13891. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, B.; Lin, S. P-Type ZnO for Photocatalytic Water Splitting. APL Mater. 2022, 10, 030901. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J. Elaborately Modified BiVO4 Photoanodes for Solar Water Splitting. Adv. Mater. 2019, 31, 1806938. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Domen, K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent Advances in Semiconductors for Photocatalytic and Photoelectrochemical Water Splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Wu, X.; Yang, J. Material Design for Photocatalytic Water Splitting from a Theoretical Perspective. Adv. Mater. 2018, 30, 1802106. [Google Scholar] [CrossRef] [PubMed]
- Moniz, S.; Shevlin, S.; Martin, D.; Guo, Z.; Tang, J. Visible-Light Driven Heterojunction Photocatalysts for Water Splitting-a Critical Review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of Interfaces in Two-Dimensional Photocatalyst for Water Splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Zha, X.; Zhou, J.; Zhou, Y.; Huang, Q.; He, J.; Francisco, J.; Luo, K.; Du, S. Promising Electron Mobility and High Thermal Conductivity in Sc2CT2 (T = F, OH) MXenes. Nanoscale 2016, 8, 6110–6117. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, M.; Zhong, X.; Qiu, K.; Bai, L.; Ma, B.; Wang, J.; Chen, Y. Theoretical Design of Sc2CF2/Ti2CO2 Heterostructure as a Promising Direct Z-Scheme Photocatalyst towards Efficient Water Splitting. Results Phys. 2024, 60, 107706. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, Y.; Li, X.; Li, Y.; Zhao, H.; Huang, L.; Yang, Z.; Zhang, X.; Li, G. 2D AlN Layers Sandwiched Between Graphene and Si Substrates. Adv. Mater. 2019, 31, 1803448. [Google Scholar] [CrossRef]
- Yu, R.; Liu, G.; Wang, G.; Chen, C.; Xu, M.; Zhou, H.; Wang, T.; Yu, J.; Zhao, G.; Zhang, L. Ultrawide-Bandgap Semiconductor AlN Crystals: Growth and Applications. J. Mater. Chem. C 2021, 9, 1852–1873. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K.; Liu, J.; Lv, T.; Wei, B.; Zhang, T.; Zeng, M.; Wang, Z.; Fu, L. Growth of 2D GaN Single Crystals on Liquid Metals. J. Am. Chem. Soc. 2018, 140, 16392–16395. [Google Scholar] [CrossRef] [PubMed]
- Al Balushi, Z.; Wang, K.; Ghosh, R.; Vilá, R.; Eichfeld, S.; Caldwell, J.; Qin, X.; Lin, Y.; DeSario, P.; Stone, G.; et al. Two-Dimensional Gallium Nitride Realized via Graphene Encapsulation. Nat. Mater 2016, 15, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Singh, A.; Hennig, R. Computational Discovery of Single-Layer III-V Materials. Phys. Rev. B 2013, 87, 165415. [Google Scholar] [CrossRef]
- Sanders, N.; Bayerl, D.; Shi, G.; Mengle, K.; Kioupakis, E. Electronic and Optical Properties of Two-Dimensional GaN from First-Principles. Nano Lett. 2017, 17, 7345–7349. [Google Scholar] [CrossRef]
- Bacaksiz, C.; Sahin, H.; Ozaydin, H.; Horzum, S.; Senger, R.; Peeters, F. Hexagonal AlN: Dimensional-Crossover-Driven Band-Gap Transition. Phys. Rev. B 2015, 91, 085430. [Google Scholar] [CrossRef]
- Bai, Y.; Deng, K.; Kan, E. Electronic and Magnetic Properties of an AlN Monolayer Doped with First-Row Elements: A First-Principles Study. RSC Adv. 2015, 5, 18352–18358. [Google Scholar] [CrossRef]
- Zhang, C. First-Principles Study on Electronic Structures and Magnetic Properties of AlN Nanosheets and Nanoribbons. J. Appl. Phys. 2012, 111, 043702. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, F. First-principles Prediction on Electronic and Magnetic Properties of Hydrogenated AlN Nanosheets. J. Comput. Chem. 2011, 32, 3122–3128. [Google Scholar] [CrossRef]
- Xu, C.; Xue, L.; Yin, C.; Wang, G. Formation and Photoluminescence Properties of AlN Nanowires. Phys. Stat. Sol. A 2003, 198, 329–335. [Google Scholar] [CrossRef]
- Xu, D.; He, H.; Pandey, R.; Karna, S. Stacking and Electric Field Effects in Atomically Thin Layers of GaN. J. Phys. Condens. Matter. 2013, 25, 345302. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hu, H.; Chen, X.; Wang, J. Tailoring Band Gap in GaN Sheet by Chemical Modification and Electric Field: Ab Initio Calculations. Appl. Phys. Lett. 2011, 98, 053102. [Google Scholar] [CrossRef]
- Taniyasu, Y.; Kasu, M.; Makimoto, T. An Aluminium Nitride Light-Emitting Diode with a Wavelength of 210 Nanometres. Nature 2006, 441, 325–328. [Google Scholar] [CrossRef] [PubMed]
- McDermott, E.; Kurmaev, E.; Boyko, T.; Finkelstein, L.; Green, R.; Maeda, K.; Domen, K.; Moewes, A. Structural and Band Gap Investigation of GaN:ZnO Heterojunction Solid Solution Photocatalyst Probed by Soft X-Ray Spectroscopy. J. Phys. Chem. C 2012, 116, 7694–7700. [Google Scholar] [CrossRef]
- Cui, Z.; Li, E.; Ke, X.; Zhao, T.; Yang, Y.; Ding, Y.; Liu, T.; Qu, Y.; Xu, S. Adsorption of Alkali-Metal Atoms on GaN Nanowires Photocathode. Appl. Surf. Sci. 2017, 423, 829–835. [Google Scholar] [CrossRef]
- Ren, K.; Wang, S.; Luo, Y.; Chou, J.; Yu, J.; Tang, W.; Sun, M. High-Efficiency Photocatalyst for Water Splitting: A Janus MoSSe/XN (X = Ga, Al) van Der Waals Heterostructure. J. Phys. D Appl. Phys. 2020, 53, 185504. [Google Scholar] [CrossRef]
- Ren, K.; Zheng, R.; Xu, P.; Cheng, D.; Huo, W.; Yu, J.; Zhang, Z.; Sun, Q. Electronic and Optical Properties of Atomic-Scale Heterostructure Based on MXene and MN (M = Al, Ga): A DFT Investigation. Nanomaterials 2021, 11, 2236. [Google Scholar] [CrossRef]
- Munawar, M.; Idrees, M.; Alrebdi, T.; Amin, B. Revealing the Electronic, Optical and Photocatalytic Properties of PN-M2CO2 (P = Al, Ga; M = Ti, Zr, Hf) Heterostructures. Nanoscale Adv. 2023, 5, 1405–1415. [Google Scholar] [CrossRef]
- Zhang, M.; Si, R.; Wu, X.; Dong, Y.; Fu, K.; Xu, X.; Zhang, J.; Li, L.; Guo, Y. Two-Dimensional Hf2CO2/GaN van Der Waals Heterostructure for Overall Water Splitting: A Density Functional Theory Study. J. Mater. Sci. Mater. Electron. 2021, 32, 19368–19379. [Google Scholar] [CrossRef]
- Bacaksiz, C.; Dominguez, A.; Rubio, A.; Senger, R.; Sahin, H. H-AlN-Mg(OH)2 van Der Waals Bilayer Heterostructure: Tuning the Excitonic Characteristics. Phys. Rev. B 2017, 95, 075423. [Google Scholar] [CrossRef]
- Li, L.; Martirez, J.; Carter, E. Prediction of Highly Selective Electrocatalytic Nitrogen Reduction at Low Overpotential on a Mo-Doped g-GaN Monolayer. ACS Catal. 2020, 10, 12841–12857. [Google Scholar] [CrossRef]
- Kadioglu, Y.; Ersan, F.; Kecik, D.; Aktürk, O.; Aktürk, E.; Ciraci, S. Chemical and Substitutional Doping, and Anti-Site and Vacancy Formation in Monolayer AlN and GaN. Phys. Chem. Chem. Phys. 2018, 20, 16077–16091. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zhu, B.; Zhang, F.; Chen, X.; Guo, H.; Qiu, J.; Liu, X.; Yu, J. Sc2CF2/Janus MoSSe Heterostructure: A Potential Z-Scheme Photocatalyst with Ultra-High Solar-to-Hydrogen Efficiency. Int. J. Hydrogen Energ. 2021, 46, 39830–39843. [Google Scholar] [CrossRef]
- Björkman, T.; Gulans, A.; Krasheninnikov, A.; Nieminen, R. Van Der Waals Bonding in Layered Compounds from Advanced Density-Functional First-Principles Calculations. Phys. Rev. Lett. 2012, 108, 235502. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Battaglia, C.; Carraro, C.; Nemsak, S.; Ozdol, B.; Kang, J.; Bechtel, H.; Desai, S.; Kronast, F.; Unal, A.; et al. Strong Interlayer Coupling in van Der Waals Heterostructures Built from Single-Layer Chalcogenides. Proc. Natl. Acad. Sci. USA 2014, 111, 6198–6202. [Google Scholar] [CrossRef]
- Guan, Y.; Li, X.; Hu, Q.; Zhao, D.; Zhang, L. Theoretical Design of BAs/WX2 (X = S, Se) Heterostructures for High-Performance Photovoltaic Applications from DFT Calculations. Appl. Surf. Sci. 2022, 599, 153865. [Google Scholar] [CrossRef]
- Nasir, S.; Ullah, H.; Ebadi, M.; Tahir, A.; Sagu, J.; Mat Teridi, M. New Insights into Se/BiVO4 Heterostructure for Photoelectrochemical Water Splitting: A Combined Experimental and DFT Study. J. Phys. Chem. C 2017, 121, 6218–6228. [Google Scholar] [CrossRef]
- Liu, B.; Long, M.; Cai, M.; Ding, L.; Yang, J. Interfacial Charge Behavior Modulation in 2D/3D Perovskite Heterostructure for Potential High-Performance Solar Cells. Nano Energy 2019, 59, 715–720. [Google Scholar] [CrossRef]
- Sanville, E.; Kenny, S.; Smith, R.; Henkelman, G. Improved Grid-based Algorithm for Bader Charge Allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef]
- Ni, J.; Quintana, M.; Jia, F.; Song, S. Using van Der Waals Heterostructures Based on Two-Dimensional InSe-XS2 (X = Mo, W) as Promising Photocatalysts for Hydrogen Production. J. Mater. Chem. C 2020, 8, 12509–12515. [Google Scholar] [CrossRef]
- Abid, A.; Haneef, M.; Ali, S.; Dahshan, A. Optoelectronic and Photocatalytic Properties of GaN, GeS and SiS Monolayers and Their vdW Heterostructures. J. Phys. Chem. Solids 2022, 161, 110433. [Google Scholar] [CrossRef]
- Idrees, M.; Amin, B.; Chen, Y.; Yan, X. Computation Insights of MoS2-CrXY (X≠Y S, Se, Te) van Der Waals Heterostructure for Spintronic and Photocatalytic Water Splitting Applications. Int. J. Hydrogen Energ. 2024, 51, 1217–1228. [Google Scholar] [CrossRef]
- Wang, S.; Ren, C.; Tian, H.; Yu, J.; Sun, M. MoS2/ZnO van Der Waals Heterostructure as a High-Efficiency Water Splitting Photocatalyst: A First-Principles Study. Phys. Chem. Chem. Phys. 2018, 20, 13394–13399. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Xu, H.; Cong, C.; Li, S.; Feng, S.; Zhang, H.; Zou, C.; Shang, J.; Yang, S.; et al. Visualizing the Anomalous Charge Density Wave States in Graphene/NbSe2 Heterostructures. Adv. Mater. 2020, 32, 2003746. [Google Scholar] [CrossRef] [PubMed]
- Toroker, M.; Kanan, D.; Alidoust, N.; Isseroff, L.; Liao, P.; Carter, E. First Principles Scheme to Evaluate Band Edge Positions in Potential Transition Metal Oxide Photocatalysts and Photoelectrodes. Phys. Chem. Chem. Phys. 2011, 13, 16644. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Huang, M.; Zhang, Z.; Wang, J.; Zhao, D.; Guo, X.; Liu, X. First-Principles Study on the Electronic Structure and Catalytic Properties of Two-Dimensional MX2N4 Systems (M = Ti, Zr; X = Si, Ge). Results Phys. 2023, 52, 106820. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, T.; Zhang, M.; Tong, Y.; Zhong, C.; Zhang, N.; Zhang, L.; Wu, C.; Xie, Y. 3D Nitrogen-Anion-Decorated Nickel Sulfides for Highly Efficient Overall Water Splitting. Adv. Mater. 2017, 29, 1701584. [Google Scholar] [CrossRef]
- Lalwani, S.; AlNahyan, M.; Al Zaabi, A.; AlMarzooqi, F.; Qurashi, A. Advances in Interfacial Engineering and Their Role in Heterostructure Formation for HER Applications in Wider pH. ACS Appl. Energy Mater. 2022, 5, 14571–14592. [Google Scholar] [CrossRef]
- Xu, L.; Tao, J.; Xiao, B.; Xiong, F.; Ma, Z.; Zeng, J.; Huang, X.; Tang, S.; Wang, L. Two-Dimensional AlN/g-CNs van Der Waals Type-II Heterojunction for Water Splitting. Phys. Chem. Chem. Phys. 2023, 25, 3969–3978. [Google Scholar] [CrossRef]
- Park, H.; Lee, E.; Lei, M.; Joo, H.; Coh, S.; Fokwa, B. Canonic-Like HER Activity of Cr1-xMoxB2 Solid Solution: Overpowering Pt/C at High Current Density. Adv. Mater. 2020, 32, 2000855. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, X.; Cong, B.; Hong, W.; Chen, G. Tailoring the D-Band Centers Endows (NixFe1-x)2P Nanosheets with Efficient Oxygen Evolution Catalysis. ACS Catal. 2020, 10, 9086–9097. [Google Scholar] [CrossRef]
- He, Y.; Yang, Y.; Zhang, Z.; Gong, Y.; Zhou, W.; Hu, Z.; Ye, G.; Zhang, X.; Bianco, E.; Lei, S.; et al. Strain-Induced Electronic Structure Changes in Stacked van Der Waals Heterostructures. Nano Lett. 2016, 16, 3314–3320. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, L.; Zhang, Z. Strain Engineering of 2D Materials: Issues and Opportunities at the Interface. Adv. Mater. 2019, 31, 1805417. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Aneesh, J.; Yadav, R.; Sanda, S.; Barik, A.; Mishra, A.; Maji, T.; Karmakar, D.; Adarsh, K. Strong Interlayer Coupling Mediated Giant Two-Photon Absorption in MoSe2/Graphene Oxide Heterostructure: Quenching of Exciton Bands. Phys. Rev. B 2016, 93, 155433. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Y.; Yu, G.; Shen, D.; Wang, Y.; Kan, E. Visible-Light-Absorption in Graphitic C3N4 Bilayer: Enhanced by Interlayer Coupling. J. Phys. Chem. Lett. 2012, 3, 3330–3334. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Yu, S.; Yang, K.; Shao, Y.; Zou, L.; Zhao, B.; Wang, Z.; Ling, Y.; Chen, Y. Ni Doping in Unit Cell of BiOBr to Increase Dipole Moment and Induce Spin Polarization for Promoting CO2 Photoreduction via Enhanced Build-in Electric Field. Appl. Catal. B Environ. 2023, 327, 122420. [Google Scholar] [CrossRef]
- Mao, J.; Yu, Y.; Wang, L.; Zhang, X.; Wang, Y.; Shao, Z.; Jie, J. Ultrafast, Broadband Photodetector Based on MoSe2/Silicon Heterojunction with Vertically Standing Layered Structure Using Graphene as Transparent Electrode. Adv. Sci. 2016, 3, 1600018. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.; Ernzerhof, M. Hybrid Functionals Based on a Screened Coulomb Potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.; Pack, J. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Nosé, S. A Unified Formulation of the Constant Temperature Molecular Dynamics Methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.; Tang, G.; Geng, W. VASPKIT: A User-Friendly Interface Facilitating High-Throughput Computing and Analysis Using VASP Code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Baroni, S.; De Gironcoli, S.; Dal Corso, A.; Giannozzi, P. Phonons and Related Crystal Properties from Density-Functional Perturbation Theory. Rev. Mod. Phys. 2001, 73, 515–562. [Google Scholar] [CrossRef]
- Gonze, X.; Lee, C. Dynamical Matrices, Born Effective Charges, Dielectric Permittivity Tensors, and Interatomic Force Constants from Density-Functional Perturbation Theory. Phys. Rev. B 1997, 55, 10355–10368. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First Principles Phonon Calculations in Materials Science. Scripta Materialia 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Ling, C.; Shi, L.; Ouyang, Y.; Zeng, X.C.; Wang, J. Nanosheet Supported Single-Metal Atom Bifunctional Catalyst for Overall Water Splitting. Nano Lett. 2017, 17, 5133–5139. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fu, C.; Hu, W.; Yang, J. Designing Direct Z-Scheme Heterojunctions Enabled by Edge-Modified Phosphorene Nanoribbons for Photocatalytic Overall Water Splitting. J. Phys. Chem. Lett. 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Zhou, S.; Bai, Y.; Zhao, J. N-Doped Graphitic Carbon Materials Hybridized with Transition Metals (Compounds) for Hydrogen Evolution Reaction: Understanding the Synergistic Effect from Atomistic Level. Carbon 2018, 133, 260–266. [Google Scholar] [CrossRef]



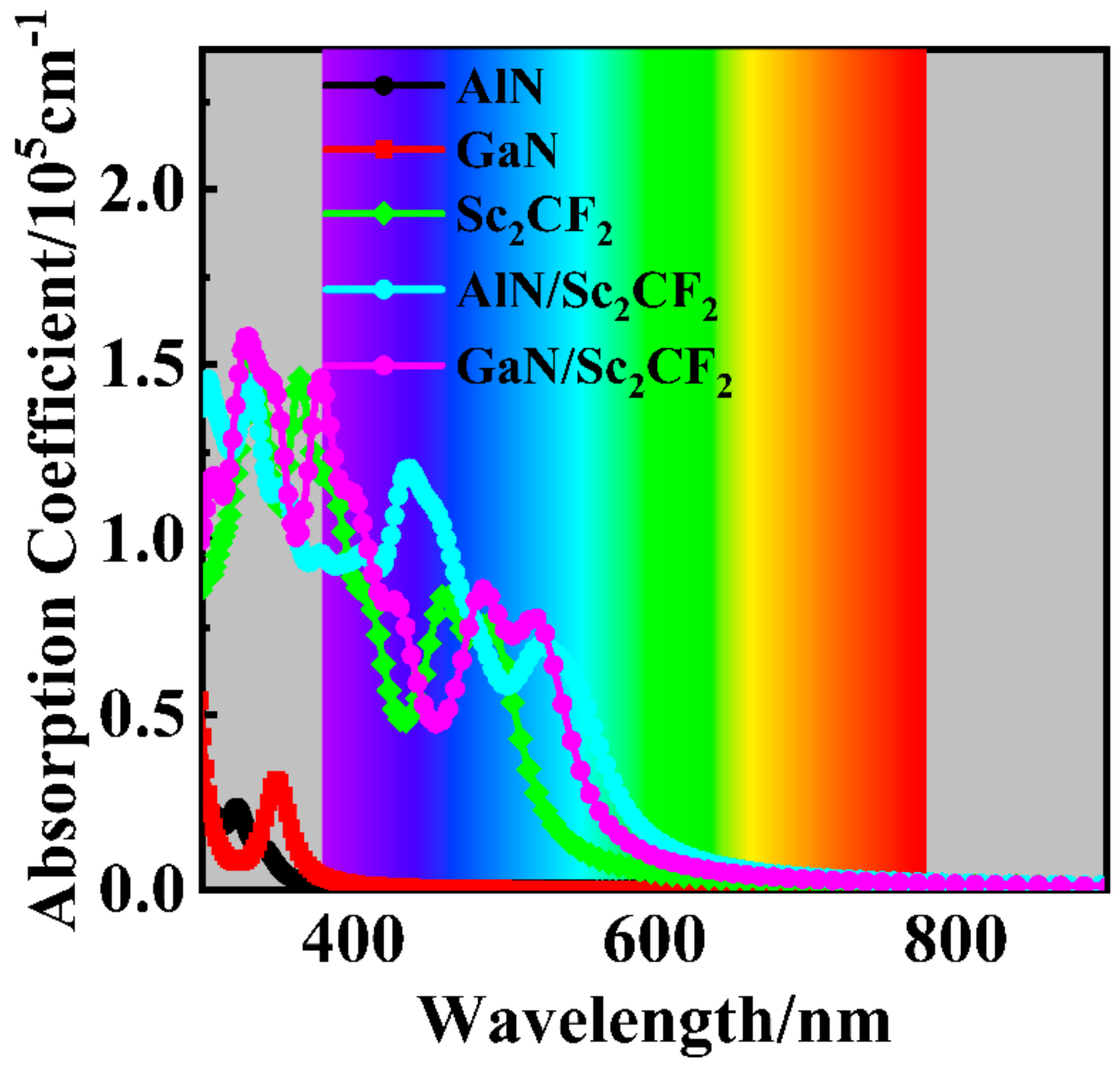
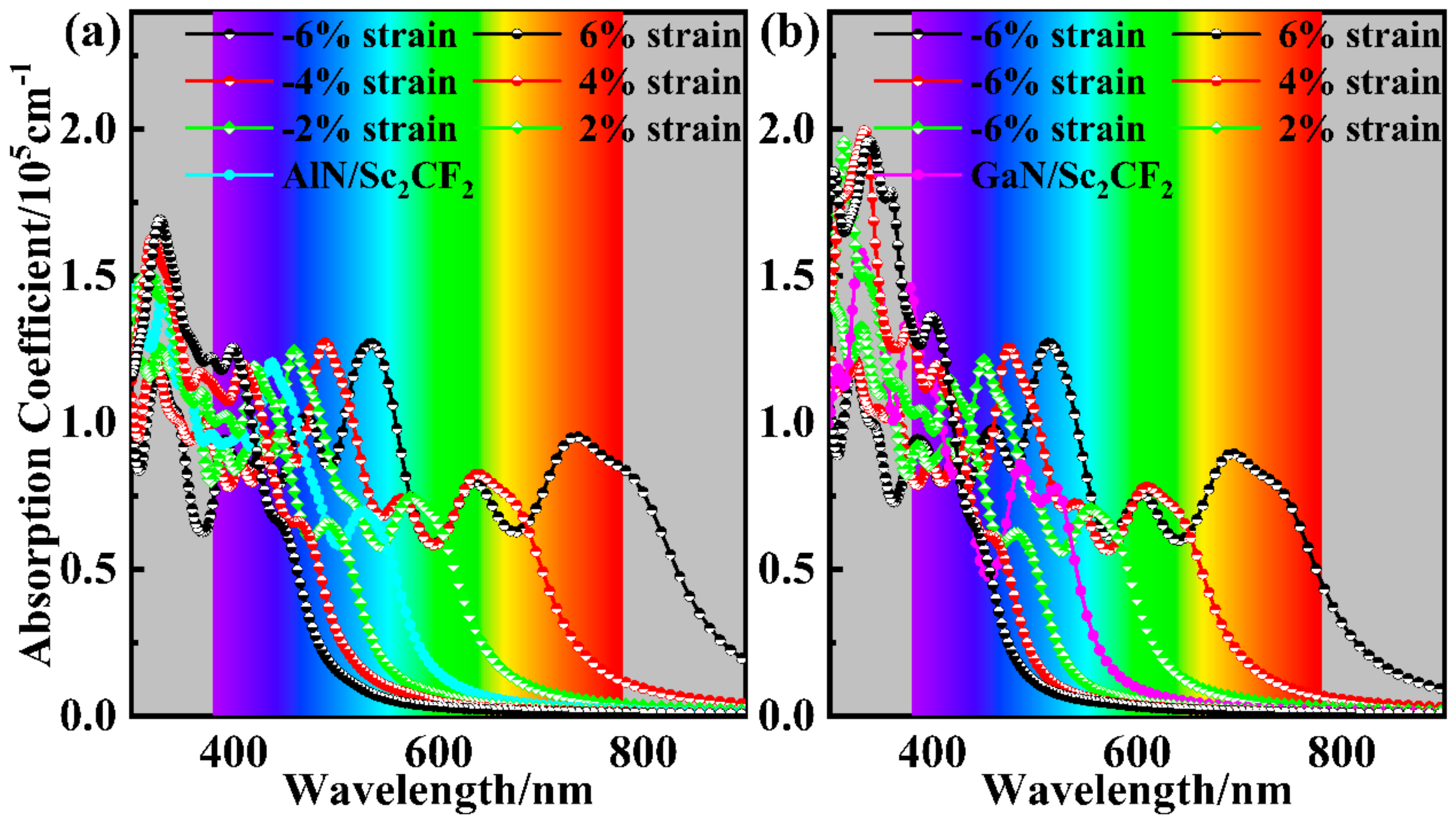
| Heterostructure | Item | SC-I | SC-II | SC-III | SC-IV | SC-V | SC-VI | |
|---|---|---|---|---|---|---|---|---|
| AlN/Sc2CF2 | a | 3.21 | 3.20 | 3.20 | 3.20 | 3.20 | 3.20 | |
| d | 2.71 | 3.38 | 2.93 | 3.37 | 2.84 | 3.03 | ||
| Eb | −22.17 | −8.74 | −13.62 | −9.69 | −18.25 | −10.54 | ||
| Eg | PBE | 0.79 | 0.85 | 0.86 | 0.86 | 0.85 | 0.85 | |
| HSE06 | 1.75 | 1.70 | 1.71 | 1.70 | 1.70 | 1.70 | ||
| GaN/Sc2CF2 | a | 3.25 | 3.22 | 3.24 | 3.22 | 3.22 | 3.21 | |
| d | 2.82 | 3.37 | 2.98 | 3.31 | 2.85 | 3.18 | ||
| Eb | −30.82 | −15.21 | −24.24 | −15.87 | −27.19 | −21.27 | ||
| Eg | PBE | 0.90 | 0.95 | 0.96 | 0.95 | 0.93 | 0.95 | |
| HSE06 | 1.84 | 1.81 | 1.82 | 1.81 | 1.82 | 1.81 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Lu, Y.; Song, J.; Ma, B.; Qiu, K.; Bai, L.; Wang, Y.; Chen, Y.; Tang, Y. First-Principles Investigation on the Tunable Electronic Structures and Photocatalytic Properties of AlN/Sc2CF2 and GaN/Sc2CF2 Heterostructures. Molecules 2024, 29, 3303. https://doi.org/10.3390/molecules29143303
Liu M, Lu Y, Song J, Ma B, Qiu K, Bai L, Wang Y, Chen Y, Tang Y. First-Principles Investigation on the Tunable Electronic Structures and Photocatalytic Properties of AlN/Sc2CF2 and GaN/Sc2CF2 Heterostructures. Molecules. 2024; 29(14):3303. https://doi.org/10.3390/molecules29143303
Chicago/Turabian StyleLiu, Meiping, Yidan Lu, Jun Song, Benyuan Ma, Kangwen Qiu, Liuyang Bai, Yinling Wang, Yuanyuan Chen, and Yong Tang. 2024. "First-Principles Investigation on the Tunable Electronic Structures and Photocatalytic Properties of AlN/Sc2CF2 and GaN/Sc2CF2 Heterostructures" Molecules 29, no. 14: 3303. https://doi.org/10.3390/molecules29143303
APA StyleLiu, M., Lu, Y., Song, J., Ma, B., Qiu, K., Bai, L., Wang, Y., Chen, Y., & Tang, Y. (2024). First-Principles Investigation on the Tunable Electronic Structures and Photocatalytic Properties of AlN/Sc2CF2 and GaN/Sc2CF2 Heterostructures. Molecules, 29(14), 3303. https://doi.org/10.3390/molecules29143303






