Abstract
Two enantiomeric pairs of new 3d–4f heterometallic clusters have been synthesized from two enantiomer Schiff base derivatives: (R/S)-2-[(2-hydroxy-1-phenylethylimino)methyl] phenol (R-/S-H2L). The formulae of the series clusters are Co3Ln(R-L)6 (Ln = Dy (1R), Gd (2R)), Co3Ln (S-L)6 (Ln = Dy (1S), Gd (2S)), whose crystal structures and magnetic properties have been characterized. Structural analysis indicated that the above clusters crystallize in the chiral P213 group space. The central lanthanide ion has a coordination geometry of D3 surrounded by three [CoIII(L)2]– anions using six aliphatic oxygen atoms of L2− featuring a star-shaped [CoIII3LnIII] configuration. Magnetic measurements showed the presence of slow magnetic relaxation with an effective energy barrier of 22.33 K in the DyIII derivatives under a zero-dc field. Furthermore, the circular dichroism (CD) spectra of 1R and 1S confirmed their enantiomeric nature.
1. Introduction
Chirality plays a significant role in various fields such as biology, chemistry, and materials science. Recent studies have demonstrated that incorporating chiral characteristics into molecular materials represents a desirable strategy for the fabrication of multifunctional molecule-based magnets [1,2,3]. Chiral molecule-based magnets are typically fabricated using enantiopure chiral organic ligands with specific coordination environments, resulting in unique properties such as magnetochiral dichroism (MChD) [4], ferroelectricity [5], magneto-optical Faraday effects [6], and second harmonic generation (SHG) [7]. In terms of molecule-based magnets, Lanthanide (III) ions, especially dysprosium ions, have been widely utilized in the construction SMMs or mononuclear single-ion magnets (SIMs) due to large spin ground states and magnetic anisotropy. Among them, the achievements of SIMs have been particularly fascinating in recent years because they do not need to consider the problem of performance degradation caused by the inconsistent arrangement of the magnetic axes of polymetallic centers [8,9,10], and a series of significant Dy-SIMs with large energy barriers and high magnetic blocking temperatures have also been developed [11,12,13,14]. Most of them, however, are organometallic compounds, which are extremely air-sensitive and should be handled under an inert atmosphere. It is worth noting that the 3d−4f SMMs containing a lanthanide metal ion, together with one or more diamagnetic 3d ions (e.g., ZnII, CaII, AlIII, low-spin CoIII or square-planar NiII), can also be regarded as SIMs, and they are often stable and exercisable [15].
Chirality is introduced to such systems in order to modulate magnetic anisotropy and also to derive multifunctional properties [16,17]. However, up to now, a limited number of chiral 3d-4f SIMS have been developed—mainly [MLn] or [M2Ln] (one or two diamagnetic 3d ion and a lanthanide metal ion) compounds, such as chiral [ZnIILnIII] [1,18,19,20], [NiIILnIII] [19,20], [CoIII2LnIII] [6,21], and [ZnII2LnIII] [2,15] constructed by enantiomeric salen or polyol Schiff base ligands. It is possible to adjust the magnetic properties by adjusting the coordination environment of the magnetic center and the magnetic dilution effect by continuously increasing the diamagnetic atom composition, but this faces challenges in design and synthesis because the magnetic center will often increase accordingly during the coordination process [22,23]. At present, as far as we know, there were only two chiral tetranuclear heterometallic [ZnII3DyIII] [24] and [ZnII3ErIII] [25] SIMs which emerged with a small energy barrier.
It has been validated that the selection of chelating ligands plays a crucial role in constructing chiral 3d-4f clusters with the desired properties. To meet the different coordination requirements in these complexes, it is widely accepted that chelating ligands should have multiple ligand sites and a flexible backbone [26,27,28,29]. The enantiomeric Schiff base derivatives, (R/S)-2-[(2-hydroxy-1-phenylethylimino)methyl] phenol (R-/S-H2L), obtained in situ during the reactions of R-/S-2-phenylglycinol and salicylaldehyde, are suitable for constructing heterometallic clusters due to the distinct abilities of coordination sites. The multiple coordination sites and abilities of the derivatives satisfy the assembly requisitions of SMMs and have been utilized in a series of chiral 3d-based magnetic clusters, such as [CuII4] [30], [FeIII4] [31], and [CoIICoIII3] [32]. But very limited 3d–4f or 4f systems have been constructed. After examining the aforementioned 3d clusters structure, we have determined that constructing 3d-4f clusters with six-coordinated 4f ions using this ligand is promising due to the 4f ions’ preference for oxygen atom coordination.
Taking into account all of the aforementioned considerations, we have chosen the chiral ligands R-/S-H2L (Scheme 1) to serve as the chelating ligands for investigating the novel 3d–4f magnetic clusters. Herein, two new pairs of chiral 3d–4f heterometallic complexes have been successfully constructed, namely, Co3Ln(R-L)6 (Ln = Dy (1R), Gd (2R)) and Co3Ln (S-L)6 (Ln = Dy (1S), Gd (2S)), which exhibit similar star-shaped structures. Magnetic studies revealed the presence of slow magnetic relaxation with an effective energy barrier of 22.33 K in the DyIII derivatives under a zero-dc field. The enantiomeric nature of 1R and 1S was confirmed by their circular dichroism (CD) spectra.
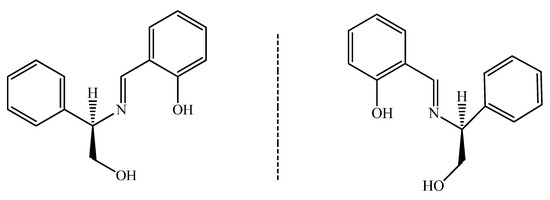
Scheme 1.
Two enantiomeric Schiff base ligands: R-H2L and S-H2L.
2. Results and Discussion
2.1. Synthesis of Chiral Heterometallic Clusters
All the chiral [CoIII3LnIII] clusters were successfully prepared through a simple one-pot method via the reaction of Ln(NO3)3·6H2O (Ln = Dy, Gd), Co(OAc)2·4H2O, salicylaldehyde-corresponding 2-phenylglycinol, and triethylamine with a rated molar ratio in methanol. The enantiomeric Schiff base ligands in the final structures are generated in situ. Moreover, the CoIII ions in the metal core must be produced by the oxidation of CoII salts in the starting materials, and a stoichiometric excess of DyIII precursor may play an important role in promoting the formation of trivalent cobalt in an aerobic reaction because it occupies a favorable position when competing with ligands for coordination [33,34]. The structures of four chiral clusters were all characterized via the analysis of single-crystal X-ray diffraction, and their crystal data and structural parameters are gathered in Table S1.
2.2. Structural Characterization
All of the four complexes are isostructural by the analysis of single-crystal and power X-ray diffraction (Table S1, Figure S2), except for the chiral configuration. Herein, only complex 1R has been structurally described in detail as the example. 1R crystallizes in the orthorhombic P213 space group, whose asymmetric unit shows the complete structure of star-shaped [CoIII3DyIII] in the cluster (Figure 1a). The DyIII ion locates in the center and is surrounded by three [CoIII(L)2]– anions coordinated by six aliphatic oxygen atoms of the ligands L2–, while the peripheral three diamagnetic CoIII ions are located in the same coordination spheres saturated with two imine-N, two phenoxy-O, and two alcohol hydroxy-O atoms from two ligands L2– (Figure 1b). Each bridging μ2-O atom originates from the amino alcohol units bind the central DyIII ion and three CoIII ions together, forming an attractive [CoIII3DyIII] core (Figure 1c). The central DyIII ion and the peripheral three CoIII ions are all six-coordinated, showing different coordination configurations. The central DyIII ion adapts the model of twisty tricapped triangular prism, but the CoIII ions locate in a slightly distorted octahedral coordination geometry (Figure 1d). Interestingly, The Dy–O bond lengths are 2.179(6) Å for Dy–O (O1, O3, O5) and 2.194(6) Å for Dy–O(O2, O4, O6); they fall within the normal range for bond lengths but are notably smaller than the Dy–O bonds found in similar structures that have been previously reported [35,36,37,38]. The O–Dy–O (O1–Dy–O2, O3–Dy–O4, O5–Dy–O6) angles are 72.1(2)°. The Co–O bond lengths range from 1.901(8) to 1.924(7) A, while the Co–N bond lengths are 1.887(10) and 1.893(9) A. In addition, the distances of adjacent Co···Co and Co···Dy are 5.539(24) Å and 3.198(19) Å. It is predicted that the DyIII ion in the center has a perfect D3 local symmetry.
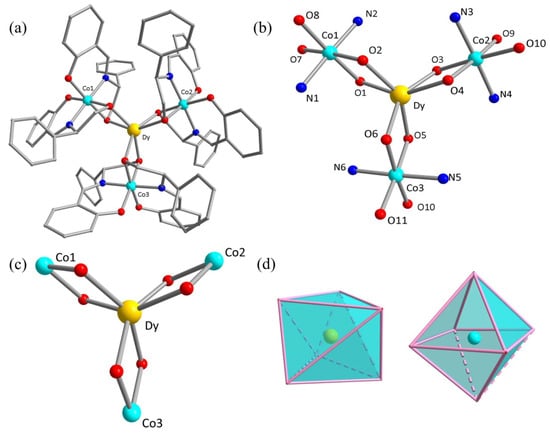
Figure 1.
(a) Single-crystal X-ray structures of complex 1R. Hydrogen atoms have been omitted for clarity; (b) the [CoIII3DyIII] skeleton with coordination environment and atomic labels; (c) the star-shaped [CoIII3DyIII] core; (d) the coordination geometry of DyIII ion (left) and CoIII (right) ions in compound 1R. Color code: DyIII yellow; CoIII turquoise; O red; N blue; C gray.
The structure of 1S is similar to that of 1R. The coordination geometry of central DyIII ion in 1S is also a twisty tricapped triangular prism configuration. The Dy–O distances are 2.163(7)Å for Dy–O (O1, O3, O5) and 2.208(6) Å for Dy–O(O2, O4, O6); the O–Dy–O (O1–Dy–O2, O3–Dy–O4, O5–Dy–O6) angles are 71.9(2)°, which are comparable to 1R with minor differences. The detailed data of bond lengths and angles for 1R are listed in Table S2.
2.3. Magnetic Properties
2.3.1. Static Magnetic Measurements
The magnetic properties of these complexes were investigated by utilizing crystalline samples from the reaction solution. Beforehand, the PXRD tests of the four complexes were carried out to verify their phase purities. The measured and simulated patterns agree well with each other (Figure S2), indicating the purities of these complexes, respectively. The two Gd- or Dy-contained enantiomers exhibited similar magnetic behaviors; and herein, the properties of 1R and 2R have been detailed. The variable-temperature magnetic susceptibility measurements have been analyzed in the temperature range of 2−300 K under an applied magnetic field of 1 kOe (Figure 2a). At 300 K, the χMT values of 1R and 2R are 10.34 and 5.79 cm3 mol–1 K, respectively, significantly less than the expected parameters of 14.17 and 7.88 cm3 mol–1 K for one uncoupled LnIII ion (DyIII, S = 5/2, g = 4/3; GdIII, S =7/2, g = 2). Upon cooling to 50 K, the χMT product of 1R remains constant and then decrease rapidly to 7.97 cm3 mol–1 K at 2 K. For complex 2R, the χMT curve shows a similar tendency. The χMT decreases smoothly along with the decreasing of the temperature to 25 K; then, it distinctly drops to 3.78 cm3 mol–1 K at 2 K. These indicate the depopulation of the Mj levels of the Dy(III) ion and, at low temperature, the possibility of weak antiferromagnetic interactions between molecules [15,39].
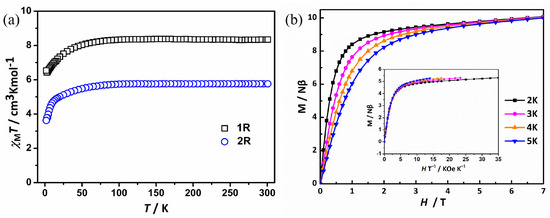
Figure 2.
(a) Plots of χMT vs. T for 1R and 2R; (b) plots of M vs. H and M vs. H/T (inset) for 1R at 2–5 K. The solid lines are a guide for the eyes.
The plots of M vs. H for 1R at 2–5 K were obtained (Figure 2b). From 0 to 1.5 T, the values of M increase, increasing rapidly, and then slowly attain values above 1.5 T. At 7 T and 2 K, the value of M reaches 5.52 Nβ, which is much smaller than the theoretical 10 Nβ, indicating the unsaturated magnetization. The plot of the M vs. H/T curves (Figure 2b, inset) shows the non-superimposed curves, implying the presence of significant magnetic anisotropy and/or low-lying excited states in these systems, as expected in DyIII-based complexes [1,40].
2.3.2. Dynamic Magnetic Measurements
To probe the magnetic dynamics for complex 1R, alternating-current (ac) susceptibility measurements were also performed under a zero-dc magnetic field during the temperature range of 1.8–10.0 K with frequencies spanning 1–997 Hz. As shown in Figure S6, a set of temperature-dependent out-of-phase (χ″) signals were obtained, indicating slow relaxation of the magnetization of SMM for 1R, but no well-defined peaks are observed above 1.8 K, which is probably caused by the rapid quantum tunneling of magnetization (QTM), typical of lanthanide magnetic molecules in distorted environments [19]. Therefore, an optimum external field of 2000 Oe was chosen to be used for further magnetic investigations. The SMM behavior of complex 1R is clearly visible after the application of the external dc field since the presence of resolved peak maxima of both χ′ and χ″ is observed (Figure 3a,b).
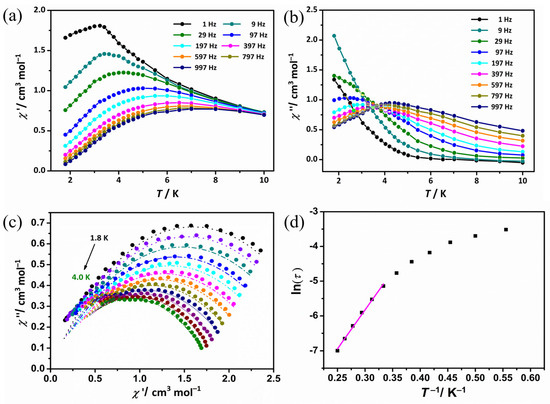
Figure 3.
Temperature dependence of the in-phase (a) and out-of-phase (b) ac susceptibility signals for 1R under the optimal dc field. The solid lines are a guide for the eyes. (c) Cole–Cole plots under the optimal dc field for 1R. The solid lines indicate the fits using a generalized Debye model. (d) The best-fitted curves employing the Arrhenius equation in the high-temperature region.
In order to further verify the magnetodynamics of complex 1R, a sweep susceptibility test was performed at a temperature range of 1.8 to 4.0 K and a frequency range of 0 to 1000 Hz. As shown in Figure S7, both χ′ and χ″ of the ac susceptibility of 1R show obvious frequency-dependent signals with observable peaks, which further indicates that complex 1R exhibits a typical slow magnetic relaxation behavior of SMM at low temperature.
The results show that QTM has a significant influence on the relaxation process in the low-temperature range but has effect on the slow relaxation process at the high-temperature range, which determines the energy barrier [33]. The Cole–Cole plots for temperatures ranging from 1.8 to 13 K were fitted by employing the generalized Debye model [41] (Figure 3c), and the obtained parameters are given in Table S3. Notably, the values of α parameters were in the range of 0.18–0.26, which indicates that there is an almost uniform relaxation process in the measured temperature range. The plots of ln(τ) versus T−1 based on the variable-frequency susceptibility data were first fitted by employing the Arrhenius equation τ = τ0exp(Ueff/kBT) [42] in the high-temperature region (T−1 < 0.35 K−1), obtaining Ueff = 22.33 K and τ0 = 3.73 × 10−6 s (Figure 3d). The value of τ0 is within the normal range (10−5–10−12 s) for the DyIII-based SMMs reported previously [43,44].
Summarizing the results of the reported chiral 3d-4f SIMs or analogues (Table 1), it is found that the Ueff value of 1R is larger than the chiral [Zn3Dy] Schiff base SIM with similar structural parameters [24], comparable with the most-reported chiral 3d-4f SIMs [1,2,18,19,20], but much smaller than the chiral [Zn2Dy] SIMs [15] with Cs symmetry of Dy ion and RRRR-Dy-D6hF12 (Ueff = 1833 K) [12]. Meanwhile, it is slightly larger than most achiral [M3Dy] SIMs [45,46,47]. These results show that the dominant role in regulating the magnetic properties of 3d-4f SIMs is still that of the axis of anisotropy for the 4f ion, and the role of chirality is limited, but the potential of chirality in inducing multifunctions is fascinating.

Table 1.
Structural and magnetic data for the chiral 3d-4f SIMs or analogues.
2.4. Circular Dichroism (CD) Spectrum
The employment of chiral ligands in coordination chemistry often leads to the phenomenon of chirality transfer, which produces structures with pre-determined supramolecular chirality. Enantiomeric R-H2L/S-H2L exhibit absorption peaks at 320 nm in DMF solution due to the intramolecular presence of the π-π* and n-π* transitions [31]. In order to study the enantiomeric nature of the above four 3d-4f complexes further, electronic circular dichroism in both solution and a solid state were studied, respectively, with 1R and 1S as representatives (Figure 4 and Figure S8). The spectrum of 1R in DMF solution exhibits a positive Cotton effect at 320 nm and negative Cotton effects at 260 and 390 nm, whereas 1S shows a mirror image at the same wavelengths. Their solid state circular dichroic chromatography also show a good mirror symmetry relationship, confirming enantiomers with each other.
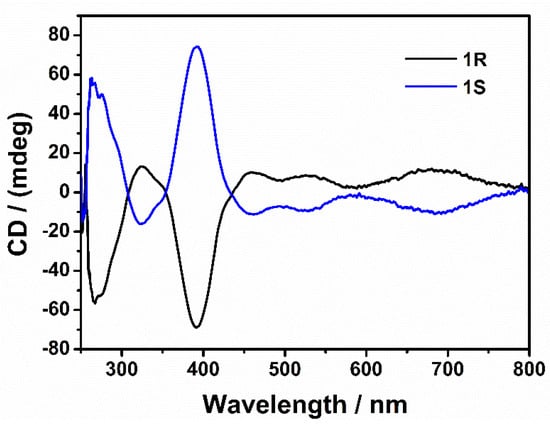
Figure 4.
CD spectra of 1R and 1S at 298 K (2 × 10−5 M, DMF).
3. Materials and Methods
3.1. Reagents and Solutions
Co(OAc)2·4H2O, Dy(NO3)3·6H2O, Gd(NO3)3·6H2O, triethylamine, salicylaldehyde, (R)-(−)-2-phenylglycinol, (S)-(+)-2-phenylglycinol, methanol, and petroleum ether were bought from Sinopharm Chemical Reagent (Shanghai, China). All starting materials were employed without further purification.
3.2. Instruments
Elemental analyses (C, H and N) were determined on a Perkin-Elmer 2400 elemental analyzer (Waltham, MA, USA). The IR spectra were recorded in range of 400–4000 cm−1 on a Nicolet 5DX spectrometer (KBr pellets, Thermo Fisher Scientific, Shanghai, China). Powder X-ray diffraction (PXRD) data were collected over the 2θ range 5–50° using a X‘Pert PRO (Cu Ka, Panalytical Company, Almelo, The Netherlands) automated diffractometer at room temperature, with a step size of 0.02° in the 2θ angle. Magnetic susceptibility measurements were carried out in the temperature range of 2–300 K with a magnetic field of 1000 Oe on a Quantum Design MPMS XL-7 magnetometer (San Diego, CA, USA).
3.3. Synthesis of 1R or 1S
A mixture of salicylaldehyde (12.2 mg, 0.1 mmol) and (R)-(−)-2-phenylglycinol (13.7 mg, 0.1 mmol)/(S)-(+)-2-phenylglycinol (13.7 mg, 0.1 mmol) was added to a methanolic solution (10 mL) of Co(OAc)2·4H2O (34.6 mg, 0.20 mmol,) and Dy(NO3)3·6H2O (45.7 mg, 0.1 mmol). After stirring for 0.5 h at room temperature, triethylamine (0.43 mL, 0.2 mmol) was added, and the resulting solution was further stirred for 4 h then filtered, and the filtrate was covered with petroleum ether carefully. After 5 days, dark red block-shaped crystals suitable for single-crystal X-ray diffraction were obtained. The crystalline samples were washed with mother liquor and then washed three times with methanol and dried in a vacuum oven at 60 °C (yield 48% for 1R and 45% for 1S based on Dy). What is more, in order to obtain high-quality single crystals, a variety of crystallization methods have been tried, including leaving the filtrate to stand at room temperature or low temperature via slow evaporation without being disturbed, the slow diffusion of ethyl ether, or the addition of n-heptane to the filtrate. All these attempts failed to achieve the desired results.
Elemental anal. Calcd (%) for 1R: C, 59.69, H, 4.56, N, 4.64; found C, 59.43, H, 4.21, N, 4.88. Elemental anal. Calcd (%) for 1S: C, 59.69, H, 4.56, N, 4.64; found C, 59.61, H, 4.46, N, 4.75. FTIR (KBr pellet, cm−1) for 1R: 3441(br), 3058(w), 3025(w), 2926(m), 2860(m), 1634(s), 1600(s), 1535(s), 1492(w), 1446(s), 1384(m), 1348(m), 1314(s), 1200(m), 1150(m), 1126(w), 1030(s), 948(w), 898(w), 800(w), 754(s), 704(s), 652(w), 595(w), 537(m), 506(s). FTIR (KBr pellet, cm−1) for 1S: 3440(br), 3058(w), 3025(w), 2926(m), 2860(m), 1634(s), 1600(s), 1535(s), 1492(w), 1445(s), 1384(m), 1348(m), 1314(s), 1201(m), 1150(m), 1126(w), 1029(s), 948(w), 898(w), 800(w), 754(s), 704(s), 653(w), 595(w), 537(m), 505(s).
3.4. Synthesis of 2R or 2S
The procedure for the synthesis of 2 was similar to 1, except using Gd(NO3)3·6H2O instead of Dy(NO3)3·6H2O. Dark red block-shaped crystals suitable for single-crystal X-ray diffraction were obtained (yield 42% for 2R and 30% for 2S based on Gd). Elemental anal. Calcd (%) for 2R: C, 59.86, H, 4.58, N, 4.65; found C, 59.63, H, 4.47, N, 4.81 Elemental anal. Calcd (%) for 2S: C, 59.86, H, 4.58, N, 4.65; found C, 59.55, H, 4.52, N, 4.79. FTIR (KBr pellet, cm−1) for 2R: 3440(br), 3058(w), 3025(w), 2922(m), 2862(m), 1634(s), 1600(s), 1535(s), 1492(w), 1445(s), 1384(w), 1348(m), 1314(s), 1201(m), 1150(m), 1127(w), 1029(s), 948(w), 899(w), 800(w), 754(s), 704(s), 653(w), 596(w), 537(m), 506(s). FTIR (KBr pellet, cm−1) for 2S: 3440(br), 3057(w), 3024(w), 2928(m), 2860(m), 1633(s), 1600(s), 1534(s), 1492(w), 1447(s), 1384(w), 1348(m), 1314(s), 1200(m), 1149(m), 1127(w), 1029(s), 947(w), 898(w), 800(w), 754(s), 704(s), 652(w), 595(w), 537(m), 504(s).
3.5. Crystallography
X-ray diffraction data of the four complexes were collected on Bruker diffractometer MD2 of BL17B beamline (MoKα radiation source, λ = 0.71073 Å) of the National Center for Protein Sciences, Shanghai (NCPSS), at Shanghai Synchrotron Radiation Facility. The HKL data were reduced via the HKL3000 program. The structures of the complexes were solved via direct methods using the SHELXT program [48,49], and the non-hydrogen atoms were located from the trial structure and then refined anisotropically with SHELXTL-2014 using a full-matrix least squares procedure based on F2 values. The hydrogen atom positions were fixed geometrically at calculated distances and allowed to ride on the parent atoms. CCDC 2348361–2348364 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
4. Conclusions
In summary, two pairs of chiral star-shaped [CoIII3LnIII] complexes derived from enantiomerically pure Schiff bases were obtained via the reaction of salicylaldehyde and 2-phenylglycinol and have been rationally designed and successfully prepared, where the LnIII ions are six-coordinated. Magnetic measurements revealed dominant antiferromagnetic exchange within all the compounds, and due to significant magnetic anisotropy of DyIII ions, the slow relaxation of the magnetization was observed in the DyIII derivatives under a zero-dc field, with the derivatives behaving as zero-field SIMs. The CD spectra confirmed that chiral features had been successfully transferred from the ligand to the entire magnetic molecular system. This work presents a feasible method for constructing multifunctional molecular magnets that contain low-coordinated LnIII ions. These chiral clusters will provide an excellent platform from which to explore synergies among different functions (e.g., magneto-optical Faraday effects, proton conduction, asymmetric catalysis). Further comprehensive theoretical studies on these family SIMs with different coordination symmetries of central ions for magnetostructural correlation remain challenges for the future.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29143304/s1: Figure S1: Coordination modes of Ligands R-/S-H2L in this work; Table S1: Crystal data and structure refinements for 1R, 1S, 2R, and 2S; Table S2 selected bond distances (Å) and angles (°) for 1R, 1S, 2R and 2S; Figure S2: FT-IR spectra of 1R (black), 1S (red), 2R (blue), and 2S (green); Figure S3: The simulated X-ray powder diffraction patterns (black) and the experimental ones (red) of compounds 1R, 1S, 2R, and 2S; Figure S4: Plots of the temperature dependence of χMT vs. T for 1S and 2S; Figure S5: Magnetization curve for compound 1S at different temperatures (2 K, 3 K, 4 K and 5 K); Figure S6: Temperature dependence of the in-phase (χ′) (left) and out-of-phase (χ″) (right) ac susceptibility data for 1R under a zero-dc field; Figure S7: Frequency dependence of the in-phase (χ′) (left) and out-of-phase (χ″) (right) ac susceptibility data for 1R under a 2000 Oe applied dc field; Table S3: The parameters obtained by fitting Cole–Cole plot under the optimal dc field for 1R; Figure S8: The solid-state CD spectra of 1R and 1S in KBr pellet at 298 K.
Author Contributions
Conceptualization, P.H.; methodology, P.H., X.Z.; software, J.W.; validation, Z.L., J.W.; formal analysis, Z.L.; investigation, L.J.; resources, L.J. and P.H.; data curation, L.J. and P.H.; writing—original draft preparation, L.J.; writing—review and editing P.H.; visualization, L.J.; supervision, P.H. and X.Z.; project administration, P.H. and X.Z.; funding acquisition, L.J. and P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (62001159), Hubei Provincial Natural Science Foundation of China (2024AFB1038), the Special Fund Projects of Hubei Key Laboratory of Radiation Chemistry and Functional Materials (2021ZX07, 2022ZX05), the Research and development Fund Project of Hubei University of Science and Technology (2023-25GP02), and the Innovation team project of Hubei University of Science and Technology (2023T09).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Huang, H.; Sun, R.; Wu, X.F.; Liu, Y.; Zhan, J.Z.; Wang, B.W.; Gao, S. Circularly polarized luminescence and magneto-optic effects from chiral Dy (III) single molecule magnets. Dalton Trans. 2023, 52, 7646–7651. [Google Scholar] [CrossRef]
- Zhu, S.D.; Hu, J.J.; Dong, L.; Wen, H.R.; Liu, S.J.; Lu, Y.B.; Liu, C.M. Multifunctional Zn(II)-Yb(III) complex enantiomers showing second-harmonic generation, near-infrared luminescence, single-molecule magnet behaviour and proton conduction. J. Mater. Chem. C 2020, 8, 16032–16041. [Google Scholar] [CrossRef]
- Marin, R.; Brunet, G.; Murugesu, M. Shining new light on multifunctional lanthanide single-molecule magnets. Angew. Chem. Int. Ed. 2021, 60, 1728–1746. [Google Scholar] [CrossRef]
- Pointillart, F.; Atzori, M.; Train, C. Magneto-chiral dichroism of chiral lanthanide complexes. Inorg. Chem. Front. 2024, 11, 1313–1321. [Google Scholar] [CrossRef]
- Liu, C.M.; Xiong, R.G.; Zhang, D.Q.; Zhu, D.B. Nanoscale homochiral C3-symmetric mixed-valence manganese cluster complexes with both ferromagnetic and ferroelectric properties. J. Am. Chem. Soc. 2010, 132, 4044–4045. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wu, G.; Xu, H.; Wang, X.; Long, L.S.; Kong, X.; Zheng, L.S. Magnetooptical Properties of Chiral [Co2Ln] Clusters. Inorg. Chem. 2019, 59, 193–197. [Google Scholar] [CrossRef]
- Train, C.; Nuida, T.; Gheorghe, R.; Gruselle, M.; Ohkoshi, S.I. Large magnetization-induced second harmonic generation in an enantiopure chiral magnet. J. Am. Chem. Soc. 2009, 131, 16838–16843. [Google Scholar] [CrossRef]
- Feltham, H.L.C.; Brooker, S. Review of purely 4f and mixed-metal nd-4f single-molecule magnets containing only one lanthanide ion. Coord. Chem. Rev. 2014, 276, 1–33. [Google Scholar] [CrossRef]
- Liu, X.; Ma, X.; Yuan, W.; Cen, P.; Zhang, Y.Q.; Ferrando-Soria, J. Concise chemistry modulation of the SMM behavior within a family of mononuclear Dy(III) complexes. Inorg. Chem. 2018, 57, 14843–14851. [Google Scholar] [CrossRef]
- Wan, Q.; Wakizaka, M.; Yamashita, M. Single-ion magnetism behaviors in lanthanide (III) based coordination frameworks. Inorg. Chem. Front. 2023, 10, 5212–5224. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Ullah, A.; Gutiérrez-Finol, G.M.; Bedoya-Pinto, A.; Gargiani, P.; Shi, D.; Yang, S.; Shi, Z.; Gaita-Arino, A.; et al. High-temperature magnetic blocking in a monometallic dysprosium azafullerene single-molecule magnet. Chem 2023, 9, 3613–3622. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, C.; Feng, T.; Liu, X.; Ying, X.; Li, X.-L.; Zhang, Y.-Q.; Tang, J. Air-stable chiral single-molecule magnets with record anisotropy barrier exceeding 1800 K. J. Am. Chem. Soc. 2021, 143, 10077–10082. [Google Scholar] [CrossRef]
- Guo, F.S.; Day, B.M.; Chen, Y.C.; Tong, M.L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef]
- Goodwin, C.A.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef]
- Liu, C.M.; Sun, R.; Wang, B.W.; Hao, X.; Li, X.L. Effects of counter ions, coordination anions, and coordination solvent molecules on single-molecule magnetic behaviors and nonlinear optical properties of chiral Zn2Dy Schiff base complexes. Inorg. Chem. 2022, 61, 18510–18523. [Google Scholar] [CrossRef]
- Liu, J.L.; Chen, Y.C.; Tong, M.L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, H.L.; Zhu, Z.H.; Wang, Y.F.; Liang, F.P.; Zou, H.H. Aggregation induced emission dynamic chiral europium(III) complexes with excellent circularly polarized luminescence and smart sensors. Nat. Commun. 2024, 15, 2896. [Google Scholar] [CrossRef]
- Long, J.; Rouquette, J.; Thibaud, J.-M.; Ferreira, R.A.S.; Carlos, L.D.; Donnadieu, B.; Vieru, V.; Chibotaru, L.F.; Konczewicz, L.; Haines, J. A high-temperature molecular ferroelectric Zn/Dy complex exhabiting single-ion-magnet behavior and lanthanide luminescence. Angew. Chem. Int. Ed. 2015, 54, 2236–2240. [Google Scholar] [CrossRef]
- Mayans, J.; Saez, Q.; Font-Bardia, M.; Escuer, A. Enhancement of magnetic relaxation properties with 3d diamagnetic cations in [ZnIILnIII] and [NiIILnIII], LnIII = Kramers lanthanides. Dalton Trans. 2019, 48, 641–652. [Google Scholar] [CrossRef]
- Wen, H.R.; Liu, S.J.; Xie, X.R.; Bao, J.; Liu, C.M.; Chen, J.-L. A family of nickel-lanthanide heterometallic dinuclear complexes derived from a chiral Schiff-base ligand exhibiting single-molecule magnet behaviors. Inorg. Chim. Acta 2015, 435, 274–282. [Google Scholar] [CrossRef]
- Aibibula, M.; Song, Y.H.; Xu, H.; Chen, M.T.; Kong, X.J.; Long, L.S.; Zheng, L.S. Magneto-optical properties of chiral Co2Ln and Co3Ln2 (Ln = Dy and Er) clusters. Inorg. Chem. 2024, 63, 8003–8007. [Google Scholar] [CrossRef]
- Miao, L.; Liu, M.J.; Zeng, M.; Kou, H.Z. Chiral Zn3Ln3 Hexanuclear Clusters of an Achiral Flexible Ligand. Inorg. Chem. 2023, 62, 12814–12821. [Google Scholar] [CrossRef]
- Li, J.; Wei, R.M.; Pu, T.C.; Cao, F.; Yang, L.; Han, Y. Tuning quantum tunnelling of magnetization through 3d-4f magnetic interactions: An alternative approach for manipulating single-molecule magnetism. Inorg. Chem. Front. 2017, 4, 114–122. [Google Scholar] [CrossRef]
- Liu, M.J.; Yuan, J.; Zhang, Y.Q.; Sun, H.L.; Liu, C.M.; Kou, H.Z. Chiral six-coordinate Dy(III) and Tb(III) complexes of an achiral ligand: Structure, fluorescence, and magnetism. Dalton Trans. 2017, 46, 13035–13042. [Google Scholar] [CrossRef]
- Yamashita, A.; Watanabe, A.; Akine, S.; Nabeshima, T.; Nakano, M.; Yamamura, T.; Kajiwara, T. Wheel-shaped ErIIIZnII3 single-molecule magnet: A macrocyclic approach to designing magnetic anisotropy. Angew. Chem. Int. Ed. 2011, 50, 4016–4019. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.Q.; Chen, J.N.; Jia, J.H.; Wang, C.; Paillot, K.; Breslavetz, I.; Long, L.S.; Zheng, L.S.; Train, C.; et al. Magnetic 3d-4f Chiral Clusters Showing Multimetal Site Magneto-Chiral Dichroism. J. Am. Chem. Soc. 2022, 144, 8837–8847. [Google Scholar] [CrossRef]
- Wang, X.; Du, M.H.; Xu, H.; Long, L.S.; Kong, X.J.; Zheng, L.S. Cocrystallization of chiral 3d-4f clusters {Mn10Ln6} and {Mn6Ln2}. Inorg. Chem. 2021, 60, 5925–5930. [Google Scholar] [CrossRef]
- Liu, C.M.; Zhang, D.Q.; Hao, X.; Zhu, D.B. Assembly of chiral 3d-4f wheel-like cluster complexes with achiral ligands: Single-molecule magnetic behavior and magnetocaloric effect. Inorg. Chem. Front. 2020, 7, 3340–3351. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, H.L.; Zhu, Z.H.; Liang, F.P.; Zou, H.H. Recent advances in the structural design and regulation of lanthanide clusters: Formation and self-assembly mechanisms. Coord. Chem. Rev. 2023, 493, 215322. [Google Scholar] [CrossRef]
- Yin, J.; Yin, T.T.; Gao, C.; Wang, B.W.; Zhu, Y.Y.; Wu, Z.Q.; Gao, S. A Pair of Enantiopure Cubane-Type CuII4O4 Clusters: Synthesis, Structure, Chirality and Magnetism. Eur. J. Inorg. Chem. 2014, 2014, 5385–5390. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Guo, X.; Cui, C.; Wang, B.W.; Wang, Z.M.; Gao, S. An enantiopure FeIII4 single-molecule magnet. Chem. Commun. 2011, 47, 8049–8051. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Zhang, Y.Q.; Yin, T.T.; Gao, C.; Wang, B.W.; Gao, S. A family of CoIICoIII3 single-ion magnets with zero-field slow magnetic relaxation: Fine tuning of energy barrier by remote substituent and counter cation. Inorg. Chem. 2015, 54, 5475–5486. [Google Scholar] [CrossRef]
- Hazra, S.; Titiš, J.; Valigura, D.; Boča, R.; Mohanta, S. Bis-phenoxido and bis-acetato bridged heteronuclear {CoIIIDyIII} single molecule magnets with two slow relaxation branches. Dalton Trans. 2016, 45, 7510–7520. [Google Scholar] [CrossRef]
- Polyzou, C.D.; Koumousi, E.S.; Lada, Z.G.; Raptopoulou, C.P.; Psycharis, V.; Rouzières, M.; Tsipis, A.C.; Mathonière, C.; Clérac, R.; Perlepes, S.P. “Switching on” the single-molecule magnet properties within a series of dinuclear cobalt(III)–dysprosium(III) 2-pyridyloximate complexes. Dalton Trans. 2017, 46, 14812–14825. [Google Scholar] [CrossRef]
- Zhu, Z.; Ying, X.; Zhao, C.; Zhang, Y.Q.; Tang, J. A new breakthrough in low-coordinate Dy(III) single-molecule magnets. Inorg. Chem. Front. 2022, 9, 6061–6066. [Google Scholar] [CrossRef]
- Canaj, A.B.; Singh, M.K.; Wilson, C.; Rajaraman, G.; Murrie, M. Chemical and in silico tuning of the magnetisation reversal barrier in pentagonal bipyramidal Dy (III) single-ion magnets. Chem. Commun. 2018, 54, 8273–8276. [Google Scholar] [CrossRef]
- Yu, S.; Hu, Z.; Chen, Z.; Li, B.; Zhang, Y.Q.; Liang, Y.; Liu, D.; Yao, D.; Liang, F. Two Dy (III) single-molecule magnets with their performance tuned by schiff base ligands. Inorg. Chem. 2019, 58, 1191–1200. [Google Scholar] [CrossRef]
- Lefeuvre, B.; Guizouarn, T.; Dorcet, V.; Cordier, M.; Pointillart, F. Single-Molecule Magnet Properties in 3d4f Heterobimetallic Iron and Dysprosium Complexes Involving Hydrazone Ligand. Molecules 2023, 28, 6359. [Google Scholar] [CrossRef]
- Xu, W.J.; Luo, Q.C.; Li, Z.H.; Zhai, Y.Q.; Zheng, Y.Z. Bis-Alkoxide Dysprosium (III) Crown Ether Complexes Exhibit Tunable Air Stability and Record Energy Barrier. Adv. Sci. 2024, 11, 2308548. [Google Scholar] [CrossRef]
- Sun, R.; Wang, C.; Wang, B.W.; Wang, Z.M.; Chen, Y.F.; Tamm, M.; Gao, S. Low-coordinate bis (imidazolin-2-iminato) dysprosium (III) single-molecule magnets. Inorg. Chem. Front. 2023, 10, 485–492. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.Q.; Zhu, Z.; Tang, J. Dysprosium compounds with hula-hoop-like geometries: The influence of magnetic anisotropy and magnetic interactions on magnetic relaxation. Inorg. Chem. 2018, 57, 12213–12221. [Google Scholar] [CrossRef]
- Li, Q.; Peng, Y.; Qian, J.; Yan, T.; Du, L.; Zhao, Q. A family of planar hexanuclear CoIII4LnIII2 clusters with lucanidae-like arrangement and single-molecule magnet behavior. Dalton Trans. 2019, 48, 12880–12887. [Google Scholar] [CrossRef]
- Bhanja, A.; Herchel, R.; Travnicek, Z.; Ray, D. Two types of hexanuclear partial tetracubane [Ni4Ln2](Ln = Dy, Tb, Ho) complexes of thioether-based schiff base ligands: Synthesis, structure, and comparison of magnetic properties. Inorg. Chem. 2019, 58, 12184–12198. [Google Scholar] [CrossRef]
- Feltham, H.L.; Clérac, R.; Ungur, L.; Vieru, V.; Chibotaru, L.F.; Powell, A.K.; Brooker, S. Synthesis and magnetic properties of a new family of macrocyclic MII3LnIII complexes: Insights into the effect of subtle chemical modification on single-molecule magnet behavior. Inorg. Chem. 2012, 51, 10603–10612. [Google Scholar] [CrossRef]
- Singh, S.K.; Beg, M.F.; Rajaraman, G. Role of Magnetic Exchange Interactions in the Magnetization Relaxation of {3d–4f} Single-Molecule Magnets: A Theoretical Perspective. Chem.-Eur. J. 2016, 22, 672–680. [Google Scholar] [CrossRef]
- Dais, T.N.; Takano, R.; Ishida, T.; Plieger, P.G. Self-assembly of non-macrocyclic triangular Ni3Ln clusters. Dalton Trans. 2022, 51, 1446–1453. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-2014, Program for Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXS-2014, Program for Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).