LVI and DI-SPME Combined with GC/MS and GC/MS for Volatile Chemical Profile Investigation and Cytotoxic Power Evaluation of Essential Oil and Hydrolate from Cannabis sativa L. cv. Carmagnola
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of C. sativa EO
2.2. LVI-GC/MS Chemical Composition of C. sativa L. Hy
2.3. DI-SPME/GC-MS Chemical Composition of C. sativa L. Hy
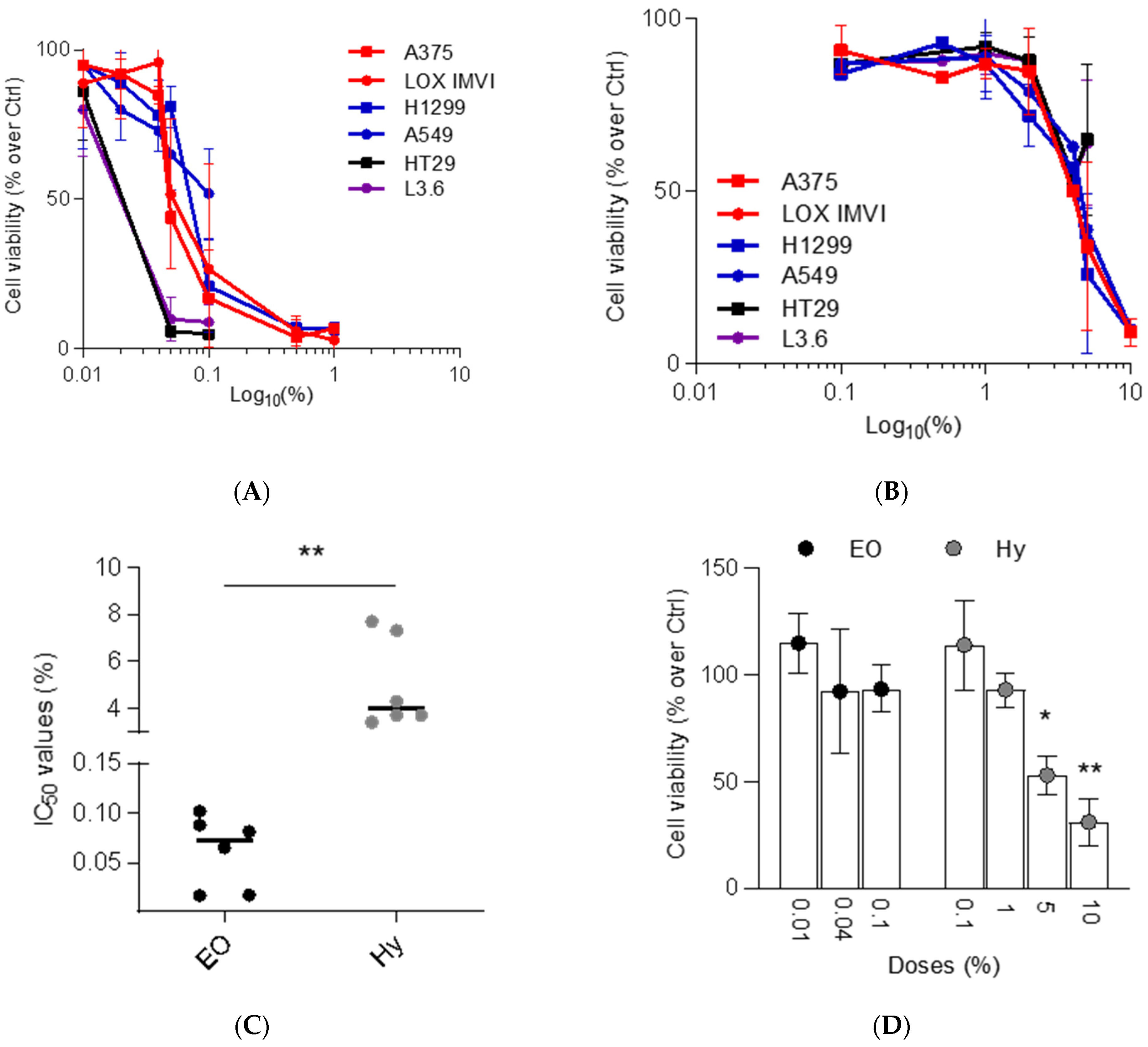
2.4. EO and Hy Differently Affected the Viability of Tumor Cell Lines
3. Materials and Methods
3.1. Plant Material
3.2. LVI-GC-MS Analysis of C. sativa Hydrolate
3.3. DI-SPME/GC-MS Analysis of C. sativa Hy
3.4. GC-MS Analysis of C. sativa EO
3.5. Cell Cultures
3.6. Treatments and Analysis of Cell Viability
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McPartland, J.M. Cannabis Systematics at the Levels of Family, Genus, and Species. Cannabis Cannabinoid Res. 2018, 3, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Hillig, K.W. Genetic evidence for speciation in Cannabis (Cannabaceae). Genet. Resour. Crop Evol. 2005, 52, 161–180. [Google Scholar] [CrossRef]
- McPartland, J.M.; Hegman, W.; Long, T. Cannabis in Asia: Its center of origin and early cultivation, based on a synthesis of subfossil pollen and archaeobotanical studies. Veg. Hist. Archaeobotany 2019, 28, 691–702. [Google Scholar] [CrossRef]
- Deitch, R. Hemp-American History Revisited: The Plant with a Divided History; Algora Publishing: New York, NY, USA, 2003; Chapter 1; pp. 7–44. [Google Scholar]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crops Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Synowiec, A.; Rys, M.; Bocianowski, J.; Wielgusz, K.; Byczyńska, M.; Heller, K.; Danuta, K. Phytotoxic Effect of Fiber Hemp Essential Oil on Germination of Some Weeds and Crops. J. Essent. Oil Bear. Plants 2016, 19, 262–276. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Iannone, M.; Cinque, G.; Marianelli, A.; Pistelli, L.; Flamini, G. “Hemping” the drinks: Aromatizing alcoholic beverages with a blend of Cannabis sativa L. flowers. Food Chem. 2020, 325, 126909. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, V.; Steinemann, S. Essential oil of Cannabis sativa L. strains. J. Int. Hemp Assoc. 1997, 4, 80. [Google Scholar]
- Fischedick, J.T. Identification of Terpenoid Chemotypes Among High (−)-trans-Δ9-Tetrahydrocannabinol-Producing Cannabis sativa L. Cultivars. Cannabis Cannabinoid Res. 2017, 2, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The Cannabis Terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Sefidkon, F.; Calagari, M.; Mousavi, A.; Mahomoodally, M.F. Impact of four hemp (Cannabis sativa L.) varieties and stage of plant growth on yield and composition of essential oils. Ind. Crops Prod. 2020, 155, 112793. [Google Scholar] [CrossRef]
- Bertoli, A.; Tozzi, S.; Pistelli, L.; Angelini, L.G. Fibre hemp inflorescences: From crop-residues to essential oil production. Ind. Crops Prod. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- Meier, C.; Mediavilla, V. Factors influencing the yield and the quality of hemp (Cannabis sativa L.) essential oil. J. Ind. Hemp. 1998, 5, 16–20. [Google Scholar]
- García-Tejero, I.F.; Durán-Zuazo, V.H.; Pérez-Álvarez, R.; Hernández, A.; Casano, S.; Morón, M.; Muriel-Fernández, J.L. Impact of plant density and irrigation on yield of hemp (Cannabis sativa L.) in a mediterranean semi-arid environment. J. Agric. Sci. Technol. 2014, 16, 887–895. [Google Scholar]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar] [CrossRef] [PubMed]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. The Anti-Inflammatory Properties of Terpenoids from Cannabis. Cannabis Cannabinoid Res. 2018, 3, 282. [Google Scholar] [CrossRef] [PubMed]
- ISO 9235:2013; Aromatic Natural Raw Materials—Vocabulary. ISO: Geneva, Switzerland, 2013.
- D’Amato, S.; Serio, A.; López, C.C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Traka, C.K.; Petrakis, E.A.; Kimbaris, A.C.; Polissiou, M.G.; Perdikis, D.C. Effects of Ocimum basilicum and Ruta chalepensis hydrosols on Aphis gossypii and Tetranychus urticae. J. Appl. Entomol. 2018, 142, 413–420. [Google Scholar] [CrossRef]
- Di Vito, M.; Bugli, F.; Cacaci, M.; Talamonti, D.; Lombardini, G.; Garzoli, S. Phytochemical analysis and evaluation of antibacterial, antifungal, antioxidant and anti-inflammatory potential of Juniperus phoenicea subsp. phoenicea L. essential oil and hydrolate from central Italy. J. Essent. Oil Res. 2024, 36, 49–59. [Google Scholar] [CrossRef]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Pieracci, Y.; Ascrizzi, R.; Terreni, V.; Pistelli, L.; Flamini, G.; Bassolino, L.; Fulvio, F.; Montanari, M.; Paris, R. Essential Oil of Cannabis sativa L: Comparison of Yield and Chemical Composition of 11 Hemp Genotypes. Molecules 2021, 26, 4080. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, C.; Perinelli, D.R.; Cespi, M.; Zeppa, L.; Mazzara, E.; Maggi, F.; Petrelli, R.; Bonacucina, G.; Nabissi, M. Encapsulation of Hemp (Cannabis sativa L.) Essential Oils into Nanoemulsions for Potential Therapeutic Applications: Assessment of Cytotoxicological Profiles. Molecules 2023, 28, 6479. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Verschut, V.; Bibb, M.J.; Bush, M.J.; Molnár, B.P.; Barane, E.; Al-Bassam, M.M.; Chandra, G.; Song, L.; Challis, G.L.; et al. Developmentally regulated volatiles geosmin and 2-methylisoborneol attract a soil arthropod to Streptomyces bacteria promoting spore dispersal. Nat. Microbiol. 2020, 5, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Paraschos, S.; Magiatis, P.; Gousia, P.; Economou, V.; Sakkas, H.; Papadopoulou, C.; Skaltsounis, A.-L. Chemical investigation and antimicrobial properties of mastic water and its major constituents. Food Chem. 2011, 129, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Braga, P.C.; Culici, M.; Dal Sasso, M.; Falch, M.; Spallino, A. Antiradical activity of sobrerol investigated by electron paramagnetic resonance (EPR). G. Ital. Mal. Torace 2009, 63, 263–267. [Google Scholar]
- Alexandrino, T.D.; Moya, A.M.T.M.; de Medeiros, T.D.M.; Morari, J.; Velloso, L.A.; Leal, R.F.; Maróstica, M.R.; Pastore, G.M.; Betim Cazarin, C.B.; Bicas, J.L.; et al. Anti-inflammatory effects of monoterpenoids in rats with TNBS-induced colitis. PharmaNutrition 2020, 14, 100240. [Google Scholar] [CrossRef]
- Leitão, S.G.; Martins, G.R.; Martínez-Fructuoso, L.; de Sousa Silva, D.; da Fonseca, T.S.; Castilho, C.V.V.; Alviano, D.S.; Alviano, C.S.; Leitão, G.G.; Pereda-Miranda, R.; et al. Absolute stereochemistry of antifungal limonene-1, 2-diols from Lippia rubella. Rev. Bras. Farm. 2020, 30, 537–543. [Google Scholar] [CrossRef]
- Sales, A.; Moreira, R.C.; Pastore, G.M.; Bicas, J.L. Establishment of culture conditions for bio-transformation of R-(+)-limonene to limonene-1,2-diol by Colletotrichum nymphaeae CBMAI 0864. Process Biochem. 2019, 78, 8–14. [Google Scholar] [CrossRef]
- Campos-Arguedas, F.; Sarrailhe, G.; Nicolle, P.; Dorais, M.; Brereton, N.J.B.; Pitre, F.E.; Pedneault, K. Different temperature and UV patterns modulate berry maturation and volatile compounds accumulation in Vitis sp. Front. Plant Sci. 2022, 13, 862259. [Google Scholar] [CrossRef]
- Zhou, Y.; He, W.; He, Y.; Chen, Q.; Gao, Y.; Geng, J.; Zhu, Z.R. Formation of 8-hydroxylinalool in tea plant Camellia sinensis var. Assamica ‘Hainan dayezhong’. Food Chem. Mol. Sci. 2023, 6, 100173. [Google Scholar] [CrossRef] [PubMed]
- Melliou, E.; Chinou, I. Chemical constituents of selected unifloral Greek bee-honeys with antimicrobial activity. Food Chem. 2011, 129, 284–290. [Google Scholar] [CrossRef]
- Kokalj Ladan, M.; Kocevar Glavac, N. GC–MS Analysis of a Helichrysum italicum Hydrosol: Sensitivity, Repeatability and Reliability of Solvent Extraction versus Direct Hydrosol Analysis. Appl. Sci. 2022, 12, 10040. [Google Scholar] [CrossRef]
- KhaleeL, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- An, Q.; Ren, J.-N.; Li, X.; Fan, G.; Qu, S.-S.; Song, Y.; Li, Y.; Pan, S.-Y. Recent updates on bioactive properties of linalool. Food Funct. 2021, 12, 10370–10389. [Google Scholar] [CrossRef] [PubMed]
- Kotana, R.; Kordali, S.; Cakird, A. Screening of Antibacterial Activities of Twenty-One Oxygenated Monoterpenes. Z. Naturforsch. C. 2007, 62, 507–513. [Google Scholar] [CrossRef]
- Ovidi, E.; Laghezza Masci, V.; Taddei, A.R.; Torresi, J.; Tomassi, W.; Iannone, M.; Tiezzi, A.; Maggi, F.; Garzoli, S. Hemp (Cannabis sativa L.; Kompolti cv.) and Hop (Humulus lupulus L.; Chinook cv.) Essential Oil and Hydrolate: HS-GC-MS Chemical Investigation and Apoptotic Activity Evaluation. Pharmaceuticals 2022, 15, 976. [Google Scholar] [CrossRef] [PubMed]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Uedo, N.; Tatsuta, M.; Iishi, H.; Baba, M.; Sakai, N.; Yano, H.; Otani, T. Inhibition by D-limonene of gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett. 1999, 137, 131–136. [Google Scholar] [CrossRef] [PubMed]
- de Araújo-Filho, H.G.; dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S.S. Anticancer activity of limonene: A systematic review of target signaling pathways. Phytot. Res. 2021, 35, 627–5329. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, Y.M.; Adu-Frimpong, M.; Xu, X.; Yu, J. Biochemical significance of limonene and its metabolites: Future prospects for designing and developing highly potent anticancer drugs. Biosci. Rep. 2018, 38, BSR20181253. [Google Scholar] [CrossRef] [PubMed]
- Sindle, A.; Martin, K. Art of Prevention: Essential Oils—Natural Products Not Necessarily Safe. Int. J. Women’s Dermatol. 2020, 7, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, D.; Yu, J. Assessing clinical relevance of limonene and linalool hydroperoxides patch test positivity: A prospective series of 247 patients. J. Am. Acad. Dermatol. 2020, 83, 946–947. [Google Scholar] [CrossRef] [PubMed]
- Inan, S.; Ward, S.J.; Baltazar, C.T.; Peruggia, G.A.; Javed, E.; Nayak, A.P. Epicutaneous Sensitization to the Phytocannabinoid β-Caryophyllene Induces Pruritic Inflammation. Int. J. Mol. Sci. 2023, 24, 14328. [Google Scholar] [CrossRef] [PubMed]
- Aragón, A.; Toledano, R.M.; Vázquez, A.; Villén, J.; Cortés, J.M. Analysis of polycyclic aromatic hydrocarbons in aqueous samples by large volume injection gas chromatography–mass spectrometry using the through oven transfer adsorption desorption interface. Talanta 2015, 139, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 2nd ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 1995. [Google Scholar]
- Garzoli, S.; Uva, C.; Iriti, M.; Vitalini, S. Viola calcarata L. and Viola dubyana Burnat ex Gremli hydrolates: DI-SPME-GC-MS analysis and biological activity evaluation. Plant Fungal Res. 2022, 5, 2–10. [Google Scholar] [CrossRef]
| N° | COMPONENT 1 | LRI 2 | LRI 3 | EO |
|---|---|---|---|---|
| 1 | 1-hexanol | 861 | 858 | 0.2 ± 0.02 |
| 2 | ethylangelate | 918 | 920 | 0.1 ± 0.00 |
| 3 | α-pinene | 935 | 942 | 35.8 ± 2.11 |
| 4 | camphene | 950 | 954 | 1.0 ± 0.03 |
| 5 | β-myrcene | 985 | 991 | 6.2 ± 0.03 |
| 6 | limonene | 1020 | 1023 | 27.6 ± 1.14 |
| 7 | α-ocimene | 1038 | 1042 | 4.1 ± 0.04 |
| 8 | linalool | 1071 | 1089 | 3.2 ± 0.03 |
| 9 | fenchol | 1091 | 1098 | 2.0 ± 0.05 |
| 10 | trans-allocimene | 1128 | 1125 | 0.1 ± 0.01 |
| 11 | L-borneol | 1160 | 1152 | 0.7 ± 0.06 |
| 12 | terpinen-4-ol | 1175 | 1170 | 0.1 ± 0.00 |
| 13 | α-terpineol | 1185 | 1190 | 1.1 ± 0.04 |
| 14 | farnesane | 1386 | 1381 | 0.1 ± 0.01 |
| 15 | hexyl caproate | 1390 | 1386 | 0.1 ± 0.00 |
| 16 | trans-α-bergamotene | 1433 | 1430 | 0.2 ± 0.02 |
| 17 | β-caryophyllene | 1439 | 1435 | 9.6 ± 0.11 |
| 18 | α-santalene | 1448 | 1449 | 0.2 ± 0.02 |
| 19 | aromadendrene | 1455 | 1451 | 0.3 ± 0.04 |
| 20 | humulene | 1457 | 1454 | 1.9 ± 0.04 |
| 21 | α-farnesene | 1462 | 1461 | 0.3 ± 0.02 |
| 22 | β-bisabolene | 1498 | 1495 | 1.3 ± 0.04 |
| 23 | trans-α-bisabolene | 1549 | 1545 | 0.7 ± 0.02 |
| 24 | trans-nerolidol | 1551 | 1547 | 0.2 ± 0.02 |
| 25 | caryophyllene oxide | 1615 | 1613 | 0.4 ± 0.02 |
| 26 | guaiol | 1622 | 1625 | 0.8 ± 0.03 |
| 27 | γ-eudesmol | 1627 | 1630 | 0.6 ± 0.02 |
| 28 | β-eudesmol | 1652 | 1649 | 0.3 ± 0.02 |
| 29 | hexaydrofarnesylacetone | 1851 | 1846 | 0.2 ± 0.02 |
| 30 | m-camphorene | 1966 | 1960 | 0.4 ± 0.02 |
| 31 | p-camphorene | 1998 | 1994 | 0.1 ± 0.01 |
| SUM | 99.9 | |||
| Monoterpenes | 82.0 | |||
| Sesquiterpenes | 17.5 | |||
| Others | 0.4 |
| N° | COMPONENT 1 | RI 2 | Hy |
|---|---|---|---|
| 1 | 4-hydroxy-butanoic acid | 15.16 | 10.0 ± 0.12 |
| 2 | hexanoic acid | 16.18 | 3.4 ± 0.09 |
| 3 | linalool | 16.67 | 3.2 ± 0.09 |
| 4 | fenchol | 17.36 | 1.3 ± 0.07 |
| 5 | α-terpineol | 19.15 | 14.5 ± 0.13 |
| 6 | linalool oxide | 22.67 | 6.7 ± 0.10 |
| 7 | limonene-1,2-diol | 24.50 | 5.2 ± 0.09 |
| 8 | 8-hydroxylinalool | 24.87 | 4.2 ± 0.12 |
| 9 | sobrerol | 25.34 | 6.4 ± 0.09 |
| 10 | 3-buten-1,2-diol-1(2-furanyl)-2-methyl | 27.24 | 16.1 ± 0.08 |
| 11 | sobrerol acetate | 27.36 | 9.6 ± 0.08 |
| 12 | 2-methylisoborneol | 29.06 | 19.2 ± 0.11 |
| SUM | 99.8 | ||
| Monoterpenes | 60.7 | ||
| Sesquiterpenes | - | ||
| Others | 29.5 |
| N° | COMPONENT 1 | LRI 2 | LRI 3 | Hy |
|---|---|---|---|---|
| 1 | 1-hexanol | 852 | 858 | 2.8 ± 0.04 |
| 2 | 5-hpten-2-one | 860 | 866 | 0.2 ± 0.02 |
| 3 | ethyl dimethylacrylate | 918 | 924 | 0.6 ± 0.03 |
| 4 | α-pinene | 937 | 942 | 0.7 ± 0.02 |
| 5 | camphene | 951 | 954 | 0.2 ± 0.02 |
| 6 | β-myrcene | 987 | 991 | 2.6 ± 0.04 |
| 7 | 1,8-cineole | 1028 | 1031 | 3.0 ± 0.03 |
| 8 | linalool oxide | 1068 | 1073 | 0.3 ± 0.02 |
| 9 | fenchone | 1072 | 1080 | 2.2 ± 0.05 |
| 10 | linalool | 1091 | 1089 | 23.2 ± 0.15 |
| 11 | fenchol | 1097 | 1098 | 21.2 ± 0.18 |
| 12 | L-borneol | 1155 | 1152 | 6.8 ± 0.06 |
| 13 | terpinen-4-ol | 1167 | 1170 | 2.0 ± 0.02 |
| 14 | α-terpineol | 1193 | 1190 | 26.4 ± 0.21 |
| 15 | β-caryophyllene | 1430 | 1435 | 1.9 ± 0.03 |
| 16 | humulene | 1461 | 1454 | 0.4 ± 0.02 |
| 17 | β-bisabolene | 1499 | 1495 | 0.3 ± 0.02 |
| 18 | epi-γ-eudesmol | 1615 | 1610 | 0.6 ± 0.03 |
| 19 | humulene epoxide II | 1617 | 1611 | 1.2 ± 0.04 |
| 20 | guaiol | 1631 | 1625 | 1.5 ± 0.02 |
| 21 | γ-eudesmol | 1634 | 1630 | 1.3 ± 0.03 |
| 22 | β-eudesmol | 1648 | 1649 | 0.6 ± 0.02 |
| SUM | 100.0 | |||
| Monoterpenes | 88.6 | |||
| Sesquiterpenes | 7.8 | |||
| Others | 3.6 |
| Tumor Cell Line | IC50 EO (%) | IC50 Hy (%) |
|---|---|---|
| A375 | 0.065 ± 0.015 | 3.72 ± 1.10 |
| LOX IMVI | 0.081 ± 0.069 | 3.71 ± 0.67 |
| H1299 | 0.088 ± 0.017 | 3.43 ± 0.88 |
| A549 | 0.101 ± 0.089 | 4.311 ± 1.07 |
| HT29 | 0.017 ± 0.001 | 7.702 ± 2.57 |
| L3.6 | 0.017 ± 0.003 | 7.314 ± 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinciguerra, V.; Di Martile, M.; Mollica Graziano, M.; Del Bufalo, D.; Garzoli, S. LVI and DI-SPME Combined with GC/MS and GC/MS for Volatile Chemical Profile Investigation and Cytotoxic Power Evaluation of Essential Oil and Hydrolate from Cannabis sativa L. cv. Carmagnola. Molecules 2024, 29, 3299. https://doi.org/10.3390/molecules29143299
Vinciguerra V, Di Martile M, Mollica Graziano M, Del Bufalo D, Garzoli S. LVI and DI-SPME Combined with GC/MS and GC/MS for Volatile Chemical Profile Investigation and Cytotoxic Power Evaluation of Essential Oil and Hydrolate from Cannabis sativa L. cv. Carmagnola. Molecules. 2024; 29(14):3299. https://doi.org/10.3390/molecules29143299
Chicago/Turabian StyleVinciguerra, Vittorio, Marta Di Martile, Monica Mollica Graziano, Donatella Del Bufalo, and Stefania Garzoli. 2024. "LVI and DI-SPME Combined with GC/MS and GC/MS for Volatile Chemical Profile Investigation and Cytotoxic Power Evaluation of Essential Oil and Hydrolate from Cannabis sativa L. cv. Carmagnola" Molecules 29, no. 14: 3299. https://doi.org/10.3390/molecules29143299
APA StyleVinciguerra, V., Di Martile, M., Mollica Graziano, M., Del Bufalo, D., & Garzoli, S. (2024). LVI and DI-SPME Combined with GC/MS and GC/MS for Volatile Chemical Profile Investigation and Cytotoxic Power Evaluation of Essential Oil and Hydrolate from Cannabis sativa L. cv. Carmagnola. Molecules, 29(14), 3299. https://doi.org/10.3390/molecules29143299







