Label-Free Assessment of Neuronal Activity Using Raman Micro-Spectroscopy
Abstract
1. Introduction
2. Results
2.1. Evaluation of Individual Neuronal Activities Using the PRESS
2.2. Acquisition of Raman Spectra of the Autonomic Neuron Ganglia
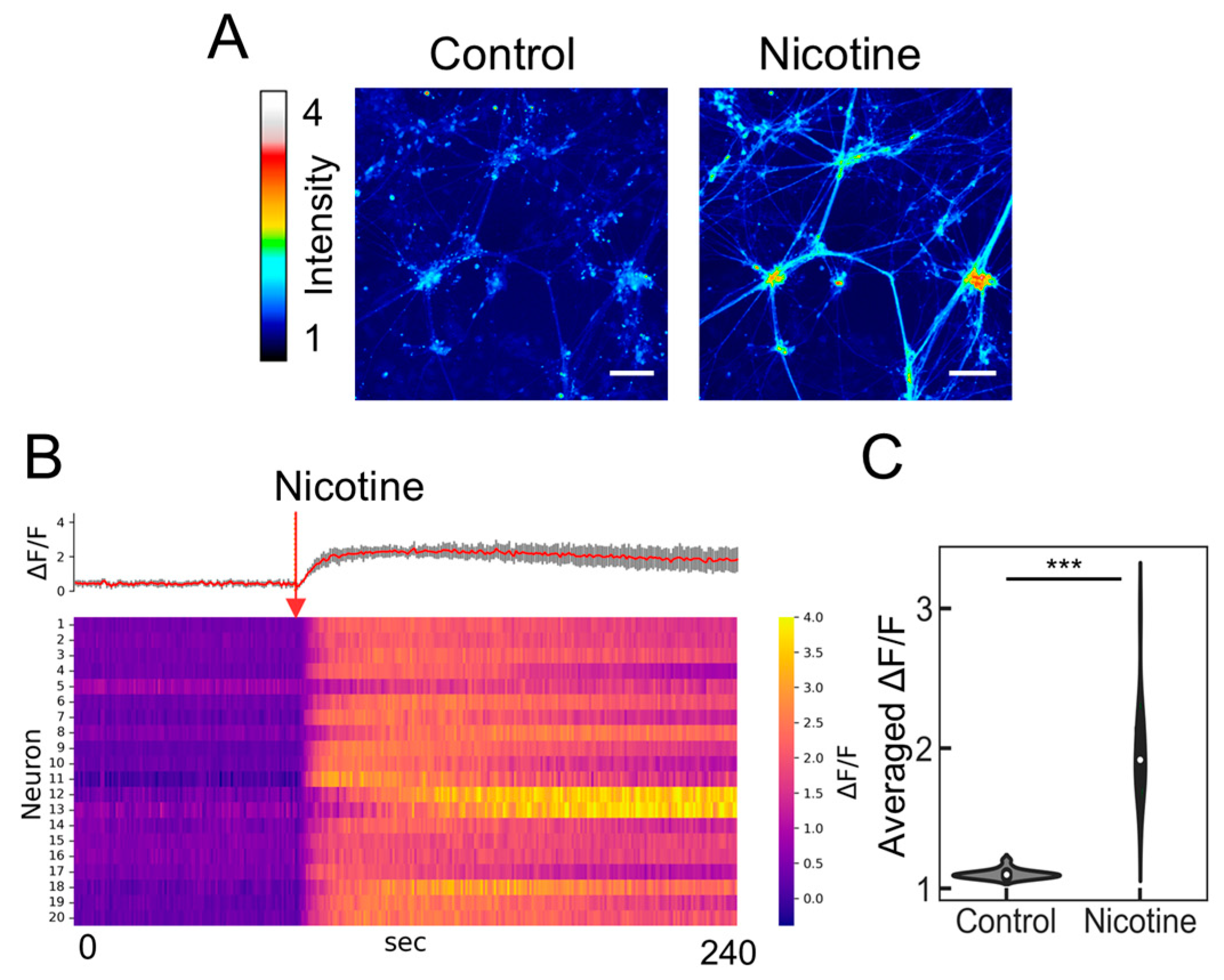
2.3. Evaluation of Nicotine Responsiveness Using Calcium Imaging
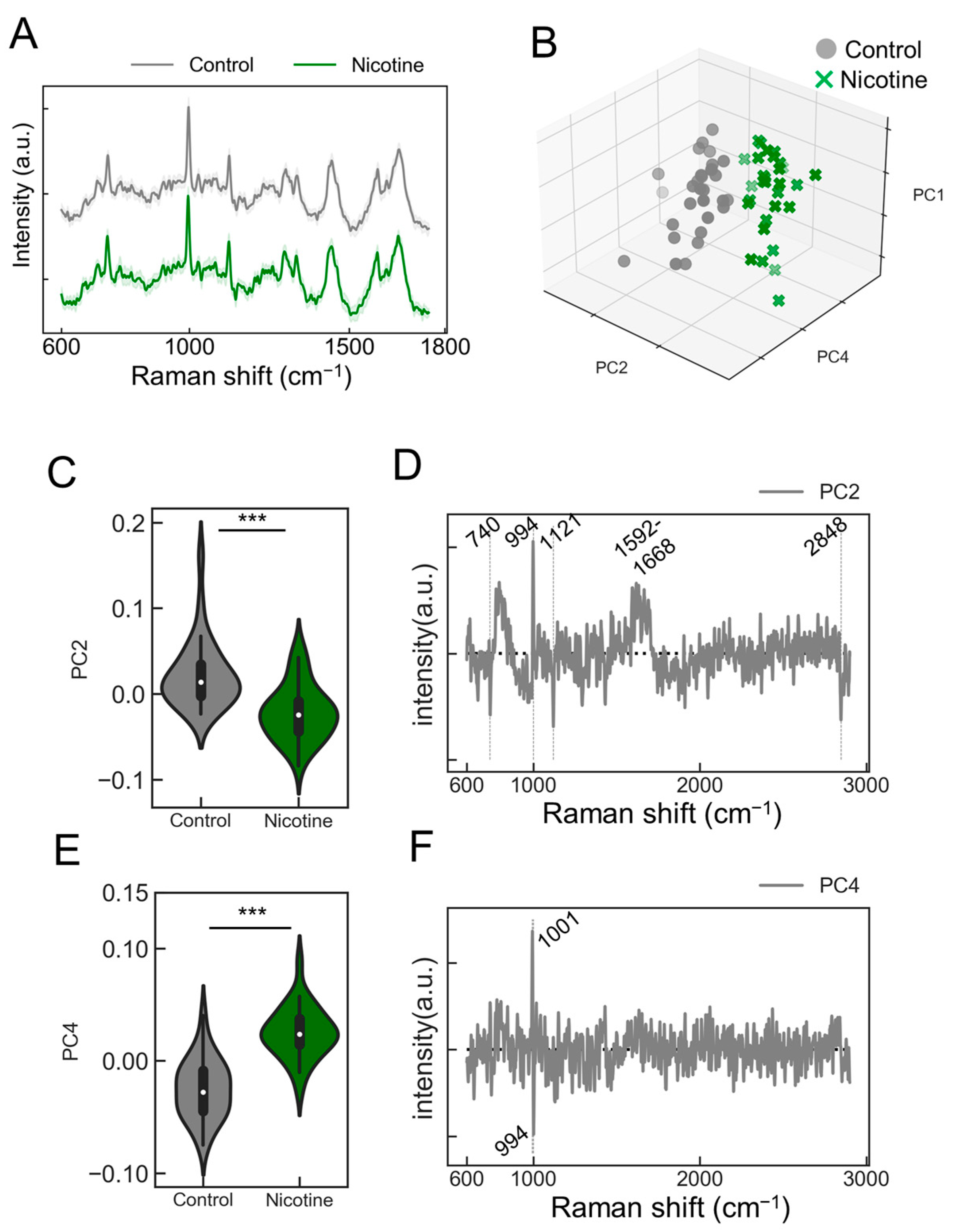
2.4. Assessment of Nicotine Responsiveness of ANs Utilizing the PRESS
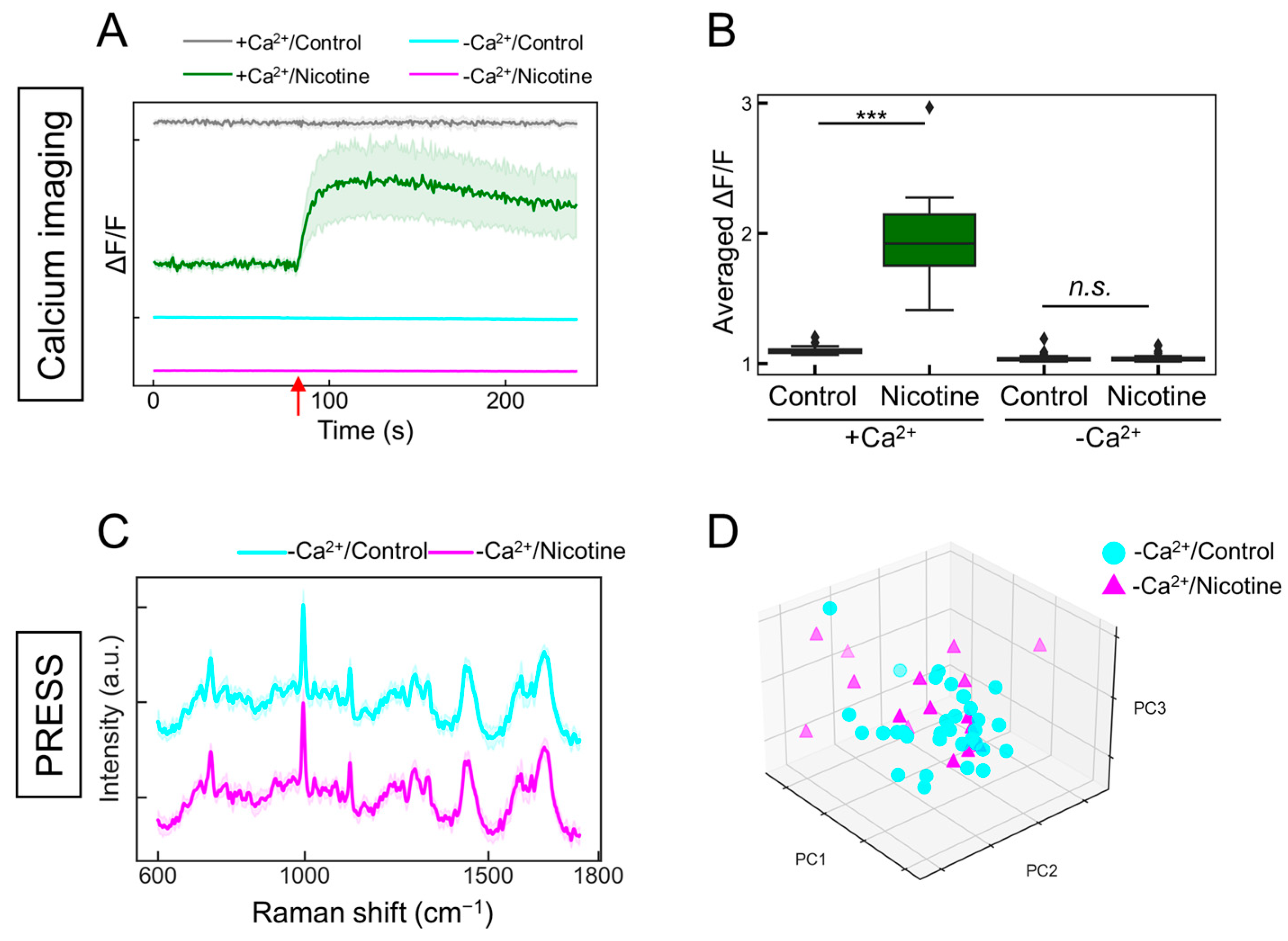
2.5. The Detection of Intracellular Calcium Ion Fluctuations
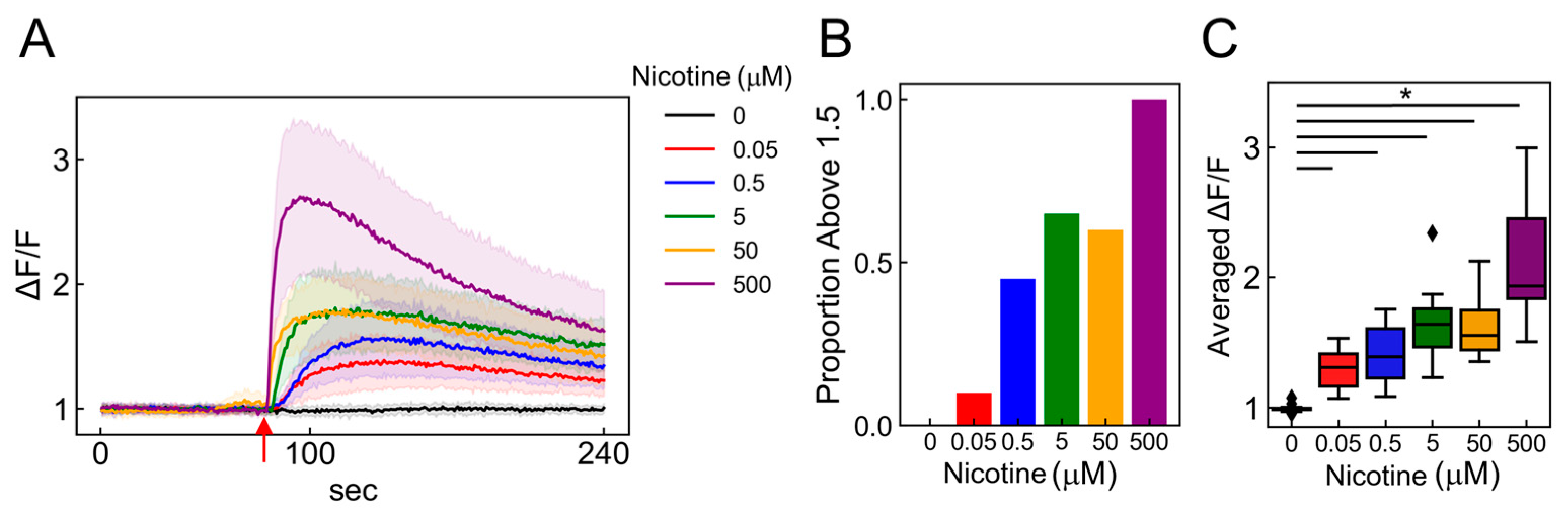
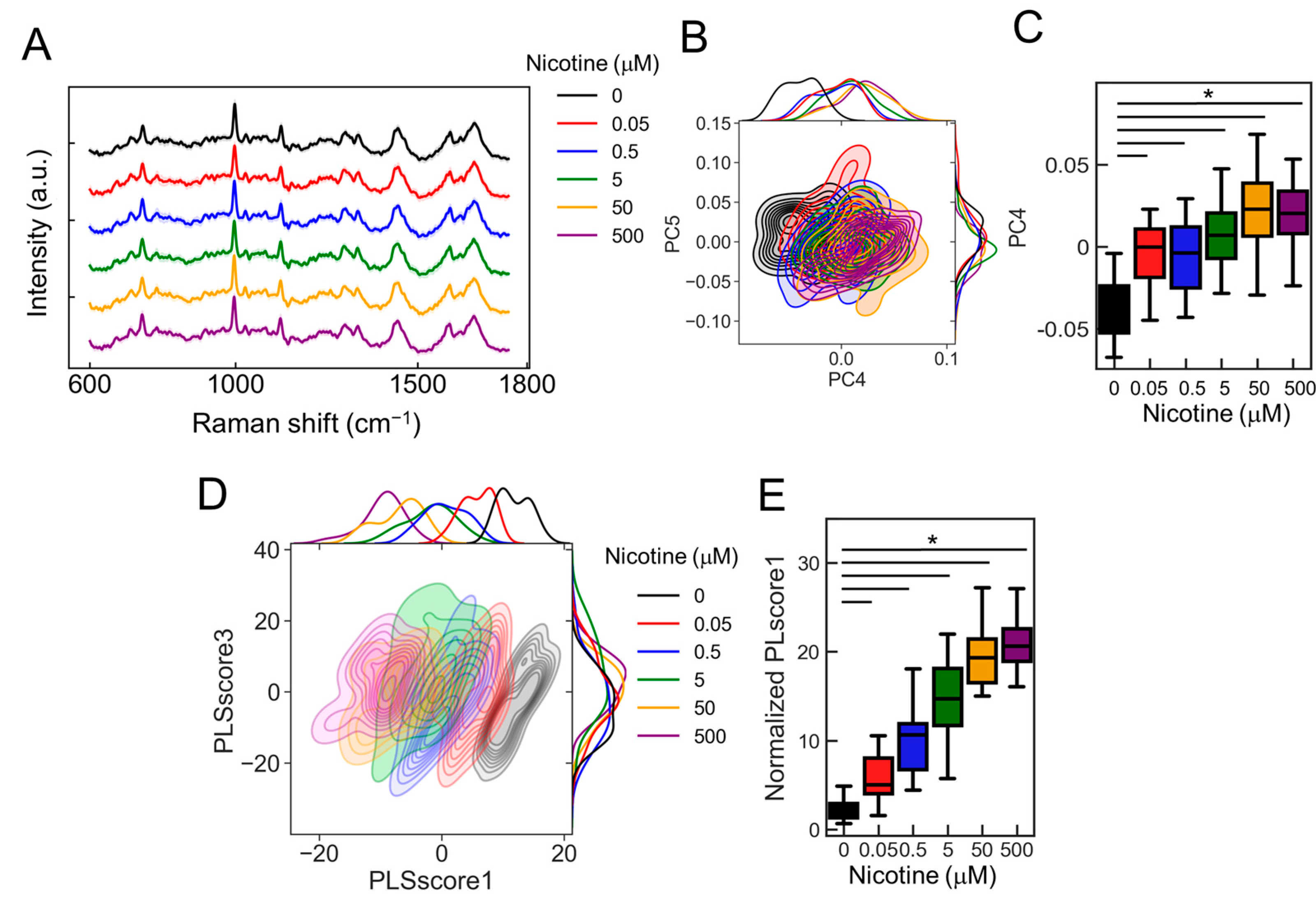
2.6. Dose-Dependent Neuronal Dynamics Detected by the PRESS
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Glutamatergic Neuron Induction
4.3. AN Induction
4.4. Calcium Imaging
4.5. The PRESS
4.6. Raman Data Acquisition
4.7. Raman Spectrum Analysis
4.8. Machine Learning Classification
4.9. Reverse Transcription (RT)-qPCR
4.10. Immunochemical Staining
4.11. Statistics and Reproducibility
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Puppels, G.J.; De Mul, F.F.M.; Otto, C.; Greve, J.; Robert-Nicoud, M.; Arndt-Jovin, D.J.; Jovin, T.M. Studying Single Living Cells and Chromosomes by Confocal Raman Microspectroscopy. Nature 1990, 347, 301–303. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, L.; Li, Q.; Qi, X.; Zhou, A. Microfluidic Chip for Non-Invasive Analysis of Tumor Cells Interaction with Anti-Cancer Drug Doxorubicin by AFM and Raman Spectroscopy. Biomicrofluidics 2018, 12, 024119. [Google Scholar] [CrossRef]
- Morita, S.I.; Takanezawa, S.; Hiroshima, M.; Mitsui, T.; Ozaki, Y.; Sako, Y. Raman and Autofluorescence Spectrum Dynamics along the HRG-Induced Differentiation Pathway of MCF-7 Cells. Biophys. J. 2014, 107, 2221–2229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, D.; Feng, S.; Pan, J.; Chen, Y.; Lin, J.; Chen, G.; Xie, S.; Zeng, H.; Chen, R. Colorectal Cancer Detection by Gold Nanoparticle Based Surface-Enhanced Raman Spectroscopy of Blood Serum and Statistical Analysis. Opt. Express 2011, 19, 13565. [Google Scholar] [CrossRef]
- Jermyn, M.; Mok, K.; Mercier, J.; Desroches, J.; Pichette, J.; Saint-Arnaud, K.; Bernstein, L.; Guiot, M.C.; Petrecca, K.; Leblond, F. Intraoperative Brain Cancer Detection with Raman Spectroscopy in Humans. Sci. Transl. Med. 2015, 7, 274ra19. [Google Scholar] [CrossRef]
- Cordero, E. In-Vivo Raman Spectroscopy: From Basics to Applications. J. Biomed. Opt. 2018, 23, 071210. [Google Scholar] [CrossRef] [PubMed]
- Brauchle, E.; Thude, S.; Brucker, S.Y.; Schenke-Layland, K. Cell Death Stages in Single Apoptotic and Necrotic Cells Monitored by Raman Microspectroscopy. Sci. Rep. 2014, 4, 4698. [Google Scholar] [CrossRef] [PubMed]
- Akagi, Y.; Mori, N.; Kawamura, T.; Takayama, Y.; Kida, Y.S. Non-Invasive Cell Classification Using the Paint Raman Express Spectroscopy System (PRESS). Sci. Rep. 2021, 11, 8818. [Google Scholar] [CrossRef]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly Efficient Neural Conversion of Human ES and iPS Cells by Dual Inhibition of SMAD Signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef]
- Telias, M.; Segal, M.; Ben-Yosef, D. Electrical Maturation of Neurons Derived from Human Embryonic Stem Cells. F1000Res 2014, 3, 196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takayama, Y.; Kushige, H.; Akagi, Y.; Suzuki, Y.; Kumagai, Y.; Kida, Y.S. Selective Induction of Human Autonomic Neurons Enables Precise Control of Cardiomyocyte Beating. Sci. Rep. 2020, 10, 9464. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Akagi, Y.; Kida, Y.S. Deciphering the Molecular Mechanisms of Autonomic Nervous System Neuron Induction through Integrative Bioinformatics Analysis. Int. J. Mol. Sci. 2023, 24, 9053. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Binoy, J.; Abraham, J.P.; Joe, I.H.; Jayakumar, V.S.; Pettit, G.R.; Nielsen, O.F. NIR-FT Raman and FT-IR Spectral Studies and Ab Initio Calculations of the Anti-cancer Drug combretastatin-A4. J. Raman Spectrosc. 2004, 35, 939–946. [Google Scholar] [CrossRef]
- Malini, R.; Venkatakrishna, K.; Kurien, J.; Pai, K.M.; Rao, L.; Kartha, V.B.; Krishna, C.M. Discrimination of Normal, Inflammatory, Premalignant, and Malignant Oral Tissue: A Raman Spectroscopy Study. Biopolymers 2006, 81, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Mayo, D.W.; Miller, F.A.; Hannah, R. Course Notes on the Interpretation of Infrared and Raman Spectra; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; ISBN 978-0-471-24823-1. [Google Scholar]
- Fischer, W.B.; Eysel, H.H. Polarized Raman Spectra and Intensities of Aromatic Amino Acids Phenylalanine, Tyrosine and Tryptophan. Spectrochim. Acta Part A Mol. Spectrosc. 1992, 48, 725–732. [Google Scholar] [CrossRef]
- Chan, J.W.; Taylor, D.S.; Zwerdling, T.; Lane, S.M.; Ihara, K.; Huser, T. Micro-Raman Spectroscopy Detects Individual Neoplastic and Normal Hematopoietic Cells. Biophys. J. 2006, 90, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Shetty, G.; Kendall, C.; Shepherd, N.; Stone, N.; Barr, H. Raman Spectroscopy: Elucidation of Biochemical Changes in Carcinogenesis of Oesophagus. Br. J. Cancer 2006, 94, 1460–1464. [Google Scholar] [CrossRef]
- Silveira, L.; Sathaiah, S.; Zngaro, R.A.; Pacheco, M.T.T.; Chavantes, M.C.; Pasqualucci, C.A.G. Correlation between Near-Infrared Raman Spectroscopy and the Histopathological Analysis of Atherosclerosis in Human Coronary Arteries. Lasers Surg. Med. 2002, 30, 290–297. [Google Scholar] [CrossRef]
- Lakshmi, R.J.; Kartha, V.B.; Krishna, C.M.; Solomon, J.G.R.; Ullas, G.; Devi, P.U. Tissue Raman Spectroscopy for the Study of Radiation Damage: Brain Irradiation of Mice. Radiat. Res. 2002, 157, 175–182. [Google Scholar] [CrossRef]
- Notingher, I.; Green, C.; Dyer, C.; Perkins, E.; Hopkins, N.; Lindsay, C.; Hench, L.L. Discrimination between Ricin and Sulphur Mustard Toxicity in Vitro Using Raman Spectroscopy. J. R. Soc. Interface 2004, 1, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Dukor, R.K. Vibrational Spectroscopy in the Detection of Cancer. In Handbook of Vibrational Spectroscopy; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; pp. 3335–3359. [Google Scholar]
- Cheng, W.T.; Liu, M.T.; Liu, H.N.; Lin, S.Y. Micro-Raman Spectroscopy Used to Identify and Grade Human Skin Pilomatrixoma. Microsc. Res. Tech. 2005, 68, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Faoláin, E.Ó.; Hunter, M.B.; Byrne, J.M.; Kelehan, P.; McNamara, M.; Byrne, H.J.; Lyng, F.M. A Study Examining the Effects of Tissue Processing on Human Tissue Sections Using Vibrational Spectroscopy. Vib. Spectrosc. 2005, 38, 121–127. [Google Scholar] [CrossRef]
- Katainen, E.; Elomaa, M.; Laakkonen, U.; Sippola, E.; Niemelä, P.; Suhonen, J.; Järvinen, K. Quantification of the Amphetamine Content in Seized Street Samples by Raman Spectroscopy. J. Forensic Sci. 2007, 52, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Perevedentseva, E.; Krivokharchenko, A.; Karmenyan, A.V.; Chang, H.H.; Cheng, C.L. Raman Spectroscopy on Live Mouse Early Embryo While It Continues to Develop into Blastocyst in Vitro. Sci. Rep. 2019, 9, 6636. [Google Scholar] [CrossRef] [PubMed]
- Naumann, D. Infrared and NIR Raman Spectroscopy in Medical Microbiology. Proc. SPIE 1998, 3257, 245–257. [Google Scholar] [CrossRef]
- Koljenović, S.; Schut, T.B.; Vincent, A.; Kros, J.M.; Puppels, G.J. Detection of Meningioma in Dura Mater by Raman Spectroscopy. Anal. Chem. 2005, 77, 7958–7965. [Google Scholar] [CrossRef]
- Skok, V.I. Mini Review Nicotinic Acetylcholine Receptors in Autonomic Ganglia. Auton Neurosci. 2002, 97, 1–11. [Google Scholar] [CrossRef]
- Akagi, Y.; Takayama, Y.; Nihashi, Y.; Yamashita, A.; Yoshida, R.; Miyamoto, Y.; Kida, Y.S. Functional Engineering of Human iPSC-Derived Parasympathetic Neurons Enhances Responsiveness to Gastrointestinal Hormones. FEBS Open Bio 2024, 14, 63–78. [Google Scholar] [CrossRef]
- Kubasek, W.L.; Hudson, B.; Peticolas, W.L. Ultraviolet Resonance Raman Excitation Profiles of Nucleic Acid Bases with Excitation from 200 to 300 Nanometers. Proc. Natl. Acad. Sci. USA 1985, 82, 2369–2373. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; McWilliams, A.; Lui, H.; McLean, D.I.; Lam, S.; Zeng, H. Near-Infrared Raman Spectroscopy for Optical Diagnosis of Lung Cancer. Int. J. Cancer 2003, 107, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, S.; Szostak, R. Quantitative Determination of Captopril and Prednisolone in Tablets by FT-Raman Spectroscopy. J. Pharm. Biomed. Anal. 2006, 40, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.X.; Yakel, J.L. Nicotinic Acetylcholine Receptor-Mediated Calcium Signaling in the Nervous System. Acta Pharmacol. Sin. 2009, 30, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Xu, J.; Brinkhof, B.; Wang, H.; Cui, Z.; Huang, W.E.; Ye, H. A Single-Cell Raman-Based Platform to Identify Developmental Stages of Human Pluripotent Stem Cell-Derived Neurons. Proc. Natl. Acad. Sci. USA 2020, 117, 18412–18423. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.K.; Conroy, W.G. Nicotinic A7 Receptors: Synaptic Options and Downstream Signaling in Neurons. J. Neurobiol. 2002, 53, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Dunckley, T.; Lukas, R.J. Nicotine Modulates the Expression of a Diverse Set of Genes in the Neuronal SH-SY5Y Cell Line. J. Biol. Chem. 2003, 278, 15633–15640. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.E.; Ziff, E.B.; Greene, L.A. Stimulation of Neuronal Acetylcholine Receptors Induces Rapid Gene Transcription. Science 1986, 234, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi-Kirschvink, K.J.; Nakaoka, H.; Oda, A.; Kenichiro, F.K.; Nosho, K.; Fukushima, H.; Kanesaki, Y.; Yajima, S.; Masaki, H.; Ohta, K.; et al. Linear Regression Links Transcriptomic Data and Cellular Raman Spectra. Cell Syst. 2018, 7, 104–117.e4. [Google Scholar] [CrossRef]
- Kobayashi-Kirschvink, K.J.; Comiter, C.S.; Gaddam, S.; Joren, T.; Grody, E.I.; Ounadjela, J.R.; Zhang, K.; Ge, B.; Kang, J.W.; Xavier, R.J.; et al. Prediction of Single-Cell RNA Expression Profiles in Live Cells by Raman Microscopy with Raman2RNA. Nat. Biotechnol. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Chung, C.Y.; Potma, E.O. Biomolecular Imaging with Coherent Nonlinear Vibrational Microscopy. Annu. Rev. Phys. Chem. 2013, 64, 77–99. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman Spectra of Pyridine Adsorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Kneipp, K. Surface-Enhanced Raman Scattering. Phys. Today 2007, 60, 40–46. [Google Scholar] [CrossRef]
- Feng, S.; Chen, R.; Lin, J.; Pan, J.; Chen, G.; Li, Y.; Cheng, M.; Huang, Z.; Chen, J.; Zeng Haishan, H. Nasopharyngeal Cancer Detection Based on Blood Plasma Surface-Enhanced Raman Spectroscopy and Multivariate Analysis. Biosens. Bioelectron. 2010, 25, 2414–2419. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Kida, Y.S. In Vitro Reconstruction of Neuronal Networks Derived from Human iPS Cells Using Microfabricated Devices. PLoS ONE 2016, 11, e0148559. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- E1840-96; Standard Guide for Raman Shift Standards for Spectrometer Calibration. ASTM International: West Conshohocken, PN, USA, 2014. [CrossRef]
- Brézillon, S.; Untereiner, V.; Mohamed, H.T.; Hodin, J.; Chatron-Colliet, A.; Maquart, F.X.; Sockalingum, G.D. Probing Glycosaminoglycan Spectral Signatures in Live Cells and Their Conditioned Media by Raman Microspectroscopy. Analyst 2017, 142, 1333–1341. [Google Scholar] [CrossRef]
- Stone, N.; Kendall, C.; Shepherd, N.; Crow, P.; Barr, H. Near-Infrared Raman Spectroscopy for the Classification of Epithelial Pre-Cancers and Cancers. J. Raman Spectrosc. 2002, 33, 564–573. [Google Scholar] [CrossRef]
- Krafft, C.; Neudert, L.; Simat, T.; Salzer, R. Near Infrared Raman Spectra of Human Brain Lipids. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Gniadecka, M.; Wulf, H.C.; Mortensen, N.N.; Nielsen, O.F.; Christensen, D.H. Diagnosis of Basal Cell Carcinoma by Raman Spectroscopy. J. Raman Spectrosc. 1997, 28, 125–129. [Google Scholar] [CrossRef]


| Peak (cm−1) | Assignment | Reference |
|---|---|---|
| 748 | DNA | [15] |
| 1000 | Phenylalanine Bound and free of nicotinamide adenine dinucleotide hydrogen (NADH) Breathing mode in benzene ring | [16,17,18] |
| 1126 | C-C stretching in lipid acyl backbone C-N stretching in proteins | [19,20] |
| 1301 | Triglycerides (fatty acids) C-H stretching in lipids CH2 twisting in lipids | [21,22,23] |
| 1337 | Amide III CH2 wagging from the glycine backbone and proline side chain A, G ring breathing in DNA bases C-H bending in proteins | [19,24,25] |
| 1447 | CH2 bending in proteins and lipids | [19,26] |
| 1585 | C=C stretching in proteins cytochrome c | [26,27,28] |
| 1660 | Amide I stretching in structural proteins C=C stretching in cis lipids and fatty acids | [20,21,23,29] |
| 2930 | CH2 asymmetric stretching | [30] |
| Peak (cm−1) | Assignment | Reference |
|---|---|---|
| 740 | Nucleotide conformation | [19] |
| 994 | O-P-O symmetric stretching | [33] |
| 1001 | Phenylalanine Bound and free NADH Breathing mode in the benzene ring | [16,17,18] |
| 1121 | C-O stretching in ribose | [24] |
| 1592–1668 | C=C stretching Tryptophan Protein (Amid I) Bound and free NADH | [16,34] |
| 2848 | CH2 and CH3 symmetric stretching in lipids | [17,30,35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akagi, Y.; Norimoto, A.; Kawamura, T.; Kida, Y.S. Label-Free Assessment of Neuronal Activity Using Raman Micro-Spectroscopy. Molecules 2024, 29, 3174. https://doi.org/10.3390/molecules29133174
Akagi Y, Norimoto A, Kawamura T, Kida YS. Label-Free Assessment of Neuronal Activity Using Raman Micro-Spectroscopy. Molecules. 2024; 29(13):3174. https://doi.org/10.3390/molecules29133174
Chicago/Turabian StyleAkagi, Yuka, Aya Norimoto, Teruhisa Kawamura, and Yasuyuki S. Kida. 2024. "Label-Free Assessment of Neuronal Activity Using Raman Micro-Spectroscopy" Molecules 29, no. 13: 3174. https://doi.org/10.3390/molecules29133174
APA StyleAkagi, Y., Norimoto, A., Kawamura, T., & Kida, Y. S. (2024). Label-Free Assessment of Neuronal Activity Using Raman Micro-Spectroscopy. Molecules, 29(13), 3174. https://doi.org/10.3390/molecules29133174





