Recent Advances in the Determination of Major and Trace Elements in Plants Using Inductively Coupled Plasma Optical Emission Spectrometry
Abstract
1. Introduction
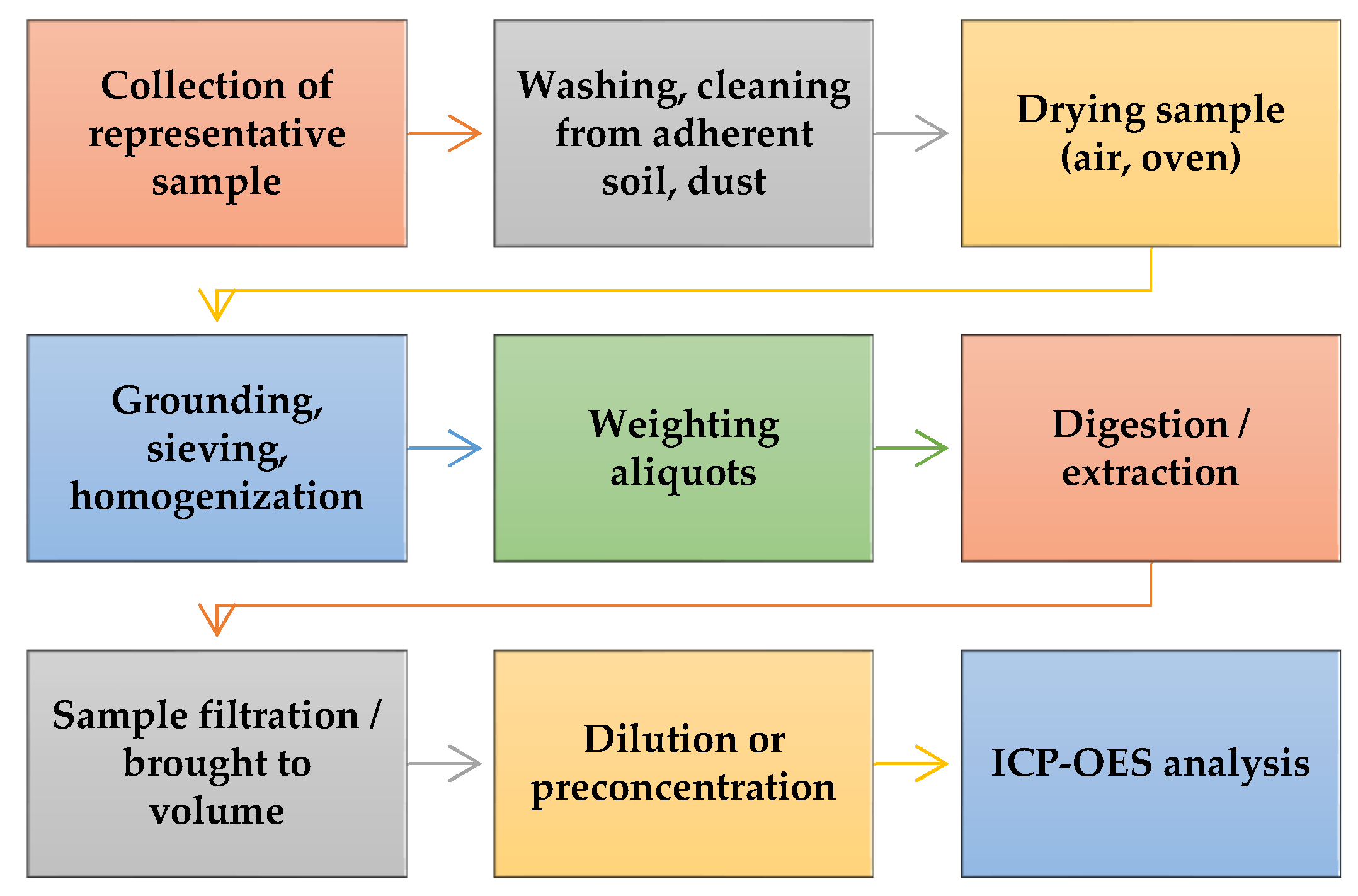
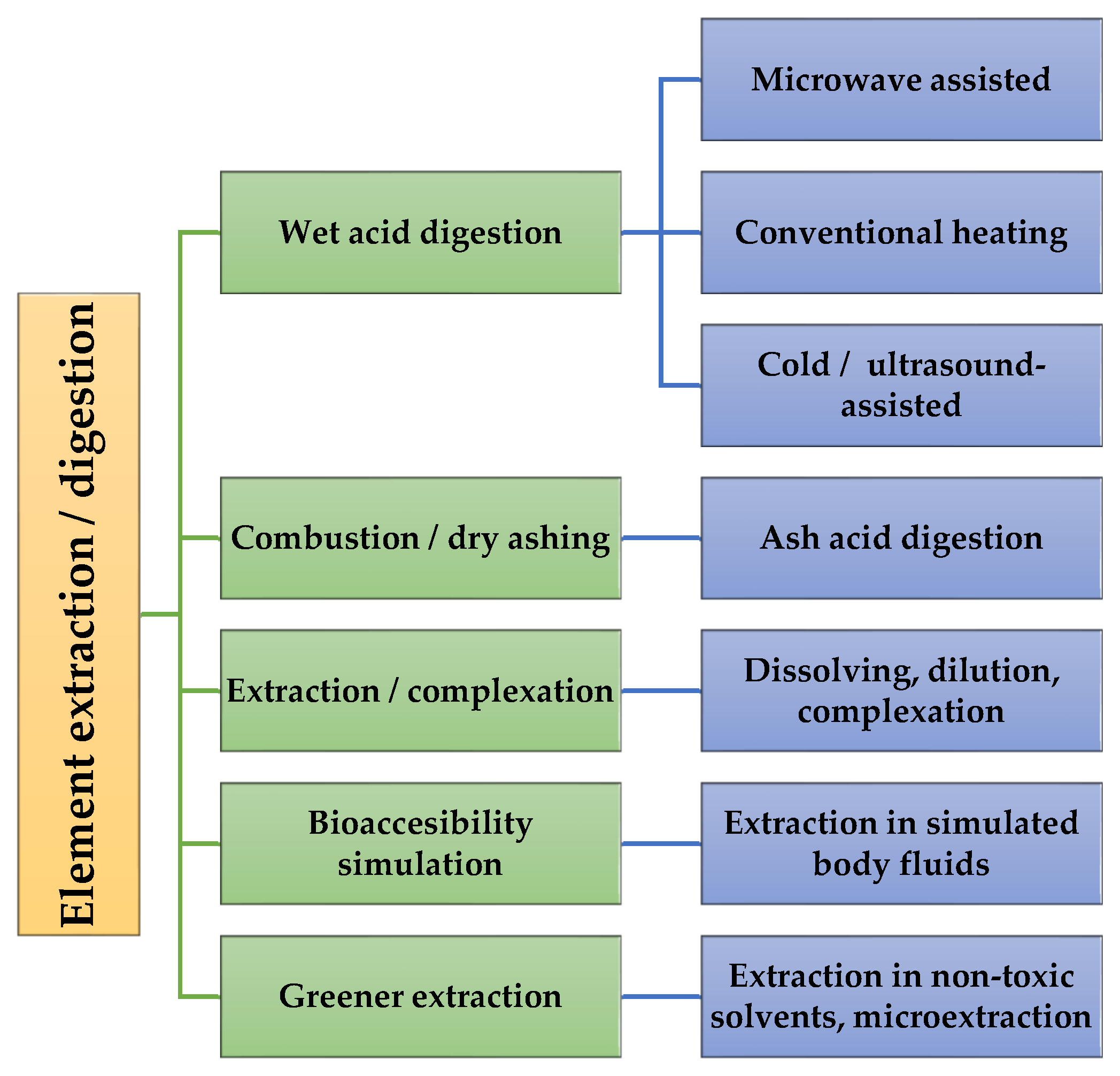
2. Plants Samples Preparation for ICP-OES Analysis
2.1. Wet Acid Digestion of Plant Samples for Metals Determination
2.2. Combustion and Acid Digestion
2.3. Dissolving, Complexing, and Green Extraction Methods
2.4. Extraction for Bioaccesibility Studies on Plant Samples
3. Advantages, Limitations and Advances in Plasma Viewing, Sample Introduction Systems and Miniaturization of Optical Emission Spectrometry Instrumentation
4. Method Validation and Performance Parameters for ICP-OES Used in Plant Sample Analysis
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Welna, M.; Szymczycha-Madeja, A.; Pohl, P. Novel ICP-OES-based method for the reliable determination of the total content of 15 elements in yerba mate drinks along with the determination of caffeine and the in vitro bioaccessibility of the compounds. Molecules 2023, 28, 3374. [Google Scholar] [CrossRef] [PubMed]
- Senila, M.; Cadar, O.; Senila, L.; Angyus, B.S. Simulated bioavailability of heavy metals (Cd, Cr, Cu, Pb, Zn) in contaminated soil amended with natural zeolite using diffusive gradients in thin-films (DGT) technique. Agriculture 2022, 12, 321. [Google Scholar] [CrossRef]
- Sarkar, M.I.U.; Islam, S.; Hosain, M.T.; Naidu, R.; Rahman, M.M. Distribution of essential and non-essential elements in rice-based products sold in Australian markets: Exposure assessment. J. Food Compos. Anal. 2023, 120, 105339. [Google Scholar] [CrossRef]
- Frentiu, T.; Ponta, M.; Senila, M.; Mihaltan, A.I.; Darvasi, E.; Frentiu, M.; Cordos, E. Evaluation of figures of merit for Zn determination in environmental and biological samples using EDL excited AFS in a new radiofrequency capacitively coupled plasma. J. Anal. At. Spectrom. 2010, 25, 739–742. [Google Scholar] [CrossRef]
- Senila, M.; Levei, E.; Miclean, M.; Senila, L.; Stefanescu, L.; Mărginean, S.; Ozunu, A.; Roman, C. Influence of pollution level on heavy metals mobility in soil from NW Romania. Environ. Eng. Manag. J. 2011, 10, 59–64. [Google Scholar] [CrossRef]
- Biolé, F.G.; Llamazares Vegh, S.; Bavio, M.; Tripodi, P.; Volpedo, A.V.; Thompson, G. Essential and non-essential elements in marine silverside Odontesthes argentinensis from southwestern Atlantic coast: Tissues bioaccumulation, biomagnification and public health risk assessment. Food Chem. Toxicol. 2024, 185, 114452. [Google Scholar] [CrossRef] [PubMed]
- Zeiner, M.; Šoltic, M.; Nemet, I.; Juranovic Cindric, I. Multielement determination in turmeric (Curcuma longa L.) using different digestion methods. Molecules 2022, 27, 8392. [Google Scholar] [CrossRef] [PubMed]
- Senila, M.; Cadar, O.; Senila, L.; Hoaghia, A.; Miu, I. Mercury determination in natural zeolites by thermal decomposition atomic absorption spectrometry: Method validation in compliance with requirements for use as dietary supplements. Molecules 2019, 24, 4023. [Google Scholar] [CrossRef]
- Hlodák, M.; Matúš, P.; Urík, M.; Korenková, L.; Mikusová, P.; Senila, M.; Diviš, P. Evaluation of various inorganic and biological extraction techniques suitability for soil mercury phytoavailable fraction assessment. Water Air Soil Pollut. 2015, 226, 198. [Google Scholar] [CrossRef]
- Douvris, C.; Vaughan, T.; Bussan, D.; Bartzas, G.; Thomas, R. How ICP-OES changed the face of trace element analysis: Review of the global application landscape. Sci. Total Environ. 2023, 905, 167242. [Google Scholar] [CrossRef] [PubMed]
- Gajek, M.; Wysocki, P.; Pawlaczyk, A.; Sac, Ł.; Szynkowska-Józwik, M.I. The elemental profile of beer available on Polish market: Analysis of the potential impact of type of packaging material and risk assessment of consumption. Molecules 2022, 27, 2962. [Google Scholar] [CrossRef] [PubMed]
- Leśniewicz, A.; Kurowska, D.; Pohl, P. Mineral constituents profiling of ready-to-drink nutritional supplements by Inductively Coupled Plasma Optical Emission Spectrometry. Molecules 2020, 25, 851. [Google Scholar] [CrossRef] [PubMed]
- Senila, M.; Resz, M.A.; Torok, I.; Senila, L. Nutritional composition and health risk of toxic metals of some edible wild mushrooms growing in a mining area of Apuseni Mountains, Western Carpathians. J. Food Compos. Anal. 2024, 128, 106061. [Google Scholar] [CrossRef]
- Petrean, I.A.; Micle, V.; Sur, I.M.; Șenilă, M. Characterization of sterile mining dumps by the ICP-OES analytical method: A case study from Baia Mare mining area (Maramures, Romania). Sustainability 2023, 15, 1158. [Google Scholar] [CrossRef]
- Senila, M. Real and simulated bioavailability of lead in contaminated and uncontaminated soils. J. Environ. Health Sci. Eng. 2014, 12, 108. [Google Scholar] [CrossRef]
- Anastas, P.T. Green Chemistry and the role of analytical methodology development. Crit. Rev. Anal. Chem. 1999, 29, 167–175. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An approach to reconcile the principles of Green Analytical Chemistry and functionality. Trends Anal. Chem. 2021, 138, 116223. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namiesnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Senila, M. Metal and metalloid monitoring in water by passive sampling—A review. Rev. Anal. Chem. 2023, 42, 20230065. [Google Scholar] [CrossRef]
- Roma, A.; Abete, M.C.; Brizio, P.; Picazio, G.; Caiazzo, M.; D’auria, J.L.; Esposito, M. Evaluation of trace elements in potatoes (Solanum tuberosum) from a suburban area of Naples, Italy: The “Triangle of Death”. J Food Prot. 2017, 80, 1167–1171. [Google Scholar] [CrossRef]
- Can, H.; Ozyigit, I.I.; Can, M.; Hocaoglu-Ozyigit, A.; Yalcin, I.E. Multidimensional scaling of the mineral nutrient status and health risk assessment of commonly consumed fruity vegetables marketed in Kyrgyzstan. Biol. Trace Elem. Res. 2022, 200, 1902–1916. [Google Scholar] [CrossRef] [PubMed]
- Baumgärtel, C.; Götzke, L.; Weigand, J.J.; Christoph, N.; Panzo, M.H.G.; Afonso, F.; Lautenschläger, T. Beneficial or hazardous? A comprehensive study of 24 elements from wild edible plants from Angola. J. Appl. Bot. Food Qual. 2023, 96, 30–40. [Google Scholar] [CrossRef]
- Bashir, S.; Hussain, Q.; Shaaban, M.; Hu, H. Efficiency and surface characterization of different plant derived biochar for cadmium (Cd) mobility, bioaccessibility and bioavailability to Chinese cabbage in highly contaminated soil. Chemosphere 2018, 211, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, Y.; Zeng, C.; Zhong, Q.; Xiao, Z.; Mao, X.; Cao, F. Selenium accumulation in plant foods and selenium intake of residents in a moderately selenium-enriched area of Mingyueshan, Yichun, China. J. Food Compos. Anal. 2023, 116, 105089. [Google Scholar] [CrossRef]
- Butorova, L.; Polovka, M.; Porizka, J.; Vitova, E. Multi-experimental characterization of selected medical plants growing in the Czech Republic. Chem. Pap. 2017, 71, 1605–1621. [Google Scholar] [CrossRef]
- Ghasemidehkordi, B.; Malekirad, A.A.; Nazem, H.; Fazilati, M.; Salavati, H.; Shariatifar, N.; Rezaei, M.; Fakhri, Y.; Khaneghah, A.M. Concentration of lead and mercury in collected vegetables and herbs from Markazi province, Iran: A non-carcinogenic risk assessment. Food Chem. Toxicol. 2018, 113, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Mahlangeni, N.T.; Moodley, R.; Jonnalagadda, S.B. Uptake, translocation, and bioaccumulation of elements in forest nettle (Laportea alatipes). Anal. Lett. 2019, 52, 1050–1067. [Google Scholar] [CrossRef]
- Houdegbe, A.C.; Achigan-Dako, E.G.; Sogbohossou, E.O.D.; Schranz, M.E.; Odindo, A.O.; Sibiya, J. Leaf elemental composition analysis in spider plant [Gynandropsis gynandra L. (Briq.)] differentiates three nutritional groups. Front. Plant Sci. 2022, 13, 841226. [Google Scholar] [CrossRef] [PubMed]
- Bankaji, I.; Kouki, R.; Dridi, N.; Ferreira, R.; Hidouri, S.; Duarte, B.; Sleimi, N.; Caçador, I. Comparison of digestion methods using atomic absorption spectrometry for the determination of metal levels in plants. Separations 2023, 10, 40. [Google Scholar] [CrossRef]
- Kohrman, H.; Chamberlain, C.P. Heavy metals in produce from urban farms in the San Francisco Bay area. Food Addit. Contam. B 2014, 7, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Norton, G.J.; Deacon, C.M.; Mestrot, A.; Feldmann, J.; Jenkins, P.; Baskaran, C.; Meharg, A.A. Cadmium and lead in vegetable and fruit produce selected from specific regional areas of the UK. Sci. Total Environ. 2015, 533, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Bharti, V.K.; Kalia, S.; Acharya, S.; Kumar, B.; Chaurasia, O.P. Health risk assessment of heavy metals due to wheat, cabbage, and spinach consumption at cold-arid high altitude region. Biol. Trace Elem. Res. 2022, 200, 4186–4198. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Kormoker, T.; Mazumder, M.; Anika, S.E.; Islam, M.T.; Hemy, D.H.; Mimi, U.S.; Proshad, R.; Kabir, M.H.; Idris, M.A. Trace elements concentration in soil and plant within the vicinity of abandoned tanning sites in Bangladesh: An integrated chemometric approach for health risk assessment. Toxin Rev. 2022, 41, 752–767. [Google Scholar] [CrossRef]
- Barin, J.S.; Mello, P.A.; Mesko, M.F.; Duarte, F.A.; Flores, E.M. Determination of elemental impurities in pharmaceutical products and related matrices by ICP-based methods: A review. Anal. Bioanal. Chem. 2016, 408, 4547–4566. [Google Scholar] [CrossRef] [PubMed]
- Kritsananuwat, R.; Sahoo, S.K.; Arae, H.; Fukushi, M. Distribution of 238U and 232Th in selected soil and plant samples as well as soil to plant transfer factors around Southern Thailand. J. Radioanal. Nucl. Chem. 2015, 303, 2571–2577. [Google Scholar] [CrossRef]
- Alhogbi, B.G.; Al-Ansari, S.A.; El-Shahawi, M.S. A comparative study on the bioavailability and soil-to-plant transfer factors of potentially toxic element contamination in agricultural soils and their impacts: A case study of dense farmland in the western region of Saudi Arabia. Processes 2023, 11, 2515. [Google Scholar] [CrossRef]
- Anschau, K.F.; Enders, M.S.P.; Senger, C.M.; Duarte, F.A.; Dressler, V.L.; Muller, E.I. A novel strategy for medical foods digestion and subsequent elemental determination using inductively coupled plasma optical emission spectrometry. Microchem. J. 2019, 147, 1055–1060. [Google Scholar] [CrossRef]
- Bakkali, K.; Martos, N.R.; Souhail, B.; Ballesteros, E. Determination of heavy metal content in vegetables and oils from Spain and Morocco by inductively coupled plasma mass spectrometry. Anal. Lett. 2012, 45, 907–919. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Ullah, Z.; Asi, M.R.; Jinap, S.; Ahmad, M.N.; Sultan, M.T.; Malik, N. Heavy metals in selected vegetables from markets of Faisalabad, Pakistan. J. Food Prot. 2018, 81, 806–809. [Google Scholar] [CrossRef]
- Izquierdo-Díaz, M.; Holm, P.E.; Barrio-Parra, F.; De Miguel, E.; Lekfeldt, J.D.S.; Magid, J. Urban allotment gardens for the biomonitoring of atmospheric trace element pollution. J. Environ. Qual. 2019, 48, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Kohzadi, S.; Shahmoradi, B.; Ghaderi, E.; Loqmani, H.; Maleki, A. Concentration, source, and potential human health risk of heavy metals in the commonly consumed medicinal plants. Biol. Trace Elem. Res. 2019, 187, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Shen, Y.; Wang, X.; Chu, B.; Xu, T.; Liu, Y.; Zeng, Y.; Liu, J. Phytoavailability, bioaccumulation, and human health risks of metal(loid) elements in an agroecosystem near a lead-zinc mine. Environ. Sci. Pollut. Res. Int. 2018, 25, 24111–24124. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Tissot, F.; Faccio, R.; Ibáñez, F.; Pistón, M. A simple and economical ultrasound-assisted method for Cd and Pb extraction from fruits and vegetables for food safety assurance. Results Chem. 2021, 3, 100089. [Google Scholar] [CrossRef]
- Popović-Djordjević, J.B.; Kostić, A.Ž.; Rajković, M.B.; Miljković, I.; Krstić, Đ.; Caruso, G.; Siavash Moghaddam, S.; Brčeski, I. Organically vs. conventionally grown vegetables: Multi-elemental analysis and nutritional evaluation. Biol. Trace. Elem. Res. 2022, 200, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Ruchuwararak, P.; Intamat, S.; Tengjaroenkul, B.; Neeratanaphan, L. Bioaccumulation of heavy metals in local edible plants near a municipal landfill and the related human health risk assessment. Hum. Ecol. Risk Assess. 2019, 25, 1760–1772. [Google Scholar] [CrossRef]
- Sage, L.; Bassetti, O.; Johnson, E.; Shakya, K.; Weston, N. Assessment of heavy metal contamination in soil and produce of Philadelphia community gardens. Environ. Pollut. Bioavail. 2023, 35, 2209283. [Google Scholar] [CrossRef]
- Shen, M.; Chen, L.; Han, W.; Ma, A. Methods for the determination of heavy metals in indocalamus leaves after different preservation treatment using inductively-coupled plasma mass spectrometry. Microchem. J. 2018, 139, 295–300. [Google Scholar] [CrossRef]
- Silva, P.S.C.; Francisconi, L.S.; Gonçalves, R.D.M.R. Evaluation of major and trace elements in medicinal plants. J. Braz. Chem. Soc. 2016, 27, 2273–2289. [Google Scholar] [CrossRef]
- Squadrone, S.; Brizio, P.; Griglione, A.; Falsetti, S.; Curcio, A.; Abete, M.C. Aluminium occurrence in plant feed from Northwestern Italy. J. Trace Elem. Med. Biol. 2021, 68, 126850. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, A.W.; Gereslassie, T.; Yan, X.; Wang, J. Determination of heavy metal concentrations and their potential sources in selected plants: Xanthium strumarium L. (Asteraceae), Ficus exasperata Vahl (Moraceae), Persicaria attenuata (R.Br) Sojak (Polygonaceae), and Kanahia laniflora (Forssk.) R.Br. (Asclepiadaceae) from Awash River Basin, Ethiopia. Biol. Trace Elem. Res. 2019, 191, 231–242. [Google Scholar] [CrossRef]
- Tahir, M.A.; Shaheen, H.; Rathinasabapathi, B. Health risk associated with heavy metal contamination of vegetables grown in agricultural soil of Siran valley, Mansehra, Pakistan—A case study. Environ. Monit. Assess. 2022, 194, 551. [Google Scholar] [CrossRef] [PubMed]
- Uddin, A.H.; Khalid, R.S.; Alaama, M.; Abdualkader, A.M.; Kasmuri, A.; Abbas, S.A. Comparative study of three digestion methods for elemental analysis in traditional medicine products using atomic absorption spectrometry. J. Anal. Sci. Technol. 2016, 7, 6. [Google Scholar] [CrossRef]
- Ugulu, I.; Unver, M.C.; Dogan, Y. Potentially toxic metal accumulation and human health risk from consuming wild Urtica urens sold on the open markets of Izmir. Euro-Mediterr. J. Environ. Integr. 2019, 4, 36. [Google Scholar] [CrossRef]
- Usman, K.; Al Jabri, H.; Abu-Dieyeh, M.H.; Alsafran, M.H.S.A. Comparative Assessment of Toxic Metals Bioaccumulation and the Mechanisms of Chromium (Cr) Tolerance and Uptake in Calotropis procera. Front. Plant Sci. 2020, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, Z.; Zheng, J.; Yang, X.; Shen, K.; Zhou, T.; Zhang, Y. Multielemental analysis of botanical samples by ICP-OES and ICP-MS with focused infrared lightwave ashing for sample preparation. Microchem. J. 2017, 134, 68–77. [Google Scholar] [CrossRef]
- Zhuang, M.; Zhao, J.; Li, S.; Liu, D.; Wang, K.; Xiao, P.; Yu, L.; Jiang, Y.; Song, J.; Zhou, J.; et al. Concentrations and health risk assessment of rare earth elements in vegetables from mining area in Shandong, China. Chemosphere 2017, 168, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Pannico, A.; Zarrelli, A.; Kyriacou, M.C.; De Pascale, S.; Rouphael, Y. Macro and trace element mineral composition of six hemp varieties grown as microgreens. J. Food Compos. Anal. 2022, 114, 104750. [Google Scholar] [CrossRef]
- Dobrowolska-Iwanek, J.; Zagrodzki, P.; Galanty, A.; Fołta, M.; Kryczyk-Kozioł, J.; Szlósarczyk, M.; Rubio, P.S.; Saraiva de Carvalho, I.; Pasko, P. Determination of essential minerals and trace elements in edible sprouts from different botanical families—Application of chemometric analysis. Foods 2022, 11, 371. [Google Scholar] [CrossRef]
- Abdi, L.; Aghaee, E.M.; Nazmara, S.; Alipour, M.R.; Fakhri, Y.; Khaneghah, A.M. Potentially toxic elements (PTEs) in corn (Zea mays) and soybean (Glycine max) samples collected from Tehran, Iran: A health risk assessment study. Int. J. Environ. Anal. Chem. 2020, 102, 4640–4651. [Google Scholar] [CrossRef]
- Giacomino, A.; Inaudi, P.; Silletta, G.; Diana, A.; Bertinetti, S.; Gaggero, E.; Malandrino, M.; Stilo, F.; Abollino, O. Analytical methods for the characterization of vegetable oils. Molecules 2023, 28, 153. [Google Scholar] [CrossRef] [PubMed]
- Karaś, K.; Zioła-Frankowska, A.; Bartoszewicz, M.; Krzyśko, G.; Frankowski, M. Investigation of chocolate types on the content of selected metals and non-metals determined by ICP-OES analytical technique. Food Addit. Contam. Part A 2020, 38, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Khaleeq, A.; Ahmed, M.; Huma, R.; Mujtaba, A.; Noor, S.; Rehman, R.; Sheikh, T.A.; Qamar, S.; Iqbal, D.N.; Alharthy, R.D.; et al. Evaluation of trace and heavy metals in different varieties of sauces to characterize their impact on human health. J. Food Compos. Anal. 2022, 114, 104789. [Google Scholar] [CrossRef]
- Manousi, N.; Isaakidou, E.; Zachariadis, G.A. An inductively coupled plasma optical emission spectrometric method for the determination of toxic and nutrient metals in spices after pressure-assisted digestion. Appl. Sci. 2022, 12, 534. [Google Scholar] [CrossRef]
- Mehri, F.; Heshmati, A.; Ghane, E.T.; Mahmudiono, T.; Fakhri, Y. Concentration of Heavy Metals in Traditional and Industrial Fruit Juices from Iran: Probabilistic Risk Assessment Study. In Biological Trace Element Research; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Pastor, K.; Nastić, N.; Ilić, M.; Skendi, A.; Stefanou, S.; Ačanski, M.; Rocha, J.M.; Papageorgiou, M. A screening study of elemental composition in legume (Fabaceae sp.) cultivar from Serbia: Nutrient accumulation and risk assessment. J. Food Compos. Anal. 2024, 130, 106127. [Google Scholar] [CrossRef]
- Sharafi, K.; Mansouri, B.; Omer, A.K.; Bashardoust, P.; Ebrahimzadeh, G.; Sharifi, S.; Massahi, T.; Soleimani, H. Investigation of health risk assessment and the effect of various irrigation water on the accumulation of toxic metals in the most widely consumed vegetables in Iran. Sci. Rep. 2022, 12, 20806. [Google Scholar] [CrossRef] [PubMed]
- Jacimovic, S.; Popovic-Djordjevic, J.; Saric, B.; Krstic, A.; Mickovski-Stefanovic, V.; Pantelic, N.Ð. Antioxidant activity and multi-elemental analysis of dark chocolate. Foods 2022, 11, 1445. [Google Scholar] [CrossRef] [PubMed]
- Senila, M.; Resz, M.-A.; Senila, L.; Torok, I. Application of Diffusive Gradients in Thin-films (DGT) for assessing the heavy metals mobility in soil and prediction of their transfer to Russula virescens. Sci. Total Environ. 2024, 909, 168591. [Google Scholar] [CrossRef] [PubMed]
- Aldakheel, R.K.; Gondal, M.A.; Alsayed, H.N.; Almessiere, M.A.; Nasr, M.M.; Shemsi, A.M. Rapid determination and quantification of nutritional and poisonous metals in vastly consumed ayurvedic herbal medicine (Rejuvenator Shilajit) by humans using three advanced analytical techniques. Biol. Trace Elem. Res. 2022, 200, 4199–4216. [Google Scholar] [CrossRef] [PubMed]
- Al-Juhaimi, F.; Kulluk, D.A.; Mohamed Ahmed, I.A.; Özcan, M.M.; Adiamo, O. Quantitative determination of macro and micro elements and heavy metals accumulated in wild fruits analyzed by ICP-OES method. Environ. Monit. Assess. 2023, 195, 1370. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.; Sánchez, R.; Lefevre, J.; Todoli Torro, J. The accurate direct elemental analysis of fats and oils is accomplished according to a dilution and shot analysis method. J. Anal. At. Spectrom. 2020, 35, 1897–1909. [Google Scholar] [CrossRef]
- Taghizadeh, S.F.; Rezaee, R.; Boskabady, M.; Sardoo, H.M.; Karimi, G. Exploring the carcinogenic and non-carcinogenic risk of chemicals present in vegetable oils. Int. J. Environ. Anal. Chem. 2022, 102, 5756–5784. [Google Scholar] [CrossRef]
- Covaci, E.; Angyus, S.B.; Senila, M.; Ponta, M.; Darvasi, E.; Frentiu, M.; Frentiu, T. Eco-scale non-chromatographic method for mercury speciation in fish using formic acid extraction and UV–Vis photochemical vapor generation capacitively coupled plasma microtorch optical emission spectrometry. Microchem. J. 2018, 141, 155–162. [Google Scholar] [CrossRef]
- de la Guardia, M.; Garrigues, S. Challenges in Green Analytical Chemistry, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2020. [Google Scholar]
- Keith, L.H.; Gron, L.U.; Young, J.L. Green analytical methodologies. Chem. Rev. 2007, 107, 2695–2708. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Wojnowski, W. Complementary green analytical procedure index (ComplexGAPI) and software. Green Chem. 2021, 23, 8657. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.M.; Konieczka, P.; Namieśnik, J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 2012, 37, 61–72. [Google Scholar] [CrossRef]
- Hicks, M.B.; Farrell, W.; Aurigemma, C.; Lehmann, L.; Weisel, L.; Nadeau, K.; Lee, H.; Moraff, C.; Wong, M.; Huang, Y.; et al. Making the move towards modernized greener separations: Introduction of the analytical method greenness score (AMGS) calculator. Green Chem. 2019, 21, 1816–1826. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. Agree—Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep–Analytical greenness metric for sample preparation. TrAC Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Kaya, S.I.; Ozkan, S.A. An overview of the current progress in green analytical chemistry by evaluating recent studies using greenness assessment tools. TrAC Trends Anal. Chem. 2023, 168, 117330. [Google Scholar] [CrossRef]
- Pereira Junior, J.B.; Carvalho, V.S.; Ferreira, W.Q.; Araujo, R.G.O.; Ferreira, S.L.C. Green sample preparation of medicinal herbs in closed digester block for elemental determination by ICP OES. J. Pharm. Biomed. Anal. 2024, 238, 115810. [Google Scholar] [CrossRef] [PubMed]
- Ncube, N.; Tancu, Y.; Mketo, N. A greener, rapid and accurate microwave-assisted hydrogen peroxide digestion method for ICP-OES determination of heavy metals in pet food samples. J. Food Compos. Anal. 2024, 131, 106201. [Google Scholar] [CrossRef]
- Igual, M.; Fernandes, Â.; Dias, M.I.; Pinela, J.; García-Segovia, P.; Martínez-Monzó, J.; Barros, L. The in vitro simulated gastrointestinal digestion affects the bioaccessibility and bioactivity of beta vulgaris constituents. Foods 2023, 12, 338. [Google Scholar] [CrossRef] [PubMed]
- Kfle, G.; Asgedom, G.; Goje, T.; Abbebe, F.; Habtom, L.; Hanes, H. The level of heavy metal contamination in selected vegetables and animal feed grasses grown in wastewater irrigated area, around Asmara, Eritrea. J. Chem. 2020, 2020, 1359710. [Google Scholar] [CrossRef]
- Tremlová, J.; Sehnal, M.; Száková, J.; Goessler, W.; Steiner, O.; Najmanová, J.; Horáková, T.; Tlustoš, P. A Profile of arsenic species in different vegetables growing in arsenic-contaminated soils. Arch. Agron. Soil Sci. 2017, 63, 918–927. [Google Scholar] [CrossRef]
- González-Suárez, S.; Paz-Montelongo, S.; Niebla-Canelo, D.; Alejandro-Vega, S.; González-Weller, D.; Rubio-Armendáriz, C.; Hardisson, A.; Gutiérrez-Fernández, Á.J. Baby food jars as a dietary source of essential (K, Na, Ca, Mg, Fe, Zn, Cu, Co, Mo, Mn) and toxic elements (Al, Cd, Pb, B, Ba, V, Sr, Li, Ni). Appl. Sci. 2022, 12, 8044. [Google Scholar] [CrossRef]
- Koch, W.; Czop, M.; Iłowiecka, K.; Nawrocka, A.; Wiacek, D. Dietary intake of toxic heavy metals with major groups of food products—Results of analytical determinations. Nutrients 2022, 14, 1626. [Google Scholar] [CrossRef] [PubMed]
- Padrón, P.; Paz, S.; Rubio, C.; Gutiérrez, Á.J.; González-Weller, D.; Hardisson, A. Trace element levels in vegetable sausages and burgers determined by ICP-OES. Biol. Trace Elem. Res. 2020, 194, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Czop, M.; Nawrocka, A.; Wiacek, D. Contribution of major groups of food products to the daily intake of selected elements—Results from analytical determinations supported by chemometric analysis. Nutrients 2020, 12, 3412. [Google Scholar] [CrossRef]
- Shishov, A.; Savinov, S.; Volodina, N.; Gurev, I.; Bulatov, A. Deep eutectic solvent-based extraction of metals from oil samples for elemental analysis by ICP-OES. Microchem. J. 2022, 179, 107456. [Google Scholar] [CrossRef]
- Yilmaz, E.; Soylak, M. A novel and simple deep eutectic solvent based liquid phase microextraction method for rhodamine B in cosmetic products and water samples prior to its spectrophotometric determination. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 202, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Shishov, A.; Gerasimov, A.; Bulatov, A. Deep eutectic solvents based on carboxylic acids for metals separation from plant samples: Elemental analysis by ICP-OES. Food Chem. 2022, 366, 130634. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Romero, I.; Sánchez, R.; Beltrán, A.; Guillena, G.; Todolí, J.L. Multielemental analysis of oils and animal fat by using deep eutectic solvents assisted by an aerosol phase extraction procedure. Talanta Open 2023, 7, 100234. [Google Scholar] [CrossRef]
- Santana, A.P.R.; Andrade, D.F.; Mora-Vargas, J.A.; Amaral, C.D.B.; Oliveira, A.; Gonzalez, M.H. Natural deep eutectic solvents for sample preparation prior to elemental analysis by plasma-based techniques. Talanta 2019, 199, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Altunay, N.; Yildirim, E.; Gürkan, R. Extraction and preconcentration of trace Al and Cr from vegetable samples by vortex-assisted ionic liquid-based dispersive liquid-liquid microextraction prior to atomic absorption spectrometric determination. Food Chem. 2018, 245, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Öner, M.; Bodur, S.; Demir, C.; Yazici, E.; Erarpat, S.; Bakirdere, S. An effective and rapid magnetic nanoparticle based dispersive solid phase extraction method for the extraction and preconcentration of cadmium from edible oil samples before ICP OES measurement. J. Food Compos. Anal. 2021, 101, 103978. [Google Scholar] [CrossRef]
- Askarpour, S.A.; Molaee-Aghaee, E.; Ghaderi-Ghahfarokhi, M.; Shariatifar, N.; Mahmudiono, T.; Sadighara, P.; Fakhri, Y. Potentially toxic elements (PTEs) in refined and cold-pressed vegetable oils distributed in Ahvaz, Iran: A probabilistic health risk assessment. Biol. Trace Elem. Res. 2023, 201, 4567–4575. [Google Scholar] [CrossRef] [PubMed]
- Nyaba, L.; Nomngongo, P.N. Determination of trace metals in vegetables and water samples using dispersive ultrasound-assisted cloud point-dispersive μ-solid phase extraction coupled with inductively coupled plasma optical emission spectrometry. Food Chem. 2020, 322, 126749. [Google Scholar] [CrossRef] [PubMed]
- Abellán-Martín, S.J.; Pérez, J.; Pinheiro, F.C.; Nóbrega, J.A.; Aguirre, M.Á.; Vidal, L.; Canals, A. Synergistic combination of natural deep eutectic solvent and chemical vapor generation for trace determination of As, Cd, Hg and Pb in drug samples by inductively coupled plasma optical emission spectrometry. Adv. Sample Prep. 2023, 7, 100084. [Google Scholar] [CrossRef]
- Sihlahla, M.; Mpupa, A.; Sojka, M.; Saeid, A.; Nomngongo, P.N. Determination of selenium in cereal and biofortified samples by ICP-OES using an alcohol-based deep eutectic solvent in digestion procedure. Adv. Sample Prep. 2023, 8, 100092. [Google Scholar] [CrossRef]
- Khoja, K.K.; Buckley, A.; Aslam, M.F.; Sharp, P.A.; Latunde-Dada, G.O. In vitro bioaccessibility and bioavailability of iron from mature and microgreen fenugreek, rocket and broccoli. Nutrients 2020, 12, 1057. [Google Scholar] [CrossRef] [PubMed]
- Tokalioğlu, Ş. Bioaccessibility of Cu, Mn, Fe, and Zn in fruit and vegetables by the in vitro UBM and statistical evaluation of the results. Biol. Trace Elem. Res. 2023, 201, 1538–1546. [Google Scholar] [CrossRef]
- Altundag, H.; Mutlu, E.; Altintig, E.; Tuzen, M. Effect of Cu, Fe, Mn, Ni, and Zn and bioaccessibilities in the hazelnuts growing in Sakarya, Turkey using in-vitro gastrointestinal extraction method. Biol. Trace Elem. Res. 2020, 194, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.A.; Scheffler, G.L.; Pozebon, D. Straightforward determination of U, Th, and Hf at trace levels using ultrasonic nebulization and axial view ICP OES. Anal. Methods 2016, 8, 504–509. [Google Scholar] [CrossRef]
- Özcan, M.M.; Yılmaz, F.G.; Kulluk, D.A. The accumulation of element and heavy metal concentrations in different parts of some carrot and radish types. Environ. Monit. Assess. 2023, 195, 754. [Google Scholar] [CrossRef] [PubMed]
- Beller, E.; Roessler, C.; Probst, U.; Thueringer, R.; Volkmar, C. A radio-frequency generator for ion thrusters based on a Class-E power circuit. J. Electr. Propuls. 2022, 1, 8. [Google Scholar] [CrossRef]
- Horiba Scientific. Available online: https://www.horiba.com/aut/scientific/technologies/inductively-coupled-plasma-optical-emission-spectroscopy-icp-oes/excitation-source/ (accessed on 7 June 2024).
- Perkin Elmer. Available online: https://resources.perkinelmer.com/lab-solutions/resources/docs/TCH_Avio-Series-ICP-OES-Vertical-Dual-wiew_013462_01.pdf (accessed on 7 June 2024).
- de Andrade, M.F.; da Silva, I.J.S.; Pimentel, M.F.; Paim, A.P.S.; Cervera, M.L.; de la Guardia, M. Ultrasonic nebulization inductively coupled plasma optical emission spectrometry method for wine analysis. Spectrochim. Acta Part B At. Spectrosc. 2020, 170, 105924. [Google Scholar] [CrossRef]
- García, M.; Aguirre, M.Á.; Canals, A. Determination of As, Se, and Hg in fuel samples by in-chamber chemical vapor generation ICP OES using a Flow Blurring® multinebulizer. Anal. Bioanal. Chem. 2017, 409, 5481–5490. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, Z. Evaluation of solution anode glow discharge as a vapor generator in ICP-OES procedures: Application to highly sensitive determination of Cd and Hg. Anal. Chim. Acta 2022, 1203, 339724. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zheng, C.; Jiang, X.; Wu, X.; Hou, X. Integration of hydride generation and photochemical vapor generation for multi-element analysis of traditional Chinese medicine by ICP-OES. Microchem. J. 2015, 123, 164–169. [Google Scholar] [CrossRef]
- Fornieles, A.C.; de Torres, A.G.; Alonso, E.V.; Pavón, J.M.C. Simultaneous determination of traces of Pt, Pd, and Ir by SPE-ICP-OES. Test for chemical vapor generation. Microchem. J. 2016, 124, 82–89. [Google Scholar] [CrossRef]
- Butaciu, S.; Senila, M.; Sarbu, C.; Ponta, M.; Tanaselia, C.; Cadar, O.; Roman, M.; Radu, E.; Sima, M.; Frentiu, T. Chemical modeling of groundwater in the Banat Plain, southwestern Romania, with elevated As content and co-occurring species by combining diagrams and unsupervised multivariate statistical approaches. Chemosphere 2017, 172, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Zioła-Frankowska, A.; Frankowski, M. Determination of metals and metalloids in wine using inductively coupled plasma optical emission spectrometry and minitorch. Food Anal. Methods 2017, 10, 180–190. [Google Scholar] [CrossRef]
- Frentiu, T.; Butaciu, S.; Darvasi, E.; Ponta, M.; Senila, M.; Petreus, D.; Frentiu, M. Analytical characterization of a method for mercury determination in food using cold vapour capacitively coupled plasma microtorch optical emission spectrometry–compliance with European legislation requirements. Anal. Methods 2015, 7, 747–752. [Google Scholar] [CrossRef]
- Covaci, E.; Senila, M.; Tanaselia, C.; Angyus, S.B.; Ponta, M.; Darvasi, E.; Frentiu, M.; Frentiu, T. A highly sensitive eco-scale method for mercury determination in water and food using photochemical vapor generation and miniaturized instrumentation for capacitively coupled plasma microtorch optical emission spectrometry. J. Anal. At. Spectrom. 2018, 33, 799–808. [Google Scholar] [CrossRef]
- Drava, G.; Minganti, V. Influence of an internal standard in axial ICP OES analysis of trace elements in plant materials. J. Anal. At. Spectrom. 2020, 35, 301–306. [Google Scholar] [CrossRef]
- Giraldo, Y.R.; Sánchez, E.R.; Torres, L.G.; Montenegro, A.C.; Pichimata, M.A. Development of validation methods to determine cadmium in cocoa almond from the beans by ICP-MS and ICP-OES. Talanta Open 2022, 5, 100078. [Google Scholar] [CrossRef]
- Ul-Haq, I.; Ahmed, E.; Sharif, A.; Ahmed, M.; Ahmad, W. Optimization of ultrasound-assisted extraction of essential and non-essential/toxic trace metals in vegetables and their determination by FAAS and ICP-OES: An evaluation of human health risk. Food Anal. Methods 2021, 14, 2262–2275. [Google Scholar] [CrossRef]
- CAC/GL, 71, 22; Guidelines for the Design and Implementation of National Regulatory Food Safety Assurance Program Associated with the Use of Veterinary Drugs in Food Producing Animals. Codex Alimentarius. United Nations: New York, NY, USA, 2009.
- Liu, H.; Meng, Q.; Zhao, X.; Ye, Y.; Tong, H. Inductively coupled plasma mass spectrometry (ICP-MS) and inductively coupled plasma optical emission spectrometer (ICP-OES)-based discrimination for the authentication of tea. Food Control 2021, 123, 107735. [Google Scholar] [CrossRef]
- Khan, S.R.; Sharma, B.; Chawla, P.A.; Bhatia, R. Inductively coupled plasma optical emission spectrometry (ICP-OES): A powerful analytical technique for elemental analysis. Food Anal. Methods 2022, 15, 666–688. [Google Scholar] [CrossRef]
| Analytes | Type of Samples | Digestion Method | References |
|---|---|---|---|
| Ag, As, Al, Ba, Bi, Be, Cd, Ca, Cr, Co, Cu, K, Mn, Mg, Na, Fe, Pb, Li, Ga, Mo, Ni, Rb, Sr, Se, Tl, Te, V, Zn | Curcuma | A: HNO3 (3 mL), B: HNO3 (6 mL) + H2O2 1 M (2 mL), C: HNO3 (1 mL) + H2O (4 mL) + H2O2 1 M (2 mL), D: HNO3 (6 mL) + H2O2 1 M (2 mL), E: HNO3 (1 mL) + HCl (3 mL) | [7] |
| As, Cd, Be, Cr, Co, Cu, Mn, Fe, Mo, Ni, Sb, Pb, Sn, Se, V, Tl, Zn | Potatoes | 0.65 g sample digested with HNO3 70% and H2O2 30%, microwave oven, heating program up to 200 °C, total time 23 min | [20] |
| B, Cd, Ca, Cu, Cr, Fe, K, Mn, Mg, Na, Pb, Ni, Zn | Ten fruit-type plants belonging to the Solanaceae and Cucurbitaceae families | 8 mL HNO3 65% added to 0.2 g of plant, digestion in microwave oven, heating program up to 155 °C, total time 20 min | [21] |
| Al, Mg, Ca, Na, K, As, Co, Cd, Cu, Cr, Li, Fe, Mo, Mn, Pb, Ni, Se, Sb, Sr, Si, Tl, Ti, V Zn | 43 plant species | 10 mL HNO3 added to 0.1–2.1 g plant, digestion in microwave oven | [22] |
| Cd | Chinese cabbage | Mixture of HNO3-HClO4, 9:4 (v/v) ratio, hotplate at temperature of 150–180 °C, until obtaining clear liquid | [23] |
| Hg, Pb | Different vegetables and herbs | 1 g of dried plant digested with 15 mL mixture of HNO3 (70%)—H2SO4 (65%)—HClO4 (70%) = 5:1:1 ratio | [26] |
| As, Co, Ca, Cu, Cr, Fe, Mn, Mg, Pb, Ni, Se, Zn | Laportea alatipes | 0.25 g dried plant sample digested with 10 mL HNO3 70%, microwave oven | [27] |
| Cd, Mn, Al and Mg | Atriplex portulacoides, Ulva lactuca, Arthrocnemum indicum | Cold extraction HNO3 (1% and 10%) Heating with mixtures of acids: HCl-HNO3, HNO3-H2SO4, HNO3-HCl-H2SO4, HNO3-H2SO4-HClO4, HNO3-HClO4, HNO3-HCl-HClO4 | [29] |
| Pb, Cd | Different fruit and vegetable produce (~1300 samples) | 0.2–0.3 g dried plant sample digested with 2.5 mL HNO3 conc., then incubated overnight. After, 2.5 mL of H2O2 was added, microwave-assisted digestion | [31] |
| Al, As, B, Cd, Co, Cu, Fe, Pb, Se, Zn | Wheat, cabbage, spinach | 0.5 g of dried plant mixed with 70% HNO3 and heated up to 70 °C. Then 2 mL of HClO4 and 2 mL of HNO3 was added and heat up at 135 °C for 25 min. After cool down, 2 mL of HNO3 and 5 mL of HCl were added, then diluted at 50 mL with water | [32] |
| As, Cd, Cr, Cu, Ni, Mn, Fe, Pb, Zn | Sweet potato | 0.2 g of dried sample digested with 6 mL HNO3 69% and 2 mL of H2O2 30%, microwave oven | [33] |
| Pb, Cr, Cu, Fe, Zn, and Ni | Coriander, parsley, dill, arugula | 0.5 g of sample digested with 8 mL HNO3 69%, and let overnight at room temperature. After, 2 mL of H2O2 30% were added, and heated on hot plate at 90–120 °C | [36] |
| Ca, Cu, Fe, Mg, Mn, K, Na and Zn) | Medical foods | 1 g of sample + 6 mL H2O2 50% at 250 °C and 160 bar, in a single reaction chamber system | [37] |
| As, Cd, Co, Cr, Cu, Mn, Mo, Ni, Pb, Sb, and Sn | Tomato, onion, pepper, spinach, carrots, lettuce, marrow squash | 0.5 g of sample digested with 6 mL HNO3 conc. + 2 mL H2O2 30%, in microwave oven, five-steps digestion program | [38] |
| Cd, Pb, As, Hg | Okra, tomato, pumpkin, potato, cabbage, eggplant, spinach | 10 mL HNO3 conc. added to 5–10 g sample, on hotplate at temperature of 120 °C for 6 h. About 1 mL H2O2 was periodically added, until a clear solution was obtained | [39] |
| Ca, K, Mg, P, Na, B, Fe, Mn, Zn, Al, Sr, Co, Cu, Ni, Se, V, As, Cd, Cr, Pb, Sb, Sc, Y | Kale, rapeseed | 0.1 g of dried sample digested with 2.5 mL HNO3 (70%) and 1 mL H2O2 (15%), microwave oven | [40] |
| Al, Cd, Cu, Co, Cr, Fe, Mg, Mn, Ni, Pb, Zn | Medicinal herbs | 0.2 g of dried sample digested with 10 mL of HNO3:HClO4 (2:5 v/v) mixture. Few hours cold digestion, then heated on a hot plate, until colorless solution | [41] |
| As, Cd, Cr, Cu, Mn, Pb, Zn | Coriander, celery, C. coronarium, spinach, leek | 0.5 g of dried sample digested with 5 mL HNO3 and 1 mL of H2O2, kept overnight. Then were heated in oven at 150 °C near dryness. Next, 5 mL of HNO3 and 2 mL of H2O2 were added, then kept at 150 °C for 4 h | [42] |
| Pb, Cd | Lettuce, apples, carrots, tomatoes | 0.5 g of vegetable powder mixed with 20 mL of 2.5 M HNO3. The mixture was immersed in ultrasonic bath for 15 min. For comparison, microwave digestion was applied: 0.5 g of sample digested with 10 mL of 4.8 mol L−1 HNO3 | [43] |
| Ca, Mg, K, Na, S, P, Al, As, Ba, Co, Cd, Cr, Cu, Fe, Hg, Ni, Mn, Pb, V, Se, Zn | Brussels sprout, cabbage, potato, onion, kohlrabi, carrot, beetroot | 0.5 g of dried sample digested with a mixture HNO3:H2O2 (7:1), heating at 80 °C in a water bath, for 5 h | [44] |
| As, Cd, Pb, Cr | Ten species of edible plant samples | 0.5 g of dried sample digested with 5 mL HNO3 and 3 mL H2O2, heating at 120 °C on a hot plate to near dryness, then was diluted with water to 25 mL | [45] |
| As, Cr, Cd, Cu, Co, Pb, Ni | Kale, collard greens, basil, romaine lettuce, carrot, potato, radish, tomato, squash, pepper | 0.5 g of dried sample digested with 5 mL HNO3, microwave oven, at a temperature of 175 °C | [46] |
| Cd, Cu, Cr, Mn, Pb, Hg | Indocalamus leaves | 0.3 g of dried sample digested in two ways: (1) 5 mL HNO3 + 1 mL H2O2; (2) 5 mL HNO3 + 1 mL HF, microwave oven | [47] |
| Cu, Cd, Ni, Pb | 59 medicinal plants | 0.3 g of dried sample digested with a mix of acids (HNO3, HClO4, HCl) and H2O2, microwave oven | [48] |
| Al | Rice, corn, wheat, rye, barley, triticale, soy, oats | 0.5 g of dried sample digested with 1.5 mL H2O2 and 7 mL HNO3, microwave oven | [49] |
| Al, As, Cd, Cr, Cu, Ni, Zn, Hg, Pb | Xanthium strumarium L., Ficus exasperata, Persicaria attenuata, Kanahia laniflora | 0.25 g of dried sample digested with 7 mL HNO3 (63%) and 2 mL H2O2 (30%) microwave oven, four-step digestion procedure. After digestion, the samples were evaporated down to 1 mL on a hot plate, then diluted with water to 50 mL and filtered | [50] |
| As, Cr, Cd, Pb | 16 species of edible vegetables | 0.2 g of dried sample digested with mixture of 2 mL 1:1 (v/v) HNO3:H2O, heated at 90 °C on a hotplate; 1 mL HNO3 was repeatedly added until brown fumes disappeared. Sample was evaporated to 1 mL, then 0.4 mL H2O and 0.6 mL 30% H2O2 were added and heated again until effervescence stopped | [51] |
| As, Cd, Pb, Ni, Fe, Zn | Traditional medicine samples | 0.5 g of dried sample digested three ways: (1) 5 mL HNO3 + 2.5 mL HClO4; (2) 5 mL HNO3 + 2.5 mL HNO3; (3) 9 mL mixture HNO3:HCl (1:3), heating until total dissolving | [52] |
| Cu, Cd, Cr, Ni, Fe, Pb, Mn, Zn | Urtica urens | 2 g of dried sample digested with 25 mL 5% HNO3 heating by induction then cooled down. Afterward, 15 mL of 5% HClO4 was added and boiled 1 until the solution became colorless | [53] |
| Cd, Cu, Cr, Ni, Pb | Atriplex leucoclada, Salsola imbricata, Typha augustifolia, Calotropis procera, Phragmites australis | 0.5 g of dried sample digested with HNO3 and H2O2, using a large-capacity HotBlock digestion system by heating until clear solutions were obtained | [54] |
| Ba, Be, Bi, Ca, Co, Cs, Cu, Mg, Mn, Na, K, P, Pb, Ni, Rb, Sr, Mo, Th, U, Zn, REEs | Botanical samples | 0.2 g sample digested in microwave-assisted conditions. 2.5 mL of conc. HNO3 was added for predigestion 4 h. Then, 2 mL of 30% H2O2 was added for digestion in a microwave oven | [55] |
| 14 rare earth elements (REEs) | Chinese cabbage, long bean, towel gourd, scallion, radish, white gourd, eggplant, potato, tomato, carrot, red pepper, pumpkin | 0.50 g of dried sample digested with 8 mL of HNO3 (65%), microwave oven, cooled down, and then diluted to 10 mL with ultrapure water | [56] |
| Ca, Mg, K, Na, Al, B, Ba, Cd, Cu, Cr, S, Se, Sn, Fe, Mn, Mo, Ni, P, Zn | Hemp varieties | 1.0 g of lyophilized sample, ground, digested in microwave system with 10 mL of 69% HNO3. The digestion program was from 20 °C to 140 °C for 30 min, then kept for 50 min at 140 °C | [57] |
| Ca, Cu, Fe, Mn, Mg, Zn | Sprouts | 0.5 g of freeze-dried sprouts, ground, digested in microwave system with 10 mL of concentrated HNO3. After cooling down, 6 M HCl were added | [58] |
| As, Pb, Hg, Ni, Cd, Cu, Cr, Zn | Corn and soybean | 1 g of grounded sample digested with 15 mL of a mixture of HNO3 65%, H2SO4 98%, and HCl 36% (5:1:1 v/v) heated at 80 °C until obtaining a clear solution | [59] |
| Al, Ba, Ca, Cu, K, Fe, Li, P, Mg, Mn, Na, Sb, Se, Zn | Vegetable oils | 0.5 g of sample digested with 3 mL of HNO3 65% and 3 mL of H2O2 30% in microwave-assisted conditions | [60] |
| Al, Ba, Cu, Ca, K, Fe, Na, Ni, Mg, Mn, S, P, Sr, Zn | Chocolate and cocoa | 1 g of sample mixed with 9 mL of HNO3 65% and then heated in a water bath at 95 °C for 1 h, transferred, and diluted to 25 mL with deionized water | [61] |
| Cd, Cr, Cu, Co, Mn, Ni, Zn, Pb | Sauces from different ingredients | 10 mL of sample mixed with 10–15 mL aqua regia, kept 1 h. Then, it was added to 100 mL water and heated on the hot plate at 150 °C. | [62] |
| Al, Ag, Ba, B, Bi, Ca, Co, Cd, Cu, Cr, Fe, Mn, Mg, Pb, Ni, Tl, Zn | Spices | 0.2 mg of sample mixed with 6 mL of HNO3 65% and 1 mL of H2O2 30% and heated for 90 min at 120 °C in a heating block. After cooling down at room temperature, the digested sample was diluted 25 mL | [63] |
| Pb, As, Cd, Cu, Zn | Fruit juices (apple, grape, peach, orange, mango, pineapple) | 2 mL of sample added to 20 mL mixture of HNO3 65% and H2O2 30% at a ratio of 9:1, v/v, stirred 10 min at room temperature, and then heated at 180 °C for 15 min. Samples were digested in microwave oven at 1800 W for 27 min | [64] |
| As, Ca, Cu, Cr, Cd, P, K, Fe, Mg, Mn, Pb, Ni, Zn | Legumes (Phaseolus spp., Vicia spp., Pisum spp. and Lathyrus spp.) | 0.5 g of flour sample mixed with 5 mL of HNO3 65% and 2 mL of H2O2 30%. A three-step microwave digestion program with a total time of 40 min at 800 W was applied | [65] |
| As, Cd, Cr, Cu, Pb, Fe, Mn, Ni, Zn, | Vegetables | 0.2 g of sample mixed with 4 mL of conc. HNO3, heated in a water bath for 150 min up to 100 °C. Then the sample was cooled at room temperature and 0.2 mL of H2O2 30%, and let to react 30 min | [66] |
| Analytes | Type of Samples | Digestion Method | References |
|---|---|---|---|
| Ca, Cu, K, Mg, Mn, Na, P, Zn Fe | Gynandropsis gynandra | 0.5 g of powdered sample was burned for 2 h in a furnace at 550 °C. The ashes were digested with 10 mL HNO3:HCl, 1:3 ratio mixture on a hot plate | [28] |
| Ba, Be, Bi, Ca, Co, Cs, Cu, Mg, Mn, Na, K, P, Pb, Ni, Rb, Sr, Mo, Th, U, Zn, REEs | Botanical samples | 0.2 g sample incinerated using infrared assisted heating in quartz tubes. 10 mL of 10% HNO3 was added to dissolve the ash | [55] |
| P, K, Na, Ca, Mg, Fe | Beetroot (Beta vulgaris L.) | Sample was burned in an oven at 550 °C for 24 h. The incineration residue was then extracted with HCl (50%, v/v) and HNO3 (50%, v/v) | [85] |
| Al, Co, Cd, Cu, Cr, Fe, Mo, Mn, V, Pb, Zn | Medicago sativa L., Cynodon dactylon L., Corchorus olitorius L., Avena sativa L., Cynara scholymus L. | 2.0 g of powdered sample was burned for 3 h in a furnace at 550 °C. 60 mL aqua-regia was added and heated on hot plate at 100 °C | [86] |
| As | Black radish, lettuce, black salsify, savoy cabbage, parsnip, swede turnip | 1 g of powdered sample decomposed in an oxidizing gas mixture at 400 °C. 20 mL 1.5% HNO3 was added to dissolve the ash | [87] |
| Al, As, Zn, B, Cd, Cu, Co, Pb, Fe, Se | Baby food | 10 g of sample mixed with 5 mL of 65% HNO3, heated on hot plate until acid evaporation, then burned at 450 °C 24 h. The ash dissolved in 1.5% HNO3 | [88] |
| Analytes | Type of Samples | Digestion Method | References |
|---|---|---|---|
| Al, Ca, As, Co, Cd, Cr, Fe, Cu, K, Mg, Na, Mn, P, Si, Pb, Zn | 10 medicinal plant species | 0.5 g of plant dried sample extracted with 20 mL ethanol/water solution (50% (v/v)) | [25] |
| Ag, Al, Ba, B, Ca, Co, Cu, Cr, Fe, Mg, Mo, Mn, Ni, Na, Pb, Ti, Sn, V, K, Zn | Oil samples | 5 g oil mixed with 0.5 g of DES (choline chloride and hydrogen donors: tartaric, citric, benzoic, oxalic, acetic, malonic, malic, formic, maleic, succinic, adipic, boric, lactic, ascorbic, gallic, and mandelic acids; 1;4-butanediol; glycerol; sorbitol; ethylene glycol; triethylene glycol; benzamide; urea; thiourea; fructose; glucose; sucrose; maltose | [92] |
| Ca, Cu, Ba, Na, K, Fe, Mn, Mg, Mo, Pb, Ni, Sn, V, Zn, | Tobacco, lettuce | 100 mg of plant sample mixed with 0.5 g of DES (choline chloride, and malic acid, 1:1) at 70 °C | [94] |
| Al, Ag, Ba, Cd, Cu, Cr, Fe, Li, K, Ni, Mg, Pb, Mn | Oil samples | DES (ethylene glycol and choline chloride, ratio (2:1)) and aerosol phase extraction method | [95] |
| As, Cd, Ca, Cu, K, Fe, Na, Mg, Mn, P, Zn | Vegetables | 90 mg sample mixed with 9 mL natural deep eutectic solvents (xylitol, citric acid, malic acid) in ultrasound-assisted conditions | [96] |
| Al, Cr | Vegetables | Ionic liquid dispersive liquid–liquid microextraction, based on anionic chelate complexes formation between Al(III) and Cr(VI) with o-hydroxy azo dye, and extraction of the ternary complexes | [97] |
| Cd | Oil samples | Dispersive solid phase extraction with stearic acid coated with Fe3O4 nanoparticle as adsorbent | [98] |
| As, Cd, Cu, Fe, Pb, Mn, Ni, Zn | Oil samples | 0.1 g oil mixed with 10 mL of diluted acids mixture 1% HNO3/0.2% HCl. Shaken by vortex, then ultrasound extraction | [99] |
| As, Cd, Co, Cr, Sb, Tl, Pb | Vegetables | Ultrasound-assisted cloud point extraction (UA-CPE) combined with dispersive μ-solid phase extraction (D-μ-SPE) for preconcentration of metals. A nanocomposite compound Mg/Al-LDH@CNTs was synthetized and used as solid phase | [100] |
| As, Cd, Hg, Pb | Drug samples | A combination of dispersive liquid–liquid microextraction using deep eutectic solvent (NADES) as extractant combined chemical vapor generation | [101] |
| Se | Cereal and biofortified samples | DES (choline chloride (ChCl) as hydrogen bond acceptor, and phenol (PhOH) as hydrogen bond donator) at different mole ratios of ChCl: PhOH = 1:1, 1:2, 1:3 and 1:4 | [102] |
| Analytes | Type of Samples | Digestion Method | References |
|---|---|---|---|
| P, K, Na, Ca, Mg, Fe | Beetroot (Beta vulgaris L.) | Sample digestibility assessed using the in vitro digestion method: oral (pH 7), gastric (pH 3), intestinal (pH 7), and digested (D) phases. | [85] |
| Cu, Mn, Fe, Ni, Zn | Hazelnut | Sample digestibility assessed using the in vitro digestion method: stomach (pH 2.5), Intestine 1 (pH 7), Intestine 2 (pH 7) | [105] |
| Analytes | Type of Samples | Analytical Performances | References |
|---|---|---|---|
| Hg, Pb | Vegetables, herbs | Vegetable and/or herb samples spiked at concentrations of 15, 25, 75, 150, 250, 500, and 750 μg/mL for recovery test | [26] |
| As, Co, Ca, Cu, Cr, Fe, Mn, Mg, Pb, Ni, Se, Zn | Laportea alatipes | CRM White clover (BCR 402) analyzed for quality assurance | [27] |
| As, Cd, Cr, Cu, Ni, Mn, Fe, Pb, Zn | Sweet potato | CRM INCT-CF-3–corn flour, analyzed for quality assurance | [33] |
| As, Cd, Co, Cr, Cu, Mn, Mo, Ni, Pb, Sb, and Sn | Tomato, onion, pepper, spinach, carrots, lettuce, marrow squash | CRM NCS ZC85006 tomato analyzed. Student’s t-test at the 95% level, indicated the results consistent with the certified values (recoveries between 93–103%) | [38] |
| Cd, Pb, As, Hg | Okra, tomato, pumpkin, potato, cabbage, eggplant, spinach | Fortified potato samples analyzed. Obtained recoveries varied in the range 83–103%, RSD, varied between 7–14% | [39] |
| Ca, K, Mg, P, Na, B, Fe, Mn, Zn, Al, Sr, Co, Cu, Ni, Se, V, As, Cd, Cr, Pb, Sb, Sc, Y | Kale, rapeseed | CRM apple (Malus domestica Borkh) leaves NIST-SRM 1515 was analyzed. Recoveries varied from 84–118% of certified values | [40] |
| As, Cd, Pb, Cr | Ten species of edible plant samples | CRM analyzed, recoveries in the recoveries were in the range of 96–100% | [45] |
| As, Pb, Hg, Ni, Cd, Cu, Cr, Zn | Corn, soybean | LODs 0.001–0.005 mg/kg; LOQs 0.003–0.015 mg/kg Inter-day precision between 3.2–6.4% | [59] |
| Al, Ba, Ca, Cu, K, Fe, Li, P, Mg, Mn, Na, Sb, Se, Zn | CRM tomato leaves | Recoveries in the range of 75–101.5%. | [60] |
| Al, Ba, Cu, Ca, K, Fe, Na, Ni, Mg, Mn, S, P, Sr, Zn | Cacao | Use of spiked solutions for percentage recovery | [61] |
| Cd, Cr, Cu, Co, Mn, Ni, Zn, Pb | SRM baking chocolate | Recoveries ranged from 98.6–101.2% | [62] |
| Al, Ag, Ba, B, Bi, Ca, Co, Cd, Cu, Cr, Fe, Mn, Mg, Pb, Ni, Tl, Zn | Spices | Two emission lines were verified for each element for selectivity evaluation. LOQs were in the range of 0.27 to 19.83 mg/kg. Recoveries were between 82.0 and 117.5% | [63] |
| Pb, As, Cd, Cu, Zn | Fruit juices (apple, grape, peach, orange, mango, pineapple) | LODs between 0.03 and 0.92 µg/L Recoveries between 93 and 99% | [64] |
| As, Ca, Cu, Cr, Cd, P, K, Fe, Mg, Mn, Pb, Ni, Zn | Legumes | LODs in the range 0.027 mg/L (Cd)–0.076 mg/L (P) Recovery 88–106% | [65] |
| As, Cd, Cr, Cu, Pb, Fe, Mn, Ni, Zn | Vegetables | LODs in the range 0.049 ppm (Cd)–0.564 mg/L (Cr) | [66] |
| Al, As, Zn, B, Cd, Cu, Co, Pb, Fe, Se | CRMs with plants and food matrices | LODs 0.001–3.655 mg/L; recoveries in the range of 93.1–102.7% | [88] |
| Cd, Ni, Pb, Hg | Food samples | Recoveries ranged from 95.0–106.0% LOQ between 2.1 and 14.8 µg/kg | [89] |
| Al, Ba, B, Cd, Co, Cu, Cr, Fe, Pb, Mn, Li, Mo, Ni, Sr, V, Zn | Vegetable sausages | LOQs between 0.001 mg/L (Cd, Pb) and 0.013 mg/L (Li) | [90] |
| As, Cd, Cu, Fe, Pb, Mn, Ni, Zn | Oil | LODs 0.002–0.036 mg/L | [99] |
| As, Cd, Co, Cr, Sb, Tl, Pb | Vegetables | LODs in the range of 90–150 ng/L by preconcentration using micro-solid phase extraction. Recoveries between 97 and 99.3% | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senila, M. Recent Advances in the Determination of Major and Trace Elements in Plants Using Inductively Coupled Plasma Optical Emission Spectrometry. Molecules 2024, 29, 3169. https://doi.org/10.3390/molecules29133169
Senila M. Recent Advances in the Determination of Major and Trace Elements in Plants Using Inductively Coupled Plasma Optical Emission Spectrometry. Molecules. 2024; 29(13):3169. https://doi.org/10.3390/molecules29133169
Chicago/Turabian StyleSenila, Marin. 2024. "Recent Advances in the Determination of Major and Trace Elements in Plants Using Inductively Coupled Plasma Optical Emission Spectrometry" Molecules 29, no. 13: 3169. https://doi.org/10.3390/molecules29133169
APA StyleSenila, M. (2024). Recent Advances in the Determination of Major and Trace Elements in Plants Using Inductively Coupled Plasma Optical Emission Spectrometry. Molecules, 29(13), 3169. https://doi.org/10.3390/molecules29133169






