1. Introduction
The main gases responsible for the phenomenon known as the greenhouse effect are steam, carbon dioxide, methane and ozone. Carbon dioxide amounts to about 79% of the total and its concentration is increasing dramatically because of human activities, for instance: massive deforestation, coal burning, natural gas, petroleum fuels and the industrialization of most economic sectors. In order to reduce and mitigate the negative effects of CO
2, research has focused on obtaining materials with adsorption–desorption properties to capture and store CO
2 through different technologies [
1].
The chemical properties of various oxides (of alkaline, transition and alkaline earth metals) have led to extensive research of their use in the adsorption–desorption of CO
2. Their thermodynamic properties are used to calculate the thermodynamic equilibrium of the CO
2 absorption–desorption cycle according to the values of the heat of reaction and chemical potential; however, this is not the only criterion for validating a material, as other factors must also be taken into account such as cost, regeneration temperature, durability, etc. [
2,
3,
4]. Although metal oxides have good interactions with CO
2, there are also a number of disadvantages to their use, such as quite difficult absorbent regeneration, the use of high temperatures and reduced durability in some cases. Utilizing these important selection criteria put forward adsorbents based on MgO, showing that they have fast adsorption speed, stability and good reversibility of regeneration during 10 cycles without decreasing work capacity, but they also a significant influence regarding the content of carbonate and nitrate salts [
5].
Mesoporous and microporous materials based on SiO
2 are the most often used in CO
2 adsorption–desorption studies. The adsorption of CO
2 is higher in the case of microporous materials, but mesoporous ones are preferred because they can be used as a support which can be functionalized by different amines. Amino groups favor CO
2 adsorption due to the physical and chemical interactions between functionalized species and CO
2 molecules [
6,
7,
8,
9].
In the last three decades, the synthesis of mesoporous materials has gained considerable momentum and the main morphologies in this family are: MCM-41 [
10,
11] with hexagonal symmetry P6mm and mesochannels that are interconnected by micropores, MCM-48 [
12] with Ia3d cubic symmetry and MCM-50 [
13], which is characterized by a lamellar structure.
One of the most studied mesoporous materials is SBA-15, which has a hexagonal structure and P6mm symmetry with thicker walls compared to the other mesoporous materials, leading to the ability to have clearly superior mechanical and hydrothermal resistances due to the large pores. Thus, SBA-15 mesoporous sieves are among the most widely studied as a catalytic support for active phase dispersion [
14,
15,
16]. Mesoporous silica of the KIT-6 type, characterized by a three-dimensional cubic Ia3d structure with large interconnected pores and large specific surfaces, is also being studied more and more intensively [
8,
17]. KIT-6 silica has a great advantage given by the silanols groups that can be functionalized post-synthesis, leading to modification of the surface properties to favor the adsorption–desorption of gases.
Silica materials functionalized with amines, named aminosilica, are some of the most important and widely studied materials with adsorbent properties used for CO
2 capture [
18]. Amine functionalization of silica can be performed by the impregnation method, when the amines are physically load onto the silica support, or by the grafting method, through covalent bonding. At the equlibrium point, amine-impregnated silica presents higher CO2 uptake because of its higher amine loading compared with amine-grafted samples. Therefore, amine-impregnated silicas may have slower adsorption kinetics and lower thermal and hydrothermal stability, leading to amine evaporation.
The most used adsorbents in post-combustion capture (PCC) of CO
2 are based on amines, like monoethanolamine (MEA), diethanolamine (DEA), 3-aminopropyl triethoxysilane (APTES), tetraethylenepentamine (TPEA), polyethylenimine (PEI), etc. [
19,
20,
21,
22]. Using these amines for high CO
2 capture efficiency results in some disadvantages regarding the high levels of equipment corrosion and the high energy consumption needed for amine regeneration. All of these requirements represent additional costs for the application of this method [
23].
The functionalization of mesoporous sieves with amines with different number of nitrogen atoms led to obtaining materials with varying CO
2 adsorption capacities. Vilarrasa-Garcia E et al. [
24] studied MCF-type porous silica by grafting with 3-aminopropyl triethoxysilane (APTES) and by impregnation with polymers rich in amine groups PEI or TEPA, and found that grafting with APTES improves CO
2 adsorption and physisorption has a main role compared to chemosorption. The increase in temperature had a negative effect for MCFs grafted with APTES, while in the case of samples impregnated with PEI and TEPA, the adsorption was assigned to chemical interactions between the amine species and carbon dioxide. In the case of MCF materials impregnated with PEI or TEPA, the chemisorption process controlled the CO
2 retention over the physisorption process and as a result, CO
2 uptake was enhanced by increasing the temperature. The authors concluded that the CO
2/N molar ratio was considerably lower for the PEI and TEPA grafted samples compared to the APTES-grafted samples because of the lesser availability of the amino groups. The stability of the investigated materials was reflected the nine CO
2 adsorption–desorption cycles.
Runa Dey et al. [
14] studied adsorption–desorption kinetics and the stability of a mesoporous SBA-15 material impregnated with polyethylenimine (PEI), obtaining an optimal amine loading of 50% with a CO
2 adsorption capacity between 2.79 and 3.09 mmol∙g
−1. In order to improve CO
2 adsorption by adding moisture to the atmosphere, they used 10% pre-humidified CO
2 at 75 °C. The authors concluded that the CO
2/N molar ratio was considerably lower for the PEI- and TEPA-grafted samples compared to the APTES-grafted samples because of the lesser availability of the amino groups. The stability of the investigated materials was reflected by nine CO
2 adsorption–desorption cycles.
The researchers in the field directed their research towards a series of mesoporous materials that were functionalized with various amines in order to increase the adsorption–desorption capacity of carbon dioxide, studying various parameters such as the reaction medium, temperature, molar ratios, the influence of pore sizes, etc. Liu Y. et al. [
25] functionalized KIT-6 mesoporous silica with pentaethylenehexamine (PEHA) and the obtained results showed that the pore size, pore volume and surface area of the adsorbents decreased after loading with PEHA, while the basic structure of the KIT-6 pores remained unchanged. The amount of adsorbed carbon dioxide increased with increasing temperature, obtaining a maximum adsorption at 70 °C, which remained almost constant even after 10 cycles of adsorption–desorption, while above this temperature the adsorption capacity decreased.
Son W.J. [
26] et al. synthesized the mesoporous silicas MCM-41, MCM-48, SBA-15, SBA-16 and KIT-6 and impregnated all of them with 50% polyethyleneamine (PEI) in methanol. They studied CO
2 adsorption–desorption in cycles of 150 min at a temperature of 75 °C and obtained reversible adsorption–desorption behavior for all tested types of mesoporous silica. The KIT-6 mesoporous silica presented the best stability in three consecutive tests over a duration of 900 min and the highest CO
2 adsorption capacity of 135 mg/g due to its larger pores in a 3D arrangement; the order of effectiveness was: KIT-6 > SBA-16 ≈ SBA-15 > MCM-48 > MCM-41.
The influence of the reaction environment on amine-grafted adsorbents was investigated by Anyanwu et al. [
27], who found that the presence of water had much better effects vis a vis alcohol on SBA-15 loading and on other adsorbents. A continuous increase in water concentration led to polymerization and a higher loading of amines without signs of a plateau and also showed superior stability after several cycles of adsorption–desorption.
In addition to the mesoporous silicas previously described, there are many novel nanoporous materials for CO
2 capture which show good performance, such as porous carbon materials, heteroatom-doped biomass carbon materials, MOFs, COFs and so on. Rehman et al. [
28] made a series of heteroatom-enriched porous carbons using KOH as a porogen and activating agent, while urea/thiourea was used as an N/N and S dopant. A cellulose-based highly porous carbon framework (1026 m
2/g, 0.7135 cm
3/g) with a narrow micropore stucture (<0.94 nm) significantly contributed to an efficient CO
2 uptake of ~297.1 mg/g at 0 °C and 193.7 mg/g at 25 °C/1 bar. An excellent CO
2/N
2 selectivity was obtained for Cell-TK at ~110 at 25 °C/1 bar. These results were achieved due to the large number of narrow micropores with high adsorption potential, together with high N and S contents, which produced a better affinity for CO
2 molecules through Lewis acid–base interactions. Sani et al. [
29] presented recent progress in the development of different types of covalent organic frameworks (COFs), which are a class of crystalline porous materials built entirely from light elements (C, H, O and N), as well as their application as catalysts for different types of CO
2 fixation reactions. These thermally stable COFs with excellent surface areas and volumes are now used for the conversion of CO
2 to CO by photochemical or electrochemical reduction.
Muchan et al. [
18] studied CO
2 adsorption–desorption on mesoporous MCM-41, SBA-15 and KIT-6 silicas functionalized with APTES. The adsorption was carried out under conditions of normal temperature and atmospheric pressure using 15% CO
2, and desorption was carried out at 100 °C under N
2 balance. The three silicas modified with APTES presented an improvement in adsorption capacity, demonstrating that the primary amine present in the structure can carry out a rapid chemical reaction with CO
2 in the mesopore channels, but the results diminished in the middle and final stages due to CO
2 transport through smaller pore channels. KIT-6 can be considered a promissing adsorbent material due to its large pore size and large pore volume. Investigations show that KIT-6 presents a reasonable adsorption capacity with a high adsorption rate. In addition, it can also be regenerated with an efficiency of 99.72% using a 12.07 kJ/mmol CO
2 heat load for regeneration.
In this work, mesoporous cubic Ia3d KIT-6 was prepared and functionalized with 3-aminopropyltriethoxysilane (APTES) by the grafting method. The composites were prepared with three different concentrations of APTES: 20, 30 and 40 wt.%. The obtained materials were studied by thermogravimetric analysis (TG/DTA), FT-IR spectroscopy and X-ray diffraction at low angles, and surface areas were determined by the BET method. The adsorption capacity (mg CO2/g ads) and the efficiency of amino groups (mol CO2/mol NH2) were measured using a thermo analyzer system coupled with mass spectrometry (MS) in the temperature range of 40–70 °C.
In our work, we performed a detailed study of the influence of temperature and functionalization agent concentration on the adsorption capacity (mg CO2/g ads) and the efficiency of amino groups (mol CO2/mol NH2) for mesoporous silica KIT-6 composites. We combined two methods, thermal analysis and mass spectrometry, for the investigation of CO2 adsorption–desorption. It is important to underline that the change in the amount of adsorbed and desorbed CO2 was determined from the MS spectra for all stages of the CO2 adsorption–desorption process. The stability and recyclability of the studied amino-functionalized materials were also tested for a longer period of time, in order to demonstrate their usability for practical applications.
2. Results and Discussion
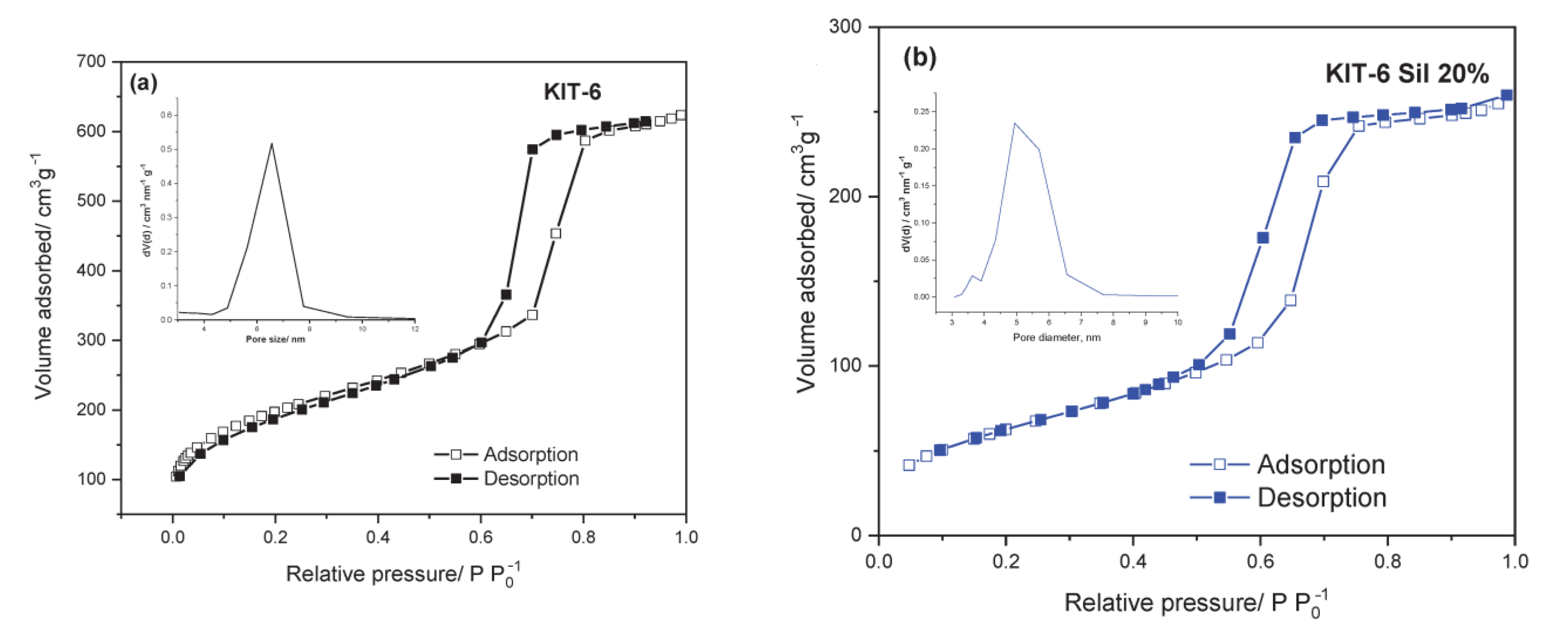
The textural parameters of the parent and amino-functionalized materials are presented in
Table 1. The results show that the surface area and the other textural parameters of KIT-6 decreased after adding APTES in three different concentrations: 20, 30 and 40 wt.%. The modified KIT-6 samples are denoted as KIT-6 Sil. The reduction in surface area may be due to the intensification of the silica particles’ agglomeration and/or the filling of pores after the addition of APTES. All composites show a type IV isotherm with H1 hysteresis and a sharp increase in volume adsorbed at p/p
0 = 0.5–0.9, characteristic of highly ordered mesoporous materials (
Figure 1a–d). The micropore volume and micropore area parameters were calculated by the V-t method, and the results for KIT-6 were as follows: V
mp = 0.07 cm
3 and A
mp = 147.9 m
2/g, respectively (
Table 1). After functionalization with APTES, for the KIT-6 Sil 20–40% samples, the values of the textural parameters of the micropores decreased, probably as a consequence of the micropores filling or the agglomeration of silica particles.
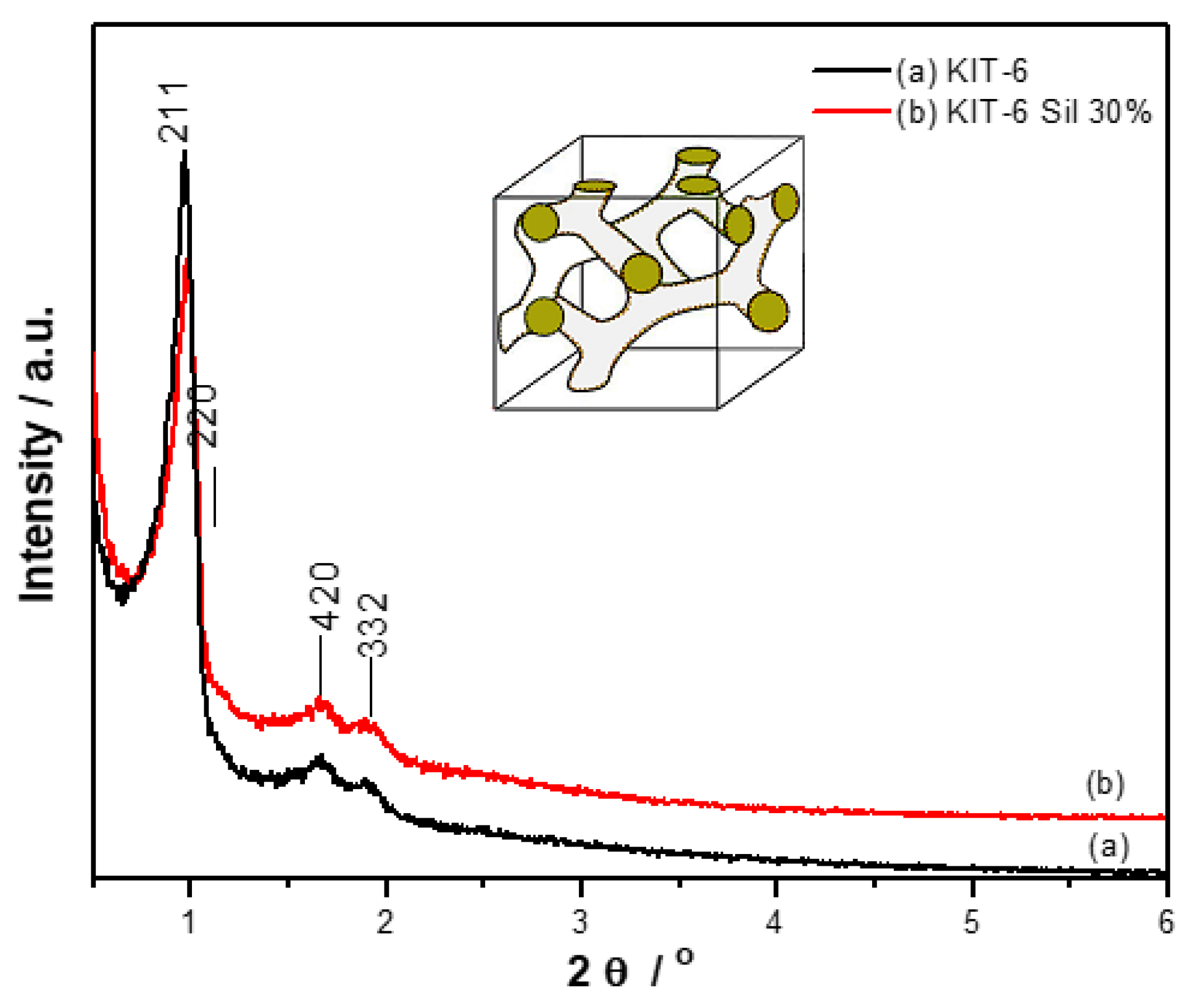
Figure 2 shows the diffractograms for KIT-6 (a) and KIT-6 Sil 30% (b) obtained at low angles in the range 2θ = 0–6°. The presence of four diffraction peaks at 0.96°, 1.16°, 1.69° and 1.92° corresponds to the Miller indices (211), (220), (420) and (332), confirming the
Ia3d cubical mesoporous structure specific to KIT-6 sieves [
30,
31]. With the introduction of APTES, a slight decrease in the tipping points was observed, which suggests that the grafting operation was successful.
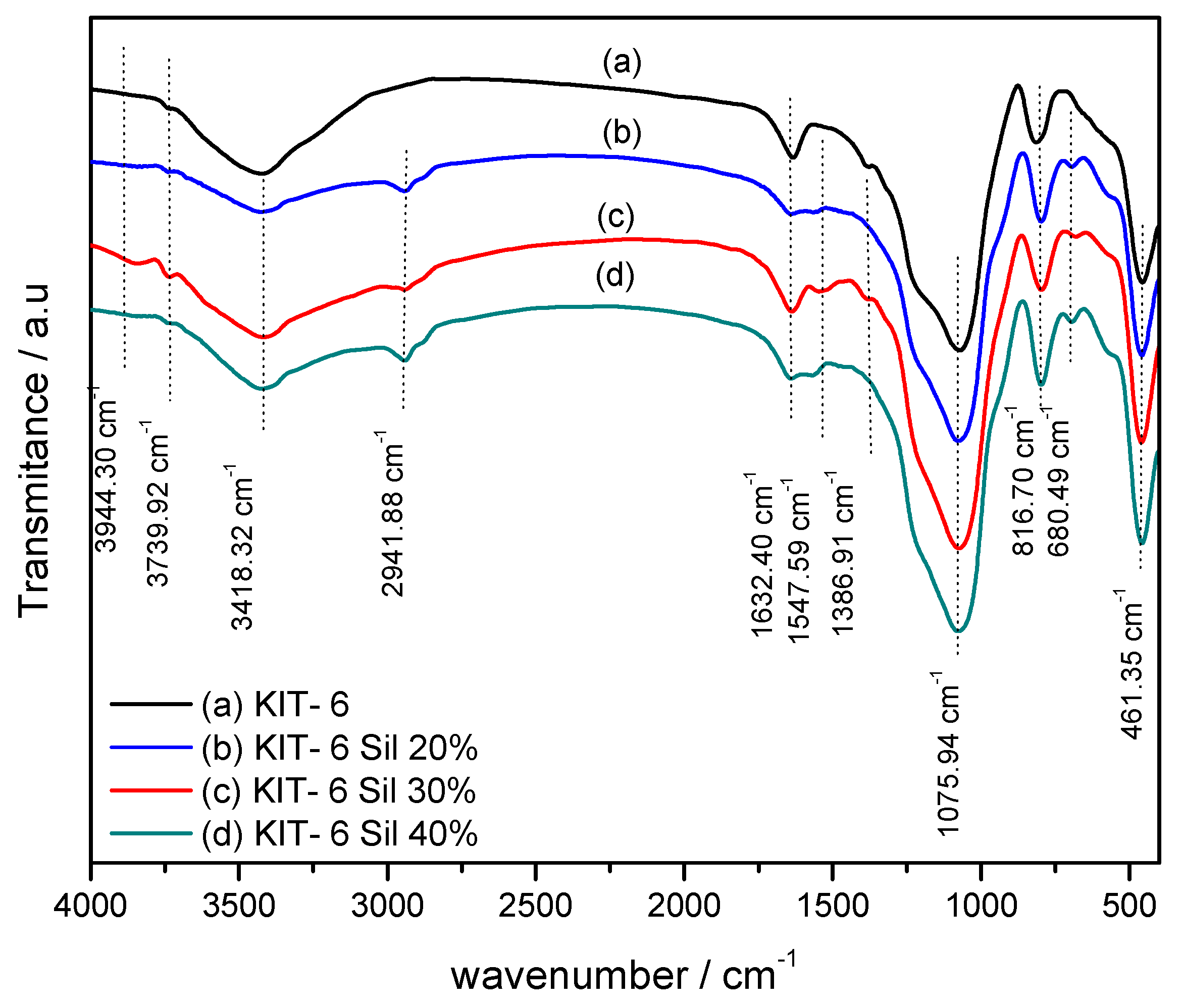
As is illustrated in
Figure 3, the vibration bands of the parent KIT-6 and KIT-6 Sil 20–40% were determined by Fourier-transform infrared analysis. Generally, the IR absorption bands correspond to the stretching frequencies of the inorganic functional groups in all samples. It can be seen that all samples have absorption bands around 3739, 3418, 1632, 1386, 1075, 816 and 461 cm
−1.
The band at 1632 cm
−1 is due to water deformation modes and the respective OH stretching modes are responsible for the band at 3418 cm
−1. A contribution to the latter band also comes from the OH of some silanols interacting with adsorbed H
2O, leading to a red shift of their OH modes. The band at 3739 cm
−1 is typical on internal silanols [
31,
32]. The bands that can be observed at 1075 cm
−1 and the shoulder present at 1386 cm
−1 on the FT-IR spectra can be atributed to asymmetric stretches of the Si–O–Si bonds. The smaller peak observed at 816 cm
−1 coresponds to symmetrical stretches of the Si–O–Si bonds. The band present at 461 cm
−1 refer to the symmetrical and asymmetric stretches of the Si–O bonds of the Si–OH groups [
32].
The FT-IR spectra of KIT-6 Sil 20–40% (
Figure 3b–d) show the presence of peaks around 680 cm
−1 and 1547 cm
−1, corresponding to –NH- and –NH
2 bending vibrations that are absent in the parent KIT-6. Also, N-H stretching modes are expected around 3200 cm
−1 and contribute to the band at 3418 cm
−1. The adsorption band at 2941 cm
−1 corresponds to the asymmetric deformation of the -CH
2 groups present in the propyl chain of APTES. The FT-IR results indicate the successful loading and grafting of APTES onto the surface.
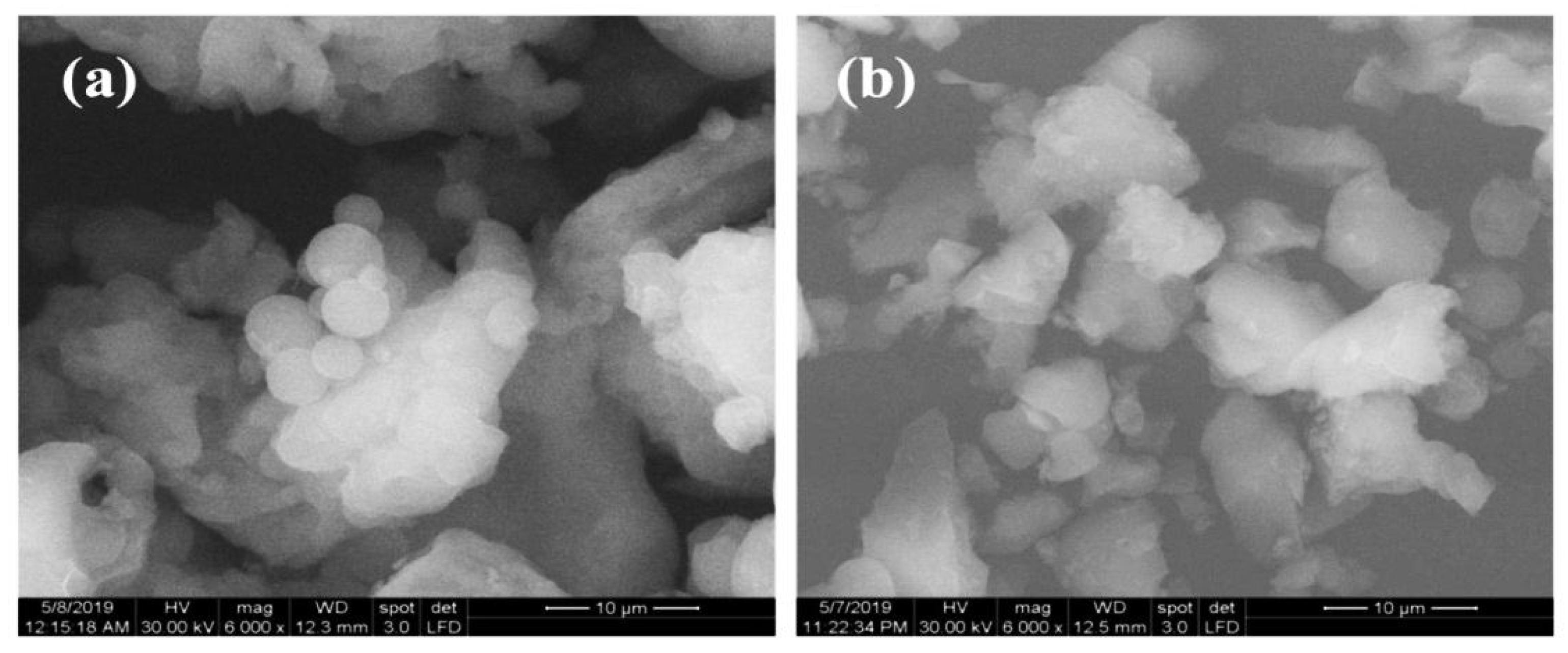
Scanning electron micrograph (SEM) images were used to analyze the morphology of the KIT-6 and KIT-6 Sil 30% particles (
Figure 4). The picture of KIT-6 (
Figure 4a) shows an agglomeration of spherical particles characteristic of an orderly network with an Ia3d structure type [
14,
33]. It can be seen that the spherical morphology is modified by the adding of APTES, with the spheres becoming rougher and more dispersed, which indicates that the grafting has taken place successfully (
Figure 4b).
2.1. Thermal Analysis
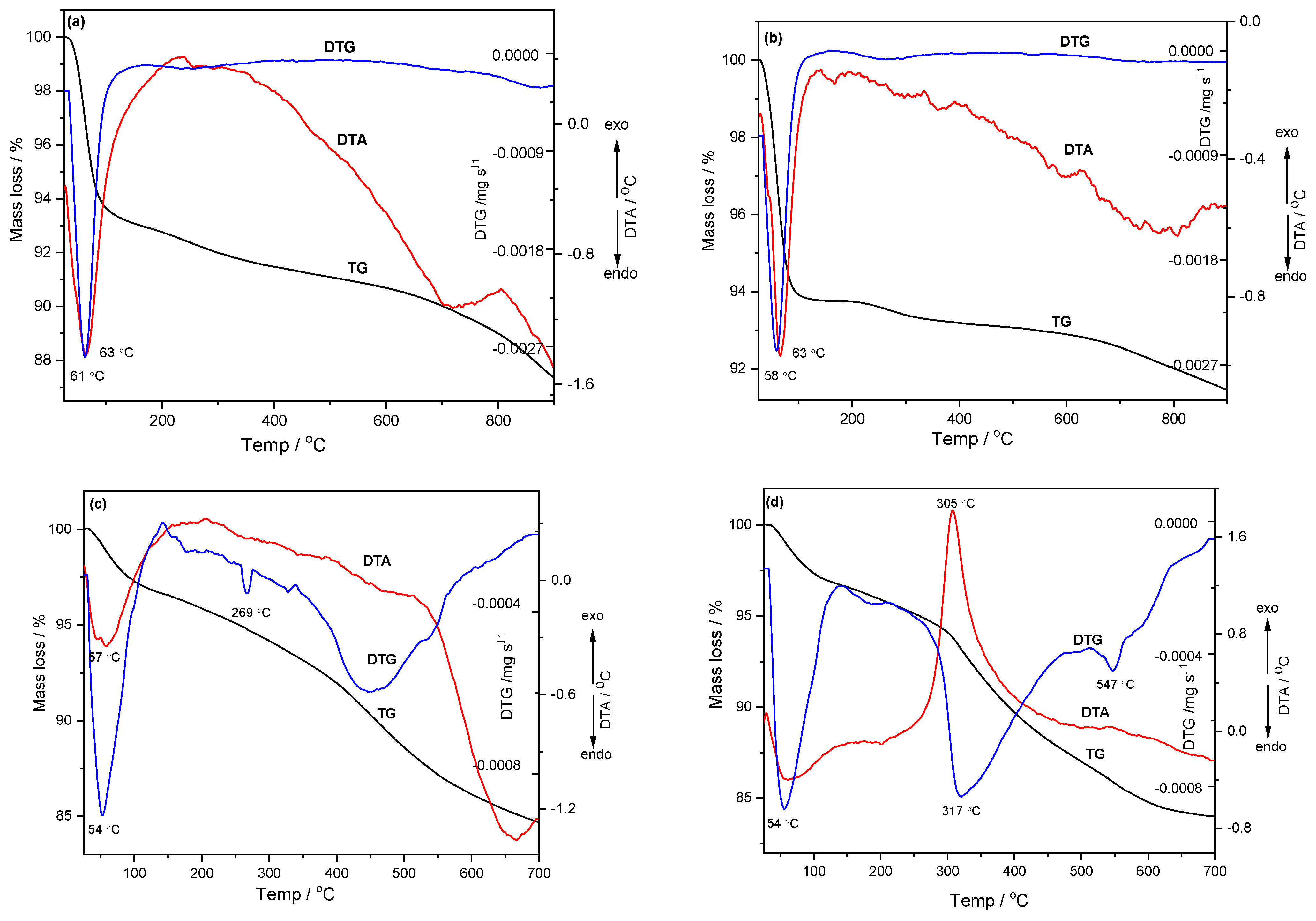
The thermal stability was investigated by TG-DTA-DTG methods in the temperature range of 25–900 °C in the case of the KIT-6 mesoporous sieve, and the mesoporous sieves functionalized with 3-aminopropyl triethoxysilane (APTES) were tested in the temperature range of 25–700 °C. For the KIT-6 and KIT-6 Sil 20–40% mesoporous sieves, the thermal stability studies were performed in an inert atmosphere (nitrogen), as well as in an oxidizing atmosphere (air), with a heating rate of 10 °C /min. From the thermal analysis of KIT-6 in the oxidative as well as inert atmospheres, three stages of mass loss could be found (
Figure 5a,b). In the first stage, the mass loss was due to the elimination of physically adsorbed water and residual solvents from the synthesis process, a fact supported by the endothermal effect at 63 °C. In the second stage, a significant mass loss was observed, which was due to the degradation of the organic matrix and the elimination of chemosorbed water, while in the third stage, the mass loss corresponded to the condensation of silanol groups remaining on the surface and in the material pores [
34].
From the thermal analysis of KIT-6 Sil 30% in the inert and oxidizing atmosphere, the endothermic effects at 54 °C (
Figure 5c,d) corresponded to the elimination of physically adsorbed water and residual solvents. At temperatures above 200 °C, it can be observed that the exothermic effect at 305 °C in the oxidizing atmosphere (
Figure 5d) was much stronger than the one obtained in the inert atmosphere (
Figure 5c), which suggests that the oxidation and decomposition of the APTES amination agent occurred faster, with a faster mass loss and a noticeably lower thermal stability.
These results demonstrate the relatively good thermal stability of the investigated KIT-6 and KIT-6 Sil materials. In agreement with these results, TPD measurements regarding the adsorption–desorption process of CO2 were performed in a narrower temperature range, between 40 and 70 °C.
The mass loss between temperatures 250 and 700 °C in the case of the KIT-6 Sil 30% sieve was significantly higher (11% in N2 and 13% in air) than in the case of the parent KIT-6, which suggests that the functionalization of the KIT-6 sieve was successfully achieved.
2.2. The Adsorption–Desorption Process of CO2
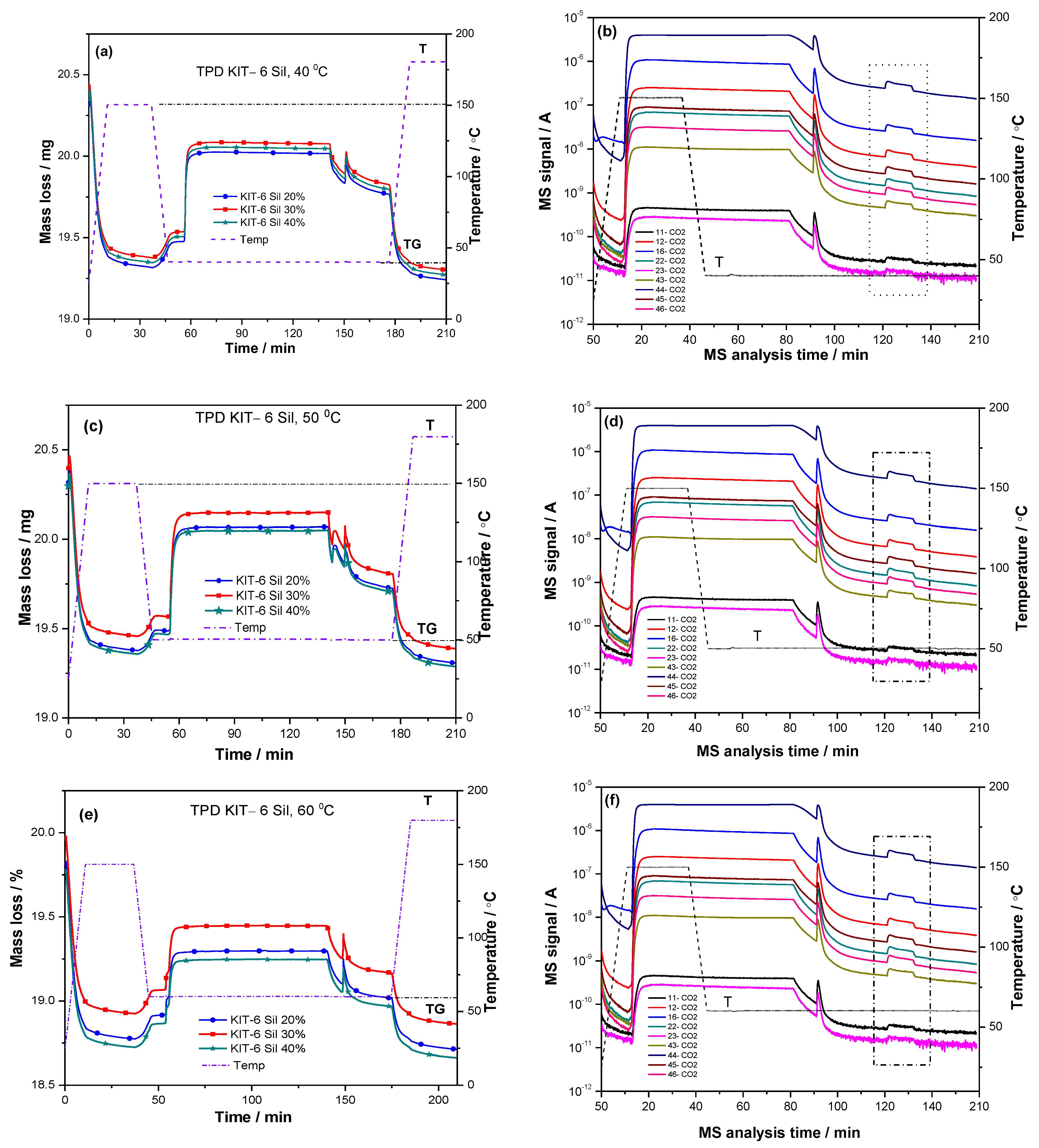
The adsorption–desorption process of CO
2 was studied for amine-grafted KIT-6 Sil 20–40% using the TPD program. Since the non-amine-functionalized KIT-6 mesoporous sieve did not show a carbon dioxide capture activity, the CO
2 adsorption–desorption process was studied for amine-grafted KIT-6 Sil 20–40% at different temperatures (
Figure 6a–f) by thermogravimetric analysis. The mass gain of the samples in mmol of CO
2 per gram of adsorbent during the adsorption process represents the carbon dioxide adsorption capacity. The steps of the adsorption–desorption processes at temperatures of 40, 50, 60 and 70 °C for KIT-6 Sil 20–40% are the following: first, each sample was pretreated in a stream of N
2 at 150 °C for 30 min to remove physically adsorbed water, as well as possible impurities, then, the temperature was reduced to the desired adsorption temperature and maintained under the same nitrogen flow. The next step was the exposure of the samples to the adsorption gas mixture, 30% CO
2/N
2 (70 mL/min), which was maintained for 1.5 h. The last step after the completion of the adsorption process was the maintenance of the samples for 30 min in N
2 for the removal of the physically adsorbed CO
2.
After cleaning the surface of physically adsorbed CO
2, the next stage was the desorption of chemisorbed CO
2 from the amine-grafted samples of KIT-6 Sil 20–40%. This step was carried out from the adsorption temperature up to 180 °C, with a temperature increase of 10 °C/min and with an isotherm at 180 °C for 30 min. The change in the amount of desorbed CO
2 was determined (at certain mass-to-charge ratios, m/z = 46, 45, 44, 43, 23, 22, 16, 12 and 11) from the MS spectra (
Figure 6b,d,f,h) for all stages of the CO
2 adsorption–desorption process.
The signal characteristic for CO
2 on the MS spectra increased during the adsorption process and was maintained at a constant for 90 min during the exposure process of the CO
2/N
2 gas mixture. When the exposure to the gas mixture was interrupted, the MS signal characteristic for CO
2 decreased. Then, the sample was kept for 30 min under a continuous N
2 atmosphere. In this stage of the adsorption–desorption process, the physisorbed CO
2 was removed from the sample. Increasing the temperature up to 180 °C (the selected area in the Origin plots in
Figure 6b,d,f,h), the signal characteristic for CO
2 increased again during the desorption step of the chemisorbed CO
2.
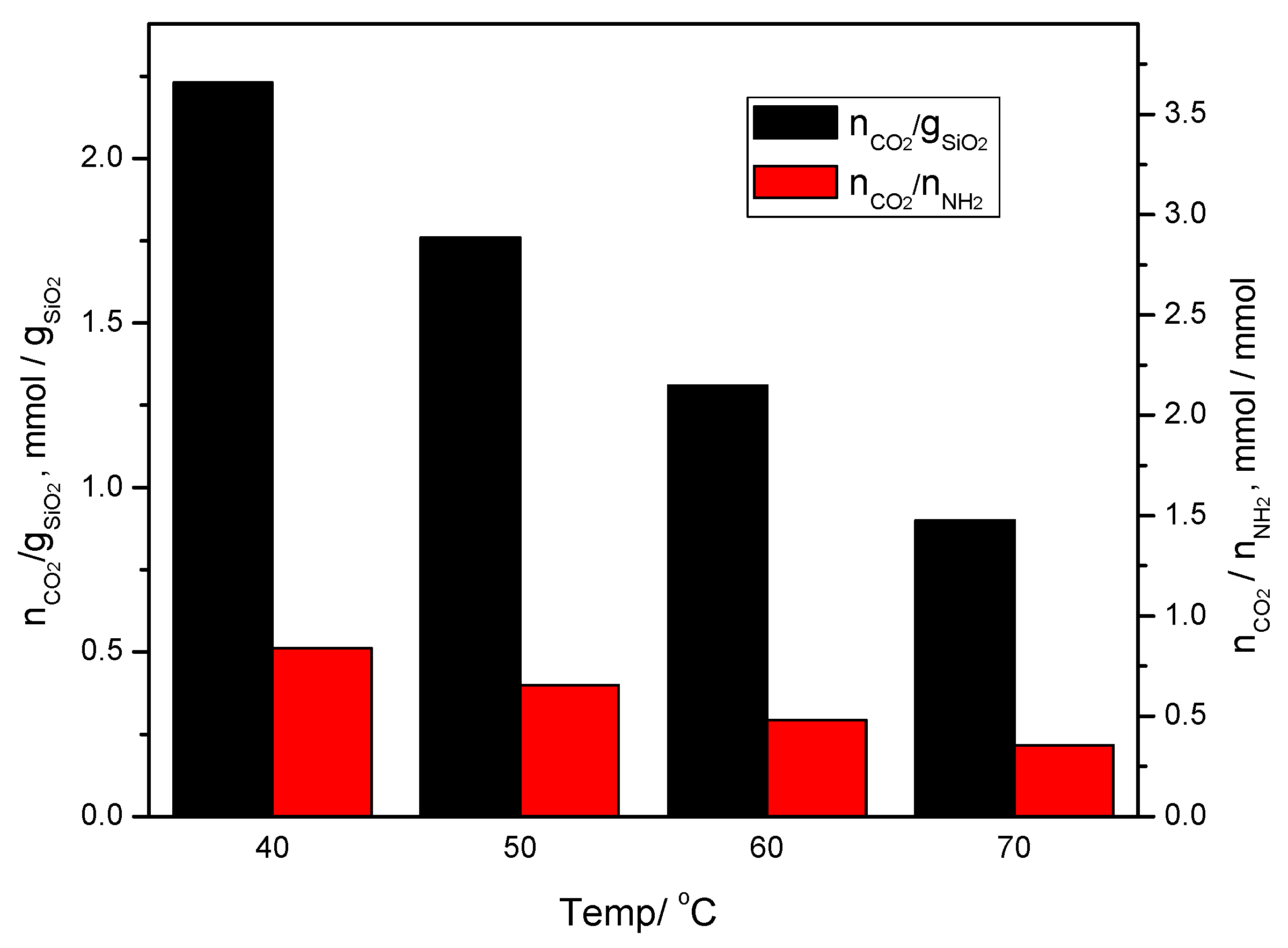
The amounts of CO
2 captured on the KIT-6 Sil 20–40% adsorbents at different temperatures are shown in
Figure 6a,c,e,g. It can be seen that with an increase in the temperature, the adsorption capacity and the efficiency of the amino groups decreased for all concentration of APTES: 20, 30 and 40 wt.%. In the case of KIT-6 Sil 30%, the adsorption capacity and the efficiency of the amino groups decreased from 2.23 to 0.95 mmol CO
2/g SiO
2 and from 0.51 to 0.22 mmol CO
2/mmol NH
2, respectively. At 40 °C, an adsorption capacity of 2.23 mmol CO
2/g SiO
2 and an efficiency of the amino groups of 0.51 mmol CO
2/mmol NH
2 represent the best results for KIT-6 Sil 30%; these results are clearly superior to those at the other temperatures of 50, 60 and 70 °C, as can be seen in
Figure 6a,b. The formulae used to calculate the above parameters, including adsorption capacity and the efficiency of amino groups are published elsewhere [
15].
The influence of functionalization agent concentration was studied for all concentrations of APTES: 20, 30 and 40 wt.%. The adsorption capacity and the efficiency of the amino groups of the captured CO
2 at 40 °C for all composites are summarized in
Table 2. At the lowest temperature (40 °C), an adsorption capacity of 2.23 mmol CO
2/g SiO
2 and an efficiency of amino groups of 0.51 mmol CO
2/mmol NH
2 represent the best results for KIT-6 Sil 30%, which was clearly superior to the other concentrations of APTES.
From the obtained results, it can be concluded that the values for both adsorption capacity (mmol CO
2/g adsorbent) and the efficiency of the amino groups (mmol CO
2/mmol NH
2) strictly depend on the investigated temperature and, to a lesser extent, on the functionalization agent concentration. At lower investigated temperatures, 40 and 50 °C, respectively, higher values of CO
2 adsorption were obtained. By increasing the temperature investigation range to 60 and 70 °C, the values for CO
2 adsorption decreased. The achieved results for mesoporous silica KIT-6 are comparable or even superior to results existing in the literature. Some of these results regarding the adsorption capacities obtained for different functionalized mesoporous molecular sieves using different amines (APTES, PEI and PEHA) are summarized in
Table 3.
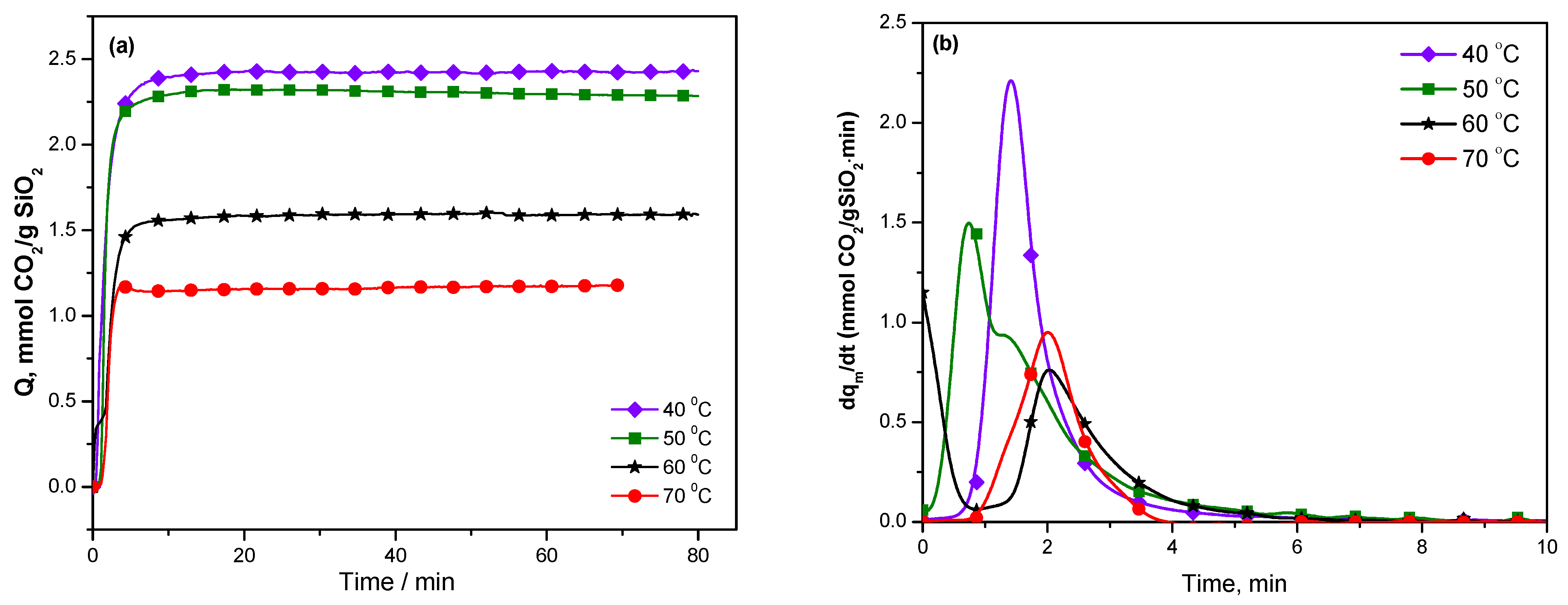
The variations of the CO
2 adsorption with time and its time derivative—which is considered as a measure of adsorption rate—are shown in
Figure 7 for KIT-6 Sil 30% at temperatures between 40 and 70 °C. The CO
2 adsorption rate reached the maximum values for KIT-6 Sil 30% at 40 and 50 °C and then decreased at higher temperatures.
Álvarez et al. [
39] studied the sources of activation of the CO
2 molecule on catalytic surfaces such that electricity, light and/or temperature can be external activators. We can say that a temperature of 40 °C is the most suitable for the KIT-6 type mesoporous sieve functionalized with APTES, as it is the most thermally stable and can be used as an adsorbent for CO
2. The type of amines used can influence the reaction mechanism for CO
2 adsorption [
40]. By incorporating some basic organic groups (in this case, APTES) into KIT-6 type mesoporous silica, surface chemistry changes occur, leading to an increase in the CO
2 adsorption capacity.
According to the literature data [
41], the interaction of amines with CO
2 generates a reversible reaction of carbamates by the formation of 1,3-zwitterion, R-NH2
+COO
−, which is often characteristic under dry conditions. The reaction mechanism with the formation of 1,3-zwitterion resulting from the interaction between CO
2 and amines can be expressed as follows:
This type of reaction requires two amine groups per one CO2 molecule, so the CO2/N ratio (amine efficiency) is found to be in accordance with the density of the amine groups.
The regeneration reaction of the KIT-6 mesoporous molecular sieve and CO
2 desorption can be expressed by the following reaction:
From the mass loss during the desorption process measured in mmol CO
2/mmol NH
2, the efficiency of the adsorbent KIT-6 Sil mesoporous molecular sieve functionalized with APTES was calculated at different temperatures. As can be seen from
Figure 8, the adsorption capacity (mmol CO
2/g ads) and the amino group efficiency (mmol CO
2/mmol NH
2) were higher in the case of the KIT-6 Sil functionalized mesoporous molecular sieve at 40 and 50 °C.
In order to be able to say that a material has a practical utility (value), several criteria must be taken into account, such as high adsorption–desorption capacity, good selectivity and, last but not least, the greatest possible stability during prolonged operation for CO2 capture.
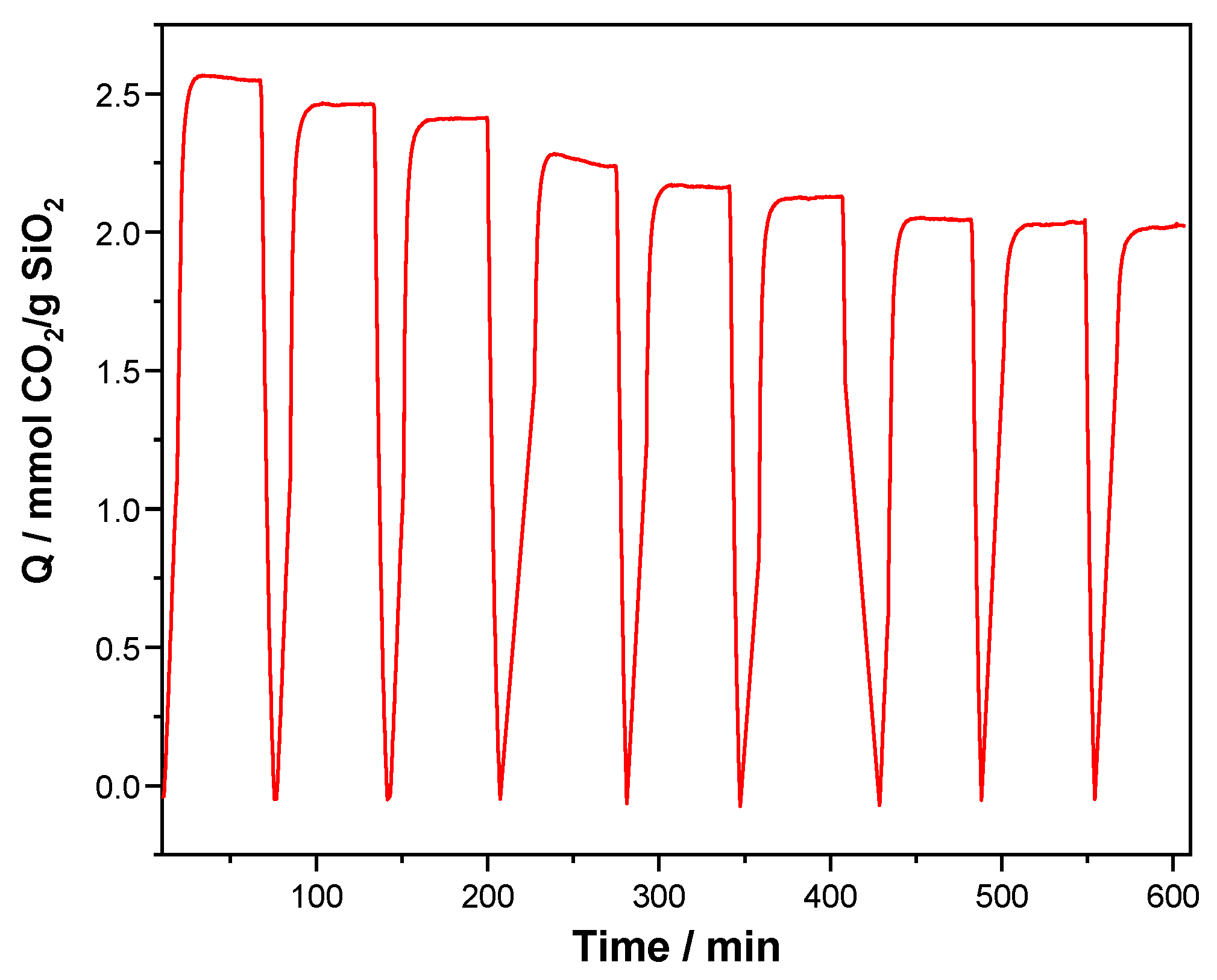
Figure 9 shows the behavior of KIT-6 Sil 30% with the highest CO
2 adsorption rate at 40 °C for a duration of nine cycles. The KIT-6 Sil 30% sample was pretreated in flowing N
2 at 120 °C. The samples were kept in this environment for 10 min, then cooled down to the adsorption temperature, 40 °C, and exposed further to 30% CO
2 in N
2 for 40 min. The CO
2 desorption was achieved by heating the sample to 120 °C at a rate of 10 °C/min.
As can be seen in
Figure 9, the KIT-6 Sil 30% adsorbent (40 °C) showed a high adsorption capacity and good stability over nine cycles of adsorption and desorption. After nine cycles of experimental tests, the adsorption capacities of KIT-6 Sil 30% showed only a 1.46% decrease. This decrease of 1.46% can be assigned to the regeneration conditions (120 °C), which can be responsible for removing small proportions of N-species that are not bound strongly enough to the KIT-6 type mesoporous molecular sieve surface through electrostatic interactions.