The Impact of Phospholipid-Based Liquid Crystals’ Microstructure on Stability and Release Profile of Ascorbyl Palmitate and Skin Performance
Abstract
1. Introduction
2. Results and Discussion
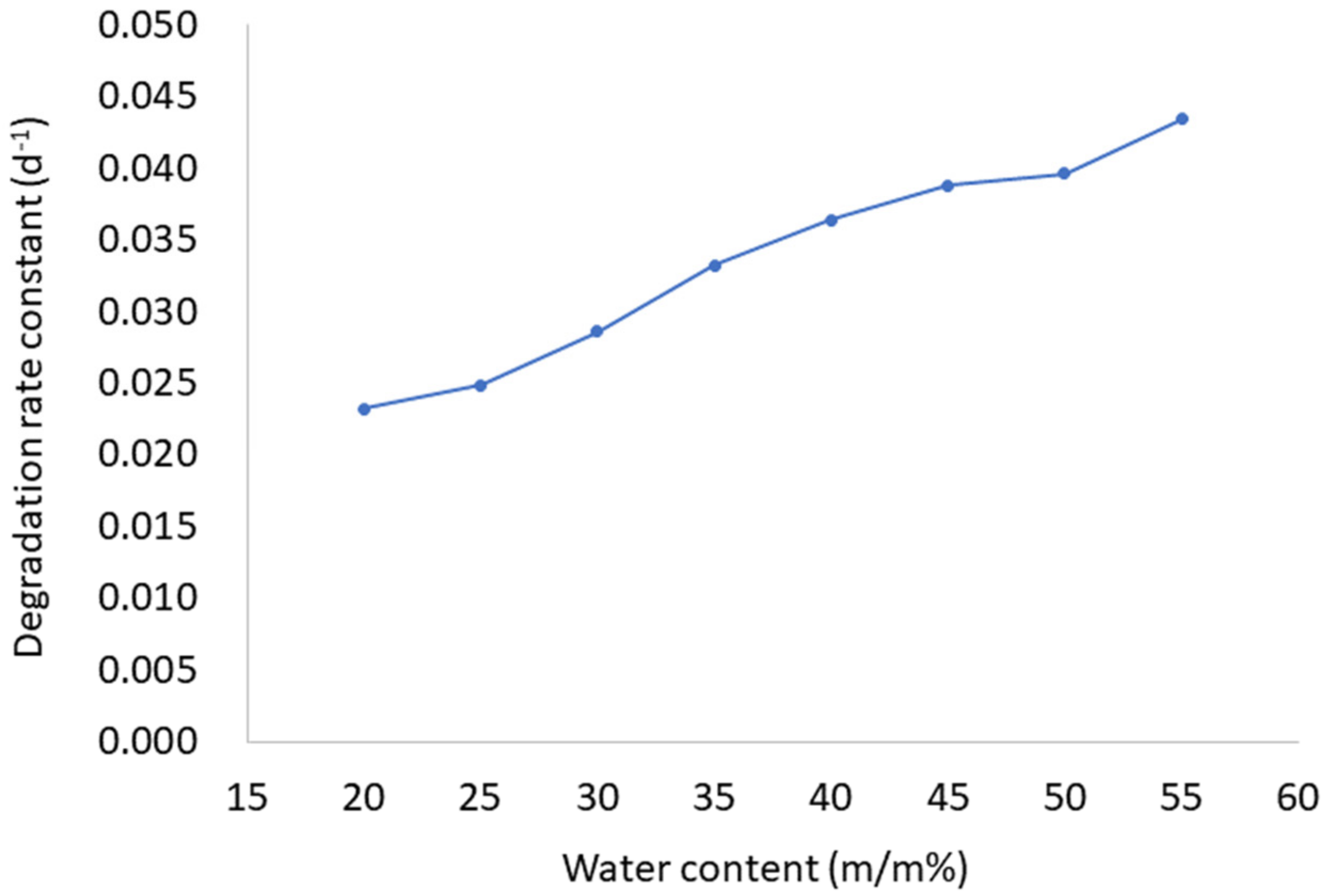
2.1. Ascorbyl Palmitate Stability
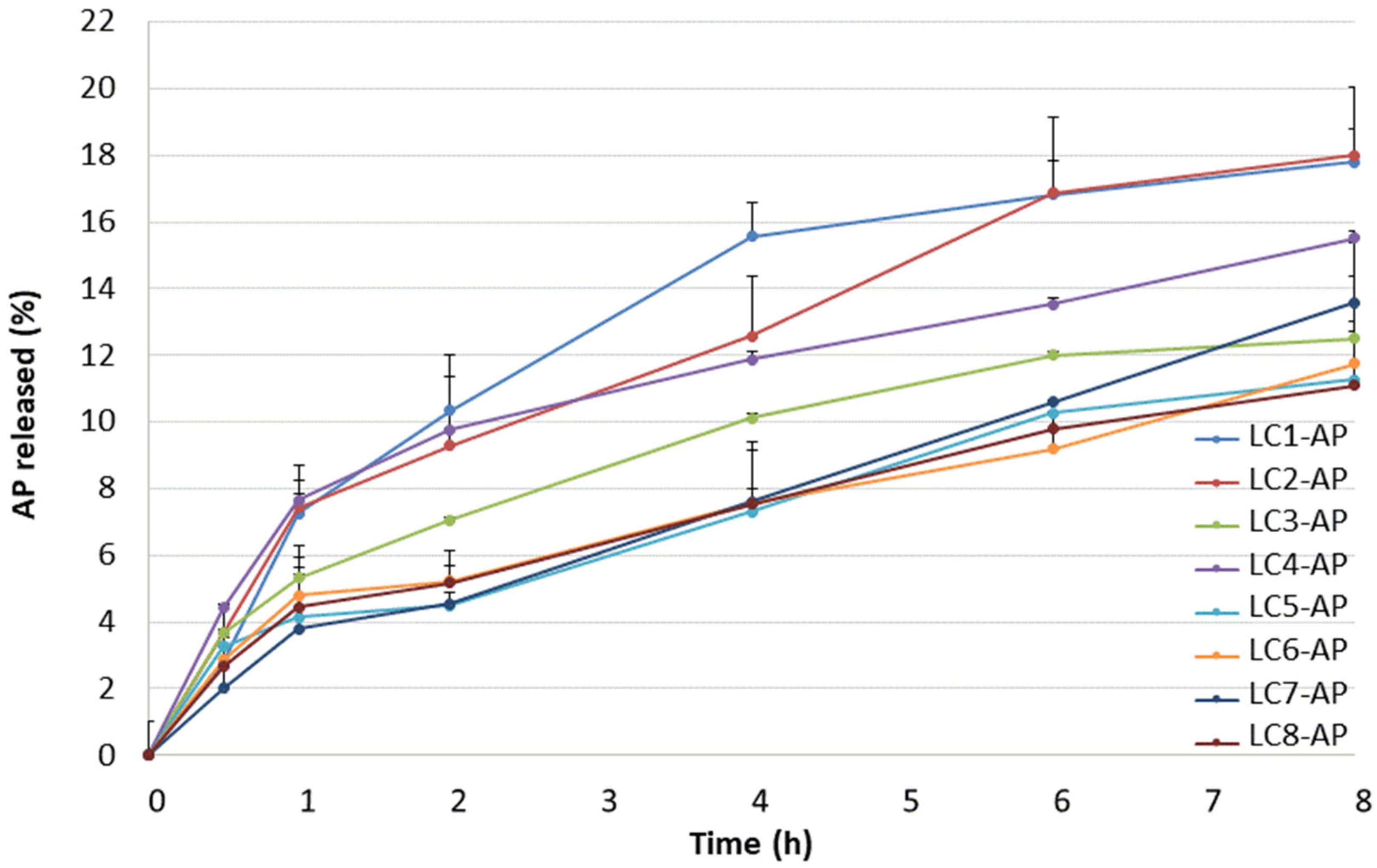
2.2. In Vitro Release Profile of Ascorbyl Palmitate
2.3. In Vitro Skin Performance
2.4. Structural Characterisation of LCs
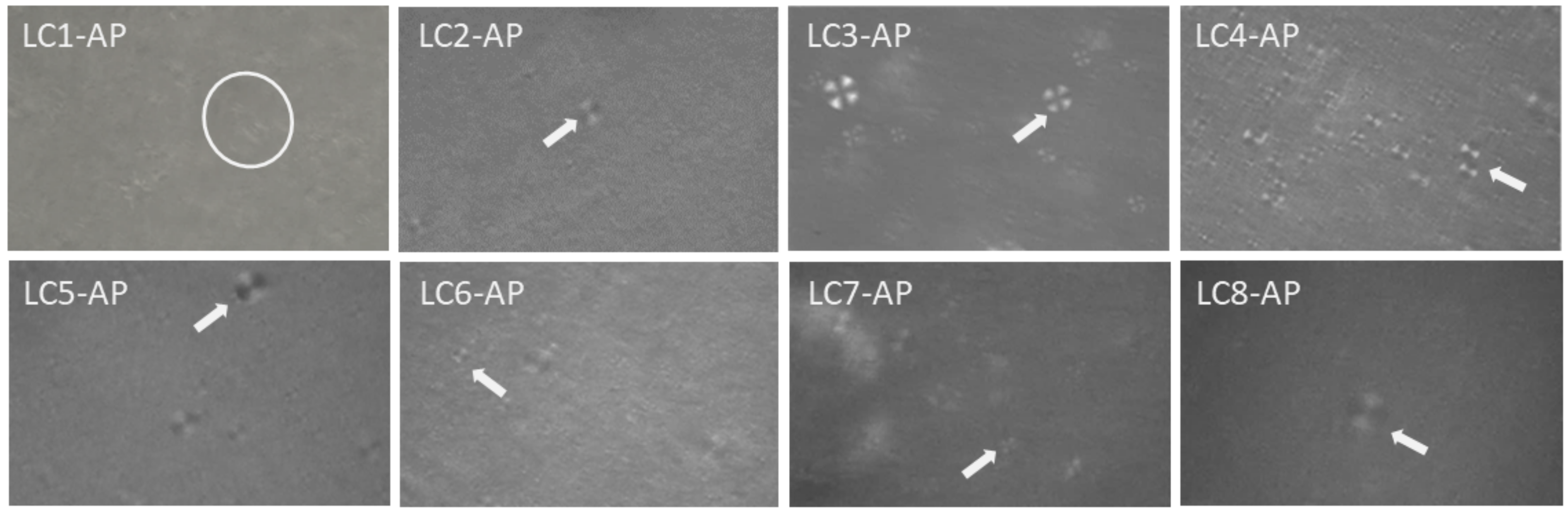
2.4.1. Polarized Light Microscopy Investigations
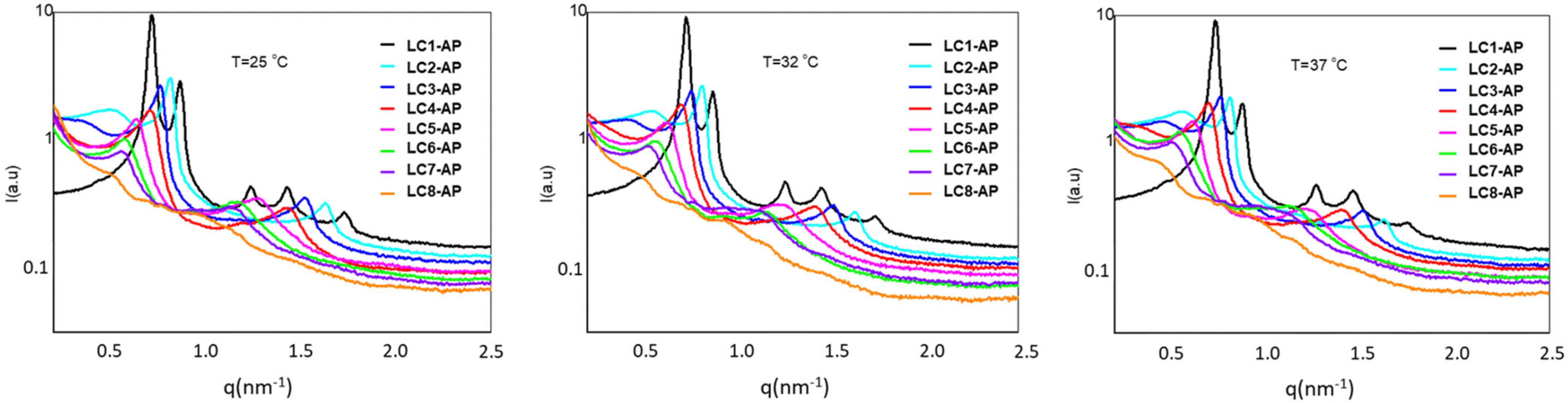
2.4.2. SAXS Analysis
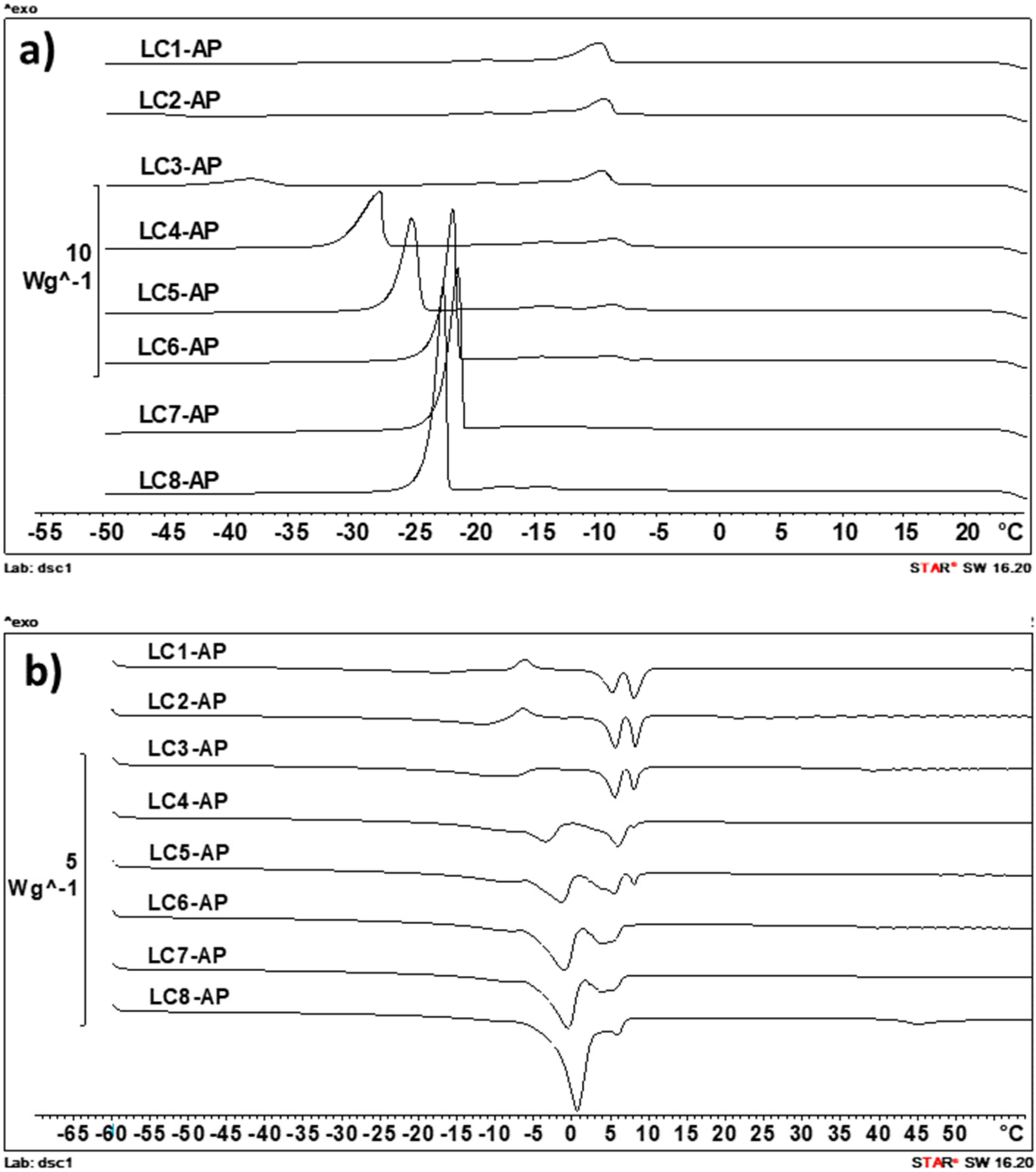
2.4.3. DSC Analysis
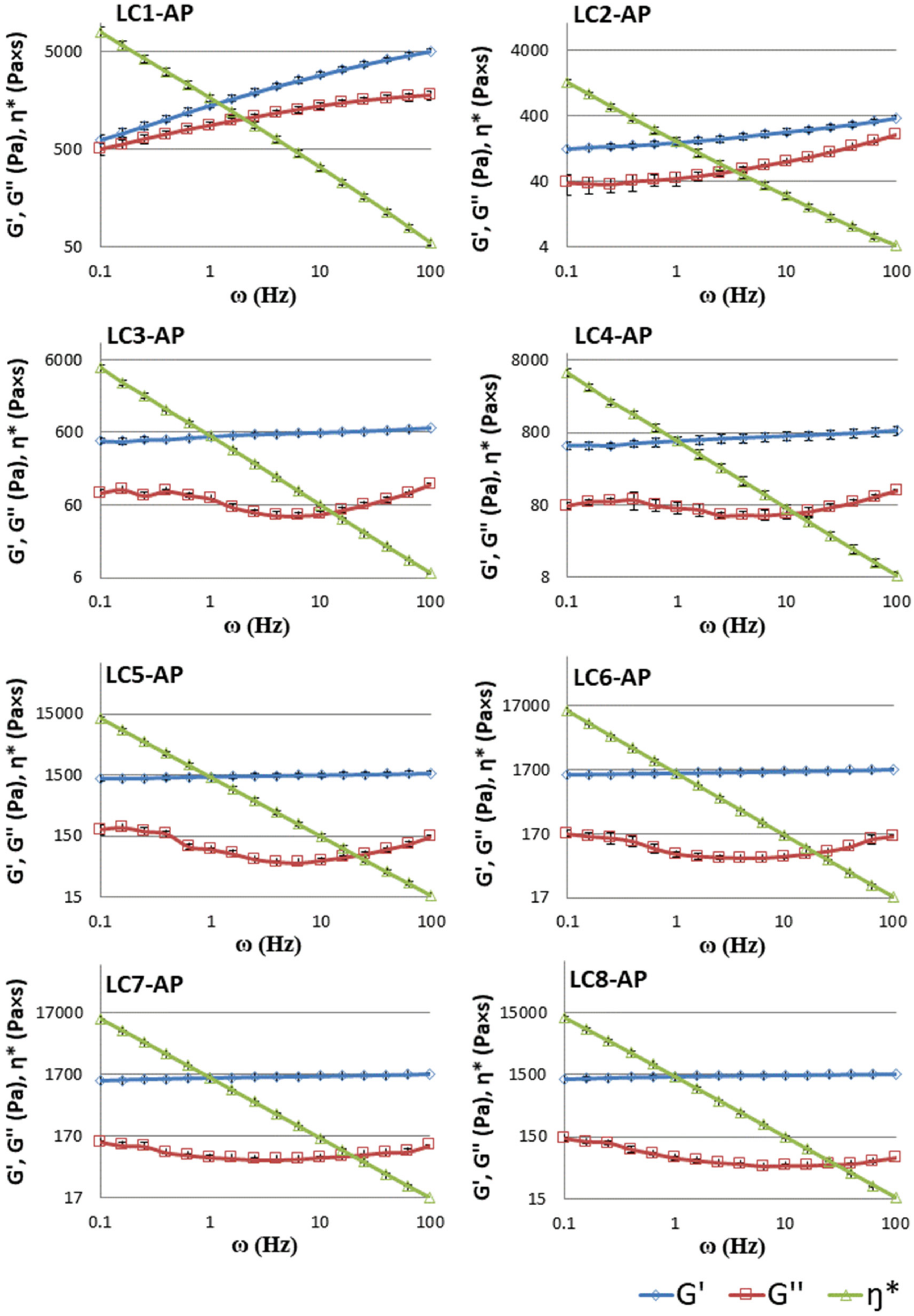
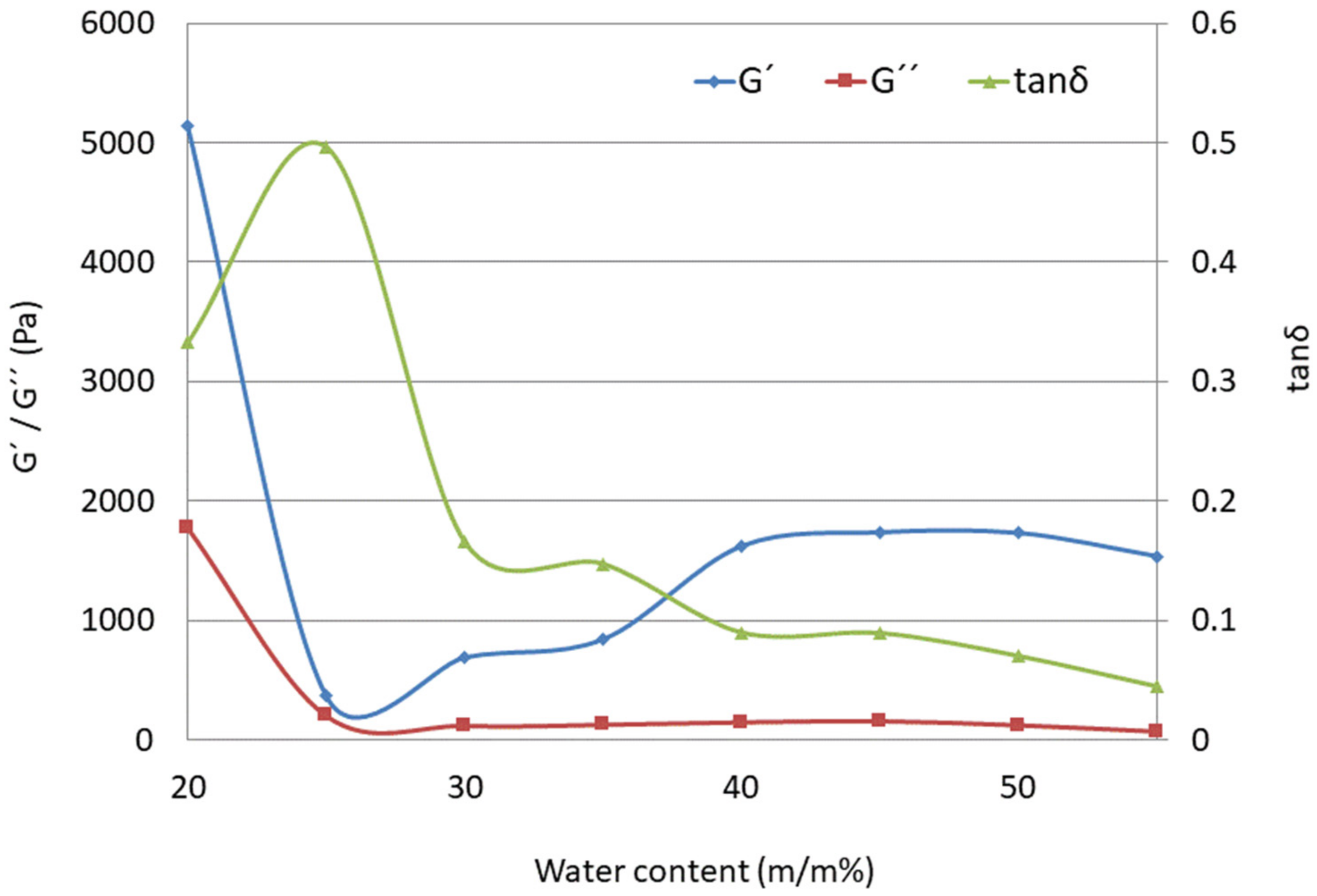
2.4.4. Rheological Behavior
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Sample Preparation
3.2.2. Stability Study
3.2.3. In Vitro Drug Release Study
3.2.4. Skin Performance Testing
3.2.5. Structural Characterization
Polarizing Light Microscopy
Small-Angle X-ray Scattering
Differential Scanning Calorimetry
Rheological Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ. Int. 2024, 185, 108535. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Dahmane, R. Free Radicals and Extrinsic Skin Aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Mota, S.; Torres, A.; Cruz, M.T.; Sousa, E.; Almeida, I.F.; Cidade, H. Antioxidants in Sunscreens: Which and What For? Antioxidants 2023, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Ramos-e-Silva, M.; Carneiro, S.C. Cosmetics for the elderly. Clin. Dermatol. 2001, 19, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Oresajo, C.; Pillai, S.; Manco, M.; Yatskayer, M.; McDaniel, D. Antioxidants and the skin: Understanding formulation and efficacy. Dermatol. Ther. 2012, 25, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Gašperlin, M.; Gosenca, M. Main approaches for delivering antioxidant vitamins through the skin to prevent skin ageing. Expert Opin. Drug Deliv. 2011, 8, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, D.V.; Ankola, D.D.; Bhardwaj, V.; Sahana, D.K.; Kumar, M.N. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control Release 2006, 113, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Nageen, A.; Shilpi, A.; Rayasa, M.R.S. Latest Technology Advances in Cosmaceuticals. Int. J. Pharm. Sci. Drug Res. 2012, 4, 168–182. [Google Scholar]
- Costa, R.; Santos, L. Delivery systems for cosmetics—From manufacturing to the skin of natural antioxidants. Powder Technol. 2017, 322, 402–416. [Google Scholar] [CrossRef]
- Kouassi, M.C.; Grisel, M.; Gore, E. Multifunctional active ingredient-based delivery systems for skincare formulations: A review. Colloids Surf. B Biointerfaces 2022, 217, 112676. [Google Scholar] [CrossRef] [PubMed]
- Rapalli, V.K.; Waghule, T.; Hans, N.; Mahmood, A. Insight into lyotropic liquid crystals in topical drug delivery for targeting various skin disorders. J. Mol. Liquids 2020, 315, 1137712020. [Google Scholar] [CrossRef]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.; Abd, E.; Leite-Silva, V.; Benson, H.; et al. Topical and Cutaneous Delivery Using Nanosystems. J. Control Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Jurkovič, P.; Šentjurc, M.; Gašperlin, M.; Kristl, J.; Pečar, S. Skin protection against ultraviolet induced free radicals with ascorbyl palmitate in microemulsions. Eur. J. Pharm. Biopharm. 2003, 56, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kristl, J.; Volk, B.; Gašperlin, M.; Šentjurc, M.; Jurkovič, P. Effect of colloidal carriers on ascorbyl palmitate stability. Eur. J. Pharm. Sci. 2003, 19, 181–189. [Google Scholar] [CrossRef]
- Bhowmick, M.; Sengodan, T.; Erode, S. Evaluation and characterisation of transdermal therapeutic systems: An exhaustive pictorial and figurative review. J. Drug. Deliv. Ther. 2014, 4, 9–22. [Google Scholar]
- Ji, J. Chemical synthesis of ascorbyl palmitate in [BMIM]BF4. Adv. Mat. Res. 2011, 236, 1962–1965. [Google Scholar]
- Üner, M.; Wissig, S.; Yener, G.; Müller, R. Solid lipid nanoparticles (SLN) and nanostructured lipid carreirs (NLC) for application of ascorbyl palmitate. Pharmazie 2005, 60, 577–582. [Google Scholar] [PubMed]
- Špiclin, P.; Gašperlin, M.; Kmetec, V. Stability of ascorbyl palmitate in topical microemulsions. Int. J. Pharm. 2001, 222, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Gosenca, M.; Bešter-Rogač, M.; Gašperlin, M. Lecithin based lamellar liquid crystals as a physiologically acceptable dermal delivery system for ascorbyl palmitate. Eur. J. Pharm. Sci. 2013, 50, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K.K.; Kannojia, P.; Mishra, N. Liquid Crystal Systems in Drug Delivery. In Novel Approaches for Drug Delivery; Keservani, R.K., Sharma, A.K., Kesharwani, R.K., Eds.; IGI Global: Hershey, PA, USA, 2016; pp. 217–243. [Google Scholar]
- Pupo Silvestrini, A.V.; Caron, A.L.; Viegas, J.S.R.; Lopes Badra Bentley, M.V. Advances in lyotropic liquid crystal systems for skin drug delivery. Expert Opin. Drug Deliv. 2020, 17, 1781–1805. [Google Scholar] [CrossRef] [PubMed]
- Vitek, M.; Gosenca Matjaž, M. Clinical application of hempseed or flaxseed oil-based lyotropic liquid crystals: Evaluation of their impact on skin barrier function. Acta Pharm. 2024, 74, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Müller-Goymann, C.C. Drug delivery. Liquid crystals. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Ed.; Informa Healthcare: New York, NY, USA, 2007; Volume 2, pp. 1115–1131. [Google Scholar]
- Sharma, S.C.; Warr, G.G. Phase behavior, self-assembly, and emulsification of Tween 80/water mixtures with limonene and perfluoromethyldecalin. Langmuir 2012, 28, 11707–11713. [Google Scholar] [CrossRef] [PubMed]
- Garti, N.; Libster, D.; Aserin, A. Solubilization and delivery of drugs from GMO-based lyotropic liquid crystals. In Nanoscience with Liquid Crystals, from Self-Organized Nanostructures to Applications; Li, Q., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 355–414. [Google Scholar]
- Burducea, G. Lyotropic liquid crystals I. Specific structures. Rom. Rep. Phys. 2004, 56, 66–86. [Google Scholar]
- Stevenson, C.L.; Bennett, D.B.; Lechuga-Ballesteros, D. Pharmaceutical liquid crystals: The relevance of partially ordered systems. J. Pharm. Sci. 2005, 9, 1861–1880. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jahn, A.; Cho, S.J.; Kim, J.S.; Ki, M.-H.; Kim, D.-D. Lyotropic liquid crystal systems in drug delivery: A review. J. Pharm. Investig. 2015, 45, 1–11. [Google Scholar] [CrossRef]
- Makai, M.; Csanyi, E.; Németh, Z.; Pálinkás, J.; Erős, I. Structure and drug release of lamellar liquid crystals containing glycerol. Int. J. Pharm. 2003, 256, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Martiel, I.; Baumann, N.; Vallooran, J.J.; Bergfreund, J.; Sagalowicz, L.; Mezzenga, R. Oil and drug control the release rate from lyotropic liquid crystals. J. Control. Release 2015, 204, 78–84. [Google Scholar] [CrossRef]
- Gosenca Matjaž, M.; Škarabot, M.; Gašperlin, M.; Janković, B. Lamellar liquid crystals maintain keratinocytes′ membrane fluidity: An AFM qualitative and quantitative study. Int. J. Pharm. 2019, 572, 118712. [Google Scholar] [CrossRef] [PubMed]
- Tsengam, I.K.M.; Omarova, M.; Kelley, E.G.; McCormick Tsengam, A. Transformation of Lipid Vesicles into Micelles by Adding Nonionic Surfactants: Elucidating the Structural Pathway and the Intermediate Structures. J. Phys. Chem. B 2022, 126, 2208–2216. [Google Scholar] [CrossRef] [PubMed]
- Benedini, L.; Schulz, E.P.; Messina, P.V.; Palma, S.D.; Allemandi, D.A.; Schulz, P.C. The ascorbyl palpitate-water system: Phase diagram and state of the water. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 178–185. [Google Scholar] [CrossRef]
- Mackeben, S.; Müller, M.; Müller-Goymann, C.C. The influence of water on phase transition of a drug-loaded reverse micellar solution into lamellar liquid crystal. Colloids Surf. A Physicochem. Eng. Asp. 2001, 183–185, 699–713. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Manciu, M. On the Stability of Lyotropic Lamellar Liquid Crystals and the Thicknesses of Their Lamellae. Langmuire 2001, 17, 546–5475. [Google Scholar] [CrossRef]
- Huang, Y.; Gui, S. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. RSC Adv. 2018, 8, 6978–6987. [Google Scholar] [CrossRef] [PubMed]
- Gosenca Matjaž, M.; Mravljak, J.; Bešter Rogač, M.; Šentjurc, M.; Gašperlin, M.; Zvonar Pobirk, A. Microstructure evaluation of dermally applicable liquid crystals as a function of water content and temperature: Can electron paramagnetic resonance provide complementary data? Int. J. Pharm. 2017, 533, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Austria, R.; Semenzato, A.; Bettero, A. Stability of vitamin C derivatives in solution and topical formulations. J. Pharm. Biomed. Anal. 1997, 15, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Vitek, M.; Gosenca Matjaž, M.; Roškar, R.; Gašperlin, M.; Zvonar Pobirk, A. A comparative study of lipid-based drug delivery systems with different microstructure for combined dermal administration of antioxidant vitamins. J. Disper. Sci. Technol. 2022, 44, 1711–1724. [Google Scholar] [CrossRef]
- Gradzielski, M.; Hoffmann, H. Rheological Properties of Microemulsions. In Handbook of Microemulsion Science and Technology, 1st ed.; Kumar, P., Mittal, K.L., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1999; p. 375. [Google Scholar]
- Podlogar, F.; Bešter-Rogač, M.; Gašperlin, M. The effect of internal structure of selected water–Tween 40®–Imwitor 308®–IPM microemulsions on ketoprofene release. Int. J. Pharm. 2005, 302, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, L.; Primorac, M.; Stupar, M. In vitro release of diclofenacdiethylamine from caprylocaproyl macrogolglycerides based micro-emulsions. Int. J. Pharm. 2005, 296, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Ngwuluka, N.C.; Lawal, K.; Olorunfemi, P.O.; Ochekpe, N.A. Post-market in vitro bioequivalence study of six brands of ciprofloxacin tablets/caplets in Jos, Nigeria. Sci. Res. Essays 2009, 4, 298–305. [Google Scholar]
- van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The Important Role of Stratum Corneum Lipids for the Cutaneous Barrier Function. Biochim. Biophys. Acta 2014, 1841, 295–313. [Google Scholar] [CrossRef]
- Baker, P.; Huang, C.; Radi, R.; Moll, S.B.; Jules, E.; Arbiser, J.L. Skin Barrier Function: The Interplay of Physical, Chemical, and Immunologic Properties. Cells 2023, 12, 2745. [Google Scholar] [CrossRef] [PubMed]
- Bing, J.; Qianjie, Z.; Zheng, Z.; Minghua, C.; Wanping, Z. Preparation of liquid crystal emulsion and its application performance study. J. Dispers. Sci. Technol. 2017, 39, 100–105. [Google Scholar] [CrossRef]
- Zhuang, W.; Chen, X.; Cai, J.; Zhang, G.; Qiu, H. Characterisation of lamellar phases fabricated from Brij-30/water/1-butyl-3-methylimidazolium salts ternary systems by small-angle X-ray scattering. Colloids Surf. A Physicochem. Eng. Asp. 2008, 318, 175–183. [Google Scholar] [CrossRef]
- Garti, N.; Aserin, A.; Tiunova, I.; Fanun, M. A DSC study of water behaviour in water-in-oil microemulsions stabilized by sucrose esters and butanol. Colloids Surf. A Physicochem. Eng. Asp. 2000, 170, 1–18. [Google Scholar] [CrossRef]
- Sato, K. Crystallization behaviour of fats and lipids: A review. Chem. Eng. Sci. 2001, 56, 2255–2265. [Google Scholar] [CrossRef]
- Kodama, M.; Aoki, H. Water in phospholipid bilayer systems. In Thermal Behaviour of Dispersed System; Garti, N., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2001; pp. 59–181. [Google Scholar]
- Omidian, H.; Park, K. Hydrogels. In Fundamentals and Applications of Controlled Release Drug Delivery; Siepmann, J., Siegel, R.A., Rathbone, M.J., Eds.; Springer Science&Business Media, LLC: New York, NY, USA, 2012; pp. 75–105. [Google Scholar]
- Carvalho, F.C.; Barbi, M.S.; Sarmento, V.H.; Chiavacci, L.A.; Netto, F.M.; Gremião, M.P. Surfactant systems for nasal zidovudine delivery: Structural, rheological and mucoadhesive properties. J. Pharm. Pharmacol. 2010, 62, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, J.; Reed, R.; Bentley, M.V.; Nornoo, A.; Lopes, L.B. Microemulsions containing medium-chain glycerides as transdermal delivery systems for hydrophilic and hydrophobic drugs. AAPS PharmSciTech 2009, 10, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.; Alfaro, M.C. Rheological and phase behaviour of amphiphilic lipids. Grasas Aceites 2000, 51, 6–25. [Google Scholar] [CrossRef]
- Moros, J.E.; Franco, J.M.; Gallegos, C. Rheology of Spray-Dried Egg Yolk-Stabilized Emulsions. Int. J. Food Sci. Technol. 2002, 37, 297–307. [Google Scholar] [CrossRef]
- Montalvo, G.; Valiente, M.; Rodenas, E. Rheological properties of the L phase and the hexagonal, lamellar, and cubic liquid crystals of the CTAB/benzyl alcohol/water system. Langmuir 1996, 125, 202–5208. [Google Scholar] [CrossRef]
- Németh, Z.; Halász, L.; Pálinkás, J.; Bóta, A.; Horányi, T. Rheological behaviour of a lamellar liquid crystalline surfactant–water system. Colloids Surf. A Physicochem. Eng. Asp. 1998, 145, 107–119. [Google Scholar] [CrossRef]
- Dürrschmidt, T.; Hoffmann, H. Organogels from ABA triblock copolymers. Colloid Polym. Sci. 2001, 279, 1005–1012. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Z.; Liu, F.; Mi, Q.; Wu, J. Study on the microstructure and rheological property of fish oil lyotropic liquid crystal. Colloids Surf. A Physicochem. Eng. Asp. 2011, 385, 47–54. [Google Scholar] [CrossRef]
| % of Nondegraded AP over Time (Days) | |||||||
|---|---|---|---|---|---|---|---|
| Sample | t = 0 | t = 1 | t = 7 | t = 14 | t = 28 | t = 47 | t = 56 |
| LC1-AP | 100.0 | 97.8 ± 0.5 | 75.7 ± 0.1 | 66.6 ± 0.1 | 52.1 ± 0.8 | 28.0 ± 0.3 | 14.8 ± 0.4 |
| LC2-AP | 100.0 | 101.6 ± 0.1 | 78.7 ± 0.0 | 67.0 ± 0.1 | 51.0 ± 1.1 | 19.8 ± 0.3 | 10.6 ± 0.1 |
| LC3-AP | 100.0 | 100.5 ± 0.5 | 78.9 ± 0.1 | 65.0 ± 0.3 | 45.6 ± 1.2 | 12.4 ± 0.1 | 6.4 ± 0.2 |
| LC4-AP | 100.0 | 100.4 ± 0.6 | 78.1 ± 0.4 | 64.7 ± 0.1 | 39.8 ± 0.1 | 4.7 ± 0.1 | 3.0 ± 0.1 |
| LC5-AP | 100.0 | 96.8 ± 1.2 | 73.6 ± 0.2 | 59.9 ± 0.1 | 35.9 ± 0.3 | 4.5 ± 0.0 | 2.7 ± 0.1 |
| LC6-AP | 100.0 | 97.6 ± 0.7 | 69.0 ± 0.7 | 56.7 ± 0.8 | 33.6 ± 0.4 | 6.7 ± 0.0 | 4.2 ± 0.1 |
| LC7-AP | 100.0 | 97.1 ± 1.0 | 67.5 ± 1.7 | 57.3 ± 0.4 | 32.5 ± 0.3 | 5.8 ± 0.2 | 4.2 ± 0.0 |
| LC8-AP | 100.0 | 94.5 ± 0.1 | 62.3 ± 0.6 | 49.7 ± 1.3 | 29.3 ± 0.2 | 6.7 ± 0.1 | 4.3 ± 0.0 |
| SMEDDS | 100.0 | 98.1 ± 18.2 | 53.2 ± 9.3 | 43.6 ± 0.8 | 30.1 ± 5.8 | 13.7 ± 0.6 | 10.0 ± 0.6 |
| W/O ME | 100.0 | 95.5 ± 1.9 | 68.7 ± 0.1 | 61.2 ± 0.2 | 40.1 ± 0.3 | 18.2 ± 0.5 | 13.4 ± 0.3 |
| LC1-AP | LC2-AP | LC3-AP | LC4-AP | LC5-AP | LC6-AP | LC7-AP | LC8-AP | |
|---|---|---|---|---|---|---|---|---|
| Zero-order | 0.8489 | 0.9064 | 0.8674 | 0.8248 | 0.9339 | 0.9209 | 0.9818 | 0.9237 |
| First-order | 0.8635 | 0.9214 | 0.8794 | 0.8432 | 0.9411 | 0.9298 | 0.9854 | 0.9325 |
| Higuchi | 0.9640 | 0.9890 | 0.9884 | 0.9714 | 0.9777 | 0.9840 | 0.9673 | 0.9939 |
| Korsmeyer–Peppas | 0.9171 | 0.9651 | 0.9933 | 0.9569 | 0.9430 | 0.9689 | 0.9819 | 0.9830 |
| Hixon–Crowell | 0.8587 | 0.9165 | 0.8755 | 0.8372 | 0.9388 | 0.9269 | 0.9843 | 0.9296 |
| Sample | Baseline | Absolute Value after 30 min | Absolute Value after 90 min | Change # from Baseline after 30 min | Change # from Baseline after 90 min | p-Value * | p-Value ** |
|---|---|---|---|---|---|---|---|
| LC1 | 37.90 ± 5.68 | 30.87 ± 5.81 | 30.19 ± 3.92 | −7.03 (18.6%) | −7.71 (20.3%) | 0.12 | 0.06 |
| 7.9 ± 5.0 | 22.6 ± 9.6 | 15.0 ± 3.6 | +14.7 (186.1%) | +7.1 (89.9%) | 0.06 | 0.09 | |
| LC2 | 33.36 ± 5.06 | 30.45 ± 3.41 | 28.14 ± 4.33 | −2.91 (8.7%) | −5.22 (15.6%) | 0.44 | 0.22 |
| 6.1 ± 1.6 | 15.6 ± 4.2 | 11.0 ± 4.8 | +9.5 (155.7%) | +4.9 (80.3%) | 0.04 | 0.25 | |
| LC3 | 31.91 ± 5.97 | 27.73 ± 6.04 | 26.90 ± 7.67 | −4.18 (13.1%) | −5.01 (15.7%) | 0.35 | 0.33 |
| 5.5 ± 4.2 | 11.8 ± 6.9 | 14.6 ± 5.9 | +6.3 (114.6%) | +9.1 (165.5%) | 0.16 | 0.03 | |
| LC4 | 28.29 ± 7.85 | 24.68 ± 7.06 | 23.75 ± 8.74 | −3.61 (12.8%) | −4.54 (16.1%) | 0.58 | 0.53 |
| 15.4 ± 4.2 | 33.3 ± 7.9 | 29.6 ± 11.0 | +17.9 (116.2%) | +14.2 (92.2%) | 0.01 | 0.08 | |
| LC5 | 36.76 ± 4.10 | 32.49 ± 4.47 | 28.54 ± 5.44 | −4.27 (11.6%) | −8.22 (22.4%) | 0.27 | 0.08 |
| 5.3 ± 1.6 | 6.0 ± 3.6 | 5.9 ± 3.7 | +0.7 (13.2%) | +0.6 (11.3%) | 0.75 | 0.79 | |
| LC6 | 31.51 ± 6.90 | 26.29 ± 6.67 | 25.52 ± 6.05 | −5.22 (16.6%) | −5.99 (19.0%) | 0.48 | 0.41 |
| 4.2 ± 2.7 | 4.8 ± 0.3 | 4.5 ± 0.7 | +0.6 (14.2%) | +0.3 (7.1%) | 0.86 | 0.92 | |
| LC7 | 30.22 ± 2.53 | 29.00 ± 3.85 | 28.82 ± 2.53 | −1.22 (4.0%) | −1.40 (4.6%) | 0.66 | 0.52 |
| 8.9 ± 4.7 | 15.6 ± 3.7 | 11.6 ± 5.6 | +6.6 (73.3%) | +2.6 (28.9%) | 0.09 | 0.54 | |
| LC8 | 31.43 ± 5.94 | 28.10 ± 6.97 | 27.37 ± 8.27 | −3.33 (10.6%) | −4.06 (12.9%) | 0.55 | 0.52 |
| 6.8 ± 4.7 | 8.5 ± 5.7 | 8.3 ± 4.8 | +1.7 (25.0%) | +1.5 (22.1%) | 0.76 | 0.77 |
| Sample | d (nm) | ||
|---|---|---|---|
| 25 °C | 32 °C | 37 °C | |
| LC1-AP | 8.76 | 8.64 | 8.58 |
| LC2-AP | 7.74 | 7.75 | 7.76 |
| LC3-AP | 8.27 | 8.34 | 8.28 |
| LC4-AP | 8.87 | 8.96 | 9.02 |
| LC5-AP | 9.87 | 10.10 | 10.22 |
| LC6-AP | 10.99 | 11.13 | 11.63 |
| LC7-AP | 11.24 | 12.00 | 12.50 |
| LC8-AP | / | / | / |
| Sample | W/S Ratio | η (Pas) 25 °C | η (Pas) 32 °C | η (Pas) 37 °C | tan δ (100 Hz) |
|---|---|---|---|---|---|
| LC1-AP | 0.36 | 47.3 | 44.8 | 43.4 | 0.333 |
| LC2-AP | 0.48 | 18.9 | 9.2 | 8.3 | 0.497 |
| LC3-AP | 0.61 | 34.3 | 30.6 | 22.2 | 0.166 |
| LC4-AP | 0.78 | 26.6 | 17.5 | 16.0 | 0.147 |
| LC5-AP | 0.95 | 81.2 | 37.4 | 28.7 | 0.090 |
| LC6-AP | 1.18 | 73.3 | 42.2 | 32.3 | 0.089 |
| LC7-AP | 1.43 | 155.0 | 108.9 | 81.1 | 0.070 |
| LC8-AP | 1.77 | 70.4 | 59.1 | 52.4 | 0.044 |
| Sample # | (Tween 80/Lecithin) * | IPM | Bidistilled Water | AP |
|---|---|---|---|---|
| LC1-AP | 55.44 | 23.76 | 19.80 | 1 |
| LC2-AP | 51.48 | 22.77 | 24.75 | 1 |
| LC3-AP | 48.51 | 20.79 | 29.70 | 1 |
| LC4-AP | 44.55 | 19.80 | 34.65 | 1 |
| LC5-AP | 41.58 | 17.82 | 39.60 | 1 |
| LC6-AP | 37.62 | 16.83 | 44.55 | 1 |
| LC7-AP | 34.65 | 14.85 | 49.50 | 1 |
| LC8-AP | 30.69 | 13.86 | 54.45 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zvonar Pobirk, A.; Roškar, R.; Bešter-Rogač, M.; Gašperlin, M.; Gosenca Matjaž, M. The Impact of Phospholipid-Based Liquid Crystals’ Microstructure on Stability and Release Profile of Ascorbyl Palmitate and Skin Performance. Molecules 2024, 29, 3173. https://doi.org/10.3390/molecules29133173
Zvonar Pobirk A, Roškar R, Bešter-Rogač M, Gašperlin M, Gosenca Matjaž M. The Impact of Phospholipid-Based Liquid Crystals’ Microstructure on Stability and Release Profile of Ascorbyl Palmitate and Skin Performance. Molecules. 2024; 29(13):3173. https://doi.org/10.3390/molecules29133173
Chicago/Turabian StyleZvonar Pobirk, Alenka, Robert Roškar, Marija Bešter-Rogač, Mirjana Gašperlin, and Mirjam Gosenca Matjaž. 2024. "The Impact of Phospholipid-Based Liquid Crystals’ Microstructure on Stability and Release Profile of Ascorbyl Palmitate and Skin Performance" Molecules 29, no. 13: 3173. https://doi.org/10.3390/molecules29133173
APA StyleZvonar Pobirk, A., Roškar, R., Bešter-Rogač, M., Gašperlin, M., & Gosenca Matjaž, M. (2024). The Impact of Phospholipid-Based Liquid Crystals’ Microstructure on Stability and Release Profile of Ascorbyl Palmitate and Skin Performance. Molecules, 29(13), 3173. https://doi.org/10.3390/molecules29133173








