Designing Organic Spin-Gapless Semiconductors via Molecular Adsorption on C4N3 Monolayer
Abstract
1. Introduction
2. Results and Discussion
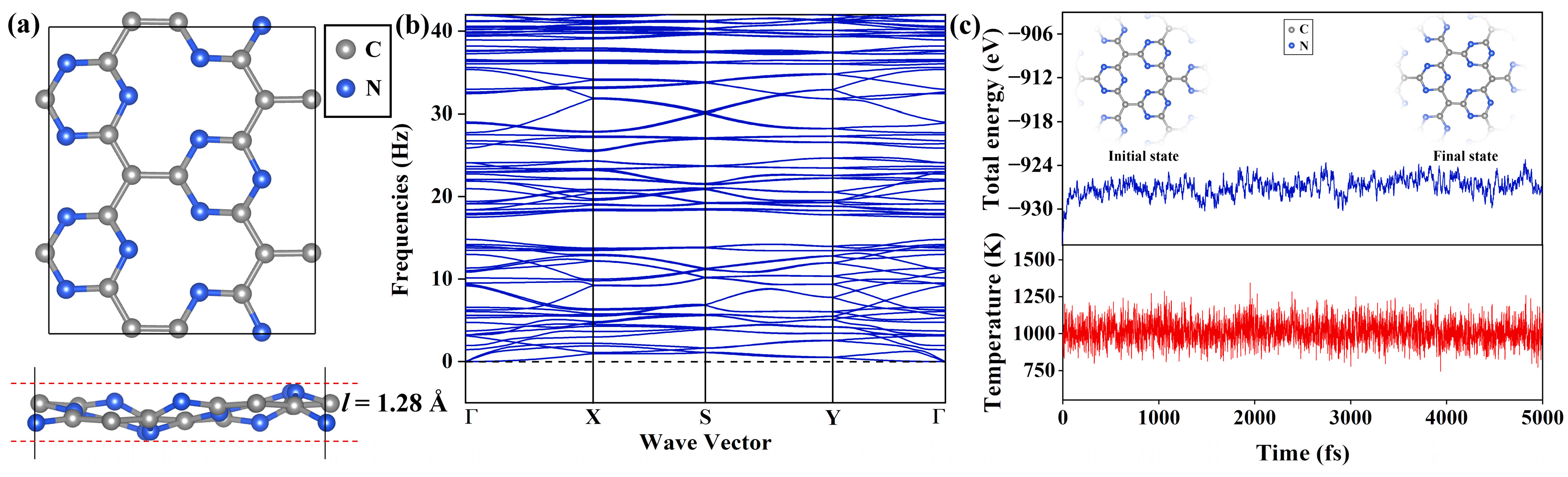
2.1. Geometric Structure and Stability of Pure Pca21 C4N3 Monolayer
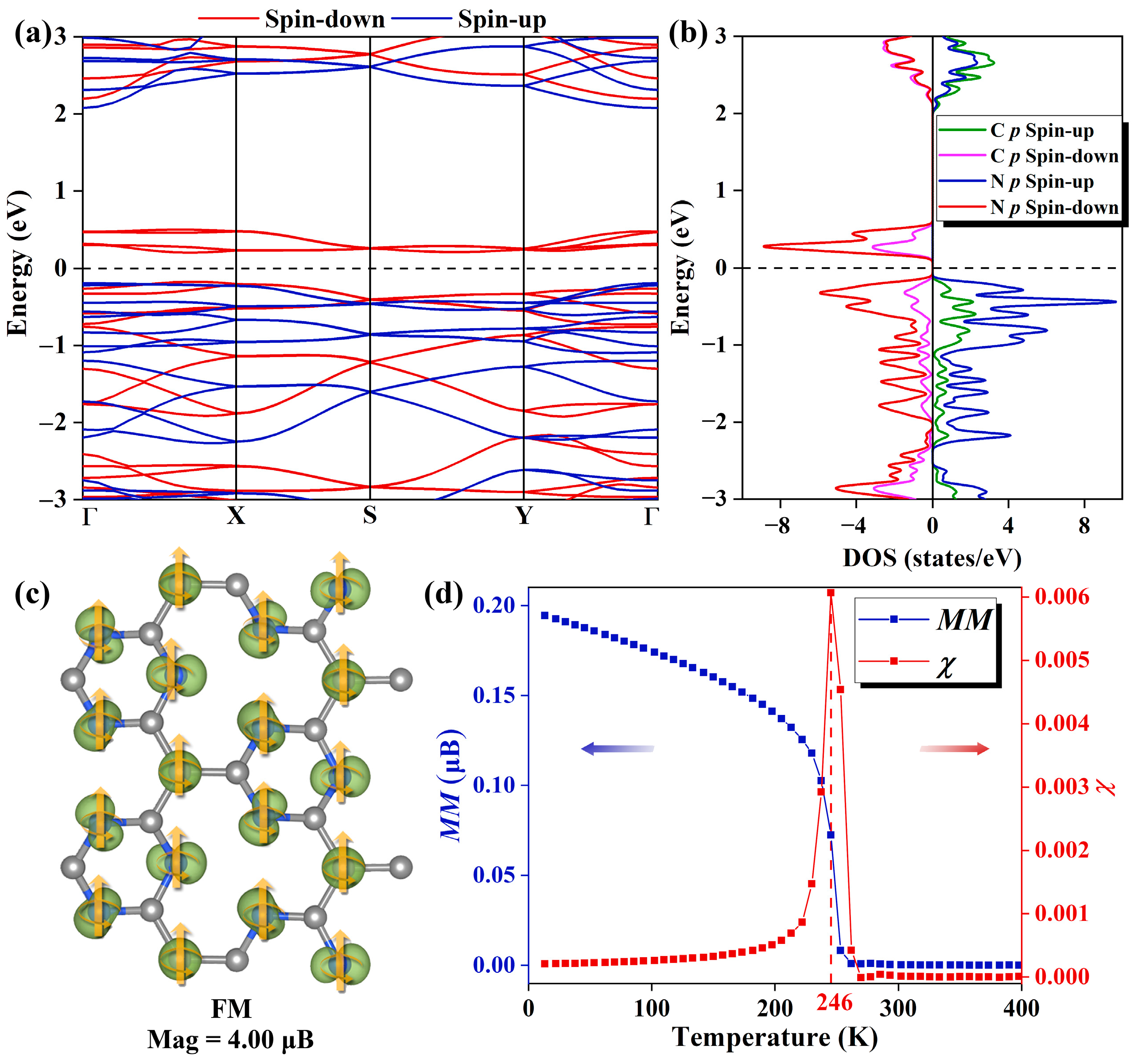
2.2. Electronic Structure and Magnetic Properties of Pure Pca21 C4N3 Monolayer
2.3. Magnetic Property of Pure Pca21 C4N3 Bilayer
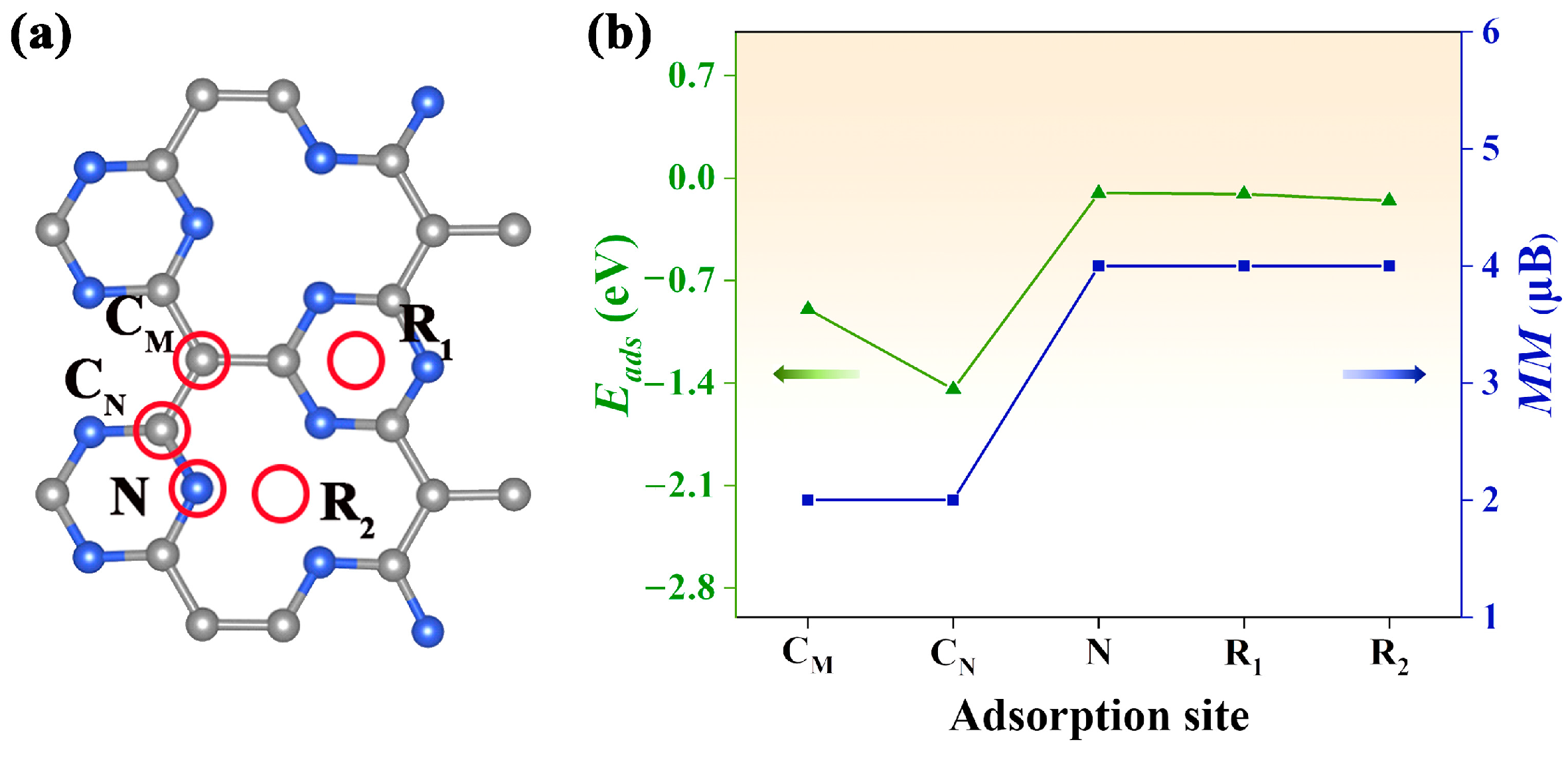
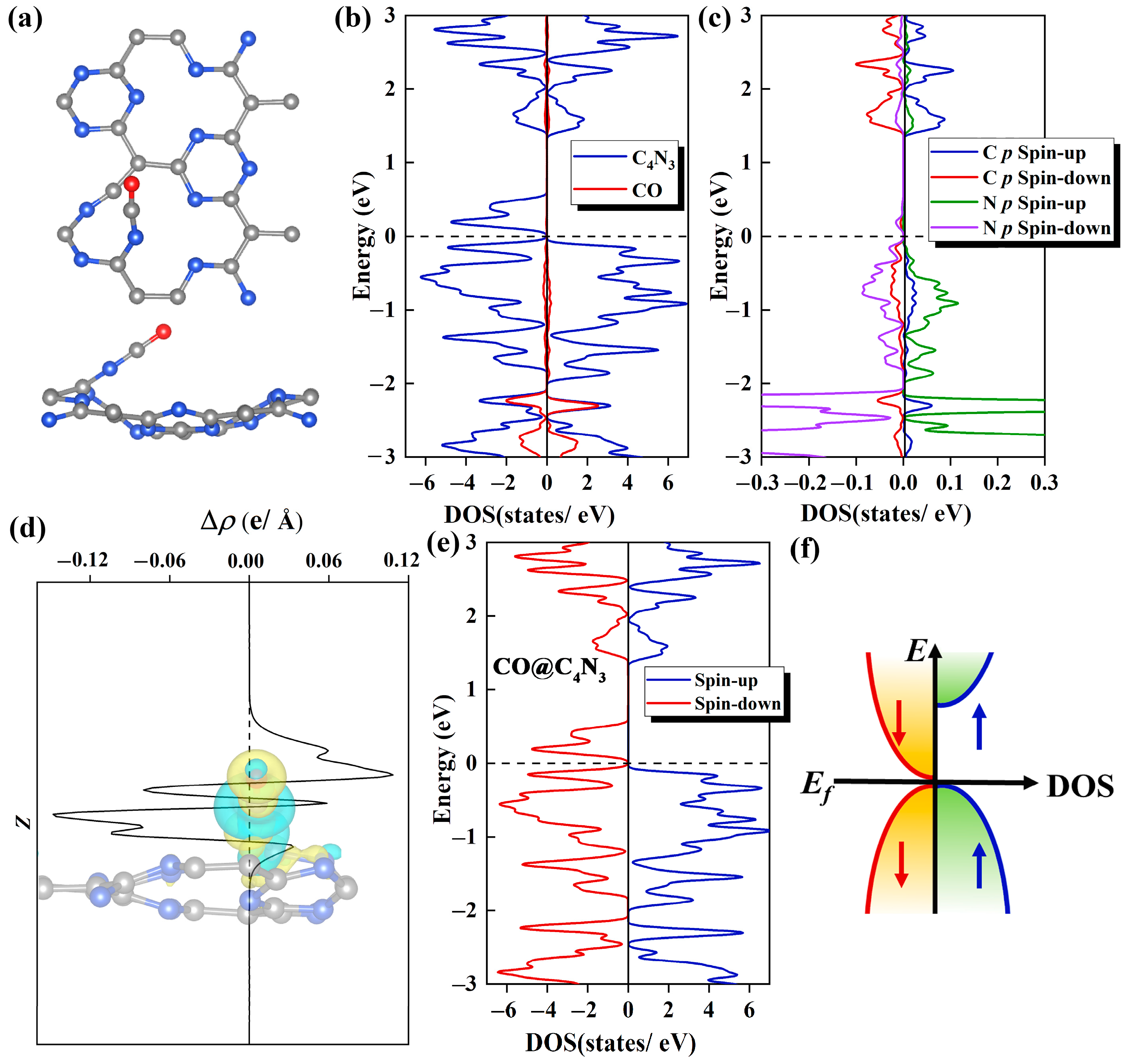
2.4. Improving SGS Property with Electron Injection through CO Adsorption
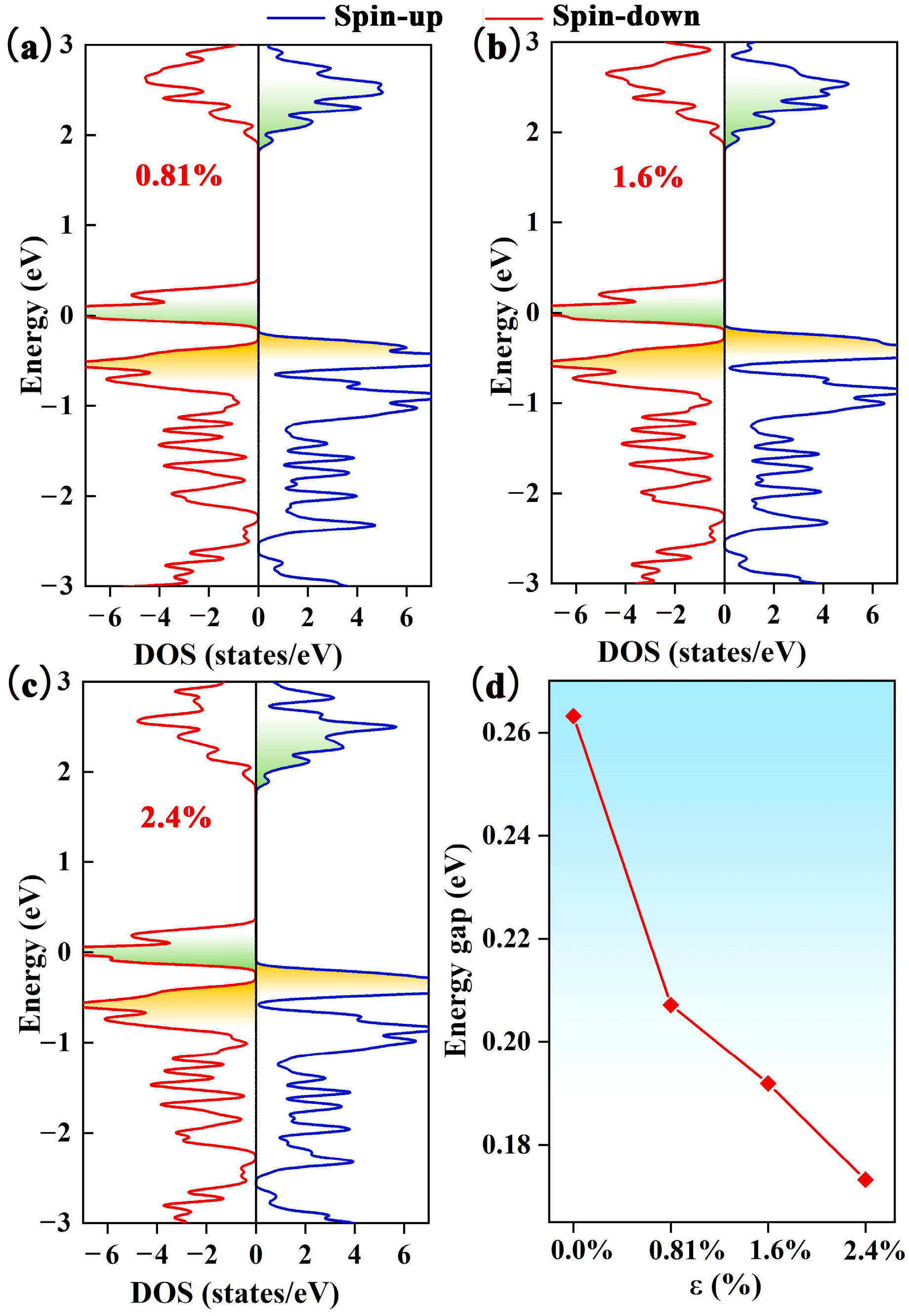
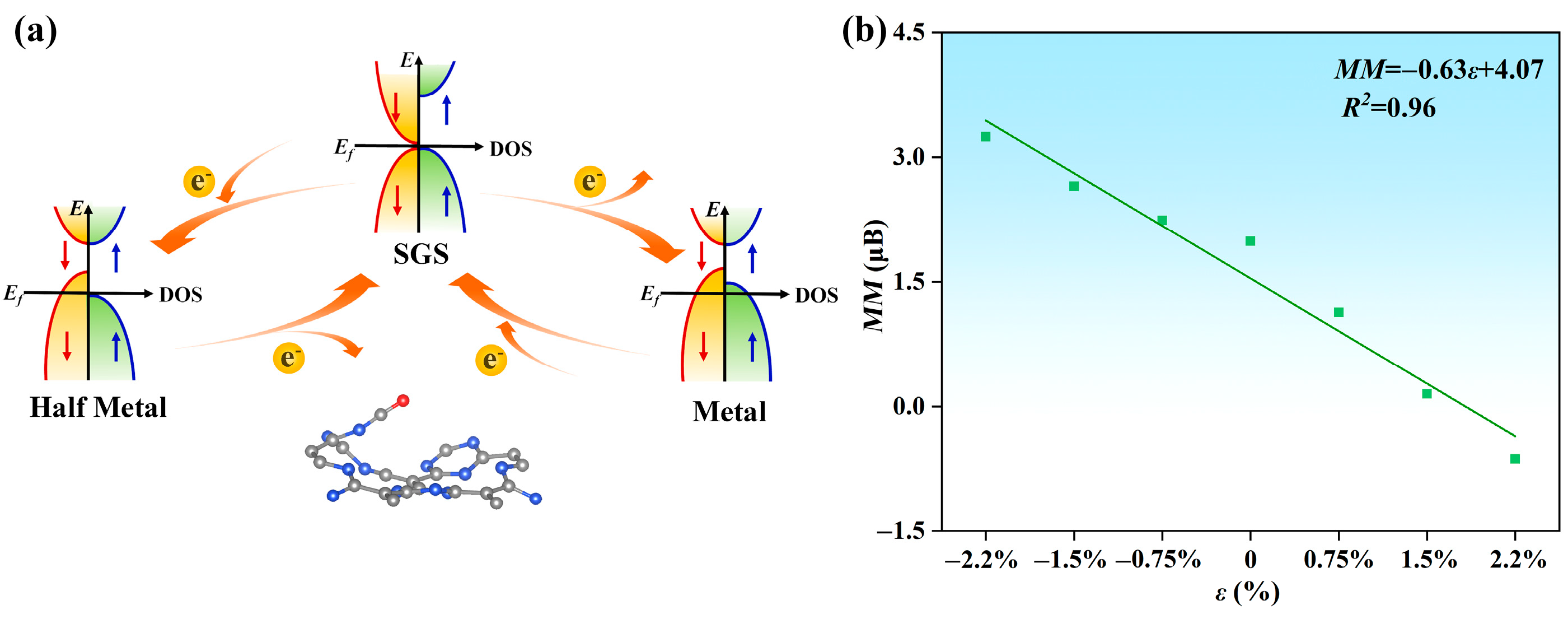
2.5. Adjusting the Magnetic Moment and Electrical Conductivity of CO@C4N3 through Electron/Hole Injection
3. Computation Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolf, S.; Awschalom, D.; Buhrman, R.; Daughton, J.; von Molnár, V.S.; Roukes, M.; Chtchelkanova, A.Y.; Treger, D. Spintronics: A spin-based electronics vision for the future. Science 2001, 294, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Awschalom, D.D.; Flatté, M.E. Challenges for semiconductor spintronics. Nat. Phys. 2007, 3, 153–159. [Google Scholar] [CrossRef]
- Felser, C.; Fecher, G.H.; Balke, B. Spintronics: A challenge for materials science and solid-state chemistry. Angew. Chem. Int. Ed. 2007, 46, 668–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Dou, S.X.; Zhang, C. Zero-gap materials for future spintronics, electronics and optics. NPG Asia. Mater. 2010, 2, 31–38. [Google Scholar] [CrossRef]
- Groves, S.; Brown, R.; Pidgeon, C. Interband magnetoreflection and band structure of HgTe. Phys. Rev. 1967, 161, 779. [Google Scholar] [CrossRef]
- Tang, E.; Mei, J.-W.; Wen, X.-G. High-temperature fractional quantum Hall states. Phys. Rev. Lett. 2011, 106, 236802. [Google Scholar] [CrossRef] [PubMed]
- Kane, C.L.; Mele, E.J. Z2 topological order and the quantum spin Hall effect. Phys. Rev. Lett. 2005, 95, 146802. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L. Proposal for a New Class of Materials: Spin Gapless Semiconductors. Phys. Rev. Lett. 2008, 100, 156404. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, H.; Motome, Y. Dirac Half-Metal in a Triangular Ferrimagnet. Phys. Rev. Lett. 2012, 109, 237207. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Han, D.; Li, X.-B.; Xie, S.-Y.; Chen, N.-K.; Tian, W.Q.; West, D.; Sun, H.-B.; Zhang, S.B. Determination of Formation and Ionization Energies of Charged Defects in Two-Dimensional Materials. Phys. Rev. Lett. 2015, 114, 196801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.W.; Wang, A.Z.; Zhang, X.M. Half-metallicity of a kagome spin lattice: The case of a manganese bis-dithiolene monolayer. Nanoscale 2013, 5, 10404–10408. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.Z.; Liu, E.K.; Du, Y.; Li, G.J.; Liu, G.D.; Wang, W.H.; Wu, G.H. A new spin gapless semiconductors family: Quaternary Heusler compounds. Europhys. Lett. 2013, 102, 17007. [Google Scholar] [CrossRef]
- Ouardi, S.; Fecher, G.H.; Felser, C.; Kübler, J. Realization of Spin Gapless Semiconductors: The Heusler Compound Mn2CoAl. Phys. Rev. Lett. 2013, 110, 100401. [Google Scholar] [CrossRef] [PubMed]
- Skaftouros, S.; Özdoğan, K.; Şaşıoğlu, E.; Galanakis, I. Search for spin gapless semiconductors: The case of inverse Heusler compounds. Appl. Phys. Lett. 2013, 102, 022402. [Google Scholar] [CrossRef]
- Ma, D.W.; Lu, Z.S.; Ju, W.W.; Tang, Y.N. First-principles studies of BN sheets with absorbed transition metal single atoms or dimers: Stabilities, electronic structures, and magnetic properties. J. Phys.-Condens. Mat. 2012, 24, 145501. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.F.; Yang, Z.Q. Electronic structures and spin gapless semiconductors in BN nanoribbons with vacancies. Phys. Rev. B 2010, 82, 195308. [Google Scholar] [CrossRef]
- Wang, Z.F.; Jin, S.; Liu, F. Spatially Separated Spin Carriers in Spin-Semiconducting Graphene Nanoribbons. Pyhs. Rev. Lett. 2013, 111, 096803. [Google Scholar] [CrossRef]
- Ju, L.; Liu, C.; Shi, L.; Sun, L. The high-speed channel made of metal for interfacial charge transfer in Z-scheme g–C3N4/MoS2 water-splitting photocatalyst. Mater. Res. Express 2019, 6, 115545. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tang, X.; He, C.; Wang, T.; Shang, L.; Wang, M.; Yang, S.; Tang, Z.; Ju, L. Sc-Modified C3N4 Nanotubes for High-Capacity Hydrogen Storage: A Theoretical Prediction. Molecules 2024, 29, 1966. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Liu, J.; Wang, M.; Yang, S.; Liu, S. Modulation of charge in C9N4 monolayer for a high-capacity hydrogen storage as a switchable strategy. Front. Phys. 2024, 19, 43208. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Antonietti, M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: From photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, X.; Zhao, M. Topological insulator states in a honeycomb lattice of s-triazines. Nanoscale 2014, 6, 11157–11162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, A.; Zhao, M. Spin-gapless semiconducting graphitic carbon nitrides: A theoretical design from first principles. Carbon 2015, 84, 1–8. [Google Scholar] [CrossRef]
- He, C.; Liao, Y.; Ouyang, T.; Zhang, H.; Xiang, H.; Zhong, J. Flat-band based high-temperature ferromagnetic semiconducting state in the graphitic C4N3 monolayer. Fund. Res. 2023. [Google Scholar] [CrossRef]
- Ma, F.; Lu, Z.Y.; Xiang, T. Arsenic-bridged antiferromagnetic superexchange interactions in LaFeAsO. Phys. Rev. B 2008, 78, 224517. [Google Scholar] [CrossRef]
- Torelli, D.; Thygesen, K.S.; Olsen, T. High throughput computational screening for 2D ferromagnetic materials: The critical role of anisotropy and local correlations. 2D Mater. 2019, 6, 045018. [Google Scholar] [CrossRef]
- Ju, L.; Xu, T.; Zhang, Y.; Shi, C.; Sun, L. Ferromagnetism of Na0.5Bi0.5TiO3 (1 0 0) surface with O2 adsorption. Appl. Surf. Sci. 2017, 412, 77–84. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yin, H.; Liu, P.-F. Strain-dependent electronic and mechanical properties in one-dimensional topological insulator Nb4 SiTe4. Phys. Rev. B 2023, 108, 045411. [Google Scholar] [CrossRef]
- Parlinski, K.; Li, Z.Q.; Kawazoe, Y. First-Principles Determination of the Soft Mode in Cubic ZrO2. Phys. Rev. Lett. 1997, 78, 4063–4066. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Chen, Y.-B.; Zhou, K.-G.; Liu, C.-H.; Zeng, J.; Zhang, H.-L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Ju, W.; Li, T.; Zhang, X.; He, C.; Ma, B.; Lu, Z.; Yang, Z. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016, 383, 98–105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Tang, X.; Xing, W.; Zhang, Y.; Gao, X.; Zhang, M.; Xie, Z.; Yan, X.; Ju, L. Designing Organic Spin-Gapless Semiconductors via Molecular Adsorption on C4N3 Monolayer. Molecules 2024, 29, 3138. https://doi.org/10.3390/molecules29133138
Zhao D, Tang X, Xing W, Zhang Y, Gao X, Zhang M, Xie Z, Yan X, Ju L. Designing Organic Spin-Gapless Semiconductors via Molecular Adsorption on C4N3 Monolayer. Molecules. 2024; 29(13):3138. https://doi.org/10.3390/molecules29133138
Chicago/Turabian StyleZhao, Dongqiu, Xiao Tang, Wanyan Xing, Yixin Zhang, Xueying Gao, Mengrui Zhang, Zhengao Xie, Xunwang Yan, and Lin Ju. 2024. "Designing Organic Spin-Gapless Semiconductors via Molecular Adsorption on C4N3 Monolayer" Molecules 29, no. 13: 3138. https://doi.org/10.3390/molecules29133138
APA StyleZhao, D., Tang, X., Xing, W., Zhang, Y., Gao, X., Zhang, M., Xie, Z., Yan, X., & Ju, L. (2024). Designing Organic Spin-Gapless Semiconductors via Molecular Adsorption on C4N3 Monolayer. Molecules, 29(13), 3138. https://doi.org/10.3390/molecules29133138







