Air-Assisted Electrospinning of Dihydromyricetin-Loaded Dextran/Zein/Xylose Nanofibers and Effects of the Maillard Reaction on Fiber Properties
Abstract
1. Introduction
2. Results and Discussion
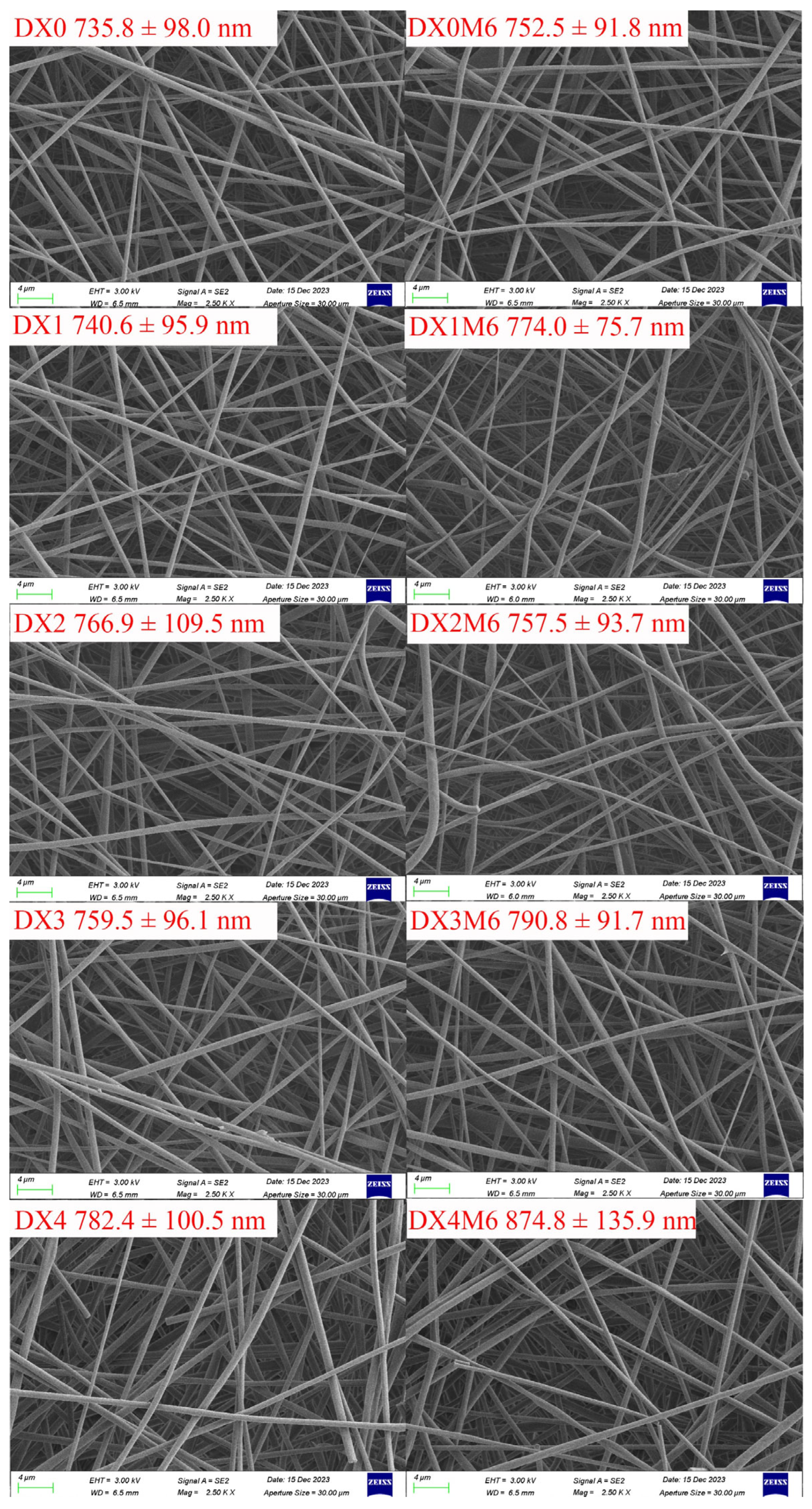
2.1. Fiber Morphologies
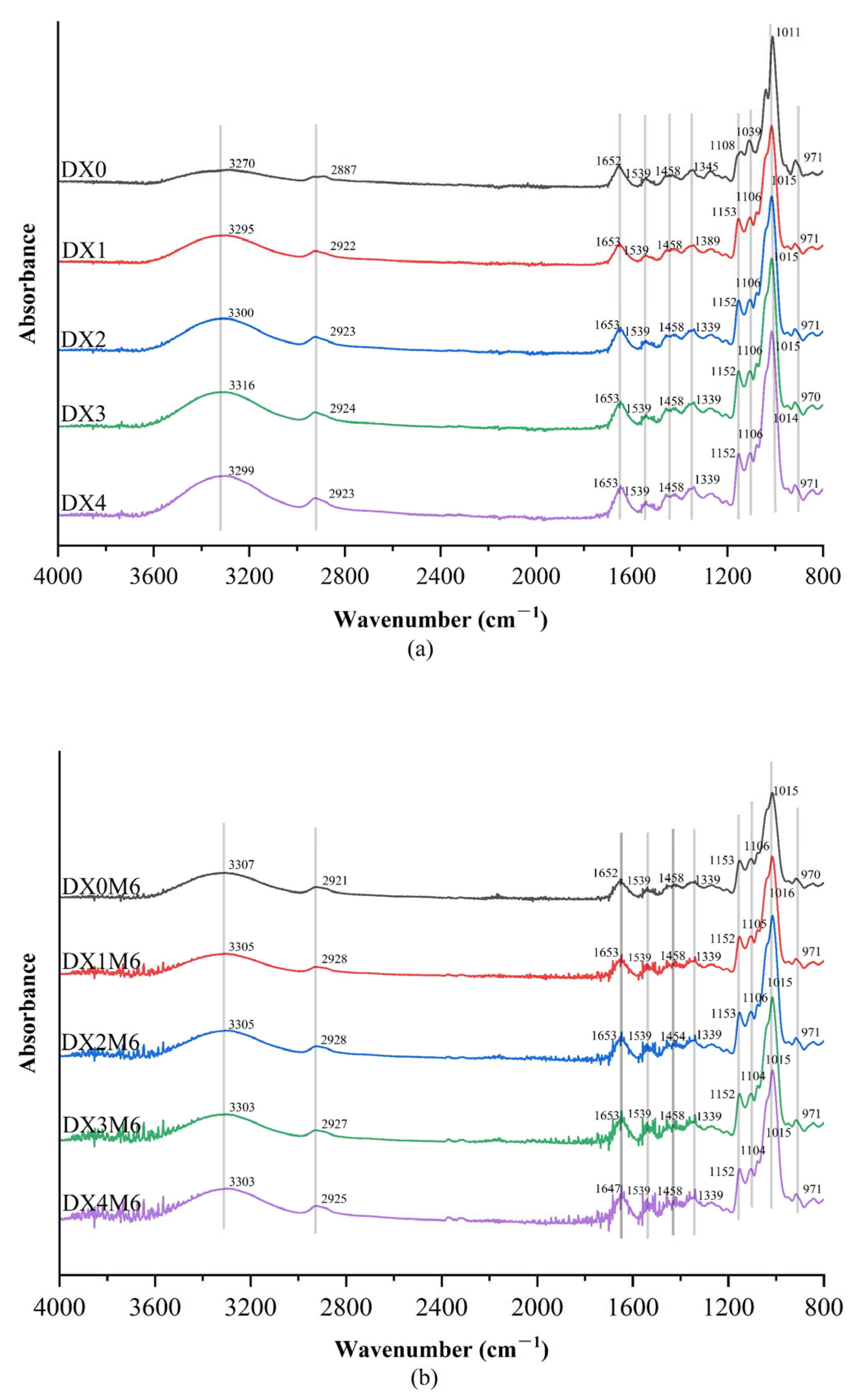
2.2. FTIR Spectroscopy
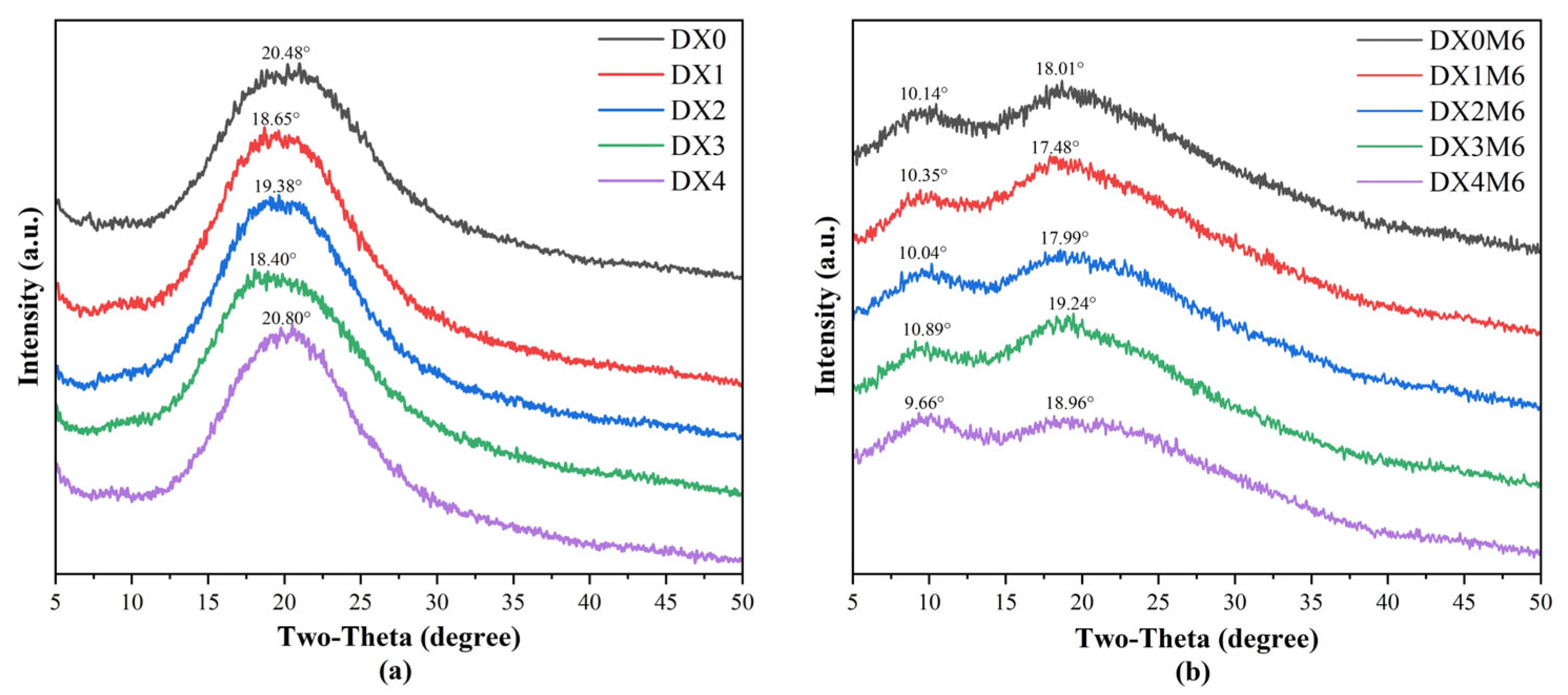
2.3. XRD Analysis
2.4. Thermal Analysis
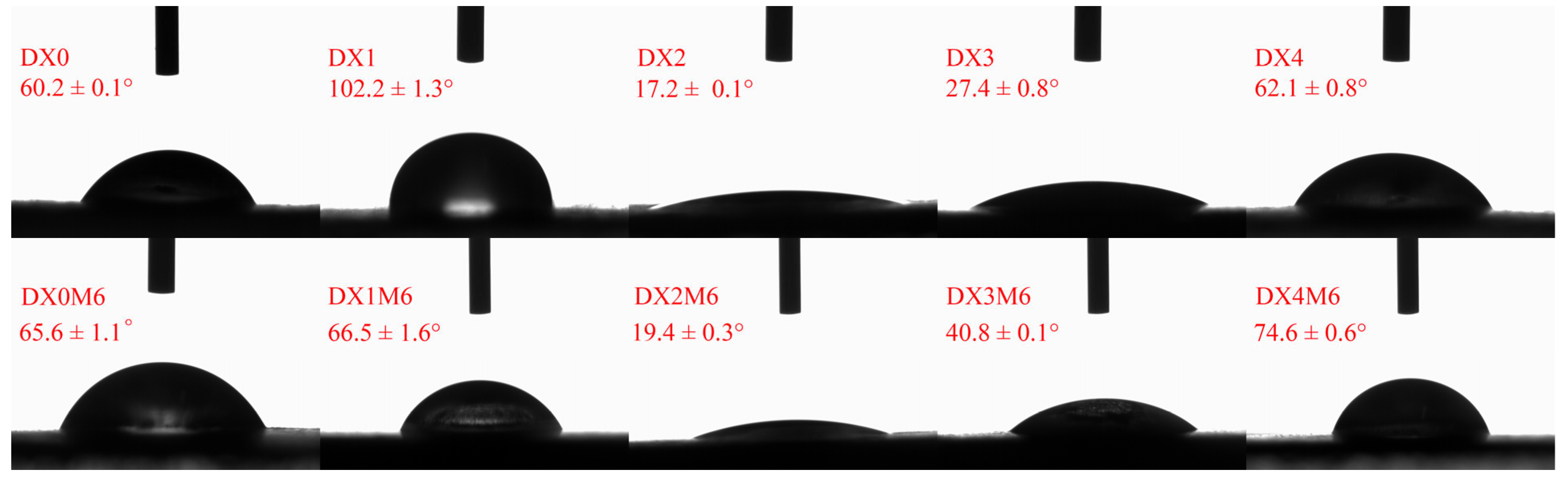
2.5. Water Contact Angle Analysis
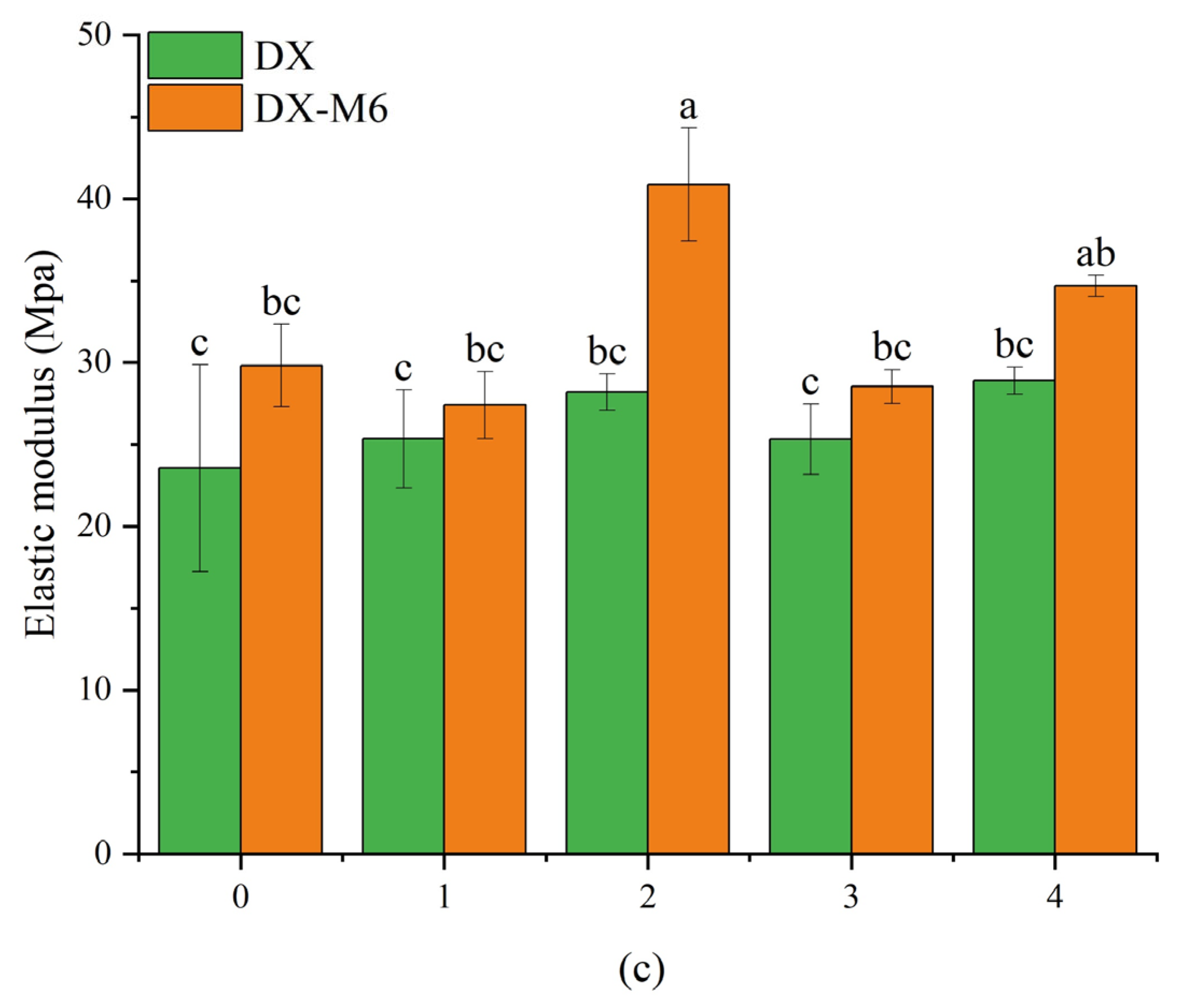
2.6. Mechanical Properties
2.7. Water Vapor Permeability
2.8. Antioxidant Activities
3. Materials and Methods
3.1. Chemicals
3.2. Solution Preparation
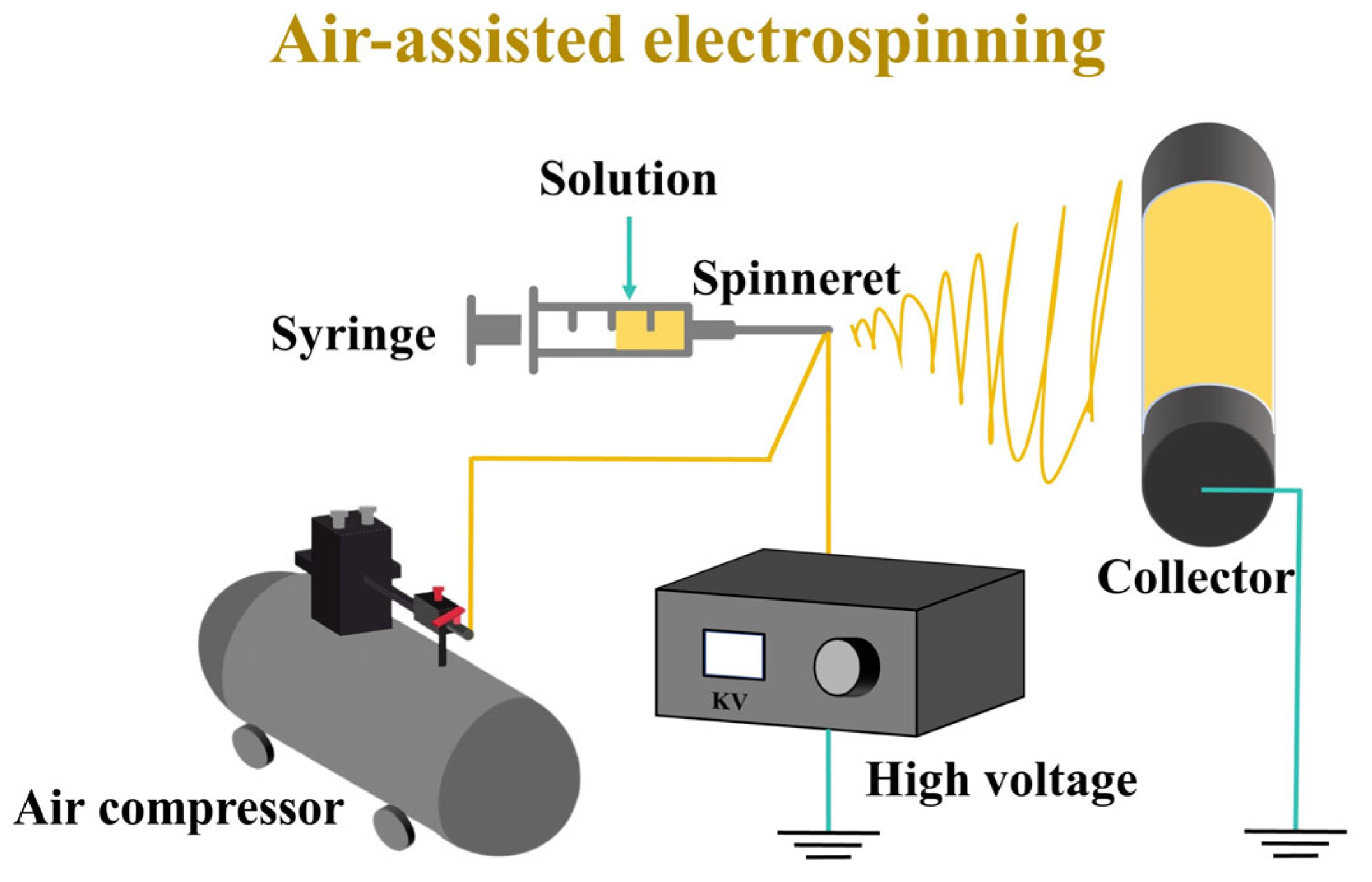
3.3. Air-Assisted Electrospinning
3.4. Maillard Reaction
3.5. Fiber Morphologies
3.6. FTIR Spectroscopy
3.7. XRD Analysis
3.8. Analysis of Thermal Properties
3.9. Water Contact Angle Tests
3.10. Mechanical Properties
3.11. Water Vapor Permeability
3.12. Antioxidant Activity
3.12.1. DPPH Assay
3.12.2. ABTS Assay
3.12.3. FRAP Assay
3.12.4. CUPRAC Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Feng, X.; Wang, Q.; Liu, D.; Zhang, S.; Chu, L. Fabrication and characterization of dihydromyricetin-loaded microcapsules stabilized by glyceryl monostearate and whey protein–xanthan gum. Int. J. Biol. Macromol. 2024, 254, 128039. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, Z.; Li, H.; Zhu, N.; Gu, J.; Wang, W.; Liu, C.; Wang, W.; Qin, L. Present Status, Challenges, and Prospects of Dihydromyricetin in the Battle against Cancer. Cancers 2022, 14, 3487. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Xiang, H.; Ding, P.; Wu, T.; Ji, G. Recent update on application of dihydromyricetin in metabolic related diseases. Biomed. Pharmacother. 2022, 148, 112771. [Google Scholar] [CrossRef]
- Yan, Q.; Li, M.; Dong, L.; Luo, J.; Zhong, X.; Shi, F.; Ye, G.; Zhao, L.; Fu, H.; Shu, G.; et al. Preparation, characterization and protective effect of chitosan—Tripolyphosphate encapsulated dihydromyricetin nanoparticles on acute kidney injury caused by cisplatin. Int. J. Biol. Macromol. 2023, 245, 125569. [Google Scholar] [CrossRef]
- Zhen, S.; Geng, S.; Ma, H.; Liu, B. Interaction of α-lactalbumin with dihydromyricetin and its application in nano-emulsion. Int. J. Food Sci. Technol. 2023, 58, 1120–1129. [Google Scholar] [CrossRef]
- Lu, N.; Wu, L.; Zhen, S.; Liu, B. Characterization of a Dihydromyricetin/α-Lactoalbumin Covalent Complex and Its Application in Nano-emulsions. Foods 2023, 12, 2783. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Yuan, Y.; Jiang, X.; Zhang, R.; Ma, H.; Liang, G.; Liu, B. An investigation on pickering nano-emulsions stabilized by dihydromyricetin/high-amylose corn starch composite particles: Preparation conditions and carrier properties. Curr. Res. Food Sci. 2023, 6, 100458. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Chen, L.; Lin, S.; Cao, H.; Teng, H. A designed self-microemulsion delivery system for dihydromyricetin and its dietary intervention effect on high-fat-diet fed mice. Food Chem. 2022, 390, 132954. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Peng, X.; Zheng, Y.; Cheng, Z.; Sun, S.; Ding, Q.; Liu, W.; Ding, C. A poloxamer/hyaluronic acid/chitosan-based thermosensitive hydrogel that releases dihydromyricetin to promote wound healing. Int. J. Biol. Macromol. 2022, 216, 475–486. [Google Scholar] [CrossRef]
- Liu, X.; Ding, Q.; Liu, W.; Zhang, S.; Wang, N.; Chai, G.; Wang, Y.; Sun, S.; Zheng, R.; Zhao, Y.; et al. A Poloxamer 407/chitosan-based thermosensitive hydrogel dressing for diabetic wound healing via oxygen production and dihydromyricetin release. Int. J. Biol. Macromol. 2024, 263, 130256. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, T.; Huang, F.; Yang, M.; Jiang, X.; Chen, X. Dihydromyricetin encapsulated gelatin-dialdehyde-β-cyclodextrin hydrogels for antibacterial application. J. Appl. Polym. Sci. 2023, 140, e54594. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Ding, C.; Zhao, Y.; Zhang, S.; Sun, S.; Zhang, L.; Ma, S.; Ding, Q.; Liu, W. Polyvinylpyrrolidone/chitosan-loaded dihydromyricetin-based nanofiber membrane promotes diabetic wound healing by anti-inflammatory and regulating autophagy-associated protein expression. Int. J. Biol. Macromol. 2024, 259, 129160. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Z.; Zhang, H.; Li, Y.; Maierhaba, T.; An, J.; Zhou, Z.; Deng, L. Comparison of eugenol and dihydromyricetin loaded nanofibers by electro-blowing spinning for active packaging. Food Biosci. 2023, 51, 102294. [Google Scholar] [CrossRef]
- Li, M.; Luo, X.; Zhu, R.; Zhong, K.; Ran, W.; Wu, Y.; Gao, H. Development and characterization of active bilayer film incorporated with dihydromyricetin encapsulated in hydroxypropyl-β-cyclodextrin for food packaging application. Food Hydrocoll. 2022, 131, 107834. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, T.; Liu, F.; Zhang, Y.; Xie, Y.; Xiao, X.; Zhang, Y. Dihydromyricetin solid dispersion: Preparation, characterization, and preservative effects on sturgeon fillets stored at 4 °C. LWT 2023, 174, 114387. [Google Scholar] [CrossRef]
- Kim, A.A.; Poudel, M.B. Spiral Structured Cellulose Acetate Membrane Fabricated by One-Step Electrospinning Technique with High Water Permeation Flux. J. Compos. Sci. 2024, 8, 127. [Google Scholar] [CrossRef]
- Li, L.; Kang, W.; Zhuang, X.; Shi, J.; Zhao, Y.; Cheng, B. A comparative study of alumina fibers prepared by electro-blown spinning (EBS) and solution blowing spinning (SBS). Mater. Lett. 2015, 160, 533–536. [Google Scholar] [CrossRef]
- Ahmed, S.B.; Doğan, N.; Doğan, C.; Akgul, Y. A Novel Approach to Crosslink Gelatin Nanofibers Through Neutralization-Induced Maillard Reaction. Food Bioprocess Technol. 2024, 17, 489–503. [Google Scholar] [CrossRef]
- Dou, Y.; Wang, N.; Zhang, S.; Sun, C.; Chen, J.; Qu, Z.; Cui, A.; Li, J. Electroactive nanofibrous membrane with antibacterial and deodorizing properties for air filtration. J. Hazard. Mater. 2024, 469, 134064. [Google Scholar] [CrossRef]
- Zhao, T.; Xu, Y.; Wu, M.; Li, Y.; Ma, J.; Li, H.; Zheng, Y.; Zeng, Y. Highly efficient fabrication of biomimetic nanoscaled tendrils for high-performance PM0. 3 air filters. Nano Lett. 2024, 24, 1385–1391. [Google Scholar] [CrossRef]
- Zeng, J.; Chen, Y.; Xu, G.; Shi, Z.; Luo, Z.; Liu, F.; Chen, X.; Zou, Z.; Wang, H.; Du, Z. Fabrication of nanofiber yarns via electro-blown and hang-fiber process. AIP Adv. 2024, 14, 045330. [Google Scholar] [CrossRef]
- Deng, N.; Zeng, Q.; Feng, Y.; Gao, H.; Wang, G.; Yan, J.; Zheng, T.; Liu, Y.; Kang, W.; Cheng, B. CoP nanoparticles embedded in three-dimensional porous network-like structured N, O co-doped carbon nanofibers as an effective bi-functional electrocatalyst for rechargeable zinc–air batteries. Catal. Sci. Technol. 2023, 13, 4823–4838. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, H.; Cao, L.; Dai, C.; Ren, J.; Liang, J.; Ling, S. Functionalization of structural materials through electro-blown spinning of ultrathin and transparent silk fibroin ionotronic nanofiber skin. Nano Today 2023, 50, 101873. [Google Scholar] [CrossRef]
- Hui, C.; Gao, Y.; Yan, B.-Y.; Ding, L.-Q.; Sun, T.-C.; Liu, Z.; Ramakrishna, S.; Long, Y.-Z.; Zhang, J. Collocalia birds inspired Janus-structured bandage with strong wet tissue adhesion for rapid hemostasis and wound healing. Chem. Eng. J. 2023, 464, 142458. [Google Scholar] [CrossRef]
- Aminyan, R.; Bazgir, S. Fabrication and characterization of nanofibrous polyacrylic acid superabsorbent using gas-assisted electrospinning technique. React. Funct. Polym. 2019, 141, 133–144. [Google Scholar] [CrossRef]
- Duan, G.; Greiner, A. Air-blowing-assisted coaxial electrospinning toward high productivity of Core/sheath and hollow fibers. Macromol. Mater. Eng. 2019, 304, 1800669. [Google Scholar] [CrossRef]
- Luo, S.; Saadi, A.; Fu, K.; Taxipalati, M.; Deng, L. Fabrication and characterization of dextran/zein hybrid electrospun fibers with tailored properties for controlled release of curcumin. J. Sci. Food Agric. 2021, 101, 6355–6367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Azizi-Lalabadi, M.; Roy, S.; Salim, S.A.; Castro-Muñoz, R.; Jafari, S.M. Maillard-reaction (glycation) of biopolymeric packaging films; principles, mechanisms, food applications. Trends Food Sci. Technol. 2023, 138, 523–538. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Feng, F.; Zhang, H. Study on wettability, mechanical property and biocompatibility of electrospun gelatin/zein nanofibers cross-linked by glucose. Food Hydrocoll. 2019, 87, 1–10. [Google Scholar] [CrossRef]
- Kchaou, H.; Benbettaïeb, N.; Jridi, M.; Abdelhedi, O.; Karbowiak, T.; Brachais, C.-H.; Léonard, M.-L.; Debeaufort, F.; Nasri, M. Enhancement of structural, functional and antioxidant properties of fish gelatin films using Maillard reactions. Food Hydrocoll. 2018, 83, 326–339. [Google Scholar] [CrossRef]
- Kwak, H.W.; Park, J.; Yun, H.; Jeon, K.; Kang, D.-W. Effect of crosslinkable sugar molecules on the physico-chemical and antioxidant properties of fish gelatin nanofibers. Food Hydrocoll. 2021, 111, 106259. [Google Scholar] [CrossRef]
- Siimon, K.; Reemann, P.; Poder, A.; Pook, M.; Kangur, T.; Kingo, K.; Jaks, V.; Mäeorg, U.; Jaervekuelg, M. Effect of glucose content on thermally cross-linked fibrous gelatin scaffolds for tissue engineering. Mater. Sci. Eng. C 2014, 42, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Kchaou, H.; Benbettaieb, N.; Jridi, M.; Nasri, M.; Debeaufort, F. Influence of Maillard reaction and temperature on functional, structure and bioactive properties of fish gelatin films. Food Hydrocoll. 2019, 97, 105196. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, X.; Li, B.; Tian, J. Konjac glucomannan-dihydromyricetin complex improves viscosity and hydration capacity of konjac glucomannan as well as the thermal stability of dihydromyricetin. Int. J. Biol. Macromol. 2023, 242, 124666. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, S.; Li, Y.; Zhang, H.; Yuan, Z.; Shang, L.; Deng, L. Influence of the Maillard Reaction on Properties of Air-Assisted Electrospun Gelatin/Zein/Glucose Nanofibers. Foods 2023, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- Davidov-Pardo, G.; McClements, D.J. Resveratrol encapsulation: Designing delivery systems to overcome solubility, stability and bioavailability issues. Trends Food Sci. Technol. 2014, 38, 88–103. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Feng, F.; Wu, D.; Zhang, H. Encapsulation of allopurinol by glucose cross-linked gelatin/zein nanofibers: Characterization and release behavior. Food Hydrocoll. 2019, 94, 574–584. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, K.; Qin, Z.; Zou, Y.; Zhong, H.; Zhang, H. Cross-linked gluten/zein nanofibers via Maillard reaction with the loading of star anise essential oil/β-cyclodextrin inclusions for food-active packaging. Food Packag. Shelf Life 2022, 34, 100950. [Google Scholar] [CrossRef]
- Wang, Z.; You, W.; Wang, W.; Tian, W.; Chen, F.; Xiao, Y.; Chen, Y.; Wang, X. Dihydromyricetin-Incorporated Multilayer Nanofibers Accelerate Chronic Wound Healing by Remodeling the Harsh Wound Microenvironment. Adv. Fiber Mater. 2022, 4, 1556–1571. [Google Scholar] [CrossRef]
- Qin, Z.; Jia, X.; Liu, Q.; Kong, B.; Wang, H. Enhancing physical properties of chitosan/pullulan electrospinning nanofibers via green crosslinking strategies. Carbohydr. Polym. 2020, 247, 116734. [Google Scholar] [CrossRef]
- Xie, W.; Du, Y.; Yuan, S.; Pang, J. Dihydromyricetin incorporated active films based on konjac glucomannan and gellan gum. Int. J. Biol. Macromol. 2021, 180, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Turan, D. Water vapor transport properties of polyurethane films for packaging of respiring foods. Food Eng. Rev. 2021, 13, 54–65. [Google Scholar] [CrossRef]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-K.; Park, P.-J.; Ahn, C.-B.; Je, J.-Y. Preparation and antioxidant potential of maillard reaction products from (MRPs) chitooligomer. Food Chem. 2014, 145, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, S.; Xie, R.; Zhong, W.; Wang, H.; Farag, M.A.; Hussain, H.; Arroo, R.R.J.; Chen, X.; Xiao, J. Thermal degradation of (2R, 3R)-dihydromyricetin in neutral aqueous solution at 100 °C. Food Chem. 2024, 435, 137560. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Sani, M.; Tavassoli, M.; McClements, D.J.; Hamishehkar, H. Multifunctional halochromic packaging materials: Saffron petal anthocyanin loaded-chitosan nanofiber/methyl cellulose matrices. Food Hydrocoll. 2021, 111, 106237. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, L.; Zhong, H.; Zou, Y.; Qin, Z.; Li, Y.; Zhang, H. Impact of glycation on physical properties of composite gluten/zein nanofibrous films fabricated by blending electrospinning. Food Chem. 2022, 366, 130586. [Google Scholar] [CrossRef]
- Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Improvement of barrier properties of fish gelatin films promoted by gelatin glycation with lactose at high temperatures. LWT—Food Sci. Technol. 2015, 63, 315–321. [Google Scholar] [CrossRef]





| DSC | TGA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| T (°C) | ∆H (J/g) | Peak 1 (°C) | Weight Loss (%) | Peak 2 (°C) | Weight Loss (%) | Peak 3 (°C) | Weight Loss (%) | Residue at 600 °C (%) | |
| DX0 | 80.6 | −29.086 | 73.3 | 5.14 | 216.0 | 4.61 | 296.1 | 63.39 | 26.86 |
| DX1 | 80.8 | −25.474 | 69.4 | 5.11 | 214.7 | 6.50 | 297.9 | 60.97 | 27.42 |
| DX2 | 83.1 | −31.134 | 70.9 | 10.24 | 215.1 | 5.53 | 297.6 | 60.77 | 23.45 |
| DX3 | 86.3 | −28.642 | 72.8 | 5.63 | 213.2 | 4.66 | 290.9 | 62.25 | 27.42 |
| DX4 | 83.3 | −28.798 | 72.4 | 5.28 | 210.1 | 5.25 | 294.3 | 61.08 | 28.39 |
| DX0M6 | 69.4 | −6.303 | 59.8 | 8.55 | 211.7 | 7.35 | 319.1 | 71.41 | 12.69 |
| DX1M6 | 67.1 | −4.748 | 53.9 | 8.35 | 210.6 | 6.53 | 324.4 | 68.74 | 16.38 |
| DX2M6 | 67.9 | −8.643 | 59.8 | 8.18 | 218.2 | 5.66 | 297.6 | 68.60 | 17.56 |
| DX3M6 | 67.5 | −7.732 | 60.9 | 6.70 | 217.6 | 5.50 | 298.6 | 68.06 | 19.74 |
| DX4M6 | 70.2 | −8.491 | 63.4 | 6.59 | 210.8 | 5.36 | 300.2 | 66.34 | 21.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; An, J.; Tian, C.; Shang, L.; Tao, Y.; Deng, L. Air-Assisted Electrospinning of Dihydromyricetin-Loaded Dextran/Zein/Xylose Nanofibers and Effects of the Maillard Reaction on Fiber Properties. Molecules 2024, 29, 3136. https://doi.org/10.3390/molecules29133136
Ren Y, An J, Tian C, Shang L, Tao Y, Deng L. Air-Assisted Electrospinning of Dihydromyricetin-Loaded Dextran/Zein/Xylose Nanofibers and Effects of the Maillard Reaction on Fiber Properties. Molecules. 2024; 29(13):3136. https://doi.org/10.3390/molecules29133136
Chicago/Turabian StyleRen, Yupeng, Jianhui An, Cheng Tian, Longchen Shang, Yexing Tao, and Lingli Deng. 2024. "Air-Assisted Electrospinning of Dihydromyricetin-Loaded Dextran/Zein/Xylose Nanofibers and Effects of the Maillard Reaction on Fiber Properties" Molecules 29, no. 13: 3136. https://doi.org/10.3390/molecules29133136
APA StyleRen, Y., An, J., Tian, C., Shang, L., Tao, Y., & Deng, L. (2024). Air-Assisted Electrospinning of Dihydromyricetin-Loaded Dextran/Zein/Xylose Nanofibers and Effects of the Maillard Reaction on Fiber Properties. Molecules, 29(13), 3136. https://doi.org/10.3390/molecules29133136







