Effects of Different pH Levels on the Structural and Functional Properties of Proteins of Phaeodactylum tricornutum
Abstract
1. Introduction
2. Results and Discussion
2.1. Amino Acid Composition of the PTP
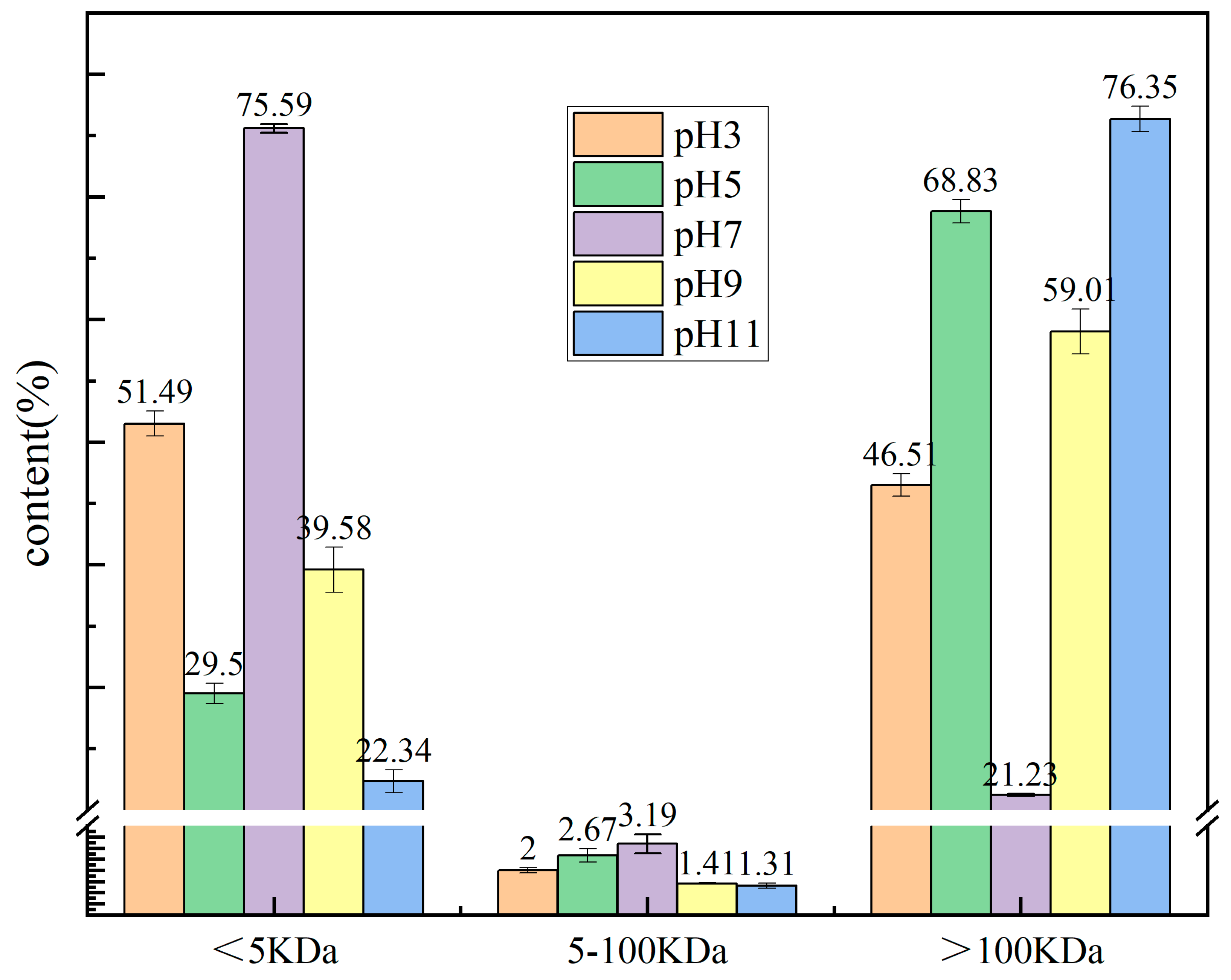
2.2. Molecular Weight (MW)t Analysis of the PTP
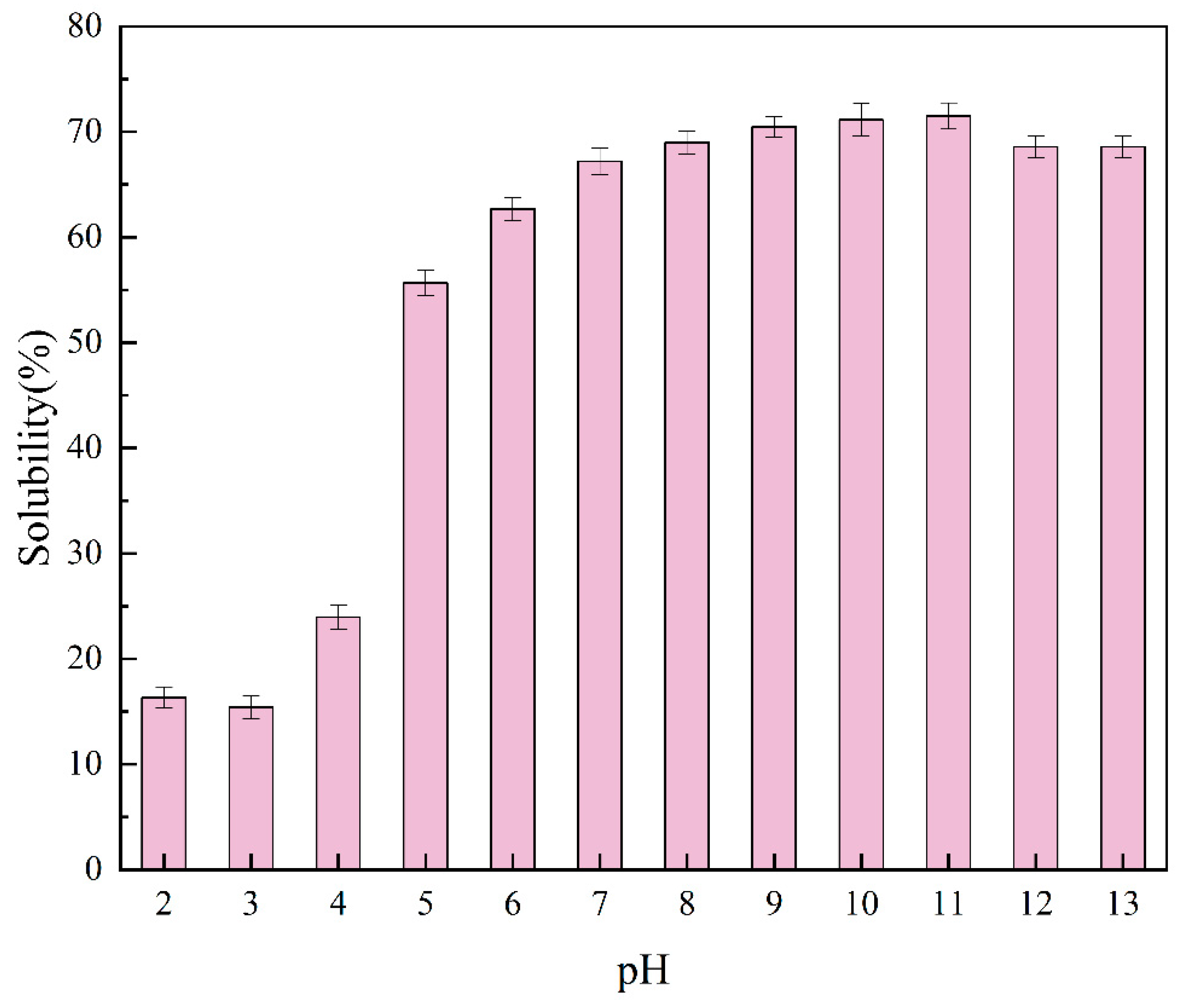
2.3. Solubility
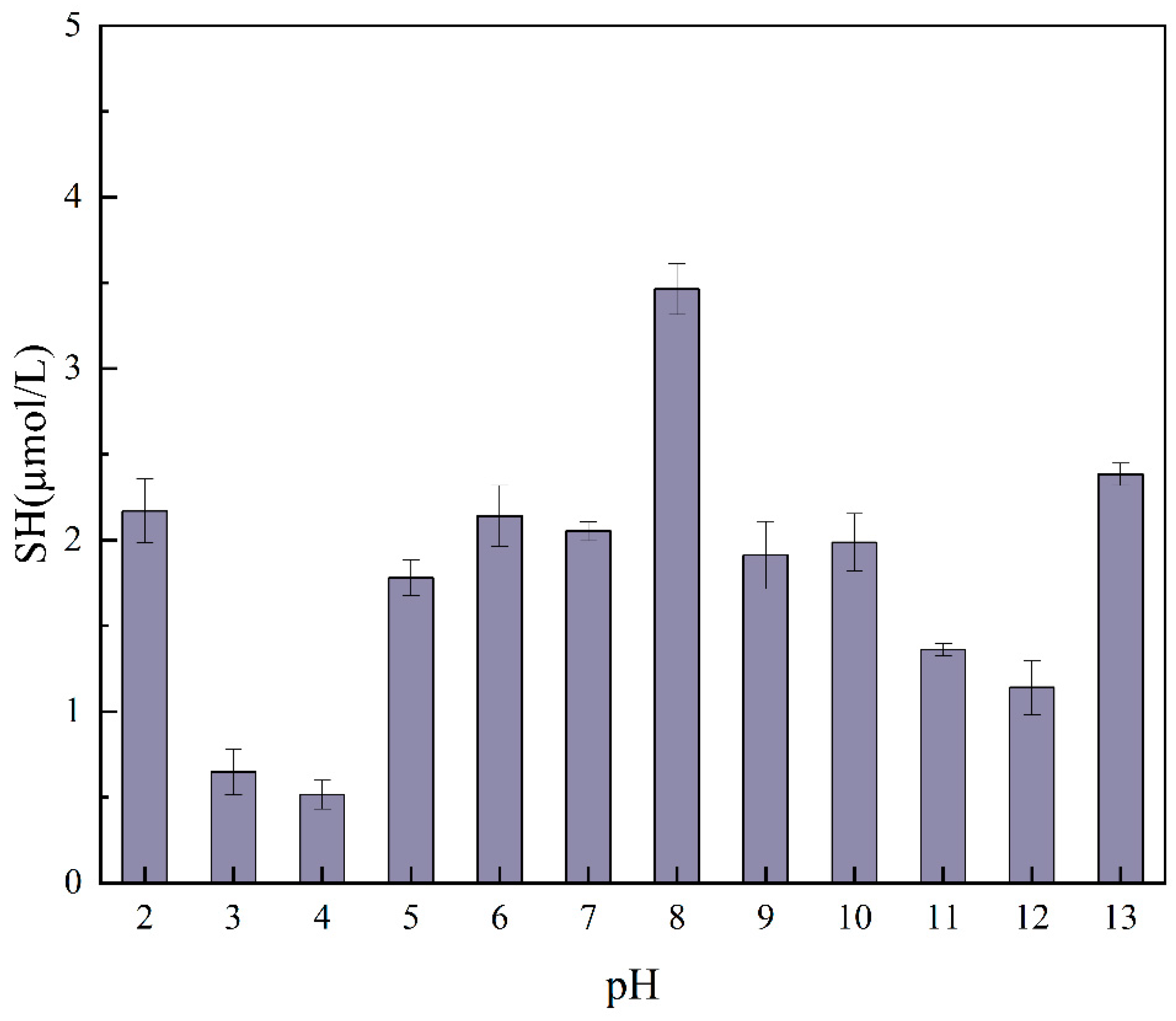
2.4. Free Sulfhydryl Groups
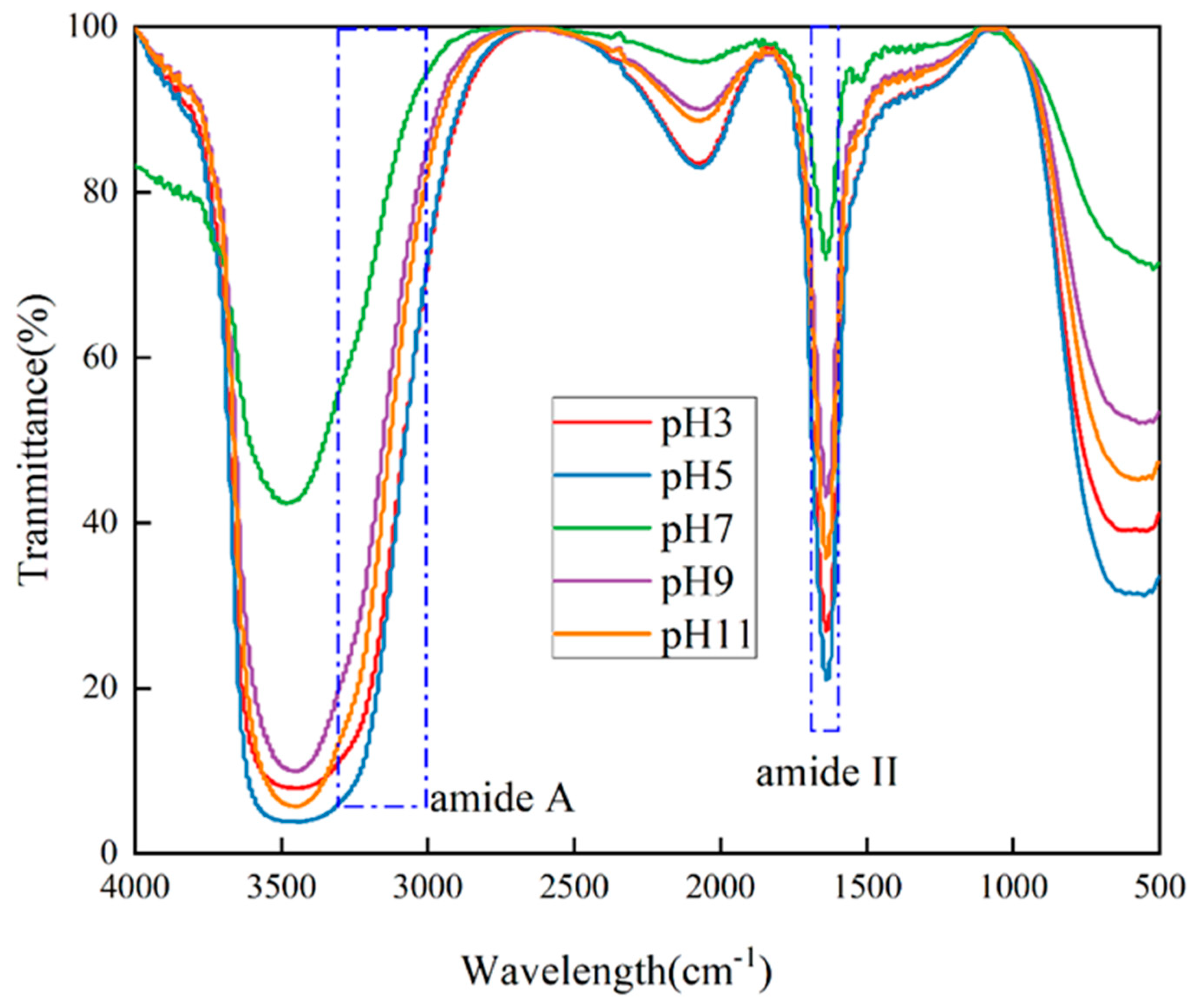
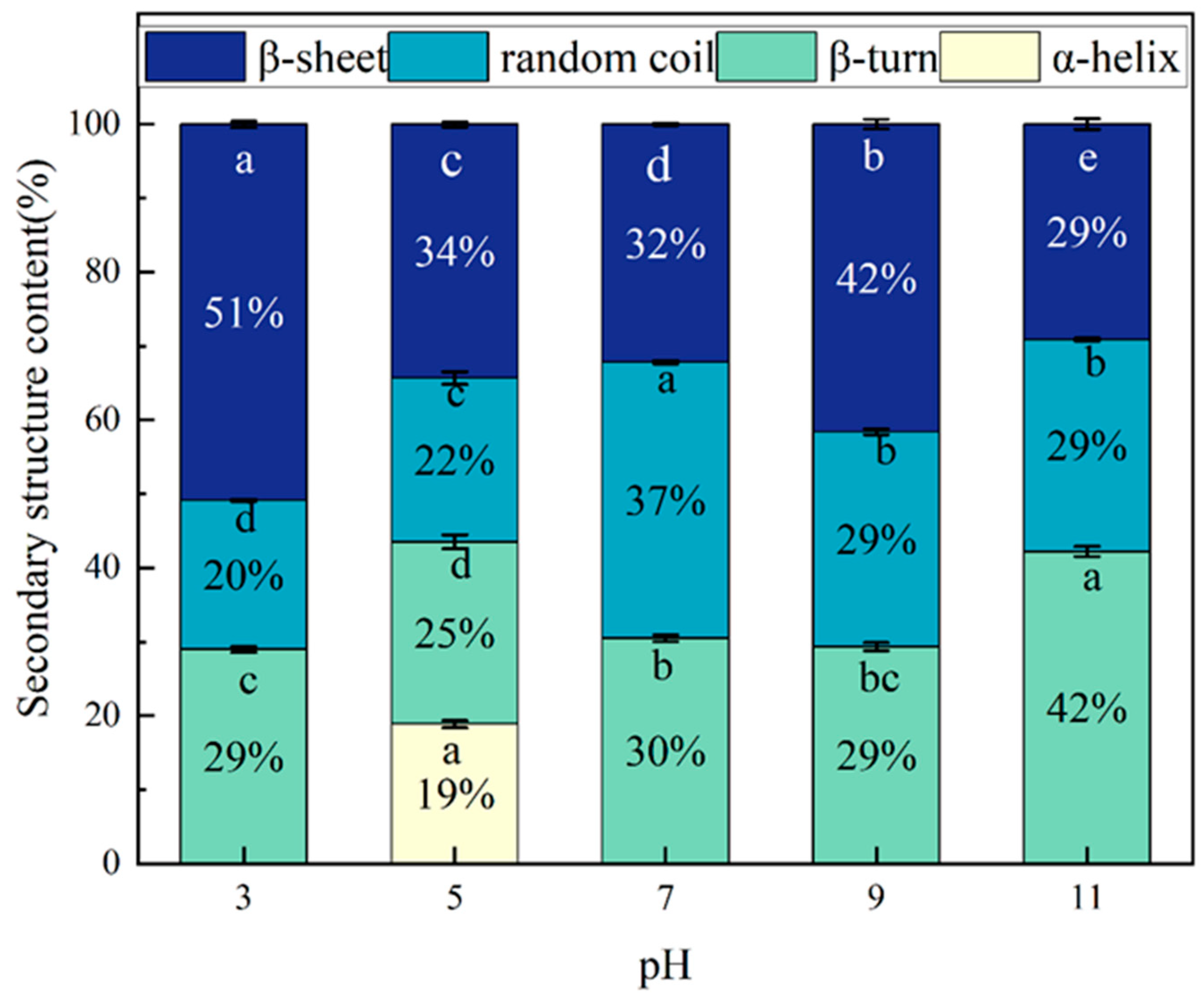
2.5. Secondary Structure
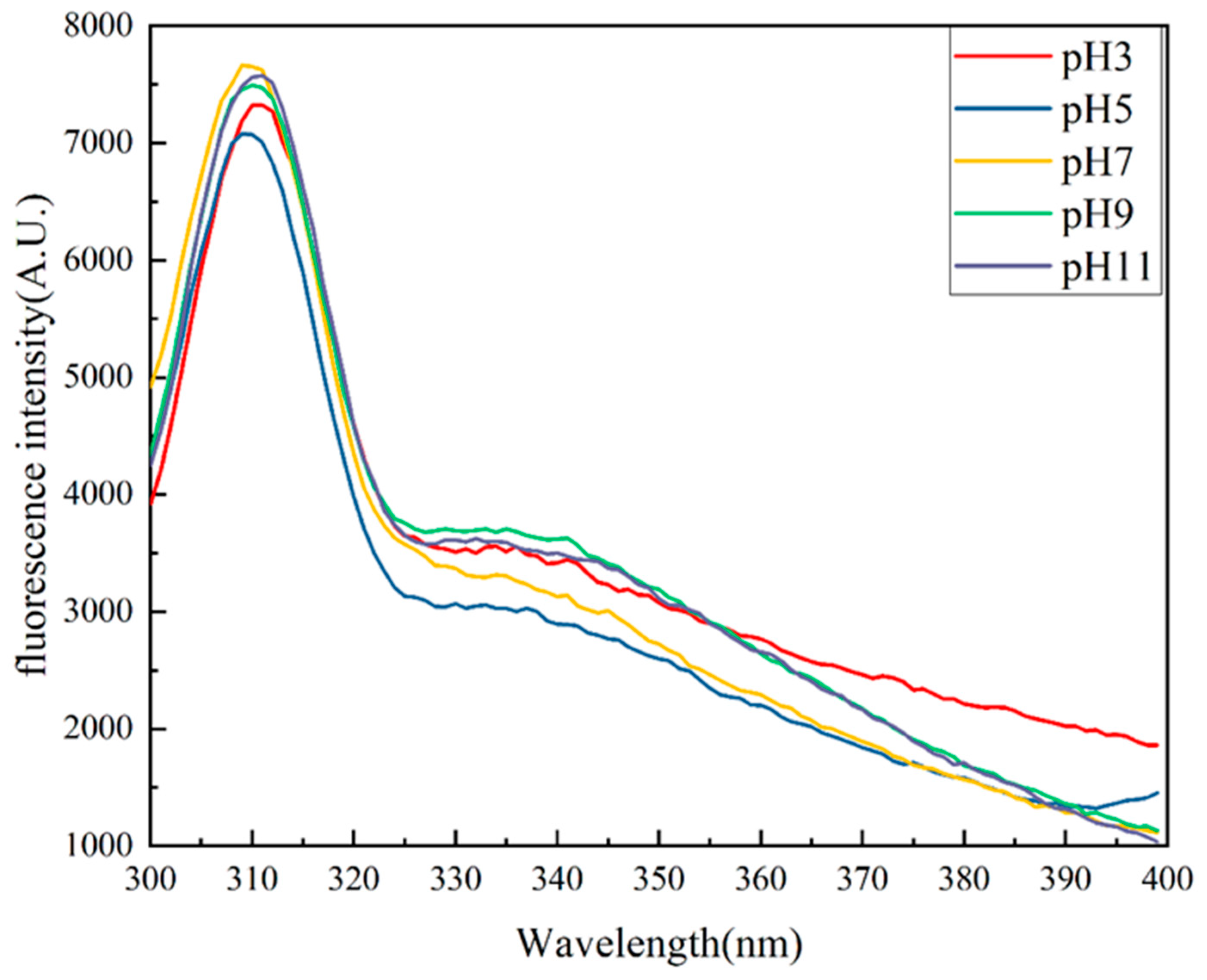
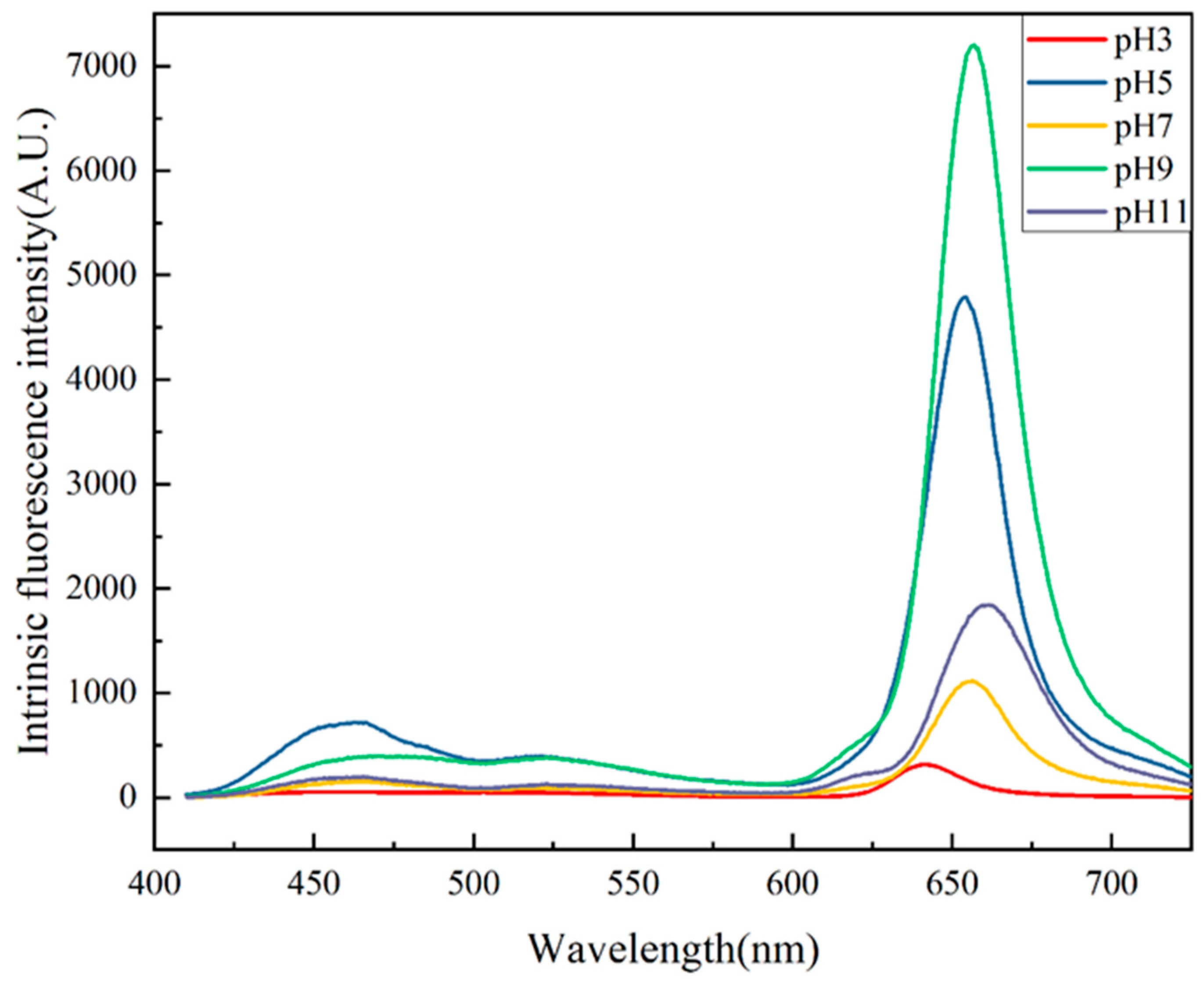
2.6. Intrinsic Fluorescence Spectroscopy
2.7. Surface Hydrophobicity
2.8. Thermal Properties
2.9. Particle Size and Zeta Potential
2.10. Foaming Capacity and Foaming Stability
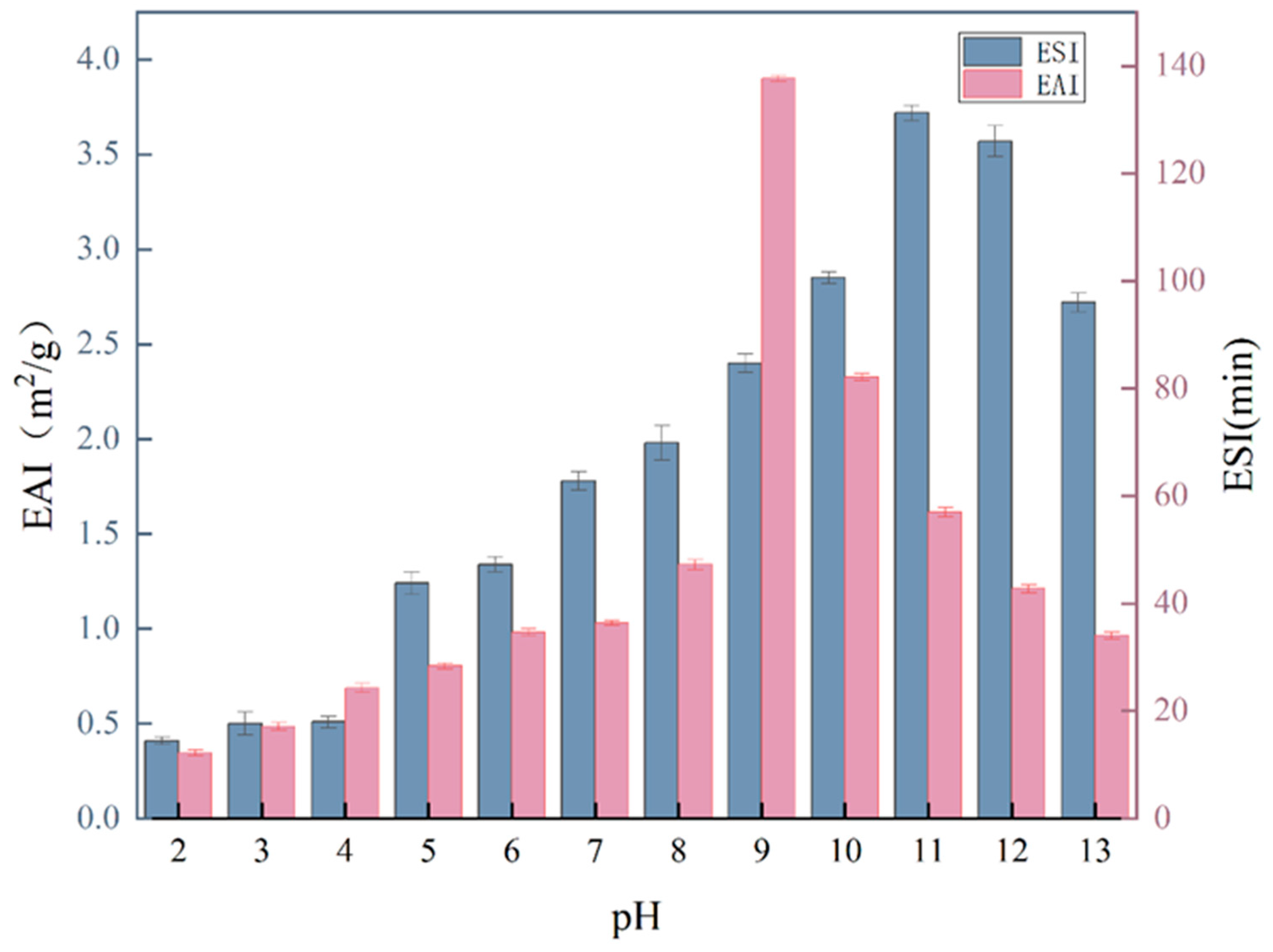
2.11. Emulsifying Activity and Emulsion Stability
2.12. Antioxidant Activity
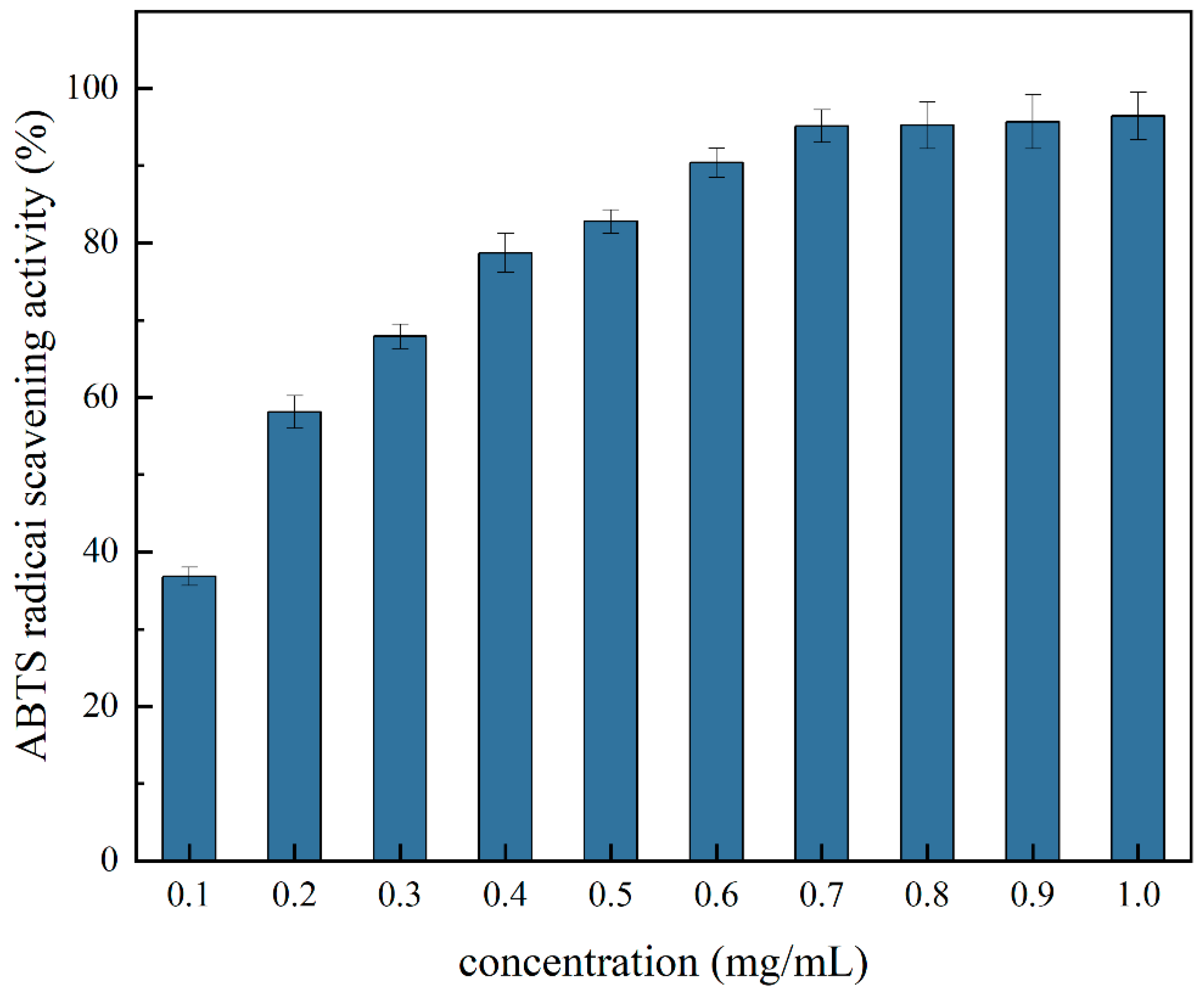
2.12.1. ABTS Free Radical Scavenging Activity
2.12.2. DPPH Free Radical Scavenging Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Phaeodactylum Tricornutum Protein (the PTP)
3.3. Amino Acid Analysis
3.4. Molecular Weight Distribution (MW) of the PTP
3.5. Fourier Transform Infrared Spectroscopy (FTIR) of the PTP
3.6. Intrinsic Fluorescence Spectroscopy
3.7. Surface Hydrophobicity
3.8. Free Sulfhydryl Groups (SH) of the PTP
3.9. Particle Size Distribution and Zeta Potential
3.10. Solubility
3.11. Foaming Properties
3.12. Emulsification Properties
3.13. Thermal Properties of the PTP
3.14. Antioxidant Properties of the PTP
3.14.1. ABTS Radical Scavenging Activity
3.14.2. DPPH Radical Scavenging Activity
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grasso, A.C.; Hung, Y.; Olthof, M.R.; Verbeke, W.; Brouwer, I.A. Older Consumers’ Readiness to Accept Alternative, More Sustainable Protein Sources in the European Union. Nutrients 2019, 11, 1904. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tang, T.; Shi, Q.; Zhou, Z.; Fan, J. The potential and challenge of microalgae as promising future food sources. Trends Food Sci. Technol. 2022, 126, 99–112. [Google Scholar] [CrossRef]
- Celi, C.; Fino, D.; Savorani, F. Phaeodactylum tricornutum as a source of value-added products: A review on recent developments in cultivation and extraction technologies. Bioresour. Technol. Rep. 2022, 19, 101122. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, A Carotenoid Derived from Phaeodactylum tricornutum Exerts Antiproliferative and Antioxidant Activities In Vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Lupette, J.; Benning, C. Human health benefits of very-long-chain polyunsaturated fatty acids from microalgae. Biochimie 2020, 178, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Castro-Ferreira, C.; Gomes-Dias, J.S.; Ferreira-Santos, P.; Pereira, R.N.; Vicente, A.A.; Rocha, C.M.R. Phaeodactylum tricornutum extracts as structuring agents for food applications: Physicochemical and functional properties. Food Hydrocoll. 2022, 124, 107276. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, Q.; Xiong, Y.L. A pH shift approach to the improvement of interfacial properties of plant seed proteins. Curr. Opin. Food Sci. 2018, 19, 50–56. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Hultin, H.O. Effect of Low and High pH Treatment on the Functional Properties of Cod Muscle Proteins. J. Agric. Food Chem. 2003, 51, 5103–5110. [Google Scholar] [CrossRef]
- Xu, H.; Pan, J.; Dabbour, M.; Kumah Mintah, B.; Chen, W.; Yang, F.; Zhang, Z.; Cheng, Y.; Dai, C.; He, R.; et al. Synergistic effects of pH shift and heat treatment on solubility, physicochemical and structural properties, and lysinoalanine formation in silkworm pupa protein isolates. Food Res. Int. 2023, 165, 112554. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Du, Y.; Wang, Q.; Xu, X.; Li, J.; Tao, J.; Gao, F.; Yang, P.; Feng, B.; Gao, J. Effects of nitrogen fertilizer on the physicochemical, structural, functional, thermal, and rheological properties of mung bean (Vigna radiata) protein. Int. J. Biol. Macromol. 2024, 260, 129616. [Google Scholar] [CrossRef] [PubMed]
- Dietary Protein Quality Evaluation in Human Nutrition; Food and Agriculture Organization of the United Nations, World Health Organization: Rome, Italy, 2013; pp. 28–29.
- Xu, Y.; Yang, Y.; Ma, C.-m.; Bian, X.; Liu, X.-f.; Wang, Y.; Chen, F.-l.; Wang, B.; Zhang, G.; Zhang, N. Characterization of the structure, antioxidant activity and hypoglycemic activity of soy (Glycine max L.) protein hydrolysates. Food Res. Int. 2023, 173, 113473. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, L.; Hinrichs, J.; Weiss, J. Solubility of extracted proteins from Chlorella sorokiniana, Phaeodactylum tricornutum, and Nannochloropsis oceanica: Impact of pH-value. LWT 2019, 105, 408–416. [Google Scholar] [CrossRef]
- Phat, C.; Moon, B.; Lee, C. Evaluation of umami taste in mushroom extracts by chemical analysis, sensory evaluation, and an electronic tongue system. Food Chem. 2016, 192, 1068–1077. [Google Scholar] [CrossRef]
- Yao, M.; Gai, X.; Zhang, M.; Liu, X.; Cui, T.; Liu, C.; Liu, D.; Jia, A. Two proteins prepared from defatted Antarctic krill (Euphausia superba) powder: Composition, structure and functional properties. Food Hydrocoll. 2023, 145, 109009. [Google Scholar] [CrossRef]
- Xi, C.; Kang, N.; Zhao, C.; Liu, Y.; Sun, Z.; Zhang, T. Effects of pH and different sugars on the structures and emulsification properties of whey protein isolate-sugar conjugates. Food Biosci. 2020, 33, 100507. [Google Scholar] [CrossRef]
- López-Monterrubio, D.I.; Lobato-Calleros, C.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Huauzontle (Chenopodium nuttalliae Saff.) protein: Composition, structure, physicochemical and functional properties. Food Hydrocoll. 2020, 108, 106043. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Chang, C.; Chen, J.; Cao, F.; Zhao, J.; Zheng, Y.; Zhu, J. Physicochemical and functional properties of proteins extracted from three microalgal species. Food Hydrocoll. 2019, 96, 510–517. [Google Scholar] [CrossRef]
- Lupatini Menegotto, A.L.; Souza, L.E.S.d.; Colla, L.M.; Costa, J.A.V.; Sehn, E.; Bittencourt, P.R.S.; Moraes Flores, É.L.d.; Canan, C.; Colla, E. Investigation of techno-functional and physicochemical properties of Spirulina platensis protein concentrate for food enrichment. LWT 2019, 114, 108267. [Google Scholar] [CrossRef]
- He, S.; Zhao, J.; Cao, X.; Ye, Y.; Wu, Z.; Yue, J.; Yang, L.; Jin, R.; Sun, H. Low pH-shifting treatment would improve functional properties of black turtle bean (Phaseolus vulgaris L.) protein isolate with immunoreactivity reduction. Food Chem. 2020, 330, 127217. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, M.; Wang, Y.; Li, K.; Du, J.; Bai, Y. Effect of pH-shifting treatment on structural and heat induced gel properties of peanut protein isolate. Food Chem. 2020, 325, 126921. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Li, R.; Wang, Y.; Xiang, Q.; Li, K.; Bai, Y. Effects of combined treatment with ultrasound and pH shifting on foaming properties of chickpea protein isolate. Food Hydrocoll. 2022, 124, 107351. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Structural modification in album (Chenopodium album) protein isolates due to controlled thermal modification and its relationship with protein digestibility and functionality. Food Hydrocoll. 2020, 103, 105708. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Cui, C.; Sun-Waterhouse, D. Structural, functional properties, and in vitro digestibility of sunflower protein concentrate as affected by extraction method: Isoelectric precipitation vs ultrafiltration. Food Chem. 2024, 439, 138090. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, X.; Li, J.; Pan, D.; Du, L. Enhancing the functionalities of chickpea protein isolate through a combined strategy with pH-shifting and cold plasma treatment. Innov. Food Sci. Emerg. Technol. 2024, 93, 103607. [Google Scholar] [CrossRef]
- Ai, M.; Tang, T.; Zhou, L.; Ling, Z.; Guo, S.; Jiang, A. Effects of different proteases on the emulsifying capacity, rheological and structure characteristics of preserved egg white hydrolysates. Food Hydrocoll. 2019, 87, 933–942. [Google Scholar] [CrossRef]
- Yin, S.-W.; Tang, C.-H.; Cao, J.-S.; Hu, E.-K.; Wen, Q.-B.; Yang, X.-Q. Effects of limited enzymatic hydrolysis with trypsin on the functional properties of hemp (Cannabis sativa L.) protein isolate. Food Chem. 2008, 106, 1004–1013. [Google Scholar] [CrossRef]
- Yu, Y.; Guan, Y.; Liu, J.; Hedi, W.; Yu, Y.; Zhang, T. Molecular structural modification of egg white protein by pH-shifting for improving emulsifying capacity and stability. Food Hydrocoll. 2021, 121, 107071. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Ma, X.; Lv, R.; Balaso Watharkar, R.; Ding, T.; Ye, X.; Liu, D. Effect of pH-shifting treatment on structural and functional properties of whey protein isolate and its interaction with (−)-epigallocatechin-3-gallate. Food Chem. 2019, 274, 234–241. [Google Scholar] [CrossRef]
- Pereira, A.M.; Lisboa, C.R.; Costa, J.A.V. High protein ingredients of microalgal origin: Obtainment and functional properties. Innov. Food Sci. Emerg. Technol. 2018, 47, 187–194. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, L.; Wu, G.; Liu, T.; Li, X.; Wang, X.; Zhang, H. Comparative study of various methods used for bitterness reduction from pea (Pisum sativum L.) protein hydrolysates. LWT 2022, 159, 113228. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Y.; Ma, C.; Julian McClements, D.; Liu, F.; Liu, X. Pea protein isolate-inulin conjugates prepared by pH-shift treatment and ultrasonic-enhanced glycosylation: Structural and functional properties. Food Chem. 2022, 384, 132511. [Google Scholar] [CrossRef]
- Lyu, S.; Chen, M.; Wang, Y.; Zhang, D.; Zhao, S.; Liu, J.; Pan, F.; Zhang, T. Foaming properties of egg white proteins improved by enzymatic hydrolysis: The changes in structure and physicochemical properties. Food Hydrocoll. 2023, 141, 108681. [Google Scholar] [CrossRef]
- Han, W.; Shi, W.; Gong, D.; Zhang, G. Improvement of solubility, emulsification property and stability of potato protein by pH-shifting combined with microwave treatment and interaction with pectin. Food Biosci. 2023, 56, 103301. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; He, J.; Xu, Y.; Guo, X. Effects of high-pressure homogenization on the physicochemical, foaming, and emulsifying properties of chickpea protein. Food Res. Int. 2023, 170, 112986. [Google Scholar] [CrossRef]
- Miranda, M.P. Comparison of the effect of Sodium Chloride concentration on protein determination: Bradford and Biuret methods. Anal. Biochem. 2024, 687, 115450. [Google Scholar] [CrossRef]
- Yeasmin, F.; Prasad, P.; Sahu, J.K. Effect of ultrasound on physicochemical, functional and antioxidant properties of red kidney bean (Phaseolus vulgaris L.) proteins extract. Food Biosci. 2024, 57, 103599. [Google Scholar] [CrossRef]
- Fan, Y.; Peng, G.; Pang, X.; Wen, Z.; Yi, J. Physicochemical, emulsifying, and interfacial properties of different whey protein aggregates obtained by thermal treatment. LWT 2021, 149, 111904. [Google Scholar] [CrossRef]
- Zhu, K.-X.; Sun, X.-H.; Chen, Z.-C.; Peng, W.; Qian, H.-F.; Zhou, H.-M. Comparison of functional properties and secondary structures of defatted wheat germ proteins separated by reverse micelles and alkaline extraction and isoelectric precipitation. Food Chem. 2010, 123, 1163–1169. [Google Scholar] [CrossRef]


| Amino Acid | (g/100 g) | FAO/WHO Recommendation for Children (g/100 g) | FAO/WHO Recommendation for Adults (g/100 g) |
|---|---|---|---|
| Threonine (Thr) | 4.83 ± 0.21 | 3.10 | 2.50 |
| Valine (Val) | 5.39 ± 0.17 | 4.30 | 4.00 |
| Cysteine (Cys) | 2.92 ± 0.30 | ||
| Methionine (Met) | 3.29 ± 0.20 | ||
| Isoleucine (Ile) | 4.13 ± 0.04 | 3.20 | 3.00 |
| Leucine (Leu) | 6.62 ± 0.08 | 6.60 | 6.10 |
| Tyrosine (Tyr) | 4.58 ± 0.13 | ||
| Phenylalanine (Phe) | 5.66 ± 0.09 | ||
| Lysine (Lys) | 4.87 ± 0.11 | 5.70 | 4.80 |
| Histidine (His) | 3.12 ± 0.06 | 2.00 | 1.60 |
| Asparagine (Asp) | 8.09 ± 0.31 | - | - |
| Serine (Ser) | 4.98 ± 0.20 | - | - |
| Glutarnine (Glu) | 8.26 ± 0.16 | - | - |
| Glycine (Gly) | 4.57 ± 0.24 | - | - |
| Alaine (Ala) | 5.28 ± 0.14 | - | - |
| Arginine (Arg) | 4.65 ± 0.18 | - | - |
| Proline (Pro) | 4.31 ± 0.07 | - | - |
| Essential amino acid | 45.44 | ||
| Nonessential amino acid | 40.15 | ||
| Hydrophobic | 17.33 | ||
| Hydrophilic | 39.25 | ||
| Acidic | 16.36 | ||
| Basic | 12.65 | ||
| Aromatic | 10.24 | 5.2 | 4.1 |
| Branched-chain | 16.15 | ||
| Negatively charged | 12.65 | ||
| Positively charged | 26.17 | ||
| SAA(Cys+Met) | 6.21 | 2.7 | 2.3 |
| TO (°C) | TP (°C) | ΔH (J/g) | |
|---|---|---|---|
| pH3 | 43.54 ± 0.32 a | 68.44 ± 0.12 d | 42.48 ± 0.47 d |
| pH5 | 40.57 ± 2.04 b | 71.19 ± 0.67 c | 90.52 ± 0.09 c |
| pH7 | 42.60 ± 1.79 ab | 71.07 ± 0.17 c | 35.17 ± 0.70 e |
| pH9 | 41.51 ± 1.79 a | 74.36 ± 0.93 b | 137.59 ± 0.54 a |
| pH11 | 43.82 ± 0.85 a | 77.66 ± 0.14 a | 93.34 ± 0.31 b |
| Average Particle Size (nm) | Zeta Potential (mV) | |
|---|---|---|
| pH3 | 3199.84 ± 0.47 e | −6.03 ± 0.47 a |
| pH5 | 1841.70 ± 0.73 d | −26.78 ± 0.22 b |
| pH7 | 1463.05 ± 0.46 c | −34.23 ± 0.67 c |
| pH9 | 1245.21 ± 0.91 b | −36.60 ± 0.86 d |
| pH11 | 1115.11 ± 0.57 a | −45.33 ± 0.54 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhu, L.; Zhu, Z.; Liu, M.; Zhao, X. Effects of Different pH Levels on the Structural and Functional Properties of Proteins of Phaeodactylum tricornutum. Molecules 2024, 29, 3139. https://doi.org/10.3390/molecules29133139
Wang Y, Zhu L, Zhu Z, Liu M, Zhao X. Effects of Different pH Levels on the Structural and Functional Properties of Proteins of Phaeodactylum tricornutum. Molecules. 2024; 29(13):3139. https://doi.org/10.3390/molecules29133139
Chicago/Turabian StyleWang, Yanli, Laijing Zhu, Zhunyao Zhu, Meng Liu, and Xiangzhong Zhao. 2024. "Effects of Different pH Levels on the Structural and Functional Properties of Proteins of Phaeodactylum tricornutum" Molecules 29, no. 13: 3139. https://doi.org/10.3390/molecules29133139
APA StyleWang, Y., Zhu, L., Zhu, Z., Liu, M., & Zhao, X. (2024). Effects of Different pH Levels on the Structural and Functional Properties of Proteins of Phaeodactylum tricornutum. Molecules, 29(13), 3139. https://doi.org/10.3390/molecules29133139






