Designing Dual-State and Aggregation-Induced Emissive Luminogens from Lignocellulosic Biosourced Molecules
Abstract
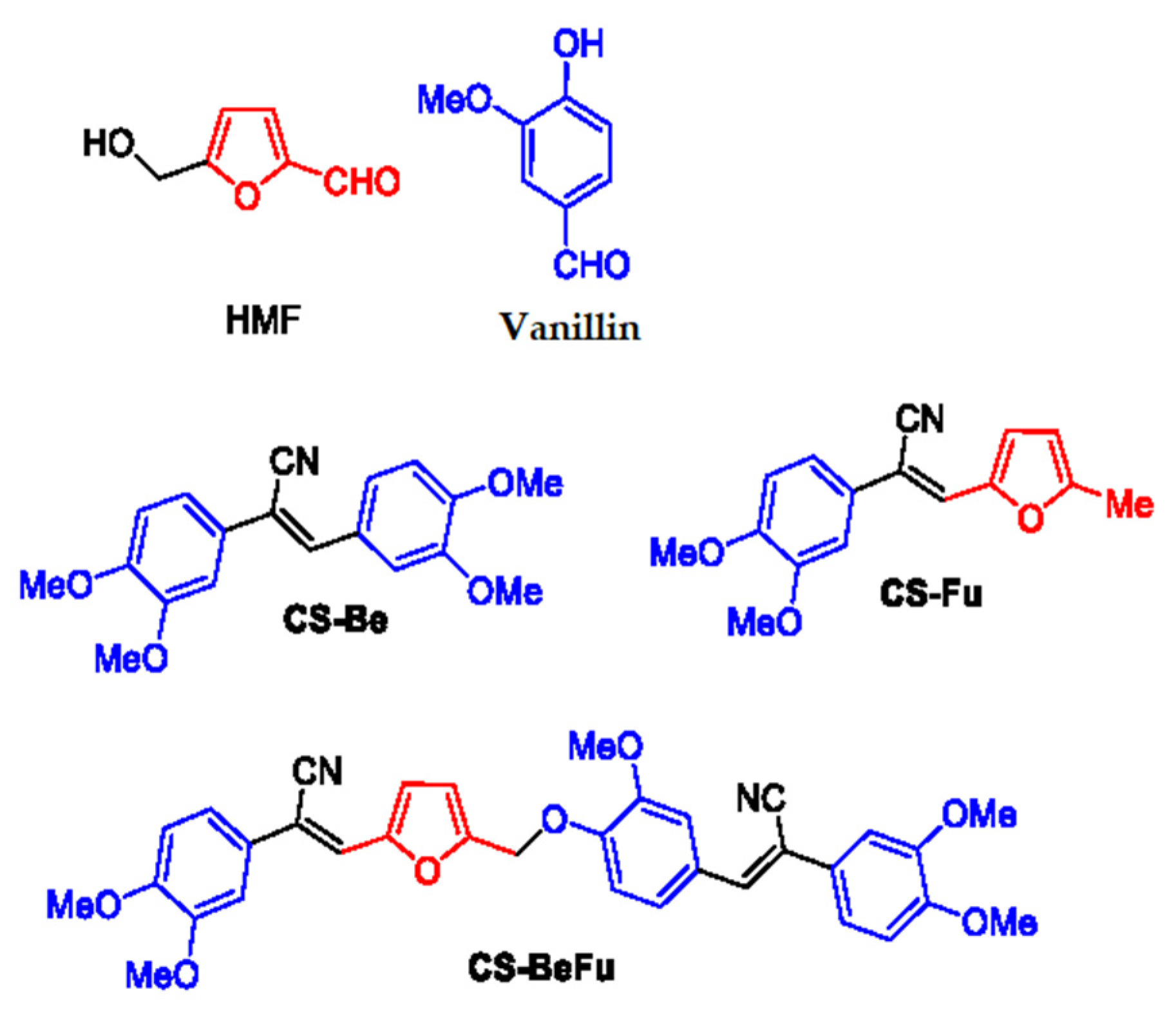
1. Introduction
2. Results and Discussion
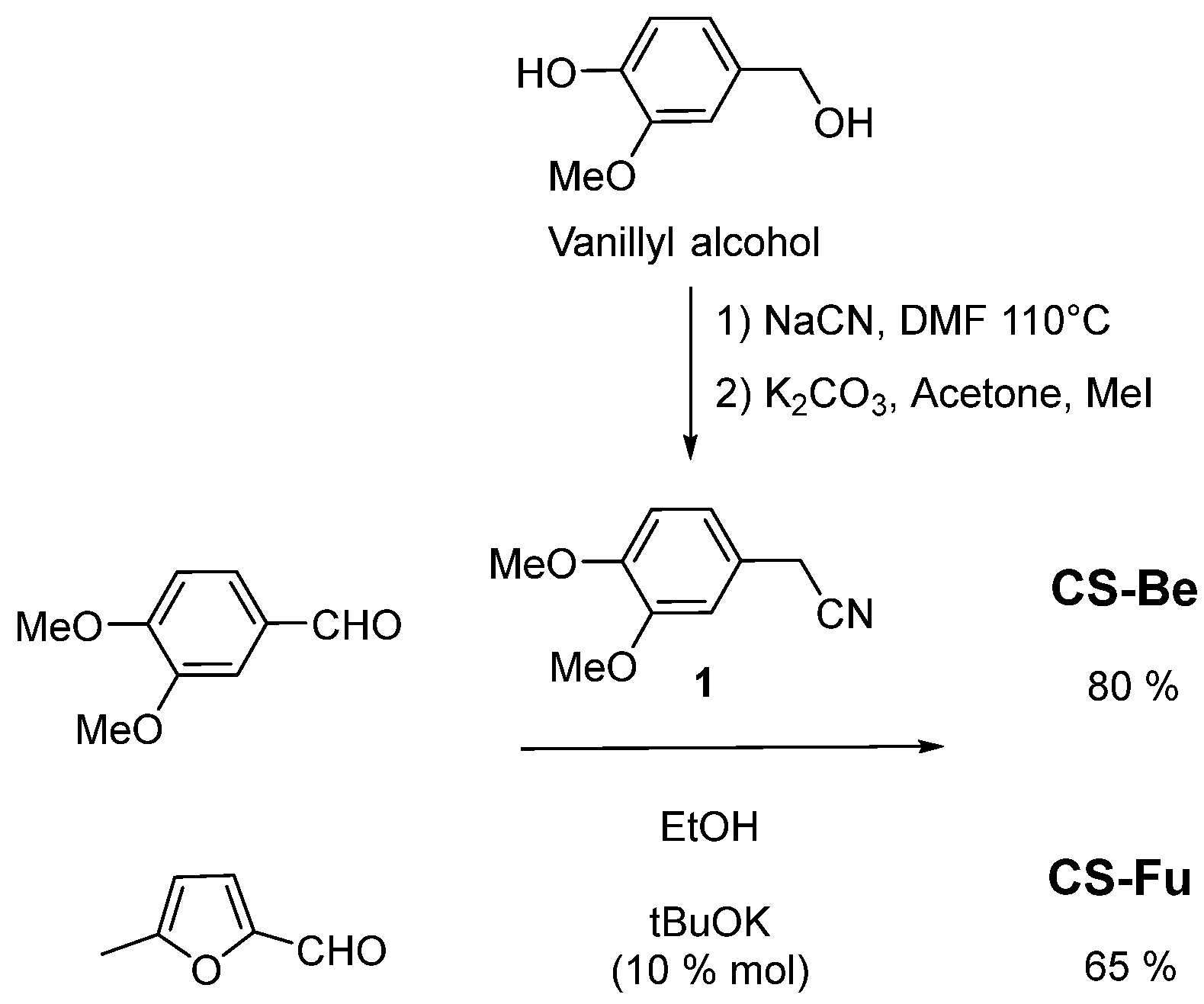
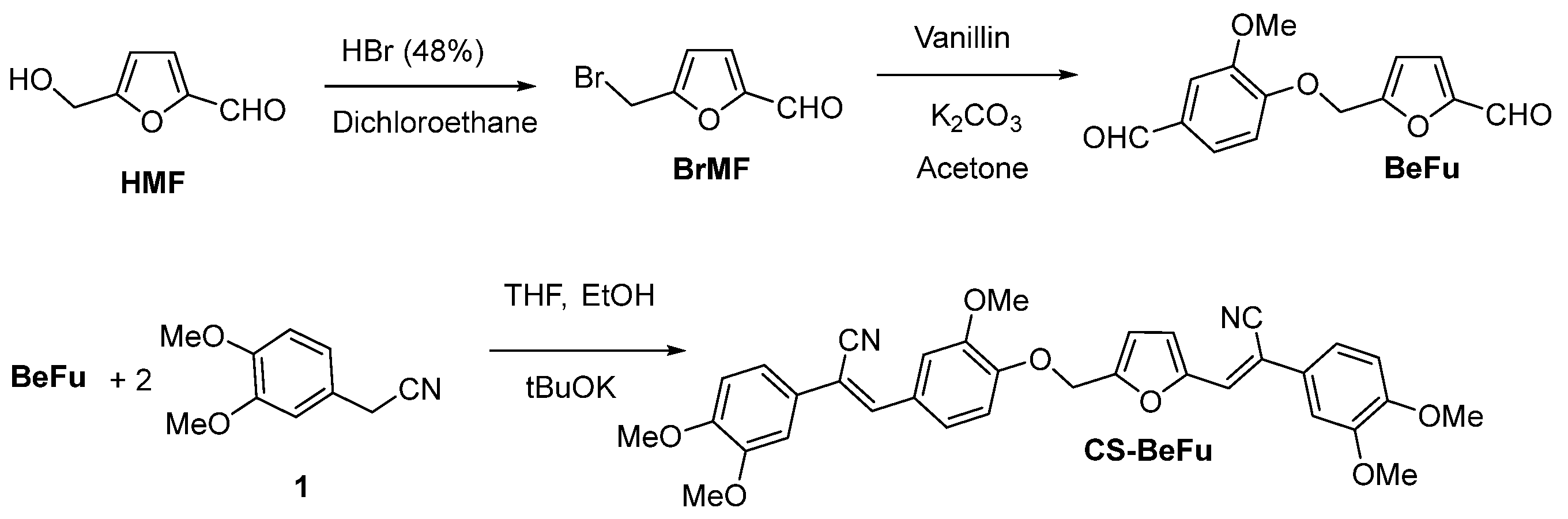
2.1. Synthesis
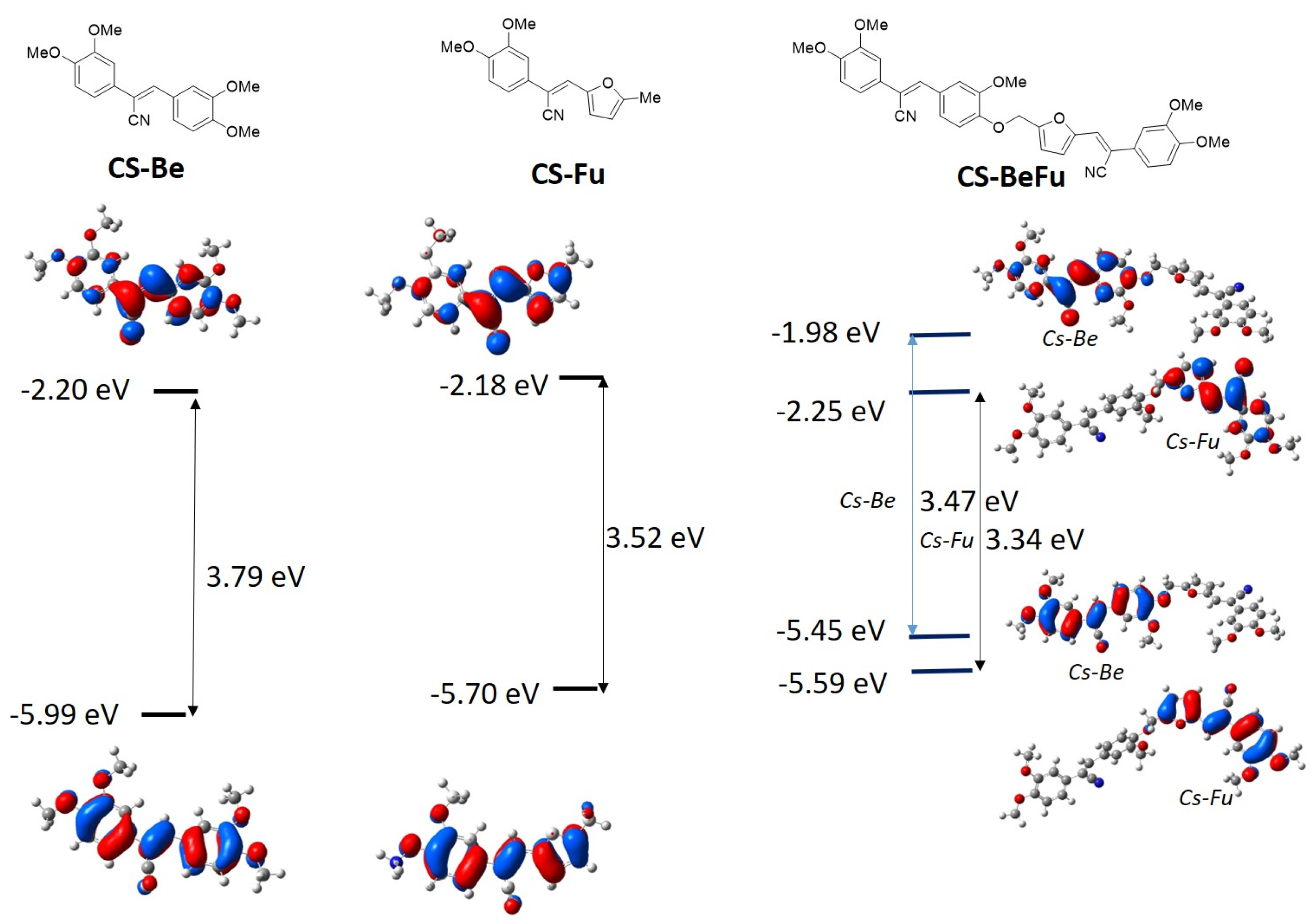
2.2. Electronic Properties
2.3. Optical Data
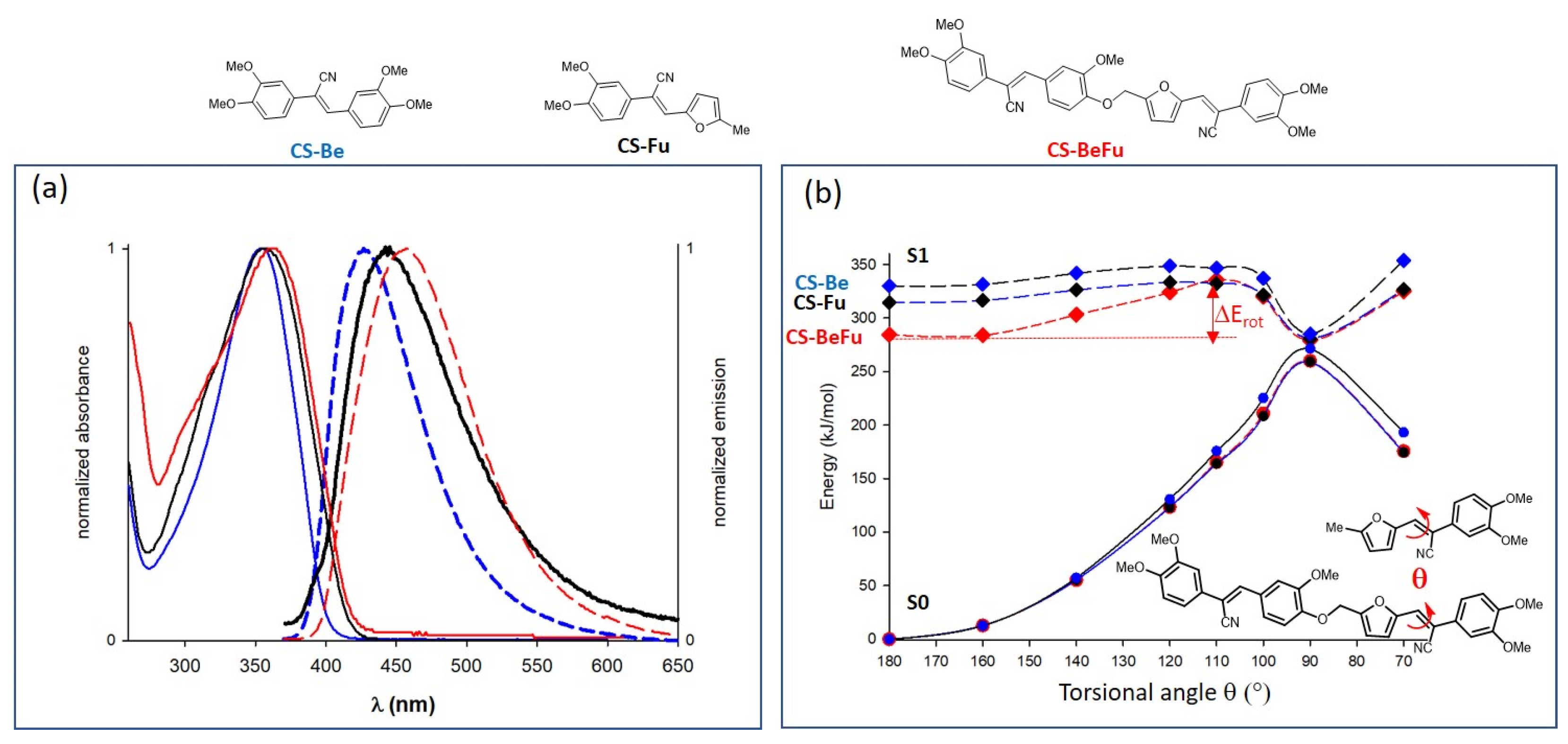
2.3.1. Optical Properties in Solution
2.3.2. Emission in THF/Water Mixtures
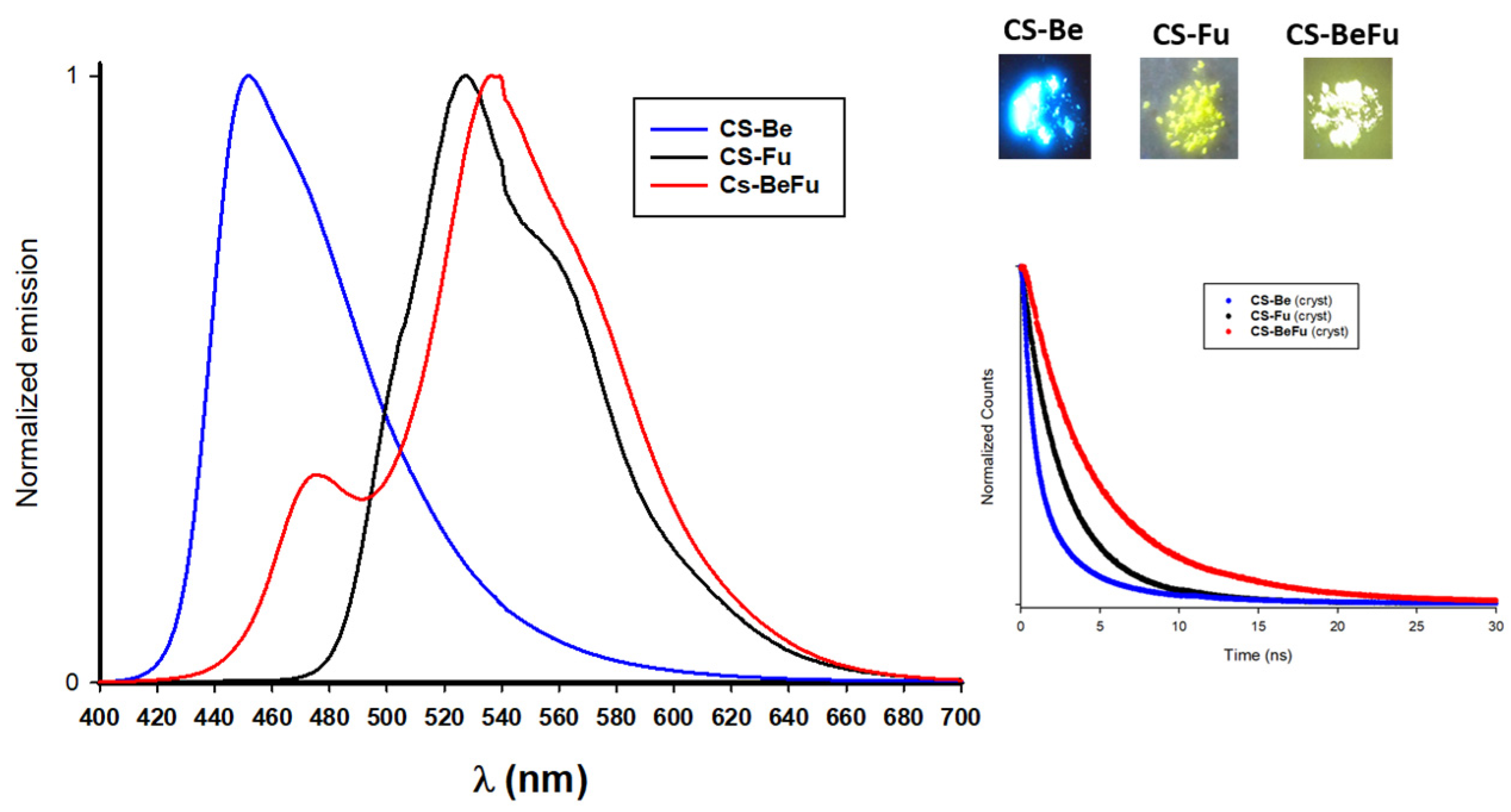
2.3.3. Emission in the Solid State for the Crystals
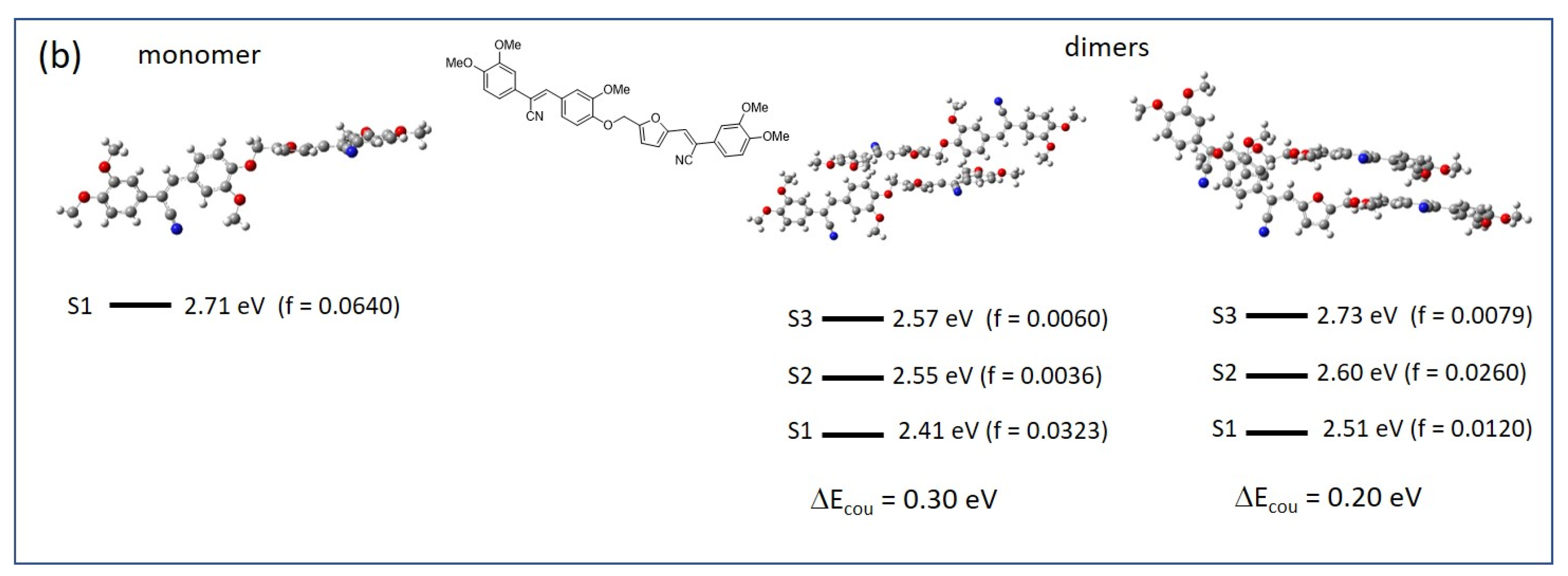
2.4. Structure–Property Relationships
3. Experimental Section
3.1. Materials and Methods
3.1.1. UV–Vis and Fluorescent Spectroscopy
3.1.2. X-ray Analysis
3.2. Syntheses and Characterization of Compounds
3.2.1. Synthesis of BeFu: 5-((4-formyl-2-methoxyphenoxy)methyl)furan-2-carbaldehyde
3.2.2. Procedure for Knoevenagel Monocondensation
3.2.3. Synthesis of CS-BeFu: (Z)-3-(5-((4-((Z)-2-cyano-2-(3,4-dimethoxyphenyl)vinyl)-2-methoxyphenoxy)methyl)furan-2-yl)-2-(3,4-dimethoxyphenyl)acrylonitrile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gierschner, J.; Shi, J.; Milián-Medina, B.; Roca-Sanjuán, D.; Varghese, S.; Park, S. Luminescence in Crystalline Organic Materials: From Molecules to Molecular Solids. Adv. Opt. Mater. 2021, 9, 2002251. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Voskuhl, J.; Giese, M. Mesogens with aggregation-induced emission properties: Materials with a bright future. Aggregate 2022, 3, e124. [Google Scholar] [CrossRef]
- Würthner, F. Aggregation-Induced Emission (AIE): A Historical Perspective. Angew. Chem. Int. Ed. 2020, 59, 14192–14196. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Turelli, M.; Ciofini, I.; Wang, Q.; Ottochian, A.; Labat, F.; Adamo, C. Organic compounds for solid state luminescence enhancement/aggregation induced emission: A theoretical perspective. Phys. Chem. Chem. Phys. 2023, 25, 17769–17786. [Google Scholar] [CrossRef]
- Belmonte-Vázquez, J.L.; Amador-Sánchez, Y.A.; Rodríguez-Cortés, L.A.; Rodríguez-Molina, B. Dual-State Emission (DSE) in Organic Fluorophores: Design and Applications. Chem. Mater. 2021, 33, 7160–7184. [Google Scholar] [CrossRef]
- Huber, A.; Dubbert, J.; Scherz, T.D.; Voskuhl, J. Design Concepts for Solution and Solid-State Emitters—A Modern Viewpoint on Classical and Non-Classical Approaches. Chem. Eur. J. 2023, 29, e202202481. [Google Scholar] [CrossRef]
- Rodríguez-Cortés, L.A.; Navarro-Huerta, A.; Rodríguez-Molina, B. One molecule to light it all: The era of dual-state emission. Matter 2021, 4, 2622–2624. [Google Scholar] [CrossRef]
- Xia, G.; Si, L.; Wang, H. Dual-state emission: The compatible art of substantial rigidity and twisting conformation within a single molecule. Mater. Today Chem. 2023, 30, 101596. [Google Scholar] [CrossRef]
- Stoerkler, T.; Pariat, T.; Laurent, A.D.; Jacquemin, D.; Ulrich, G.; Massue, J. Excited-State Intramolecular Proton Transfer Dyes with Dual-State Emission Properties: Concept, Examples and Applications. Molecules 2022, 27, 2443. [Google Scholar] [CrossRef]
- Ma, Z.; Tu, J.; Yang, D.; Zhang, Q.; Wu, J. Recent advances in organic small-molecular dual-state emission probes. J. Mol. Struct. 2024, 1312, 138478. [Google Scholar] [CrossRef]
- Boivin, L.-P.; Dupont, W.; Gendron, D.; Leclerc, M. Biosourced Monomers: Toward Sustainable Conjugated Polymers for Organic Electronics. Macromol. Chem. Phys. 2023, 224, 2200378. [Google Scholar] [CrossRef]
- Giraud, L.; Grelier, S.; Grau, E.; Hadziioannou, G.; Brochon, C.; Cramail, H.; Cloutet, E. Upgrading the chemistry of π-conjugated polymers toward more sustainable materials. J. Mater. Chem. C 2020, 8, 9792–9810. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cui, Q.; Feng, Y.; Xuan, J. Composition of Lignocellulose Hydrolysate in Different Biorefinery Strategies: Nutrients and Inhibitors. Molecules 2024, 29, 2275. [Google Scholar] [CrossRef]
- Decostanzi, M.; Auvergne, R.; Boutevin, B.; Caillol, S. Biobased phenol and furan derivative coupling for the synthesis of functional monomers. Green Chem. 2019, 21, 724–747. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Zheng, B.; Huo, L. Recent Advances of Furan and Its Derivatives Based Semiconductor Materials for Organic Photovoltaics. Small Methods 2021, 5, 2100493. [Google Scholar] [CrossRef] [PubMed]
- Karlinskii, B.Y.; Ananikov, V.P. Recent advances in the development of green furan ring-containing polymeric materials based on renewable plant biomass. Chem. Soc. Rev. 2023, 52, 836–862. [Google Scholar] [CrossRef] [PubMed]
- Fache, M.; Boutevin, B.; Caillol, S. Vanillin Production from Lignin and Its Use as a Renewable Chemical. ACS Sustain. Chem. Eng. 2016, 4, 35–46. [Google Scholar] [CrossRef]
- Xu, L.; Liaqat, F.; Sun, J.; Khazi, M.I.; Xie, R.; Zhu, D. Advances in the vanillin synthesis and biotransformation: A review. Renew. Sustain. Energy Rev. 2024, 189, 113905. [Google Scholar] [CrossRef]
- Martău, G.A.; Călinoiu, L.-F.; Vodnar, D.C. Bio-vanillin: Towards a sustainable industrial production. Trends Food Sci. Technol. 2021, 109, 579–592. [Google Scholar] [CrossRef]
- D’Arrigo, P.; Rossato, L.A.M.; Strini, A.; Serra, S. From Waste to Value: Recent Insights into Producing Vanillin from Lignin. Molecules 2024, 29, 442. [Google Scholar] [CrossRef] [PubMed]
- Mallet, C.; Moussallem, C.; Faurie, A.; Allain, M.; Gohier, F.; Skene, W.G.; Frère, P. Rational Topological Design for Fluorescence Enhancement upon Aggregation of Distyrylfuran Derivatives. Chem. Eur. J. 2015, 21, 7944–7953. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Moussallem, C.; Allain, M.; Segut, O.; Gohier, F.; Frère, P. Exploring the Electronic Properties of Extended Benzofuran-Cyanovinyl Derivatives Obtained from Lignocellulosic and Carbohydrate Platforms Raw Materials. ChemPlusChem 2021, 86, 475–482. [Google Scholar] [CrossRef]
- Mallet, C.; Frère, P. Furylenevinylene Oligomers as Extended Conjugated Systems from Renewable Materials. Eur. J. Org. Chem. 2022, 2022, e202201276. [Google Scholar] [CrossRef]
- Gao, A.; Wang, Q.; Wu, H.; Zhao, J.-W.; Cao, X. Research progress on AIE cyanostilbene-based self-assembly gels: Design, regulation and applications. Coord. Chem. Rev. 2022, 471, 214753. [Google Scholar] [CrossRef]
- Mahalingavelar, P.; Kanvah, S. α-Cyanostilbene: A multifunctional spectral engineering motif. Phys. Chem. Chem. Phys. 2022, 24, 23049–23075. [Google Scholar] [CrossRef]
- Martínez-Abadía, M.; Giménez, R.; Ros, M.B. Self-Assembled α-Cyanostilbenes for Advanced Functional Materials. Adv. Mater. 2018, 30, 1704161. [Google Scholar] [CrossRef]
- Zhao, J.; Chi, Z.; Zhang, Y.; Mao, Z.; Yang, Z.; Ubba, E.; Chi, Z. Recent progress in the mechanofluorochromism of cyanoethylene derivatives with aggregation-induced emission. J. Mater. Chem. C 2018, 6, 6327–6353. [Google Scholar] [CrossRef]
- Lin, S.; Gutierrez-Cuevas, K.G.; Zhang, X.; Guo, J.; Li, Q. Fluorescent Photochromic α-Cyanodiarylethene Molecular Switches: An Emerging and Promising Class of Functional Diarylethene. Adv. Funct. Mater. 2021, 31, 2007957. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Chi, W.; Bindra, A.K.; Gu, L.; Qian, C.; Wu, B.; Yue, B.; Liu, G.; Yang, G.; et al. Structural Engineering of Luminogens with High Emission Efficiency Both in Solution and in the Solid State. Angew. Chem. Int. Ed. 2019, 58, 11419–11423. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Tang, F.; He, F.; Kong, L.; Huang, J.; Yang, J.; Ding, A. Pyrene and triphenylamine substituted cyanostyrene and cyanostilbene derivatives with dual-state emission for high-contrast mechanofluorochromism and cell imaging. Org. Chem. Front. 2022, 9, 5118–5124. [Google Scholar] [CrossRef]
- Xue, J.; Tang, F.; Ding, A.; He, F.; Huang, J.; Kong, L.; Yang, J. α-Cyanostilbene functionalized carbazole derivatives exhibiting dual-state emission and multi-stimuli responsive fluorescent switching. J. Lumin. 2022, 250, 119119. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, L.; Cao, S.; Wang, J.; Niu, G. Donor–Acceptor–Acceptor-Conjugated Dual-State Emissive Acrylonitriles: Investigating the Effect of Acceptor Unit Order and Biological Imaging. Org. Lett. 2022, 24, 8305–8309. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Yang, L.; Kong, L.; Wang, C.; Zhang, Q.; Yang, J. Highly efficient dual-state emission and two-photon absorption of novel naphthalimide functionalized cyanostilbene derivatives with finely tuned terminal alkoxyl groups. Mater. Chem. Front. 2022, 6, 3522–3530. [Google Scholar] [CrossRef]
- Shi, Q.; Ni, Y.; Yang, L.; Kong, L.; Gu, P.; Wang, C.; Zhang, Q.; Zhou, H.; Yang, J. Fluorination of naphthalimide–cyanostilbene derivatives to achieve dual-state emission luminogens with strong fluorescence in highly polar environments for bioimaging. J. Mater. Chem. B 2023, 11, 6859–6867. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Loumaigne, M.; Grolleau, J.; Allain, M.; Frère, P. Dual-state emission versus no emission by manipulating the molecular structures of cyanovinyl–benzofuran derivatives. Mol. Syst. Des. Eng. 2022, 7, 1119–1128. [Google Scholar] [CrossRef]
- Schwartz, M.A.; Zoda, M.; Vishnuvajjala, B.; Mami, I. A convenient synthesis of o-and p-hydroxy substituted phenylacetonitriles and phenethylamines. J. Org. Chem. 1976, 41, 2502–2503. [Google Scholar] [CrossRef]
- Shi, J.; Aguilar Suarez, L.E.; Yoon, S.-J.; Varghese, S.; Serpa, C.; Park, S.Y.; Lüer, L.; Roca-Sanjuán, D.; Milián-Medina, B.; Gierschner, J. Solid State Luminescence Enhancement in π-Conjugated Materials: Unraveling the Mechanism beyond the Framework of AIE/AIEE. J. Phys. Chem. C 2017, 121, 23166–23183. [Google Scholar] [CrossRef]
- Shi, J.; Izquierdo, M.A.; Oh, S.; Park, S.Y.; Milián-Medina, B.; Roca-Sanjuán, D.; Gierschner, J. Inverted energy gap law for the nonradiative decay in fluorescent floppy molecules: Larger fluorescence quantum yields for smaller energy gaps. Org. Chem. Front. 2019, 6, 1948–1954. [Google Scholar] [CrossRef]
- Rivera, M.; Stojanović, L.; Crespo-Otero, R. Role of Conical Intersections on the Efficiency of Fluorescent Organic Molecular Crystals. J. Phys. Chem. A 2021, 125, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, Z. The Strong Light-Emission Materials in the Aggregated State: What Happens from a Single Molecule to the Collective Group. Adv. Sci. 2017, 4, 1600484. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z. Molecular conformation and packing: Their critical roles in the emission performance of mechanochromic fluorescence materials. Mater. Chem. Front. 2017, 1, 2174–2194. [Google Scholar] [CrossRef]
- Wilbraham, L.; Louis, M.; Alberga, D.; Brosseau, A.; Guillot, R.; Ito, F.; Labat, F.; Métivier, R.; Allain, C.; Ciofini, I. Revealing the Origins of Mechanically Induced Fluorescence Changes in Organic Molecular Crystals. Adv. Mater. 2018, 30, 1800817. [Google Scholar] [CrossRef]
- Ding, Z.; Lu, T.; Bi, C.; Li, B.; Zhang, S.-T.; Xu, W.; Jiang, S. A multifunctional material with distinct mechanochromic and piezochromic properties: π-stacking in play. Mater. Chem. Front. 2022, 6, 86–93. [Google Scholar] [CrossRef]


| CS-Be | CS-Fu | CS-BeFu | |
|---|---|---|---|
| Solution 1 | |||
| λabs (nm) | 352 | 354 | 358 |
| ε (mol L−1cm−1) | 23,000 | 25,600 | 33,000 |
| ΔEopt (eV) 2 | 3.03 | 2.95 | 2.91 |
| λem-solu (nm) | 426 | 453 | 457 |
| Φsolu (%) 3 | <1 | <1 | 15 |
| ΔErot (kJ/mol) 4 | 19 | 20 | 39 |
| Solid 5 | |||
| λem-solid (nm) | 450 | 527 | 476 538 |
| Φsolid (%) 3 | 46 | 5 | 25 |
| Δλemi (eV) 6 | 0.16 | 0.38 | 0.41 |
| ΔEcou (eV) 7 | 0.21 | 0.38 | 0.20 0.30 |
| τ (ns) | 2.3 (85.4%) 4.6 (14.6%) | 2.2 (71.0%) 5.1 (29.0%) | 1.9 (12.4%) 4.3 (45.7%) 7.2 (41.9%) |
| τaver (ns) | 2.6 | 3.1 | 5.2 |
| kr (ns−1) 8 | 0.177 | 0.016 | 0.048 |
| knr (ns−1) 9 | 0.21 | 0.31 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonnot, M.; Ibrahim, N.; Allain, M.; Frère, P. Designing Dual-State and Aggregation-Induced Emissive Luminogens from Lignocellulosic Biosourced Molecules. Molecules 2024, 29, 3135. https://doi.org/10.3390/molecules29133135
Bonnot M, Ibrahim N, Allain M, Frère P. Designing Dual-State and Aggregation-Induced Emissive Luminogens from Lignocellulosic Biosourced Molecules. Molecules. 2024; 29(13):3135. https://doi.org/10.3390/molecules29133135
Chicago/Turabian StyleBonnot, Maelys, Nagham Ibrahim, Magali Allain, and Pierre Frère. 2024. "Designing Dual-State and Aggregation-Induced Emissive Luminogens from Lignocellulosic Biosourced Molecules" Molecules 29, no. 13: 3135. https://doi.org/10.3390/molecules29133135
APA StyleBonnot, M., Ibrahim, N., Allain, M., & Frère, P. (2024). Designing Dual-State and Aggregation-Induced Emissive Luminogens from Lignocellulosic Biosourced Molecules. Molecules, 29(13), 3135. https://doi.org/10.3390/molecules29133135






