Potential Use of Tomato Peel, a Rich Source of Lycopene, for Cancer Treatment
Abstract
1. Introduction
2. Methodology
3. Tomato
| Content | Unit | Ref. |
|---|---|---|
| Energy | (8351–15,375) J/s | [9,30] |
| Proteins | (5.4–17.71) g/100 g d.m. | [9] |
| Lipids | (3.22–23.44) g/100 g d.m. | [31] |
| Ash | (0.02–0.5) g/100 g d.m. | [32] |
| Carbohydrates | (3.9–5.92) g/100 g d.m. | [33] |
| Fiber | (1.2–11.44) g/100 g d.m. | [34] |
| pH | (4.68–3.38) | [35] |
| Glucose | (0.48–2.45) g/100 g d.m. | [36] |
| Sucrose | (0.02–0.05) g/100 g d.m. | [37] |
| Acidity | (0.07–0.48) g/100 g d.m. | [38] |
| Water content | (68.03–96.17) g/100 g d.m. | [39] |
| Lycopene | (410.6–445.2) mg/100 g d.m. | [40] |
| Vitamin C (ascorbic acid) | (255.7–39.4) mg/100 g d.m. | [41] |
| Vitamin E | (0.17–0.1) mg/100 g d.m. | [42] |
| β-carotene | (0.62–1.2) mg/100 g d.m. | [43] |
| Phenolic acids | 0.07372 g/100 g d.m. | [44] |
3.1. World Tomato Production
3.2. Tomato Processing
3.3. Waste Production during Tomato Processing
3.3.1. Tomato Peel
3.3.2. Chemical Composition of Tomato Peel
3.3.3. Carotenoids
3.3.4. Carotenoid Extraction
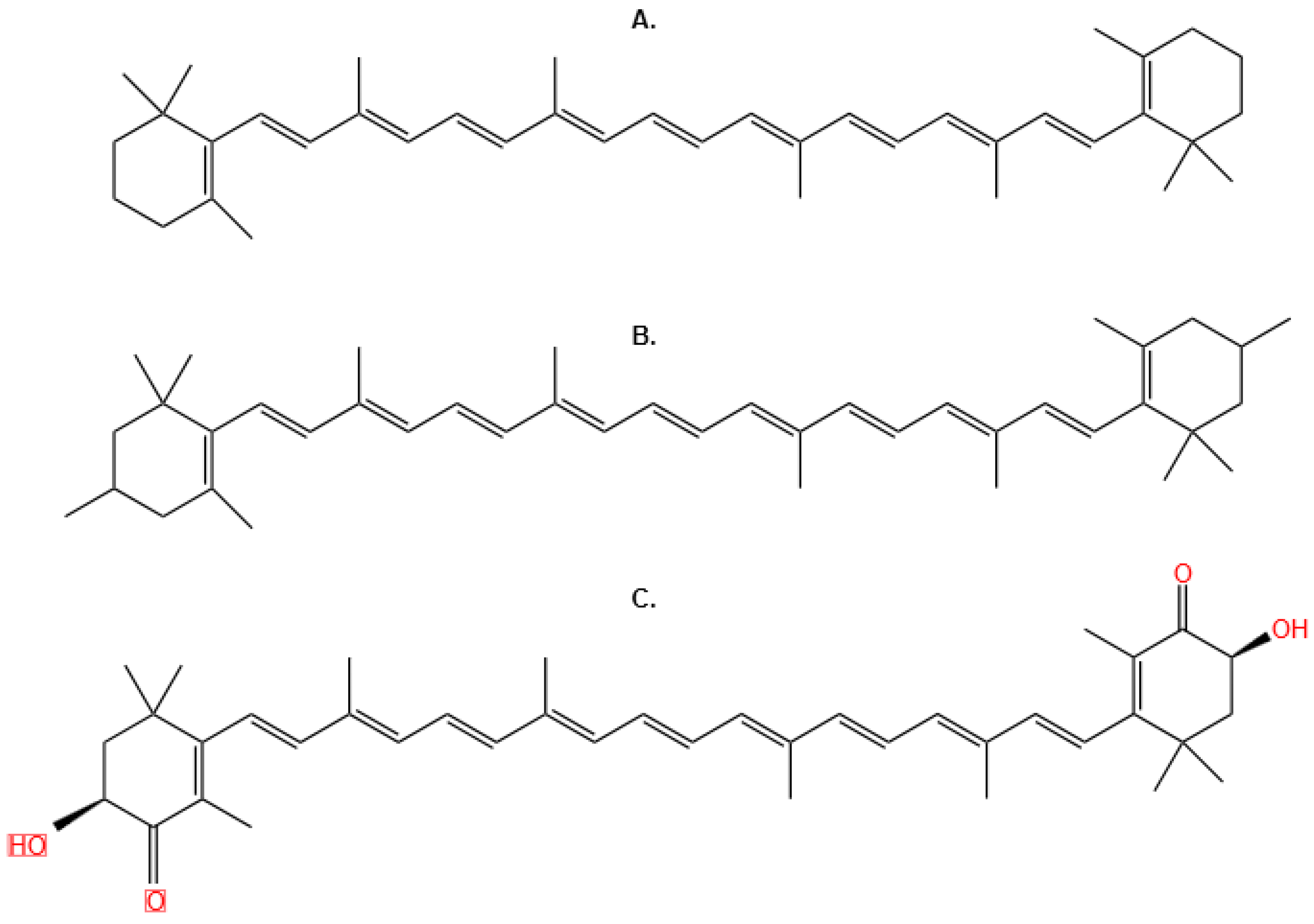
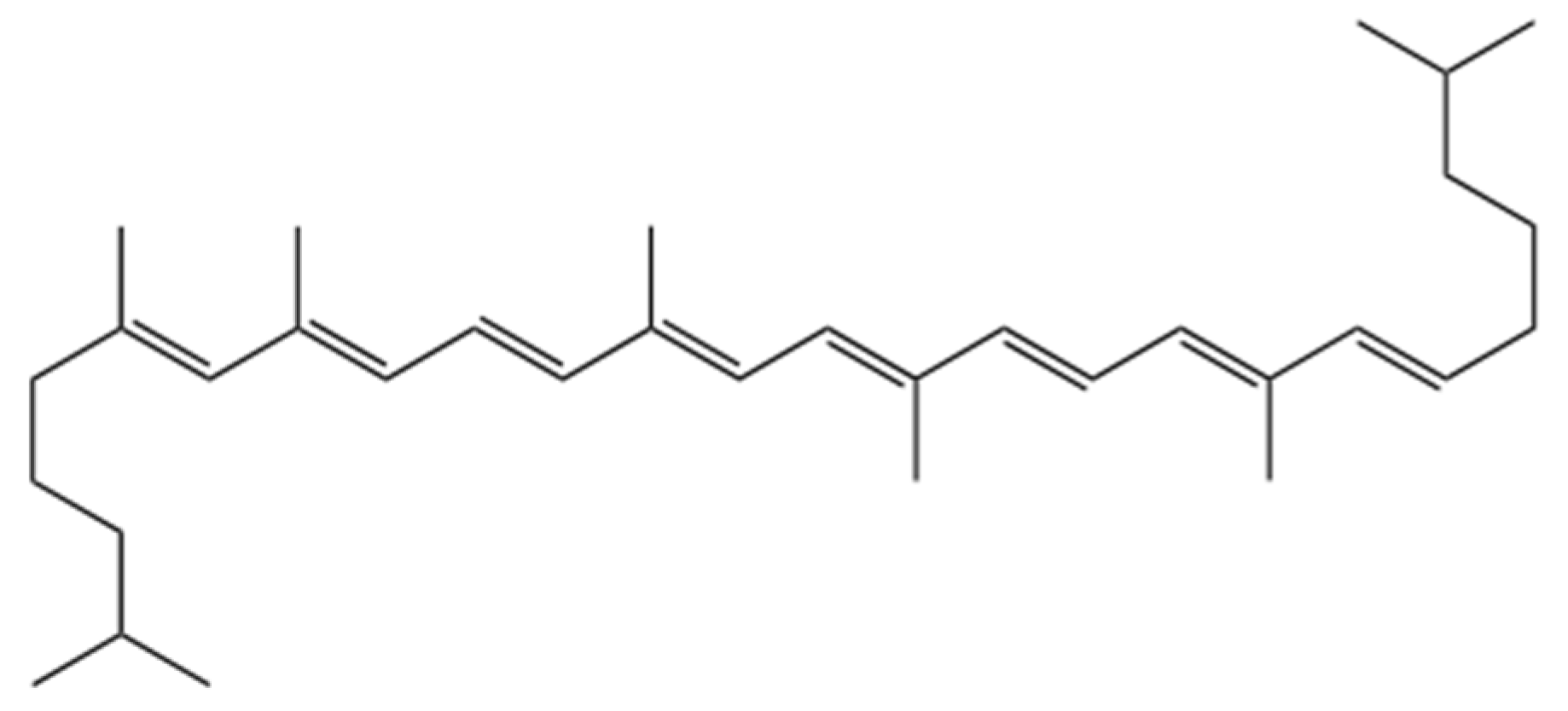
3.3.5. Lycopene
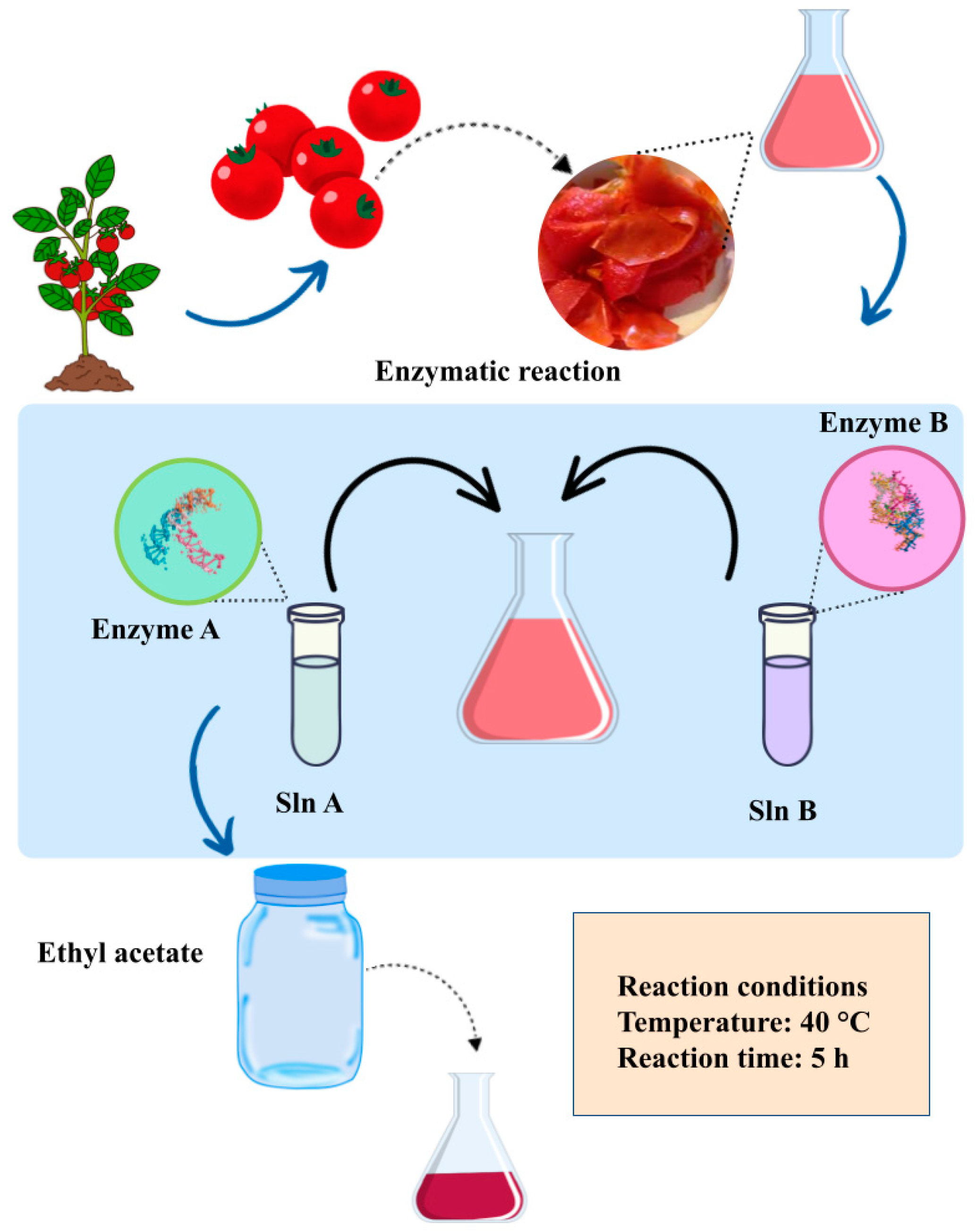
3.3.6. Lycopene Extraction from Tomato Peel
| Technique | Principle of Operation | Operating Conditions | Lycopene Extraction | Environmental Considerations | Refs. |
|---|---|---|---|---|---|
| Supercritical fluid extraction with CO2 (SC-CO2) | Employing carbon dioxide within supercritical extraction methods implements the supercritical fluid extraction (SFE) technique. This separation method utilizes a solvent fluid in a supercritical state to conduct the extraction process. | Pressure, temperature, CO2 flow rate, and extraction time. | Lycopene extraction uses supercritical carbon dioxide (SC-CO2) as a solvent. This process takes advantage of the supercritical properties of CO2, acting as a highly efficient solvent to extract the lycopene-containing tomato oleoresin. SC-CO2 penetrates the plant material during extraction and selectively dissolves the lycopene from the tomato matrix. | Employs environmentally friendly methods, eliminating the need for organic solvents and reducing storage, disposal, and environmental risks. | [91,92,93] |
| Enzyme-assisted extraction (EAE) | This method entails employing enzymes to enhance the effectiveness and specificity of extraction procedures. Through collaborative action with the enzymes inherent in the matrix, EAE enables a more effective breakdown of cellular structures, thereby aiding the liberation of the desired compounds. | Optimal enzyme conditions of temperature, pH, and dosage; optimal time-temperature conditions; plant material such as particle size, water content, chemical composition, and solvent-to-solid ratio. | Enzyme-assisted extraction is used to obtain lycopene. Enzymes such as cellulases, pectinases, and glucanases, individually or in combination, hydrolyze the bonds present in plant cell wall polysaccharides. | Mild conditions, extract quality, higher extraction yields, and higher quality. | [94,95,96] |
| Microwave-assisted extraction (MAE) | A technique in which microwave radiation is utilized to warm solvents in contact with a sample, facilitating the extraction of analytes from the sample matrix into the solvent. | Solid-to-solvent ratio, extraction time, and microwave power for extraction yield. | The application is the optimization of lycopene extraction from tomato peels using the microwave-assisted extraction (MAE) technique. The main objective is to improve the extraction efficiency of lycopene, a carotenoid present in tomato peel, which can be used as a natural colorant or bioactive ingredient. | MAE exploits a small number of solvents, so it is considered a “green” technique. Moreover, heating occurs selectively with much less energy loss in the environment. | [97,98,99,100] |
| Optimized mixed-polarity solvent mixtures | This method is frequently utilized in the food and pharmaceutical sectors due to its efficacy in extracting lipophilic compounds such as lycopene. | Extraction temperature, type of solvent used, stirring time, sample volume, and filtration method. | It is based on using homogeneous mixtures of solvents that exhibit two distinct properties: (a) high affinity for lycopene and (b) ability to swell the plant material and thus improve solvent penetration. | By optimizing solvent combinations, it is feasible to reduce the total amount required to perform an extraction or separation, reducing natural resource use and waste production. | [101] |
| Ultrasonic-assisted extraction | The ultrasound-assisted extraction (UAE) technique is based on acoustic cavitation, which occurs due to the propagation of mechanical waves generated by alternating high- and low-pressure cycles, known as compressions and rarefactions. | Properties of the solvent involved in extraction, such as viscosity and surface tension, alongside environmental factors like temperature and pressure. | Recent studies suggest that ultrasonic extraction enhances the extraction speed and boosts the yield of lycopene by approximately 10%. | Allow for the practical, cost-efficient, and eco-friendly extraction of bioactive components from plant sources. These technologies provide a sustainable and efficient means of producing top-quality plant-based products and offer a substantial competitive edge to businesses in the field. | [43,102,103,104] |
Supercritical Fluid Extraction with CO2 (SC-CO2)
Enzyme-Assisted Extraction (EAE)
Microwave-Assisted Extraction
Optimized Mixed-Polarity Solvent Mixtures
Ultrasonic-Assisted Extraction
3.3.7. Antioxidant Activity of Lycopene
3.3.8. Anticancer Activity of Lycopene
3.3.9. Lycopene Contribution to Cancer Treatment
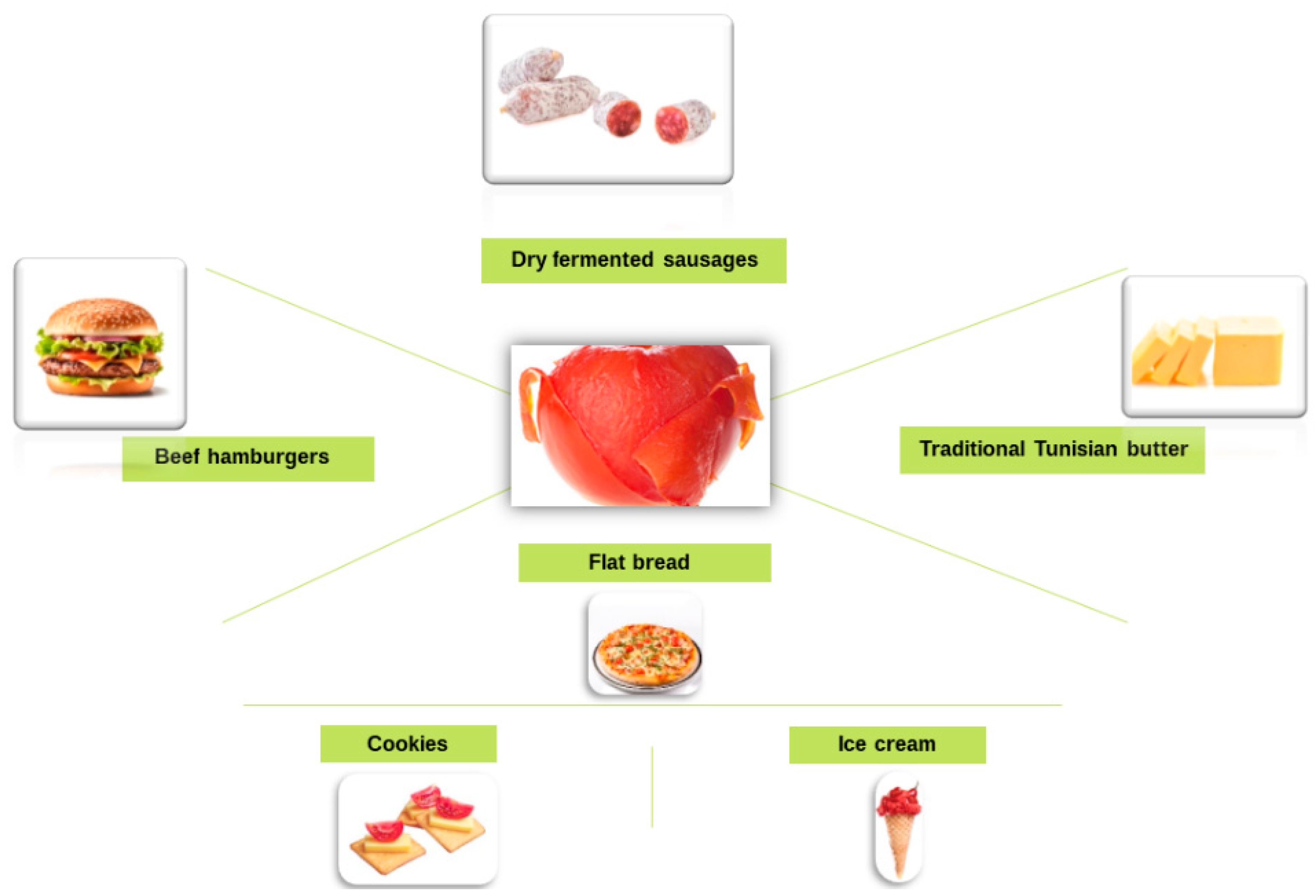
3.4. Other Uses of Tomato Peel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Madia, V.N.; De Vita, D.; Ialongo, D.; Tudino, V.; De Leo, A.; Scipione, L.; Di Santo, R.; Costi, R.; Messore, A. Recent advances in recovery of lycopene from tomato waste: A potent antioxidant with endless benefits. Molecules 2021, 26, 4495. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.; Mickan, B.S.; Siddique, K.H.M.; Rinklebe, J.; Kirkham, M.B.; Bolan, N.S. Physical, chemical, and microbial contaminants in food waste management for soil application: A review. Environ. Pollut. 2022, 300, 118860. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Neupane, N.; Adhikari, D.P. Climatic change and its impact on tomato (Lycopersicum esculentum L.) production in plain area of Nepal. Environ. Chall. 2021, 4, 100129. [Google Scholar] [CrossRef]
- Amrith, V.; Rai, B.S.; Antony, B.; Dsouza, J.G. Characterization and Semi-quantitation of Microorganism Present in the Partially Spoiled Tomatoes. Int. J. Multidiscip. Res. 2024, 6. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone, E. Valorization of Tomato Waste as a Source of Carotenoids. Molecules 2021, 26, 5062. [Google Scholar] [CrossRef] [PubMed]
- Løvdal, T.; Van Droogenbroeck, B.; Eroglu, E.C.; Kaniszewski, S.; Agati, G.; Verheul, M.; Skipnes, D. Valorization of Tomato Surplus and Waste Fractions: A Case Study Using Norway, Belgium, Poland, and Turkey as Examples. Foods 2019, 8, 229. [Google Scholar] [CrossRef]
- Kumar, M.; Chandran, D.; Tomar, M.; Bhuyan, D.J.; Grasso, S.; Sá, A.G.A.; Carciofi, B.A.M.; Radha; Dhumal, S.; Singh, S.; et al. Valorization Potential of Tomato (Solanum lycopersicum L.) Seed: Nutraceutical Quality, Food Properties, Safety Aspects, and Application as a Health-Promoting Ingredient in Foods. Horticulturae 2022, 8, 265. [Google Scholar] [CrossRef]
- Guan, Z.; Biswas, T.; Wu, F. The U.S. Tomato Industry: An Overview of Production and Trade. EDIS 2018, 2018. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2020, 10, 45. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Wang, Q.; Shi, R.; Yang, X.; Zhang, Q. Determination of soluble solids content of multiple varieties of tomatoes by full transmission visible-near infrared spectroscopy. Front. Plant Sci. 2024, 15, 1324753. [Google Scholar] [CrossRef]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef] [PubMed]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration—Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Irina, Z.; Irina, P.; Dmitriy, E.; Inessa, P.; Alla, C.; Alena, P.; Natalya, H. Assessment of tomato fruits’ vitamin- and mineral-content stability as a potential raw material to produce functional food. Funct. Foods Health Dis. 2024, 14, 14. [Google Scholar] [CrossRef]
- Elbadrawy, E.; Sello, A. Evaluation of nutritional value and antioxidant activity of tomato peel extracts. Arab. J. Chem. 2016, 9, S1010–S1018. [Google Scholar] [CrossRef]
- Begliţa, V.; Ungureanu-Iuga, M.; Mironeasa, S. Assessing the Features of Tomato Pomace Powder in Suspensions. Appl. Sci. 2023, 13, 2235. [Google Scholar] [CrossRef]
- Sangeetha, K.; Ramyaa, R.B.; Mousavi Khaneghah, A.; Radhakrishnan, M. Extraction, characterization, and application of tomato seed oil in the food industry: An updated review. J. Agric. Food Res. 2023, 11, 100529. [Google Scholar] [CrossRef]
- Fritsch, C.; Staebler, A.; Happel, A.; Márquez, M.A.C.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; López, M.J.; et al. Processing, Valorization and Application of Bio-Waste Derived Compounds from Potato, Tomato, Olive and Cereals: A Review. Sustainability 2017, 9, 1492. [Google Scholar] [CrossRef]
- Mahmoodi-Eshkaftaki, M.; Ghani, A. An efficient process for improvement of biohydrogen and biomethane production from tomato waste: Inhibitory effects of ultrasonic pretreatment. Fuel 2022, 328, 125273. [Google Scholar] [CrossRef]
- Gusenbauer, M.; Haddaway, N.R. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res. Synth. Methods 2020, 11, 181–217. [Google Scholar] [CrossRef]
- Burnham, J.F. Scopus database: A review. Biomed. Digit. Libr. 2006, 3, 1. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Collins, A.M.; Coughlin, D.; Kirk, S. The Role of Google Scholar in Evidence Reviews and Its Applicability to Grey Literature Searching. PLoS ONE 2015, 10, e0138237. [Google Scholar] [CrossRef]
- Knapp, S.; Peralta, I.E. The Tomato (Solanum lycopersicum L., Solanaceae) and Its Botanical Relatives. In The Potato Genome; Springer: Berlin/Heidelberg, Germany, 2016; pp. 7–21. [Google Scholar] [CrossRef]
- Wu, F.; Tanksley, S.D. Chromosomal evolution in the plant family Solanaceae. BMC Genom. 2010, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Swamy, K.R.M. Origin, distribution, taxonomy, botanical description, genetic diversity and breeding of tomato (Solanum lycopersicum L.). Int. J. Dev. Biol. 2023, 13, 62364–62387. [Google Scholar]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens. Food Chem. Toxicol. 2012, 50, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R. Chemical and nutritional quality changes of tomato during postharvest transportation and storage. J. Saudi Soc. Agric. Sci. 2021, 20, 401–408. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of phytochemicals present in tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.A.; Fontana, L. Vitamin C. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2023; pp. 504–514. [Google Scholar] [CrossRef]
- Jin, N.; Jin, L.; Wang, S.; Meng, X.; Ma, X.; He, X.; Zhang, G.; Luo, S.; Lyu, J.; Yu, J.; et al. A Comprehensive Evaluation of Effects on Water-Level Deficits on Tomato Polyphenol Composition, Nutritional Quality and Antioxidant Capacity. Antioxidants 2022, 11, 1585. [Google Scholar] [CrossRef]
- Kaboré, K.; Konaté, K.; Sanou, A.; Dakuyo, R.; Sama, H.; Santara, B.; Compaoré, E.W.R.; Dicko, M.H. Tomato By-Products, a Source of Nutrients for the Prevention and Reduction of Malnutrition. Nutrients 2022, 14, 2871. [Google Scholar] [CrossRef]
- Afify, A.; Abdalla, A.; Elsayed, A.; Gamuhay, B.; Abu-Khadra, A.; Hassan, M.; Ataalla, M.; Mohamed, A. Survey on the Moisture and Ash Contents in Agricultural Commodities in Al-Rass Governorate, Saudi Arabia in 2017. Assiut J. Agric. Sci. 2017, 48, 55–62. [Google Scholar] [CrossRef]
- Chen, J.; Vercambre, G.; Kang, S.; Bertin, N.; Gautier, H.; Génard, M. Fruit water content as an indication of sugar metabolism improves simulation of carbohydrate accumulation in tomato fruit. J. Exp. Bot. 2020, 71, 5010–5026. [Google Scholar] [CrossRef] [PubMed]
- Torbica, A.; Belović, M.; Mastilović, J.; Kevrešan, Ž.; Pestorić, M.; Škrobot, D.; Hadnadev, T.D. Nutritional, rheological, and sensory evaluation of tomato ketchup with increased content of natural fibres made from fresh tomato pomace. Food Bioprod. Process. 2016, 98, 299–309. [Google Scholar] [CrossRef]
- Anthon, G.E.; LeStrange, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Food Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, S.; Zhu, X.; Chang, Y.; Wang, C.; Ma, N.; Wang, J.; Zhang, X.; Lyu, J.; Xie, J.; et al. A Comprehensive Evaluation of Tomato Fruit Quality and Identification of Volatile Compounds. Plants 2023, 12, 2947. [Google Scholar] [CrossRef] [PubMed]
- Kasim, M.U.; Kasim, R. Postharvest UV-B treatments increased fructose content of tomato (Solanum lycopersicon L. cv. Tayfun F1) harvested at different ripening stages. Food Sci. Technol. 2015, 35, 742–749. [Google Scholar] [CrossRef]
- Subramaniyan, L.; Veerasamy, R.; Prabhakaran, J.; Selvaraj, A.; Algarswamy, S.; Karuppasami, K.M.; Thangavel, K.; Nalliappan, S. Biostimulation Effects of Seaweed Extract (Ascophyllum nodosum) on Phytomorpho-Physiological, Yield, and Quality Traits of Tomato (Solanum lycopersicum L.). Horticulturae 2023, 9, 348. [Google Scholar] [CrossRef]
- Torkian Boldaji, M.; Borghei, A.M.; Beheshti, B.; Hosseini, S.E. The process of producing tomato paste by ohmic heating method. J. Food Sci. 2015, 52, 3598–3606. [Google Scholar] [CrossRef]
- Kang, D.M.; Kwon, J.M.; Jeong, W.J.; Jung, Y.J.; Kang, K.K.; Ahn, M.J. Antioxidant Constituents and Activities of the Pulp with Skin of Korean Tomato Cultivars. Molecules 2022, 27, 8741. [Google Scholar] [CrossRef]
- Rivero, A.G.; Keutgen, A.J.; Pawelzik, E. Antioxidant Properties of Tomato Fruit (Lycopersicon esculentum Mill.) as Affected by Cultivar and Processing Method. Horticulturae 2022, 8, 547. [Google Scholar] [CrossRef]
- Raiola, A.; Tenore, G.; Barone, A.; Frusciante, L.; Rigano, M. Vitamin E Content and Composition in Tomato Fruits: Beneficial Roles and Bio-Fortification. Int. J. Mol. Sci. 2015, 16, 29250–29264. [Google Scholar] [CrossRef]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Perea-Domínguez, X.P.; Hernández-Gastelum, L.Z.; Olivas-Olguin, H.R.; Espinosa-Alonso, L.G.; Valdez-Morales, M.; Medina-Godoy, S. Phenolic composition of tomato varieties and an industrial tomato by-product: Free, conjugated and bound phenolics and antioxidant activity. J. Food Sci. Technol. 2018, 55, 3453–3461. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Diaconeasa, Z.; Cătoi, A.F.; Vodnar, D.C. Screening of Ten Tomato Varieties Processing Waste for Bioactive Components and Their Related Antioxidant and Antimicrobial Activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- De las Mercedes Capobianco-Uriarte, M.; Aparicio, J.; De Pablo-Valenciano, J.; del Pilar Casado-Belmonte, M. The European tomato market. An approach by export competitiveness maps. PLoS ONE 2021, 16, e0250867. [Google Scholar] [CrossRef]
- Aksoy, A. Outlook on Turkish Tomato Sector. J. Inst. Sci. Technol. 2016, 6, 121. [Google Scholar] [CrossRef]
- Li, W.; Ren, L.; Li, Q.; Zhang, D.; Jin, X.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Cao, A.; et al. Evaluation of ethylicin as a potential soil fumigant in commercial tomato production in China. Sci. Total Environ. 2023, 854, 158520. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.; Fischer, G.; Barrientos, J.; Carranza, C.; Rodríguez, M.; Lanchero, O. Characterization of productive systems of tomato (Solanum lycopersicum L.) in producing zones of Colombia. Acta Hortic. 2009, 821, 35–46. [Google Scholar] [CrossRef]
- Jaramillo, L.R.; Tobio, J.W.; Escamilla, M.J. Efecto de la sacarosa en la producción de celulosa por Gluconacetobacter xylinus en cultivo estático. Rev. MVZ Cordoba 2012, 17, 3004–3013. [Google Scholar] [CrossRef]
- Laranjeira, T.; Costa, A.; Faria-Silva, C.; Ribeiro, D.; de Oliveira, J.M.P.F.; Simões, S.; Ascenso, A. Sustainable Valorization of Tomato By-Products to Obtain Bioactive Compounds: Their Potential in Inflammation and Cancer Management. Molecules 2022, 27, 1701. [Google Scholar] [CrossRef]
- Ma, M.; Taylor, P.W.J.; Chen, D.; Vaghefi, N.; He, J.Z. Major Soilborne Pathogens of Field Processing Tomatoes and Management Strategies. Microorganisms 2023, 11, 263. [Google Scholar] [CrossRef]
- Eslami, E.; Abdurrahman, E.; Ferrari, G.; Pataro, G. Enhancing resource efficiency and sustainability in tomato processing: A comprehensive review. J. Clean. Prod. 2023, 425, 138996. [Google Scholar] [CrossRef]
- Wu, X.; Yu, L.; Pehrsson, P.R. Are Processed Tomato Products as Nutritious as Fresh Tomatoes? Scoping Review on the Effects of Industrial Processing on Nutrients and Bioactive Compounds in Tomatoes. Adv. Nutr. 2022, 13, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Vidyarthi, S.K.; Simmons, C.W. Characterization and management strategies for process discharge streams in California industrial tomato processing. Sci. Total Environ. 2020, 723, 137976. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Nelson, P.E. Quality attributes of processed tomato products: A review. Food Rev. Int. 1996, 12, 375–401. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Bhuyan, D.J.; Punia, S.; Grasso, S.; Sá, A.G.A.; Mekhemar, M. Tomato (Solanum lycopersicum L.) seed: A review on bioactives and biomedical activities. Biomed. Pharmacother. 2021, 142, 112018. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wu, Z.; Ma, Q.; Lu, Z.; Ye, F.; Zhao, G. Effects of Breaking Methods on the Viscosity, Rheological Properties and Nutritional Value of Tomato Paste. Foods 2021, 10, 2395. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H.; Selli, S.; Kadiroğlu, P.; Kola, O.; Kesen, S.; Uçar, B.; Çetiner, B. Bioactive compounds and antioxidant potential in tomato pastes as affected by hot and cold break process. Food Chem. 2017, 220, 31–41. [Google Scholar] [CrossRef]
- Mangut, V.; Sabio, E.; Gañán, J.; González, J.F.; Ramiro, A.; González, C.M.; Román, S.; Al-Kassir, A. Thermogravimetric study of the pyrolysis of biomass residues from tomato processing industry. Fuel Process. Technol. 2006, 87, 109–115. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef]
- Nour, V.; Panaite, T.D.; Ropota, M.; Turcu, R.; Trandafir, I.; Corbu, A.R. Nutritional and bioactive compounds in dried tomato processing waste. CyTA—J. Food 2018, 16, 222–229. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, J.; Gao, R.; Ye, F.; Zhao, G. Sustainable valorisation of tomato pomace: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 172–187. [Google Scholar] [CrossRef]
- Koçak, E.; Esmer, O.K.; Sahiner, A. Optimization of the conditions of alkaline extraction of tomato peels and characterization of tomato peel extracts obtained under atmospheric and oxygen free conditions. An. Acad. Bras. Ciênc. 2023, 95. [Google Scholar] [CrossRef] [PubMed]
- Midhun Prasad, K.; Murugavelh, S. Experimental investigation and kinetics of tomato peel pyrolysis: Performance, combustion and emission characteristics of bio-oil blends in diesel engine. J. Clean. Prod. 2020, 254, 120115. [Google Scholar] [CrossRef]
- Conti, V.; Romi, M.; Guarnieri, M.; Cantini, C.; Cai, G. Italian Tomato Cultivars under Drought Stress Show Different Content of Bioactives in Pulp and Peel of Fruits. Foods 2022, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Ferrari, G.; Velikov, K.P.; Donsì, F. High-pressure homogenization treatment to recover bioactive compounds from tomato peels. J. Food Eng. 2019, 262, 170–180. [Google Scholar] [CrossRef]
- Anđelini, M.; Major, N.; Išić, N.; Kovačević, T.K.; Ban, D.; Palčić, I.; Goreta Ban, S. Sugar and Organic Acid Content Is Dependent on Tomato (Solanum Lycoperiscum L.) Peel Color. Horticulturae 2023, 9, 313. [Google Scholar] [CrossRef]
- Mellinas, C.; Solaberrieta, I.; Pelegrín, C.J.; Jiménez, A.; Garrigós, M.C. Valorization of Agro-Industrial Wastes by Ultrasound-Assisted Extraction as a Source of Proteins, Antioxidants and Cutin: A Cascade Approach. Antioxidants 2022, 11, 1739. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Martinez-Garcia, J.F. Plant colours come to light. Biochemist 2020, 42, 46–50. [Google Scholar] [CrossRef]
- Orsi, B.; Sestari, I.; Preczenhak, A.P.; de Abreu Vieira, A.P.; Tessmer, M.A.; da Silva Souza, M.A.; Hassimotto, N.M.A.; Kluge, R.A. Fruits from tomato carotenoid mutants have altered susceptibility to grey mold. Plant Physiol. Biochem. 2023, 204, 108100. [Google Scholar] [CrossRef]
- Strati, I.F.; Oreopoulou, V. Recovery of carotenoids from tomato processing by-products—A review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- Blejan, A.M.; Nour, V. Physico-Chemical Characteristics, Sensory Attributes and Oxidative Stability of Soy Milk Mayonnaise Enriched in Carotenoids from Tomato By-Products. Appl. Sci. 2023, 13, 7101. [Google Scholar] [CrossRef]
- Meng, F.; Li, Y.; Li, S.; Chen, H.; Shao, Z.; Jian, Y.; Mao, Y.; Liu, L.; Wang, Q. Carotenoid biofortification in tomato products along whole agro-food chain from field to fork. Trends Food Sci. Technol. 2022, 124, 296–308. [Google Scholar] [CrossRef]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Moradi, M.; Ezati, P.; O’Higgins, L.; Meléndez-Martínez, A.J.; Frleta Matas, R.; Šimat, V.; McClements, D.J.; Jakobsen, A.N.; Lerfall, J.; et al. Encapsulation of microalgal-based carotenoids: Recent advances in stability and food applications. Trends Food Sci. Technol. 2023, 138, 382–398. [Google Scholar] [CrossRef]
- De Queiroz, J.L.C.; Medeiros, I.; Piuvezam, G.; De França Nunes, A.C.; Gomes, C.C.; MacIel, B.L.L.; De Araújo Morais, A.H.; Passos, T.S. Effect of carotenoid encapsulation on antioxidant activities. Medicine 2020, 99, e19772. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, J.; Kay, H.P.; Krishnamurthy, N.P.; Ramakrishnan, N.R.; Aldawoud, T.M.S.; Galanakis, C.M.; Wei, O.C. Extraction of Carotenoids from Tomato Pomace via Water-Induced Hydrocolloidal Complexation. Biomolecules 2020, 10, 1019. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, X.; Zhang, L.; Shao, P.; Wu, W.; Chen, Z.; Li, J.; Renard, C.M. An overview of carotenoid extractions using green solvents assisted by Z-isomerization. Trends Food Sci. Technol. 2022, 123, 145–160. [Google Scholar] [CrossRef]
- Viñas-Ospino, A.; López-Malo, D.; Esteve, M.J.; Frígola, A.; Blesa, J. Green Solvents: Emerging Alternatives for Carotenoid Extraction from Fruit and Vegetable By-Products. Foods 2023, 12, 863. [Google Scholar] [CrossRef]
- Vlachoudi, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Lalas, S.I. Enhanced Extraction of Carotenoids from Tomato Industry Waste Using Menthol/Fatty Acid Deep Eutectic Solvent. Waste 2023, 1, 977–992. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Salama, Z.A.; Salem, S.H.; Aly, H.F.; Nassrallah, A.; Abou-Elella, F.; Aboul-Enein, A.M. Lycopene Nanoparticles Ameliorate The Antioxidants, Antimicrobial And Anticancer Potencies Of Tomato Pomace. Egypt. J. Chem. 2021, 64, 3739–3749. [Google Scholar] [CrossRef]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- van Breemen, R.B.; Pajkovic, N. Multitargeted therapy of cancer by lycopene. Cancer Lett. 2008, 269, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G. What is apoptosis, and why is it important? BMJ 2001, 322, 1536–1538. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, A.J.; Oliveira, F.L.; Martins, N.B.; de Maia, G.; Martucci, R.B.; Borojevic, R. Effect of lycopene on cell viability and cell cycle progression in human cancer cell lines. Cancer Cell Int. 2012, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.T.N.; Nguyen, H.V.H. Optimization of enzyme-assisted lycopene extraction from tomato (Lycopersicon esculentum) peel using rice bran oil. J. Food Meas. Charact. 2023, 17, 5154–5162. [Google Scholar] [CrossRef]
- Choksi, P.M.; Joshi, V.Y. A Review on Lycopene—Extraction, Purification, Stability and Applications. Int. J. Food Prop. 2007, 10, 289–298. [Google Scholar] [CrossRef]
- Hatami, T.; Meireles, M.A.A.; Ciftci, O.N. Supercritical carbon dioxide extraction of lycopene from tomato processing by-products: Mathematical modeling and optimization. J. Food Eng. 2019, 241, 18–25. [Google Scholar] [CrossRef]
- Nobre, B.P.; Gouveia, L.; Matos, P.G.S.; Cristino, A.F.; Palavra, A.F.; Mendes, R.L. Supercritical Extraction of Lycopene from Tomato Industrial Wastes with Ethane. Molecules 2012, 17, 8397–8407. [Google Scholar] [CrossRef]
- Salgado-Ramos, M.; Martí-Quijal, F.J.; Huertas-Alonso, A.J.; Sánchez-Verdú, M.P.; Cravotto, G.; Moreno, A.; Barba, F.J. Sequential extraction of almond hull biomass with pulsed electric fields (PEF) and supercritical CO2 for the recovery of lipids, carbohydrates and antioxidants. Food Bioprod. Process. 2023, 139, 216–226. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. 2020, 60, 1826–1841. [Google Scholar] [CrossRef] [PubMed]
- Shinwari, K.J. Emerging technologies for the recovery of bioactive compounds from saffron species. Saffron 2021, 143–182. [Google Scholar] [CrossRef]
- Costa, R. The Chemistry of Mushrooms: A Survey of Novel Extraction Techniques Targeted to Chromatographic and Spectroscopic Screening. Nat. Prod. Res. 2016, 49, 279–306. [Google Scholar] [CrossRef]
- Eskilsson, C.S.; Björklund, E. Analytical-scale microwave-assisted extraction. J. Chromatogr. A 2000, 902, 227–250. [Google Scholar] [CrossRef]
- Yi, Z.; Su, Y.; Brynjolfsson, S.; Olafsdóttir, K.; Fu, W. Bioactive polysaccharides and their derivatives from microalgae: Biosynthesis, applications, and challenges. Nat. Prod. Res. 2021, 71, 67–85. [Google Scholar] [CrossRef]
- Ho, K.K.H.Y.; Ferruzzi, M.G.; Liceaga, A.M.; San Martín-González, M.F. Microwave-assisted extraction of lycopene in tomato peels: Effect of extraction conditions on all-trans and cis-isomer yields. LWT—Food Sci. Technol. 2015, 62, 160–168. [Google Scholar] [CrossRef]
- Zuorro, A. Enhanced Lycopene Extraction from Tomato Peels by Optimized Mixed-Polarity Solvent Mixtures. Molecules 2020, 25, 2038. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Pino, V. Extraction With Ionic Liquids-Organic Compounds. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; Volume 17, pp. 499–537. [Google Scholar] [CrossRef]
- Li, J.; Pettinato, M.; Casazza, A.A.; Perego, P. A Comprehensive Optimization of Ultrasound-Assisted Extraction for Lycopene Recovery from Tomato Waste and Encapsulation by Spray Drying. Processes 2022, 10, 308. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem 2023, 101, 106646. [Google Scholar] [CrossRef]
- Larocca, V.; Martino, M.; Trupo, M.; Magarelli, R.A.; Spagnoletta, A.; Ambrico, A. Evaluation of carbon dioxide supercritical fluid extraction (CO2-SFE) on carotenoids recovery from red yeast cells. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- MacHmudah, S.; Zakaria; Winardi, S.; Sasaki, M.; Goto, M.; Kusumoto, N.; Hayakawa, K. Lycopene extraction from tomato peel by-product containing tomato seed using supercritical carbon dioxide. J. Food Eng. 2012, 108, 290–296. [Google Scholar] [CrossRef]
- Tizón Alba, A.; Aliaño-González, M.J.; Palma, M.; Fernández Barbero, G.; Carrera, C. Enhancing Efficiency of Enzymatic-Assisted Extraction Method for Evaluating Bioactive Compound Analysis in Mulberry: An Optimization Approach. Agronomy 2023, 13, 2548. [Google Scholar] [CrossRef]
- Majik, M.S.; Gawas, U.B. Recent advances in extraction of natural compounds. In New Horizons in Natural Compound Research; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 2; pp. 17–33. [Google Scholar] [CrossRef]
- Catalkaya, G.; Kahveci, D. Optimization of enzyme assisted extraction of lycopene from industrial tomato waste. Sep. Purif. Technol. 2019, 219, 55–63. [Google Scholar] [CrossRef]
- Kapoore, R.; Butler, T.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.V.B.; Moniruzzaman, M.; Madhavi, V.; Jaafar, J. Recent improvements in the extraction, cleanup and quantification of bioactive flavonoids. Stud. Nat. Prod. Chem. 2020, 66, 197–223. [Google Scholar] [CrossRef]
- Baskar, G.; Kalavathy, G.; Aiswarya, R.; Abarnaebenezer Selvakumari, I. Advances in bio-oil extraction from nonedible oil seeds and algal biomass. In Advances in Eco-Fuels for a Sustainable Environment; Elsevier: Amsterdam, The Netherlands, 2019; pp. 187–210. [Google Scholar] [CrossRef]
- Chahardoli, A.; Jalilian, F.; Memariani, Z.; Farzaei, M.H.; Shokoohinia, Y. Analysis of organic acids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 767–823. [Google Scholar] [CrossRef]
- Villamil-Galindo, E.; Gastélum-Estrada, A.; Chuck-Hernandez, C.; Antunes-Ricardo, M.; Reza-Zaldivar, E.E.; Piagentini, A.; Jacobo-Velázquez, D.A. Kinetic Ultrasound-Assisted Extraction as a Sustainable Approach for the Recovery of Phenolics Accumulated through UVA Treatment in Strawberry By-Products. Foods 2023, 12, 2989. [Google Scholar] [CrossRef]
- Kobus, Z.; Pecyna, A.; Buczaj, A.; Krzywicka, M.; Przywara, A.; Nadulski, R. Optimization of the Ultrasound-Assisted Extraction of Bioactive Compounds from Cannabis sativa L. Leaves and Inflorescences Using Response Surface Methodology. Appl. Sci. 2022, 12, 6747. [Google Scholar] [CrossRef]
- Kumcuoglu, S.; Yilmaz, T.; Tavman, S. Ultrasound assisted extraction of lycopene from tomato processing wastes. J. Food Sci. Technol. 2014, 51, 4102–4107. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-haq, I.; Ur-rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Bacanli, M.; Başaran, N.; Başaran, A.A. Lycopene: Is it Beneficial to Human Health as an Antioxidant? Turk. J. Pharm. Sci. 2017, 14, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Günal-Köroğlu, D.; Karadag, A.; Capanoglu, E.; Cardoso, S.M.; Al-Omari, B.; Cho, W.C. A mechanistic updated overview on lycopene as potential anticancer agent. Biomed. Pharmacother. 2023, 161, 114428. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, Y.J.; Shin, Y. Estimation of daily intake of lycopene, antioxidant contents and activities from tomatoes, watermelons, and their processed products in Korea. Appl. Biol. Chem. 2020, 63, 50. [Google Scholar] [CrossRef]
- Hraishawi, R.M.O.; Abdul-Razak, A.S.; Al-Hayder, M.N.; Al-Wafi, H. Investigation the antimicrobial and antioxidant activity of lycopene extraction from Solanum Lycopersicum. EurAsian J. BioSci. 2020, 14, 5305–5310. [Google Scholar]
- Hidayat, D.F.; Mahendra, M.Y.N.; Kamaludeen, J.; Pertiwi, H. Lycopene in Feed as Antioxidant and Immuno-Modulator Improves Broiler Chicken’s Performance under Heat-Stress Conditions. Vet. Med. Int. 2023, 2023, 418081. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Ishtiaque, G.M.A.; Rahat, S.A.; Hossain, M.A.; Islam, M.R.; Maeesa, S.K.; Rauf, A. Multifunctional Role of Natural Products for Therapeutic Approaches of Prostate Cancer: An Updated Review. J. Herb. Med. 2023, 42, 100803. [Google Scholar] [CrossRef]
- Song, X.; Luo, Y.; Ma, L.; Hu, X.; Simal-Gandara, J.; Wang, L.S.; Chen, F. Recent trends and advances in the epidemiology, synergism, and delivery system of lycopene as an anti-cancer agent. Semin. Cancer Biol. 2021, 73, 331–346. [Google Scholar] [CrossRef]
- Marzocco, S.; Singla, R.K.; Capasso, A. Multifaceted Effects of Lycopene: A Boulevard to the Multitarget-Based Treatment for Cancer. Molecules 2021, 26, 5333. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.R.; Silva, J.R.V. Mechanisms of action of non-enzymatic antioxidants to control oxidative stress during in vitro follicle growth, oocyte maturation, and embryo development. Anim. Reprod. Sci. 2023, 249, 107186. [Google Scholar] [CrossRef] [PubMed]
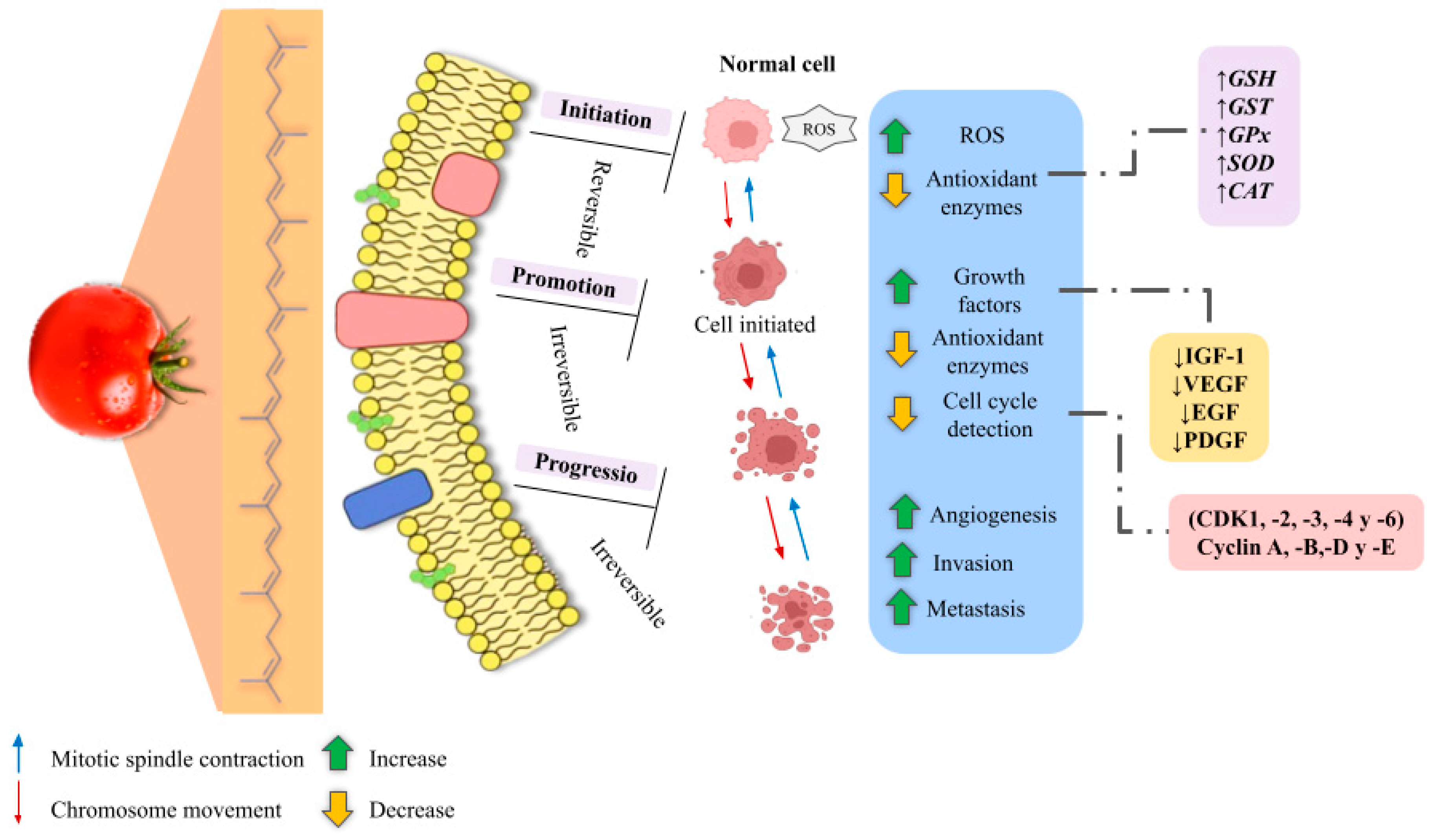
- Poillet-Perez, L.; Despouy, G.; Delage-Mourroux, R.; Boyer-Guittaut, M. Interplay between ROS and autophagy in cancer cells, from tumor initiation to cancer therapy. Redox Biol. 2015, 4, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Lim, J.; Kim, H. Lycopene Inhibits Reactive Oxygen Species-Mediated NF-κB Signaling and Induces Apoptosis in Pancreatic Cancer Cells. Nutrients 2019, 11, 762. [Google Scholar] [CrossRef] [PubMed]
- Ataseven, D.; Öztürk, A.; Özkaraca, M.; Joha, Z. Anticancer activity of lycopene in HT-29 colon cancer cell line. Med. Oncol. 2023, 40, 127. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, Y.; Mariya, Y.; Yamada, K.; Yoshida, K.; Sasa, A.; Saito, H.; Hirai, A.; Suzuki, S.; Aizawa, K.; Suganuma, H.; et al. Tomato Juice Consumption Could Improve Breast Skin Adverse Effects of Radiotherapy in Breast Cancer Patients. In Vivo 2020, 34, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Kapała, A.; Szlendak, M.; Motacka, E. The Anti-Cancer Activity of Lycopene: A Systematic Review of Human and Animal Studies. Nutrients 2022, 14, 5152. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xu, F.; Wu, K.; Li, L.; Qiao, T.; Li, Z.; Chen, T.; Sun, C. Anticancer effects and possible mechanisms of lycopene intervention on N-methylbenzylnitrosamine induced esophageal cancer in F344 rats based on PPARγ1. Eur. J. Pharmacol. 2020, 881, 173230. [Google Scholar] [CrossRef] [PubMed]
- Mirahmadi, M.; Azimi-Hashemi, S.; Saburi, E.; Kamali, H.; Pishbin, M.; Hadizadeh, F. Potential inhibitory effect of lycopene on prostate cancer. Biomed. Pharmacother. 2020, 129, 110459. [Google Scholar] [CrossRef]
- Tjahjodjati; Sugandi, S.; Umbas, R.; Satari, M. The Protective Effect of Lycopene on Prostate Growth Inhibitory Efficacy by Decreasing Insulin Growth Factor-1 in Indonesian Human Prostate Cancer Cells. Res. Rep. Urol. 2020, 12, 137–143. [Google Scholar] [CrossRef]
- Amorim, A.D.G.N.; Vasconcelos, A.G.; Souza, J.; Oliveira, A.; Gullón, B.; de Souza de Almeida Leite, J.R.; Pintado, M. Bio-Availability, Anticancer Potential, and Chemical Data of Lycopene: An Overview and Technological Prospecting. Antioxidants 2022, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Simone, R.E.; Catalano, A.; Mele, M.C. Tomato Lycopene and Lung Cancer Prevention: From Experimental to Human Studies. Cancers 2011, 3, 2333–2357. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Kucuk, O. Lycopene in Cancer Prevention. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3875–3922. [Google Scholar] [CrossRef]
- Sahin, K.; Yenice, E.; Tuzcu, M.; Orhan, C.; Mizrak, C.; Ozercan, I.H.; Sahin, N.; Yilmaz, B.; Bilir, B.; Ozpolat, B.; et al. Lycopene Protects Against Spontaneous Ovarian Cancer Formation in Laying Hens. J. Cancer Prev. 2018, 23, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, N.P.; Shokoohmand, A.; Wagner, F.; Landgraf, M.; Champ, S.; Holzapfel, B.M.; Clements, J.A.; Hutmacher, D.W.; Loessner, D. Lycopene reduces ovarian tumor growth and intraperitoneal metastatic load. Am. J. Cancer Res. 2017, 7, 1322–1336. [Google Scholar] [PubMed]
- Kim, M.J.; Kim, H. Anticancer Effect of Lycopene in Gastric Carcinogenesis. J. Cancer Prev. 2015, 20, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Puah, B.P.; Jalil, J.; Attiq, A.; Kamisah, Y. New Insights into Molecular Mechanism behind Anti-Cancer Activities of Lycopene. Molecules 2021, 26, 3888. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Dulf, F.V.; Diaconeasa, Z.; Vodnar, D.C. Antimicrobial and antioxidant properties of tomato processing byproducts and their correlation with the biochemical composition. LWT 2019, 116, 108558. [Google Scholar] [CrossRef]
- Panthee, D.R.; Gardner, R.G. Genetic Improvement of Fresh Market Tomatoes for Yield and Fruit Quality Over 35 Years in North Carolina: A Review. Int. J. Veg. Sci. 2011, 17, 259–273. [Google Scholar] [CrossRef]
- Solaberrieta, I.; Mellinas, C.; Jiménez, A.; Garrigós, M.C. Recovery of Antioxidants from Tomato Seed Industrial Wastes by Microwave-Assisted and Ultrasound-Assisted Extraction. Foods 2022, 11, 3068. [Google Scholar] [CrossRef]
- Ponce Fernández, N.E.; Pollorena López, G.; Rosas Domínguez, C.; Osuna Izaguirre, S.C.; López Peñuelas, V.M. Effect of the addition of dehydrated peel and seed on the antioxidant capacity of a tomato paste produced in Sinaloa. Biotecnia 2021, 23, 135–140. [Google Scholar] [CrossRef]
- Zanón, M.J.; Font, M.I.; Jordá, C. Use of tomato crop residues into soil for control of bacterial wilt caused by Ralstonia solanacearum. Crop Prot. 2011, 30, 1138–1143. [Google Scholar] [CrossRef]
- Rizk, E.M.; El-Kady, A.T.; El-Bialy, A.R. Charactrization of carotenoids (lyco-red) extracted from tomato peels and its uses as natural colorants and antioxidants of ice cream. Ann. Agric. Sci. 2014, 59, 53–61. [Google Scholar] [CrossRef]
- Barbulova, A.; Colucci, G.; Apone, F. New Trends in Cosmetics: By-Products of Plant Origin and Their Potential Use as Cosmetic Active Ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Calvo, M.M.; García, M.L.; Selgas, M.D. Dry fermented sausages enriched with lycopene from tomato peel. Meat Sci. 2008, 80, 167–172. [Google Scholar] [CrossRef]
- Szabo, K.; Cătoi, A.F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]




| Taxonomy | |
|---|---|
| Division | Angiosperms |
| Class | Magnoliopsida |
| Order | Solanales |
| Family | Solanaceae |
| Genus | Solanum |
| Species | Solanum lycopersicum |
| Type of Cancer | Function of Lycopene | Administration | Studies | Type of Study | Refs. |
|---|---|---|---|---|---|
| Prostate cancer | Lycopene inhibits DNA synthesis, which could significantly decrease the proliferation and growth of cancer cells in primary epithelial prostate cancer. | Lycopene could be used as a therapeutic adjunct in patients with prostate cancer to improve apoptosis and prevent the progression of cancer cells. | Lycopene has demonstrated efficacy in treating locally advanced prostate cancer, reducing mortality in high-risk men, and slowing the progression of the disease. | In vitro | [134,136,137] |
| Breast cancer | Lycopene decreased cell growth, induced cell cycle arrest, and caused changes in mitochondrial membranes and DNA fragmentation. It showed no hemolytic activity and had low toxicity against peritoneal macrophages. | Supplementation with lycopene complexes and other antioxidants reduces skin toxicity during radical radiation therapy. | In an animal model, lycopene supplementation and other antioxidants demonstrated the potential to reduce skin toxicity during radical radiotherapy treatment for breast cancer. | In vitro | [134,138] |
| Lung cancer | Inhibits induced pulmonary toxicity by preventing inflammation and macrophage infiltration. | Adjuvant therapy. | Lycopene has been studied in laboratory and animal models of lung cancer cells. It shows cell growth-inhibiting properties and promotes apoptosis. | In vitro | [139,140] |
| Ovarian cancer | The consumption of lycopene through diet has been associated with a lower risk of ovarian cancer, indicating its potential as a preventive agent against ovarian carcinogenesis. | Lycopene, administered orally as a preventive measure, significantly reduced intraperitoneal metastatic burden and, when given as a treatment, significantly reduced the tumor burden of ovarian cancer. | Lycopene intake has decreased the occurrence and size of ovarian tumors in laying hens. This effect is attributed to its antioxidant and anti-inflammatory properties, which regulate signaling pathways in ovarian cells. | In vitro | [141,142] |
| Stomach cancer | Treatment with lycopene suppresses the proliferation of gastric cancer cells by inducing cell cycle arrest in the G0–G1 phase. Moreover, lycopene prevents the upregulation of p53 expression in gastric mucosa exposed to cigarette smoke. | The administration of lycopene at doses of 50, 100, or 150 mg/kg of body weight led to an anticipated increase in antioxidant enzymes (SOD, CAT, GSH-Px). It also increased cytokine levels (IL-2, IL-4, IL-10, TNF-α) and antibodies (IgG, IgA, IgM). | Lycopene intake protects against stomach cancer, regardless of Helicobacter pylori. Its beneficial effect in animal models of gastric and esophageal cancer lies in modulating the proliferation and apoptosis of tumor cells induced by carcinogens. | In vitro | [143,144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez Bolaño, D.C.; Insuasty, D.; Rodríguez Macías, J.D.; Grande-Tovar, C.D. Potential Use of Tomato Peel, a Rich Source of Lycopene, for Cancer Treatment. Molecules 2024, 29, 3079. https://doi.org/10.3390/molecules29133079
Jiménez Bolaño DC, Insuasty D, Rodríguez Macías JD, Grande-Tovar CD. Potential Use of Tomato Peel, a Rich Source of Lycopene, for Cancer Treatment. Molecules. 2024; 29(13):3079. https://doi.org/10.3390/molecules29133079
Chicago/Turabian StyleJiménez Bolaño, Diana Carolina, Daniel Insuasty, Juan David Rodríguez Macías, and Carlos David Grande-Tovar. 2024. "Potential Use of Tomato Peel, a Rich Source of Lycopene, for Cancer Treatment" Molecules 29, no. 13: 3079. https://doi.org/10.3390/molecules29133079
APA StyleJiménez Bolaño, D. C., Insuasty, D., Rodríguez Macías, J. D., & Grande-Tovar, C. D. (2024). Potential Use of Tomato Peel, a Rich Source of Lycopene, for Cancer Treatment. Molecules, 29(13), 3079. https://doi.org/10.3390/molecules29133079