Reflectivity and Angular Anisotropy of Liquid Crystal Microcapsules with Different Particle Sizes by Complex Coalescence
Abstract
1. Introduction
2. Results and Discussions
2.1. Effect of Spin Speed and Rotor Size
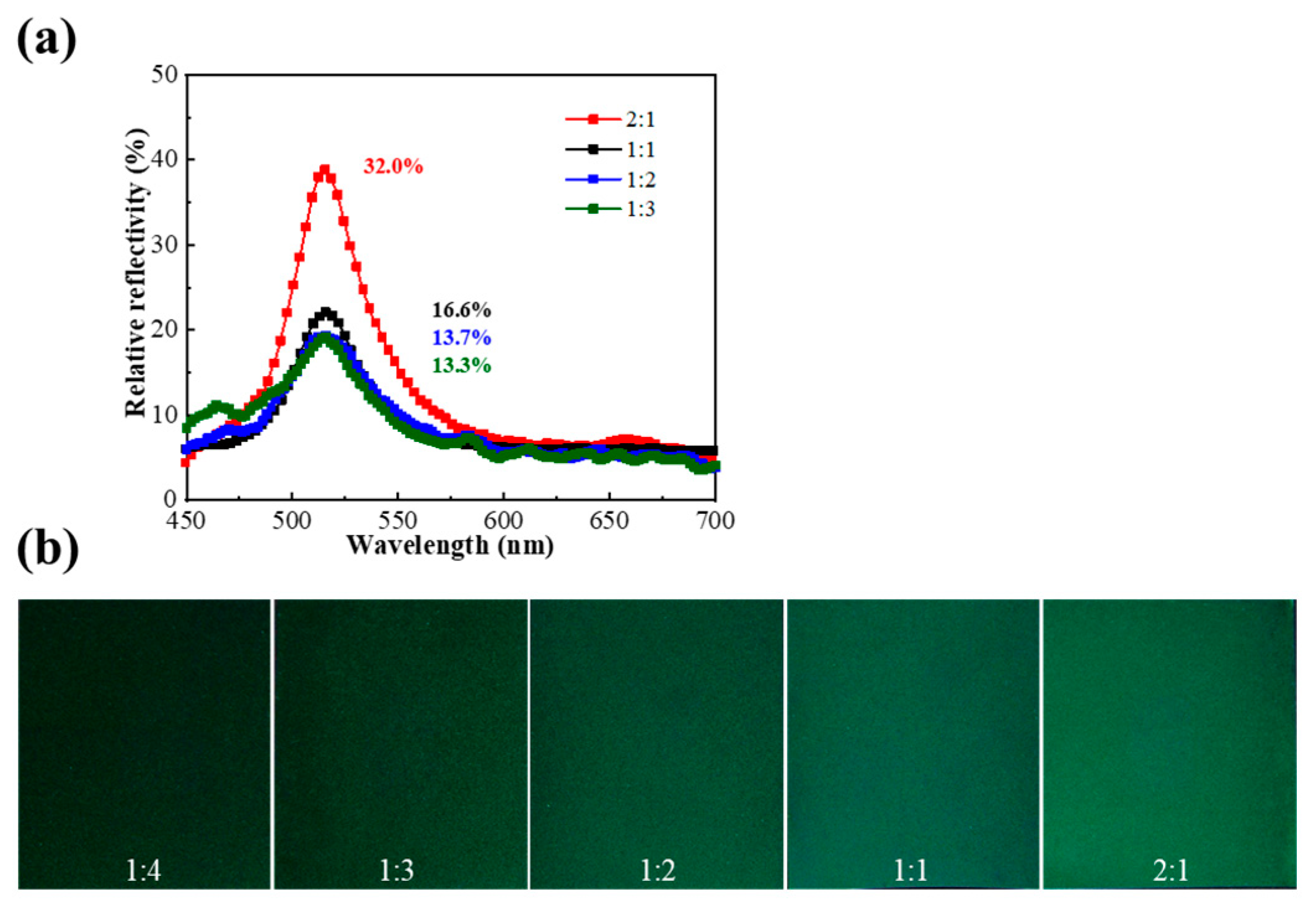
2.2. Effect of Core-to-Shell Ratio
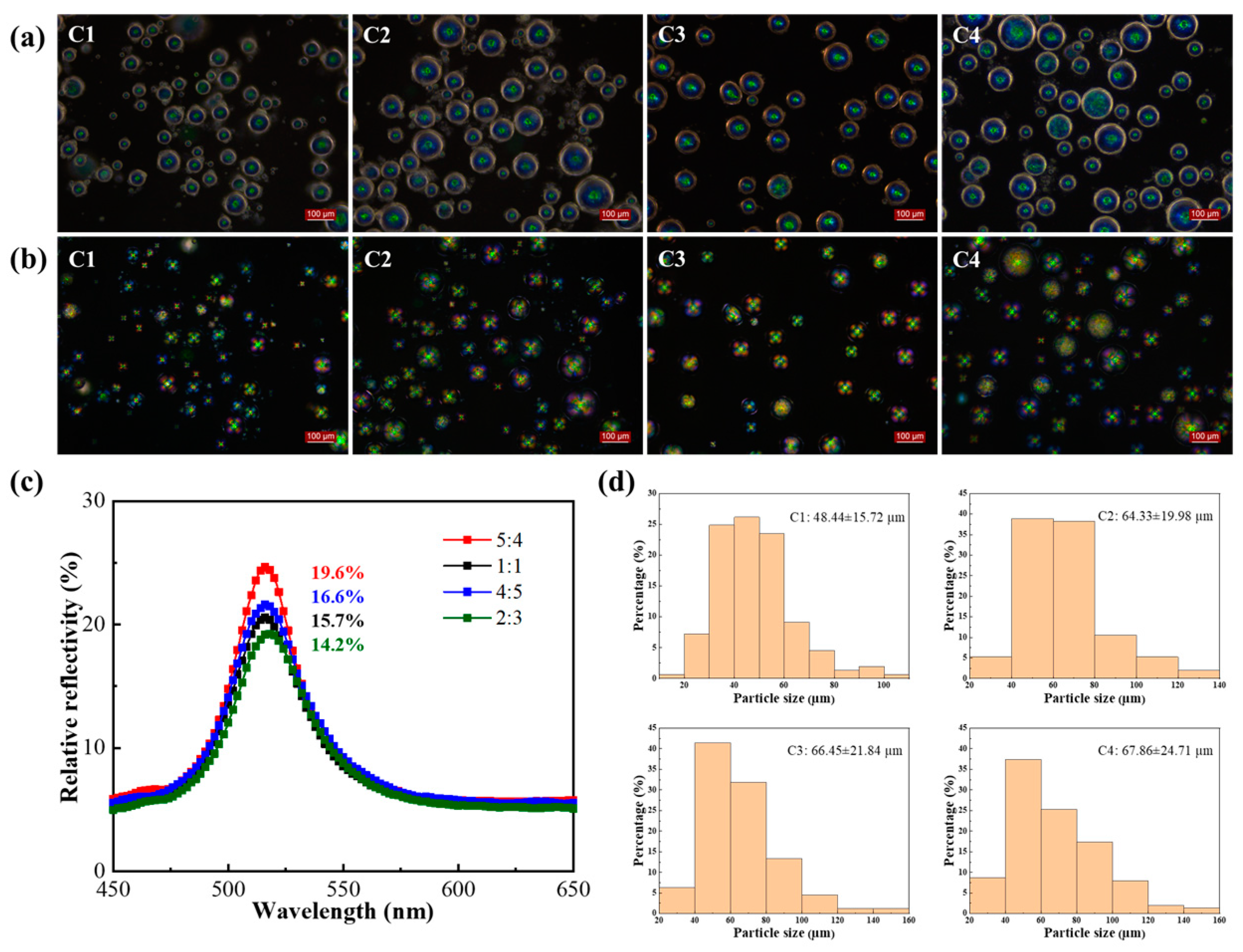
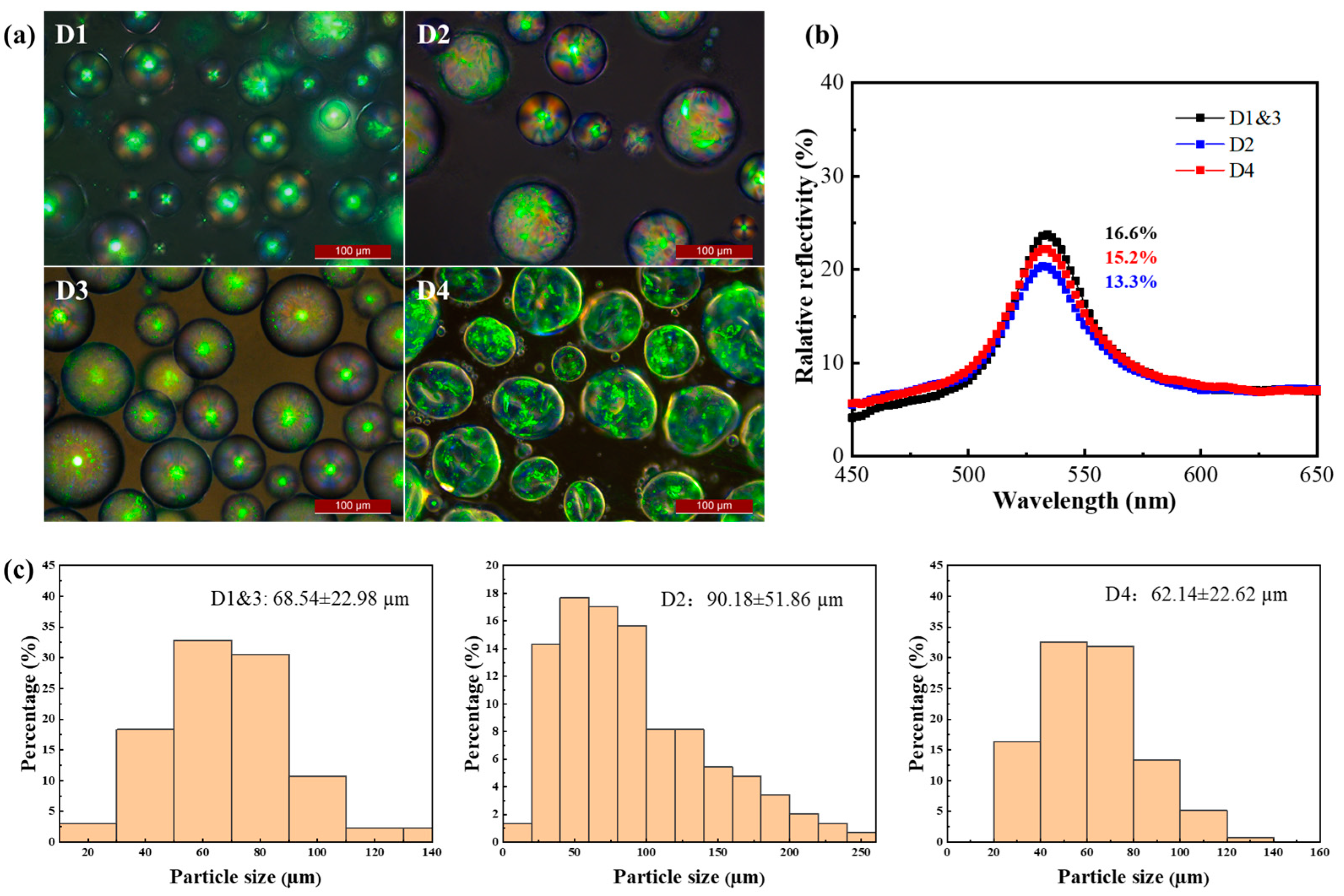
2.3. Effect of Emulsifiers and Curing Agent
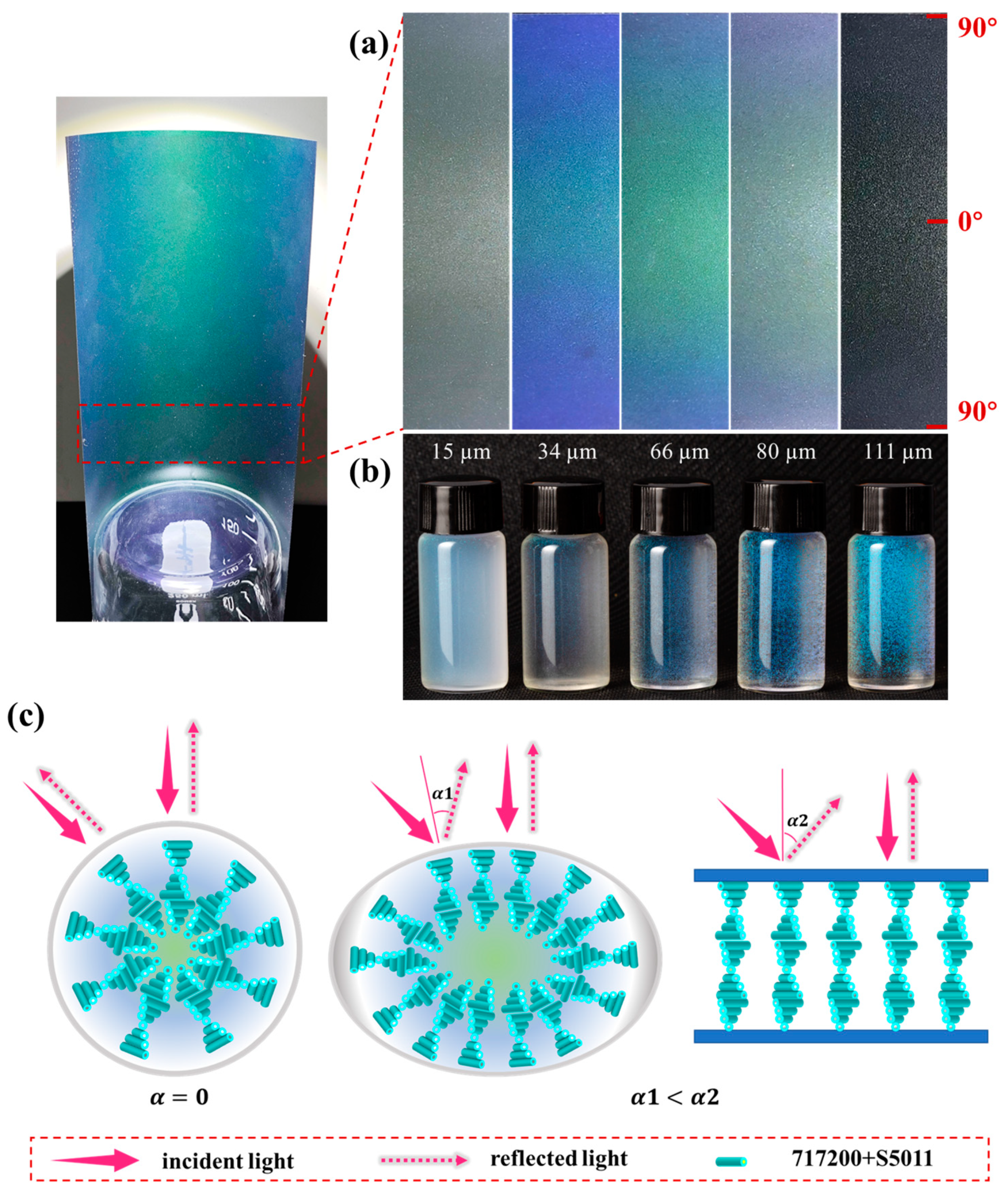
2.4. Optical Property
3. Experiment
3.1. Materials
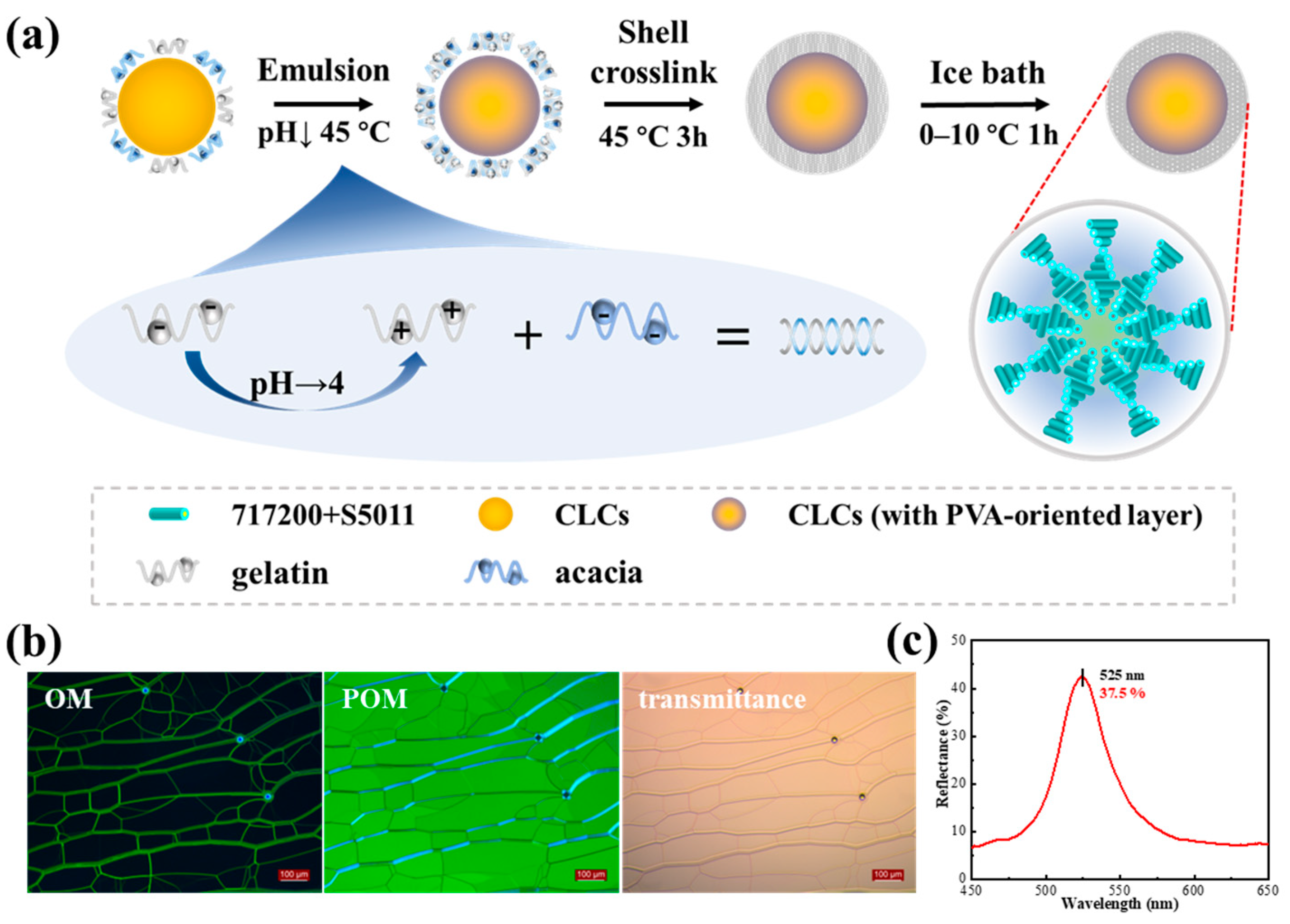
3.2. Preparations of CLCMs
3.3. Preparation of Coatings
3.4. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, S.; Lee, S.S.; Kim, S.H. Photonic multishells composed of cholesteric liquid crystals designed by controlled phase separation in emulsion drops. Adv. Mater. 2020, 32, 2002166. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, C.; Zhang, Y.; Qiao, Z.; Yuan, Z.; Gong, Y.; Chang, G.-E.; Tu, W.-C.; Chen, Y.-C. Programmable rainbow-colored optofluidic fiber laser encoded with topologically structured chiral droplets. ACS Nano 2021, 15, 11126–11136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.; Chen, Y.; Yu, H. Progress in Fabrication and Applications of Cholesteric Liquid Crystal Microcapsules. Chem. Eur. J. 2024, 30, e202303198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, H.; He, W.; Yang, Z.; Cao, H.; Wang, D.; Li, Y. Research progress of cholesteric liquid crystals with broadband reflection. Molecules 2022, 27, 4427. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Wang, J.; Ikeda, T.; Jiang, L. Bio-inspired liquid crystal actuator materials. J. Mater. Chem. C 2019, 7, 3413–3428. [Google Scholar] [CrossRef]
- Park, G.; Choi, Y.-S.; Yun, H.S.; Yoon, D.K. Fabrication of bilayer dichroic films using liquid crystal materials for multiplex applications. ACS Appl. Mater. Interfaces 2020, 12, 45315–45321. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Liang, H.-L.; Drevensek-Olenik, I.; Lagerwall, J.P. Tuneable multicoloured patterns from photonic cross-communication between cholesteric liquid crystal droplets. J. Mater. Chem. C 2014, 2, 806–810. [Google Scholar] [CrossRef]
- Mikhailenko, V.; Krivoshey, A.; Pozhidaev, E.; Popova, E.; Fedoryako, A.; Gamzaeva, S.; Barbashov, V.; Srivastava, A.K.; Kwok, H.S.; Vashchenko, V. The nano-scale pitch ferroelectric liquid crystal materials for modern display and photonic application employing highly effective chiral components: Trifluoromethylalkyl diesters of p-terphenyldicarboxylic acid. J. Mol. Liq. 2019, 281, 186–195. [Google Scholar] [CrossRef]
- Lu, H.; Wu, S.; Zhang, C.; Qiu, L.; Wang, X.; Zhang, G.; Hu, J.; Yang, J. Photoluminescence intensity and polarization modulation of a light emitting liquid crystal via reversible isomerization of an α-cyanostilbenic derivative. Dye. Pigment. 2016, 128, 289–295. [Google Scholar] [CrossRef]
- El-Rabiaey, M.A.; Areed, N.F.; Obayya, S.S. Novel plasmonic data storage based on nematic liquid crystal layers. J. Light. Technol. 2016, 34, 3726–3732. [Google Scholar] [CrossRef]
- Belmonte, A.; Bus, T.; Broer, D.J.; Schenning, A.P. Patterned full-color reflective coatings based on photonic cholesteric liquid-crystalline particles. ACS Appl. Mater. Interfaces 2019, 11, 14376–14382. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Zhang, L.; West, J.L.; Fu, S. Multicolor electrochromic dye-doped liquid crystal yolk–shell microcapsules. ACS Appl. Mater. Interfaces 2020, 12, 29728–29736. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Zhang, L.; Wang, D.; Li, M.; Li, L.; West, J.L.; Fu, S. Fabrication of dye-doped liquid crystal microcapsules for electro-stimulated responsive smart textiles. Dye. Pigment. 2018, 158, 1–11. [Google Scholar] [CrossRef]
- Sheng, M.; Zhang, L.; Jiang, S.; Yang, L.; Zaaboul, F.; Fu, S. Bioinspired Electro-Responsive Multispectral Controllable Dye-Doped Liquid Crystal Yolk–Shell Microcapsules for Advanced Textiles. ACS Appl. Mater. Interfaces 2021, 13, 13586–13595. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Li, J.; Jiang, X.; Wang, C.; Li, J.; Zhang, L.; Fu, S. Biomimetic solid–liquid transition structural dye-doped liquid crystal/phase-change-material microcapsules designed for wearable bistable electrochromic fabric. ACS Appl. Mater. Interfaces 2021, 13, 33282–33290. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yan, Q.; Wei, Z.; Chen, Y.; Chen, S.; Wang, H.; Huang, Z.; Wang, X.; Cheng, Z. Chiral photonic crystalline microcapsules with strict monodispersity, ultrahigh thermal stability, and reversible response. ACS Appl. Mater. Interfaces 2018, 10, 18289–18299. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Chen, H.; Li, A.; Zhuang, H.; Chen, Z.; Xie, Y.; Zhou, H.; Mo, S.; Chen, Y.; Lu, X. Bioinspired Multiple Stimuli-Responsive Optical Microcapsules Enabled by Microfluidics. ACS Appl. Mater. Interfaces 2020, 12, 46788–46796. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Kim, B.; Han, S.W.; Seo, M.; Choi, S.-E.; Yang, H.; Kim, S.-H.; Jeong, S.; Kim, J.W. 2-Dimensional colloidal micropatterning of cholesteric liquid crystal microcapsules for temperature-responsive color displays. J. Ind. Eng. Chem. 2018, 68, 393–398. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, S.-H. Controlled encapsulation of cholesteric liquid crystals using emulsion templates. Macromol. Res. 2018, 26, 1054–1065. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, B.; Kim, S.K.; Won, J.C.; Kim, Y.H.; Kim, S.H. Robust microfluidic encapsulation of cholesteric liquid crystals toward photonic ink capsules. Adv. Mater. 2015, 27, 627–633. [Google Scholar] [CrossRef]
- Iwai, Y.; Maeda, T.; Uchida, Y.; Araoka, F.; Nishiyama, N. Controlled Release of Photoresponsive Nematic Liquid Crystalline Microcapsules. Adv. Photonics 2021, 2, 2000079. [Google Scholar] [CrossRef]
- Hao, H.; Liu, X. Preparation and characterization of thermotropic liquid crystal microcapsules and application in textile. Fibers Polym. 2017, 18, 246–252. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, L.; Wang, D.; West, J.L.; Fu, S. Preparation of thermochromic liquid crystal microcapsules for intelligent functional fiber. Mater. Des. 2018, 147, 28–34. [Google Scholar] [CrossRef]
- Guan, Y.; Agra-Kooijman, D.M.; Fu, S.; Jákli, A.; West, J.L. Responsive Liquid-Crystal-Clad Fibers for Advanced Textiles and Wearable Sensors. Adv. Mater. 2019, 31, 1902168. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Lan, R.; Shen, C.; Zhang, Z.; Wang, Z.; Bao, J.; Wang, Z.; Zhang, L.; Hu, W.; Yu, Z. Remotely Controlling Drug Release by Light-Responsive Cholesteric Liquid Crystal Microcapsules Triggered by Molecular Motors. ACS Appl. Mater. Interfaces 2021, 13, 59221–59230. [Google Scholar] [CrossRef]
- Green, B.K.; Lowell, S. Oil-Containing Microscopic Capsules and Method of Making Them. U.S. Patent US2800457A, 23 July 1957. [Google Scholar]
- Chen, C.P.; Kim, D.S.; Jhun, C.G. Electro-optical effects of a color polymer-dispersed liquid crystal device by micro-encapsulation with a pigment-doped shell. Crystals 2019, 9, 364. [Google Scholar] [CrossRef]
- Yang, T.; Yuan, D.; Liu, W.; Zhang, Z.; Wang, K.; You, Y.; Ye, H.; de Haan, L.T.; Zhang, Z.; Zhou, G. Thermochromic cholesteric liquid crystal microcapsules with cellulose nanocrystals and a melamine resin hybrid shell. ACS Appl. Mater. Interfaces 2022, 14, 4588–4597. [Google Scholar] [CrossRef]
- Seo, H.J.; Lee, S.S.; Noh, J.; Ka, J.-W.; Won, J.C.; Park, C.; Kim, S.-H.; Kim, Y.H. Robust photonic microparticles comprising cholesteric liquid crystals for anti-forgery materials. J. Mater. Chem. C 2017, 5, 7567–7573. [Google Scholar] [CrossRef]
- Lin, P.; Wei, Z.; Yan, Q.; Chen, Y.; Wu, M.; Xie, J.; Zeng, M.; Wang, W.; Xu, J.; Cheng, Z. Blue phase liquid crystal microcapsules: Confined 3D structure inducing fascinating properties. J. Mater. Chem. C 2019, 7, 4822–4827. [Google Scholar] [CrossRef]
- Sheng, M.; Zhang, L.; Lei, Q.; Hu, A.; Li, L.; Fu, S. Dye-doped liquid crystals under confinement in microcapsules. Dye. Pigment. 2020, 180, 108544. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Zhang, Q.; Jiang, N.; Wei, J. Fabrication of cholesteric liquid crystal microcapsulates by interfacial polymerization and potential as photonic materials. RSC Adv. 2013, 3, 21620–21627. [Google Scholar] [CrossRef]
- Lv, K.; Liu, D.; Li, W.; Tian, Q.; Zhou, X. Reflection characteristics of cholesteric liquid crystal microcapsules with different geometries. Dye. Pigment. 2012, 94, 452–458. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, S.K.; Won, J.C.; Kim, Y.H.; Kim, S.H. Reconfigurable photonic capsules containing cholesteric liquid crystals with planar alignment. Angew. Chem. 2015, 127, 15481–15485. [Google Scholar] [CrossRef]
- Beltran-Gracia, E.; Parri, O. A new twist on cholesteric films by using reactive mesogen particles. J. Mater. Chem. C 2015, 3, 11335–11340. [Google Scholar] [CrossRef]
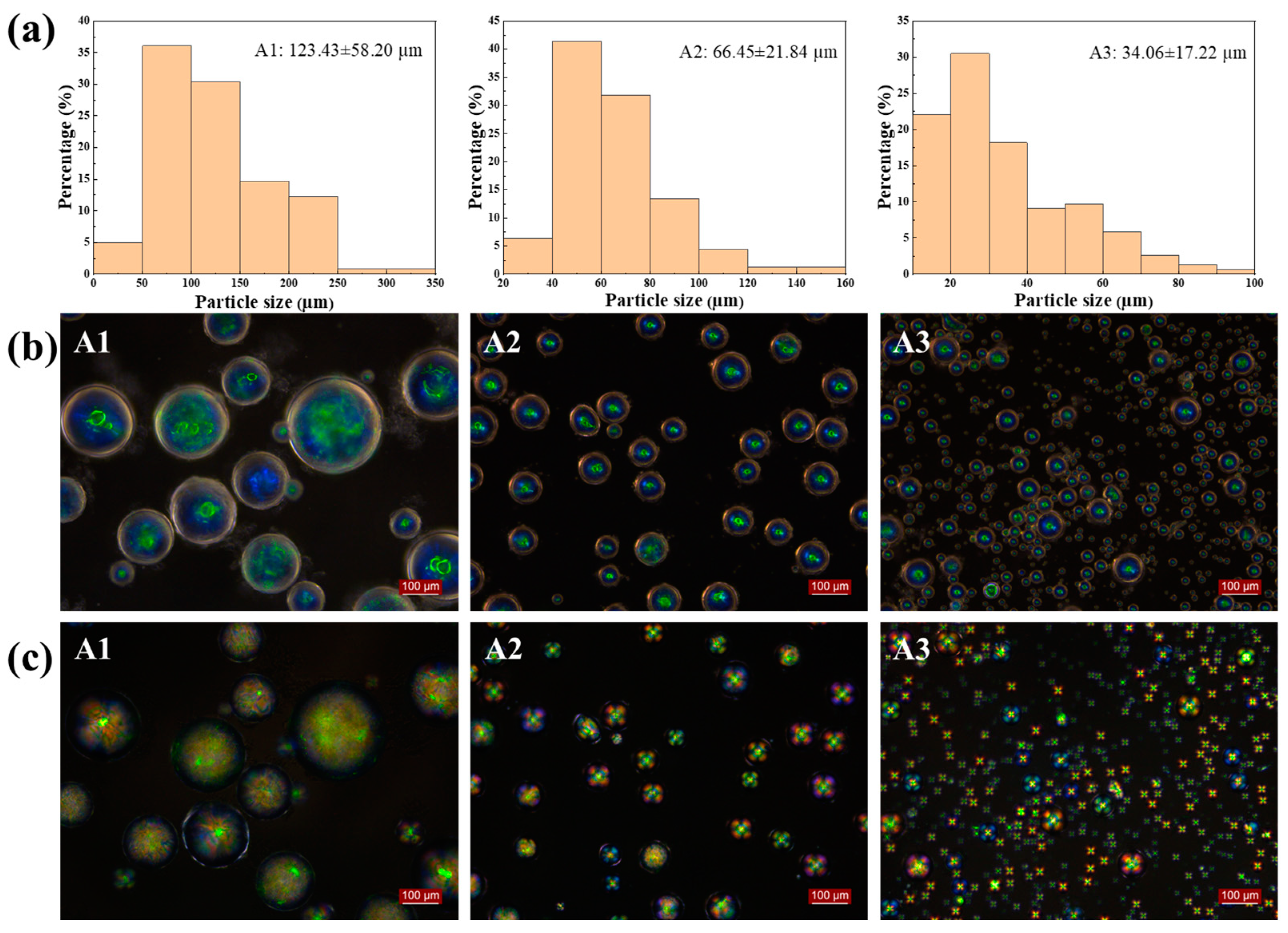
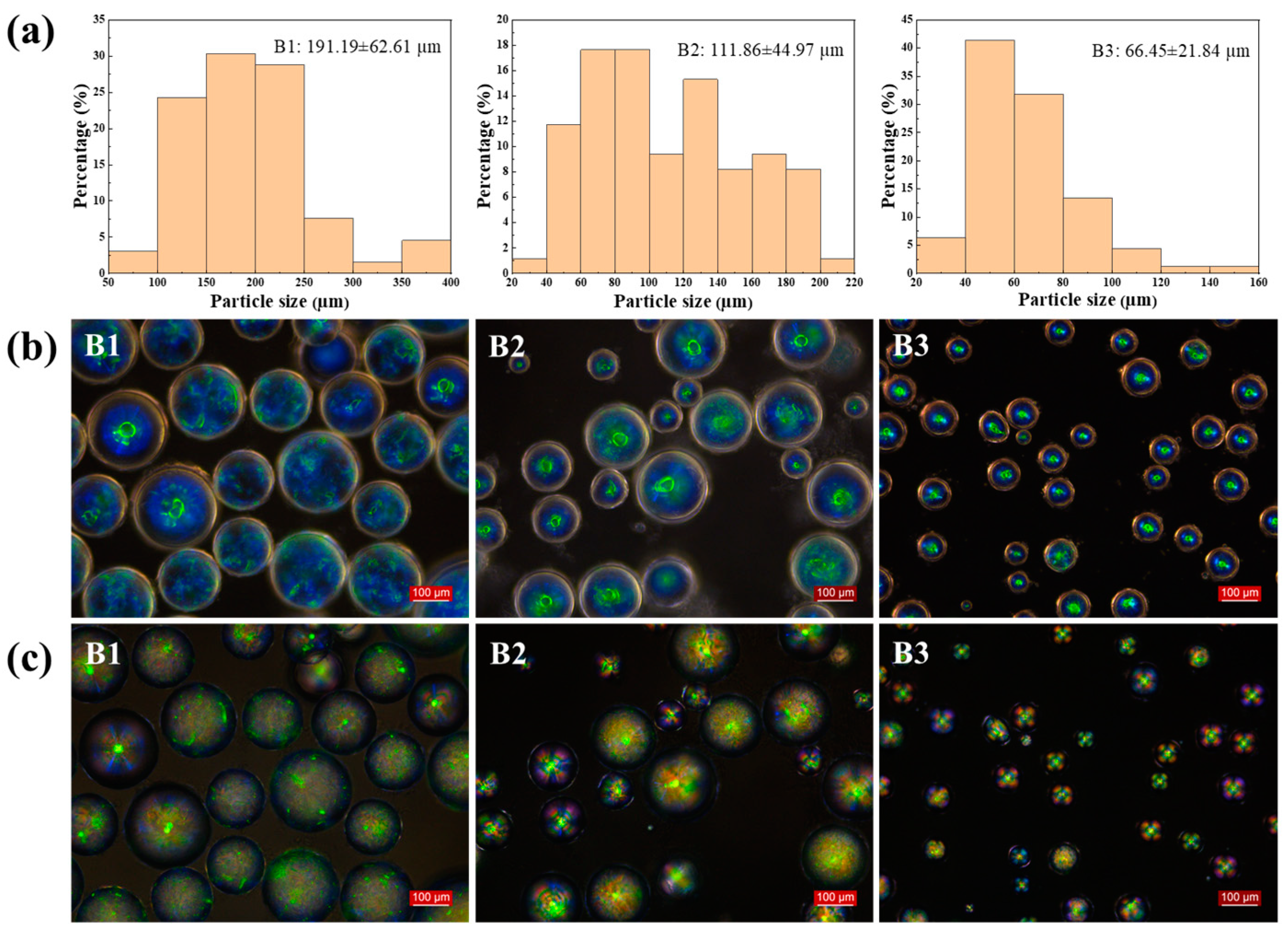
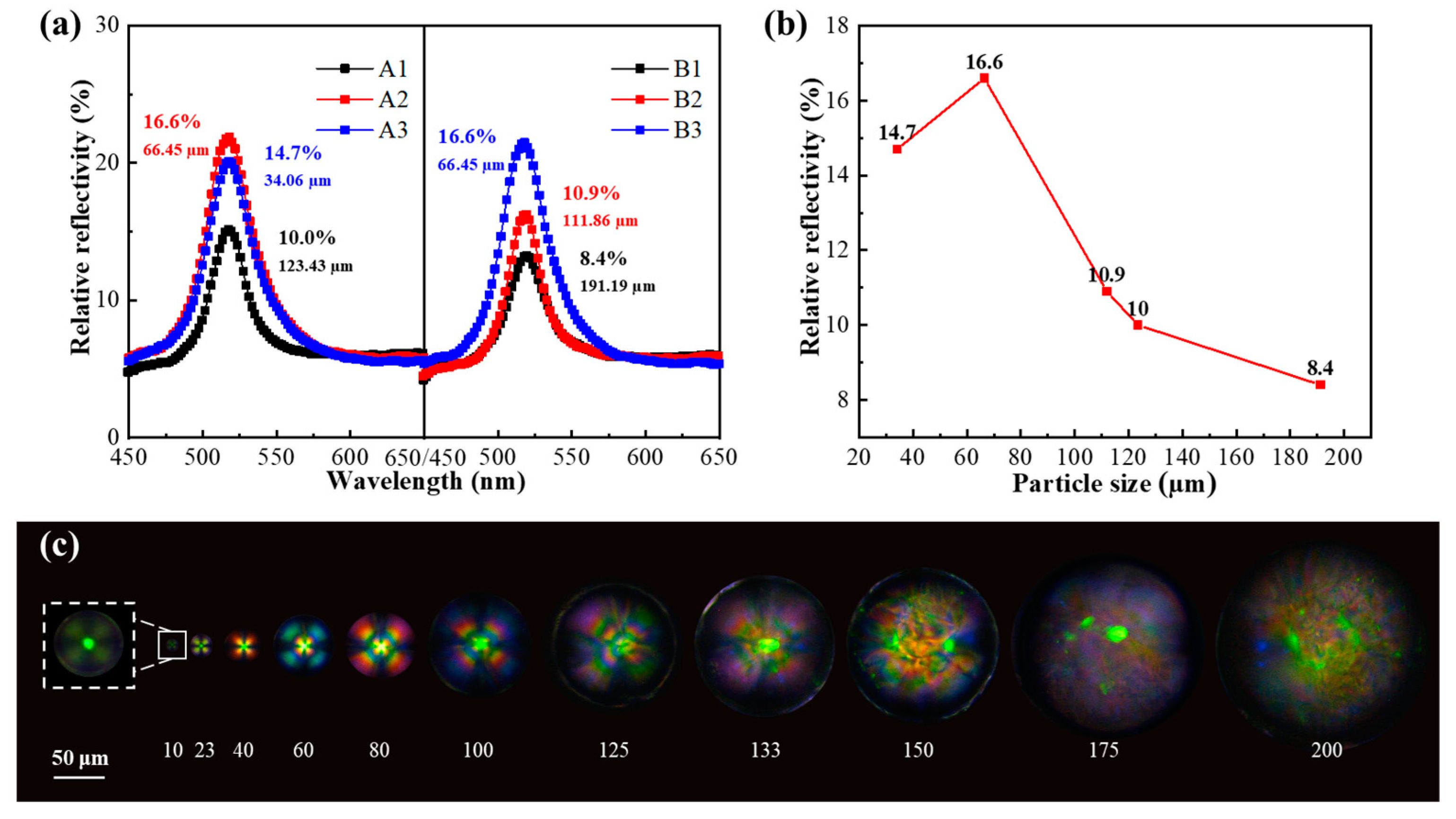

| Samples | Percentage (wt%) | Raw Material Input (g) | Spin Speed (rpm) | Rotor Size (cm) | |||
|---|---|---|---|---|---|---|---|
| 717200 | S5011 | CLC | Gelatine | Gums | |||
| A1 | 97.30 | 2.70 | 2 | 1.25 | 1.25 | 500 | 3.0 |
| A2 | 97.30 | 2.70 | 2 | 1.25 | 1.25 | 1000 | 3.0 |
| A3 | 97.30 | 2.70 | 2 | 1.25 | 1.25 | 1500 | 3.0 |
| B1 | 97.30 | 2.70 | 2 | 1.25 | 1.25 | 1000 | 2.0 |
| B2 | 97.30 | 2.70 | 2 | 1.25 | 1.25 | 1000 | 2.5 |
| B3 | 97.30 | 2.70 | 2 | 1.25 | 1.25 | 1000 | 3.0 |
| C1 | 97.30 | 2.70 | 2 | 0.80 | 0.80 | 1000 | 3.0 |
| C2 | 97.30 | 2.70 | 2 | 1.00 | 1.00 | 1000 | 3.0 |
| C3 | 97.30 | 2.70 | 2 | 1.25 | 1.25 | 1000 | 3.0 |
| C4 | 97.30 | 2.70 | 2 | 1.50 | 1.50 | 1000 | 3.0 |
| R | 97.75 | 2.25 | 2 | 1.25 | 1.25 | 1000 | 3.0 |
| G | 97.30 | 2.70 | 2 | 1.25 | 1.25 | 1000 | 3.0 |
| B | 96.90 | 3.10 | 2 | 1.25 | 1.25 | 1000 | 3.0 |
| Sample | Emulsifiers | Curing Agent |
|---|---|---|
| D1&3 | PVA 1788 | glutaraldehyde |
| D2 | - | glutaraldehyde |
| D4 | PVA 1788 | formaldehyde |
| Core | Shell | Fabrication Methods | Reflectivity | Particle Size | Testing Instrumentation | Ref. |
|---|---|---|---|---|---|---|
| RM1, RM2, NRM | Polyvinyl pyrrolidone (PVP) | Interfacial polymerization | 4% | 15 ± 10 μm | ultraviolet–visible spectroscopy (Lambda 750) | [11] |
| SLC-1717, R811 | PU | Interfacial polymerization | 35% | 9 µm | optical fiber spectrometer (AvaSpec-2048) | [32] |
| E7, S5011, S811 | MF | In situ polymerization | 35% | 10.9 µm | ultraviolet-visible spectrometer (Lambda 950) | [28] |
| R-CLC, G-CLC | PMMA | Solvent evaporation | 25% | 5–30 µm | UV/VIS/NIR spectrophotometer (UV3600) | [33] |
| BHR-59001, S811 | Silicone methacrylate | Microfluidics | 30.3% | ≈160 µm | fiber-coupled spectrometer (USB 4000) | [34] |
| JK-1001, S811 | Toluene-2,4- diisocyanate (TDI) and Tetraethylenepentamine (TEPA) | Microfluidics | 45% | ≈90 µm | UV-Vis-NIR spectrophotometer (Lambda 950) | [16] |
| CLC, chiral RM894 | RM257, RM520 | Reactive mesogen polymerization | 23–25% | 4.7 ± 2 µm | UV spectrophotometer with an integrating sphere (x-Rite color i5) | [35] |
| E7, R5011, S5011 | RM257 | Reactive mesogen polymerization | 3% | 60–180 µm | fiber-coupled spectrometer (USB 2000) | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Cui, Y.; Chen, Y.; Guo, Y.; Liu, X.; Chen, X.; Liu, J.; Liu, Y.; Liu, Z. Reflectivity and Angular Anisotropy of Liquid Crystal Microcapsules with Different Particle Sizes by Complex Coalescence. Molecules 2024, 29, 3030. https://doi.org/10.3390/molecules29133030
Yang Y, Cui Y, Chen Y, Guo Y, Liu X, Chen X, Liu J, Liu Y, Liu Z. Reflectivity and Angular Anisotropy of Liquid Crystal Microcapsules with Different Particle Sizes by Complex Coalescence. Molecules. 2024; 29(13):3030. https://doi.org/10.3390/molecules29133030
Chicago/Turabian StyleYang, Yonggang, Yuchen Cui, Yinjie Chen, Yanan Guo, Xiaoqi Liu, Xia Chen, Jianghao Liu, Yu Liu, and Zhengfeng Liu. 2024. "Reflectivity and Angular Anisotropy of Liquid Crystal Microcapsules with Different Particle Sizes by Complex Coalescence" Molecules 29, no. 13: 3030. https://doi.org/10.3390/molecules29133030
APA StyleYang, Y., Cui, Y., Chen, Y., Guo, Y., Liu, X., Chen, X., Liu, J., Liu, Y., & Liu, Z. (2024). Reflectivity and Angular Anisotropy of Liquid Crystal Microcapsules with Different Particle Sizes by Complex Coalescence. Molecules, 29(13), 3030. https://doi.org/10.3390/molecules29133030




