A Synthetic Derivative SH 66 of Homoisoflavonoid from Liliaceae Exhibits Anti-Neuroinflammatory Activity against LPS-Induced Microglial Cells
Abstract
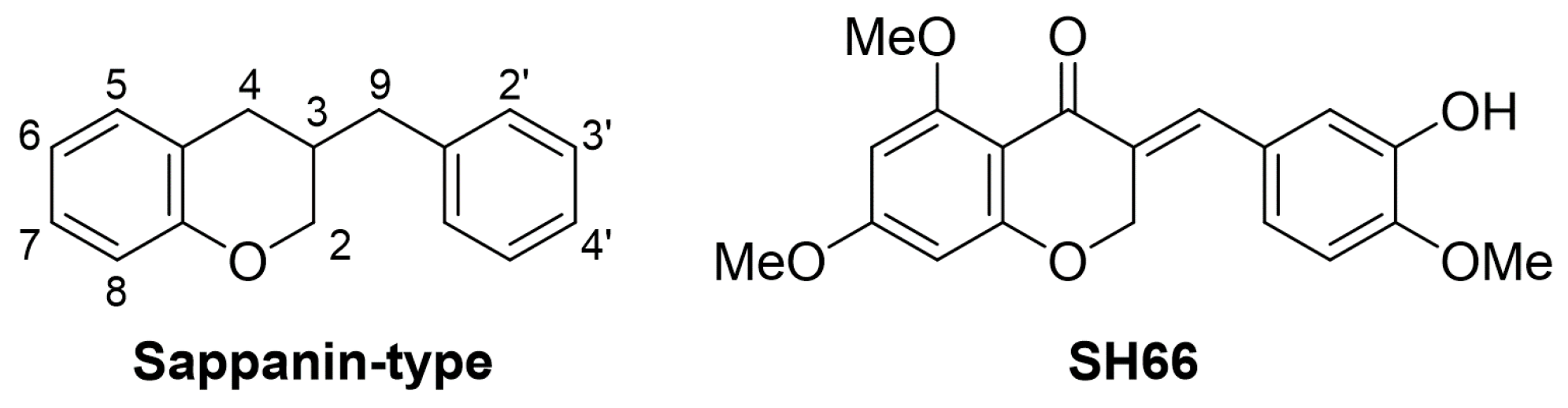
1. Introduction
2. Results
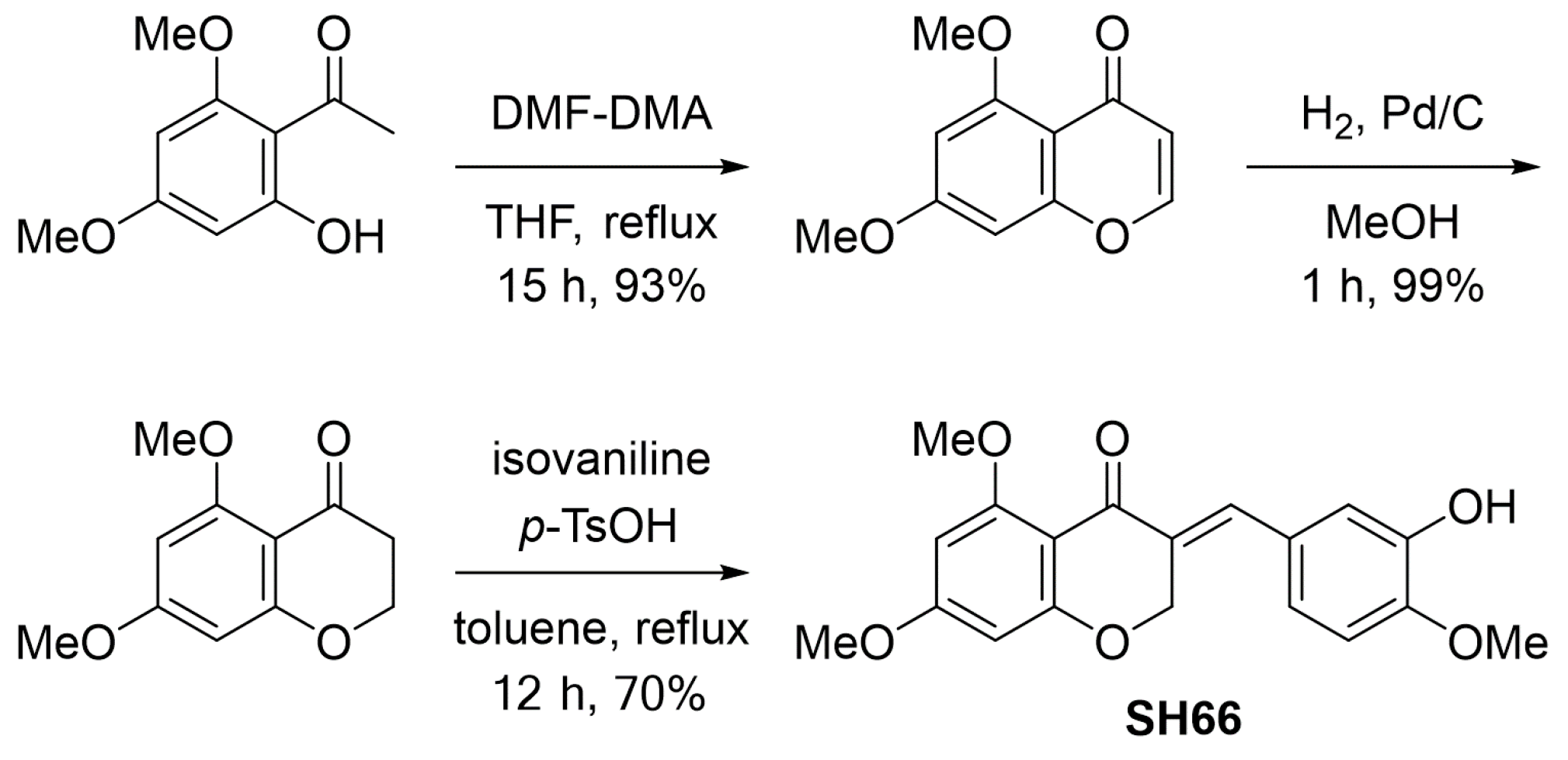
2.1. SH66 Synthesis
2.2. SH66 Lowers Nitric Oxide (NO) Production and Protein Levels of iNOS and COX-2 in TLR-Activated BV2 Microglia Cells
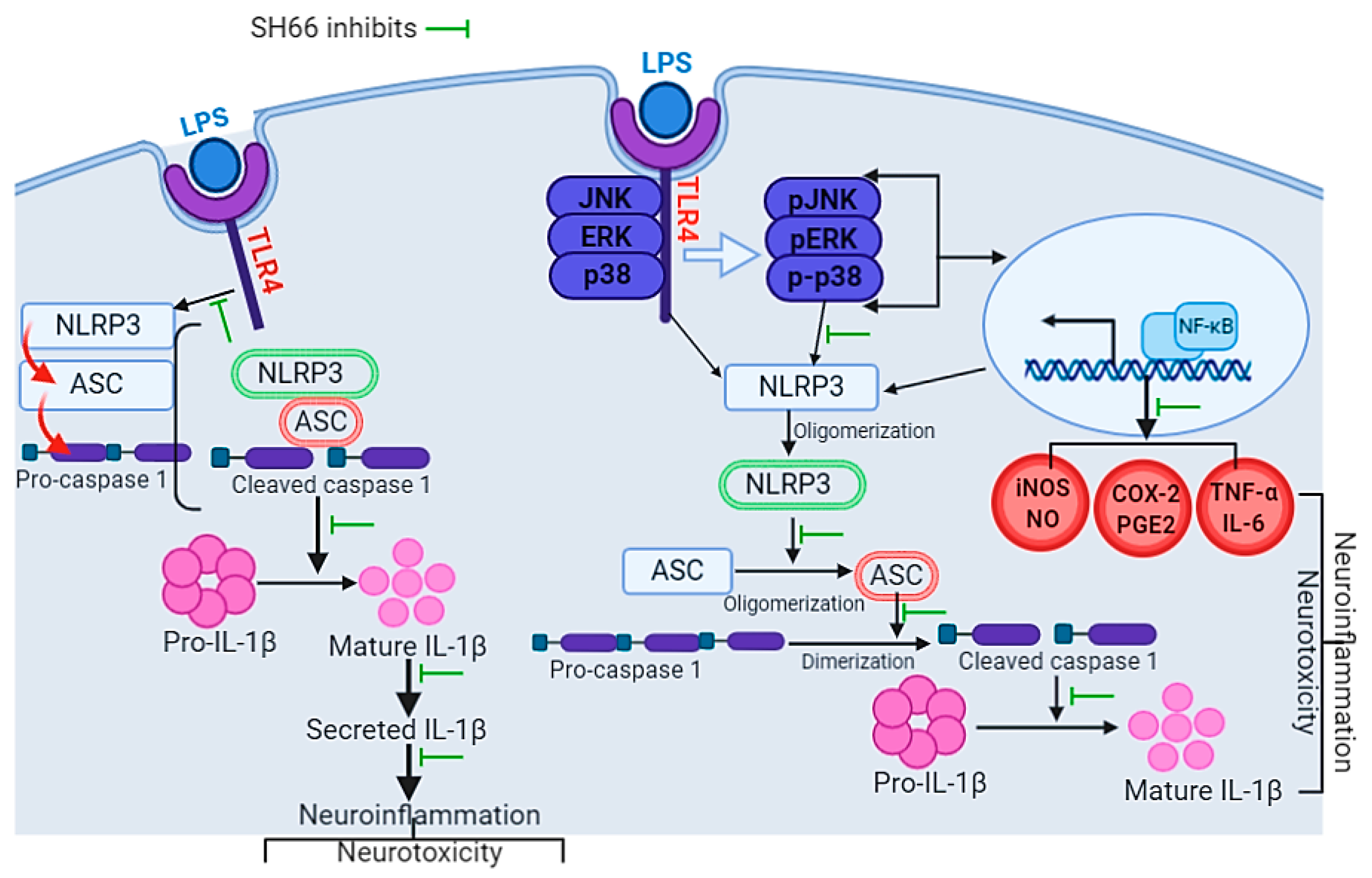
2.3. SH66 Controls MAPK-Mediated Effector Signaling to Inhibit Inflammatory Cascades in LPS-Activated BV2 Microglia Cells
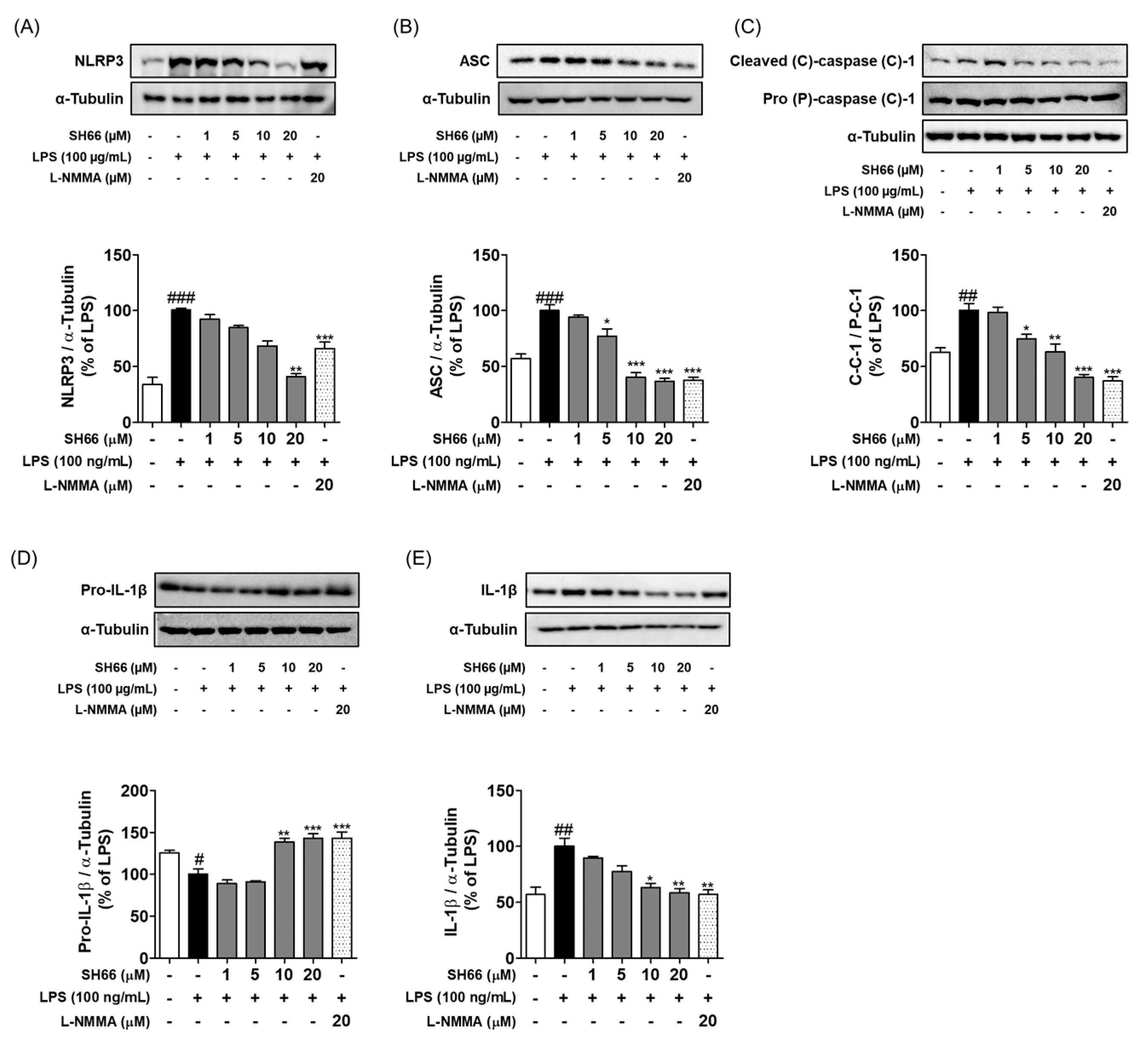
2.4. SH66 Treatment Inhibited the NLRP3 Inflammasome Complex and Subsequent IL-1β Activation in LPS-Activated BV2 Microglia Cells
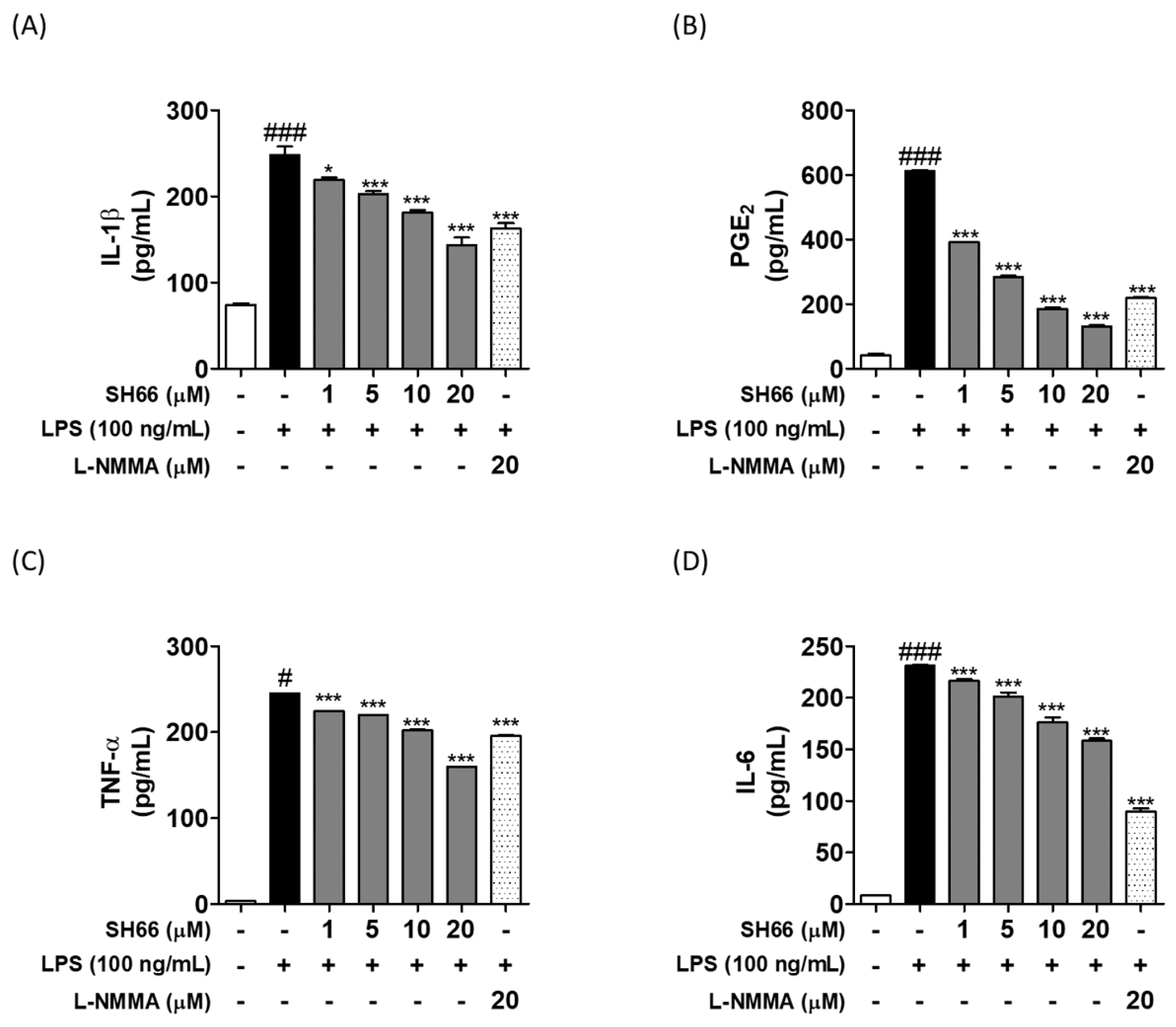
2.5. SH66 Treatment Inhibited the Production of Pro-Inflammatory Cytokines in LPS-Activated BV2 Microglia Cells
2.6. SH66 Treatment Induced Neuroprotection by Increasing the Level of Nerve Growth Factor (NGF) and Neurite Outgrowth, and Decreased Activated Microglia-Mediated Neuronal Death
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. NO Production and Cell Viability Assays
4.3. Western Blot Analysis
4.4. Preparation of Conditioned Medium (CM) from BV2 Microglia Cells
4.5. Neurite Outgrowth Assay
4.6. ELISA Kit Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Subedi, L.; Gaire, B.P. Phytochemicals as regulators of microglia/macrophages activation in cerebral ischemia. Pharmacol. Res. 2021, 165, 105419. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Stuppner, H. A Comprehensive Review on Chemotaxonomic and Phytochemical Aspects of Homoisoflavonoids, as Rare Flavonoid Derivatives. Int. J. Mol. Sci. 2021, 22, 2735. [Google Scholar] [CrossRef]
- du Toit, K.; Drewes, S.E.; Bodenstein, J. The chemical structures, plant origins, ethnobotany and biological activities of homoisoflavanones. Nat. Prod. Res. 2010, 24, 457–490. [Google Scholar] [CrossRef]
- Lin, L.G.; Liu, Q.Y.; Ye, Y. Naturally occurring homoisoflavonoids and their pharmacological activities. Planta Med. 2014, 80, 1053–1066. [Google Scholar] [CrossRef]
- Basavarajappa, H.D.; Lee, B.; Lee, H.; Sulaiman, R.S.; An, H.; Magana, C.; Shadmand, M.; Vayl, A.; Rajashekhar, G.; Kim, E.Y.; et al. Synthesis and Biological Evaluation of Novel Homoisoflavonoids for Retinal Neovascularization. J. Med. Chem. 2015, 58, 5015–5027. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.H.; Wu, J.J.; Li, F.; Cai, P.; Yang, X.L.; Kong, L.Y.; Wang, X.B. Synthesis and pharmacological evaluation of multi-functional homoisoflavonoid derivatives as potent inhibitors of monoamine oxidase B and cholinesterase for the treatment of Alzheimer’s disease. Medchemcomm 2017, 8, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Yan, W.; Gu, Z.; Li, Y.; Chen, L.; He, B. Anti-Neuroinflammatory Potential of Natural Products in the Treatment of Alzheimer’s Disease. Molecules 2023, 28, 1486. [Google Scholar] [CrossRef]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact with the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Zeng, Z.J.; Lin, X.; Yang, L.; Li, Y.; Gao, W. Activation of Inflammasomes and Relevant Modulators for the Treatment of Microglia-mediated Neuroinflammation in Ischemic Stroke. Mol. Neurobiol. 2024, 1–13. [Google Scholar] [CrossRef]
- Rodriguez-Gomez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.R.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Yang, X.; Wang, J.; Wang, Z.; Wang, Q.; Ding, Y.; Yu, A. H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer’s disease through the NFkappaB signaling pathway. J. Neuroinflamm. 2023, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.; Lee, J.H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef] [PubMed]
- Skrzypczak-Wiercioch, A.; Salat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. [Google Scholar] [CrossRef] [PubMed]
- Ayata, P.; Amit, I.; Cuda, C.M. Editorial: Microglia in neuroinflammation. Front. Immunol. 2023, 14, 1227095. [Google Scholar] [CrossRef] [PubMed]
- McManus, R.M.; Latz, E. NLRP3 inflammasome signalling in Alzheimer’s disease. Neuropharmacology 2024, 252, 109941. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Sapkota, A.; Gaire, B.P.; Choi, J.W. NLRP3 Inflammasome Activation Is Involved in LPA(1)-Mediated Brain Injury after Transient Focal Cerebral Ischemia. Int. J. Mol. Sci. 2020, 21, 8595. [Google Scholar] [CrossRef] [PubMed]
- Damodar, K.; Lee, J.T.; Kim, J.K.; Jun, J.G. Synthesis and in vitro evaluation of homoisoflavonoids as potent inhibitors of nitric oxide production in RAW-264.7 cells. Bioorganic Med. Chem. Lett. 2018, 28, 2098–2102. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.Y.; Zeng, K.W.; Zhang, L.; Che, Y.Y.; Tu, P.F. Anti-inflammatory homoisoflavonoids from the tuberous roots of Ophiopogon japonicus. Fitoterapia 2012, 83, 1042–1045. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, S.; Heo, M.; Lee, B.; Fei, X.; Corson, T.W.; Seo, S.Y. Total Synthesis of Naturally Occurring 5,7,8-Trioxygenated Homoisoflavonoids. ACS Omega 2020, 5, 11043–11057. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Han, B.; Hai, Y.; Liu, Y.; Liu, X.; Yang, J.; Sun, D.; Yin, P. The Role of Microglia/Macrophages Activation and TLR4/NF-kappaB/MAPK Pathway in Distraction Spinal Cord Injury-Induced Inflammation. Front. Cell Neurosci. 2022, 16, 926453. [Google Scholar] [CrossRef] [PubMed]
- Pike, A.F.; Varanita, T.; Herrebout, M.A.C.; Plug, B.C.; Kole, J.; Musters, R.J.P.; Teunissen, C.E.; Hoozemans, J.J.M.; Bubacco, L.; Veerhuis, R. alpha-Synuclein evokes NLRP3 inflammasome-mediated IL-1beta secretion from primary human microglia. Glia 2021, 69, 1413–1428. [Google Scholar] [CrossRef]
- Subedi, L.; Ji, E.; Shin, D.; Jin, J.; Yeo, J.H.; Kim, S.Y. Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro. Nutrients 2017, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Abdo Qaid, E.Y.; Abdullah, Z.; Zakaria, R.; Long, I. Minocycline mitigates tau pathology via modulating the TLR-4/NF-small ka, Cyrillicbeta signalling pathway in the hippocampus of neuroinflammation rat model. Neurol. Res. 2024, 46, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Bao, K.; Zhou, X.; Deng, Y.; Li, X.; Zhang, J.; Lan, X.; Zhao, J.; Lu, D.; Xu, Y.; et al. PSMC5 regulates microglial polarization and activation in LPS-induced cognitive deficits and motor impairments by interacting with TLR4. J. Neuroinflamm. 2023, 20, 277. [Google Scholar] [CrossRef] [PubMed]
- Fann, D.Y.; Lim, Y.A.; Cheng, Y.L.; Lok, K.Z.; Chunduri, P.; Baik, S.H.; Drummond, G.R.; Dheen, S.T.; Sobey, C.G.; Jo, D.G.; et al. Evidence that NF-kappaB and MAPK Signaling Promotes NLRP Inflammasome Activation in Neurons Following Ischemic Stroke. Mol. Neurobiol. 2018, 55, 1082–1096. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Al Kury, L.T.; Atzaz, N.; Alattar, A.; Alshaman, R.; Shah, F.A.; Li, S. Carvacrol Alleviates Hyperuricemia-Induced Oxidative Stress and Inflammation by Modulating the NLRP3/NF-kappaB Pathwayt. Drug Des. Dev. Ther. 2022, 16, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Liu, C.C.; Li, Y.S.; Lee, P.Y.; Liu, P.L.; Wu, P.C.; Lin, T.C.; Chen, C.S.; Chiu, C.C.; Lai, Y.H.; et al. Punicalagin Attenuates LPS-Induced Inflammation and ROS Production in Microglia by Inhibiting the MAPK/NF-kappaB Signaling Pathway and NLRP3 Inflammasome Activation. J. Inflamm. Res. 2022, 15, 5347–5359. [Google Scholar] [CrossRef]
- Huang, L.; Gong, L.; Huo, X.; Lei, L.; Zhang, Q.; Hu, Y.; Kuang, Q.; Gui, Y.; Dai, Y.; Gu, Y.; et al. N-acetyldopamine dimer inhibits neuroinflammation through the TLR4/NF-kappaB and NLRP3/Caspase-1 pathways. Acta Biochim. Biophys. Sin. 2022, 55, 23–33. [Google Scholar]
- Hara, H.; Tsuchiya, K.; Kawamura, I.; Fang, R.; Hernandez-Cuellar, E.; Shen, Y.; Mizuguchi, J.; Schweighoffer, E.; Tybulewicz, V.; Mitsuyama, M. Phosphorylation of the adaptor ASC acts as a molecular switch that controls the formation of speck-like aggregates and inflammasome activity. Nat. Immunol. 2013, 14, 1247–1255. [Google Scholar] [CrossRef]
- Araki, T.; Ikegaya, Y.; Koyama, R. The effects of microglia- and astrocyte-derived factors on neurogenesis in health and disease. Eur. J. Neurosci. 2021, 54, 5880–5901. [Google Scholar] [CrossRef]
- Gaire, B.P.; Kwon, O.W.; Park, S.H.; Chun, K.H.; Kim, S.Y.; Shin, D.Y.; Choi, J.W. Neuroprotective effect of 6-paradol in focal cerebral ischemia involves the attenuation of neuroinflammatory responses in activated microglia. PLoS ONE 2015, 10, e0120203. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsuzzaman, M.; Subedi, L.; Hong, S.-M.; Lee, S.; Gaire, B.P.; Ko, E.-J.; Choi, J.-W.; Seo, S.-Y.; Kim, S.-Y. A Synthetic Derivative SH 66 of Homoisoflavonoid from Liliaceae Exhibits Anti-Neuroinflammatory Activity against LPS-Induced Microglial Cells. Molecules 2024, 29, 3037. https://doi.org/10.3390/molecules29133037
Samsuzzaman M, Subedi L, Hong S-M, Lee S, Gaire BP, Ko E-J, Choi J-W, Seo S-Y, Kim S-Y. A Synthetic Derivative SH 66 of Homoisoflavonoid from Liliaceae Exhibits Anti-Neuroinflammatory Activity against LPS-Induced Microglial Cells. Molecules. 2024; 29(13):3037. https://doi.org/10.3390/molecules29133037
Chicago/Turabian StyleSamsuzzaman, Md, Lalita Subedi, Seong-Min Hong, Sanha Lee, Bhakta Prasad Gaire, Eun-Ji Ko, Ji-Woong Choi, Seung-Yong Seo, and Sun-Yeou Kim. 2024. "A Synthetic Derivative SH 66 of Homoisoflavonoid from Liliaceae Exhibits Anti-Neuroinflammatory Activity against LPS-Induced Microglial Cells" Molecules 29, no. 13: 3037. https://doi.org/10.3390/molecules29133037
APA StyleSamsuzzaman, M., Subedi, L., Hong, S.-M., Lee, S., Gaire, B. P., Ko, E.-J., Choi, J.-W., Seo, S.-Y., & Kim, S.-Y. (2024). A Synthetic Derivative SH 66 of Homoisoflavonoid from Liliaceae Exhibits Anti-Neuroinflammatory Activity against LPS-Induced Microglial Cells. Molecules, 29(13), 3037. https://doi.org/10.3390/molecules29133037








